Abstract
Symbiotic nitrogen-fixing associations between Casuarina trees and the actinobacteria Frankia are widely used in agroforestry in particular for salinized land reclamation. The aim of this study was to analyze the effects of salinity on the establishment of the actinorhizal symbiosis between C. glauca and two contrasting Frankia strains (salt sensitive; CcI3 vs. salt tolerant; CeD) and the role of these isolates in the salt tolerance of C. glauca and C. equisetifolia plants. We show that the number of root nodules decreased with increasing salinity levels in both plants inoculated with CcI3 and CeD. Nodule formation did not occur in seedlings inoculated with CcI3 and CeD, at NaCl concentrations above 100 and 200 mM, respectively. Salinity also affected the early deformation of plant root hairs and reduced their number and size. In addition, expression of symbiotic marker Cg12 gene, which codes for a subtilase, was reduced at 50 mM NaCl. These data suggest that the reduction of nodulation in C. glauca under salt stress is in part due to inhibition of early mechanisms of infection. We also show that prior inoculation of C. glauca and C. equisetifolia with Frankia strains CcI3 and CeD significantly improved plant height, dry biomass, chlorophyll and proline contents at all levels of salinity tested, depending on the Casuarina-Frankia association. There was no correlation between in vitro salt tolerance of Frankia strains and efficiency in planta under salt-stressed conditions. Our results strongly indicate that increased N nutrition, photosynthesis potential and proline accumulation are important factors responsible for salt tolerance of nodulated C. glauca and C. equisetifolia.
Keywords: salinity, Frankia, Casuarina glauca, Casuarina equisetifolia, root hair deformation, CgNIN, Cg12, proline
Introduction
Soil salinization is a major problem worldwide. Indeed, high levels of salt in soil limit crop production and increase the loss of arable land. More than 800 million hectares of land worldwide are salt-affected (Munns and Tester, 2008). By the year 2050, 50% of all arable lands could be affected by salinity (Wang et al., 2003). There is therefore a need to design strategies to rehabilitate salinized areas.
Actinorhizal plants belonging to Casuarinaceae family such as Casuarina glauca and C. equisetifolia are able to grow under saline environments (El-Lakany and Luard, 1983; Girgis et al., 1992; Tani and Sasakawa, 2003). They are fast-growing trees, originated from Australia and Pacific islands, widely used in agroforestry systems for several purposes (Diem and Dommergues, 1990). In many tropical and sub-tropical countries, Casuarina species play a major role in land reclamation, crop protection and as windbreaks (National Research Council, 1984). In Senegal, a green barrier of C. equisetifolia was established on the northern Atlantic fringe between Dakar and Saint-Louis to stabilize sand dunes and protect the vegetable and fruit producing so-called “Niayes” area (Maheut and Dommergues, 1961; Mailly et al., 1994). C. equisetifolia is also appreciated for source of poles, firewood and charcoal (Diagne et al., 2013; Potgieter et al., 2014). Thus, this family of plants is of high importance for salinized land reclamation.
Casuarina species are pioneer plants, able to colonize poor and degraded lands and increase their fertility (Duhoux and Franche, 2003). Therefore, they promote development of pedogenetic processes leading to the formation of a more suitable microclimate for the installation of other plants species (Moiroud, 1996). This property is mainly due to the tremendous plasticity of their root system allowing them, among other things, to establish a nitrogen-fixing actinorhizal symbiosis with a filamentous soil bacterium called Frankia. Nitrogen is one of the main factors limiting plant growth and crop production worldwide, despite being the most abundant element in the atmosphere (80%). Unlike nitrogen-fixing plants, the majority of plant species are unable to directly utilize atmospheric nitrogen and rely on poor nitrogen sources in soils for their nutrition (Santi et al., 2013).
Among Casuarina species, C. glauca and C. equisetifolia display a high salt tolerance (El-Lakany and Luard, 1983). In addition, C. glauca is a model tree for basic and fundamental research in actinorhizal symbiosis with the development of many tools including genetic transformation of C. glauca and transcriptome analyses (Smouni et al., 2002; Gherbi et al., 2008; Tromas et al., 2012; Svistoonoff et al., 2013, 2014; Diédhiou et al., 2014; Champion et al., 2015). They are therefore good models to study the mechanisms involved in tolerance to salt stress in actinorhizal trees. One important question is how salt stress impacts actinorhizal symbioses establishment. The early steps of the infection process leading to the development of root nodules of Casuarina tree starts with the induction of root hair curling by Frankia, as early as 24 h after inoculation (Callaham et al., 1979; Perrine-Walker et al., 2011). Frankia hyphae proceed to penetrate the host plant through a deformed root hair (Franche et al., 1998) and induce the expression of several plant genes involved in actinorhizal nodule formation and functioning. Among them are the CgNIN, encoding a transcriptional factor, expressed at pre-infection stages in root hairs competent for Frankia infection (Clavijo et al., 2015) and Cg12, encoding a subtilase, whose expression is linked to the infection of root hairs and cortical cells by Frankia (Laplaze et al., 2000; Svistoonoff et al., 2003).
Frankia is a genus of soil actinobacteria (Normand et al., 2014). These Gram+, aerobic, heterotrophic bacteria are able to fix nitrogen both under free-living conditions and inside symbiotic root nodule (Simonet et al., 1989). The first pure culture of a Frankia strain was isolated from Comptonia peregrina nodules (Callaham et al., 1978). Since then, several Frankia strains have been isolated from different actinorhizal species (Diem et al., 1982, 1983; Gomaa et al., 2008; Gtari et al., 2015). They are grouped into four major clusters (Normand et al., 1996). Frankia strains in cluster 1 form nodules either with members of Betulaceae and Myricaceae (cluster 1a) or Casuarinaceae (cluster 1c). Cluster 2 includes Frankia strains able to infect the Coriariaceae, Datiscaceae, Rosaceae, and Ceanothus of the Rhamnaceae. Frankia strains in cluster 3 form effective nodules with the Myricaceae, Rhamnaceae, Elaeagnaceae, and Gymnostoma belonging to Casuarinaceae. Cluster 4 includes atypical Frankia strains, which are non-infective and/or non-nitrogen-fixing. Casuarina isolates show contrasting responses in their salt tolerance (Ngom et al., 2016). Indeed, some strains are more tolerant in vitro to salt stress than others even though they were isolated from the same host plant (Dawson and Gibson, 1987; Fauzia, 1999; Tani and Sasakawa, 2003; Oshone et al., 2013). Nevertheless, the symbiotic performance under salt stress of diverse Frankia strains toward Casuarinaceae species remains poorly understood.
In this study we aim to analyze (i) the effects of salinity on the establishment of symbiosis between C. glauca and two Frankia strains: CcI3 (a salt sensitive strain) vs. CeD (a salt tolerant strain) and (ii) the role of these isolates in salt tolerance in C. glauca and C. equisetifolia.
Materials and methods
Bacterial material and growth conditions
Two contrasting Frankia strains were used in this study. CcI3 strain, whose isolation was reported by Zhang et al. (1984) is sensitive to salt stress while CeD, isolated by Diem et al. (1982) is salt tolerant in our cultivation conditions. Both Frankia isolates were grown in liquid BAP medium which contained (at final concentration) 1.4 mM CaCl2·2H2O, 0.2 mM MgSO4·7H2O, 0.195 mM FeNaEDTA, 5.6 mM KH2PO4, 3.2 mM K2HPO4, trace elements (H3BO4, MnCl2·4H2O, ZnSO4·7H2O, CuSO4·5H2O, Na2MO O4·2H2O, and CoSO4·7H2O) and vitamins (thiamine-HCL, pyridoxine-HCL, folic acid, Ca panthotenate, nicotinic acid, biotin, and riboflavin) at a final pH of 6.7 (Murry et al., 1984). Sodium propionate (5 mM) and NH4Cl (5 mM) were used as carbon and nitrogen sources, respectively. For rapid hyphal growth, this nutrient BAP medium was modified and supplemented with phosphatidyl choline (3.33 g/L) and MES-Tris buffer (0.5 M, pH 6.8; Schwencke, 1991). Cultures were maintained at 28 ± 1°C, in darkness under stirring conditions.
Plant material, plant transformation growth conditions
C. glauca seeds (seed lot 15,934, ref. 086-5929) were collected at the Myall Lakes National Park in Australia and provided by the Australian Tree Seed Centre (ATSC, CSIRO). C. equisetifolia seeds (seedlot SN/2011/0014/D) were collected in Louga area in Senegal and provided by the National Tree Seed Program (PRONASEF).
For experiments with non-trangenic plants, C. glauca and C. equisetifolia seeds were germinated under semi axenic conditions in a plastic tray (53.5 × 27.5 cm) containing a sterile mixture of compost (ref EN 12580) and sandy soil (v/v; 120°C, 60 min). They were watered daily with a quarter-strength Hoagland liquid medium (Hoagland and Arnon, 1950) to promote germination and initial growth of the seedlings.
Genetic transformation of C. glauca was performed using an Agrobacterium tumefaciens strain containing a ProCg12:GFP construct (Svistoonoff et al., 2003). Six independent C. glauca transgenic lines were generated as described previously (Smouni et al., 2002). For each transgenic line, GFP expression was analyzed. All plants showed the expression pattern described in Svistoonoff et al. (2003). The ProCg12:GFP line showing the highest expression levels of GFP was clonally propagated as described (Svistoonoff et al., 2010). Similarly for ProCgNIN:GFP, we used the transgenic line previously described (Clavijo et al., 2015) which showed the highest GFP expression.
Effect of salinity on nodulation of C. glauca plants
One month after seed germination, C. glauca seedlings were uprooted from the soil, gently washed 5 times with distilled water. Seedlings were individually transferred in hydroponic conditions, into Gibson glass tubes filled with a 50 mL liquid BD medium supplemented with KNO3 (5 mM) as nitrogen source, at pH 6.7 (Broughton and Dilworth, 1971). They were incubated in a growth chamber at 28 ± 1°C with 16 h day/8 h night photoperiod and a 74 μmol m−2 s−1 light intensity. The BD medium was renewed every 2 weeks to avoid nutrient depletion and pH drift. After 1 month, salt stress was applied gradually through the weekly increment of one concentration of NaCl at 0, 50, 100, 200, 300, 400, and 500 mM. When 500 mM NaCl was reached, the plants were placed in nitrogen free-BD medium before being inoculated separately either with CcI3 or CeD Frankia strains.
C. glauca nodulation was performed as described previously (Ngom et al., 2015). Before inoculation, homogenized cells of CcI3 and CeD were suspended in sterile water with a final absorbance of 0.2, measured at λ = 595 nm for each strain. To establish actinorhizal symbiosis, inoculum of each strain was first brought into contact with the root system for 2 h. Plants were replaced back into Gibson tubes replenished with a 45 mL of nitrogen free-BD medium +5 mL of each bacterial suspension. Nodulation rate or the percentage of nodulated plants (total number of nodulated plants/total number of inoculated plants × 100) and the mean nodule number (average number of nodules per plant) were followed for about 2 months after inoculation. All experiments were repeated twice and 22 plants were used for each salt treatment per experiment.
Effect of salinity on C. glauca root hair deformation
C. glauca seedlings were placed in hydroponic culture. Salt stress was applied gradually and plants were inoculated separately with either Frankia strain CcI3 or Frankia strain CeD, as described above. Two days after inoculation, root hair deformation was evaluated through micrographs of small lateral roots acquired with a Micro Publisher 3.3 RTV digital camera (QImaging) and a BX50F microscope (Olympus). For each treatment, five plants were used and three lateral roots were analyzed per plant. A total of 180 lateral roots and 12,217 root hairs were observed. Root hair deformation intensity was evaluated as described in Clavijo et al. (2015). For each micrograph, root hairs were observed and the following scoring was used: 0, no deformation; 1, straight root hair with tip swelling; 2, only one change in growth direction; 3, more than one change in growth direction but no bifurcation; 4, one or more bifurcations. At least two independent experiments were performed.
Analysis of CgNIN and Cg12 activation under salinity
Transgenic lines expressing ProCgNIN:GFP or ProCg12:GFP fusions were propagated and grown hydroponically in BD medium as described previously (Svistoonoff et al., 2010). Two NaCl concentrations (0 and 50 mM) were applied for 7 days. Plants were inoculated either with Frankia strain CcI3 or CeD, as described above. For each transgenic line, four plants per treatment were used. Activation of ProCgNIN:GFP was monitored 24, 48, and 72 h after inoculation. Activation of ProCg12:GFP was observed 3, 7, and 14 days after inoculation and nodule sections were examined for GFP fluorescence. GFP expression was observed using an AZ100 epifluorescence microscope (Nikon) and a GFP filter.
Effects of prior inoculation with Frankia on the salt tolerance of C. glauca
C. glauca seedlings were cultivated in hydroponic conditions and were nodulated with Frankia strains CcI3 or CeD, as described above. A batch of 22 uninoculated plants was used as controls. After inoculation, nodule formation was monitored weekly. Twenty-five days after inoculation, all of the plants were nodulated and treatment with NaCl was initiated. Salt stress (0, 50, 100, 200, 300, 400, and 500 mM NaCl) was applied gradually, as described above, to avoid osmotic shock. Morphological and physiological parameters of growth such as length of aerial parts, shoot and root dry weight, chlorophyll, and proline contents were evaluated as described below. Independent experiments were performed twice with 22 plants each treatment per experiment.
Effects of prior inoculation with Frankia on the salt tolerance of C. equisetifolia
One month after seed germination, C. equisetifolia seedlings were transplanted into plastic bags containing sterile sandy soil (120°C, 1 h). The experiments were conducted in a nethouse (Bel-Air experimental station, 14°44′N–17°30′W, Dakar, Senegal). Seedlings were watered daily and inoculation was applied 1 month after transplantation. Suspension of crushed nodule was used as inoculum. Nodules (20 g) were collected from C. glauca plants grown in hydroponic conditions and inoculated separately with Frankia strains CcI3 or CeD. Nodules were surface-sterilized with 5% sodium hypochlorite for 20 min then rinsed 3 times in sterile distilled water as described by Ng (1987). Grounded nodules were resuspended in 500 mL sterile distilled water. A 5 mL suspension was added into each bag according to the Frankia strain except for uninoculated plants. A batch of 8 plants was used for each treatment. As for C. glauca, the establishment of the symbiosis was monitored before gradually applying salt stress (0, 50, 100, 200, 300, 400, and 500 mM NaCl), as described above. Morphological and physiological parameters of growth such as length of aerial parts, shoot and root dry weight, chlorophyll, and proline contents were evaluated as described below.
Growth of aerial part and dry weight determination
Length of aerial parts were measured every 2 weeks. Four months after inoculation, plants were harvested. Shoot and root systems were collected, washed in deionized water, surface-wiped with blotting paper, and dried at 70°C for 72 h. The dried biomasses of each samples (C. glauca n = 22, C. equisetifolia n = 8 per sample) were weighed separately.
Measurement of chlorophyll content
Chlorophyll content was determined using Arnon's method (1949). Fresh leaves (100 mg) were crushed in 10 mL of acetone at 80%. Samples were incubated overnight at 4°C and centrifuged at 6000 g for 10 min. The absorbance of chlorophyll (a) and (b) was measured using a UV-1800 spectrophotometer (UVisco) at λ = 663 and 645 nm, respectively. Total chlorophyll content (C. glauca n = 5, C. equisetifolia n = 4 per sample) was calculated according to Arnon (1949).
Extraction and measurement of proline content
Fresh leaves (100 mg) were crushed in 2 mL of methanol at 40%, and the samples were immerged in a water bath at 85°C for 1 h. After cooling, 1 mL of leaf extract was mixed with 1 mL of ninhydrin at 2.5% and 1 mL of the reaction mixture (48 mL distilled water, 32 mL acetic acid, and 120 mL orthophosphoric acid). A second incubation was done in a water bath at 100°C for 30 min. Samples were cooled on ice, then a 5 mL toluene was added to the mixture. The upper phase was collected after vortexing and dehydrated with anhydrous sodium sulfate. Absorbance of leave samples was measured using a spectrophotometer at λ = 520 nm, as described by Monneveux and Nemmar (1986). Proline contents (C. glauca n = 5, C. equisetifolia n = 4 per sample) were calculated and determined through a calibration straight graph constructed from a standard range of proline concentrations (Monneveux and Nemmar, 1986).
Acetylene reduction assay (ARA)
Nitrogen fixation was measured using the acetylene reduction assay described by Hardy et al. (1973). C. glauca plants were placed in tightly closed 150 mL jars. In each jar, 10% of the air (15 mL) was removed and replaced with acetylene. Plants were incubated at 28°C for 3 h. From each jar, 1 mL was withdrawn and assayed for ethylene using a gas chromatograph (Agilent 6850, GC System). Nodules were removed from plant roots and dried at 70°C for 72 h. Nitrogenase activity was calculated per nodule dry weight and expressed as nmoles ethylene/nodule (g).
Statistical analysis
Statistical analyses were performed on dry weight, chlorophyll and proline data. Statistical tests were performed using the XLSTAT 7.2 software. The Student-Newman–Keuls test at p < 0.05 was used to evaluate the differences between inoculated and uninoculated plants and between NaCl treatments.
Results
Effects of salt and osmotic stresses on the growth of Frankia strains CcI3 and CeD
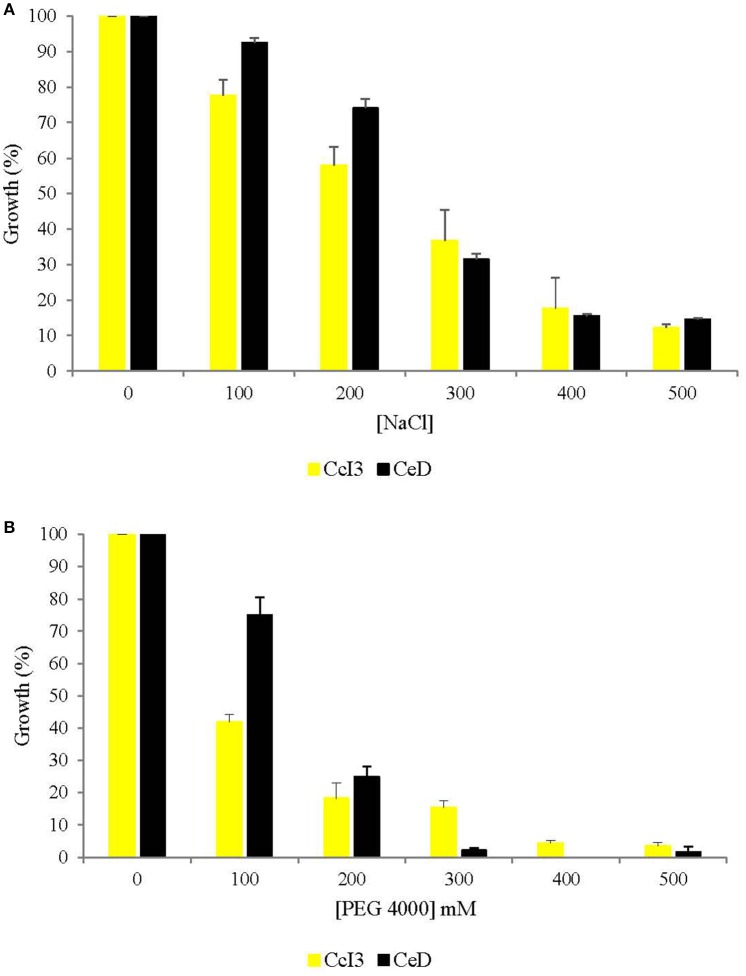
First we analyzed the growth of 8 Frankia strains isolated from several Casuarina species under saline conditions. All of the strains showed a reduced growth in response to salt treatment (data not shown). Two Frankia strains (CcI3 and CeD) were selected on the basis of their different sensitivity to salt and osmotic stresses. As shown in Figure 1, the growth of both Frankia isolates was reduced by increasing the NaCl and PEG concentrations in the medium. At 100 and 200 mM NaCl or PEG, the growth of CeD was significantly less impacted than the growth of CcI3 (Figures 1A,B). At high concentrations of NaCl and PEG (300, 400, and 500 mM), no or reduced growth was observed for both strains, with a more pronounced effect in presence of PEG 4000 in the medium. Altogether, our data indicated that CeD is more tolerant to salt and osmotic stresses than CcI3.
Figure 1.
Effect of salt and osmotic stresses on the growth of Frankia strains Ccl3 and CeD. Cultures were grown under several concentrations of NaCl (A) and Polyethylene Glycol 4000 (B) for 7 days. Growth of each Frankia strain was estimated by measuring the turbidity at λ = 595 nm. The growth of Frankia in absence of salt and osmotic stresses (100% growth) was compared with those in the presence of NaCl or PEG 4000. Vertical bars indicate the standard error of mean (2 biological and 8 technical replicates). The absence of error bars indicates that the size of the error does not exceed the size of the symbol.
Salinity inhibits C. glauca plant nodulation
The impact of different NaCl concentrations on the nodulation of C. glauca plants by Frankia strains CcI3 and CeD was studied (Table 1). In both plants inoculated with Frankia CcI3 and CeD, the control plants (0 mM NaCl) had higher mean nodule number and rate of nodulation than NaCl-treated plants. The number of nodules formed increased over time at different rates with more nodules on seedlings inoculated with Frankia strain CeD for 63 days. At 50 mM NaCl, the mean nodule number declined by 66.6 and 60.3% in plants inoculated with CcI3 and CeD, respectively, compared to control plants. Nodule formation did not occur in seedlings inoculated with strain CcI3 at NaCl concentrations above 100 mM, whereas some plants inoculated with strain CeD were still forming nodules at 200 mM NaCl after 49 days of inoculation. Mean nodule number and nodulation rate of seedlings were reduced by increasing the salinity level.
Table 1.
Effects of several concentrations of NaCl on the nodulation of C. glauca plants inoculated with Frankia strains CcI3 and CeD.
| Frankia strains | NaCl treatments (mM) | Nodulation kinetic (Days after inoculation) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 14 | 21 | 28 | 35 | 42 | 49 | 56 | 63 | |||
| CcI3 | 0 | Nodulation rate (%) | 72.7 | 95.5 | 100 | 100 | 100 | 100 | 100 | 100 |
| Mean nodule number | 4.4 ± 1.1 | 9.9 ± 1.7 | 27.9 ± 3.4 | 36.1 ± 4.2 | 40.4 ± 4.4 | 42.2 ± 4.1 | 44.1 ± 4.2 | 45.8 ± 4.2 | ||
| 50 | Nodulation rate (%) | 36.4 | 59.1 | 86.4 | 86.4 | 90.9 | 90.9 | 90.9 | 95.5 | |
| Mean nodule number | 0.8 ± 0.3 | 3 ± 0.8 | 7.4 ± 1.4 | 9.6 ± 1.9 | 10.2 ± 2 | 11.7 ± 1.9 | 13.4 ± 2.2 | 15.3 ± 2.3 | ||
| 100 | Nodulation rate (%) | 4.5 | 18.2 | 40.9 | 40.9 | 40.9 | 40.9 | 40.9 | 40.9 | |
| Mean nodule number | 0.05 ± 0.05 | 0.4 ± 0.2 | 2.4 ± 0.9 | 3.6 ± 1.3 | 3.7 ± 1.4 | 4.2 ± 1.6 | 4.5 ± 1.7 | 4.9 ± 1.8 | ||
| ≥200 | Nodulation rate (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mean nodule number | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| CeD | 0 | Nodulation rate (%) | 50 | 90.9 | 100 | 100 | 100 | 100 | 100 | 100 |
| Mean nodule number | 2.3 ± 0.7 | 11 ± 2 | 28 ± 3.3 | 37.1 ± 3.3 | 38.8 ± 3.3 | 44.8 ± 3.4 | 48.8 ± 3.5 | 50.3 ± 3.5 | ||
| 50 | Nodulation rate (%) | 18.2 | 50 | 68.2 | 68.2 | 68.2 | 81.8 | 81.8 | 81.8 | |
| Mean nodule number | 0.5 ± 0.3 | 2.7 ± 0.8 | 7.1 ± 1.5 | 12.2 ± 2.5 | 13.5 ± 2.6 | 16.7 ± 2.8 | 18 ± 3 | 20 ± 3.2 | ||
| 100 | Nodulation rate (%) | 4.5 | 9.1 | 22.7 | 40.9 | 40.9 | 59.1 | 63.6 | 68.2 | |
| Mean nodule number | 0.05 ± 0.05 | 0.1 ± 0.1 | 3 ± 1.2 | 4.6 ± 1.6 | 4.7 ± 1.6 | 7.9 ± 2.3 | 9.1 ± 2.4 | 9.7 ± 2.5 | ||
| 200 | Nodulation rate (%) | 0 | 0 | 0 | 0 | 0 | 9.1 | 9.1 | 22.7 | |
| Mean nodule number | 0 | 0 | 0 | 0 | 0 | 0.5 ± 0.3 | 0.6 ± 0.4 | 0.8 ± 0.5 | ||
| ≥300 | Nodulation rate (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Mean nodule number | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
Salt stress was first gradually applied then plants were inoculated separately with each Frankia strain. Nodulation rate is the percentage of plant nodulated and mean nodule number is the average number of nodule per plant. Values represent the mean ± standard deviation of plants used in each treatment (n = 22).
Salinity severely affects root hair deformation response to Frankia inoculation
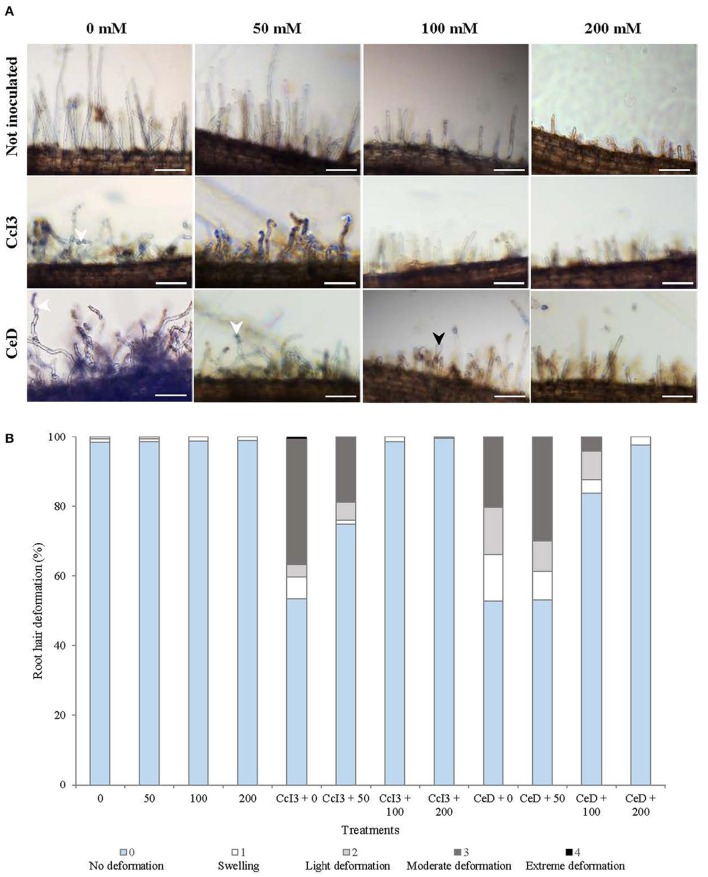
Because salinity inhibited nodulation, the effects of salt stress during the early stages of the actinorhizal symbiosis establishment was investigated. Root hair deformation responses in C. glauca plants treated with several concentrations of NaCl and inoculated with Frankia strains CcI3 and CeD was first analyzed. Regardless of the presence of Frankia, salt treatment reduced the number and size of root hairs in both uninoculated and inoculated C. glauca plants (Figure 2A). In C. glauca plants inoculated with Frankia strains CcI3 and CeD, extensive root hair deformation was detected 2 days after inoculation, in small lateral roots of no salt-treated plants. Increased salinity reduced the amount of deformation in seedlings inoculated by both strains with a more pronounced effect for plants inoculated with CcI3 (Figure 2B). Deformation was particularly low at 200 mM NaCl, which also showed previously the smallest number of nodules in plants inoculated with CeD and no nodule development in plants inoculated with CcI3. No or few deformation were observed on uninoculated plants treated with various levels of NaCl.
Figure 2.
Effect of various levels of NaCl on C. glauca root hair deformation. (A) Root hairs under salt-stressed conditions from uninoculated and inoculated C. glauca plants, observed 2 days after inoculation. White arrows indicate moderate deformation and black arrows swelling root hairs (Bars, 100 μm). (B) Quantification of root hair deformation showing the proportion of deformed root hairs in short lateral roots 2 days after inoculation with Frankia strains Ccl3 and CeD. For each treatment, 5 plants were used and 3 lateral roots were observed per plant.
Effects of salinity on CgNIN and Cg12 expression
To further investigate the effects of salinity during the early stages of the establishment of symbiosis, we studied the impact of salt stress on the expression of two early symbiotic marker genes: CgNIN (Clavijo et al., 2015) and Cg12 (Svistoonoff et al., 2003) using transgenic plants of C. glauca expressing ProCgNIN:GFP and ProCg12:GFP. CgNIN gene is a pre-infection marker which is early expressed in root hairs competent for Frankia infection (Clavijo et al., 2015) and Cg12, an infection marker associated with root hairs and cortical cells infection by Frankia (Svistoonoff et al., 2003).
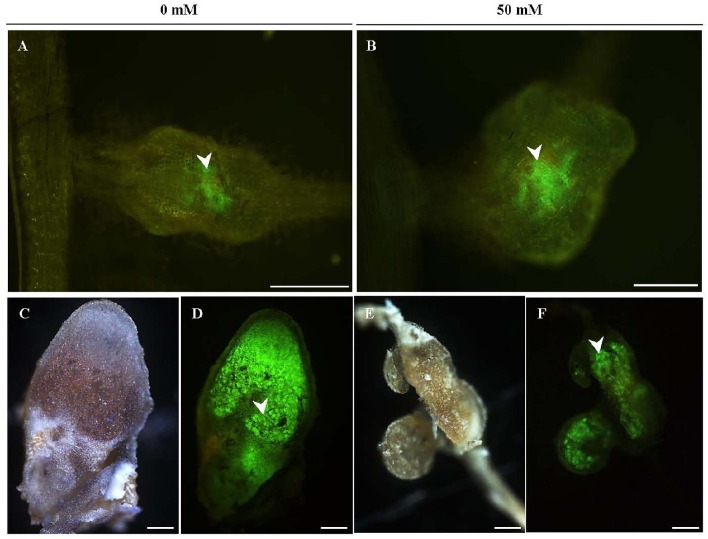
Observations revealed that ProCgNIN:GFP was activated in both control and 50 mM NaCl treated plants, from 24 to 72 h after inoculation with Frankia strains CcI3 and CeD (Table 2A), suggesting that CgNIN expression was not repressed by salt treatment (50 mM NaCl). Expression of ProCg12:GFP was observed 14 days after inoculation in both control and 50 mM NaCl treated plants. A lower number of fluorescent spots per plant were detected in NaCl treated plants (Table 2B), pointing to a possible inhibition of infection and ProCg12 expression by salinity. We were not able to detect any differences regarding the pattern or the intensity of ProCg12 activation in prenodules or nodules when comparing control and NaCl-treated plants, as shown in Figure 3.
Table 2.
Effect of salt stress on ProCgNIN and ProCg12 genes activation.
| (A) | |||||
|---|---|---|---|---|---|
| Transgenic line | Frankia strains | NaCl treatments (mM) | Hours after inoculation | ||
| 24 | 48 | 72 | |||
| ProCgNIN: GFP | CcI3 | 0 | + | + | + |
| 50 | + | + | + | ||
| CeD | 0 | + | + | + | |
| 50 | + | + | + | ||
| (B) | |||||
| Transgenic line | Frankia strains | NaCl treatments (mM) | Days after inoculation | ||
| 3 | 7 | 14 | |||
| ProCg12: GFP | CcI3 | 0 | − | − | + + + |
| 50 | − | − | + | ||
| CeD | 0 | − | − | + + + | |
| 50 | − | − | + | ||
Activation of ProCgNIN:GFP (A) and ProCg12:GFP (B) genes in presence of 0 and 50 mM of NaCl. Reporter gene expression (GFP) was detected using an epifluorescence microscopy. More sign + indicate more fluorescent spots observed per plant in transgenic lines.
+, gene activation; −, inactivation of gene.
Figure 3.
ProCg12 is active in saline condition during infection by Frankia. Salt stress (50 mM NaCl) was first applied then C. glauca plants were inoculated separately with Frankia strains Ccl3 (A–D) and CeD (E,F). ProCg12 is activated in prenodules (A,B) and nodules (C–F) of control and NaCl treated C. glauca plants, 14 days after inoculation. (C–F) Sections of matures nodules expressing green fluorescent protein (GFP). White arrows indicate reporter gene expression. (C,E) Bright field microscopy. (A,B,D,F) Epifluorescence microscopy. Bars 100 μm.
Nodulated C. glauca and C. equisetifolia plants are more tolerant to salt stress
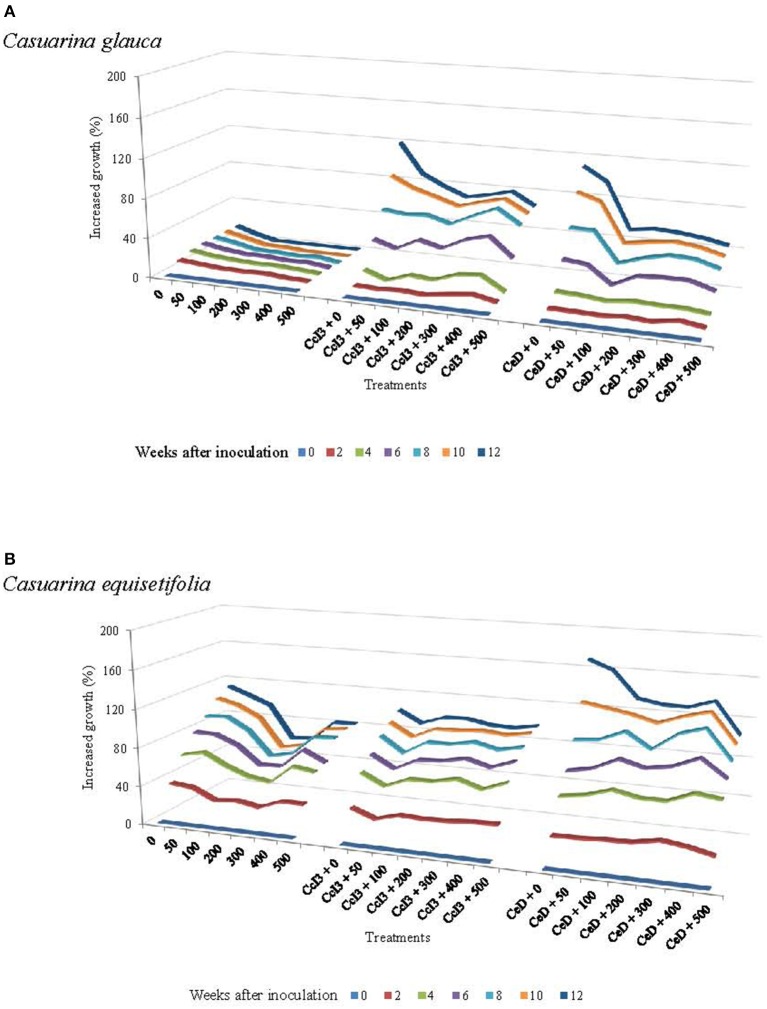
In addition to C. glauca, C. equisetifolia was studied because it is the most introduced Casuarina species worldwide for land reclamation and reforestation programs including Senegal (LADA, 2003; National Research Council, 1984). Furthermore, C. equisetifolia is also highly tolerant to salt stress (El-Lakany and Luard, 1983). The effects of prior inoculation with Frankia strains CcI3 and CeD on the salt tolerance of C. glauca and C. equisetifolia was studied to see if these nitrogen-fixing bacteria could be used to increase the salt tolerance of Casuarina species. Both Frankia strains CcI3 and CeD improved the growth of C. glauca at all concentrations of NaCl tested compared to the control plants (Figure 4A). In control plants, growth decreased with increased NaCl concentrations. There was no growth above 50 mM NaCl, 12 weeks after inoculation. Positive effect of inoculation with Frankia CcI3 and CeD on plant height started to be observed 4 weeks after inoculation. Plant height increased progressively at all NaCl concentrations in inoculated plants compared to the control, but growth gradually decreased with increasing NaCl concentrations. For instance, an increase by 65.5 and 44.5% was observed in 500 mM NaCl treated plants inoculated with Frankia strains CcI3 and CeD, respectively compared to controls. In contrast, in C. equisetifolia plants only Frankia strain CeD increased plant growth at all NaCl concentrations as compared to the control (Figure 4B).
Figure 4.
Shoot growth of non-nodulated and nodulated C. glauca (A) and C. equisetifolia (B) plants treated with various salt concentrations. Plants were inoculated separately with Frankia strains Ccl3 and CeD. Salt stress was applied gradually after the establishment of symbiosis. For each treatment, height growth was measured every 2 weeks from the day of inoculation and the increased growth was calculated from this time (0% of growth). Each value represents the mean of plants used in each treatment (C. glauca n = 22, C. equisetifolia n = 8).
Inoculation with Frankia strains CcI3 and CeD improved C. glauca shoot and total biomass significantly in all NaCl treatments compared to control (Table 3A). Compared to control, Frankia strains CcI3 and CeD significantly increased root biomass in all NaCl treatments except for 0, 50, and 100 mM salt-treated plants inoculated with CeD. In both control and inoculated plants, root, shoot, and total biomass decreased with increasing salt concentration, but the change was not significant in plants inoculated with strain CeD and in root dry biomass of control plants. On the other hand, only CeD increased significantly shoot and total dry biomass of C. equisetifolia plants for some NaCl treatments (0–200 mM) compared to the control and the plants inoculated with CcI3 (Table 3B). As observed for C. glauca plants, root, shoot, and total dry biomass decreased with increasing salt concentration in both control and inoculated plants. The change was significant between NaCl treatments in general and between low (0 and 50 mM NaCl) and high salinity (300 and 500 mM NaCl) in particular.
Table 3.
Mean comparison of shoot and root dry weight of non-nodulated and nodulated C. glauca (A) and C. equisetifolia (B) plants treated with several salt concentrations.
| (A) C. glauca | ||||
|---|---|---|---|---|
| NaCl treatments (mM) | Dry weight (g) | |||
| Control | CcI3 | CeD | ||
| Shoot | 0 | 0.449 c A | 1.322 a A | 0.876 b A |
| 50 | 0.364 c AB | 1.103 a AB | 0.800 b A | |
| 100 | 0.320 c B | 1.111 a AB | 0.694 b A | |
| 200 | 0.347 c AB | 0.924 a B | 0.708 b A | |
| 300 | 0.330 c B | 0.920 a B | 0.660 b A | |
| 400 | 0.291 c B | 0.921 a B | 0.661 b A | |
| 500 | 0.244 c B | 0.836 a B | 0.697 b A | |
| Root | 0 | 0.238 b A | 0.422 a A | 0.272 b A |
| 50 | 0.222 b A | 0.359 a AB | 0.264 b A | |
| 100 | 0.204 b A | 0.354 a AB | 0.241 b A | |
| 200 | 0.192 b A | 0.284 a B | 0.258 a A | |
| 300 | 0.190 b A | 0.291 a B | 0.259 a A | |
| 400 | 0.177 c A | 0.309 a B | 0.230 b A | |
| 500 | 0.174 b A | 0.256 a B | 0.272 a A | |
| Total biomass | 0 | 0.687 c A | 1.744 a A | 1.148 b A |
| 50 | 0.586 c AB | 1.462 a AB | 1.064 b A | |
| 100 | 0.524 c AB | 1.465 a AB | 0.935 b A | |
| 200 | 0.539 c AB | 1.208 a BC | 0.966 b A | |
| 300 | 0.520 c AB | 1.211 a BC | 0.919 b A | |
| 400 | 0.468 c B | 1.230 a BC | 0.891 b A | |
| 500 | 0.418 b B | 1.092 a C | 0.969 a A | |
| (B) C. equisetifolia | ||||
| Shoot | 0 | 2.813 b A | 3.220 b A | 5.252 a A |
| 50 | 2.336 b AB | 2.351 b B | 3.432 a B | |
| 100 | 2.159 b ABC | 2.106 b BC | 2.804 a BC | |
| 200 | 1.828 b BC | 1.882 b CD | 2.518 a CD | |
| 300 | 1.538 a BC | 1.790 a CD | 1.918 a D | |
| 400 | 1.531 a BC | 1.496 a D | 1.838 a D | |
| 500 | 1.349 a C | 1.384 a D | 1.696 a D | |
| Root | 0 | 1.136 a A | 1.375 a A | 1.249 a A |
| 50 | 0.730 b B | 1.026 ab B | 1.170 a A | |
| 100 | 0.850 a B | 0.778 a C | 0.940 a B | |
| 200 | 0.614 a B | 0.689 a CD | 0.788 a B | |
| 300 | 0.553 a B | 0.595 a CD | 0.544 a C | |
| 400 | 0.595 a B | 0.544 a CD | 0.503 a C | |
| 500 | 0.575 a B | 0.395 a D | 0.464 a C | |
| Total biomass | 0 | 3.949 b A | 4.595 b A | 6.501 a A |
| 50 | 3.066 b B | 3.377 b B | 4.602 a B | |
| 100 | 3.009 b BC | 2.884 b C | 3.744 a BC | |
| 200 | 2.442 b BC | 2.571 b CD | 3.306 a CD | |
| 300 | 2.091 a BC | 2.385 a CD | 2.462 a DE | |
| 400 | 2.126 a BC | 2.040 a DE | 2.341 a DE | |
| 500 | 1.924 a C | 1.779 a E | 2.160 a E | |
Each value represents the mean of plants used in each treatment (C. glauca n = 22, C. equisetifolia n = 8). For each salt concentration, different lowercase letters (a–c) indicate significant difference between control and plants inoculated separately with Frankia CcI3 and CeD. For each condition (control/plants inoculated with each strain), different capital letters (A–E) indicate significant difference between NaCl treatments according to the Student-Newman-Keuls (SNK) test at P < 0.05.
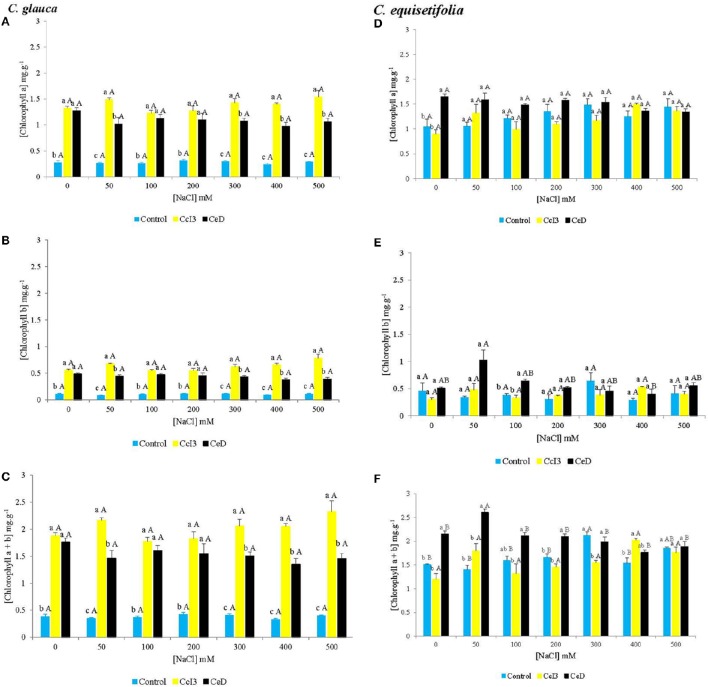
Chlorophyll and proline contents were determined in order to appreciate physiological state of non-nodulated and nodulated plants in saline conditions. The chlorophyll content (a, b, and total) was significantly increased in C. glauca plants inoculated with Frankia strains CcI3 and CeD, as compared to control (Figures 5A–C). However, there was no significant difference between NaCl treatments in C. glauca plants. In C. equisetifolia plants, the chlorophyll a was significantly increased by strain CeD only in no salt-treated plants (Figures 5D–F). No significant difference was observed between NaCl treatments. However, Frankia strain CeD increased the total chlorophyll content in 0, 50, 100, and 200 mM NaCl treated plants, compared to control and plants inoculated with CcI3.
Figure 5.
Mean comparison of chlorophyll contents in non-nodulated and nodulated C. glauca (A–C) and C. equisetifolia (D–F) plants under saline conditions. Each value represents the mean of plants used in each treatment (C. glauca n = 5, C. equisetifolia n = 4). For each salt concentration, different lowercase letters (a–c) indicate significant difference between control and plants inoculated separately with Frankia Ccl3 and CeD. For each condition (control/plants inoculated with each strain), different capital letters (A,B) indicate significant difference between NaCl treatments according to the Student-Newman-Keuls (SNK) test at P < 0.05.
As what was observed in chlorophyll content, there were significant changes in proline content between control and inoculated plants (Figure 6). Frankia strains CcI3 and CeD increased the proline content of C. glauca at all concentrations of salt tested (Figure 6A). Proline content increased with increasing salt concentrations in the control and inoculated plants, but the change was not significant in C. glauca plants inoculated with strain CeD. Similarly, in C. equisetifolia plants, proline content increased with increasing salinity (Figure 6B). There were significant differences between NaCl treatments in both control and plants inoculated with strains CcI3 and CeD. Only Frankia strain CeD increased proline contents significantly in 0, 50, and 300 mM NaCl treated plants.
Figure 6.
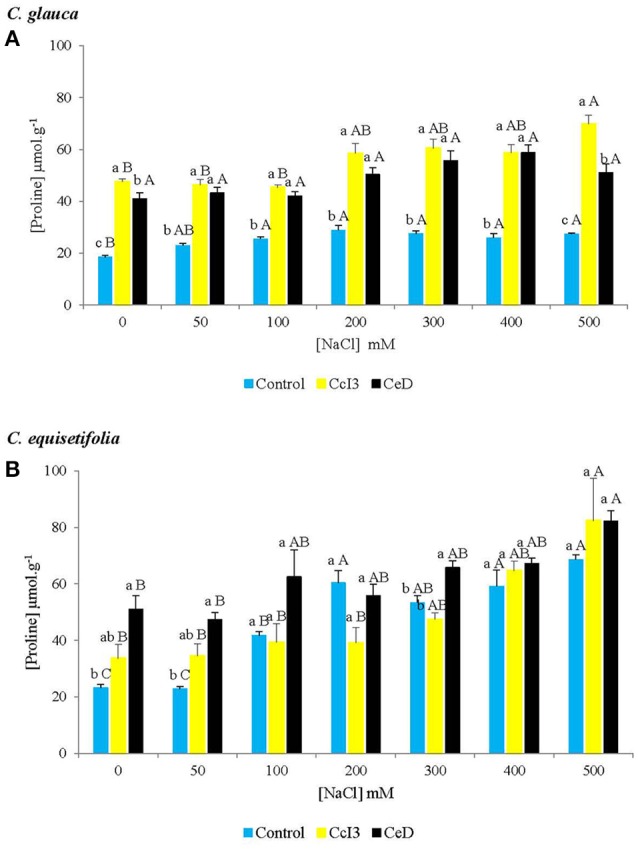
Mean comparison of proline contents in non-nodulated and nodulated C. glauca (A) and C. equisetifolia (B) plants treated with several salt concentrations. Each value represents the mean of plants used in each treatment (C. glauca n = 5, C. equisetifolia n = 4). For each salt concentration, different lowercase letters (a–c) indicate significant difference between control and plants inoculated separately with Frankia Ccl3 and CeD. For each condition (control/plants inoculated with each strain), different capital letters (A,B) indicate significant difference between NaCl treatments according to the Student-Newman-Keuls (SNK) test at P < 0.05.
Discussion
Among Casuarina tree species, C. glauca and C. equisetifolia have been shown to be highly salt-tolerant (Hyland, 1983; Luard and El-Lakany, 1984; Aswathappa and Bachelard, 1986; Van der Moezel et al., 1989) and are widely planted outside of their native habitat (National Research Council, 1984). However, salinity could affect plant growth and the establishment of actinorhizal symbiosis which could be thus a limit to salinized land reclamation (Reddell et al., 1986). In this study, we first investigated the effect of salinity on the symbiotic relationship between C. glauca and two contrasting Frankia strains CcI3 (salt sensitive) and CeD (salt tolerant).
Our results indicate that nodule formation in C. glauca is inhibited by salt stress regardless of the salt tolerance of the Frankia strain. However, the most salt tolerant strain, CeD, is still able to infect C. glauca up to 200 mM NaCl while the salt-sensitive strain CcI3 is not. Nitrogen fixation was not measured in this study, although it has been reported a significant correlation between nodule number per plant in C. glauca and the acetylene reduction activity (ARA) under salt stress (Girgis et al., 1992). A decrease in nodulation under saline conditions has been previously reported for C. equisetifolia (Ng, 1987; Tani and Sasakawa, 2003) and C. obesa (Reddell et al., 1986) depending on the Frankia source, culture conditions and duration of the experiment. Nodulation did not occur in C. equisetifolia inoculated with Ceq1 strain and cultured in 500 mM NaCl for 6 weeks (Tani and Sasakawa, 2003), while nodules were formed in C. equisetifolia seedlings cultured for 24 weeks at 500 mM NaCl and inoculated with a nodule suspension (Ng, 1987). With C. obesa, increased salinity reduced nodule dry weight with both Casuarina–Frankia associations having a more pronounced effect with one of the inoculum source (Reddell et al., 1986).
The effects of salinity on C. glauca nodulation could be due to an inhibition of nodule initiation and/or infection processes. These processes leading to the development of root nodules of Casuarina involve various responses such as root hair deformation (Torrey, 1976) and early expression of several genes like CgNIN and Cg12 (Laplaze et al., 2000; Svistoonoff et al., 2003; Clavijo et al., 2015). Extensive deformation of root hairs occurs in the zone of root hair elongation within the first 24 h after inoculation (Torrey, 1976). Frankia hyphae infect plants through the intracellular infection pathway in Casuarina trees (Callaham et al., 1979; Perrine-Walker et al., 2011). We observed that an increase in salt concentration reduced the percentage of root hairs deformed in C. glauca plants, 48 h after inoculation with both strains and with a more pronounced effect in plants inoculated with the salt-sensitive strain CcI3. Root hair deformation is dependent on the production of diffusible signals by Frankia (Cérémonie et al., 1999). The observed results might be due to the fact that the salt tolerant strain CeD is able to maintain growth and production of symbiotic factors at higher salt concentration than CcI3. This effect could explain in part the impact of salt on nodule formation. Indeed, there is a positive correlation between the extent of root hair deformation and the number of nodules which subsequently developed (Callaham et al., 1979). In addition, salt stress decreased the number and size of root hairs regardless of the presence of Frankia, which could reduce their availability and susceptibility. Therefore, Frankia colonization may decrease and the establishment of the symbiosis is thus impaired. A similar reduction in root hair deformation by salt stress was reported in legumes Vicia faba (Zahran and Sprent, 1986), Glycine max (Tu, 1981), and Medicago sativa (Lakshmi-Kumari et al., 1974) in response to rhizobial inoculation. The extent of the deformation depends on the association Rhizobium-Legume and was correlated to the number or dry weight of nodules. Morphological symptoms of damage by NaCl such as reduction in the number and size of root hairs was observed in Medicago sativa (Lakshmi-Kumari et al., 1974).
CgNIN is a transcription factor which plays a central role in the nodulation of actinorhizal hosts and is induced by diffusible symbiotic signals produced by Frankia (Clavijo et al., 2015; Chabaud et al., 2016). Cg12 is a subtilisin gene isolated from C. glauca and its expression is associated with Frankia infection (Laplaze et al., 2000; Svistoonoff et al., 2003). In this study, we showed that CgNIN was activated in both control and 50 mM NaCl treated plants (Supplementary Figure 1), from 24 to 72 h after inoculation. This effect suggests that the production of symbiotic diffusible signals by Frankia or its perception is not perturbed by mild salt stress for both strains. On the other hand, expression of Cg12 was observed 14 days after inoculation in both treatments with low number of fluorescent spots in NaCl treated plants. This effect suggests Cg12 expression is negatively affected by salinity that is possibly related to a perturbation of plant cell infection. This result is in accordance with those of Duro et al. (2016) which showed that Cg12 was down-regulated with increasing salt concentration. However, this study used higher levels of NaCl (200, 400, and 600 mM) that what we used in this experiment (50 mM).
Altogether, our results indicate that salt stress alters actinorhizal symbiosis formation in C. glauca. This effect could be due at least in part to a negative impact of salt stress on the infection process that might be related to a reduction of potential infection sites (root hairs) or reduced perception of infection signals. Furthermore, the salt-tolerant strain CeD is able to infect at higher concentrations of salt than the salt sensitive strain CcI3. This result indicates that the use of appropriate strains is necessary for efficient nodulation of trees in salinized soils.
The impact of Frankia inoculation on salt tolerance in C. glauca and C. equisetifolia was tested. Our results indicate that inoculation of C. glauca and C. equisetifolia by Frankia strains CcI3 and CeD significantly improved plants growth under salt stress, depending on the specific Casuarina-Frankia association. For C. glauca, both Frankia strains significantly increased plant height, shoot, root and total dry weight at all concentrations of NaCl, as compared to uninoculated plants. This positive effect was more pronounced in plants inoculated with strain CcI3. In contrast, only Frankia strain CeD increased C. equisetifolia height at all NaCl treatments, and significantly elevated plant shoot, root, and total dry weight from 0 to 200 mM NaCl, as compared to control. These results suggest that the effectiveness of the symbiosis in saline conditions depends on the appropriate Casuarina-Frankia association. Indeed, according to Girgis et al. (1992), there is no correlation between in vitro salt tolerance of Frankia strains and their effectiveness in association with plants under salt-stressed conditions. However, it is important to emphasize that the experiment with C. glauca was conducted in hydroponic conditions, whereas C. equisetifolia was grown in soil. The improvement of morphological parameters (height, shoot, root and total dry weight) may be due to the increased N nutrition and photosynthesis potential in Casuarina inoculated with Frankia compared to the uninoculated controls. This conclusion was supported by our results for chlorophyll (a, b, and a + b) content and nitrogenase activity under saline conditions. Under all NaCl concentrations, chlorophyll content was significantly increased in C. glauca plants inoculated with both strains, as compared to control. With C. equisetifolia, only CeD increased significantly total chlorophyll content from 0 to 200 mM NaCl. Salinity decreased nitrogenase activity in C. glauca (Supplementary Figure 2). However, N2 fixation occurred even at the highest NaCl concentration (Supplementary Figure 2). This implies that increased N nutrition and potential photosynthesis allow inoculated Casuarina plants to grow better than uninoculated controls plants under saline conditions. These results are in agreement with a previous report showing that the actinorhizal tree Alnus glutinosa inoculated with Frankia and cultivated in alkaline and saline anthropogenic sediment, had better plant growth, leaf N and chlorophyll a + b content than the control (Oliveira et al., 2005). Several studies have shown that inoculation with selected microsymbionts like Frankia can enhance the development of actinorhizal plants and their resistance to other abiotic stresses such as heavy metals and extreme pH and temperature (Reviewed by Ngom et al., 2016). Symbiotic associations with arbuscular mycorrhizal fungi (AMF) and nitrogen-fixing bacteria called rhizobia can also enhance plant salinity tolerance, leading to better plant growth and yield, nutrient acquisition and chlorophyll content in several species including Medicago sativa (Azcon and El-Atrash, 1997), Acacia nilotica, Leucaena leucocephela, Prosopis juliphora (Bala et al., 1990), Phaseolus vulgaris (Dardanelli et al., 2008), and soybean (Elsheikh and Wood, 1995), under saline conditions. The benefits of these microsymbionts in saline environments depend also on the symbiotic associations.
Compatibles solutes or osmolytes such as glycine betaine, mannitol, or proline are accumulated in organisms in response to salt and osmotic stresses (Delauney and Verma, 1993; Wang et al., 2003). They play important roles in maintaining cell turgor and thus the driving gradient for water (Wang et al., 2003). Compatible solutes can also act as free-radical scavengers or chemical chaperones by directly stabilizing membranes and/or proteins (Lee et al., 1997; Bohnert and Shen, 1998; McNeil et al., 1999; Diamant et al., 2001). Proline, an amino acid, is the most common osmolyte accumulated under salinity and drought stress in plants (Watanabe et al., 2000; Tani and Sasakawa, 2003). In our study, a significantly higher proline content was observed in all inoculated C. glauca plants at all NaCl concentrations, as compared to the control. Significant improvement of proline content was also observed in 0, 50, and 300 NaCl treated C. equisetifolia plants inoculated with strain CeD. In both control and inoculated plants, proline content increased with increasing salinity. These results suggest that, in addition to better N nutrition and potential photosynthesis, proline accumulation adjusts the osmotic pressure and maintain cell homeostasis in inoculated C. glauca and C. equisetifolia plants, under saline conditions. These results are in agreement with those of Diouf et al. (2005) which showed that inoculation with both Rhizobium and AMF induced higher proline content in legumes such as Acacia auriculiformis and Acacia mangium, compared to uninoculated plants, at all levels of salinity tested (0, 50, and 100 mM NaCl). Proline accumulation under salt stress has been previously described in C. equisetifolia seedlings not infected by Frankia (Tani and Sasakawa, 2006).
In conclusion, our results strongly indicate that the beneficial effects of Frankia inoculation are due to improved N nutrition, photosynthesis potential and proline accumulation in inoculated plants under salt stress conditions. There was no correlation between in vitro salt tolerance of Frankia strains and efficiency in planta in salt stress conditions. Hence, the success of planting Casuarina in saline sites will require appropriate salt-tolerant Casuarina-Frankia associations that will form an efficient N2-fixing symbiosis. In vitro salt tolerance of Frankia strains should be considered if they are introduced in saline soils, otherwise, the screening should be done with both symbiotic partners.
Author contributions
MN conducted some experiments, analyzed the data, interpreted the results, and prepared the manuscript. KG and JF conducted some experiments and prepared the manuscript. ND, HG, VH, SS conducted some experiments, interpreted the results, and improved the manuscript. RO, LL, and LT interpreted the results and improved the manuscript. AC and MS designed and coordinated the experiments, analyzed the data, interpreted the results, and improved the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported in part by the IRD (Institut de Recherche pour le Développement) and the AUF (Agence Universitaire de la Francophonie) through the inter-regional doctoral college in food and plant biotechnology (CD-BIOVEGAGRO) and grants from the USDA National Institute of Food and Agriculture (Hatch 022821) and USDA AFRI (A1151 2014-03765). MN was supported by the MERS (Ministère de l'Enseignement Supérieur et de la Recherche du Sénégal, national grant), the WFS (Word Federation of Scientists, research allowance) and the IRD (ARTS PhD grant).
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01331
References
- Arnon D. I. (1949). Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswathappa N., Bachelard E. P. (1986). Ion regulation in the organs of Casuarina species differing in salt tolerance. Funct. Plant Biol. 13, 533–545. [Google Scholar]
- Azcon R., El-Atrash F. (1997). Influence of arbuscular mycorrhizae and phosphorus fertilization on growth, nodulation and N2 fixation (15N) in Medicago sativa at four salinity levels. Biol. Fertil. Soils 24, 81–86. 10.1007/BF01420225 [DOI] [Google Scholar]
- Bala N., Sharma P. K., Lakshminarayana K. (1990). Nodulation and nitrogen fixation by salinity-tolerant rhizobia in symbiosis with tree legumes. Agric. Ecosyst. Environ. 33, 33–46. 10.1016/0167-8809(90)90142-Z [DOI] [Google Scholar]
- Bohnert H. J., Shen B. O. (1998). Transformation and compatible solutes. Sci. Hortic. 78, 237–260. 10.1016/S0304-4238(98)00195-2 [DOI] [Google Scholar]
- Broughton W. J., Dilworth M. J. (1971). Control of leghaemoglobin synthesis in snake beans. Biochem. J. 125, 1075–1080. 10.1042/bj1251075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaham D., Deltredici P., Torrey J. G. (1978). Isolation and cultivation in vitro of the actinomycete causing root nodulation in Comptonia. Science 199, 899–902. 10.1126/science.199.4331.899 [DOI] [PubMed] [Google Scholar]
- Callaham D., Newcomb W., Torrey J. G., Peterson R. L. (1979). Root hair infection in actinomycete-induced root nodule initiation in Casuarina, Myrica, and Comptonia. Bot. Gaz. 140, S1–S9. 10.1086/337028 [DOI] [Google Scholar]
- Cérémonie H., Debellé F., Fernandez M. P. (1999). Structural and functional comparison of Frankia root hair deforming factor and rhizobia Nod factor. Can. J. Bot. 77, 1293–1301. 10.1139/cjb-77-9-1293 [DOI] [Google Scholar]
- Chabaud M., Gherbi H., Pirolles E., Vaissayre V., Fournier J., Moukouanga D., et al. (2016). Chitinase-resistant hydrophilic symbiotic factors secreted by Frankia activate both Ca2+ spiking and NIN gene expression in the actinorhizal plant Casuarina glauca. New Phytol. 209, 86–93. 10.1111/nph.13732 [DOI] [PubMed] [Google Scholar]
- Champion A., Lucas M., Tromas A., Vaissayre V., Crabos A., Diédhiou I., et al. (2015). Inhibition of auxin signaling in Frankia species-infected cells in Casuarina glauca nodules leads to increased nodulation. Plant Physiol. 167, 1149–1157. 10.1104/pp.114.255307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo F., Diedhiou I., Vaissayre V., Brottier L., Acolatse J., Moukouanga D., et al. (2015). The Casuarina NIN gene is transcriptionally activated throughout Frankia root infection as well as in response to bacterial diffusible signals. New Phytol. 208, 887–903. 10.1111/nph.13506 [DOI] [PubMed] [Google Scholar]
- Dardanelli M. S., de Cordoba F. J. F., Espuny M. R., Carvajal M. A. R., Díaz M. E. S., Serrano A. M. G., et al. (2008). Effect of Azospirillum brasilense coinoculated with Rhizobium on Phaseolus vulgaris flavonoids and Nod factor production under salt stress. Soil Biol. Biochem. 40, 2713–2721. 10.1016/j.soilbio.2008.06.016 [DOI] [Google Scholar]
- Dawson J. O., Gibson A. H. (1987). Sensitivity of selected Frankia isolates from Casuarina, Allocasuarina and North American host plants to sodium chloride. Physiol. Plant. 70, 272–278. 10.1111/j.1399-3054.1987.tb06144.x [DOI] [Google Scholar]
- Delauney A. J., Verma D. P. S. (1993). Proline biosynthesis and osmoregulation in plants. Plant J. 4, 215–223. 10.1046/j.1365-313X.1993.04020215.x [DOI] [Google Scholar]
- Diagne N., Diouf D., Svistoonoff S., Kane A., Noba K., Franche C., et al. (2013). Casuarina in Africa: distribution, role and importance of arbuscular mycorrhizal, ectomycorrhizal fungi and Frankia on plant development. J. Environ. Manage. 128, 204–209. 10.1016/j.jenvman.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Diamant S., Eliahu N., Rosenthal D., Goloubinoff P. (2001). Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J. Biol. Chem. 276, 39586–39591. 10.1074/jbc.M103081200 [DOI] [PubMed] [Google Scholar]
- Diédhiou I., Tromas A., Cissoko M., Gray K., Parizot B., Crabos A., et al. (2014). Identification of potential transcriptional regulators of actinorhizal symbioses in Casuarina glauca and Alnus glutinosa. BMC Plant Biol. 14:1. 10.1186/s12870-014-0342-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem H. G., Dommergues Y. R. (1990). Current and potential uses and management of Casuarinaceae in the tropics and subtropics, in Biology of Frankia and Actinorhizal Plants, eds Schwintzer C. R., Tjepkema J. D. (Academic Press; ), 317–342. 10.1016/B978-0-12-633210-0.50021-6 [DOI] [Google Scholar]
- Diem H. G., Gauthier D., Dommergues Y. (1983). An effective strain of Frankia from Casuarina sp. Can. J. Bot. 61, 2815–2821. 10.1139/b83-312 [DOI] [Google Scholar]
- Diem H. G., Gauthier D., Dommergues Y. R. (1982). Isolation of Frankia from nodules of Casuarina equisetifolia. Can. J. Microbiol. 28, 526–530. 10.1139/m82-079 [DOI] [Google Scholar]
- Diouf D., Duponnois R., Ba A. T., Neyra M., Lesueur D. (2005). Symbiosis of Acacia auriculiformis and Acacia mangium with mycorrhizal fungi and Bradyrhizobium spp. improves salt tolerance in greenhouse conditions. Funct. Plant Biol. 32, 1143–1152. 10.1071/FP04069 [DOI] [PubMed] [Google Scholar]
- Duhoux E., Franche C. (2003). Les nodules actinorhiziens de Casuarina. Biofutur 235, 45–49. [Google Scholar]
- Duro N., Batista-Santos P., da Costa M., Maia R., Castro I. V., Ramos M., et al. (2016). The impact of salinity on the symbiosis between Casuarina glauca Sieb. ex Spreng. and N2-fixing Frankia bacteria based on the analysis of Nitrogen and Carbon metabolism. Plant Soil 398, 327–337. 10.1007/s11104-015-2666-3 [DOI] [Google Scholar]
- El-Lakany M. H., Luard E. J. (1983). Comparative salt tolerance of selected Casuarina species [Australia; Egypt]. Aust. For. Res. Aust. 13, 11–20. [Google Scholar]
- Elsheikh E. A. E., Wood M. (1995). Nodulation and N2 fixation by soybean inoculated with salt-tolerant rhizobia or salt-sensitive bradyrhizobia in saline soil. Soil Biol. Biochem. 27, 657–661. 10.1016/0038-0717(95)98645-5 [DOI] [Google Scholar]
- Fauzia Y. H. (1999). Frankia and Rhizobium strains as inoculum for fast growing trees in saline environment. Pak. J. Bot. 31, 173–182. [Google Scholar]
- Franche C., Laplaze L., Duhoux E., Bogusz D. (1998). Actinorhizal symbioses: recent advances in plant molecular and genetic transformation studies. Crit. Rev. Plant Sci. 17, 1–28. 10.1016/S0735-2689(98)00356-6 [DOI] [Google Scholar]
- Gherbi H., Markmann K., Svistoonoff S., Estevan J., Autran D., Giczey G., et al. (2008). SymRK defines a common genetic basis for plant root endosymbioses with arbuscular mycorrhiza fungi, rhizobia, and Frankia bacteria. Proc. Natl. Acad. Sci. U.S.A. 105, 4928–4932. 10.1073/pnas.0710618105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis M. G. Z., Ishac Y. Z., Diem H. G., Dommergues Y. R. (1992). Selection of salt tolerant Casuarina glauca and Frankia. Acta Oecologica 13, 443–451. [Google Scholar]
- Gomaa A. M., Abo-Aba S. E. M., Awad N. S. (2008). Isolation, characterization and genetic differentiation of Frankia sp. isolated from ecologically different Egyptian locations. Res. J. Cell Mol. Biol. 2, 6–17. [Google Scholar]
- Gtari M., Ghodhbane-Gtari F., Nouioui I., Ktari A., Hezbri K., Mimouni W., et al. (2015). Cultivating the uncultured: growing the recalcitrant cluster-2 Frankia strains. Sci. Rep. 5:13112. 10.1038/srep13112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R., Burns R. C., Holsten R. D. (1973). Applications of the acetylene-ethylene assay for measurement of nitrogen fixation. Soil Biol. Biochem. 5, 47–81. 10.1016/0038-0717(73)90093-X [DOI] [Google Scholar]
- Hoagland D. R., Arnon D. I. (1950). The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 347, 1–32. [Google Scholar]
- Hyland B. P. M. (1983). Germination, growth and mineral ion concentrations of Casuarina species under saline conditions. Aust. J. Bot. 31, 1–164. 10.1071/BT9830001 [DOI] [Google Scholar]
- LADA (2003). L'évaluation de la dégradation des terres au Sénégal. Projet FAO Land Degradation Assessment. Rapport préliminaire.
- Lakshmi-Kumari M., Singh C. S., Rao N. S. (1974). Root hair infection and nodulation in lucerne (Medicago sativa L.) as influenced by salinity and alkalinity. Plant Soil 40, 261–268. 10.1007/BF00011509 [DOI] [Google Scholar]
- Laplaze L., Ribeiro A., Franche C., Duhoux E., Auguy F., Bogusz D., et al. (2000). Characterization of a Casuarina glauca nodule-specific subtilisin-like protease gene, a homolog of Alnus glutinosa ag12. Mol. Plant. Microbe Interact. 13, 113–117. 10.1094/MPMI.2000.13.1.113 [DOI] [PubMed] [Google Scholar]
- Lee G. J., Roseman A. M., Saibil H. R., Vierling E. (1997). A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 16, 659–671. 10.1093/emboj/16.3.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luard E. J., El-Lakany M. H. (1984). Effects on Casuarina and Allocasuarina species of increasing sodium chloride concentrations in solution culture. Funct. Plant Biol. 11, 471–481. [Google Scholar]
- Maheut J., Dommergues Y. (1961). La fixation par le reboisement des dunes de la presqu'île du Cap-Vert. Centre Technique Forestier Tropical. Available online at: http://horizon.documentation.ird.fr/exl-doc/pleins_textes/divers11-05/08933.pdf (Accessed March 23, 2016).
- Mailly D., Ndiaye P., Margolis H. A., Pineau M. (1994). Fixation des dunes et reboisement avec le filao (Casuarina equisetifolia) dans la zone du littoral nord du Senegal. For. Chron. 70, 282–290. 10.5558/tfc70282-3 [DOI] [Google Scholar]
- McNeil S. D., Nuccio M. L., Hanson A. D. (1999). Betaines and related osmoprotectants. Targets for metabolic engineering of stress resistance. Plant Physiol. 120, 945–949. 10.1104/pp.120.4.945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiroud A. (1996). Diversité et écologie des plantes actinorhiziennes. Acta Bot. Gallica 143, 651–661. 10.1080/12538078.1996.10515366 [DOI] [Google Scholar]
- Monneveux P., Nemmar M. (1986). Contribution à l'étude de la résistance à la sécheresse chez le blé tendre (Triticum aestivum L.) et chez le blé dur (Triticum durum Desf.): étude de l'accumulation de la proline au cours du cycle de développement. Agronomie 6, 583–590. 10.1051/agro:19860611 [DOI] [Google Scholar]
- Munns R., Tester M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. 10.1146/annurev.arplant.59.032607.092911 [DOI] [PubMed] [Google Scholar]
- Murry M. A., Fontaine M. S., Torrey J. G. (1984). Growth kinetics and nitrogenase induction in Frankia sp. HFPArI 3 grown in batch culture, in Frankia Symbioses (Springer; ), 61–78. Available online at: http://link.springer.com/chapter/10.1007/978-94-009-6158-6_7 (Accessed September 24, 2014). [Google Scholar]
- National Research Council (1984). Casuarinas: Nitrogen-Fixing Trees for Adverse Sites. Washington, DC: National Academy Press. [Google Scholar]
- Ng B. H. (1987). The effects of salinity on growth, nodulation and nitrogen fixation of Casuarina equisetifolia. Plant Soil 103, 123–125. 10.1007/BF02370676 [DOI] [Google Scholar]
- Ngom M., Diagne N., Laplaze L., Champion A., Sy M. O. (2015). Symbiotic ability of diverse Frankia strains on Casuarina glauca plants in hydroponic conditions. Symbiosis 70, 79–86. 10.1007/s13199-015-0366-7 [DOI] [Google Scholar]
- Ngom M., Oshone R., Diagne N., Cissoko M., Svistoonoff S., Tisa L. S., et al. (2016). Tolerance to environmental stress by the nitrogen-fixing actinobacterium Frankia and its role in actinorhizal plants adaptation. Symbiosis 70, 17–29. 10.1007/s13199-016-0396-9 [DOI] [Google Scholar]
- Normand P., Benson D. R., Berry A. M., Tisa L. S. (2014). Family Frankiaceae, in Prokaryote–Actinobacteria, 4th Edn., eds Rosenberg E., DeLong E. F., Lory S., Stackebrandt E., Thompson F. (Berlin: Springer-Verlag; ), 339–356. [Google Scholar]
- Normand P., Orso S., Cournoyer B., Jeannin P., Chapelon C., Dawson J., et al. (1996). Molecular phylogeny of the genus Frankia and related genera and emendation of the family Frankiaceae. Int. J. Syst. Bacteriol. 46, 1–9. 10.1099/00207713-46-1-1 [DOI] [PubMed] [Google Scholar]
- Oliveira R. S., Castro P. M. L., Dodd J. C., Vosátka M. (2005). Synergistic effect of Glomus intraradices and Frankia spp. on the growth and stress recovery of Alnus glutinosa in an alkaline anthropogenic sediment. Chemosphere 60, 1462–1470. 10.1016/j.chemosphere.2005.01.038 [DOI] [PubMed] [Google Scholar]
- Oshone R., Mansour S. R., Tisa L. S. (2013). Effect of salt stress on the physiology of Frankia sp strain CcI6. J. Biosci. 38, 699–702. 10.1007/s12038-013-9371-2 [DOI] [PubMed] [Google Scholar]
- Perrine-Walker F., Gherbi H., Imanishi L., Hocher V., Ghodhbane-Gtari F., Lavenus J., et al. (2011). Symbiotic signaling in actinorhizal symbioses. Curr. Protein Pept. Sci. 12, 156–164. 10.2174/138920311795684896 [DOI] [PubMed] [Google Scholar]
- Potgieter L. J., Richardson D. M., Wilson J. R. (2014). Casuarina cunninghamiana in the Western Cape, South Africa: determinants of naturalisation and invasion, and options for management. South Afr. J. Bot. 92, 134–146. 10.1016/j.sajb.2014.02.013 [DOI] [Google Scholar]
- Reddell P., Foster R. C., Bowen G. D. (1986). The effects of sodium chloride on growth and nitrogen fixation in Casuarina obesa Miq. New Phytol. 102, 397–408. 10.1111/j.1469-8137.1986.tb00817.x [DOI] [PubMed] [Google Scholar]
- Santi C., Bogusz D., Franche C. (2013). Biological nitrogen fixation in non-legume plants. Ann. Bot. 111, 743–767. 10.1093/aob/mct048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwencke J. (1991). Rapid, exponential growth and increased biomass yield of some Frankia strains in buffered and stirred mineral medium (BAP) with phosphatidyl choline. Plant Soil 137, 37–41. 10.1007/BF02187429 [DOI] [Google Scholar]
- Simonet P., Normand P., Hirsch A. M., Akkermans A. D. (1989). The Genetics of Frankia-Actinorhizal Symbiosis. Available online at: http://library.wur.nl/WebQuery/wurpubs/10415 (Accessed February 17, 2015).
- Smouni A., Laplaze L., Bogusz D., Guermache F., Auguy F., Duhoux E., et al. (2002). Research note: the 35S promoter is not constitutively expressed in the transgenic tropical actinorhizal tree Casuarina glauca. Funct. Plant Biol. 29, 649–656. 10.1071/PP01121 [DOI] [PubMed] [Google Scholar]
- Svistoonoff S., Benabdoun F. M., Nambiar-Veetil M., Imanishi L., Vaissayre V., Cesari S., et al. (2013). The independent acquisition of plant root nitrogen-fixing symbiosis in fabids recruited the same genetic pathway for nodule organogenesis. PLoS ONE 8:e64515. 10.1371/journal.pone.0064515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svistoonoff S., Hocher V., Gherbi H. (2014). Actinorhizal root nodule symbioses: what is signalling telling on the origins of nodulation? Curr. Opin. Plant Biol. 20C, 11–18. 10.1016/j.pbi.2014.03.001 [DOI] [PubMed] [Google Scholar]
- Svistoonoff S., Laplaze L., Auguy F., Runions J., Duponnois R., Haseloff J., et al. (2003). cg12 expression is specifically linked to infection of root hairs and cortical cells during Casuarina glauca and Allocasuarina verticillata actinorhizal nodule development. Mol. Plant. Microbe Interact. 16, 600–607. 10.1094/MPMI.2003.16.7.600 [DOI] [PubMed] [Google Scholar]
- Svistoonoff S., Sy M. O., Diagne N., Barker D. G., Bogusz D., Franche C. (2010). Infection-specific activation of the Medicago truncatula Enod11 early nodulin gene promoter during actinorhizal root nodulation. Mol. Plant. Microbe Interact. 23, 740–747. 10.1094/MPMI-23-6-0740 [DOI] [PubMed] [Google Scholar]
- Tani C., Sasakawa H. (2003). Salt tolerance of Casuarina equisetifolia and Frankia Ceq1 strain isolated from the root nodules of C. equisetifolia. Soil Sci. Plant Nutr. 49, 215–222. 10.1080/00380768.2003.10410000 [DOI] [Google Scholar]
- Tani C., Sasakawa H. (2006). Proline accumulates in Casuarina equisetifolia seedlings under salt stress. Soil Sci. Plant Nutr. 52, 21–25. 10.1111/j.1747-0765.2006.00005.x [DOI] [Google Scholar]
- Torrey J. G. (1976). Initiation and development of root nodules of Casuarina (Casuarinaceae). Am. J. Bot. 63, 335–344. 10.2307/2441579 [DOI] [Google Scholar]
- Tromas A., Parizot B., Diagne N., Champion A., Hocher V., Cissoko M., et al. (2012). Heart of endosymbioses: transcriptomics reveals a conserved genetic program among arbuscular mycorrhizal, actinorhizal and legume-rhizobial symbioses. PLoS ONE 7:e44742. 10.1371/journal.pone.0044742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu J. C. (1981). Effect of salinity on Rhizobium-root-hair interaction, nodulation and growth of soybean. Can. J. Plant Sci. 61, 231–239. 10.4141/cjps81-035 [DOI] [Google Scholar]
- Van der Moezel P. G., Walton C. S., Pearce-Pinto G. V. N., Bell D. T. (1989). Screening for salinity and waterlogging tolerance in five Casuarina species. Landsc. Urban Plan. 17, 331–337. 10.1016/0169-2046(89)90087-X [DOI] [Google Scholar]
- Wang W., Vinocur B., Altman A. (2003). Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218, 1–14. 10.1007/s00425-003-1105-5 [DOI] [PubMed] [Google Scholar]
- Watanabe S., Kojima K., Ide Y., Sasaki S. (2000). Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tissue Organ Cult. 63, 199–206. 10.1023/A:1010619503680 [DOI] [Google Scholar]
- Zahran H. H., Sprent J. I. (1986). Effects of sodium chloride and polyethylene glycol on root-hair infection and nodulation of Vicia faba L. plants by Rhizobium leguminosarum. Planta 167, 303–309. 10.1007/BF00391332 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Lopez M. F., Torrey J. G. (1984). A comparison of cultural characteristics and infectivity of Frankia isolates from root nodules of Casuarina species, in Frankia Symbioses (Springer; ), 79–90. Available at: http://link.springer.com/chapter/10.1007/978-94-009-6158-6_8 (Accessed September 24, 2014). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.