Abstract
Background:
Mastocytosis is either cutaneous (with skin-limited proliferation of mast cells) or systemic (with mast cells in extracutaneous sites). The onset of solitary mastocytoma in an adult is rare.
Purpose:
A woman with the new onset of solitary mastocytoma is described. The clinical features of patients with adult-onset solitary mastocytoma are summarized. Recommendations for the evaluation and treatment of individuals with adult-onset solitary mastocytoma are proposed.
Methods:
PubMed was searched with the key words: adult, CD2, CD25, cell, cutaneous, disease, KIT, KIT D816V, mast, mastocytoma, mutation, pigmentosa, solitary, tryptase, and urticarial. The papers generated by the search, and their references, were reviewed.
Results:
A 38-year-old Taiwanese woman presented with an asymptomatic brown patch, which morphologically mimicked a dysplastic nevus, on her right abdomen; biopsy demonstrated a solitary mastocytoma. Comprehensive evaluation (including serologic and bone marrow examination) excluded systemic mastocytosis and her residual mastocytoma is being monitored. Adult-onset solitary mastocytoma has been described in 16 patients. Lesions were either on the head and neck (5/14), torso (5/14) or extremities (4/14). Urtication following lesion rubbing was noted in 79% (11/14) of patients. Excision of the mastocytoma [75% (9/12)] was the most common treatment. Other management approaches included corticosteroids (topical or intralesional), antihistamines (systemic) or observation. Systemic symptoms were noted in 5 patients: flushing (3 women) and pruritus (3 women); gastrointestinal symptoms and headaches, flushing and/or anaphylaxis were each noted in one woman. None of the patients with adult-onset solitary mastocytoma had systemic mastocytosis; however, only 3 women were evaluated for systemic mastocytosis.
Conclusions:
Systemic mastocytosis is common in adults with new onset cutaneous mastocytosis. Therefore, a conservative work up for new onset solitary mastocytosis in adults is proposed to include complete blood cell counts, serum chemistries (including liver function tests), and serum tryptase level and bone marrow biopsy to evaluate for mast cell clusters, morphology and immunophenotype and KIT gene mutation in codon 816. Similar serologic testing should be considered annually for adult-onset solitary mastocytosis patients without systemic disease.
Keywords: adult, CD2, CD25, cell, cutaneous, disease, KIT, KIT D816V, mast, mastocytoma, mutation, pigmentosa, solitary, tryptase, urticaria
Introduction
Cutaneous mastocytosis can present as either urticarial pigmentosa (also referred to as maculopapular cutaneous mastocytosis), diffuse cutaneous mastocytosis, telangiectasia macularis perstans, and mastocytoma [1–5]. Mastocytomas are most commonly seen in infants (under 2 years of age) and children (ranging in age from 2 year to less than 16 years); many of these lesions improve spontaneously over several years duration [2,3,5,6]. The adult-onset of a solitary mastocytoma has seldom been reported [2]. A woman who developed a solitary mastocytoma is described and the characteristics of other men and women with adult-onset mastocytoma are reviewed. Evaluation of these individuals for systemic mastocytosis and treatment options for the solitary mast cell skin lesion are discussed.
Case report
A healthy 38-year-old Taiwanese woman noticed four new asymptomatic pigmented lesions that had appeared during the prior year. She has no prior history of itching, flushing, fevers, fatigue, night sweats, weight loss or skin rashes. She had experienced diarrhea whenever she ate wheat, several years earlier, but this subsided once she eliminated gluten from her diet. Her bowel movements are normal and she has no abdominal cramping or reflux.
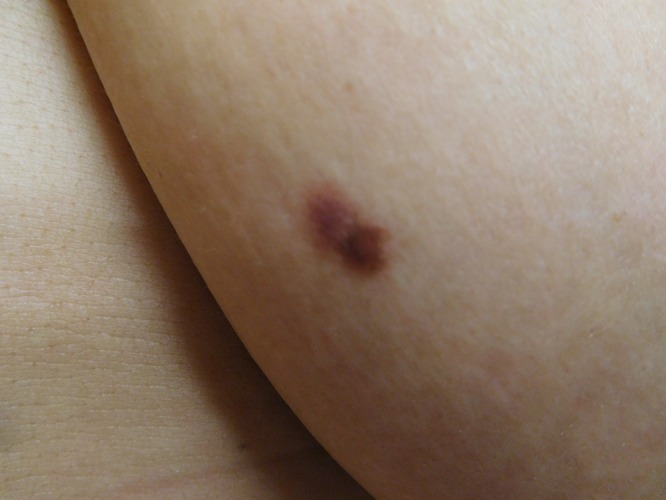
Cutaneous examination of her right abdomen showed an 8 x 4 mm brown patch with irregular borders (Figure 1). Her left breast showed a 9 x 5 mm, 2-toned brown patch with irregular borders (Figure 2). Dark brown 2 x 2 mm macules were noted superiorly and inferiorly on the deltoid area of her right arm (Figure 3).
Figure 1.
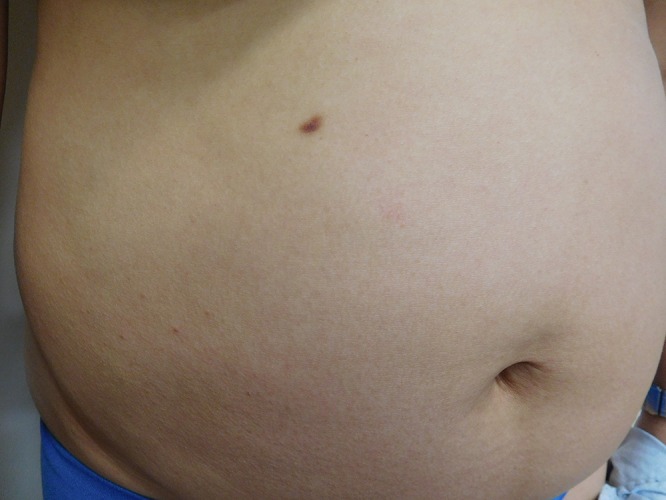
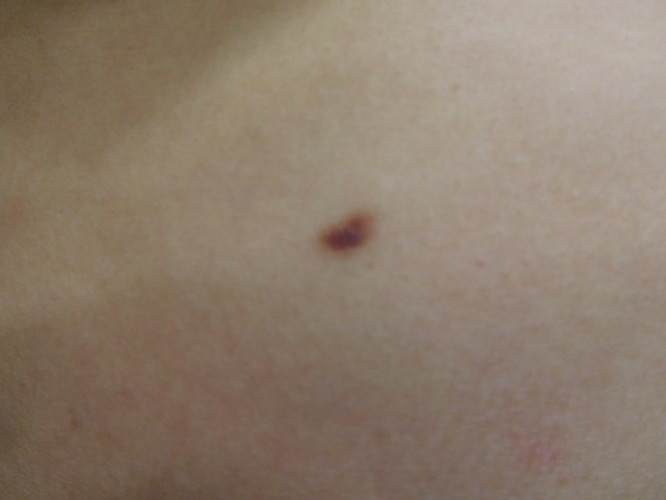
Distant (a) and closer (b) views of the right abdomen show a solitary mastocytoma presenting as a brown patch with irregular borders. [Copyright: ©2016 Cohen.]
Figure 2.
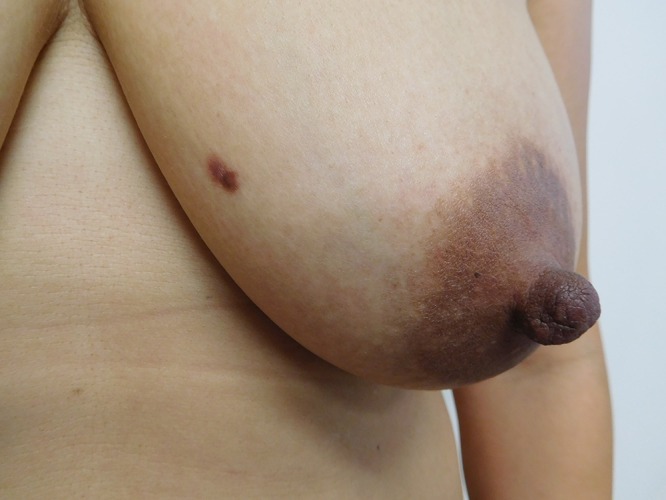
Distant (a) and closer (b) views of the left breast show a dermatofibroma presenting as a two-toned brown patch with irregular borders. [Copyright: ©2016 Cohen.]
Figure 3.
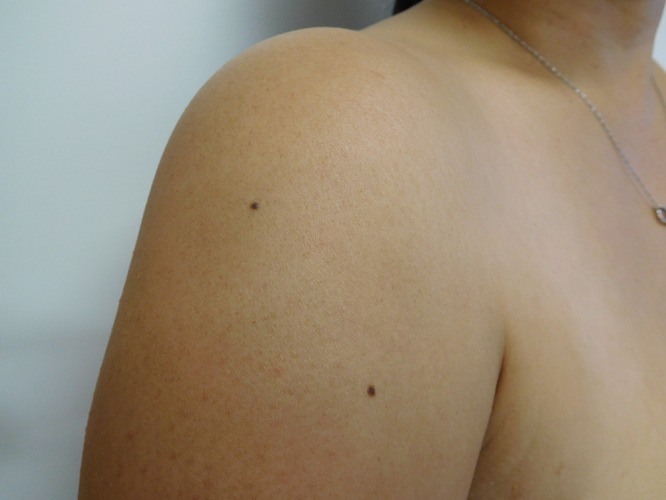
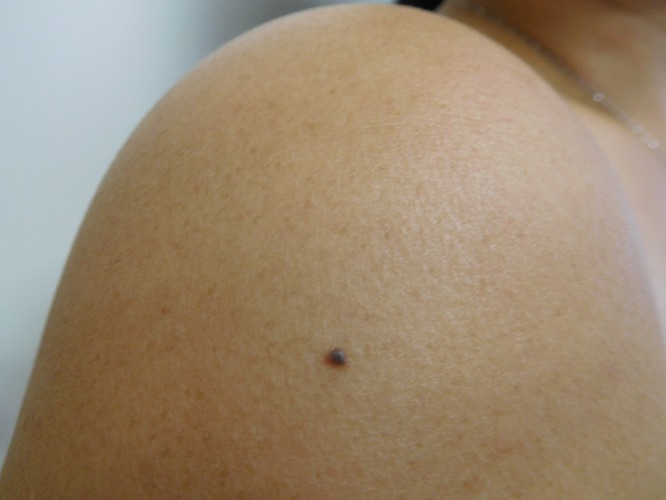
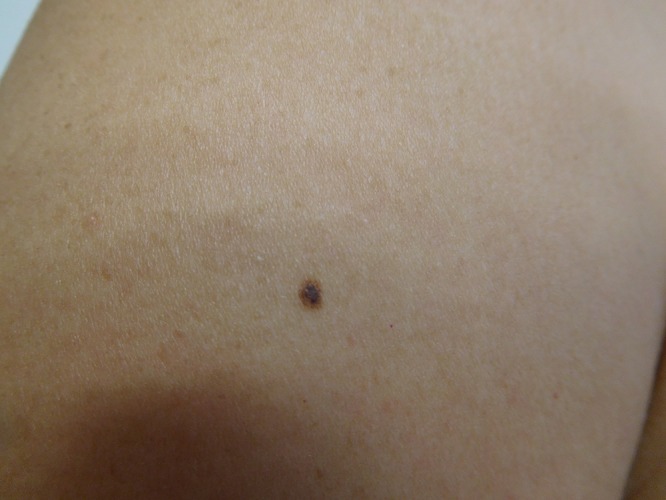
Distant (a) and closer (b and c) views of the right deltoid area show a compound nevus (superior) (a and b) and a junctional dysplastic nevus with mild atypia (inferior) (a and c); both presented as dark brown macules. [Copyright: ©2016 Cohen.]
Microscopic examination of a biopsy from the right abdomen lesion showed an infiltrate of monomorphous mononuclear cells in the upper dermis (Figure 4). Tryptase highlights the cells comprising the infiltrate, consistent with cutaneous mastocytosis (Figure 5). Correlation of the clinical presentation and the pathologic findings establishes the diagnosis of an adult-onset solitary mastocytoma. The biopsy specimens from the left breast, superior right arm and inferior right arm showed dermatofibroma, compound nevus and junctional dysplastic nevus with mild atypia.
Figure 4.
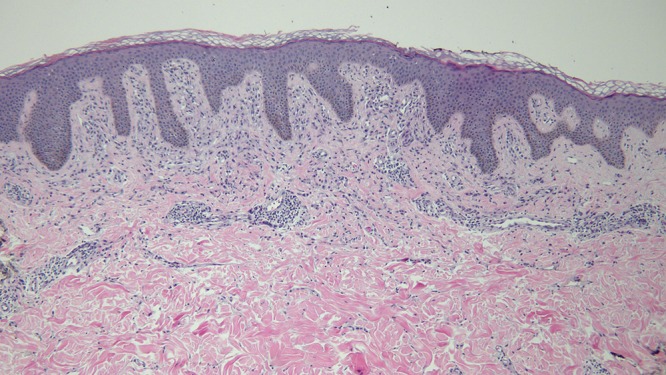
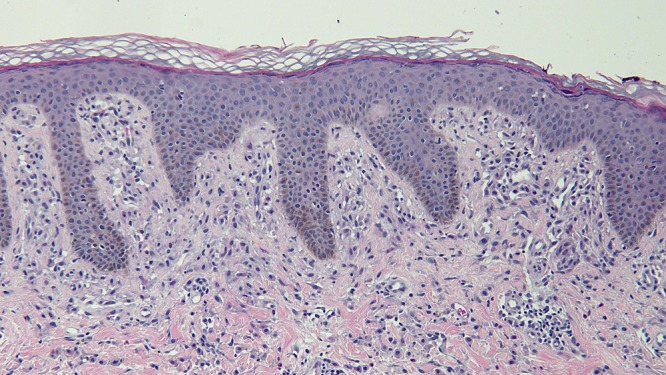
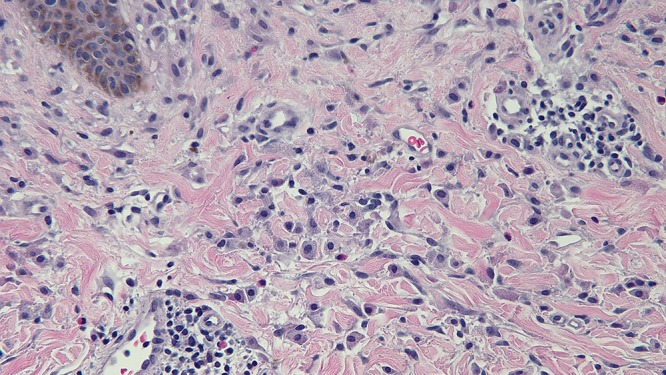
Distant (a) and closer (b and c) views of the skin biopsy of the mastocytoma on the right abdomen, stained with hematoxylin and eosin, show a monomorphous mononuclear cell infiltrate in the upper dermis (hematoxylin and eosin, a = x4; b = x20; c= x 40). [Copyright: ©2016 Cohen.]
Figure 5.
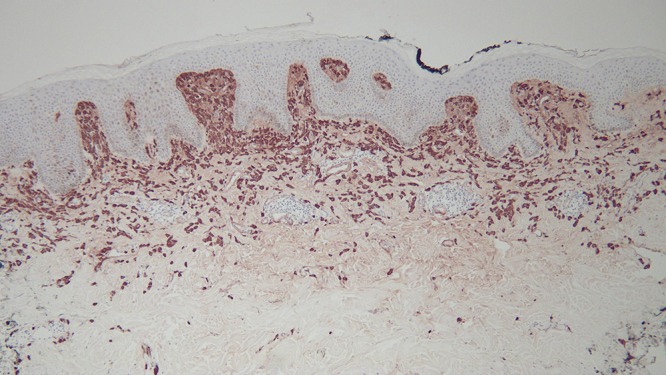
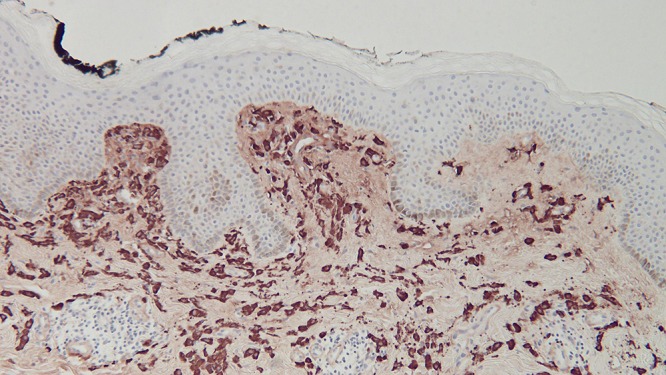
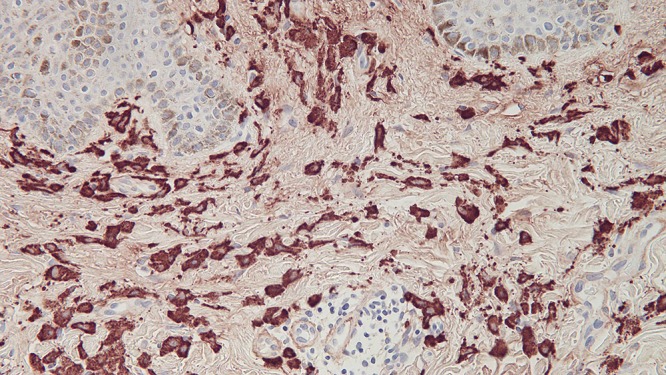
Distant (a) and closer (b and c) views of the right abdomen mastocytoma skin biopsy, stained with tryptase, show that the cells in the upper dermis are highlighted by the immunoperoxidase stain [tryptase, a = x4; b = x20; c=x40). [Copyright: ©2016 Cohen.]
Laboratory studies demonstrated normal complete blood counts (including eosinophils) and platelet count, normal serum chemistries (including liver function tests), and normal serum tryptase level of 2.2 ug/L (normal, < 10.9 ug/L). KIT (D816V) mutation by polymerase chain reaction was not detected in her peripheral blood. A bone marrow biopsy examination did not show foci of greater than or equal to 15 mast cells, abnormal morphology of the mast cells, nor expression of CD2 and/or CD25 by the bone marrow mast cells. In addition, the bone marrow specimen did not demonstrate a KIT mutation at codon 816. Therefore, she did not fulfill the any of the criteria for systemic mastocytosis.
Subsequent examination of her skin did not reveal any similar appearing lesions. The biopsy site at her left abdomen had healed. There was urtication of the residual lesion after rubbing, demonstrating a positive Darier’s sign. Since the mastocytoma was otherwise asymptomatic and she had no mastocytosis-associated general symptoms, she elected to monitor the residual lesion.
Discussion
Mastocytosis, a disease in which there is an accumulation of mast cells in tissues, has two variants: cutaneous mastocytosis and systemic mastocytosis [1–5]. Cutaneous mastocytosis occurs when the mast cell proliferation is limited to the skin; evaluation for systemic mast cell disease is negative [3,4]. Adult-onset cutaneous mast cell disease is usually associated with systemic mastocytosis [1,3,4].
Systemic mastocytosis is characterized by increased mast cells in extracutaneous sites, such as the bone marrow, gastrointestinal tract, lymph nodes, liver or spleen [1,3] Skin involvement may also be present [1]. The World Health Organization has established major and minor diagnostic criteria for systemic mastocytosis (Table 1) [1,3]
TABLE 1.
Diagnostic criteria for systemic mastocytosis [a] [Copyright: ©2016 Cohen.]
| Major criteria |
| The presence of multifocal dense infiltrates of mast cells in the bone marrow or other extracutaneous organs, confirmed by special stains such as mast cell tryptase (greater than15 mast cells aggregating) |
| Minor criteria |
| Atypical mast cell morphology: in mast cell infiltrates in the bone marrow or other extracutaneous organs, greater than 25% of the mast cells are spindle-shaped or otherwise atypical; or in bone marrow smears, greater than 25% of the mast cells are spindle-shaped or otherwise atypical |
| Aberrant mast cell immunophenotype: mast cells in extracutaneous organs (CD117) co-express either CD2 or CD25 or both, as determined by flow cytometry |
| Activating point mutation of KIT in codon 816 is present in extracutaneous organs |
| Baseline serum tryptase level is persistently elevated (greater than 20 ng/ml); this does not count in patients who have an associated clonal hematologic non-mast cell disease (AHNMD) |
[a] The diagnosis of systemic mastocytosis is fulfilled either by: (1) the presence of the major criterion plus one minor criterion or (2) the presence of at least three minor criterion.
A mastocytoma is a localized dermal accumulation of mast cells that clinically presents as a reddish-brown macule or slightly raised papule; the lesion can be as large as 8 cm, thereby developing into a patch or nodule [7]. It usually presents as a single lesion; therefore, it has previously been referred to as a ‘solitary mastocytoma’; this designation shall be used in this paper. However, in a recent consensus report on the cutaneous manifestations in patients with mastocytosis, the investigators recommended that the term ‘cutaneous mastocytoma’ be used to describe mastocytosis of the skin in individuals with 3 or less isolated mast cell skin lesions. If 4 or more lesions are noted, the patient was classified as having urticaria pigmentosa [4].
The occurrence of a mastocytoma in an adult—a person 16 years of age or older—is rare [2]. It was originally described in 2 of the 14 patients reported by Johnson and Helwig in 1961 [8]. To the best of my knowledge, including the currently reported woman, adult-onset solitary mastocytoma has only been described in 16 individuals (Table 2) [2,7–16].
TABLE 2.
Characteristics of individuals with adult-onset solitary mastocytoma [a,b] [Copyright: ©2016 Cohen.]
| Case | Age On Dx | Ra S | Size (mm) | Color | Site | Symptoms Lesion General | Treatment | Ref |
|---|---|---|---|---|---|---|---|---|
| 1 | 16 18 |
Ca M |
5 x 5 | Pink | L thigh | I,U - |
Excision | 15 |
| 2 | 17 21 |
NS M |
15 x 15 | Brown-Red | R cheek | B,T,U - |
Excision | 8c14 |
| 3 | 19 24 |
In M |
25 x 25 | Hypopigmented | Nape of neck | I,U - |
Excison | 14c1 |
| 4 | 20 30 |
In M |
30 x 25 | Hypopigmented | Nape of neck | I,U - |
ILS [c] | 14c2 |
| 5 | 20 50 |
In M |
78 x 82 | Brown-Red | L back | B,I,U - |
NS | 13 |
| 6 | 27 27 |
NS M |
6 x 6 | Brown-Red | R thigh | B,U - |
Excision | 8c12 |
| 7 [d] | 39 39 |
Ca M |
10 x 7 | Red | L forearm | - - |
NS | 9 |
| 8 [e] | 46 46 |
Ca M |
5 x 4 | Non-pigmented | R medial canthus | - - |
Excison | 12 |
| 9 | 18 19 |
In W |
125 x 125 | Red-Purple | R chest | B,I,T,U + [f] | Excison | 16c2 |
| 10 | 20 22 |
In W |
25 x 25 | Yellow, Red-Purple | L shoulder | B,I,T,U + [g] | POA, Topical steroid, ILS | 16c1 |
| 11 | 23 33 |
Ca W |
50 x 30 | Brown-Red | L supra-clavicular | I,U +[h] | Excision | 11 |
| 12 | 24 24 |
In W |
15 x 15 | Red | L forearm | I,T,U +[i] | POA, Topical steroid, then Excision [i] | 10 |
| 13 [j] | 30 30 |
NS W |
33 x 15 | Flesh-colored | Scalp | − +[j] |
Excision | 7 |
| 14 | 37 38 |
Tw W |
8 x 4 | Brown | R abdomen | U − |
None | CR |
[a] Abbreviations: B, blister; c; case; Ca, Caucasian; Dx, diagnosis age when lesion was biopsied or excised (years); I, Itch (after rubbing); ILS, intralesional corticosteroid; In, Indian, L, left; M, man; mm, millimeters; NS, not stated; T, tender; Tw, Taiwanese; U, urtication (positive Darier’s sign) after rubbing lesion; On, onset age when lesion initially was noted by patient (years); POA, oral antihistamine; R, right; Ra, race; Ref, references; S, sex; W, woman; +, present; −, absent
[b] Cases 15 and 16 were cited in a manuscript that provided a comparison of mastocytosis with onset in children and adults. Adult onset solitary mastocytoma was listed in 2 of the individuals; neither had systemic symptoms (pruritus, flushing, abdominal pain, diarrhea or headache). Complete resolution of the skin lesion was observed in one of the individuals; the other person was not followed for at least 10 years [2].
[c] He received 20 mg/ml of kenalog into the lesion every 3 weeks for 2 injections; there was decreased itching of the lesion, but no change in its size. Also, the positive urtication (Darier’s sign) of the lesion persisted.
[d] The pathology report read: “The H. and E. stain is not diagnostic. With toluidine blue the upper cutis shows about 10 mast cells per high power field, somewhat concentrated around the blood vessels.” The patient was presented by Dr. Jesse A. Tolmach as “urticaria pigmentosa (solitary lesion in adult) at the NewYork Academy of Medicine, Section of Dermatology and Syphilology meeting on March 6, 1962 . The unopposed comments of Dr. Arthur Bernard Hyman were: “ . . . where no other pathology is found and there are 10 mast cells per high-power field, it is considered, according to our present arbitrary standards, as more than sufficient to make a diagnosis and confirms a diagnosis of urticarial pigmentosa.
[e] The patient also had severe prolidase deficiency. He had 2 adjacent solitary mastocytomas; the larger (inferior) measured 5 x 4 mm and the superior lesion was smaller.
[f] The patient had facial flushing and generalized itching.
[g] The patient had facial flushing and generalized itching every 2–3 months; the “itching was suppressed only with continuous use of systemic antihistaminics and topical steroids, later intralesional steroids relieved pruritus for 6 months.”
[h] The patient had one episode of gastrointestinal distress with severe nausea and vomiting, diarrhea, and abdominal pain. Complete blood cell counts, platelet counts and liver function tests were normal and stool guiac was negative.
[i] She had intense bouts of pruritus every 3–4 weeks. She was initially treated symptomatically with systemic antihistamines and topical corticosteroids with a partial response. Subsequently, an excisional biopsy was performed.
[j] She had a 10-year history of headaches, flushing, and anaphylaxis triggered by exercise, stress, heat or scalp friction. The patient experienced no flushing or anaphylaxis while pregnant, but her symptoms returned after delivery. The subcutaneous scalp mass was excised; postoperatively, she had no further symptoms at 27 months follow-up.
Adult onset solitary mastocytoma has occurred in 8 men and 6 women. Two additional adults are included in a report that provided a comparison of mastocytosis with onset in children and adults; however, no additional epidemiologic features were provided. The adults ranged from 16 to 46 years of age (median, 22 years). The age at which the mastocytoma was discovered in men ranged from 16 to 46 years (median, 20 years). In women, the onset of discovery ranged from 18 to 37 years (median, 24 years). Six of the patients were Indian and four were Caucasian; one woman was Taiwanese.
The onset age of the mastocytoma was the same age as the diagnosis of the lesion in five individuals: three men and two women. A delay from 1 year to 30 years (median, 4 years) occurred between the age when the other nine patients initially recognized the skin lesion and the diagnosis was established. For the five men, between 2 to 30 years (median, 5 years) passed between the onset age and diagnosis age of their mastocytoma. For the four women, between 1 to 10 years (median, 2 years) passed between the onset age and diagnosis age of their mastocytoma.
The clinical appearance of the mastocytoma varied from a patch to a plaque to a nodule. The color varied from brown to brown-red to red or pink; part of one woman’s lesion was yellow. Three of the men had lesions that were either hypopigmented or non-pigmented.
The women in this report had a brown patch that was clinically suspected to be a dysplastic nevus; solitary [11] and multiple [17,18] mastocytomas have previously been misinterpreted as a melanocytic nevus. The hyperpigmentation associated with mastocytomas has been postulated to be secondary to induction of melanocytes by increased local concentrations of soluble mast cell growth factors from the mast cell lesions of cutaneous mastocytosis [13,19–21]. In addition to a melanocytic nevus (or a dysplastic nevus [current report] or Spitz nevus [11], the clinical differential diagnosis of solitary mastocytoma includes chronic arthropod bite reaction [15], cyst [8], granuloma [8], hidrocystoma [15], leukemia cutis [11], and xanthogranuloma [11].
The solitary mastocytomas ranged in size from 5 x 4 mm to 12.5 x 12.5 cm. One man, who had severe prolidase deficiency, had two adjacent cutaneous mastocytomas located on his right medial canthus; the inferior lesion was 5 x 4 mm and the superior lesion was smaller. All of the other adults only had a single lesion.
A positive Darier’s sign (observed as urtication of the lesion after rubbing) was noted in 79% (11/14) of the patients. Therefore, the absence of urtication after stimulation of the lesion does not exclude the possibility of a mastocytoma. Other lesional symptoms included blistering (36%, 5/14), pruritus (64%, 9/14), and tenderness (29%, 4/14).
The mastocytomas were located on the head and neck (36%, 5/14), the torso (36%, 5/14) and the extremities (29%, 4/14). The most common locations—each with two individuals—were the arms, legs, and posterior neck. All other sites only had one person: abdomen, back, cheek, chest, medial canthus, scalp, shoulder, and supraclavicular area.
Generalized symptoms associated with the solitary mastocytoma were observed in 31% (5/16) of the individuals [7,10,11,16]. All of the patients were women: three Indian [10,16], one Caucasian [11], and one whose race was not reported [7]. The symptoms most commonly occurring included flushing (three women) [7,16] and pruritus (three women) [10,16]. One of the women had experienced only one episode of gastrointestinal distress characterized by severe nausea and vomiting, diarrhea and abdominal pain [11]. Another woman had generalized symptoms of 10 years duration consisting of headaches, flushing and anaphylaxis that were triggered by exercise, stress, heat or scalp friction; her mastocytoma presented as a subcutaneous scalp nodule [7].
All patients had hematoxylin and eosin stained tissue biopsy confirmation of their mastocytoma. The mast cells were typically present in the upper dermis—in either nodular or interstitial or sheet-like infiltrates and/or around blood vessels. They presented as monomorphic mononuclear cells with abundant pale basophilic staining, granule containing, cytoplasm. Special histopathologic and/or immunohistochemical stains were used to confirm the suspected diagnosis of mast cells: azure A [15], CD25 (inerleukin-2 receptor alpha chain) [12], Giemsa [7,8], kit (CD117, the receptor for stem cell factor) [12], toluidine blue [8,9,14] and tryptase [7,12,current report]; the granules in the cytoplasm were clearly demonstrated using either Giemsa and toluidine blue stain [8]. In some of the patients lymphocytes [7] or eosinophils [14,15] were also present in the dermal infiltrate.
The management of the solitary mastocytoma was described for 12 patients. Excision (9/12, 75%) was the most common treatment. This was either performed as an excisional biopsy during the initial evaluation [8,10–12,15,16] or subsequently [7,14]. Two individuals were initially treated with oral antihistamines and topical corticosteroids [10,16]; the lesions were subsequently treated with either excision [10] or an intralesional corticosteroid injection [16]. The woman in the current report received no additional treatment following her biopsy; her lesion was asymptomatic and she remains free of symptoms.
Intralesional corticosteroids have been used to successfully treat solitary mastocytomas in infants [22]. The mastocytoma was treated with intralesional corticosteroids in two patients [14,16]. A 30-year-old Indian man had two injections—three weeks apart—of 20 mg/ml of Kenalog®, that resulted in decreased itching but neither size change nor ceasing of urtication of the lesion [14]. Also, a 22–year-old Indian woman whose itching was relieved for six months following intralesional corticosteroids; her pruritus previously has only been suppressed with continuous use of systemic antihistamines and topical corticosteroids [16].
In addition to the reported patient, evaluation for systemic mastocytosis was only preformed in 2 patients [7,11]. One patient was a 33-year-old Caucasian woman who had experienced one episode of gastrointestinal distress (associated with severe nausea and vomiting, diarrhea, and abdominal pain) two years earlier. She had a complete blood cell count, platelet count and liver function tests that were normal and a stool guaiac examination that was negative [11].
Systemic mastocytosis was suspected in a 30-year-old woman with a 10-year history of anaphylaxis, headaches and flushing triggered by exercise, heat, scalp friction or stress [7]. She had a major criterion for the diagnosis of systemic mastocytosis (a few clusters of greater than 15 mast cells were present on the bone marrow biopsy specimen), but did not fulfill any of the diagnostic minor criteria (Table 1) [1,3]. Although her serum tryptase level was elevated (ranging from 11 to 14 ng/mL), it was below the 20 ng/mL threshold established by the World Health Organization. In addition, there was neither D816V mutation in the KIT gene nor evidence of the FEP1L1-PDGFRA fusion transcript on her molecular studies [7].
The reported patient, a 38-year-old Taiwanese woman, had no local or systemic symptoms of mastocytosis. However, since adult onset cutaneous mastocytosis is commonly associated with systemic mastocytosis [1,3,4], she received an evaluation for systemic disease. Her complete blood cell and platelet counts, serum chemistries (including liver function tests), and tryptase level were normal. In addition, c-KIT D816V mutation was not detected in her peripheral blood. Her bone marrow aspirate and biopsy were negative for clusters of mast cells, c-KIT mutation at codon 816, and mast cell expression of CD2 and/or CD25 by flow cytometry or immunohistochemistry. Hence, she did not fulfill either the major or any of the minor criteria for diagnosis of systemic mastocytosis.
Recommendations for the treatment and evaluation of patients with adult onset solitary mastocytoma remain to be established. Similar to the successful treatment of infant-onset solitary mastocytoma [23], an excisional biopsy or subsequent excision of a biopsy-confirmed cutaneous mastocytoma was performed in 75% (9/12) of the patients with adult onset solitary mastocytomas. Some symptomatic relief was provided with oral antihistamines, topical corticosteroids or intralesional corticosteroids; however, these interventions were only performed in a small number of patients. It is not unreasonable to observe the residual, biopsy-confirmed, mastocytoma in patients whose skin lesion is asymptomatic and who does not have systemic disease, similar to the patient in this report.
To date, none of the patients with adult onset solitary mastocytoma have systemic mastocytosis. However, the number of patients in the literature is small and comprehensive evaluation for systemic disease was only performed in two women. Since adult onset mastocytosis has such a high correlation with systemic disease (albeit indolent systemic mastocytosis in some of these individuals), a conservative recommendation to evaluate adults with the new onset of a solitary mastocytoma for systemic mastocytosis is reasonable. This should include a complete blood cell and platelet count, serum chemistries that include liver function tests, and a serum tryptase. Peripheral blood and/or the bone marrow should be evaluated for c-KIT mutation at codon 816; in addition the bone marrow aspirate and biopsy should be examined for foci of greater than or equal to 15 mast cells, morphology of the mast cells, and expression of CD2 and/or CD25 by bone marrow mast cells [3,4]. If the initial evaluation for systemic mastocytosis is negative, annual testing for complete blood cell and platelet counts, serum chemistries including liver function tests, and a serum tryptase level should be considered.
Conclusions
A mastocytoma presents as a reddish-brown papule that consists of an accumulation of mast cells in the dermis. A solitary mastocytoma can be a single lesion of cutaneous mastocytosis or represent the cutaneous stigmata of systemic mastocytosis. Solitary mastocytomas typically appear in infants or children, are not associated with systemic disease, and occasionally resolve spontaneously.
Albeit less common, solitary mastocytomas have been described in 16 adults: 8 men and 6 women; 2 individuals did not have any epidemiologic features reported. The onset age for appearance of adult-onset solitary mastocytoma ranged from 16 to 46 years (median, 22 years). However, confirmation of the mastocytoma diagnosis was the same as the lesion onset age in five patients; there was a delay between 1 to 30 years (median, 4 years) between recognizing the skin lesion and establishing the diagnosis in the other nine patients. Routine and special stains of a lesional biopsy confirmed the diagnosis.
The head and neck (5/14), the torso (5/14) and the extremities (4/14) were the sites of the mastocytomas. They ranged in size from 5 x 4 mm to 12.5 x 12.5 cm. Their color varied from brown to brown-red to red or pink; yet three lesions were either hypopigmented or non-pigmented. Post-rubbing urtication (signifying a positive Darier’s sign) was noted in 79% (11/14) of the mastocytomas.
None of the patients had systemic mastocytosis. However, five patients (31%of 16 individuals) had generalized symptoms. Flushing (3 women) and pruritus (3 women) were the most common symptoms. Gastrointestinal symptoms (one episode in one woman) and a 10-year duration of headaches, flushing and anaphylaxis were symptoms in other women.
Treatment of solitary mastocytoma was excision of the lesion in 75% (9/12) of the patients either as past of the diagnosis evaluation or subsequently. Other modalities, with varying success, included topical or intralesional corticosteroids and systemic antihistamines. Observation of the residual lesion was also conducted for one patient who had no general or lesion symptoms.
Evaluation for systemic mastocytosis was only performed in three individuals. Diagnostic tests were limited to routine serologic laboratory tests in one woman. The other women had a more comprehensive evaluation including serum tryptase level and bone marrow biopsy searching for clusters of mast cells, aberrant mast cell morphology or immunopheno-type, and KIT gene mutation in codon 816.
Although none of the patients with adult-onset solitary mastocytoma had systemic mastocytosis, only a small number of these individuals have been described. Yet, the new onset of cutaneous mastocytosis in adults is commonly associated with systemic disease—albeit the incidence of indolent systemic mastocytosis remains to be established in this group of patients. Therefore, until further data is accumulated, conservative recommendations with regards to the work up of adults with new onset solitary mastocytosis are reasonable.
In summary, it is proposed that the initial work up for adult-onset solitary mastocytoma would include a complete blood cell count, serum chemistries (including liver function tests), and serum tryptase level; serologic evaluation for D816V mutation in the KIT gene should also be considered. In addition, a bone marrow biopsy to assess, not only mast cell clusters, morphology and immunophenotype, but also KIT gene mutation in codon 816, should be performed. In patients with adult-onset solitary mastocytoma without systemic mastocytosis, yearly blood tests to evaluate complete blood cell counts, chemistries (including liver function tests), and tryptase level should be considered.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
All authors have contributed significantly to this publication.
References
- 1.Berezowska S, Flaig MJ, Rueff F, et al. Adult-onset mastocytosis in the skin is highly suggestive of systemic mastocytosis. Mod Pathol. 2014;27:19–29. doi: 10.1038/modpathol.2013.117. [DOI] [PubMed] [Google Scholar]
- 2.Middelkamp Hup MA, Heide R, Tank B, Mulder PG, Orate AP. Comparison of mastocytosis with onset in children and adults. J Eur Acad Dermatol Venereol. 2002;16:115–20. doi: 10.1046/j.1468-3083.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- 3.Lange M, Nedoszytko B, Gorska A, et al. Mastocytosis in children and adults: clinical disease heterogeneity. Arch Med Sci. 2012;8:533–41. doi: 10.5114/aoms.2012.29409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann K, Escribano L, Grattan C, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016;137:35–45. doi: 10.1016/j.jaci.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 5.Lange M, Lugowska-Umer H, Niedoszytko M, et al. Diagnosis of mastocytosis in children and adults in daily clinical practice. Acta Derm Venereol. 2015 Aug 13; doi: 10.2340/00015555-2210. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Caplan RM. The natural course of urticaria pigmentosa: analysis an followup of 112 cases. Arch Dermatol. 1963;87:146–57. doi: 10.1001/archderm.1963.01590140008002. [DOI] [PubMed] [Google Scholar]
- 7.Khan K, Kupferman ME, Gardner JM, Ivan D. Solitary mastocytoma in an adult with an unusual clinical presentation [letter] J Am Acad Dermatol. 2011;65:683–4. doi: 10.1016/j.jaad.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Johnson WC, Helwig EB. Solitary mastocytosis (urticaria pigmentosa) Arch Dermatol. 1961;84:806–15. doi: 10.1001/archderm.1961.01580170100014. [DOI] [PubMed] [Google Scholar]
- 9.Tolmach JA. Urticaria pigmentosa. Arch Derm Syphilol. 1946;54:479. [PubMed] [Google Scholar]
- 10.Jain VK, Dayal S. Solitary mastocytoma in an adult. J Dermatol Venereol Leprol. 1997;63:310–311. [PubMed] [Google Scholar]
- 11.Ashinoff R, Soter NA, Freedberg IM. Solitary mastocytoma in an adult: treatment by excision. J Dermatol Surg Oncol. 1993;19:487–8. doi: 10.1111/j.1524-4725.1993.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 12.Ma SP, Hardy TG. Solitary mastocytoma of the eyelid in an adult patient with prolidase deficiency. Ophthal Plast Reconstr Surg. 2015;20:e1–e3. doi: 10.1097/IOP.0000000000000376. [DOI] [PubMed] [Google Scholar]
- 13.Harman M, Akdeniz S, Balci G, Uzunlar AK. A brownish-red plaque in an adult. Indian J Dermatol Venereol Leprol. 2009;75:101. doi: 10.4103/0378-6323.45244. [DOI] [PubMed] [Google Scholar]
- 14.Pandhi D, Singal A, Aggarwal S. Adult onset, hypopigmented solitary mastocytoma: Report of two cases. Indian J Dermatol Venereol Leprol. 2008;74:41–3. doi: 10.4103/0378-6323.38407. [DOI] [PubMed] [Google Scholar]
- 15.Baraf CS, Shapiro L. Solitary mastocytoma: case report in an adult. Arch Dermatol. 1969;99:589–90. doi: 10.1001/archderm.99.5.589. [DOI] [PubMed] [Google Scholar]
- 16.Mittal RR, Goyal DK. Solitary mastocytoma in adults. Indian J Dermatol Venereol Leprol. 1990;56:315–6. [Google Scholar]
- 17.Novy FG., Jr Single lesion of urticaria pigmentosa (nevus?) Arch Dermatol Syphilol. 1950;61:514–5. [Google Scholar]
- 18.Jacobs PH. Urticaria pigmentosa; solitary lesions occurring in a mother and her daughter. AMA Arch Dermatol. 1958;77:112–4. doi: 10.1001/archderm.1958.01560010114020. [DOI] [PubMed] [Google Scholar]
- 19.Okun MR. Mast cells and melanocytes. Int J Dermatol. 1976;15:711–22. doi: 10.1111/j.1365-4362.1976.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 20.Okun MR. Mast cells, melanocytes, balloon cells [letter] Am J Dermatopathol. 1982;4(5):478–9. [PubMed] [Google Scholar]
- 21.Okun MR, Bhawan J. Combined melanocytoma-mastocytoma in a case of nodular mastocytosis. J Am Acad Dermatol. 1979;1:338–47. doi: 10.1016/s0190-9622(79)70027-2. [DOI] [PubMed] [Google Scholar]
- 22.Kang N-G, Kim T-H. Solitary mastocytoma improved by intralesional injections of steroid. J Dermatol. 2002;29:536–8. doi: 10.1111/j.1346-8138.2002.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 23.Birt AR, Nickerson M. Generalized flushing of the skin with urticarial pigmentosa. Arch Dermatol. 1959;80:311–7. doi: 10.1001/archderm.1959.01560210053010. [DOI] [PubMed] [Google Scholar]


