Abstract
Objective:
The safety of endoscopic skull base surgery can be enhanced by accurate navigation in preoperative computed tomography (CT) and magnetic resonance imaging (MRI). Here, we report our initial experience of real-time intraoperative CT-guided navigation surgery for pituitary tumors in childhood.
Materials and Methods:
We report the case of a 15-year-old girl with a huge growth hormone-secreting pituitary adenoma with supra- and perisellar extension. Furthermore, the skull base was infiltrated. In this case, we performed an endonasal transsphenoidal approach for debulking the adenoma and for chiasma decompression. We used an MRI neuronavigation (Medtronic Stealth Air System) which was registered via intraoperative CT scan (Siemens CT Somatom). Preexisting MRI studies (navigation protocol) were fused with the intraoperative CT scans to enable three-dimensional navigation based on MR and CT imaging data. Intraoperatively, we did a further CT scan for resection control.
Results:
The intraoperative accuracy of the neuronavigation was excellent. There was an adjustment of <1 mm. The navigation was very helpful for orientation on the destroyed skull base in the sphenoid sinus. After opening the sellar region and tumor debulking, we did a CT scan for resection control because the extent of resection was not credible evaluable in this huge infiltrating adenoma. Thereby, we were able to demonstrate a sufficient decompression of the chiasma and complete resection of the medial part of the adenoma in the intraoperative CT images.
Conclusions:
The use of intraoperative CT/MRI-guided neuronavigation for transsphenoidal surgery is a time-effective, safe, and technically beneficial technique for special cases.
Keywords: Endoscopic endonasal surgery, intraoperative computed tomography, merged computed tomography-magnetic resonance neuronavigation, pituitary surgery
Introduction
Frameless neuronavigation is a meanwhile well-established tool in neurosurgery in adults.[1,2,3,4,5,6,7] Like many other new techniques, it was first extensively used in adults before it was routinely applied to the pediatric age group.[8,9,10,11,12] The neuronavigation had proven to be a great device for the surgical treatment of variable neurosurgical problems such as tumor resection or biopsy, the surgery for epilepsy, vascular neurosurgery, the localization of functional and eloquent area, spinal neurosurgery, and the operation for hydrocephalus.[13,14,15,16] Because the device helps the identification of pathologic condition and intraoperative anatomy distorted by pathologic anatomic conditions, the application of this device makes the surgery more exact, safe, fast, and less invasive due to finding the best approach and avoiding collision.[15,17]
Especially in skull base surgery, neuronavigation is very helpful for minimizing approaches and risks of intraoperative damage of essential structures. Another important factor is completeness of resection. Thus, intraoperative imaging has been introduced in the past using intraoperative computed tomography (CT)[18,19] or intraoperative magnetic resonance tomography (MRT).[20,21,22]
Although there are reports describing improved tumor resection rates of skull base lesions with intraoperative residual tumor assessment by magnetic resonance imaging (MRI),[20,23,24] its application remains complex, time-consuming, and expensive.
In this technical note, we demonstrate the ability to establish the most exact and feasible neuronavigation with merged MR images and intraoperative CT scan in case of pituitary surgery. The protocols that were used are described in detail, and the impact of intraoperative CT, MRI, and CT-MR fusion images navigated pituitary surgery is discussed.
Materials and Methods
Imaging
Intraoperative CT scan was performed with a 6-row multidetector-unit (Siemens SOMATOM Emotion 2003; Siemens healthcare, Erlangen, Germany) in high-resolution bone window level setting in strictly axial plane without inclination of gantry (150 kV, 160 mA). The CT slice thickness was 1 mm. Preoperative contrast-enhanced MRI studies (MPrage sequence with 1 mm slices without inclination) were performed. Intraoperatively, we performed a further CT scan for resection control.
Preexisting MRI studies and intraoperative CT scans were imported to the workstation (Stealth Station™, Medtronic USA). Fusion software (Stealth Merge™, Medtronic USA) was used to search automatically for optimal image-to-image registration between the CT and MRI data sets without manual correction. The CT-MRI fusion images were used to enable three-dimensional (3D) navigation based on MR and CT imaging data. For detailed information about the navigation unit Stealth Station™, we would like to refer to previous publications.[25,26] In summary, the workstation is optical-based and consists of a mobile console with a high-resolution monitor, a charge-coupled device camera unit, a referencing apparatus (dynamic reference frame) in combination with a carbon head holder and a pointer, which is operated by the surgeon. The surgeon used images in axial, sagittal, and coronal plane as well as 3D visualizations for image guidance. Intraoperatively, the accuracy of the navigation unit was measured with a software tool of the workstation by landmark checks. This accuracy was defined as the deviation between the same point in the preoperatively acquired navigation image and the real patient's anatomy.
Case illustration
A 10-year-old girl presented with bitemporal hemianopsia. A magnetic resonance image done after arrival at Pediatric Department revealed a huge enhancing lesion intra-, supra-, and peri-sellar with infiltration of the sinus cavernosus on both sides according to a macroadenoma [Figure 1]. The chiasm was clearly elevated. In the endocrinological laboratory testing, prolactin and especially growth hormone (GH) and insulin-like growth factor 1 (IGF-1) had high serum levels.
Figure 1.
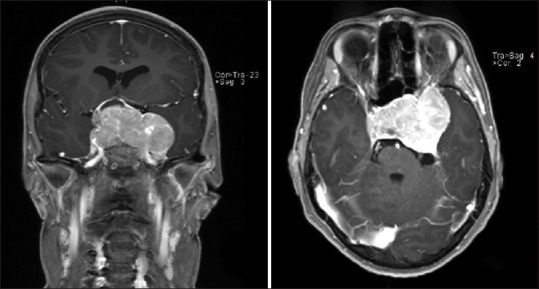
Preoperative magnetic resonance images revealed intra- and large supra-sellar tumor mass with invasion of sphenoidal sinus and sinus cavernosus in coronal and axial images
The decision was made to offer decompression of the chiasm and debulking of the tumor mass because of the visual deficits and to obtain histopathological diagnosis of the adenoma with ambiguous characteristics. Consideration was given to approach the lesion using an endoscopic, mononostril, and transsphenoidal approach.
Surgical procedure and real-time neuronavigation
The patient was placed supine with the upper part of the body slightly elevated to about 20° and the head tilted to the left. The patient's head was fixed with a three-pin carbon-head-fixation system in general anesthesia under orotracheal intubation. An intraoperative CT-scan with Siemens CT-suite Somatom was used for CT- and MRI merged-neuronavigation with Stealth Station™, Medtronic, USA.
The nose and the nasal cavities were prepared with the application of a nasal decongestant and an alcohol-based disinfectant. Mepivacaine with 1:100 000 epinephrine was injected into nasal mucosa for hemostasis. The authors used a right mononostril transsphenoidal approach to sellar region as described in detail in a large series before.[27] CT- and MRT-merged neuronavigation and anatomical landmarks were used to identify the carotid arteries bilaterally, sellar floor and sinus cavernosus after opening the sella. After tumor debulking and inspection of the sellar region with 30° optic, an intraoperative CT scan was performed for resection control [Figure 2a]. The CT scan revealed a sufficient decompression of the chiasm and a complete debulking of the medial part of the adenoma. The intraoperative histopathological diagnostics addicted an adenoma. Thus, we decided to finish the procedure. The sellar floor was reconstructed with bone pieces and fibrin glue. After reposition of the nasal septum, nasal packing was placed.
Figure 2.
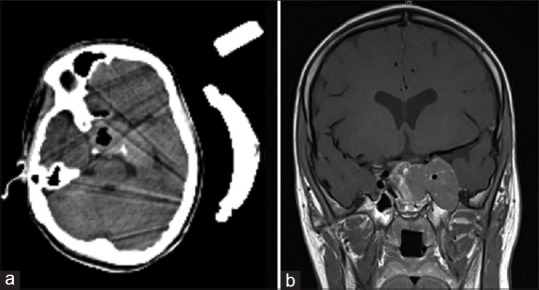
Intraoperative computed tomography resection control (a) and postoperative magnetic resonance imaging (b) 4 days after surgery
Results
Imaging
Intraoperative CT scans without contrast material enabled identification of the sphenoidal sinus and the extent of tumor infiltration of sellar floor and perisellar skull base and postoperative fenestration of sellar floor. In addition, the absence of significant intrasellar or extrasellar bleeding during the operation could be confirmed.
Merged CT- and MR-neuronavigation revealed a very good orientation during approach and in sellar region. The navigation was very helpful for orientation on the destroyed skull base in the sphenoid sinus [Figure 3]. All structures could be identified sufficient. The accuracy was excellent with a measured discrepancy of <1 mm in five landmark checks.
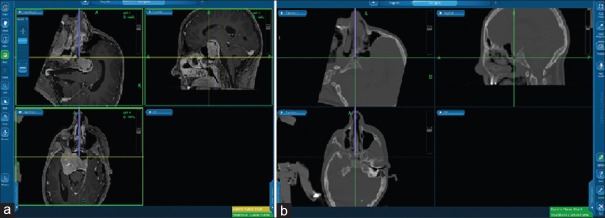
Figure 3.
Merged computed tomography-magnetic resonance neuronavigation with magnetic resonance-sequence (a) and high-resolution bone window level (b)
Postoperative course
The patient was extubated immediately after procedure and admitted to Intensive Care Unit postoperatively for neurological monitoring for 24 h. She reported about a clear reduction of bitemporal hemianopsia directly. No new neurological deficits were detected postoperatively.
No complications were appreciated. Postoperative MRI demonstrated partial resection of the macroadenoma as intended [Figure 2b]. The patient was discharged at home on postoperative day 6 in an excellent condition without any neurological deficits.
The histopathological testings revealed a GH-secreting adenoma with coexpression of prolactin. She underwent further medical treatment with octreotide 100 – 0 – 200 µg/day and cabergoline 2 mg × 0.5 mg per week. At her 12 weeks follow up, she revealed good growth without visual deficits. Serum levels of GH, IGF-1, and prolactin were decreased.
Discussion
In modern neurosurgery, intraoperative neuronavigation is an essential tool, as it projects preoperative imaging data of the patient into the operative sites and vice versa.[16] This technique was first used in the mid-eighties[6,28,29,30] in adults and has meanwhile proven to be an essential adjunct to neurosurgery in cases where topographic orientation on the basis of anatomical landmarks alone is difficult.
Furthermore, navigated skull base surgery with CT-MR image data fusion has advantages for preoperative planning and for intraoperative image guidance as described by other study groups.[31,32] In contrast to assisted surgery with either CT or MRI navigation alone, image data fusion combines and complements different benefits of CT and MR images and offers the surgeon more accurate information on the exact geometric, and volumetric relationship between the soft tissue structures seen on MRI and the bony structures observed on CT.[33,34,35,36] Whereas MRI is superior in delineating the lesion itself, the CT information is required to depict the bony structures encountered while approaching the tumor. Otherwise, with CT alone, the depth of the soft tissue pathology cannot be correctly appreciated beyond the margins of the bony defect. Merged CT-MR neuronavigation obtains all information in its images. The benefit of both diagnostic tools can be used for better orientation. Second, the surgeon has the opportunity to switch between CT and MR images during surgery dependent on actual situation and on required orientation.
The intraoperative accuracy of neuronavigation unit depends on the quality of the image fusion as well as on the accuracy of image-to-patient registration. Hence, real-time neuronavigation with merged intraoperative generated CT scans and MR images promises an excellent accuracy cause of best possible registration of the patient in the operation suite. Furthermore, the accuracy of the image fusion strongly depends on the preciseness of the obtained CT and MRI data sets.[36] The accuracy of the fusion images is not able to outreach the resolution of primary data sets and is, therefore, also dependent on the thickness of CT and MRI slices. Therefore, a slice thickness of 1 mm for the CT images and 1 mm for the MRI images was chosen. 3D T1-weighted MP-RAGE sequences prove to be very useful for image fusion. In addition, the osseous and neurovascular structures of the skull base are not subjected to relevant movements during surgical manipulation preserving the highest degree of accuracy also intraoperatively after tumor resection.
Critical aspects of navigated skull base surgery must also be considered: Neuronavigation with intraoperative image guidance represents a valuable and safe aid for the experienced surgeon, but will never be able to replace profound knowledge. Second, the surgeons must mount a learning curve to successfully apply this technology.
Although this was a single case, our experience with merged intraoperative CT-MR neuronavigation suggests a positive effect on the orientation in difficult cases of pituitary surgery. Setting up the neuronavigation requires a few minutes additional anesthesia time. However, the merged navigated image guidance during the surgery helps saving operation time compared to conventional neuronavigation images by speeding the surgeon's intraoperative orientation.
Conclusions
Real-time merged CT-MR-neuronavigation is a useful device and alternative to classical MR-based neuronavigation in special cases in neurosurgery. It is a time-effective, safe, and technically beneficial technique. The technique was very helpful for approaching the sellar floor and tumor debulking. Further studies are necessary to point out the benefit of merged real-time neuronavigation and its appliance in neurosurgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Barnett GH, Kormos DW, Steiner CP, Weisenberger J. Intraoperative localization using an armless, frameless stereotactic wand. Technical note. J Neurosurg. 1993;78:510–4. doi: 10.3171/jns.1993.78.3.0510. [DOI] [PubMed] [Google Scholar]
- 2.Golfinos JG, Fitzpatrick BC, Smith LR, Spetzler RF. Clinical use of a frameless stereotactic arm: Results of 325 cases. J Neurosurg. 1995;83:197–205. doi: 10.3171/jns.1995.83.2.0197. [DOI] [PubMed] [Google Scholar]
- 3.Kato A, Yoshimine T, Hayakawa T, Tomita Y, Ikeda T, Mitomo M, et al. A frameless, armless navigational system for computer-assisted neurosurgery. Technical note. J Neurosurg. 1991;74:845–9. doi: 10.3171/jns.1991.74.5.0845. [DOI] [PubMed] [Google Scholar]
- 4.Koivukangas J, Louhisalmi Y, Alakuijala J, Oikarinen J. Ultrasound-controlled neuronavigator-guided brain surgery. J Neurosurg. 1993;79:36–42. doi: 10.3171/jns.1993.79.1.0036. [DOI] [PubMed] [Google Scholar]
- 5.Nabavi A, Manthei G, Blömer U, Kumpf L, Klinge H, Mehdorn HM. Neuronavigation. Computer-assisted surgery in neurosurgery. Radiologe. 1995;35:573–7. [PubMed] [Google Scholar]
- 6.Reinhardt HF, Horstmann GA, Gratzl O. Sonic stereometry in microsurgical procedures for deep-seated brain tumors and vascular malformations. Neurosurgery. 1993;32:51–7. doi: 10.1227/00006123-199301000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Sipos EP, Tebo SA, Zinreich SJ, Long DM, Brem H. In vivo accuracy testing and clinical experience with the ISG viewing wand. Neurosurgery. 1996;39:194–202. doi: 10.1097/00006123-199607000-00048. [DOI] [PubMed] [Google Scholar]
- 8.Arginteanu M, Abbott R, Frempong A. ISG viewing wand-guided endoscopic catheter placement for treatment of posterior fossa CSF collections. Pediatr Neurosurg. 1997;27:319–24. doi: 10.1159/000121277. [DOI] [PubMed] [Google Scholar]
- 9.Choi KY, Seo BR, Kim JH, Kim SH, Kim TS, Lee JK. The usefulness of electromagnetic neuronavigation in the pediatric neuroendoscopic surgery. J Korean Neurosurg Soc. 2013;53:161–6. doi: 10.3340/jkns.2013.53.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCallum J. Combined frameless stereotaxy and neuroendoscopy in placement of intracranial shunt catheters. Pediatr Neurosurg. 1997;26:127–9. doi: 10.1159/000121177. [DOI] [PubMed] [Google Scholar]
- 11.Stapleton SR, Kiriakopoulos E, Mikulis D, Drake JM, Hoffman HJ, Humphreys R, et al. Combined utility of functional MRI, cortical mapping, and frameless stereotaxy in the resection of lesions in eloquent areas of brain in children. Pediatr Neurosurg. 1997;26:68–82. doi: 10.1159/000121167. [DOI] [PubMed] [Google Scholar]
- 12.Wagner W, Gaab MR, Schroeder HW, Sehl U, Tschiltschke W. Experiences with cranial neuronavigation in pediatric neurosurgery. Pediatr Neurosurg. 1999;31:231–6. doi: 10.1159/000028868. [DOI] [PubMed] [Google Scholar]
- 13.Hayhurst C, Byrne P, Eldridge PR, Mallucci CL. Application of electromagnetic technology to neuronavigation: A revolution in image-guided neurosurgery. J Neurosurg. 2009;111:1179–84. doi: 10.3171/2008.12.JNS08628. [DOI] [PubMed] [Google Scholar]
- 14.McMillen JL, Vonau M, Wood MJ. Pinless frameless electromagnetic image-guided neuroendoscopy in children. Childs Nerv Syst. 2010;26:871–8. doi: 10.1007/s00381-009-1074-5. [DOI] [PubMed] [Google Scholar]
- 15.Sangra M, Clark S, Hayhurst C, Mallucci C. Electromagnetic-guided neuroendoscopy in the pediatric population. J Neurosurg Pediatr. 2009;3:325–30. doi: 10.3171/2008.12.PEDS0888. [DOI] [PubMed] [Google Scholar]
- 16.Esposito F, Di Rocco F, Zada G, Cinalli G, Schroeder HW, Mallucci C, et al. Intraventricular and skull base neuroendoscopy in 2012: A global survey of usage patterns and the role of intraoperative neuronavigation. World Neurosurg. 2013;80:709–16. doi: 10.1016/j.wneu.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Roth J, Beni-Adani L, Biyani N, Constantini S. Classical and real-time neuronavigation in pediatric neurosurgery. Childs Nerv Syst. 2006;22:1065–71. doi: 10.1007/s00381-006-0103-x. [DOI] [PubMed] [Google Scholar]
- 18.Gasinski P, Zielinski P, Harat M, Furtak J, Rakowska J, Paczkowski D. Application of intraoperative computed tomography in a neurosurgical operating theatre. Neurol Neurochir Pol. 2012;46:536–41. doi: 10.5114/ninp.2012.32176. [DOI] [PubMed] [Google Scholar]
- 19.Tonn JC, Schichor C, Schnell O, Zausinger S, Uhl E, Morhard D, et al. Intraoperative computed tomography. Acta Neurochir Suppl. 2011;109:163–7. doi: 10.1007/978-3-211-99651-5_25. [DOI] [PubMed] [Google Scholar]
- 20.Fahlbusch R, Ganslandt O, Buchfelder M, Schott W, Nimsky C. Intraoperative magnetic resonance imaging during transsphenoidal surgery. J Neurosurg. 2001;95:381–90. doi: 10.3171/jns.2001.95.3.0381. [DOI] [PubMed] [Google Scholar]
- 21.Lipson AC, Gargollo PC, Black PM. Intraoperative magnetic resonance imaging: Considerations for the operating room of the future. J Clin Neurosci. 2001;8:305–10. doi: 10.1054/jocn.2000.0833. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann M, Seifert V, Trantakis C, Raabe A. Open MRI-guided microsurgery of intracranial tumours in or near eloquent brain areas. Acta Neurochir (Wien) 2001;143:327–37. doi: 10.1007/s007010170086. [DOI] [PubMed] [Google Scholar]
- 23.Bohinski RJ, Warnick RE, Gaskill-Shipley MF, Zuccarello M, van Loveren HR, Kormos DW, et al. Intraoperative magnetic resonance imaging to determine the extent of resection of pituitary macroadenomas during transsphenoidal microsurgery. Neurosurgery. 2001;49:1133–43. doi: 10.1097/00006123-200111000-00023. [DOI] [PubMed] [Google Scholar]
- 24.Martin CH, Schwartz R, Jolesz F, Black PM. Transsphenoidal resection of pituitary adenomas in an intraoperative MRI unit. Pituitary. 1999;2:155–62. doi: 10.1023/a:1009943700810. [DOI] [PubMed] [Google Scholar]
- 25.Ganslandt O, Behari S, Gralla J, Fahlbusch R, Nimsky C. Neuronavigation: Concept, techniques and applications. Neurol India. 2002;50:244–55. [PubMed] [Google Scholar]
- 26.Gralla J, Nimsky C, Buchfelder M, Fahlbusch R, Ganslandt O. Frameless stereotactic brain biopsy procedures using the Stealth Station: Indications, accuracy and results. Zentralbl Neurochir. 2003;64:166–70. doi: 10.1055/s-2003-44620. [DOI] [PubMed] [Google Scholar]
- 27.Linsler S, Gaab MR, Oertel J. Endoscopic endonasal transsphenoidal approach to sellar lesions: A detailed account of our mononostril technique. J Neurol Surg B Skull Base. 2013;74:146–54. doi: 10.1055/s-0033-1338258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlöndorff G, Mösges R, Meyer-Ebrecht D, Krybus W, Adams L. CAS (computer assisted surgery). A new procedure in head and neck surgery. HNO. 1989;37:187–90. [PubMed] [Google Scholar]
- 29.Watanabe E, Watanabe T, Manaka S, Mayanagi Y, Takakura K. Three-dimensional digitizer (neuronavigator): New equipment for computed tomography-guided stereotaxic surgery. Surg Neurol. 1987;27:543–7. doi: 10.1016/0090-3019(87)90152-2. [DOI] [PubMed] [Google Scholar]
- 30.Roberts DW, Strohbehn JW, Hatch JF, Murray W, Kettenberger H. A frameless stereotaxic integration of computerized tomographic imaging and the operating microscope. J Neurosurg. 1986;65:545–9. doi: 10.3171/jns.1986.65.4.0545. [DOI] [PubMed] [Google Scholar]
- 31.Nemec SF, Donat MA, Mehrain S, Friedrich K, Krestan C, Matula C, et al. CT-MR image data fusion for computer assisted navigated neurosurgery of temporal bone tumors. Eur J Radiol. 2007;62:192–8. doi: 10.1016/j.ejrad.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 32.Nemec SF, Peloschek P, Schmook MT, Krestan CR, Hauff W, Matula C, et al. CT-MR image data fusion for computer-assisted navigated surgery of orbital tumors. Eur J Radiol. 2010;73:224–9. doi: 10.1016/j.ejrad.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Kurtsoy A, Menku A, Tucer B, Oktem IS, Akdemir H. Neuronavigation in skull base tumors. Minim Invasive Neurosurg. 2005;48:7–12. doi: 10.1055/s-2004-830151. [DOI] [PubMed] [Google Scholar]
- 34.Leong JL, Batra PS, Citardi MJ. CT-MR image fusion for the management of skull base lesions. Otolaryngol Head Neck Surg. 2006;134:868–76. doi: 10.1016/j.otohns.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 35.Rohde V, Spangenberg P, Mayfrank L, Reinges M, Gilsbach JM, Coenen VA. Advanced neuronavigation in skull base tumors and vascular lesions. Minim Invasive Neurosurg. 2005;48:13–8. doi: 10.1055/s-2004-830179. [DOI] [PubMed] [Google Scholar]
- 36.Sure U, Alberti O, Petermeyer M, Becker R, Bertalanffy H. Advanced image-guided skull base surgery. Surg Neurol. 2000;53:563–72. doi: 10.1016/s0090-3019(00)00243-3. [DOI] [PubMed] [Google Scholar]