Abstract
There is increasing evidence of central hyperexcitability in chronic whiplash-associated disorders (cWAD). However, little is known about how an apparently simple cervical spine injury can induce changes in cerebral processes. The present study was designed (1) to validate previous results showing alterations of regional cerebral blood flow (rCBF) in cWAD, (2) to test if central hyperexcitability reflects changes in rCBF upon non-painful stimulation of the neck, and (3) to verify our hypothesis that the missing link in understanding the underlying pathophysiology could be the close interaction between the neck and midbrain structures. For this purpose, alterations of rCBF were explored in a case-control study using H215O positron emission tomography, where each group was exposed to four different conditions, including rest and different levels of non-painful electrical stimulation of the neck. rCBF was found to be elevated in patients with cWAD in the posterior cingulate and precuneus, and decreased in the superior temporal, parahippocampal, and inferior frontal gyri, the thalamus and the insular cortex when compared with rCBF in healthy controls. No differences in rCBF were observed between different levels of electrical stimulation. The alterations in regions directly involved with pain perception and interoceptive processing indicate that cWAD symptoms might be the consequence of a mismatch during the integration of information in brain regions involved in pain processing.
Abbreviations: WAD, whiplash associated disorders; cWAD, chronic whiplash associated disorders; rCBF, regional cerebral blood flow; CT, computed tomography; MRI, magnetic resonance imaging; PET, positron emission tomography; SPECT, single-photon emission computed tomography; PAG, periaqueductal gray; NK1, neurokinin 1; SD, standard deviation; BMI, body mass index; VAS, visual analog scale; WDQ, Whiplash Disability Questionnaire; NDI, Neck Disability Index; HADS, Hospital Anxiety and Depression Scale; mA, milliamperes; SPM, statistical parametric mapping; SwE, sandwich estimator; MNI, Montreal Neurological Institute; FWHM, full width at half maximum; gCBF, global cerebral blood flow; SVC, small volume correction; GEE, Generalized Estimating Equations; B, unstandardized coefficient; SE, standard error; CI, confidence interval; DMN, default mode network
Keywords: Whiplash associated disorders, Positron emission tomography, Non-painful electrical stimulation, Neuropsychological questionnaires
Highlights
-
•
Differences of rCBF were explored by PET in cWAD patients and healthy volunteers exposed to four conditions.
-
•
Changes in rCBF were observed in cWAD patients in regions involved in pain perception and interoceptive sensory information.
-
•
These changes might be the consequence of a mismatch in the integration of interoceptive stimuli in pain processing regions.
In the past, published work on chronic whiplash-associated disorders (cWAD) has caused much confusion and discussion, yet functional imaging methods such as positron emission tomography (PET) have demonstrated a variety of different significant alterations in the perfusion or glucose utilization of the brain. The present study, using PET and the perfusion marker, H215O, is a step forward in whiplash research. It shows changes in perfusion in regions directly involved in pain perception and interoceptive sensory information, such as the insular cortex, precuneus, and posterior cingulate, indicating a mismatch in the integration of interoceptive information in pain processing brain regions.
1. Introduction
1.1. Epidemiology and Symptoms
Whiplash-associated disorders (WAD) are a prevalent and costly problem. In fact, whiplash trauma is currently one of the most frequent consequences of motor vehicle accidents. It is estimated to have an annual cost of at least €10 billion in Europe (Report, 2005) and $29 billion in the United States (Freeman et al., 1999), affecting about 300 per 100,000 people per year in North America and Western European countries (Holm et al., 2008). While in many cases initial symptoms resolve within a few weeks, approximately half of subjects develop persistent symptoms (Carroll et al., 2008). These symptoms are heterogeneous, and subsumed under the term WAD (Spitzer et al., 1995). WAD includes neck pain and headache as the most frequent symptoms, followed by interscapular and temporomandibular pain, paresthesia in the arms and hands, and dizziness, as well as visual and psychological disturbances (Sterner and Gerdle, 2004). Although WAD includes regional neck symptoms, the common presence of psychological manifestations suggests the involvement of central nervous system processes in WAD symptom presentation. Additional historical information is supplied in Appendix A of the Supplementary Materials.
1.2. Neuroimaging
In many cases, tissue damage cannot be detected in patients with WAD leading to the question of whether a lesion is necessary or sufficient to initiate the symptoms. Several investigations performed in animals, cadavers, healthy volunteers, and patients have reported lesions in various tissues, mainly involving the zygapophysial (facet) joints (Curatolo et al., 2011). Irrespective of the presence of tissue lesions, increasing evidence indicates that a process of central hyperexcitability results in widespread lowered pain thresholds that are associated with poor recovery in patients with chronic WAD (cWAD) (Stone et al., 2013, Sterling et al., 2006, Williams et al., 2007). However, most of these alterations remain undetected by conventional imaging techniques such as radiography, computed tomography (CT), and structural magnetic resonance imaging (MRI), and are, thus, of little use in determining the prognosis of WAD (Williams et al., 2007). In this scenario, functional imaging techniques, such as new functional MRI sequences or nuclear medicine imaging techniques (e.g., positron emission tomography [PET] and single-photon emission computed tomography [SPECT]), can be helpful in understanding the underlying mechanism of cWAD as they have the ability to provide functional insight into physiological processes and biological pathways in vivo. Otte et al. performed a series of studies to visualize brain perfusion and metabolism using SPECT and PET, respectively, in cWAD patients at rest (SPECT: [99mTc]-hexamethyl propylene amine oxime and [99mTc]-ethylene biyldicysteinate dimer, PET: [18F]-fluorodeoxyglucose) (Otte et al., 1995, Otte et al., 1997a, Otte et al., 1997b, Otte et al., 1998a). In these studies, a statistically significant reduction was seen in both perfusion and metabolism in the posterior parietal occipital cortex in many cWAD patients when compared with healthy volunteers. Similar findings were observed more recently in a PET study that measured the regional cerebral blood flow (rCBF) with H215O PET. In this study, elevated rCBF was also found in cWAD patients in the posterior parahippocampal and posterior cingulate gyri in both hemispheres, and in the right medial prefrontal gyrus and right thalamus (Linnman et al., 2009). Other imaging studies in cWAD patients based on perfusion SPECT supported these results, yielding a statistically significant reduction of rCBF in the temporal lobe (Lorberboym et al., 2002, Sundström et al., 2006).
1.3. Hypotheses
The aforementioned alterations in perfusion and metabolism were suggested to be related to nociceptive afferents causing increased levels of vasoconstriction (Moskowitz and Buzzi, 1991). However, little is known regarding how whiplash injuries without significant imaging findings enhance central nervous system processes. We had previously hypothesized (Vállez García et al., 2014) that in cWAD patients there is a mismatch between aberrant information from the neck muscles and the vestibular and visual systems, which are integrated in the mesencephalic periaqueductal gray (PAG) and adjacent regions (Fig. 1). We consider that the missing key for understanding the underlying pathophysiology in cWAD is the close interaction between the neck and midbrain structures through the fibers originating from the C1–C3 spinal segments that project to the PAG and thalamus (Klop et al., 2004, Mouton et al., 2001). These fibers arise from neurons distributed throughout lamina VI–VIII, of which a specific cluster is located in the ventrolateral horn (Mouton et al., 2009). In addition, the trigeminocervical complex, constituting the trigeminal nucleus caudalis and C1–C2 dorsal horn, maintains projections to the PAG, and is known to be involved in the development of migraine and headaches (Watson and Drummond, 2016). All these ascending pathways converge in neurons located in the PAG and adjoining regions, which are essential for the normal function of multiple processes (Linnman et al., 2012). In cWAD patients, the PAG and adjoining regions have an attenuated availability of neurokinin 1 (NK1) receptors (Linnman et al., 2010)–involved in pain and anxiety behavior–and undergo gray matter changes associated with the development of headache (Obermann et al., 2009).
Fig. 1.
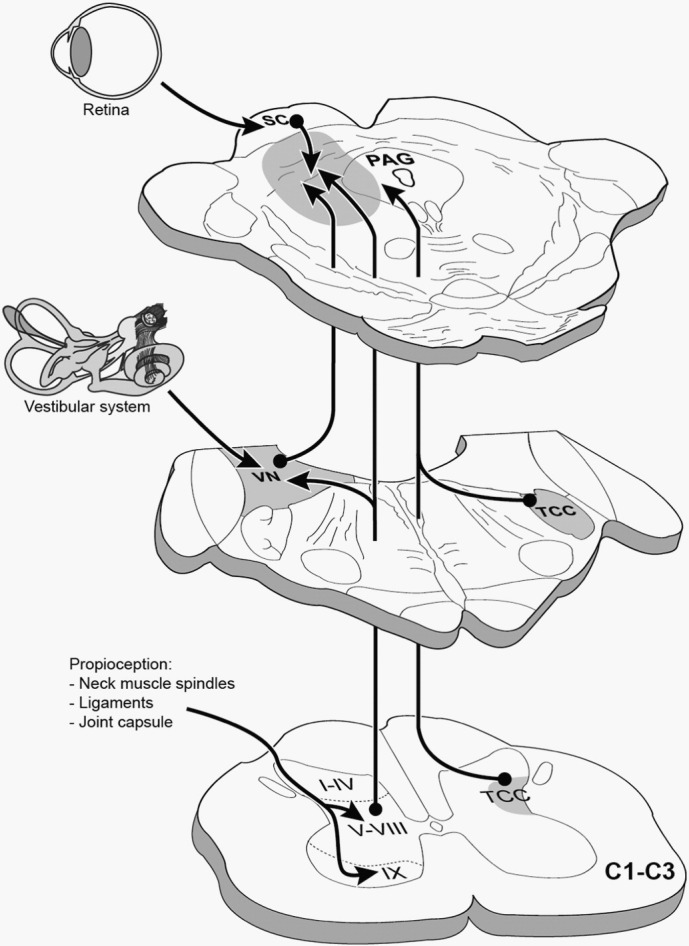
Ascending afferents from the C1–C3 spinal segments that project to the periaqueductal gray and its adjoining regions. TCC: trigeminocervical complex; VN: vestibular nuclei; PAG: periaqueductal gray; SC: superior colliculus. From Vállez García et al. (2014) (Fig. 46.5, page 957); with kind permission from Springer Business + Media, Heidelberg, Germany.
1.4. Aim and Design of This Study
The current study was designed to address three questions. First, we aimed to confirm previously reported results showing alterations in the rCBF in cWAD patients. Second, we wanted to determine if the central hyperexcitability observed in cWAD patients reflects changes in brain perfusion at rest or is a consequence of the processing of non-painful electrical stimulation of the neck. Third, we aimed to test if changes in brain perfusion are observed in the PAG and thalamus, both of which are structures with close interactions with the neck, as presented in our hypothesis. For this purpose, changes in rCBF were explored using H215O PET, where patients and healthy volunteers were each exposed to four different experimental conditions.
2. Material and Methods
2.1. Patients and Healthy Volunteers
All participants of this study were women of Dutch nationality (details in Table 1). cWAD patients (n = 12, collision 4 ± 2 [range: 1–8] years prior to the study) and healthy volunteers (n = 8) were age-matched. The population was restricted to women to prevent heterogeneity in study results, since females were found to have a higher incidence of emergency department motor vehicle-related neck injuries (Quinlan et al., 2004) and a significant risk of developing cWAD (Walton et al., 2009). The participants were recruited in the Groningen City area (The Netherlands) between 2012 and 2013, via advertisements in public buildings, local newspapers, and radio stations, as well as via the national association of whiplash patients (Whiplash Stichting Nederland) and the Royal Dutch Society for Physical Therapy. Screening procedures included a brief telephone interview and neurological examination to check if individuals met the inclusion criteria. Volunteers had to be older than 18 years, and not going through the menopause. The exclusion criterion for healthy volunteers was any past whiplash trauma. Specific exclusion criteria for cWAD patients were a classification of grade III or IV (Dutch Institute for Healthcare Improvement (CBO), 2008; Spitzer et al., 1995), loss of consciousness during the collision, neurological symptoms or pain not related to cWAD, and more than one traffic collision that could be associated with the development of cWAD. Exclusion criteria for all subjects were the use of anticoagulants, somatic or neurological disorders, left handedness, substance abuse, pregnancy, body mass index (BMI) ≥ 30 kg/m2, and a score higher than 14 in the combined anxiety and depression score using the Hospital Anxiety and Depression Scale. All volunteers were asked to refrain from analgesics and anti-inflammatory drugs for one to three days, from tobacco, alcohol and caffeine for 12 h, and food for 3 h prior to scanning (Linnman et al., 2009). The PET scan was performed between 1 and 7 weeks after the neurological examination at screening. On the day of the scan, the subjects were asked if any of their symptoms had changed in the elapsed time. All volunteers followed the requirements to avoid medication, tobacco, alcohol, caffeine, and food prior to the PET scan. The whole study was performed in a non-blinded manner.
Table 1.
Differences between chronic whiplash associated disorders (cWAD) patients and healthy volunteers.
| Healthy volunteers (n = 8) |
cWAD patients (n = 12) |
p-Values | |||
|---|---|---|---|---|---|
| Age | 25 | [23 − 30] | 35 | [28–40] | 0.14 |
| BMI | 25 | [23–28] | 24 | [21–27] | 0.49 |
| VAS | 0 | 6 | [5–7] | < 0.01 | |
| WDQ | 0 | 62 | [54–69] | < 0.01 | |
| NDI | 0 | 25 | [20–27] | < 0.01 | |
| HADS | 0 | 8 | [5–14] | < 0.01 | |
| Perception threshold (mA) | 31 | [25–41] | 30 | [20–34] | 0.32 |
| Pain threshold (mA) | 75 | [72–89] | 64 | [60–70] | 0.02 |
Median [Interquartile range]. Group difference explored using Mann-Whitney test. BMI: body mass index; VAS: last 3 months average neck pain measured with the visual analog scale; WDQ: Whiplash Disability Questionnaire; NDI: Neck Disability Index; HADS: Hospital Anxiety and Depression Scale; mA: milliamperes.
According to the Declaration of Helsinki, all participants gave written informed consent, and the study was approved by the internal ethics committee of the University Medical Center of Groningen, The Netherlands.
2.2. Subjective Ratings
During the neurological interview, all participants completed several self-reported questionnaires in Dutch. These were the Hospital Anxiety and Depression Scale (HADS), Visual Analog Scale (VAS), Neck Disability Index (NDI), and Whiplash Disability Questionnaire (WDQ). In all of these questionnaires, a low score implies the absence of pain, symptoms, or complaints, while a high value represents higher severity. In the HADS (Spinhoven et al., 1997, Zigmond and Snaith, 1983), each item is scored from 0 to 3, giving a total score ranging from 0 to 21 for either anxiety or depression. However, for the present study the combination of both scores was used as the output. The VAS was used to rate the average intensity of neck pain perceived by volunteers in the last 3 months, with a score that ranged between 0 (i.e., no pain) and 10 (i.e., worst pain imaginable). The NDI (Jorritsma et al., 2012, Vernon and Mior, 1991) comprises 10 items (rated from 0 to 5) including pain, personal care, lifting, reading, headaches, concentration, work, driving, sleeping, and recreation. Finally, an in-house non-validated Dutch translation of the WDQ (Pinfold et al., 2004) was provided to the volunteers. The WDQ is a 13-item questionnaire specifically developed for WAD.
2.3. Electrical Stimulation
For the electrical stimulation, the skin of the back part of the neck was rinsed with alcohol, and disposable auto-adhesive skin electrodes (Model ST5090 5 × 9 cm, Axelgaard Manufacturing Co., USA) were placed bilaterally at the level of the second and sixth cervical spinal processes. The following four different conditions were used during the experiment:
-
–
Rest state: no stimulation;
-
–
Low stimulation: above the perception threshold;
-
–
High stimulation: below observable muscular contraction or pain threshold; and
-
–
Placebo-like stimulation: the volunteer was informed that they received an electrical stimulation below the perception threshold, but the device did not deliver any current.
These four conditions were repeated three times, resulting in a total of twelve scans per subject. The order of stimulations was randomized between and within volunteers, with the rest state always being the first of a series. The repetition of the conditions and its randomization was expected to provide a more accurate control of within-subject variance. The stimulation consisted on a non-painful electrical constant current, delivered by Digitimer DS7A in combination with a DG2A stimulator (Digitimer Limited, UK), using a biphasic pulse, a train repetition of 50 Hz, and 100 μs pulse duration. The use of a biphasic pulse controlled for the possible habituation effect to the electrical current. Prior to the first PET scan, the individual thresholds were determined by a slow increase of the current (measured in mA). First, each volunteer was asked to make clear when the current was clearly perceived (perception threshold, considered as 0%). Subsequently, the pain threshold (100%) was set when the volunteer indicated that the current was painful, or when the researcher detected a clear muscular contraction. This procedure was repeated three times with an interval of 1–2 min between trials. The average value of these thresholds was used during the scan to define the low (15%) and high (85%) stimulation conditions, both of which were conditions within the range of perception but not painful for the volunteer. Before the injection of the radiotracer the volunteer was informed about the condition that was going to be delivered as ‘rest’, ‘low’ for the placebo, ‘medium’ for low stimulation (15%), and ‘high’ for the higher stimulation (85%). The device was turned on, providing an audible noise, for all conditions, except for the rest condition. Immediately after each scan, the device was turned off, and the subject was allowed to open their eyes.
2.4. PET Data Acquisition
During the whole procedure, lighting maintained at low intensity in a quiet room. Each PET scan was made in 3D mode (63 planes, axial field of view of 15.5 cm) using an ECAT Exact HR + camera (CTI-Siemens, USA) with a spatial resolution of 4 to 5 mm full width at half maximum (FWHM) in all three directions. For the scan, the radiotracer H215O was used to measure cerebral blood flow (CBF). Per scan, 500 MBq of activity dissolved in 32 mL of 0.9% saline was administered intravenously into the right forearm at 8 mL/s. After injection of the radiotracer, data were collected over 120 s. Consecutive scans were made with intervals of 10–15 min, resulting in a 2.5–3 h procedure. The participants were asked to keep their eyes closed during the scanning period, and their head was maintained in a steady position using a head-restraining adhesive band. Small movements of the head were manually corrected between scans using landmarks drawn on the face. A scan-specific attenuation correction was calculated to minimize inter-scan displacement induced variance (Reinders, 2002). Data acquisition involved reconstruction using a filtered back projection procedure, corrected for background radiation, and a final frame of 120 s was obtained. The rCBF was defined as the blood flow to a specific region of the brain in a given time, and was assumed to mirror the neural activity.
2.5. Statistical Analysis
2.5.1. Sample Size Calculation
Based on the assumption of a small to medium expected effect size of 0.3 for the difference in the rCBF between the cWAD and healthy volunteer groups, and analyzed with a general linear model with four correlated measurements (conditions), a two-tailed significance level of 5%, and a power of 80%, a total sample size of 18 was calculated.
2.5.2. Voxel-based Analysis
PET images were processed using the statistical parametric mapping (SPM12) software (Wellcome Department of Cognitive Neurology, United Kingdom) in combination with the Sandwich Estimator (SwE) v1.2.2 toolbox to account for repeated measures (Guillaume et al., 2014).
2.5.2.1. Image Preprocessing
Images were first aligned between acquisitions for each subject, and then normalized to the Montreal Neurological Institute's (MNI) stereotactic template. Images were then masked to remove non-cerebral signal and smoothed using a 12 mm FWHM Gaussian kernel.
2.5.2.2. Statistical Model
For the statistical analysis, the terms ‘group’, ‘condition’, and their interaction were included as variables in the model, using the SwE interface. Correction for the small samples (‘type C2’) and estimation of the degrees of freedom (‘approximate III’) were included in the design. Differences in global cerebral blood flow (gCBF) were included as covariates, separated in a between-subject component (i.e., mean subject gCBF) and within-subject component (i.e., difference with the subject mean gCBF), providing a final statistical design to explore the differences between groups in their rCBF.
2.5.2.3. Whole Brain Analysis
To explore the first and second aims of the study, the level of significance for the whole brain exploration was set to a voxel threshold of p = 0.005 uncorrected, with a minimum of k = 100 voxels in the cluster. The Talairach Daemon database atlas (Lancaster et al., 2000, Talairach and Tournoux, 1988) was used to ease the comparison with previous studies, and the anatomical localization of the results was aided by the WFU Pickatlas v3.0.5 toolbox (Maldjian et al., 2003, Maldjian et al., 2004). In those significant clusters consisting of several brain regions, each functional gray matter region was extracted and reported separately.
2.5.2.4. A Priori Volume of Interest Analysis
Additionally, to examine the third aim of the study, a separate exploration of the results was performed by restricting the research area to the periaqueductal gray (PAG), with a sphere of radius 8 mm centered at the MNI coordinates x = 0, y = − 29, z = − 12 (Linnman et al., 2012). Finally, to further examine the first aim of the study, the analysis was restricted to the parietal, temporal, and occipital lobes using a mask. For both analyses the level of significance was set to a voxel threshold of p = 0.005 uncorrected, without a minimum number of voxels (k = 0).
2.5.3. Associations Between Subjective Ratings and rCBF
The rest of the statistical analysis was performed using Stata software version 14.0 (StataCorp LP, College Station, USA). Results were considered statistically significant at p < 0.05.
2.5.3.1. Correlation Between Subjective Ratings
The correlation between variables was explored using the Spearman's rank correlation coefficient (rs). The correlations between the subjective ratings, years since the injury, and perception and pain thresholds were only performed in the cWAD group.
2.5.3.2. Subjective Ratings and rCBF
The Generalized Estimating Equations (GEE) model (Hardin and Hilbe, 2012) was used with the auto-regressive correlation matrix. The dependent variable was the rCBF measurement, including the four conditions repeated three times. The subjective ratings obtained from the self-reporting questionnaires (VAS, WDQ, NDI, and HADS) and the perception and pain thresholds were included in the model as independent variables. This model was applied to each brain region with significant differences in the rCBF between cWAD patients and healthy controls obtained from voxel-based whole brain analysis. The original values from the images were extracted and divided by the gCBF to obtain a measurement of the rCBF. These values were proportionally scaled (multiplied by 50) to resemble the average blood flow values across the whole brain in physiological conditions: 50 mL/100 g/min (Lassen, 1985).
3. Results
3.1. Subjective Ratings
When comparing the thresholds for electrical stimulation, the pain threshold in the cWAD patients was 85% (64/75) of the pain threshold in healthy subjects, however, no differences were observed in the perception threshold between the groups (Table 1).
Within the cWAD group, there was a significant positive correlation between the years since the injury and the perception and pain thresholds. Moreover, the NDI was positively correlated with the VAS, WDQ, and HADS, and the WDQ was positively correlated with the NDI and HADS (Table 2).
Table 2.
Spearman's rank correlation coefficients within the chronic whiplash associated disorders patients.
| Years | VAS | WDQ | NDI | HADS | Perception threshold (mA) | Pain threshold (mA) | |
|---|---|---|---|---|---|---|---|
| Years | 1 | − 0.03 | 0.30 | 0.00 | − 0.19 | 0.59* | 0.67* |
| VAS | 1 | 0.47 | 0.74** | 0.45 | 0.16 | 0.24 | |
| WDQ | 1 | 0.62* | 0.63* | 0.03 | − 0.03 | ||
| NDI | 1 | 0.61* | 0.07 | 0.19 | |||
| HADS | 1 | − 0.06 | − 0.09 |
Years: years subsequent to the injury, VAS: last 3 months average pain visual analog scale, WDQ: Whiplash Disability Questionnaire, NDI: Neck Disability Index, HADS: Hospital Anxiety and Depression Scale, mA: milliamperes, Significance levels by * p < 0.05 and ** p < 0.01.
3.2. Regional Cerebral Blood Flow
3.2.1. cWAD Patients and Healthy Volunteers
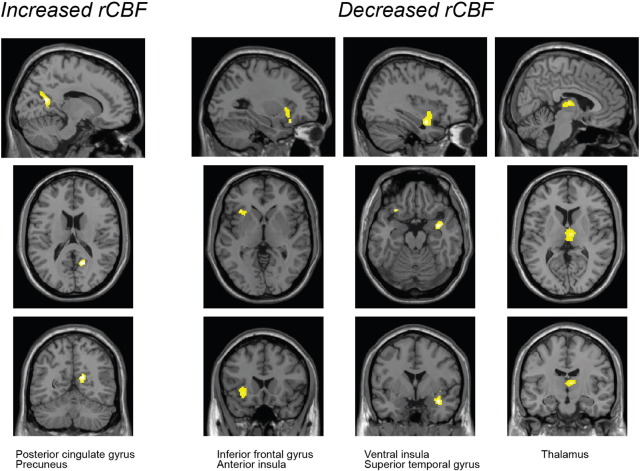
Statistically significant differences in rCBF were found in several regions of cWAD patients when compared with healthy volunteers (Table 3 and Fig. 2). A significant cluster with increased rCBF was found in parts of the right posterior cingulate and right precuneus. A decreased rCBF was observed in two clusters in the right hemisphere, comprising the superior temporal gyrus, insula, parahippocampal gyrus, and thalamus. In addition, another cluster with decreased rCBF in cWAD patients was detected in the left inferior frontal gyrus and left insula.
Table 3.
Differences in the regional cerebral blood flow in chronic whiplash associated disorders (cWAD) patients compared with healthy volunteers.
| Contrast | Cluster |
Peak |
||
|---|---|---|---|---|
| Region | Voxels | Z-value | x, y, z | |
| Healthy < cWAD (increased rCBF in cWAD patients) |
||||
| Cluster 1 | R. posterior cingulate gyrus | 46 | 3.03 ± 0.28 | |
| R. precuneus | 33 | 2.73 ± 0.11 | 15, − 63, 28 | |
| Healthy > cWAD (decreased rCBF in cWAD patients) |
||||
| Cluster 1 | R. superior temporal gyrus | 43 | 3.17 ± 0.46 | 35, − 1, − 16 |
| R. insula | 18 | 2.81 ± 0.14 | ||
| R. parahippocampal gyrus | 7 | 3.05 ± 0.36 | ||
| Cluster 2 | R. thalamus | 95 | 3.00 ± 0.32 | 7, − 11, 9 |
| Cluster 3 | L. inferior frontal gyrus | 61 | 3.21 ± 0.45 | − 33, 21, − 7 |
| L. insula | 26 | 2.88 ± 0.19 | ||
Height threshold p = 0.005 uncorrected, extent threshold k = 100, voxel size = 2 × 2 × 2 mm. Anatomical location and coordinates according to Talairach Daemon database atlas. rCBF: regional cerebral blood flow; R: right; L: left.
Fig. 2.
Voxel-based analysis of H215O PET scans. The results show the significant regions with decreased or increased regional cerebral blood flood (rCBF) in chronic whiplash associated disorders patients as compared with healthy volunteers (height threshold p = 0.005 uncorrected, extent threshold k = 100 voxels, voxel size = 2 × 2 × 2 mm).
3.2.2. rCBF After Electrical Stimulation
No statistically significant differences were found in the voxel-based analysis between any of the conditions (i.e., rest, placebo, or electrical stimulation of the neck) in either cWAD patients or healthy volunteers. However, a tendency can be observed in the results, with an increased number of clusters at the higher stimulations (see Fig. 1s and Table 1s in Appendix B of the Supplementary Material section).
3.2.3. rCBF in the PAG
No significant alterations of the rCBF were detected in the PAG during the SVC analysis.
3.2.4. rCBF in the Parietal, Occipital, and Temporal Lobes
Small clusters of hypoperfusion were observed in the posterior parietal occipital region. In addition, there were also marginal clusters of hyperperfused areas in this region (see Figs. 2s and 3s and Tables 2s and 3s, respectively, in Appendix B of the Supplementary Material section).
3.3. Association Between Subjective Ratings and Altered rCBF
In the cWAD group, the association between the subjective ratings and rCBF in the statistically significant functional gray matter regions was further explored (Table 4). The average VAS was negatively correlated with the rCBF in the left inferior frontal gyrus, and the HADS was negatively correlated with the right precuneus. The NDI was found to have a positive correlation with the rCBF in the right insula and a negative correlation with the right precuneus. The WDQ score was positively correlated with the rCBF in the right precuneus, right insula, and right superior temporal gyrus. Finally, the perception threshold was found to have a negative correlation with the rCBF in the right superior temporal gyrus, whereas the pain threshold was positively correlated with the rCBF in the right superior temporal gyrus and right parahippocampal gyrus, and negatively correlated in the right insula.
Table 4.
Differences between subjective ratings and the regional cerebral blood flow of chronic whiplash associated disorders patients.
| Parameter Estimate | B ± SE | 95% Wald CI |
Z | p-Value | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Posterior cingulate gyrus (right) | |||||
| (Intercept) | 59.96 ± 4.67 | 50.81 | 69.11 | 12.84 | < 0.01 |
| VAS | 1.48 ± 0.96 | − 0.40 | 3.36 | 1.55 | 0.12 |
| WDQ | − 0.05 ± 0.10 | − 0.25 | 0.15 | − 0.52 | 0.60 |
| NDI | − 0.44 ± 0.26 | − 0.95 | 0.08 | − 1.66 | 0.10 |
| HADS | 0.28 ± 0.31 | − 0.33 | 0.90 | 0.90 | 0.37 |
| Perception threshold | 0.77 ± 0.71 | − 0.63 | 2.16 | 1.08 | 0.28 |
| Pain threshold | − 0.23 ± 0.15 | − 0.53 | 0.07 | − 1.49 | 0.14 |
| Precuneus (right) | |||||
| (Intercept) | 58.73 ± 2.31 | 54.22 | 63.25 | 25.48 | < 0.01 |
| VAS | − 0.48 ± 0.52 | − 1.51 | 0.54 | − 0.92 | 0.36 |
| WDQ | 0.18 ± 0.05 | 0.07 | 0.28 | 3.32 | < 0.01 |
| NDI | − 0.41 ± 0.13 | − 0.67 | − 0.15 | − 3.08 | < 0.01 |
| HADS | − 0.49 ± 0.14 | − 0.77 | − 0.21 | − 3.45 | < 0.01 |
| Perception threshold | 0.12 ± 0.34 | − 0.55 | 0.79 | 0.36 | 0.72 |
| Pain threshold | 0.08 ± 0.06 | − 0.04 | 0.19 | 1.29 | 0.20 |
| Superior temporal gyrus (right) | |||||
| (Intercept) | 39.78 ± 1.89 | 36.07 | 43.48 | 21.02 | < 0.01 |
| VAS | 0.26 ± 0.54 | − 0.80 | 1.31 | 0.48 | 0.63 |
| WDQ | 0.11 ± 0.03 | 0.05 | 0.16 | 3.58 | < 0.01 |
| NDI | − 0.21 ± 0.14 | − 0.49 | 0.07 | − 1.46 | 0.14 |
| HADS | − 0.12 ± 0.11 | − 0.33 | 0.1 | − 1.06 | 0.29 |
| Perception threshold | − 0.92 ± 0.34 | − 1.58 | − 0.27 | − 2.75 | 0.01 |
| Pain threshold | 0.10 ± 0.05 | 0.00 | 0.2 | 1.88 | 0.06 |
| Insula (right) | |||||
| (Intercept) | 49.69 ± 1.70 | 46.36 | 53.03 | 29.20 | < 0.01 |
| VAS | − 0.62 ± 0.41 | − 1.42 | 0.18 | − 1.51 | 0.13 |
| WDQ | − 0.06 ± 0.02 | − 0.10 | − 0.02 | − 2.86 | < 0.01 |
| NDI | 0.66 ± 0.11 | 0.45 | 0.87 | 6.16 | < 0.01 |
| HADS | − 0.04 ± 0.06 | − 0.16 | 0.09 | − 0.59 | 0.56 |
| Perception threshold | − 0.09 ± 0.29 | − 0.66 | 0.48 | − 0.31 | 0.75 |
| Pain threshold | − 0.20 ± 0.06 | − 0.32 | − 0.09 | − 3.54 | < 0.01 |
| Parahippocampal gyrus (right) | |||||
| (Intercept) | 34.03 ± 1.89 | 30.33 | 37.73 | 18.04 | < 0.01 |
| VAS | 0.51 ± 0.62 | − 0.71 | 1.73 | 0.83 | 0.41 |
| WDQ | − 0.01 ± 0.04 | − 0.09 | 0.07 | − 0.19 | 0.85 |
| NDI | − 0.10 ± 0.15 | − 0.40 | 0.19 | − 0.69 | 0.49 |
| HADS | 0.04 ± 0.13 | − 0.22 | 0.31 | 0.32 | 0.75 |
| Perception threshold | − 0.50 ± 0.39 | − 1.27 | 0.28 | − 1.26 | 0.21 |
| Pain threshold | 0.26 ± 0.03 | 0.20 | 0.32 | 8.57 | < 0.01 |
| Thalamus (right) | |||||
| (Intercept) | 48.63 ± 4.84 | 39.15 | 58.11 | 10.05 | < 0.01 |
| VAS | 0.64 ± 0.71 | − 0.75 | 2.04 | 0.91 | 0.36 |
| WDQ | − 0.08 ± 0.09 | − 0.26 | 0.1 | − 0.85 | 0.40 |
| NDI | − 0.11 ± 0.28 | − 0.65 | 0.44 | − 0.38 | 0.70 |
| HADS | 0.38 ± 0.35 | − 0.30 | 1.06 | 1.10 | 0.27 |
| Perception threshold | 0.63 ± 0.60 | − 0.54 | 1.8 | 1.06 | 0.29 |
| Pain threshold | 0.00 ± 0.14 | − 0.28 | 0.28 | − 0.02 | 0.98 |
| Inferior Frontal gyrus (left) | |||||
| (Intercept) | 59.70 ± 2.12 | 55.53 | 63.86 | 28.10 | < 0.01 |
| VAS | − 1.17 ± 0.37 | − 1.90 | − 0.44 | − 3.13 | < 0.01 |
| WDQ | − 0.02 ± 0.06 | − 0.13 | 0.09 | − 0.32 | 0.75 |
| NDI | 0.16 ± 0.11 | − 0.05 | 0.38 | 1.49 | 0.14 |
| HADS | − 0.12 ± 0.17 | − 0.45 | 0.21 | − 0.73 | 0.47 |
| Perception threshold | − 0.33 ± 0.27 | − 0.85 | 0.2 | − 1.22 | 0.22 |
| Pain threshold | 0.09 ± 0.07 | − 0.04 | 0.23 | 1.37 | 0.17 |
| Insula (left) | |||||
| (Intercept) | 60.60 ± 2.88 | 54.96 | 66.25 | 21.05 | < 0.01 |
| VAS | − 0.98 ± 0.97 | − 2.88 | 0.92 | − 1.01 | 0.31 |
| WDQ | − 0.08 ± 0.12 | − 0.32 | 0.17 | − 0.61 | 0.54 |
| NDI | 0.23 ± 0.18 | − 0.13 | 0.58 | 1.25 | 0.21 |
| HADS | − 0.21 ± 0.35 | − 0.89 | 0.48 | − 0.59 | 0.55 |
| Perception threshold | − 0.27 ± 0.52 | − 1.29 | 0.76 | − 0.51 | 0.61 |
| Pain threshold | 0.10 ± 0.09 | − 0.08 | 0.27 | 1.06 | 0.29 |
Parameter estimates obtained using Generalized Estimating Equations. B: unstandardized coefficient, SE: standard error; CI: confidence interval; VAS: last 3 months average pain measured with visual analog scale; WDQ: Whiplash Disability Questionnaire; NDI: Neck Disability Index; HADS: Hospital Anxiety and Depression Scale. Perception and pain thresholds expressed in milliamperes.
4. Discussion
The present study shows that, compared with healthy volunteers, chronic WAD patients have increased perfusion of the right posterior cingulate gyrus and right precuneus, and decreased perfusion of the right superior temporal gyrus, right parahippocampal gyrus, left inferior frontal gyrus, right dorsomedial thalamus, and in the bilateral insular cortex. The rCBF was not affected by the different stimulation conditions in either cWAD patients or healthy volunteers. No significant differences in the rCBF were observed in the PAG between cWAD patients and healthy volunteers.
In relation with the first aim of the study, our results confirm the presence of alterations in the rCBF in cWAD patients, as reported previously in other studies. The increased rCBF in cWAD patients in the right posterior cingulate gyrus and precuneus was reported previously by Linnman et al. (2009), who found increased rCBF in the posterior cingulate and parahippocampal gyrus in cWAD patients. In addition, the decreased rCBF in the right superior temporal gyrus and right parahippocampal gyrus was consistent with the findings of Linnman et al. (2009) and Sundström et al. (2006) who found decreased rCBF in temporal cortical regions. However, the decreased rCBF in the right thalamus was not consistent with the results of study by Linnman et al. (2009), who reported increased rCBF in that region. In addition, we found decreased rCBF bilaterally in the insular cortex. No changes in the perfusion of this region were reported previously in cWAD patients compared with healthy volunteers (Bicik et al., 1998, Linnman et al., 2009, Otte et al., 1995, Otte et al., 1997a, Otte et al., 1997b, Otte et al., 1998a). Sundström et al. (2006) observed a decreased rCBF in cWAD patients in the left insula and right precuneus when compared with another patient group with chronic non-traumatic neck pain. However, these changes were not present when these groups were compared with healthy volunteers.
No significant results were obtained in relation to the second aim of our study. Although we did find changes in the rCBF between cWAD patients and healthy volunteers, the electrical stimulation of the neck did not cause changes in the rCBF. It is likely that the intensity of the stimuli was not high enough, as it did not cause any change in rCBF in the cWAD patients or healthy volunteers. The absence of changes in the rCBF in response to the stimuli may be a consequence of the non-painful stimuli used in the present design. However, a statistically significant lower pain threshold to the electrical stimulation of the neck was found in cWAD patients when compared with healthy volunteers. This lower pain threshold of cWAD patients is of interest when considering the changes in rCBF also found in the precuneus, thalamus, posterior cingulate gyrus, and insular cortex, as these regions have been previously reported to be involved in pain processing (Apkarian et al., 2005, Duerden and Albanese, 2013). In addition, the precuneus and posterior cingulate gyrus are part of the “default mode network” (DMN) (Andrews-Hanna et al., 2014, Buckner et al., 2008), which is commonly active when the subject is not focused on a goal-directed task. Several studies have found an enhanced functional connectivity between the DMN and the insula across pain populations, suggesting a disruption of pain processing (Baliki et al., 2008, Cauda et al., 2009, Napadow et al., 2010). Therefore, our results support the findings of Linnman et al. (2009) by showing rCBF alterations in the posterior cingulate gyrus, medial prefrontal cortex, and lateral temporo-parietal regions, which were considered to represent a disruption of the DMN in cWAD patients during the resting state. In addition, a recent study performed with the radioligand [11C]GR205171 (Linnman et al., 2010, Linnman et al., 2012) found significantly lower NK1 receptor availability in the left insula of cWAD patients, as well as in the frontal and cingulate cortex, hippocampus, amygdala, and PAG. NK1 receptors are widely distributed throughout the brain (Hietala et al., 2005) and are the primary receptor of the pain-modulator, substance P (Maggi, 1995). This additionally suggests that the insula might play an important role in the chronic nature of WAD, as it is assumed to be the primary reception area for interoceptive sensory information and important for the process of emotional feelings (Gasquoine, 2014).
Linnman et al. (2012) reanalyzed the NK1 receptor data in cWAD patients and healthy controls from an already published study (Linnman et al., 2010). This was done to validate if the NK1 receptor availability was altered in the posterior parietal occipital region as previously reported by others (Linnman et al., 2009, Lorberboym et al., 2002, Otte et al., 1995, Otte et al., 1997a, Otte et al., 1997b, Otte et al., 1998a, Sundström et al., 2006). Linnman restricted the analysis to the parietal, occipital, and temporal lobes, with results displayed on a MRI template at p < 0.001, not corrected for multiple comparisons, and found a significantly reduced NK1 receptor availability in the left middle occipital, right middle temporal, left superior temporal, and right superior temporal brain regions. Without this reanalysis by Linnman, the aforementioned tie to the posterior parietal occipital regions would not have been revealed. Furthermore, the studies by Otte et al., 1995, Otte et al., 1997a, Otte et al., 1997b were analyzed by visual interpretation and a region-of-interest approach. Only the study from 1998 was performed with SPM based on the Talairach atlas system (Otte et al., 1998a). The focus of all of these studies was on hypoperfusion. Hyperperfusion was not systematically analyzed, as the main clinical symptoms reported, apart from cervical complaints, were attention and memory deficits and visual disturbances such as oscillopsia. Indeed, in many patients hypoperfusion was also observed in areas that were not located in the posterior parietal occipital region. However, statistical group differences between patients and controls were only observed in the posterior parietal occipital region on both sides. Historically, previous perfusion studies were performed without a voxel-based analysis, and the use of region-of-interest analysis was the standard approach. The beginning of the 21st century paved the way for SPM in functional neuroimaging, starting with a coordinate system based on the atlas from Talairach and Tournoux (1988).
In the analysis restricted to the parietal, occipital, and temporal lobes, in our study small clusters of hypoperfusion were observed in the posterior parietal occipital region (Supplementary data). In many previous studies, patients were selected with special focus on the existence of cerebral symptoms. For example, in the study by Otte et al. (1997b), the 200 patients were suffering from a distortion of the cervical spine accompanied by neck pain, chronic headaches, visual symptoms, and deficits mainly in memory, concentration, and attention. Interestingly, in our present study, marginal areas of hyperperfusion can also be observed in the parietal, occipital, and temporal regions. This suggests that the previously reported processes in this region have been underestimated, since hyperperfused areas have not been examined in the aforementioned studies. It may be assumed that in cWAD patients, the brain is trying to compensate for hypoperfused brain conditions by hyperperfusion in the vicinity of these affected areas (brain plasticity).
In addition, the association observed between the rCBF in the regions that present statistically significant alterations in cWAD and the subjective scores are of special interest. Regions such as the right precuneus, right insula, and right superior temporal lobe were found to correlate, although to different extents, with the scores obtained from the NDI, HADS, WDQ, and the perception and pain thresholds. The existence of these correlations between subjective scores and the rCBF in cWAD patients highlights potential directions for further research in larger data sets. Moreover, the observed correlation between the years since the injury and the perception and pain thresholds in the cWAD group support the concept of an ongoing process of central hyperexcitability, which seems to change over time.
Although we found changes in rCBF in regions involved in pain perception and interoceptive sensory information, which might be involved in the development of cWAD, the link between the neck trauma and these changes in rCBF remains unclear. In other words, it is still not known why low speed rear-end traffic accidents can induce central hyperexcitability in cWAD patients. We have hypothesized (Vállez García et al., 2014) that the missing link for understanding the underlying pathophysiology in cWAD could be the close interaction between the neck and midbrain through the spino-PAG and spinothalamic fibers originating from the C1–C3 spinal segments (Klop et al., 2004, Mouton et al., 2001). In the present study, and in relation with the third aim of this work, no significant alterations in the rCBF in the PAG were detected. However, as mentioned before, the changes detected in several other brain regions might indicate that the symptoms found in cWAD might be the consequence of a mismatch between ascending information from neck structures to midbrain structures, and the later integration of this interoceptive information in pain processing brain regions.
The results of the present study contribute to an increased understanding of the mechanisms involved in cWAD. However, the results are drawn from a relatively small sample size composed only of women. While the sample size of the study is an important factor for the interpretation of the results related to neurological scores, it has relatively limited relevance for the group comparison of rCBF performed in the voxel-based analysis, since 12 repetitions of the H215O PET scan were performed per subject. These repeated measurements reinforce the statistical analysis controlling for within-subject variance. In addition, similar results were obtained in the changes of rCBF in cWAD when the group comparison was performed using only the first acquisition of each condition (Table 1s and Fig. 1s in Appendix B of the Supplementary Material section). Finally, the regional results might be influenced by sub-optimal registration of brain structures in the absence of individual MRI images, the limited spatial resolution of the PET camera (4 to 5 mm FWHM), or the absence of correction for partial volume effects. Finally, while no differences in the rCBF were observed between conditions, the complete absence of a habituation effect to the electrical stimulation of the neck cannot be completely excluded and must be considered when comparing the results with other studies at rest.
The current research data, so far, clearly show that the brain reacts to whiplash injury by changes in the perfusion of certain regions. However, these data do not reveal the underlying mechanism that produces these alterations. Are the mechanisms described by Moskowitz and Buzzi (1991) or the hypothesis proposed by Vállez García et al. (2014) the key to the disease or are both mechanisms, in combination or even triggered by each other, responsible for disease development? Is the brain reshaping its function over time after whiplash injury, a phenomenon observed after spinal cord injury, crossed cerebellar diaschisis, and other brain plasticity processes (Otte, 2001, Otte et al., 1998b)? In this context, the study by Obermann et al. (2009) on neuroplasticity in a longitudinal study following whiplash injury found adaptive gray matter changes of pain processing structures occurring in response to pain, but brain changes resolved in those where symptoms reduced, and only remained in those with persistent symptoms. Hence, possibly these symptoms relate to the persistence of the percept of pain, and given that it is possible that tissue lesions are likely in cWAD, it may be that peripheral mechanisms drive this process.
Therefore, further functional neuroimaging studies in cWAD patients are mandatory for a better understanding of the pathophysiology. As long as the pathophysiology is not understood, an evidence-based approach to any treatment option cannot be developed (Otte et al., 2014).
5. Conclusion
In conclusion, the present study demonstrated elevated rCBF in the right posterior cingulate and right precuneus, and decreased rCBF in the right superior temporal gyrus, right parahippocampal gyrus, left inferior frontal gyrus, right thalamus, and bilateral insular cortex in cWAD patients compared with healthy controls. Moreover, regions such as the insular cortex, precuneus, and posterior cingulate are directly related to pain perception and the integration of interoceptive information. Therefore, we propose that they might be linked to the close interaction between the neck and midbrain structures through the spino-PAG and spinothalamic fibers.
Author Contributions
Conceived and study designed: all. Performed and analyzed the experiments: DVG. Interpreted the data: DVG, JD, AO. Wrote the paper: DVG, AO. Edited the manuscript, contributed to the discussion: all.
Role of the Funding Source
The authors declare no competing financial interests.
Conflict of Interest Statement
The authors declare no conflicts of interests.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2016.07.008.
Contributor Information
David Vállez García, Email: d.vallez-garcia@umcg.nl.
Janine Doorduin, Email: j.doorduin@umcg.nl.
Antoon T.M. Willemsen, Email: a.t.m.willemsen@umcg.nl.
Rudi A.J.O. Dierckx, Email: r.a.dierckx@umcg.nl.
Andreas Otte, Email: andreas.otte@hs-offenburg.de.
Appendix A and B. Supplementary Data
Brief historical information on “whiplash”
Additional voxel-based analyses
References
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian A.V., Bushnell M.C., Treede R.-D., Zubieta J.-K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Baliki M.N., Geha P.Y., Apkarian A.V., Chialvo D.R. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicik I., Radanov B.P., Schafer N., Dvorak J., Blum B., Weber B., Burger C., von Schulthess G.K., Buck A. PET with 18fluorodeoxyglucose and hexamethylpropylene amine oxime SPECT in late whiplash syndrome. Neurology. 1998;51:345–350. doi: 10.1212/wnl.51.2.345. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Carroll L.J., Holm L.W., Hogg-Johnson S., Côté P., Cassidy J.D., Haldeman S., Nordin M., Hurwitz E.L., Carragee E.J., van der Velde G., Peloso P.M., Guzman J. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008;33:S83–S92. doi: 10.1097/BRS.0b013e3181643eb8. [DOI] [PubMed] [Google Scholar]
- Cauda F., Sacco K., Duca S., Cocito D., D'Agata F., Geminiani G.C., Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curatolo M., Bogduk N., Ivancic P.C., McLean S.A., Siegmund G.P., Winkelstein B.A. The role of tissue damage in whiplash-associated disorders. Spine (Phila Pa 1976) 2011;36:S309–S315. doi: 10.1097/BRS.0b013e318238842a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerden E.G., Albanese M.C. Localization of pain-related brain activation: a meta-analysis of neuroimaging data. Hum. Brain Mapp. 2013;34:109–149. doi: 10.1002/hbm.21416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutch Institute for Healthcare Improvement (CBO) Utrecht; The Netherlands: 2008. Richtlijn Diagnostiek en Behandeling van mensen met Whiplash Associated Disorder I/II. [Google Scholar]
- Freeman M.D., Croft A.C., Rossignol A.M., Weaver D.S., Reiser M. A review and methodologic critique of the literature refuting whiplash syndrome. Spine (Phila Pa 1976) 1999;24:86–96. doi: 10.1097/00007632-199901010-00022. [DOI] [PubMed] [Google Scholar]
- Gasquoine P.G. Contributions of the insula to cognition and emotion. Neuropsychol. Rev. 2014;24:77–87. doi: 10.1007/s11065-014-9246-9. [DOI] [PubMed] [Google Scholar]
- Guillaume B., Hua X., Thompson P.M., Waldorp L., Nichols T.E. Fast and accurate modelling of longitudinal and repeated measures neuroimaging data. NeuroImage. 2014;94:287–302. doi: 10.1016/j.neuroimage.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J.W., Hilbe J.M. second ed. Chapman & Hall/CRC; 2012. Generalized Estimating Equations. [Google Scholar]
- Hietala J., Nyman M.J., Eskola O., Laakso A., Grönroos T., Oikonen V., Bergman J., Haaparanta M., Forsback S., Marjamäki P., Lehikoinen P., Goldberg M., Burns D., Hamill T., Eng W.-S., Coimbra A., Hargreaves R., Solin O. Visualization and quantification of neurokinin-1 (NK1) receptors in the human brain. Mol. Imaging Biol. 2005;7:262–272. doi: 10.1007/s11307-005-7001-6. [DOI] [PubMed] [Google Scholar]
- Holm L.W., Carroll L.J., Cassidy J.D., Hogg-Johnson S., Côté P., Guzman J., Peloso P., Nordin M., Hurwitz E., van der Velde G., Carragee E., Haldeman S. The burden and determinants of neck pain in whiplash-associated disorders after traffic collisions: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine (Phila Pa 1976) 2008;33:S52–S59. doi: 10.1097/BRS.0b013e3181643ece. [DOI] [PubMed] [Google Scholar]
- Jorritsma W., de Vries G.E., Dijkstra P.U., Geertzen J.H.B., Reneman M.F. Neck pain and disability scale and neck disability index: validity of Dutch language versions. Eur. Spine J. 2012;21:93–100. doi: 10.1007/s00586-011-1920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klop E.M., Mouton L.J., Holstege G. Less than 15% of the spinothalamic fibers originate from neurons in lamina I in cat. Neurosci. Lett. 2004;360:125–128. doi: 10.1016/j.neulet.2004.02.047. [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., Liotti M., Freitas C.S., Rainey L., Kochunov P.V., Nickerson D., Mikiten S.A., Fox P.T. Automated Talairach Atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen N.A. Normal average value of cerebral blood flow in younger adults is 50 mL/100 g/min. J. Cereb. Blood Flow Metab. 1985;5:347–349. doi: 10.1038/jcbfm.1985.48. [DOI] [PubMed] [Google Scholar]
- Linnman C., Appel L., Söderlund A., Frans O., Engler H., Furmark T., Gordh T., Långström B., Fredrikson M. Chronic whiplash symptoms are related to altered regional cerebral blood flow in the resting state. Eur. J. Pain. 2009;13:65–70. doi: 10.1016/j.ejpain.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Linnman C., Appel L., Furmark T., Söderlund A., Gordh T., Långström B., Fredrikson M. Ventromedial prefrontal neurokinin 1 receptor availability is reduced in chronic pain. Pain. 2010;149:64–70. doi: 10.1016/j.pain.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Linnman C., Moulton E.A., Barmettler G., Becerra L., Borsook D. Neuroimaging of the periaqueductal gray: state of the field. NeuroImage. 2012;60:505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorberboym M., Gilad R., Gorin V., Sadeh M., Lampl Y. Late whiplash syndrome: correlation of brain SPECT with neuropsychological tests and P300 event-related potential. J. Trauma. 2002;52:521–526. doi: 10.1097/00005373-200203000-00017. [DOI] [PubMed] [Google Scholar]
- Maggi C.A. The mammalian tachykinin receptors. Gen. Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. NeuroImage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Moskowitz M.A., Buzzi M.G. Neuroeffector functions of sensory fibres: implications for headache mechanisms and drug actions. J. Neurol. 1991;238:S18–S22. doi: 10.1007/BF01642901. [DOI] [PubMed] [Google Scholar]
- Mouton L.J., Klop E.-M., Holstege G. Lamina I-periaqueductal gray (PAG) projections represent only a limited part of the total spinal and caudal medullary input to the PAG in the cat. Brain Res. Bull. 2001;54:167–174. doi: 10.1016/s0361-9230(00)00442-1. [DOI] [PubMed] [Google Scholar]
- Mouton L.J., Eggens-Meijer E., Klop E.M. The ventrolateral upper cervical cell group in cat projects to all rostrocaudal levels of the periaqueductal gray matter. Brain Res. 2009;1300:79–96. doi: 10.1016/j.brainres.2009.08.088. [DOI] [PubMed] [Google Scholar]
- Napadow V., LaCount L., Park K., As-Sanie S., Clauw D.J., Harris R.E. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann M., Nebel K., Schumann C., Holle D., Gizewski E.R., Maschke M., Goadsby P.J., Diener H.-C., Katsarava Z. Gray matter changes related to chronic posttraumatic headache. Neurology. 2009;73:978–983. doi: 10.1212/WNL.0b013e3181b8791a. [DOI] [PubMed] [Google Scholar]
- Otte A. The plasticity of the brain. Eur. J. Nucl. Med. 2001;28:263–265. doi: 10.1007/s002590000381. [DOI] [PubMed] [Google Scholar]
- Otte A., Mueller-Brand J., Fierz L. Brain SPECT findings in late whiplash syndrome. Lancet. 1995;345:1513. doi: 10.1016/s0140-6736(95)91075-1. [DOI] [PubMed] [Google Scholar]
- Otte A., Ettlin T.M., Nitzsche E.U., Wachter K., Hoegerle S., Simon G.H., Fierz L., Moser E., Mueller-Brand J. PET and SPECT in whiplash syndrome: a new approach to a forgotten brain? J. Neurol. Neurosurg. Psychiatry. 1997;63:368–372. doi: 10.1136/jnnp.63.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte A., Mueller-Brand J., Nitzsche E.U., Wachter K., Ettlin T.M. Functional brain imaging in 200 patients after whiplash injury. J. Nucl. Med. 1997;38:1002. [PubMed] [Google Scholar]
- Otte A., Goetze M., Mueller-Brand J. Statistical parametric mapping in whiplash brain: is it only a contusion mechanism? Eur. J. Nucl. Med. 1998;25:306–307. [PubMed] [Google Scholar]
- Otte A., Roelcke U., von Ammon K., Hausmann O., Maguire R.P., Missimer J., Müller-Brand J., Radü E.W., Leenders K.L. Crossed cerebellar diaschisis and brain tumor biochemistry studied with positron emission tomography, [18F]fluorodeoxyglucose and [11C]methionine. J. Neurol. Sci. 1998;156:73–77. doi: 10.1016/s0022-510x(98)00019-7. [DOI] [PubMed] [Google Scholar]
- Otte A., Vállez García D., Dierckx R.A.J.O., Holstege G. Chronic whiplash-associated disorders. Lancet. 2014;384:1346. doi: 10.1016/S0140-6736(14)61814-6. [DOI] [PubMed] [Google Scholar]
- Pinfold M., Niere K.R., O'Leary E.F., Hoving J.L., Green S., Buchbinder R. Validity and internal consistency of a whiplash-specific disability measure. Spine (Phila Pa 1976) 2004;29:263–268. doi: 10.1097/01.brs.0000107238.15526.4c. [DOI] [PubMed] [Google Scholar]
- Quinlan K., Annest J., Myers B., Ryan G., Hill H. Neck strains and sprains among motor vehicle occupants-United States, 2000. Accid. Anal. Prev. 2004;36:21–27. doi: 10.1016/s0001-4575(02)00110-0. [DOI] [PubMed] [Google Scholar]
- Reinders A. Interscan displacement-induced variance in PET activation data is excluded by a scan-specific attenuation correction. NeuroImage. 2002;17:1844–1853. doi: 10.1006/nimg.2002.1318. [DOI] [PubMed] [Google Scholar]
- Report E.W. 2005. Reining in whiplash executive director. [Google Scholar]
- Spinhoven P., Ormel J., Sloekers P.P., Kempen G.I., Speckens A.E., Van Hemert A.M. A validation study of the hospital anxiety and depression scale (HADS) in different groups of Dutch subjects. Psychol. Med. 1997;27:363–370. doi: 10.1017/s0033291796004382. [DOI] [PubMed] [Google Scholar]
- Spitzer W.O., Skovron M.L., Salmi L.R., Cassidy J.D., Duranceau J., Suissa S., Zeiss E. Scientific monograph of the Quebec Task Force on whiplash-associated disorders: redefining “whiplash” and its management. Spine (Phila Pa 1976) 1995;20:1S–73S. [PubMed] [Google Scholar]
- Sterling M., Jull G., Kenardy J. Physical and psychological factors maintain long-term predictive capacity post-whiplash injury. Pain. 2006;122:102–108. doi: 10.1016/j.pain.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Sterner Y., Gerdle B. Acute and chronic whiplash disorders—a review. J. Rehabil. Med. 2004;36:193–209. doi: 10.1080/16501970410030742. (quiz 210) [DOI] [PubMed] [Google Scholar]
- Stone A.M., Vicenzino B., Lim E.C.W., Sterling M. Measures of central hyperexcitability in chronic whiplash associated disorder - a systematic review and meta-analysis. Man. Ther. 2013;18:111–117. doi: 10.1016/j.math.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Sundström T., Guez M., Hildingsson C., Toolanen G., Nyberg L., Riklund K. Altered cerebral blood flow in chronic neck pain patients but not in whiplash patients: a 99mTc-HMPAO rCBF study. Eur. Spine J. 2006;15:1189–1195. doi: 10.1007/s00586-005-0040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme; New York: 1988. Co-planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Vállez García D., Dierckx R.A.J.O., Otte A., Holstege G. Whiplash, real or not Real? A review and new concept. In: Dierckx R.A.J.O., Otte A., de Vries E.F.J., van Waarde A., Leenders K.L., editors. PET and SPECT in Neurology. Springer; Berlin, Heidelberg: 2014. pp. 947–963. [Google Scholar]
- Vernon H., Mior S. The neck disability index: a study of reliability and validity. J. Manip. Physiol. Ther. 1991;14:409–415. [PubMed] [Google Scholar]
- Walton D.M., Pretty J., MacDermid J.C., Teasell R.W. Risk factors for persistent problems following whiplash injury: results of a systematic review and meta-analysis. J. Orthop. Sports Phys. Ther. 2009;39:334–350. doi: 10.2519/jospt.2009.2765. [DOI] [PubMed] [Google Scholar]
- Watson D.H., Drummond P.D. The role of the trigemino cervical complex in chronic whiplash associated headache: a cross sectional study. Headache. 2016;2 doi: 10.1111/head.12805. [DOI] [PubMed] [Google Scholar]
- Williams M., Williamson E., Gates S., Lamb S., Cooke M. A systematic literature review of physical prognostic factors for the development of Late Whiplash Syndrome. Spine (Phila Pa 1976) 2007;32:E764–E780. doi: 10.1097/BRS.0b013e31815b6565. [DOI] [PubMed] [Google Scholar]
- Zigmond A.S., Snaith R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Brief historical information on “whiplash”
Additional voxel-based analyses