Abstract
The currently available human tumor xenograft models permit modeling of human cancers in vivo, but in immunocompromised hosts. Here we report a humanized mouse (hu-mouse) model made by transplantation of human fetal thymic tissue plus hematopoietic stem cells transduced with a leukemia-associated fusion gene MLL-AF9. In addition to normal human lymphohematopoietic reconstitution as seen in non-leukemic hu-mice, these hu-mice showed spontaneous development of B-cell acute lymphoblastic leukemia (B-ALL), which was transplantable to secondary recipients with an autologous human immune system. Using this model, we show that lymphopenia markedly improves the antitumor efficacy of recipient leukocyte infusion (RLI), a GVHD-free immunotherapy that induces antitumor responses in association with rejection of donor chimerism in mixed allogeneic chimeras. Our data demonstrate the potential of this leukemic hu-mouse model in modeling leukemia immunotherapy, and suggest that RLI may offer a safe treatment option for leukemia patients with severe lymphopenia.
Keywords: Humanized mice, Leukemia, Lymphopenia, Mixed-lineage leukemia fusion gene, Recipient leukocyte infusion
Highlights
-
•
NSG mice grafted with thymus/oncogenic HSC develop human immune system and leukemia.
-
•
Leukemia transfer to mice with autologous immunity suffices to model immunotherapy.
-
•
Lymphopenia enhances RLI-mediated HVGR and anti-leukemia activity in mixed chimeras.
This study establishes a humanized mouse model with human immunity and autologous leukemia. Using this model, the authors demonstrate that lymphopenia promotes the rejection of donor hematopoietic chimerism and the associated anti-leukemia response by recipient leukocyte infusion in mixed allogeneic chimeras.
1. Introduction
Mouse models are the most commonly used in vivo systems for cancer studies, in which immunotherapy can be studied in immunologically intact syngeneic hosts. However, because of the many differences between rodents and humans, much of the information on both pathogenesis and immunotherapies learned from the conventional mouse models cannot be applied to humans. For this reason, xenograft models have been increasingly used to study human tumors. In this model, human tumors can be grafted and grow in immunodeficient mice that lack immune system. To study interaction between the tumor and immune system, human peripheral blood mononuclear cells (PBMCs) or T cells were transferred into the immunodeficient mice bearing human tumors. However, the immune responses are not physiological for two reasons. First, xenoantigen-reactive T cells in the injected human cells cause graft-vs.-host responses. Second, because human antigen-presenting cells (APCs) are lacking, such system can neither maintain peripheral T cell pool nor initiate human leukocyte antigen (HLA)-restricted immune responses.
Functional human hematopoietic and lymphoid systems can be established in mice by transplantation of human fetal thymic tissue (FTHY) and CD34+ fetal liver cells (FLCs) to sublethally-irradiated immunodeficient mice (Lan et al., 2006, Lan et al., 2004, Melkus et al., 2006). These hu-mice have proved to be an excellent model for assessing human innate and adaptive immune responses in vivo under normal or pathological conditions such as human immune responses to allo- and xeno-antigens and viral infection (Hu and Yang, 2012). Here, we seek to develop hu-mice with human lymphohematopoietic systems and autologous leukemia that permit exploration of human cancer immunotherapy.
2. Materials and Methods
2.1. Animals and Human Tissues and Cells
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NOD/SCID/γc−/− or NSG) mice were purchased from the Jackson Laboratory (Bar Harbor, ME), and were housed in a specific pathogen-free micro-isolator environment and used in experiments at 6 to 10 weeks of age. Human fetal thymus and liver tissues of gestational age of 17 to 20 weeks were obtained from Advanced Bioscience Resource (Alameda, CA). Thymic tissue was cut into small fragments measuring about 1 mm3; human CD34+ fetal liver cells (FLCs) were purified by a magnetic-activated cell sorter (MACS) using anti-human CD34 microbeads (Miltenyi Biotech, Aubum, CA). The prepared human thymic tissue fragments and CD34+ FLCs were then cryopreserved in liquid nitrogen until use. Protocols involving the use of human tissues and animals were approved by the Human Research Committee and the Institutional Animal Care and Use Committee of Columbia University.
2.2. Retroviral Transduction of CD34+ FLCs
Pseudo-typed retroviruses were produced by transfection using Lipofectamine 2000 (Invitrogen, San Diego, CA) of 293FT cells with a 3-plasmid system consisting of the MLL fusion vector (MSCV-MLL-AF9-human pgk-EGFP; kindly provided by Dr. John Dick) (Barabe et al., 2007) and two packaging vectors (VSV-G and Psi-Env). Viral particles were collected 48 or 72 h post-transfection and concentrated by ultracentrifugation at 50,000 g for 2 h, and were titered and stored at − 80 °C until use. For transduction, human CD34+ FLCs were stimulated overnight in medium containing 50 ng/mL rhSCF (R&D, Minneapolis, MN), 50 ng/mL Flt-3L (eBioscience, San Diego, CA), 25 ng/mL TPO (R&D, Minneapolis, MN), 10 ng/mL IL-3 (R&D, Minneapolis, MN), in 24-well plates pre-coated with Retronectin (Takara Bio Inc.), followed by incubation with retroviruses for 12 h. Cells were washed twice and injected into sublethally irradiated NSG mice to generate leukemic hu-mice (see Hu-mouse preparation below). A small aliquot of the transduced cells was cultured for 3 additional days to determine the transduction efficiency by measuring the ratio of GFP+ cells using FACS (ranged between 10 and 30% in the experiments presented).
2.3. Humanized Mouse Preparation
NSG mice were conditioned with sublethal (2 Gy) total body irradiation (TBI), and transplanted intravenously (i.v.) or intrafemorally (i.f.) with human CD34+ FLCs (0.5–2 × 105/mouse) alone or along with thymic tissue fragment measuring about 1 mm3 (under mouse kidney capsule) from the same fetal donor, as previously described (Lan et al., 2006, Tonomura et al., 2008). Levels of human hematopoietic cells in hu-mice were determined by flow cytometric analysis using various combinations of the following mAbs: anti-human CD45, CD3, CD4, CD8, CD45RA, CD45RO, CD19, CD20, CD10, IgM, IgD, CD44, CD33, CD14, CD15, CD11b, CD11c, CD56, CD34, HLA-DR, HLA-A/B/C; anti-mouse CD45 and Ter119; and isotype control mAbs (all antibodies were purchased from BD PharMingen, San Diego, CA). Analysis was performed on a LSR II (Becton Dickinson, Mountain View, CA), and dead cells were excluded from the analysis by gating out lower forward scatter and high propidium iodide or DAPI-retaining cells. For making hu-mice with autologous leukemia, NSG mice were injected with CD34+ FLCs that were transduced with retroviral vectors containing MLL-AF9, and leukemia development was assessed by FACS and histology.
2.4. Cytospin and Histology Analysis
Bone marrow or spleen cells were prepared from leukemic hu-mice and GFP+ human leukemia cells were purified by cell sorting, suspended in PBS, and centrifuged (130 g for 5 min) onto glass slides using a Cytospin centrifuge (Shandon). The slides were stained with the DipQuick Stain Kit (modified Wright Giemsa staining) from Jorgensen Laboratories. Tissues from leukemic hu-mice were fixed in 10% buffered formalin and embedded in paraffin for hematoxylin and eosin (H&E) staining. Stained slides were examined under a Zeiss microscope and photographed using a Nikon Coolpix 5000 digital color camera.
2.5. Hydrodynamic Gene Delivery
Human cytokine genes (IL-15/Flt-3L/GM-CSF/IL-3) were cloned separately into pcDNA3.1(+) vector (Invitrogen) (Chen et al., 2012, Chen et al., 2009). Plasmid DNA was purified by Maxi-prep Kit (Qiagen), and injected i.v. into hu-mice 12 days prior to RLI (5–50 μg of each plasmid in a total of 1.8-mL saline within 7 s using a 27-gauge needle) (Suda et al., 2007).
2.6. In Vivo Human T Cell Depletion
Hu-mice were treated with 6 injections (i.v.) of anti-huCD3-immunotoxin (a gift from Dr. David Neville (Woo et al., 2010)) with the dose of 5 μg/Kg BID for 3 days (6, 5 and 4 days before RLI). Right before each day injections, blood samples were collected for FACS analysis. Some hu-mice were sacrificed to confirm the depletion of human T cells in periphery and organs by FACS 3 days after the treatment was completed.
2.7. Recipient Lymphocyte Infusions
Spleen cells were prepared from RLI-cell source hu-mice and administered i.v. at a dose of 2–3 × 107 cells per mouse into hu-mouse chimeras 11–12 weeks after human CD34+ cell transplantation. In some experiments, human CD25+ cells were depleted from RLI inoculum by MACS using anti-human CD25 microbeads (Miltenyi Biotech, Aubum, CA).
2.8. Statistical Analysis
The level of significant differences in group means was determined by the Student's t-test for parametric data sets. The overall difference between groups was determined by two-way ANOVA with repeated measures. All analysis was performed using Prism 5 (GraphPad Software). A P value of ≤ 0.05 was considered significant in all analyses.
3. Results
3.1. Construction of Humanized Mice With Human Immune System and Autologous Leukemia
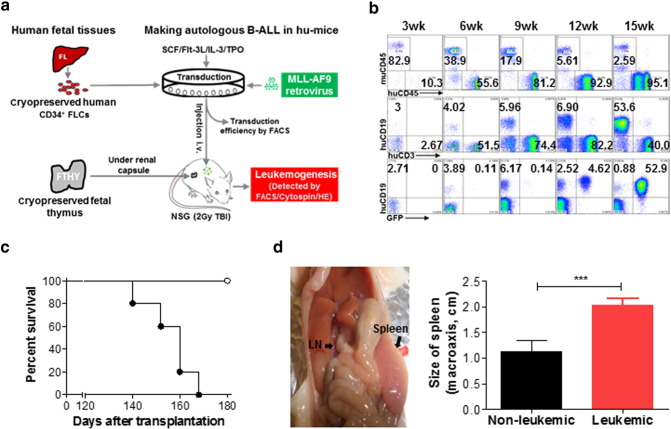
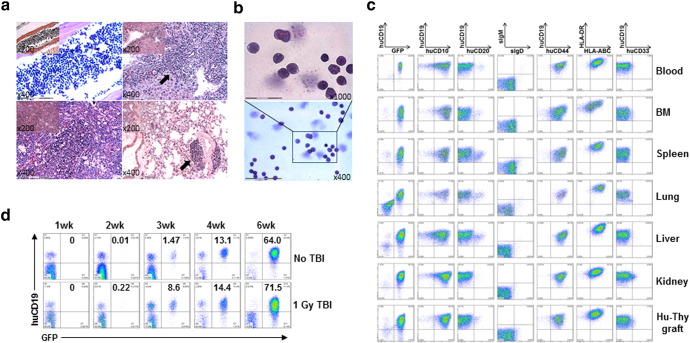
We transplanted sublethally-irradiated NSG mice with human FTHY and CD34+ FLCs that were transduced with retrovirus containing a mixed-lineage leukemia (MLL) fusion gene MLL-AF9 (Barabe et al., 2007) (Fig. 1a). FACS analysis revealed a gradual increase in the levels of human PBMCs, including T and B cells and myeloid cells (or APCs), with a similar kinetics as that seen in hu-mice receiving untransduced CD34+ FLCs (Lan et al., 2006), until 15 weeks when overt leukemia appeared (Fig. 1b). The hu-mice became moribund between 19 and 24 weeks after transplantation (Fig. 1c); autopsy revealed splenomegaly, enlarged lymph nodes, hepatomegaly, and enlarged FTHY grafts in all of these mice (Fig. 1d and data not shown). Histology demonstrated that leukemic cells infiltrated all organs/tissues examined, including bone marrow, spleen, lung, liver, kidney, and FTHY graft (Fig. 2a and data not shown). As Wright-Giemsa staining demonstrated, purified GFP+ cells exhibited a high nucleus/cytoplasm ratio (Fig. 2b), a typical leukemic blast morphology. The GFP+ leukemic cells present a B-ALL phenotype, i.e., CD19+ CD10+ CD20− sIgMlow/− sIgDlow/− CD44hiMHC-I+ MHC-IIhi and negative for other lineage markers i.e., CD33− CD15low/− CD14− CD11b− CD3− CD4− CD8− CD56− (Fig. 2c). Leukemia with a similar B-ALL phenotype also developed in mice receiving MLL-AF9-transduced CD34+ FLCs without FTHY (Table 1). Therefore, this leukemic hu-mouse model not only establishes human lymphohematopoietic system, but also develops B-ALL driven by MLL-AF9 transduction, thus providing a model to study human tumor immunology to autologous leukemia.
Fig. 1.
Generation of hu-mice with a functional human immune system and autologous leukemia. (a) Schematic showing preparation of hu-mice. (b) Representative FACS profiles showing the presence of human lymphohematopoietic cells and GFP+ leukemia cells. (c) Survival of leukemic (solid symbol; n = 5) and non-leukemic (open symbol; n = 4) hu-mice. (d) Macroscopic evidence of tumor in lymph node and spleen (left) and sizes (mean ± SDs) of spleens from leukemic and non-leukemic hu-mice. *** P < 0.001.
Fig. 2.
Characterization of leukemia developed in mice receiving MLL-AF9-transduced CD34+ cells. (a–c) Moribund leukemic hu-mice were sacrificed for histologic, morphologic and phenotypic analysis. (a) Histologic (H&E) analysis of bone marrow (BM; top left), liver (top right), spleen (bottom left), and lung (bottom right); arrow indicates tumor area. (b) Wright–Giemsa stained cytospins of purified GFP+ leukemia cells. (c) GFP+ leukemia cells characteristic of B-ALL phenotype in the indicated tissues. (d) Hu-mice were prepared by transplantation of FTHY and CD34+ FLCs from the same fetus used to make the leukemic hu-mice. Fourteen weeks later, the hu-mice were either untreated or conditioned with 1 Gy TBI, followed 1 day later by i.v. injection of 1 × 105 autologous leukemia cells (i.e., a 1:3 mixture of BM and spleen cells from the primary leukemic hu-mice; n = 4 per group). As indicated in (c), the majority of BM and spleen cells were GFP+ leukemia cells. Shown are FACS analyses of GFP+ leukemia cells in PBMCs from representative mice.
Table 1.
Human B-ALL development in hu-mice receiving MLL-AF9-transduced CD34+ FLCs alone or along with FTHY.
| Group (n) | No. of hu-mice with human engraftment | No. of hu-mice with human B-ALL |
|---|---|---|
| Hu-mice with FTHY | 6 | 5 |
| Hu-mice without FTHY | 7 | 7 |
NSG mice were sublethally (2 Gy) irradiated, followed by transplantation of MLL-AF9-transduced CD34+ FLCs (i.v.; 1 × 105 per mouse) alone or along with FTHY (under the kidney capsule). Human cell engraftment and B-ALL development were assessed by FACS and histology. Data from hu-mice made of human CD34+ FLCs and FTHY from 3 different fetuses are combined.
Bone marrow or spleen cells, when adoptively transferred into sublethally-irradiated NSG mice, induced lethal leukemia in a cell number-dependent manner (Fig. S1a–b). The leukemia cells were also transplantable in hu-mice with an established autologous human immune system (i.e., transplanted with FTHY and CD34+ FLCs from the same fetus from which CD34+ FLCs were used to make the leukemic hu-mice), in which all recipient hu-mice showed detectable GFP+ leukemia cells in blood within 2 weeks and died by 49 days following injection of 1 × 105 leukemia cells (Fig. 2d). In contrast, the leukemia cells were rejected in hu-mice with an allogeneic immune system, i.e., hu-mice made of FTHY and CD34+ FLCs from a different fetus (Fig. S1c). Together, our data demonstrate that these leukemia cells are transplantable in immune compromised recipients or recipients with autologous immune system, thereby offering another means of creating hu-mice with a functional human lymphohematopoietic system and autologous leukemia. These data also demonstrate that human alloresponses can reject leukemia in hu-mice.
3.2. Lymphopenia Enhances Anti-leukemia Responses of Recipient Leukocyte Infusion in Mixed Chimeric Hu-mice
Previous studies have shown that administration of recipient leukocyte infusion (RLI) to mixed allogeneic chimeras results in loss of donor hematopoietic chimerism and anti-host leukemia responses (Rubio et al., 2003). Although the anti-leukemia response of RLI is significantly weaker than that of alloreactive donor leukocyte infusion (DLI) (Saito et al., 2006), RLI does not induce GVHD, presenting a safe approach for use in combination with other strategies of augmenting immune responses. Lymphopenia occurs frequently in patients with hematological malignancies who receive allo-HCT (Heining et al., 2007, Small et al., 1999) and promotes both antitumor responses (Dudley et al., 2002, Quezada et al., 2010) and GVHD (Li et al., 2012, Miller et al., 2007). Here we seek to employ lymphopenia to enhance antitumor responses of RLI in a human immune system using the leukemic hu-mouse model described above.
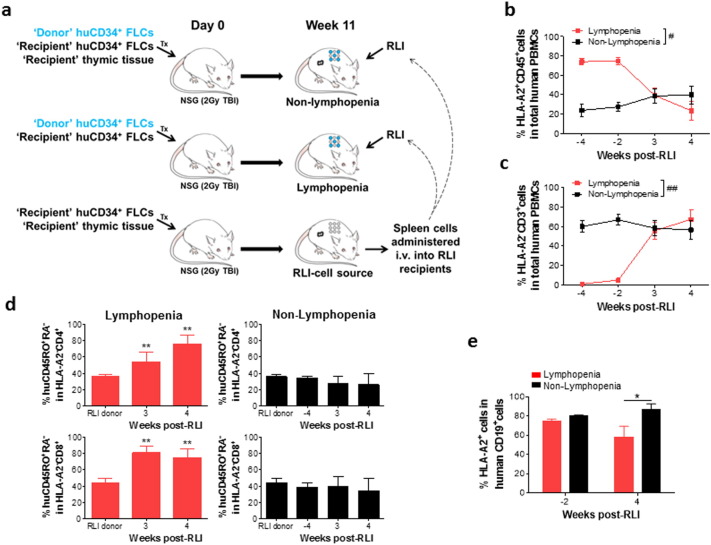
Antitumor responses of RLI in murine mixed chimeras (MCs) depend on host-vs.-graft reaction (HVGR), which in turn provokes immune responses to tumor-associated antigens expressed by recipient leukemia cells (Rubio et al., 2005, Rubio et al., 2006). Thus, we first assessed the potential of lymphopenia to promote HVGR. Non-lymphopenic MC controls were established by transplantation of a mixture of ‘recipient’ (HLA-A2−) and allogeneic ‘donor’ (HLA-A2+) CD34+ FLCs, together with implantation of ‘recipient’ FTHY. The hu-mice that received only a mixture of ‘recipient’ (HLA-A2−) and allogeneic ‘donor’ (HLA-A2+) CD34+ FLCs but no implantation of FTHY developed few T cells and were regarded lymphopenic MCs. As elucidated in Fig. 3a, RLI cells are autologous/syngeneic to the ‘recipient’ and allogeneic to the ‘donor’ origin. To establish a source of RLI cells, we created an additional group of hu-mice by transplantation of HLA-A2− ‘recipient’ FTHY and CD34+ FLCs into NSG mice. Unlike ‘recipient’ human T cells in the MCs, which are tolerant of both the ‘recipient’ and ‘donor’ cells (Hu and Yang, 2012), human T cells from the RLI-cell source hu-mice should show tolerance to only the ‘recipient’, but not the ‘donor’ alloantigens and could therefore mediate anti-‘donor’ alloresponses after injection into the MCs. Spleen cells were prepared from the RLI-cell source hu-mice 11 weeks after transplantation, when significant human T cell reconstitution was established (Fig. S2), and injected i.v. (i.e., RLI; 2 × 107/mouse) into hu-mouse MCs.
Fig. 3.
Lymphopenia promotes anti-donor alloresponses of RLI. (a) Scheme of the experimental design. Hu-mouse MCs were prepared by transplantation of a mixture of 2.5 × 104 HLA-A2− ‘recipient’ and 7.5 × 104 HLA-A2+ ‘donor’ CD34+ FLCs alone (lymphopenia), or along with HLA-A2− ‘recipient’ FTHY (non-lymphopenia) into NSG mice. RLI-cell source hu-mice were made by transplantation of HLA-A2− ‘recipient’ FTHY and CD34+ FLCs into NSG mice. (b) Levels of HLA-A2+ ‘donor’ chimerism. (c) Percentages of HLA-A2− ‘recipient’ CD3+ T cells in huCD45+ PBMCs. (d) Percentages of HLA-A2− ‘recipient’ CD4+ (Top) and CD8+ (Bottom) T cells expressing a effector/memory phenotype (i.e., CD45RA− CD45RO+). (e) The percentage of HLA-A2+ ‘donor’ cells in human CD19+ cell population. N = 4–6 per group at each time point; *P < 0.05; **P < 0.01 by the Student's t-test; #P < 0.05, ##P < 0.01 by two-way ANOVA with repeated measures.
FACS analysis revealed successful reconstitution with human hematopoietic cells and the establishment of mixed human chimerism in hu-mouse MCs (Figs. 3b and S3). Human T cells were barely detectable in MCs that did not receive FTHY (i.e., Lymphopenic group; Figs. 3c and S3). Comparing to that in the non-lymphopenic MCs, RLI T cells exhibited significantly more robust activation in the lymphopenic MCs, shown by a marked increase in the proportion of HLA-A2− ‘recipient’ CD3+ T cells (Fig. 3c), and of ‘recipient’ CD4+ and CD8+ T cells expressing a CD45RA− CD45RO+ effector/memory phenotype (Fig. 3d). After RLI, lymphopenic, but not non-lymphopenic, MCs showed a rapid decline in the levels of ‘donor’ (HLA-A2+) hematopoietic (CD45+) cells (Fig. 3b). To determine whether the decrease of ‘donor’ human chimerism resulted from dilution due to the expansion of HLA-A2− RLI T cells, we analyzed donor chimerism in the human B cell compartment. The ratios of ‘donor’ (HLA-A2+) CD19+ B cells to ‘recipient’ in the lymphopenic and non-lymphopenic MCs were comparable prior to RLI, but became significantly lower in the former than in the latter group after RLI (Fig. 3e). We conclude that lymphopenia significantly enhanced RLI-mediated HVGR in MCs.
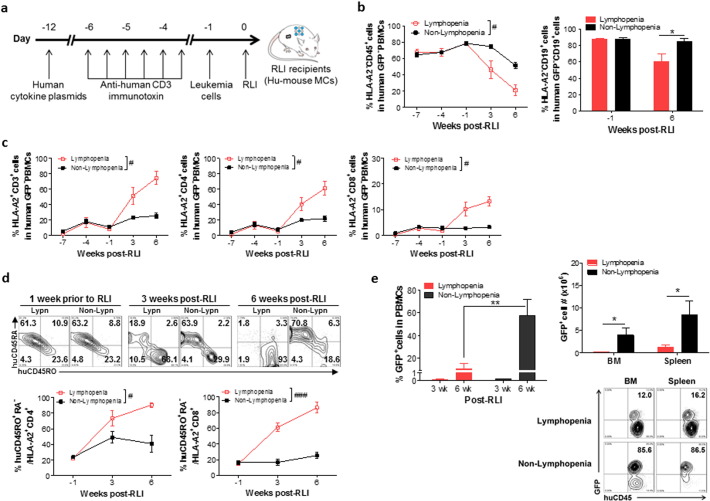
We next assessed the potential of lymphopenia to promote RLI-mediated anti-leukemia responses in MCs. Lymphopenia commonly seen in leukemia patients who need HCT is mainly induced by conditioning treatment and/or chemotherapy (Heining et al., 2007), and a lack of thymic reserve (Small et al., 1999). Thus, to simulate lymphopenia in leukemia patients, we depleted T cells by anti-huCD3-immunotoxin in the hu-mouse MCs that had received a mixture of ‘recipient’ (HLA-A2+) and allogeneic ‘donor’ (HLA-A2−) CD34+ FLCs with implantation of ‘recipient’ FTHY (Fig. 4a).
Fig. 4.
Lymphopenia promotes antitumor responses of RLI. (a) Experimental design. Hu-mouse MCs were prepared by transplantation of a mixture of 4 × 104 HLA-A2+ ‘recipient’ and 6 × 104 HLA-A2− ‘donor’ CD34+ FLCs, plus HLA-A2+ ‘recipient’ FTHY into NSG mice, and used as RLI recipients 12 weeks later. (b) Percentages of HLA-A2− ‘donor’ CD45+ cells in total human GFP− PBMCs (Left) and of HLA-A2− CD19+ ‘donor’ cells in human GFP− CD19+ cells (Right). (c) Percentages of the HLA-A2+ ‘recipient’ CD3+, CD4+ and CD8+ T cells in total human GFP− PBMCs. (d) CD45RA and CD45RO expression on HLA-A2+ ‘recipient’ CD3+ (top) and percentages of CD45RA− CD45RO+ cells in HLA-A2+ ‘recipient’ CD4+ and CD8+ (bottom) PBMCs. (e) Left, percentages of GFP+ leukemic cells in PBMCs; Right, absolute numbers of GFP+ leukemic cells in BM and spleen (top) and representative FACS profiles (bottom; numbers indicate the percentages of GFP+ leukemic cells in human CD45+ cells) at week 7 post-RLI (n ≥ 4 per group at each time point). *P < 0.05; **P < 0.01 by the Student's t-test; #P < 0.05, ###P < 0.001 by two-way ANOVA with repeated measures.
We then enhanced RLI effects by manipulating APCs and regulatory T cells (Tregs) in the hu-mouse MCs. Although data presented in Fig. 3 show that RLI mediated significantly stronger HVGR in lymphopenic than in non-lymphopenic hu-mouse MCs, the response was generally low in these mice, so as to be undetectable in the non-lymphopenic MCs. Because RLI-mediated HVGR in mouse models is largely dependent on donor APCs (Li and Sykes, 2012), the weak alloreactivity of RLI in the hu-mice may be caused by low numbers of APCs in the MCs. Thus, to boost human myeloid reconstitution, we treated hu-mouse MCs with human cytokines 12 days prior to RLI (Fig. 4a). The production of human cytokines (IL-15, Flt-3L, GM-CSF and IL-3) in hu-mouse MCs was achieved by hydrodynamic injection of the cytokine-containing plasmids as previously described (Fig. 4a) (Chen et al., 2009). Cytokine plasmid injection significantly increased reconstitution of human myeloid cells, including human CD11c+ CD14+, CD11c+ CD14− and CD11c+ HLA-DR+ cells (Fig. S4). Another factor that may inhibit RLI-mediated alloreactivity is the presence of relatively high levels of Tregs in the RLI inoculum (Fig. S5). Therefore, we also depleted CD25+ cells from RLI inoculum to enhance the RLI effect in the following experiments (see below and Fig. S7a).
T cell lymphopenia was induced in hu-mouse MCs by i.v. injection of anti-huCD3-immunotoxin (Woo et al., 2010) (5 μg/Kg BID for 3 consecutive days) starting 6 days prior to RLI (Fig. 4a). T-cell depletion in MCs was confirmed by FACS analysis (Fig. S6). RLI-cell source hu-mice were made by transplantation of ‘recipient’ FTHY and CD34+ FLCs as described above. After depletion of CD25+ human Tregs (Foxp3+ cells were mainly in the CD4+ CD25+ population, Fig. S7a), the RLI source cells contained approximately 34% human CD3+ T cells (with a CD4/CD8 ratio of about 3:1), in which most of the cells expressed a naïve phenotype (Fig. S7b). These RLI source cells were intravenously injected into hu-mouse MCs 4 days after the last injection of anti-huCD3-immunotoxin (12 weeks after FTHY/CD34+ FLC transplantation). Autologous leukemia cells were given to the hu-mouse MCs 1 day prior to RLI in order to assess the antitumor responses. Because anti-huCD3-immunotoxin has a very short in vivo half-life (approximately 45 min) (Woo et al., 2010), the conditioning treatment should not affect the function of RLI T cells given 4 days after the treatment.
FACS analysis of blood cells revealed that RLI was significantly more effective in eliminating donor hematopoietic cells in the lymphopenic than in control MCs. As shown in Fig. 4b, the levels of ‘donor’ (HLA-A2−) CD45+ cells and CD19+ cells were comparable between the control and lymphopenic MCs prior to RLI, but declined significantly more rapidly and extensively in the lymphopenic than in control MCs. The average percentages of HLA-A2− ‘donor’ CD45+ cells in GFP− PBMCs of the control and lymphopenic MCs were 79% vs. 79% 1 week prior to RLI, 75% vs. 45% 3 weeks post-RLI, and 52% vs. 21% 6 weeks post-RLI, respectively. Similar changes were seen in the CD19+ B cell population (Fig. 4b, right). The percentages of HLA-A2+ ‘recipient’ T cells (including CD4+ and CD8+ subsets) in total human GFP− PBMCs increased rapidly and extensively in MCs with lymphopenia than in those without (Fig. 4c). Furthermore, more human HLA-A2+ ‘recipient’ T cells (including both CD4+ and CD8+ subsets) expressed a CD45RA− CD45RO+ activated/memory phenotype in MC recipients with lymphopenia than in those without (Fig. 4d). We conclude that lymphopenia significantly enhances RLI induced HVGR.
The stronger HVGR in the lymphopenic MCs was associated with an increased antitumor response. FACS analysis of blood cells showed that the percentages of leukemia cells at weeks 3 and 6 post-RLI were 1.4 and 5.5 fold higher, respectively, in control MCs than in lymphopenic MCs (Fig. 4e, left). All mice were sacrificed at week 7 to measure leukemia cell counts. As shown in Fig. 4e (right), the control MCs had significantly more leukemia cells in the bone marrow and spleen than lymphopenic MCs. These data indicate that lymphopenia can significantly enhance RLI-mediated anti-leukemia responses in MCs.
4. Discussion
We report a unique hu-mouse model that permits testing human leukemia immunotherapy in recipients with functional human lymphohematopoietic systems. In addition to hu-mice having both normal human lymphohematopoietic systems and autologous leukemia, this model also enables the production of non-leukemic hu-mice with functional human lymphohematopoietic systems that are autologous or allogeneic to the leukemia. Thus, this model has potential for assessing adoptive immunotherapies using either autologous or allogeneic effectors. This hu-mouse model offers, to our knowledge, the only model available to date that permits in vivo analysis of antitumor immune responses to autologous human cancers and evaluation of anti-leukemia therapies in the presence of a human immune system. As an example, we recently confirmed the superiority of this model in testing and validating adoptive immunotherapy using autologous human T cells that are modified to express anti-CD19 chimeric antigen receptors (CARs; data not shown).
MLL-AF9 has been found to be associated with both ALL and acute myeloid leukemia (AML), but MLL-AF9 leukemia manifests more often as ALL in infants, whereas almost always as AML in adults (Meyer et al., 2013). Consistently, a previous mouse study suggests that the age of HSCs is involved in the lineage determination of MLL-AF9 leukemia, as MLL-AF9 leukemia derived from adult mouse marrow HSCs was myeloid in character with myeloperoxidase (MPO) positivity and negative for CD45R/B220, whereas MLL-AF9 leukemia derived from fetal mouse liver HSCs was positive for CD45R/B220 and negative for MPO (Chen et al., 2011). Thus, the use of fetal liver CD34+ cells might be a possible reason for the observed preference for B-ALL development in our model. However, our recent study showed that the lineage determination of MLL-AF9-driven leukemia in mice is also largely affected by the cytokine environment, as provision of human hematopoietic cytokines markedly increased the chance of AML development from MLL-AF9-transduced human CD34+ FLCs (unpublished data).
During allogeneic hematopoietic cell transplantation (allo-HCT), donor T cells mediate beneficial graft-vs.-tumor (GVT) effects. However, donor T cells also attack host normal tissues, resulting in graft-vs.-host disease (GVHD). Intriguingly, antitumor responses and sustained remissions of advanced chemo-refractory hematologic malignancies were also seen in patients who rejected donor grafts after nonmyeloablative allo-HCT (Dey et al., 2005). Mouse studies further demonstrated that administration of RLI to mixed allogeneic chimeras results in loss of donor hematopoietic chimerism and anti-host leukemia responses (Rubio et al., 2003). Although RLI mediates significantly weaker anti-leukemia response than DLI (Saito et al., 2006), RLI does not induce GVHD, presenting a safe approach for patients with a high risk of GVHD. Our data show that the antitumor response of RLI can be significantly potentiated in lymphopenic recipients, indicating that RLI may be a potential treatment option for leukemia patients with lymphopenia, an independent risk factor for GVHD following allo-HCT (Li et al., 2012).
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
J.X., Z.H., S.Y., Y.L., C-H.J., S.T., and Q.C. performed experiments; J.X., Z.H., W.L., and Y-G.Y. designed experiments and analyzed data; J.X. M.S. and Y-G.Y. wrote the paper; and Y-G.Y. conceived the research project.
Acknowledgments
The authors thank Dr. John E. Dick for providing MLL-AF9 vector, Dr. David Neville for providing anti-human CD3-immunotoxin, Dr. Kang Liu for critical review of the manuscript, and Siu-hong Ho for help with flow cytometry. This work was supported by grants from MOST of China (2015CB964400), NSFC (81273334) and NIH (P01AI045897 and R01AI064569). Flow cytometric analysis was partially performed in the CCTI Flow Cytometry Core funded in part through an NIH Shared Instrumentation Grant (1S10RR027050). The funders played no role in study design, data collection, data analysis and interpretation, or writing of the report.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.06.028.
Appendix A. Supplementary Data
Supplementary figures.
References
- Barabe F., Kennedy J.A., Hope K.J., Dick J.E. Modeling the initiation and progression of human acute leukemia in mice. Science. 2007;316:600–604. doi: 10.1126/science.1139851. [DOI] [PubMed] [Google Scholar]
- Chen Q., Khoury M., Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc. Natl. Acad. Sci. U. S. A. 2009;106:21783–21788. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., O'Sullivan M.G., Hudson W., Kersey J. Modeling human infant MLL leukemia in mice: leukemia from fetal liver differs from that originating in postnatal marrow. Blood. 2011;117:3474–3475. doi: 10.1182/blood-2010-11-317529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., He F., Kwang J., Chan J.K., Chen J. GM-CSF and IL-4 stimulate antibody responses in humanized mice by promoting T, B, and dendritic cell maturation. J. Immunol. 2012;189:5223–5229. doi: 10.4049/jimmunol.1201789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey B.R., McAfee S., Colby C., Cieply K., Caron M., Saidman S., Preffer F., Shaffer J., Tarbell N., Sackstein R. Anti-tumour response despite loss of donor chimaerism in patients treated with non-myeloablative conditioning and allogeneic stem cell transplantation. Br. J. Haematol. 2005;128:351–359. doi: 10.1111/j.1365-2141.2004.05328.x. [DOI] [PubMed] [Google Scholar]
- Dudley M.E., Wunderlich J.R., Robbins P.F., Yang J.C., Hwu P., Schwartzentruber D.J., Topalian S.L., Sherry R., Restifo N.P., Hubicki A.M. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heining C., Spyridonidis A., Bernhardt E., Schulte-Monting J., Behringer D., Grullich C., Jakob A., Bertz H., Finke J. Lymphocyte reconstitution following allogeneic hematopoietic stem cell transplantation: a retrospective study including 148 patients. Bone Marrow Transplant. 2007;39:613–622. doi: 10.1038/sj.bmt.1705648. [DOI] [PubMed] [Google Scholar]
- Hu Z., Yang Y.G. Human lymphohematopoietic reconstitution and immune function in immunodeficient mice receiving cotransplantation of human thymic tissue and CD34+ cells. Cell. Mol. Immunol. 2012;9:232–236. doi: 10.1038/cmi.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan P., Wang L., Diouf B., Eguchi H., Su H., Bronson R., Sachs D.H., Sykes M., Yang Y.G. Induction of human T-cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood. 2004;103:3964–3969. doi: 10.1182/blood-2003-10-3697. [DOI] [PubMed] [Google Scholar]
- Lan P., Tonomura N., Shimizu A., Wang S., Yang Y.G. Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood. 2006;108:487–492. doi: 10.1182/blood-2005-11-4388. [DOI] [PubMed] [Google Scholar]
- Li H.W., Sykes M. Emerging concepts in haematopoietic cell transplantation. Nat. Rev. Immunol. 2012;12:403–416. doi: 10.1038/nri3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.W., Sachs J., Pichardo C., Bronson R., Zhao G., Sykes M. Nonalloreactive T cells prevent donor lymphocyte infusion-induced graft-versus-host disease by controlling microbial stimuli. J. Immunol. 2012;189:5572–5581. doi: 10.4049/jimmunol.1200045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkus M.W., Estes J.D., Padgett-Thomas A., Gatlin J., Denton P.W., Othieno F.A., Wege A.K., Haase A.T., Garcia J.V. Humanized mice mount specific adaptive and innate immune responses to EBV and TSST-1. Nat. Med. 2006;12:1316–1322. doi: 10.1038/nm1431. [DOI] [PubMed] [Google Scholar]
- Meyer C., Hofmann J., Burmeister T., Groger D., Park T.S., Emerenciano M., Pombo de Oliveira M., Renneville A., Villarese P., Macintyre E. The MLL recombinome of acute leukemias in 2013. Leukemia. 2013;27:2165–2176. doi: 10.1038/leu.2013.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.S., Weisdorf D.J., Burns L.J., Slungaard A., Wagner J.E., Verneris M.R., Cooley S., Wangen R., Fautsch S.K., Nicklow R. Lymphodepletion followed by donor lymphocyte infusion (DLI) causes significantly more acute graft-versus-host disease than DLI alone. Blood. 2007;110:2761–2763. doi: 10.1182/blood-2007-05-090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada S.A., Simpson T.R., Peggs K.S., Merghoub T., Vider J., Fan X.Z., Blasberg R., Yagita H., Muranski P., Antony P.A. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio M.T., Kim Y.M., Sachs T., Mapara M., Zhao G., Sykes M. Antitumor effect of donor marrow graft rejection induced by recipient leukocyte infusions in mixed chimeras prepared with nonmyeloablative conditioning: critical role for recipient-derived IFN-gamma. Blood. 2003;102:2300–2307. doi: 10.1182/blood-2002-12-3949. [DOI] [PubMed] [Google Scholar]
- Rubio M.T., Saito T.I., Kattleman K., Zhao G., Buchli J., Sykes M. Mechanisms of the antitumor responses and host-versus-graft reactions induced by recipient leukocyte infusions in mixed chimeras prepared with nonmyeloablative conditioning: a critical role for recipient CD4 + T cells and recipient leukocyte infusion-derived IFN-gamma-producing CD8 + T cells. J. Immunol. 2005;175:665–676. doi: 10.4049/jimmunol.175.2.665. [DOI] [PubMed] [Google Scholar]
- Rubio M.T., Zhao G., Buchli J., Chittenden M., Sykes M. Role of indirect allo- and autoreactivity in anti-tumor responses induced by recipient leukocyte infusions (RLI) in mixed chimeras prepared with nonmyeloablative conditioning. Clin. Immunol. 2006;120:33–44. doi: 10.1016/j.clim.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Saito T.I., Rubio M.T., Sykes M. Clinical relevance of recipient leukocyte infusion as antitumor therapy following nonmyeloablative allogeneic hematopoietic cell transplantation. Exp. Hematol. 2006;34:1271–1277. doi: 10.1016/j.exphem.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Small T.N., Papadopoulos E.B., Boulad F., Black P., Castro-Malaspina H., Childs B.H., Collins N., Gillio A., George D., Jakubowski A. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood. 1999;93:467–480. [PubMed] [Google Scholar]
- Suda T., Gao X., Stolz D.B., Liu D. Structural impact of hydrodynamic injection on mouse liver. Gene Ther. 2007;14:129–137. doi: 10.1038/sj.gt.3302865. [DOI] [PubMed] [Google Scholar]
- Tonomura N., Habiro K., Shimizu A., Sykes M., Yang Y.G. Antigen-specific human T-cell responses and T cell-dependent production of human antibodies in a humanized mouse model. Blood. 2008;111:4293–4296. doi: 10.1182/blood-2007-11-121319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J.H., Lee Y.J., Neville D.M., Frankel A.E. Pharmacology of anti-CD3 diphtheria immunotoxin in CD3 positive T-cell lymphoma trials. Methods Mol. Biol. 2010;651:157–175. doi: 10.1007/978-1-60761-786-0_10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.