Abstract
OBJECTIVES
A growing body of clinical and laboratory evidence indicates that inflammation plays a crucial role in atherosclerosis. In the present study, we compared the effects of clopidogrel and prasugrel on high-sensitivity C-reactive protein (hs-CRP) in patients undergoing percutaneous coronary intervention (PCI).
METHODS
The present randomized, double-blind clinical trial included 120 patients who underwent PCI. Eligible patients were randomly assigned 2:1 to one of the two groups: 80 patients in the first group received clopidogrel (Plavix®; loading dose and maintenance dose of 300 and 75 mg daily, respectively) and 40 patients in the second group received prasugrel (Effient®; loading dose and maintenance dose of 60 and 10 mg, respectively) for 12 weeks. The hs-CRP levels between baseline and 12th week were compared.
RESULTS
Of the 120 patients, 69 patients (57.5%) were male. Pretreatment hs-CRP level was statistically comparable in clopidogrel (median, 15.10 mg/dL; interquartile range [IQR], 9.62–23.75 mg/dL) and prasugrel groups (median, 18 mg/dL; IQR, 14.25–22 mg/dL; P = 0.06). Patients taking clopidogrel showed a significant reduction in hs-CRP level compared with the baseline values (P < 0.001). Prasugrel administration also resulted in a significant reduction in hs-CRP level (P < 0.001). A significant 73% overall reduction in the hs-CRP level was seen with prasugrel compared with 39% overall reduction in hs-CRP level with clopidogrel (P = 0.002).
CONCLUSION
Prasugrel seems to be superior to clopidogrel in the reduction of hs-CRP in patients undergoing PCI.
Keywords: C-reactive protein, clopidogrel, coronary artery disease, percutaneous coronary intervention, prasugrel
Introduction
A growing body of clinical and laboratory evidence indicates that inflammation plays a crucial role in atherosclerosis.1,2 It has been demonstrated that coronary artery stenting induces a systemic inflammatory response.3–6 This inflammation has also been linked to in-stent restenosis and in-stent thrombosis.7 It has been suggested that inflammation by activating platelet activation play a central role in in-stent restenosis and in-stent thrombosis.8
Current guidelines recommend the administration of dual antiplatelet therapy consisting of aspirin and a platelet P2Y12 adenosine diphosphate (ADP) receptor antagonist (thienopyridine) to all patients undergoing percutaneous coronary intervention (PCI) to prevent recurrent ischemia and stent thrombosis.9,10 Both clopidogrel and prasugrel are noncompetitive thienopyridine antagonists of P2Y12, which inhibit the ability of ADP to induce platelet aggregation.11 The Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel – Thrombolysis in Myocardial Infarction (TRITON-TIMI 38) study is the largest study evaluating the efficacy of prasugrel compared with clopidogrel in patients with acute coronary syndromes undergoing PCI.12 However, the primary end point of this study was the composite of death resulting from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, and the effects of clopidogrel and prasugrel on inflammatory markers were not studied.
Therefore, in the present study, we compared the anti-inflammatory effects of clopidogrel and prasugrel in patients undergoing PCI, reflected as reduction in high-sensitivity C-reactive protein (hs-CRP).
Methods
Patient population
The present randomized, double-blind clinical trial included 120 consecutive patients who underwent PCI at Rajaei Cardiovascular Medical and Research Center, Tehran, Iran, during April 2014 to December 2014. Patients were eligible for enrollment if they had undergone PCI with any of the indications mentioned below:
planned PCI for stable angina or unstable angina/non-ST elevation myocardial infarction (UA/NSTEMI), or
planned PCI after medical treatment for ST-segment elevation myocardial infarction (STEMI), or
primary PCI for STEMI.
UA was defined as the presence of symptoms at rest; or symptoms that have suddenly increased in frequency, severity, or duration; or had a change from the usual pattern of angina and did not respond to rest or nitroglycerin.13
Key exclusion criteria included increased risk of major bleeding, treatment with thienopyridines, history of myocardial infarction or coronary intervention and/or revascularization, clinically assessed Killip class II/III heart failure or cardiogenic shock, liver failure, acute/chronic kidney failure with serum creatinine of more than 2.5 mg/dL, and platelet count of <100.000/mm3. Patients with malignancies, chronic inflammatory diseases, or infectious disorders were also excluded. The primary end point of this analysis was to assess the effects of a 12-week administration of prasugrel versus clopidogrel on hs-CRP serum level in patients undergoing PCI. The secondary end points were to compare the effects of prasugrel versus clopidogrel on hs-CRP in special subgroups of participants. The study was conducted according to the most recent amendments to the Declaration of Helsinki. The study protocol was approved by institutional review board, and written informed consent was obtained from all patients.
Antiplatelet administration
Eligible patients were randomly assigned to 2:1 to one of the two groups: 80 patients in the first group received a 300 mg loading dose of clopidogrel (Plavix®) 24 hours before the planned PCI or immediately before the procedure in patients with primary PCI, followed by a 75 mg daily maintenance dose for up to 12 weeks; 40 patients in the second group were administered a 60 mg loading dose of prasugrel (Effient®) 24 hours before the planned PCI or immediately before the procedure in patients with primary PCI followed by a 10 mg daily maintenance dose for up to 12 weeks. Concomitant with clopidogrel or prasugrel administration, all patients received an 80 mg loading dose of atorvastatin followed by maintenance doses of at least 20 mg daily. Patients also received a loading dose of aspirin (325 mg PO) followed by a maintenance dose of 80 mg daily. At the end of the 12th week, hs-CRP was assessed again. Decision regarding the continuation of antiplatelet therapy at this time was made for each individual patient based on the guideline.
Statistical analysis
All analyses were conducted by Statistical Package for Social Sciences (SPSS) software, version 21 (IBM Inc.). All data were initially analyzed using the Kolmogorov–Smirnov test to assess for normality. Quantitative variables were presented as mean ± standard deviation (SD) for normally distributed variables and as median (interquartile range, IQR) for variables without normal distribution. Categorical data were presented as numbers (percentages). The chi-square test was used for comparing the categorical data, and quantitative variables were compared by the Student’s t-test, the Mann–Whitney test, and the Kruskal–Wallis test, as appropriate. All P-values were two-tailed, and P < 0.05 was considered statistically significant.
Results
Baseline and procedural characteristics
Of the 120 patients randomized into the present study, 69 patients (57.5%) were male. All baseline characteristics were statistically well matched between the treatment assignments, ie, prasugrel versus clopidogrel (Table 1). Although statistically nonsignificant, the prevalence of diabetes and the use of drug-eluting stents were considerably higher in the prasugrel group compared with the clopidogrel group. Pretreatment hs-CRP level was also lower in the clopidogrel group (median, 15.10 mg/dL; IQR, 9.62–23.75 mg/dL) compared with the prasugrel group on a borderline level of statistical significance (median, 18.00 mg/dL; IQR, 14.25–22.00 mg/dL; P = 0.06).
Table 1.
Baseline characteristics of the patients.
| VARIABLE | CLOPIDOGREL GROUP (n = 80) |
PRASUGREL GROUP (n = 40) |
P VALUE |
|---|---|---|---|
| Clinical characteristics | |||
| Age (yrs) | 64 ± 9 | 63 ± 9 | 0.71 |
| Male gender | 48 (60) | 21 (52.5) | 0.43 |
| Body mass index (kg/m2) | 28.41 ± 4.51 | 27.64 ± 3.94 | 0.37 |
| Diabetes | 24 (30) | 25 (62.5) | 0. 41 |
| Hypertension | 44 (55) | 25 (62.5) | 0.55 |
| Current smoking | 42 (52.5) | 20 (50) | 0.84 |
| Clinical indication | |||
| Elective | 34 (42.5) | 16 (40) | 0.40 |
| UA | 28 (35) | 12 (30) | |
| NSTEMI | 10 (12.5) | 7 (17.5) | |
| STEMI | 8 (10) | 5 (12.5) | |
| Procedural characteristics | |||
| One-vessel PCI | 22 (27.5) | 14 (35) | 0.40 |
| Multi-vessel PCI | 58 (72.5) | 26 (65) | |
| Stent placement | |||
| POBA | 14 (17.5) | 7 (17.5) | 0.80 |
| Bare metal | 56 (70) | 20 (50) | |
| Drug-eluting | 10 (12.5) | 13 (32.5) | |
| Medications | |||
| Beta blockers | 38 (47.5) | 21 (52.5) | 0.69 |
| ACE inhibitors | 36 (45) | 19 (47.5) | 0.55 |
| Statins | 54 (57.5) | 24 (60) | 0.42 |
| Calcium channel blocker | 24 (30) | 14 (35) | 0.67 |
Notes: Data are shown as mean (±SD) for continuous variables and numbers (percentage) for dichotomous variables.
Abbreviations: ACE, angiotensin converting enzyme; NSTEMI, non-ST segment elevation myocardial infarction; STEMI, ST segment elevation myocardial infarction; PCI, percutaneous coronary intervention; POBA, plain old balloon angioplasty; UA, unstable angina.
Associations of baseline hs-CRP with the index event
Baseline median serum levels of hs-CRP were 15.00 mg/dL (IQR, 7.50–18.50 mg/dL), 15.60 mg/dL (IQR, 11.87–22.00 mg/dL), 23.00 mg/dL (IQR, 20.50–27.50 mg/dL), and 28.00 mg/L (IQR, 22.00–42.50 mg/dL) in patients with elective, UA, NSTEMI, and STEMI index events, respectively; and these differences were statistically significant (Fig. 1, P < 0.001).
Figure 1.
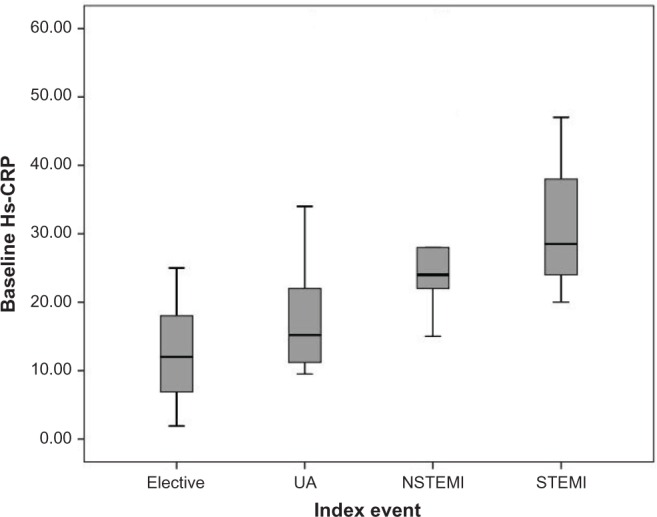
The baseline hs-CRP level in patients with various index events before percutaneous coronary intervention.
Abbreviations: UA, unstable angina; NSTEMI, non-ST segment elevation myocardial infarction; STEMI, ST segment elevation myocardial infarction.
Association of baseline hs-CRP with the index procedure
Patients who underwent single-vessel PCI had comparable baseline serum level of hs-CRP as compared with patients with multivessel PCI (median 18.00 mg/dL; IQR, 12.50–22.00 mg/dL versus median 17.00 mg/dL; IQR, 11.00–24.00 mg/dL; P = 0.07).
Association of baseline hs-CRP with baseline characteristics
Baseline hs-CRP is compared between different subgroups in both clopidogrel and prasugrel groups (Table 2). The results of multiple regression analysis indicated statistically significant relationships between baseline and index event (P < 0.001), beta blocker (P = 0.01), hypertension (P = 0.003), and statins (P = 0.01; Table 3).
Table 2.
The effects of Clopidogrel and Prasugrel on hs-CRP over 12 weeks study period.
| CLOPIDOGREL GROUP (n = 80) | PRASUGREL GROUP (n =40) | CLOPIDOGREL VERSUS PRASUGREL (WITHIN SUBGROUPS) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HS-CRP BEFORE TREATMENT (mg/dl) | P VALUE (BETWEEN SUBGROUPS) | HS-CRP AFTER TREATMENT (mg/dl) | PERCENT OF CHANGE (%) | P VALUE (BETWEEN SUBGROUPS) | HS-CRP BEFORETREATMENT (mg/dl) | P VALUE (BETWEEN |SUBGROUPS) | HS-CRP AFTER TREATMENT (mg/dl) | PERCENT OF CHANGE (%) | P VALUE (BETWEEN SUBGROUPS) | P VALUE | ||
| All | 15.10 (9.62–23.75) |
8.50 (6.00–10.75) |
39.33 (15.71–62.55) |
– | 18.00 (14.25–22.00) |
5.00 (3.00–5.00) |
72.95 (61.53–85.33) |
– | 0.002 | |||
| Gender | Male | 11.25 (8.37–19.75) |
0.03 | 8.00 (4.00–10.00) |
48.68 (21.61–62.55) |
0.18 | 18.00 (15.00–22.00) |
0.92 | 5 (3.00–5.00) |
73.68 (64.10–84.96) |
0.74 | <0.001 |
| Female | 18.00 (11.25–28.75) |
10.00 (6.00–20.00) |
33.33 (6.07–62.34) |
18.00 (12.00–23.00) |
5.00 (3.00–9.00) |
68.75 (59.09–86.36) |
<0.001 | |||||
| DM | Yes | 15.00 (9.50–21.00) |
0.91 | 11.00 (6.00–20.00) |
25.00 (4.76–33.33) |
0.007 | 15.00 (12.00–22.00) |
0.08 | 5 (3.00–5.00) |
66.66 (59.09–83.33) |
0.14 | <0.001 |
| No | 15.20 (9.25–24.25) |
8.00 (4.75–10.00) |
50.00 (21.17–64.39) |
19.00 (15.75–22.50) |
5.00 (3.00–5.00) |
73.68 (66.88–86.03) |
<0.001 | |||||
| HTN | Yes | 17.50 (11.50–24.00) |
0.01 | 10.00 (8.00–13.00) |
33.33 (20–60) |
0.77 | 15.50 (12.50–22.00) |
0.06 | 5.00 (3.00–5.00) |
67.85 (60.31–82.08) |
0.07 | <0.001 |
| No | 10.46 (6.90–23.00) |
6.00 (4.00–9.50) |
48.68 (14.28–63.40) |
19.00 (18.00–23.00) |
5.00 (3.00–5.00) |
83.33 (67.10–86.95) |
<0.001 | |||||
| Current smoking | Yes | 15.00 (9.87–25.75) |
0.72 | 9.00 (60–11.50) |
33.33 (9.58–64.39) |
0.40 | 18.00 (15.00–22.00) |
0.82 | 4.50 (3.00–5.00) |
74.34 (66.66–86.36) |
0.38 | <0.001 |
| No | 15.20 (8.00–23.00) |
8.00 (4.00–10.00) |
47.36 (25.00–58.33) |
19.00 (13.00–22.00) |
5.00 (3.00–5.00) |
70.03 (59.53–83.99) |
<0.001 | |||||
| Beta blocker | Yes | 10.20 (5.00–17.00) |
<0.001 | 6.00 (2.00–11.00) |
25.00 (4.76–50.00) |
<0.001 | 18.00 (12.50–22.00) |
0.65 | 5.00 (3.00–5.00) |
75 (61.20–83.77) |
0.90 | <0.001 |
| No | 19.00 (11.35–30.25) |
9.00 (6.00–12.00) |
55.31 (26.43–65.33) |
18.00 (15.00–22.00) |
5.00 (3.00–5.00) |
72.22 (61.53–86.36) |
<0.001 | |||||
| ACEI/ARB | Yes | 8.75 (5.00–15.00) |
<0.001 | 6.00 (2.00–10.00) |
30.55 (13.04–56.52) |
0.02 | 18.00 (15.00–22.00) |
0.79 | 4.00 (3.00–5.00) |
78.57 (66.66–85.71) |
0.68 | <0.001 |
| No | 19.00 (12.00–25.00) |
9.75 (8.00–11.00) |
50.00 (21.73–64.70) |
19.00 (12.00–22.00) |
5.00 (3.00–5.00) |
72.22 (61.20–84.84) |
<0.001 | |||||
| CCB | Yes | 9.25 (4.92–17.75) |
0.01 | 7.00 (3.00–10.00) |
32.63 (16.14–57.88) |
0.51 | 15.00 (12.00–22.00) |
0.08 | 5.00 (3.00–6.00) |
66.66 (60.42–76.83) |
0.04 | <0.001 |
| No | 17.40 (10.05–25.00) |
9.25 (6.00–16.25) |
45.58 (14.78–64.60) |
19.00 (15.42–22.00) |
4.50 (3.00–5.00) |
77.92 (66.66–86.51) |
<0.001 | |||||
| Index event | Elective | 8 (4.97–17.00) |
<0.001 | 6.00 (2.75–6.75) |
25.00 (9.58–61.17) |
0.11 | 16.25 (13.5–21.75) |
0.01 | 3.50 (3.00–5.00) |
78.40 (66.66–86.64) |
0.65 | <0.001 |
| UA | 15.10 (10.20–24.00) |
8.50 (8.00–10.00) |
44.27 (21.56–58.33) |
17 (12.00–21.50) |
5.00 (3.00–5.00) |
75.47 (59.13–85.60) |
<0.001 | |||||
| NSTEMI | 25.00 (21.00–49.50) |
18.00 (10.00–20.00 |
33.33 (24.26–63.22) |
22 (19.00–27.00) |
5.00 (3.00–9.00) |
72.22 (59.09–86.36) |
0.01 | |||||
| STEMI | 28.50 (22.00–42.50) |
10.00 (9.62–18.25) |
59.80 (51.32–66.50) |
22.00 (14.60–25.50) |
9.00 (4.00–9.00) |
67.10 (59.98–73.21) |
0.01 | |||||
| Index procedure | One-vessel | 19.00 (10.20–28.00 |
0.04 | 9.00 (6.00–10.00) |
64.28 (21.56–83.33) |
0.01 | 16.50 (13.75–22.00) |
0.36 | 5.00 (3.75–5.00) |
72.95 (65.38–79.38) |
0.60 | 0.12 |
| Multi-vessel | 15.00 (6.97–23.25) |
8.00 (5.75–14.25) |
33.33 (13.04–56.52) |
18.5 (14.65–22.25) |
4.00 (3.00–6.00) |
73.61 (60.42–86.36) |
<0.001 | |||||
| Stent | POBA | 19.00 (9.50–29.00) |
0.06 | 9.50 (3.00–10.00) |
67.24 (28–83.33) |
0.03 | 16.00 (12.00–22.00) |
0.46 | 3.00 (2.00–5.00) |
83.33 (58.33–87.50) |
0.30 | 0.07 |
| DES | 13.50 (8.50–19.75) |
8.00 (5.25–12.50) |
35.41 (13.35–56.52) |
18.00 (13.50–22.00) |
5.00 (3.25–5.00) |
67.48 (61.53–80.68) |
<0.001 | |||||
| BMS | 28.00 (9.85–40.50) |
10.00 (8.25–12.50) |
41.17 (10.69–69.04) |
19.00 (15.25–22.00) |
3.00 (3.00–5.00) |
78.57 (62.87–86.73) |
0.004 | |||||
Note: Data are shown as numbers (percentage).
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMS, bare metal stent; CCB, Calcium channel blocker; DES, drug-eluting stent; DM, diabetes mellitus; HTN, hypertension; NSTEMI, non-ST segment elevation myocardial infarction; POBA, plain old balloon angioplasty; STEMI, ST segment elevation myocardial infarction; UA, unstable angina.
Table 3.
Multiple regression model*.
| MODEL | UNSTANDARDIZED COEFFICIENTS | STANDARDIZED COEFFICIENTS | P VALUE | 95% CONFIDENCE INTERVAL FOR B | ||
|---|---|---|---|---|---|---|
| B | STD. ERROR | BETA | LOWER BOUND | UPPER BOUND | ||
| (Constant) | 1.758 | 3.788 | 0.643 | −5.745 | 9.261 | |
| Index event | 5.471 | 1.024 | 0.420 | 0.000 | 3.443 | 7.49 9 |
| Beta blocker | 4.782 | 1.826 | 0.203 | 0.010 | 1.165 | 8.398 |
| Hypertension | −5.044 | 1.687 | −0.212 | 0.003 | −8.384 | −1.703 |
| Statin | 4.739 | 1.982 | 0.192 | 0.018 | 0.813 | 8.665 |
Notes:
Dependent variable: baseline hs-CRP.
Effects of clopidogrel on hs-CRP
Patients taking clopidogrel showed a significant reduction in hs-CRP level as compared with the baseline values (P < 0.001). Clopidogrel decreased the median hs-CRP level from 15.10 mg/Dl (9.62–23.75) to 8.50 mg/dL (6.00–10.75; P < 0.001). The effects of clopidogrel on hs-CRP levels in different subgroups are shown in Table 2.
Effects of prasugrel on hs-CRP
Prasugrel administration also resulted in a significant reduction in hs-CRP level from 18.00 mg/dL (14.25–22.00) to 5.00 (3.00–5.00; P < 0.001). The effects of prasugrel on hs-CRP levels in different subgroups are demonstrated in Table 2.
Prasugrel compared with clopidogrel
A significant 73% overall reduction in the primary end point (reduction in hs-CRP) was seen with prasugrel compared with 39% overall reduction in hs-CRP level with clopidogrel (Fig. 2, P = 0.002). In all subgroups, the hs-CRP reduction was significantly higher in the prasugrel group compared with the clopidogrel group (Table 2).
Figure 2.
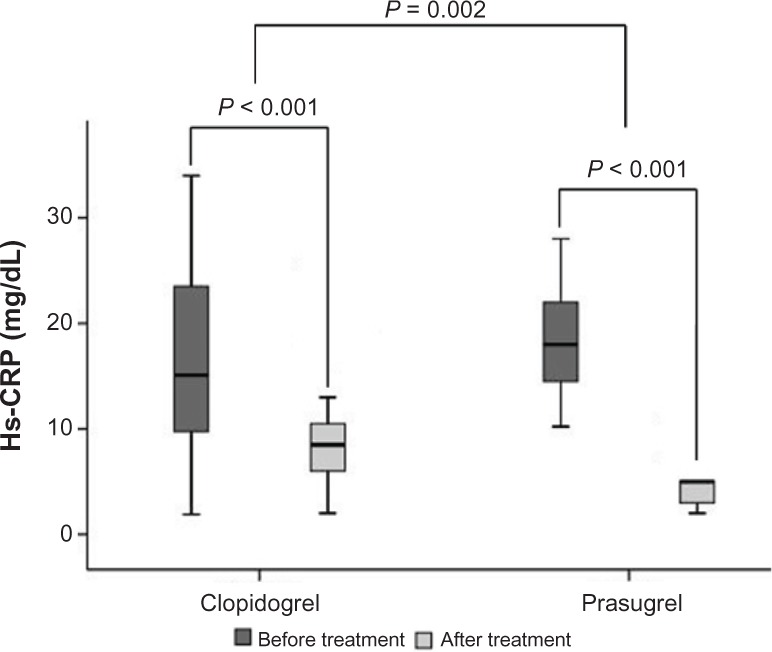
The changes in hs-CRP in patients treated with clopidogrel or prasugrel over a period of 12 weeks.
Discussion
The results of this study should be discussed in three main steps.
First, our data showed that baseline hs-CRP levels (prior PCI) were significantly different in patients with various types of index events. Patients with more advanced atherosclerosis event were more likely to have higher baseline hs-CRP (Fig. 1). We have demonstrated a similar finding in our previous study on 650 patients undergoing PCI.14 Moreover, according to our previous report,14 patients who undergo multivessel PCI have higher hs-CRP levels compared with patients with one- vessel PCI. However, in the present study, patients undergoing one-vessel PCI and multivessel PCI had comparable baseline hs-CRP, which may be due to our lower sample size in the present study. In addition, on multiple regression analysis, less serious index events, use of beta blockers and statins, and absence of hypertension were independently associated with lower baseline hs-CRP.
Second, we compared the changes in the hs-CRP levels following the administration of prasugrel and clopidogrel for 12 weeks. The main finding of the present study on 120 patients is that a 12-week administration of prasugrel to patients undergoing percutaneous coronary artery intervention results in a significantly higher decrease in serum levels of hs-CRP as compared with clopidogrel.
Third, we performed extensive subgroup analyses to characterize patients who may have higher anti- inflammatory benefits from either clopidogrel or prasugrel therapy. As patients within each subgroups were not well matched according to their baseline hs-CRP values, we sought that the assessment of net difference values might be misleading, ie, patients with lower baseline hs-CRP levels might have lower difference in their hs-CRP level with therapy. As a result, we compared the percent of change in hs-CRP levels relative to the baseline values over the study period. Using this reporting strategy, we showed 73% reduction in the hs-CRP level with prasugrel compared with 39% reduction in hs-CRP level with clopidogrel, this difference was statistically significant. According to Table 2, in both clopidogrel and prasugrel groups, male and female patients had comparable reduction in hs-CRP level. In the clopidogrel group, nondiabetic patients had approximately two times higher decrease in hs-CRP level compared with diabetic patients (50% versus 25%). In the prasugrel group, diabetic and nondiabetic patients have statistically comparable reduction in hs-CRP level (66% versus 74%). This finding shows that anti-inflammatory effects of clopidogrel is less prominent in diabetic patients, while prasugrel has comparable anti-inflammatory effects in both diabetics and nondiabetics. However, the pathophysiologic etiologies of these findings are not clear. Notably, the TRITON-TIMI 38 trial demonstrated that administration of prasugrel to patients with DM have a greater reduction in ischemic events without an increase in TIMI major bleeding.15 They concluded that the more intensive oral antiplatelet therapy with prasugrel is associated with more benefit to DM patients.
However, our results demonstrate that hypertension and current smoking did not modify anti-inflammatory properties of clopidogrel and prasugrel. Moreover, there were no significant interactions between anti-inflammatory effects of clopidogrel or prasugrel and the index event prior to PCI. Interestingly, in the clopidogrel group, patients who have undergone multivessel PCI had significantly lower decrease in hs-CRP level compared to those with single-vessel PCI (33% versus 64%). Stent implantation and its type also could affect the anti-inflammatory effects of clopidogrel.
Platelet–leukocyte interactions are suggested to induce pro-inflammatory effects. Thus, it can be presumed that platelet inhibition by antiplatelets may also induce some anti-inflammatory properties. Some previous studies have demonstrated anti-inflammatory properties of clopidogrel. It has been shown that ADP-induced P-selectin expression and platelet–leukocyte conjugate formation can be inhibited by clopidogrel, but not by aspirin.16,17 Vivekananthan et al showed that clopidogrel treatment attenuated the periprocedural increase in CRP by 65%.18 Quinn et al also showed that clopidogrel pretreatment in patients undergoing PCI reduced inflammatory markers expression on platelets.19 However, such data regarding prasugrel are still lacking.
The TRITON-TIMI 38 trial demonstrated a significant reduction in primary end point in patients randomized to prasugrel compared with those who received clopidogrel.12 In the PRASFIT-ACS study by Saito et al, efficacy and safety of prasugrel was compared with clopidogrel in Japanese patients with acute coronary syndrome.20 The primary efficacy end point was the incidence of major adverse cardiovascular events at 24 weeks, defined as a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal ischemic stroke. They showed that prasugrel with a loading dose of 20 mg and a maintenance dose of 3.75 mg was associated with a lower incidence of ischemic events compared with clopidogrel (9.4% versus 11.8%). According to their results, the incidence of noncoronary artery bypass graft-related major bleeding was similar in both groups. However, in none of these studies, inflammatory markers of atherosclerosis such as hs-CRP were not assessed.
Study Limitations
The main limitation of this study was the relatively small sample size. Our results would be more conclusive if we had measured platelet inflammatory markers such as CD40 ligand and P-selectin. The relationship between anti-inflammatory and antiplatelet properties of clopidogrel and prasugrel would also been more clarified if we could measure in vitro antiplatelet activity of these medications in each patient. The clinical importance of hs-CRP reduction would also been elucidated if we could include a clinical end point (eg, reduction in ischemic events) to our laboratory hs-CRP end point. Also the safety measures of prasugrel versus clopidogrel (eg, bleeding risk) have not been assessed in the current study.
As a result, future large-scale clinical trials overcoming the above limitations are suggested to make more comprehensive comparisons between prasugrel and clopidogrel.
Conclusion
In conclusion, prasugrel seems to be superior to clopidogrel in the reduction of hs-CRP in patients undergoing PCI, as it provides more uniform decrease in hs-CRP in different subgroups of patients. This may be related to higher inter-individual variability in response to clopidogrel compared with prasugrel (due to their metabolism) and also the higher potency, better bioavailability, and lower pharmacodynamic variability of prasugrel.
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 440 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no external funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions madeby independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE). Provenance: the authors were invited to submit this paper.
Author Contributions
Conceived and designed the experiments: SH, MC, MC. Analyzed the data: MC, MC. Wrote the first draft of the manuscript: MC, MC. Contributed to the writing of the manuscript: MC, MC. Agree with manuscript results and conclusions: SH, MC, MC, NS, AA, AAB, NB, AB, MH, SM. Jointly developed the structure and arguments for the paper: MC, MC, AA. Made critical revisions and approved final version: MC, MC, AA. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker P, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg A, Zinder O, Zdorovyak A, et al. Diagnostic coronary angiography induces a systemic inflammatory response in patients with stable angina. Am Heart J. 2003;146:819–23. doi: 10.1016/S0002-8703(03)00407-1. [DOI] [PubMed] [Google Scholar]
- 4.Almagor M, Keren A, Banai S. Increased C-reactive protein level after coronary stent implantation in patients with stable coronary artery disease. Am Heart J. 2003;145:248–53. doi: 10.1067/mhj.2003.16. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal A, Blum A, Schneider DJ, Sobel BE, Dauerman HL. Soluble CD40 ligand is an early initiator of inflammation after coronary intervention. Coron Artery Dis. 2004;15:471–5. doi: 10.1097/00019501-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal A, Schneider DJ, Terrien EF, Gilbert KE, Dauerman HL. Increase in interleukin-6 in the first hour after coronary stenting: an early marker of the inflammatory response. J Thromb Thrombolysis. 2003;15:25–31. doi: 10.1023/a:1026188200939. [DOI] [PubMed] [Google Scholar]
- 7.Park DW, Yun SC, Lee JY, Kim WJ, Kang SJ, Lee SW. C-reactive protein and the risk of stent thrombosis and cardiovascular events after drug-eluting stent implantation. Circulation. 2009;120:1987–95. doi: 10.1161/CIRCULATIONAHA.109.876763. [DOI] [PubMed] [Google Scholar]
- 8.Papp J, Kenyeres P, Toth K. Clinical importance of antiplatelet drugs in cardiovascular diseases. Clin Hemorheol Microcirc. 2013;53:81–96. doi: 10.3233/CH-2012-1578. [DOI] [PubMed] [Google Scholar]; Reinhart WH. Platelets in vascular disease. Clin Hemorheol Microcirc. 2013;53:71–9. doi: 10.3233/CH-2012-1577. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JL, Wright RS, Adams CD, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:645–81. doi: 10.1016/j.jacc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Kushner FG, Hand M, Smith SC, Jr, et al. 2009 focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction (updating the 2004 guideline and 2007 focused update) and ACC/AHA/SCAI guidelines on percutaneous coronary intervention (updating the 2005 guideline and 2007 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2009;120:2271–306. doi: 10.1161/CIRCULATIONAHA.109.192663. [DOI] [PubMed] [Google Scholar]
- 11.Sugidachi A, Asai F, Yoneda K, et al. Antiplatelet action of R-99224, an active metabolite of a novel thienopyridine-type G linked P2T antagonist, CS−747. Br J Pharmacol. 2001;132:47–54. doi: 10.1038/sj.bjp.0703761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 13.What Are the Symptoms of Heart Attack and Unstable Angina? eMedicine-Health; 2013. [Accessed May 9, 2013]. Available at: http://www.emedicinehealth.com/heart_attack_and_unstable_angina-health/page5_em.htm#Symptoms. [Google Scholar]
- 14.Hajsadeghi S, Chitsazan M, Chitsazan M, et al. Changes of high sensitivity c-reactive protein during clopidogrel therapy in patients undergoing percutaneous coronary intervention. Res Cardiovasc Med. 2016;5(1):e28997. doi: 10.5812/cardiovascmed.28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiviott SD, Braunwald E, Angiolillo DJ, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel Thrombolysis in Myocardial Infarction 38. Circulation. 2008;118:1626–36. doi: 10.1161/CIRCULATIONAHA.108.791061. [DOI] [PubMed] [Google Scholar]
- 16.Storey RF, Judge HM, Wilcox RG, Heptinstall S. Inhibition of ADP-induced P-selectin expression and platelet-leukocyte conjugate formation by clopidogrel and the P2Y12 receptor antagonist AR-C69931MX but not aspirin. Thromb Haemost. 2002;88:488–94. [PubMed] [Google Scholar]
- 17.Klinkhardt U, Graff J, Harder S. Clopidogrel, but not abciximab, reduces platelet leukocyte conjugates and Pselectin expression in a human ex vivo in vitro model. Clin Pharmacol Ther. 2002;71:176–85. doi: 10.1067/mcp.2002.122018. [DOI] [PubMed] [Google Scholar]
- 18.Vivekananthan DP, Bhatt DL, Chew DP, et al. Effect of clopidogrel pretreatment on periprocedural rise in C-reactive protein after percutaneous coronary intervention. Am J Cardiol. 2004;1(94):358–60. doi: 10.1016/j.amjcard.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 19.Quinn MJ, Bhatt DL, Zidar F, et al. Effect of clopidogrel pretreatment on inflammatory marker expression in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2004;93:679–84. doi: 10.1016/j.amjcard.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 20.Saito S, Isshiki T, Kimura T, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J. 2014;78:1684–92. doi: 10.1253/circj.cj-13-1482. [DOI] [PubMed] [Google Scholar]


