Abstract
Introduction:
Traumatic brain injury (TBI) is a leading cause of mortality, morbidity, disability, and socioeconomic losses in the Indian subcontinent. However, for policymaking and research, there is a lack of reliable and larger data in our settings.
Aims and Objectives:
To evaluate and describe the epidemiological, clinical characteristics, and outcomes of patients with TBI in a Level 1 Trauma Center in India.
Materials and Methods:
In this retrospective study, all patients with moderate or severe TBI, based on emergency department Glasgow Coma Scale, admitted to neurosurgery Intensive Care Unit (ICU) during May 2010–July 2012 were evaluated to provide detailed information on TBI-related variables and outcomes using descriptive statistics.
Results:
Among the 1527 patients with moderate or severe TBI patients with mean age 32.15 ± 16.76 years (range: 1–90) and male:female ratio 6.5:1, 1281 (83.89%) had severe TBI. The majority of cases took place in the age group of 21–40 years (50.24%) with the most common mode of injury as road traffic accidents (RTAs) (64.96%). Surgical intervention (craniotomy) was done in 49.12% of patients. About 34.58% (n = 528) patients died in hospital, and 67.21% (n = 701) had unfavorable outcome at 6 months.
Conclusions:
This is the first study of its kind from the Indian subcontinent that gives data on the admission characteristics, mortality, and 6 months outcome of such patients. Most of the injuries occurred due to RTAs, more common among the economic productive age groups and mostly in males with a high rate of mortality and unfavorable outcome.
Keywords: Epidemiology, Glasgow Outcome Scale, mortality, outcome, traumatic brain injury
Introduction
Traumatic brain injury (TBI), a significant public health problem, is a leading cause of disability and mortality in all regions of the globe despite advancement in prevention and treatments. Its global incidence is rising, and it is predicted to surpass many diseases as a major cause of death and disability by the year 2020.[1] TBI is the main cause of one-third to one-half of all trauma deaths and the leading cause of disability in people under forty, severely disabling 15–20/100,000 populations per year.[2] The World Health Organization estimates that almost 90% of deaths due to injuries occur in low- and middle-income countries (LAMICs), where 85% of population live, and this situation will continue to represent an important global health problem in the upcoming years.[3,4]
TBI is a leading cause of mortality, morbidity, disability, and socioeconomic losses in India as well. It is estimated that nearly 1.5–2 million persons are injured, and 1 million die every year in India.[5] India and other developing countries are facing the major challenges of prevention, prehospital care, and rehabilitation in their rapidly changing environments to reduce the burden of TBIs. For policymaking, there is a lack of reliable and larger data regarding TBI in our settings.[6]
The importance of continuous evaluation of TBI-related epidemiology and outcome are emphasized by trends as described in this study. This study was aimed to evaluate and describe the epidemiological, clinical characteristics, and outcomes of TBI patients admitted to Intensive Care Unit (ICU) of India's largest tertiary care, Level 1 Trauma Center, namely, Jai Prakash Narayan Apex Trauma Centre (JPNATC), All India Institute of Medical Sciences (AIIMS), New Delhi by collection of detailed data on demography, clinical, injury patterns, management, laboratory, and outcome of moderate or severe TBI.
Materials and Methods
Design
In this retrospective study, we considered all patients with moderate or severe TBI admitted to the Department of Neurosurgery of JPNATC, AIIMS during May 19, 2010–July 31, 2012, to provide detailed information on TBI-related variables and outcomes.
Patients, study site, inclusion, and exclusion criteria
Inclusion criteria
The patients whose admission Glasgow Coma Scale (GCS) ≤12 in the emergency department (ED) at JPNATC were included in the study.
Exclusion criteria
The patients who were not admitted to the ICU under the Department of Neurosurgery were excluded from the study. Presently, JPNATC, AIIMS, New Delhi, a largest full-fledged integrated Level I trauma center in the Indian subcontinent, has 30 triage and 36 ICU beds, and it is the apex referral center for TBI in New Delhi as well as for much of the other parts of the country.
Study variables, outcomes, and operational definitions
Clinical injury severity was based on the postresuscitations GCS score at admission to ED and defined as severe (GCS 3–8) or moderate (GCS 9–12). Duration before admission (hours)was the period from the time of injury to arrival at the ED of study hospital. Hypotension was considered to be present when a patient had systolic blood pressure below 90 mmHg at least for once within first 24 h of admission.
In this study, the two outcomes “in-hospital mortality” and “unfavorable functional outcome” at 6 months postadmission based on the Glasgow Outcome Scale (GOS) were measured. The GOS is a global scale for functional outcome in TBI that rates patient status into one of the five categories: (1) Dead, (2) vegetative state, (3) severe disability, (4) moderate disability, or (5) good recovery. The 6 months GOS was further dichotomized into favorable outcome (moderate disability, good recovery, or GOS = 4, 5) and unfavorable outcome (death, persistent vegetative state and severe disability, or GOS = 1, 2, 3). The 6 months follow-up data were collected through the telephonic interview of patients or their caregivers.
There were two sources of data collection: Computerized patient record system (soft copy) and patient's medical record file (hard copy). Trained research nursing and other clinical staffs including medical doctors maintained these both sources. Under the supervision of neurosurgeon, Dr. Deepak Agrawal, author Vineet Kumar Kamal collected all study variables from both these existing sources and through telephonic interview in case of 6 months follow-up outcome retrospectively in a prefixed pro forma for all treated patients enrolled during the study period. Later on, a library was created in software EPI Info 7.1.2, Centers for Disease Control and Prevention (CDC), Atlanta, Georgia (USA) for entry of collected data.
Guideline compliance
All patients were treated in ED as per ATLS protocol before being admitted to neurosurgery. Furthermore, they were treated and evaluated according to the “Guidelines for the Management of Severe Head Injury.”[7] BTF guideline compliance was assessed for intubation and intracranial pressure (ICP) monitoring. Monitoring of ICP is advised in patients with severe TBI when a computed tomography (CT) shows intracranial pathology, or in the presence of two or more of the following criteria: Age >40 years, unilateral or bilateral motor posturing (i.e., motor score ≤3), or systolic blood pressure <90 mmHg.[8] However, ICP monitors were placed variably at the discretion of the admitting neurosurgeon as it is standard practice at this trauma center.
Protocol approval
The Institute Ethics Committee/Institute Ethics Sub-committee approved this study (IESC/T-226/01.06.2012).
Statistical analysis
All the continuous and categorical variables were expressed in mean ± standard deviation (SD) (range: minimum-maximum)/median (interquartile range: 25–75th percentile) and frequency (%), respectively. We analyzed and summarized our data using descriptive statistics. All the data analysis was done using software Stata 12.1, StataCorp, 4905 Lakeway Drive, College Station, Texas 77845 USA.
Results
Demographic and clinical characteristics
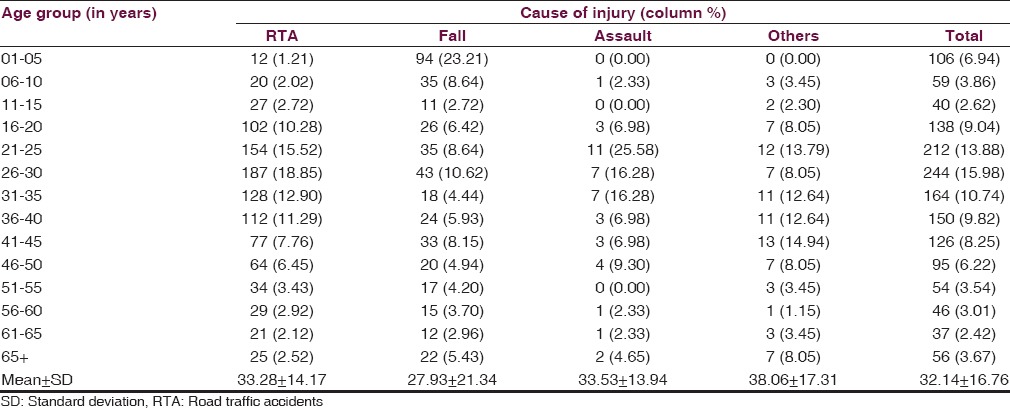
A total of 1527 patients were enrolled during the study period in which 86.64% were male with a mean ± SD age of 32.15 ± 16.76 years (median: 30, range: 1–90). Of these, based on postresuscitation GCS, 1281 (83.89%) patients had severe TBI [Table 1]. As per this study, males were at a higher risk with a male:female ratio of 6.5:1. The age distribution of patients with moderate or severe TBI revealed that the highest occurrence was in the age group of 21–30 years (29.68%), followed by 31–40 years (18.07%) [Figure 1]. On an average, 58 patients with moderate or severe TBI admitted at JPNATC, AIIMS every month. The most common mode of injury was road traffic accidents (RTAs), which accounted for about 64.96% of the patients, followed by falls (26.52%), others mode of injury (5.70%), and assaults (2.82%) [Table 1]. Table 2 depicted the age distribution of individuals sustaining the moderate or severe TBI in a traffic environment. Among the injured, road traffic injuries increased from 15 years, reached a peak in 21–30 years, gradually declined thereafter. Out of 992 RTAs, nearly 849 (85.58%) were patients with severe TBI [Table 1]. Information on age and fall-related injuries was given in Table 2. Falls occurred in greater numbers among the age group of ≤5 years (23.21%).
Table 1.
Demographic and clinical characteristics at admission
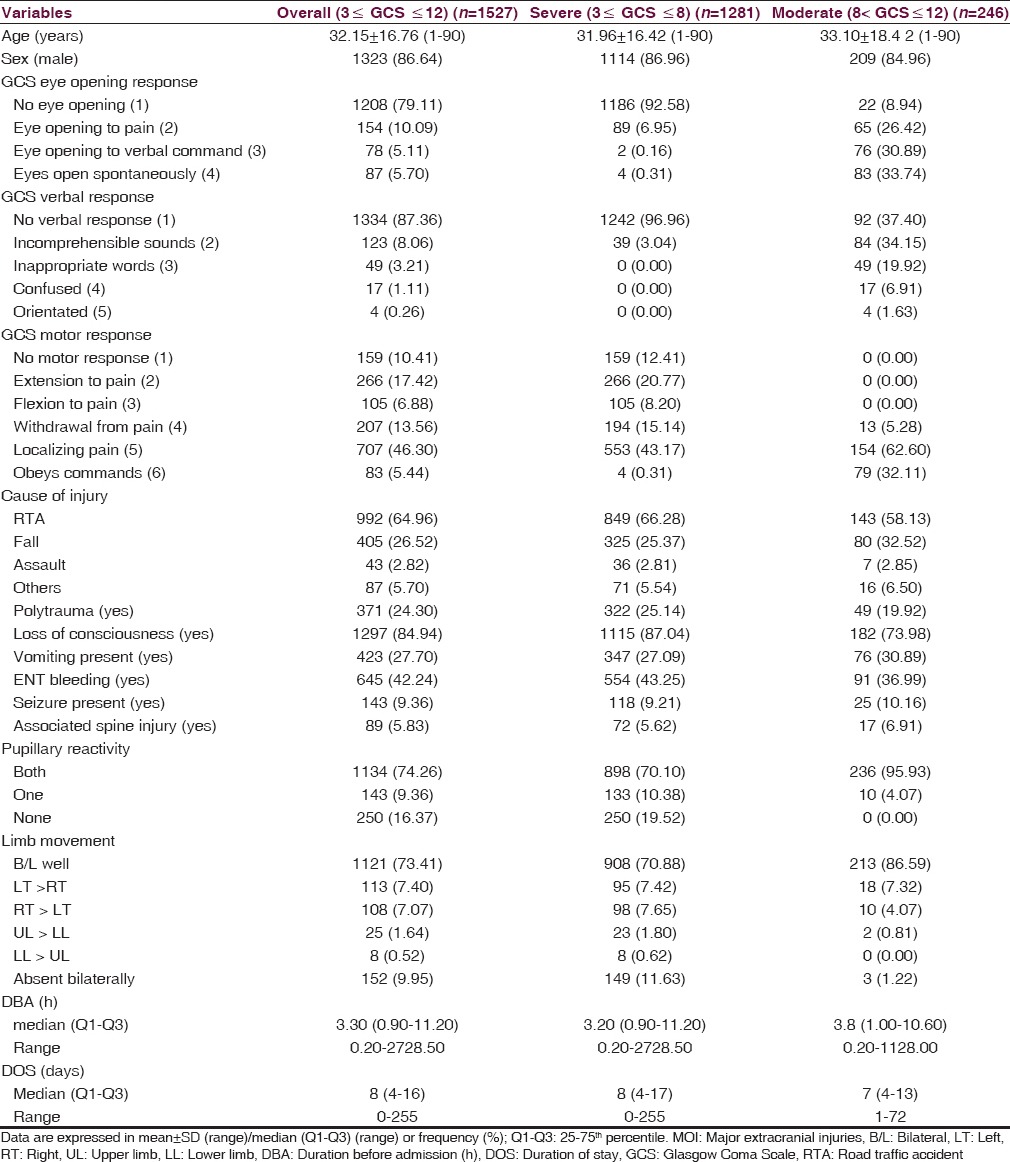
Figure 1.
Age and sex distribution of patients with moderate or severe traumatic brain injury
Table 2.
Age distribution according to causes of injuries
In addition to TBIs, nearly one-fourth patients (24.30%) had polytrauma or severe injuries to various parts of the body or major extracranial injuries. These included facial injuries, chest injuries, abdominal injuries, injuries to upper and lower limb, etc., [Table 1]. Time of the occurrence of brain injury was another crucial determinant reflecting distribution patterns in Figure 2. Highest numbers of RTA leading to severe or moderate TBI were seen between 6 pm and 12 am constituted 32% of such patients. The overall median duration of hospital stay days was 8 (range: 0–255, interquartile range: 4–16) [Table 1].
Figure 2.
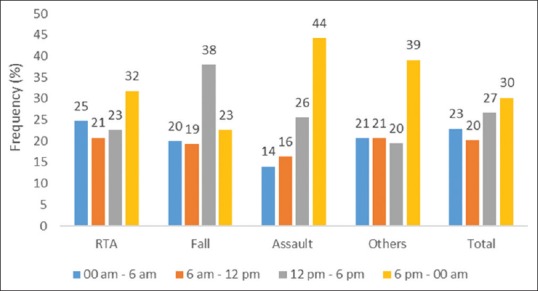
Distribution of cause of injury and time of occurrence of injury
Physiological characteristics and computed tomography findings
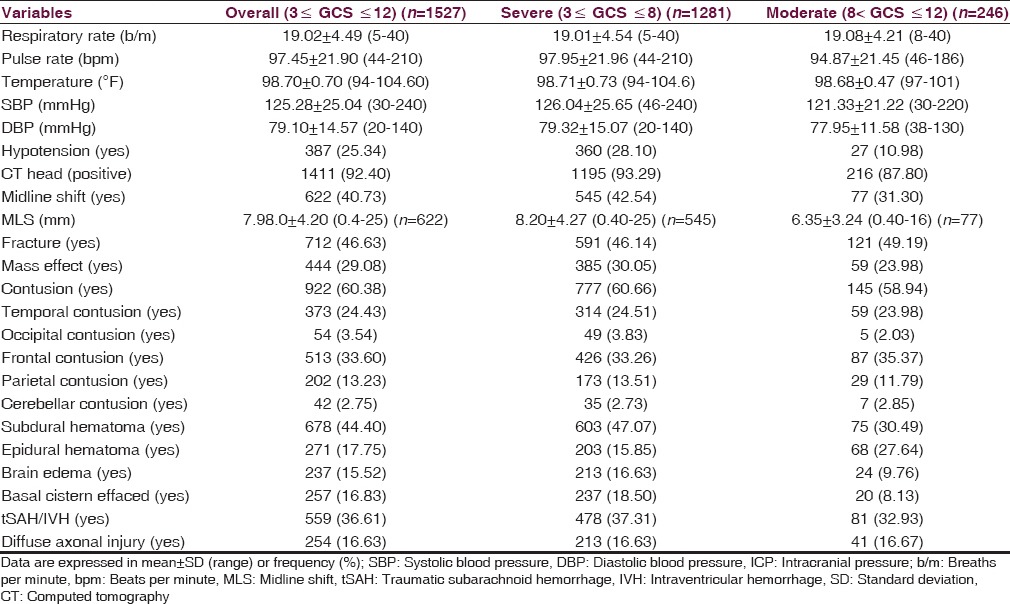
Table 3 depicted the physiological and CT characteristics at admission. Episodes of hypotension during the first 24 h of admission were present in 25.34% of all patients. This proportion was higher in patients with severe brain injury (28.10%) as compared to moderate brain injury (10.98%). Brain contusion and fracture were the most common type of intracranial pathology, detected in 60.38% (brain contusion), and 46.63% (fracture) of the patients. Traumatic subarachnoid hemorrhage or intraventricular hemorrhage and compressed or absent basal cistern was found positive in 559 (36.61%) and 257 (16.83%) patients, respectively.
Table 3.
Physiological characteristics and computed tomography scan findings at admission
Patient management and laboratory findings
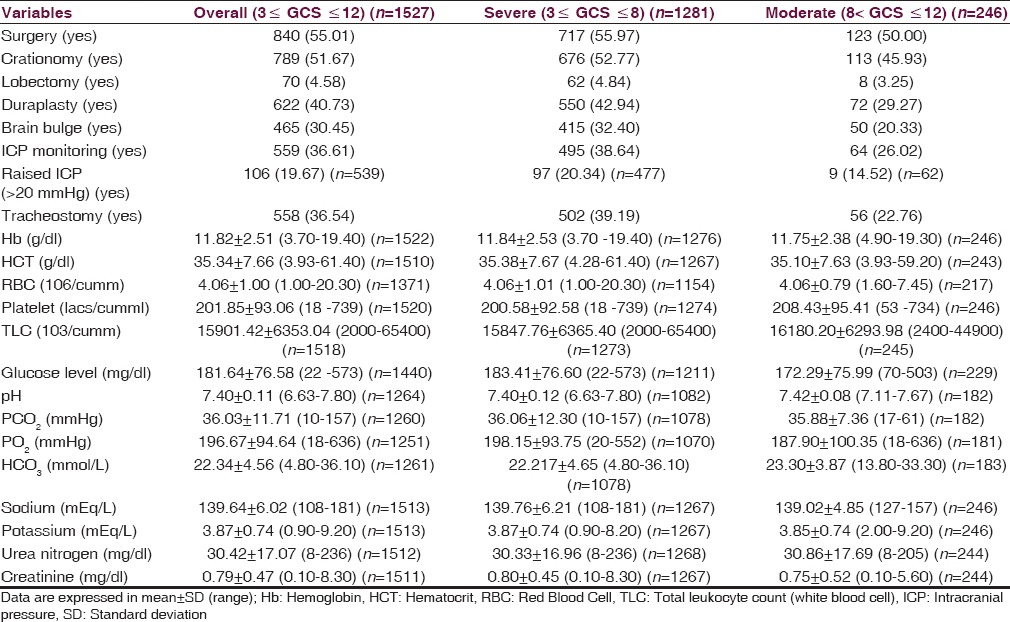
Table 4 described the general characteristics of management and laboratory-related variables in overall, severe, and moderate TBI patients. Of the patients admitted to the ICU, an ICP monitoring device using parenchymal sensor was placed in 495 patients (38.64%) with severe TBI and 64 patients (26.02) with moderate TBI, of whom 20.34% with severe TBI and 14.52% with moderate TBI had raised ICP (>20 mmHg). During the admission period, 55.01% (n = 840) of patients underwent a neurosurgical procedure, of whom 93.93% (789 of 840) involved decompressive craniotomy. Tracheostomy was done in 558 patients (36.54%), more for severe (39.19%) as compared to moderate (22.76%). The mean value of glucose level was higher in severe TBI patients as compared to moderate TBI patients (183.41 ± 76.60 vs. 172.29 ± 75.99).
Table 4.
Management-related characteristics and laboratory variables at admission
Outcome(s)
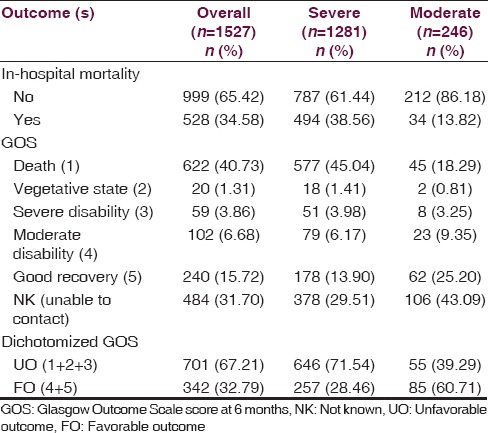
Table 5 gave the description about outcomes in overall, severe, and moderate TBI patients. Overall, 34.58% (n = 528) patients died in hospital, and in-hospital mortality rate was higher in severe TBI (38.56%) as compared to moderate (13.82%). The functional outcome according to the GOS at 6 months postadmission was available in 1043 patients as 484 (31.70%) patients were lost to follow-up. Among those who survived, 40.73% died before 6 months, either in the course of hospital stay or after discharging from hospital, 3.86% were severely disabled, and 15.72% had a good recovery.
Table 5.
Outcomes in sever and moderate traumatic brain injury patients
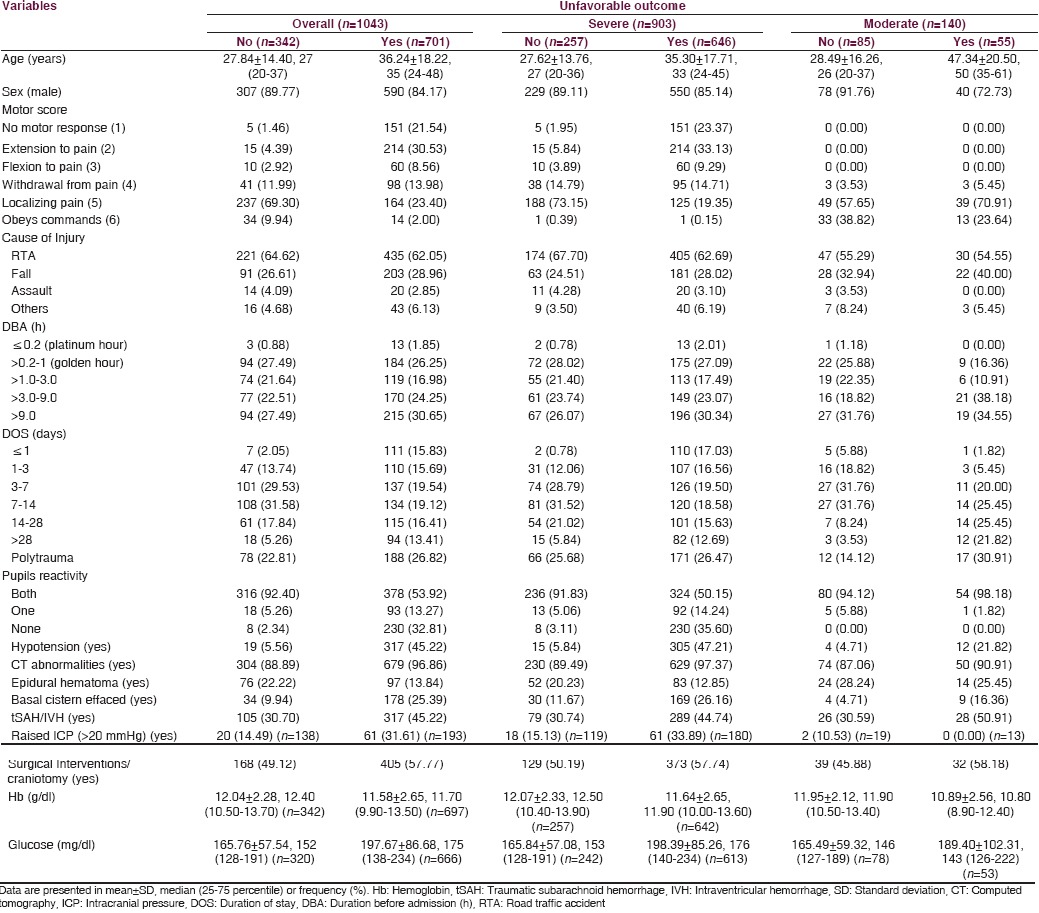
Tables 6 and 7 described the distribution of some of the important factors with respect to the outcome(s) in overall, severe, and moderate TBI. Only 1.70% and 29.03% patients in case of in-hospital mortality [Table 6] and only 0.88% and 27.49% patients in case of unfavorable outcome at 6 months [Table 7] reached hospital within platinum hour and golden hour postinjury, respectively. Most of the cases (23.86%) among those who died during hospital stay were stayed for 3–7 days, in which 23.48% and 29.41% with severe and moderate TBI, respectively. Nearly one-fourth patients had polytrauma in case of in-hospital mortality or unfavorable outcome at 6 months. Among those who died in hospital, surgical intervention was done in 56.25% cases and in the case of unfavorable outcome at 6 months, it was done in 57.77% cases.
Table 6.
Distribution of factors with respect to in-hospital mortality
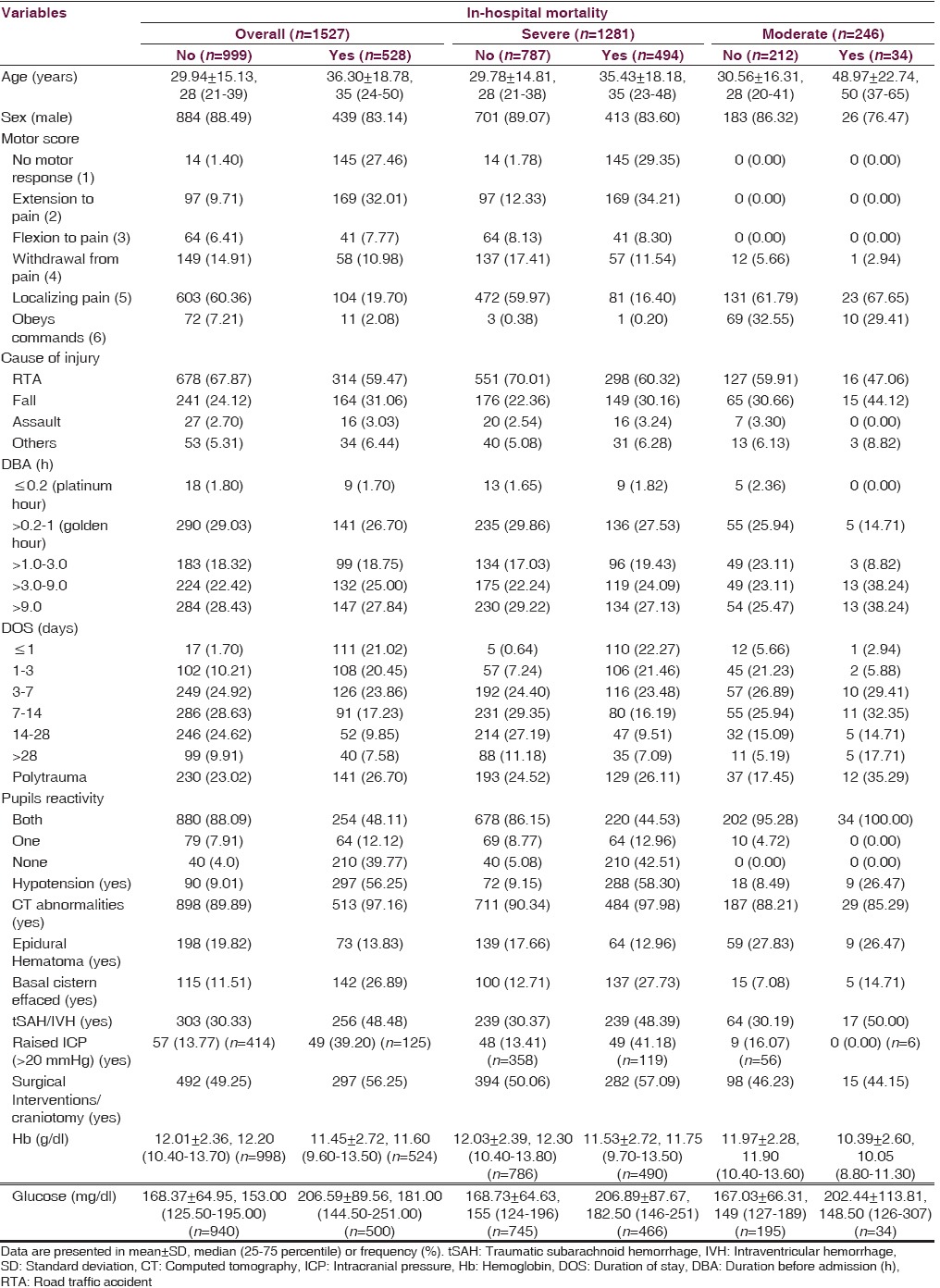
Table 7.
Distribution of factors with respect to unfavorable outcome at 6 months
Discussion
India is unenviable destination of having the highest rate of TBI's in the world. About 100,000 lives are lost every year with over 1 million suffering from serious TBIs in India.[9] The importance of the problem of TBI is always underestimated due to the lack of research and good quality data in India. This study was conducted to bridge this gap. We undertook a retrospective study of TBI admitted to ICU of the largest tertiary care, Level 1 Trauma Center of the Indian subcontinent to evaluate admission characteristics and outcome(s) of 1527 consecutive patients. According to this study, TBI predominated in young men in the form of severe TBI and mostly occurred due to RTAs. A very large number of patients with moderate or severe TBI were being admitted to ICU of JPNATC, AIIMS, and these admissions were not affected with seasonal variation. These results can also be interpreted in the context of a very low degree of public health awareness about vehicular trauma, decreased legislation regarding violations for speeding, jumping red lights, restraining devices, helmets, and drink-driving. Findings of this study suggested the high-resource requirements associated with patients of moderate and severe TBI. Patients with brain injuries, especially severe TBI, had extended ICU and hospital stays. A high proportion of patients were treated with immediate surgery, mechanical ventilation, invasive monitoring, and tracheotomy. These observations deal with the burden of injury associated with TBI in India.
The male:female ratio was 6.5:1, and this figure was also observed in other Indian studies.[5,10] The age distribution of TBIs across various Indian studies including present study found that moderate and severe injury mostly affected young men and mostly due to RTAs.[5,10] Mean age in this study was 32.15 years (31.96 years in severe and 33.10 years in moderate TBI), which was in the line of reported mean age in some previous studies in India and other developing countries, but it was far lower than the reported mean age of moderate and severe TBI patients of some developed nations.[5,10,11,12,13,14] As a result of the aging population of developed nations, the falls were suggested as the frequent emerging cause of injury.[14] However, being a world's largest youthful country, our study indicated that road traffic injuries are the leading cause of moderate and severe TBIs followed by falls. Almost similar distribution was reported in various parts of the country as well as in other parts of the world.[5,10,11,12,14,15] In this study, the second leading cause was falls, and more proportion was observed in children aged < 5 years. Other Indian studies have also highlighted the increasing role of fall, especially in children.[5,10] Surgical intervention (craniotomy) was performed in about 53% of severe TBI patients, which is similar to others previously reported results as 37–67%.[16,17,18,19,20] In other studies from developed nations, moderate TBI was given relatively little clinical attention, but in this study, we showed that a significant proportion of patients with moderate TBI required craniotomy (45.93%) which was much higher than other studies, and in-hospital mortality in such patients was also higher (13.82%), which proved that delay in treatment was detrimental to outcome.[12] Nearly, one-third moderate or severe TBIs occurred during 1800–2400 hours. As the majority of these injuries were due to RTA, it could be due to alcohol influence, greater speeds, and poor visibility factors.
The severity of brain injuries is directly associated with outcome at discharge and other subsequent outcomes at different time points. Low Glasgow coma score at admission was associated with poor outcome. This finding was similar to many other reports from India, and other parts of the world.[6,11,12,14] At 6 months, about 64% of the severe and 32% of the moderate TBI patients had died. Six-month mortality ranged between 32% and 49% in severe TBI in other reported studies[17,19,20] and between 16% and 19% in moderate TBI.[19,21] The high mortality rate in the present study may be due to late arrival of the patients from the injury site and/or primary hospital. This study also showed that about 50% of moderate or severe TBI patients reached to Level 1 trauma center after 3 h of their injury while a study from Bangalore revealed that this figure was 70%.[10] In a comparative study between India and USA, about 50% reached within 1 h, and about 88% reached within 3 h in the USA.[22] In India, 95% of trauma victims do not receive optimal care or proper management during the “golden hour” period after an injury takes place, and half of those who die from TBI do so within the first 2 h of injury in our country.[9]
At 6 months, overall 31.70% patients (29.51% for severe, and 43.09% for moderate) were lost to follow-up at JPNATC, which is very high proportion as compared to data from developed nations. Although all studies have loss of follow up and we believe that our follow was reasonably good for comparison with similar national and international studies. A study from India had reported only 34% cases for follow-up at 1-year postdischarge.[10] Out of total available patients at 6 months postadmission, this study showed different case fatality rates between severe (64%) and moderate TBI (25%), which was relatively higher than developed nations but better than what is seen in LAMICs.
On the flip side, our study showed that a significant number of severe TBI (28.46%) had favorable functional outcome at 6 months. This study also showed that a significant number of patients with poor admission GCS may have good outcome, and therefore aggressive management should be done for all severe TBI patients. Availability of early and appropriate care after an injury is a major determinant in avoiding secondary injuries and death.[23] Some of these studies have paved the way for the formulation of policies and programs, increasing public awareness, developing new action plans, and for placing neurotrauma on the public health agenda. In TBI, there are several factors that may affect outcome. The data of this study may be used for prognostication, hypothesis generation and stratification of patients, and developing prognostic models,[24] which can improve the understanding of the pathology, diagnosis, and treatment.
This study had a number of strengths. This study, based on the largest sample size, was the first to document the epidemiology, clinical characteristics, and outcomes of a large cohort of patients with TBI from a single Level 1, Tertiary Care Trauma Center from Indian subcontinent, and the first to publish 6-months functional outcome data after the foundation of India’s first full-fledged center (JPNATC) to treat victims of trauma. The author himself assessed the 6 months functional outcome. This study provided a comprehensive description of variables associated with moderate and severe TBI from the initial injury to 6 months outcome. Using this study, there is a great potential to carry out a number of secondary analyses using multivariate techniques to evaluate the predictors of outcomes in TBI.
Limitations
This study had also some limitations. Being a retrospective study, only those variables could be studied which were collected for standard clinical care. It is plausible that only a certain proportion of all traumatic brain injuries will reach the hospital, and many of those with severe injuries may have died in the prehospital setting, and many with mild injuries may not have sought clinical care. The specialized status of JPNATC, AIIMS may have introduced bias toward the inclusions of more severely injured patients. Missing data for the outcome at 6 months follow-up were prominent largely due to lack of contact information. This study did not consider the outcome of patients with TBI, who were not admitted to the ICU.
Conclusions
This is the first study of its kind from the Indian subcontinent that gave data on the admission characteristics, mortality and 6 months outcome of such patients. Most of the injuries occurred due to RTAs, more common among the economic productive age group and mostly in males. There was a very high rate of mortality and severe disability in our setting possibly due to delay in transportation of injury victims to the definite care facility. Knowledge about the causes, pattern, and distributions about TBI patients from this study will be extremely helpful in policymaking, research, health management, and rehabilitation at the national level in our country and other developing nations.
Financial support and sponsorship
ICMR, New Delhi.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Geneva: WHO; 2003. World Health Organization. World Health Report 2003-Shaping the Future. [Google Scholar]
- 2.Fleminger S, Ponsford J. Long term outcome after traumatic brain injury. BMJ. 2005;331:1419–20. doi: 10.1136/bmj.331.7530.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Cambridge (MA): Harvard University Press; 1996. Global health statistics: A compendium of incidence prevalence and mortality estimates for over 200 conditions. [Google Scholar]
- 4.Lopez AD, Murray CJ. Cambridge (MA): Harvard University Press; 1996. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. [Google Scholar]
- 5.Gururaj G. Epidemiology of traumatic brain injuries: Indian scenario. Neurol Res. 2002;24:24–8. doi: 10.1179/016164102101199503. [DOI] [PubMed] [Google Scholar]
- 6.Gururaj G. New Delhi: National Commission on Macroeconomics and Health, Ministry of Health and Family Welfare, Government of India; 2005. Injuries in India: A National Perspective. Background Papers: Burden of Disease in India Equitable Development-Healthy Future; pp. 325–47. [Google Scholar]
- 7.Bullock MR, Povlishock JT. Brain Trauma Foundation, American Association of Neurological Surgeons, Congress of Neurological Surgeons, AANS/CNS Joint Section on Neurotrauma and Critical Care. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106. [Google Scholar]
- 8.Bratton SL, Chestnut RM, Ghajar J, McConnell HF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37–44. doi: 10.1089/neu.2007.9990. [DOI] [PubMed] [Google Scholar]
- 9. [Last accessed on 2014 Nov 23]. Available from: http://www.indianheadinjuryfoundation.org/traumaticbrain-injury/
- 10.Gururaj G, Kolluri S, Chandramouli BA, Subbakrishna DK, Kraus JF. Bengaluru: National Institute of Mental Health and Neuro Sciences; 2005. Traumatic Brain Injury. Publication No. 61. [Google Scholar]
- 11.Agrawal A, Galwankar S, Kapil V, Coronado V, Basavaraju SV, McGuire LC, et al. Epidemiology and clinical characteristics of traumatic brain injuries in a rural setting in Maharashtra, India 2007-2009. Int J Crit Illn Inj Sci. 2012;2:167–71. doi: 10.4103/2229-5151.100915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andriessen TM, Horn J, Franschman G, van der Naalt J, Haitsma I, Jacobs B, et al. Epidemiology, severity classification, and outcome of moderate and severe traumatic brain injury: A prospective multicenter study. J Neurotrauma. 2011;28:2019–31. doi: 10.1089/neu.2011.2034. [DOI] [PubMed] [Google Scholar]
- 13.Bajracharya A, Agrawal A, Yam B, Agrawal C, Lewis O. Spectrum of surgical trauma and associated head injuries at a university hospital in Eastern Nepal. J Neurosci Rural Pract. 2010;1:2–8. doi: 10.4103/0976-3147.63092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marmarou A, Lu J, Butcher I, McHugh GS, Murray GD, Steyerberg EW, et al. Prognostic value of the Glasgow Coma Scale and pupil reactivity in traumatic brain injury assessed pre-hospital and on enrollment: An IMPACT analysis. J Neurotrauma. 2007;24:270–80. doi: 10.1089/neu.2006.0029. [DOI] [PubMed] [Google Scholar]
- 15.Bruns J, Jr, Hauser WA. The epidemiology of traumatic brain injury: A review. Epilepsia. 2003;44(Suppl 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 16.Foulkes MA, Eisenberg HM, Jane JA, Marmarou A, Marshall LF. The Traumatic Coma Data Bank: Design, methods, and baseline characteristics. J Neurosurg. 1991;75:S8–13. [Google Scholar]
- 17.Jennett B, Teasdale G, Galbraith S, Pickard J, Grant H, Braakman R, et al. Severe head injuries in three countries. J Neurol Neurosurg Psychiatry. 1977;40:291–8. doi: 10.1136/jnnp.40.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leitgeb J, Erb K, Mauritz W, Janciak I, Wilbacher I, Rusnak M. Severe TBI Study Investigators. Severe traumatic brain injury in Austria V: CT findings and surgical management. Wien Klin Wochenschr. 2007;119:56–63. doi: 10.1007/s00508-006-0764-1. [DOI] [PubMed] [Google Scholar]
- 19.Murray GD, Teasdale GM, Braakman R, Cohadon F, Dearden M, Iannotti F, et al. The European brain injury consortium survey of head injuries. Acta Neurochir (Wien) 1999;141:223–36. doi: 10.1007/s007010050292. [DOI] [PubMed] [Google Scholar]
- 20.Myburgh JA, Cooper DJ, Finfer SR, Venkatesh B, Jones D, Higgins A, et al. Epidemiology and 12-month outcomes from traumatic brain injury in Australia and New Zealand. J Trauma. 2008;64:854–62. doi: 10.1097/TA.0b013e3180340e77. [DOI] [PubMed] [Google Scholar]
- 21.Compagnone C, d’Avella D, Servadei F, Angileri FF, Brambilla G, Conti C, et al. Patients with moderate head injury: A prospective multicenter study of 315 patients. Neurosurgery. 2009;64:690–6. doi: 10.1227/01.NEU.0000340796.18738.F7. [DOI] [PubMed] [Google Scholar]
- 22.Colohan AR, Alves WM, Gross CR, Torner JC, Mehta VS, Tandon PN, et al. Head injury mortality in two centers with different emergency medical services and intensive care. J Neurosurg. 1989;71:202–7. doi: 10.3171/jns.1989.71.2.0202. [DOI] [PubMed] [Google Scholar]
- 23.Murthy TV, Bhatia P, Sandhu K, Prabhakar T, Gogna RL. Secondary brain injury: Prevention and intensive care management. Indian J Neurotrauma. 2005;2:7–12. [Google Scholar]
- 24.Kamal VK, Agrawal D, Pandey RM. Prognostic models for prediction of outcomes after traumatic brain injury based on patients admission characteristics. Brain Inj. 2016;30:393–406. doi: 10.3109/02699052.2015.1113568. [DOI] [PubMed] [Google Scholar]


