Abstract
Leber's hereditary optic neuropathy (LHON) is a disease that leads to blindness. Gene therapy has been investigated with some success, and could lead to important advancements in treating LHON. This was a prospective, open-label trial involving 9 LHON patients at Tongji Hospital, Wuhan, China, from August 2011 to December 2015. The purpose of this study was to evaluate the long-term outcomes of gene therapy for LHON. Nine LHON patients voluntarily received an intravitreal injection of rAAV2-ND4. Systemic examinations and visual function tests were performed during the 36-month follow-up period to determine the safety and efficacy of this gene therapy. Based on successful experiments in an animal model of LHON, 1 subject also received an rAAV2-ND4 injection in the second eye 12 months after gene therapy was administered in the first eye. Recovery of visual acuity was defined as the primary outcome of this study. Changes in the visual field, visual evoked potential (VEP), optical coherence tomography findings, liver and kidney function, and antibodies against AAV2 were defined as secondary endpoints. Eight patients (Patients 2–9) received unilateral gene therapy and visual function improvement was observed in both treated eyes (Patients 4, 6, 7, and 8) and untreated eyes (Patients 2, 3, 4, 6 and 8). Visual regression fluctuations, defined as changes in visual acuity greater than or equal to 0.3 logMAR, were observed in Patients 2 and 9. Age at disease onset, disease duration, and the amount of remaining optic nerve fibers did not have a significant effect on the visual function improvement. The visual field and pattern reversal VEP also improved. The patient (Patient 1) who received gene therapy in both eyes had improved visual acuity in the injected eye after the first treatment. Unfortunately, visual acuity in this eye decreased 3 months after he received gene therapy in the second eye. Animal experiments suggested that ND4 expression remains stable in the contralateral eye after intravitreal injections. No serious safety problem was observed in the 3-year follow-up of the 9 participants enrolled in this virus-based gene therapy. Meanwhile, our results support the use of intravitreal rAAV2-ND4 as an aggressive maneuver in our clinical trial. Further study in additional patients and in these 9 subjects is needed to better understand the effects of rAAV2-ND4 gene therapy on LHON and to increase the applications of this technique.
Abbreviations: AAV, adeno-associated virus; BCVA, best corrected visual acuity; CF, counting fingers; ERG, electroretinogram; HM, hand movement; IOP, intraocular pressure; LHON, Leber's hereditary optic neuropathy; MD, mean defect; MtDNA, mitochondrial DNA; ND4, NADH–ubiquinone oxidoreductase, subunit 4; OCT, optical coherence tomography; rAAV2-ND4, recombinant adeno-associated virus carrying the ND4 gene; RNFL, retinal nerve fiber layer; VEP, visual evoked potential; VFI, visual field index
Keywords: Leber's hereditary optic neuropathy, Gene therapy, Best-corrected visual acuity
Highlights
-
•
A long-term study of efficacy of gene therapy for Leber's hereditary optic neuropathy.
-
•
No serious adverse effects were noted in the 9 participants over a 3-year period.
-
•
Five patients experienced an improvement in visual function.
-
•
Gene therapy is a promising treatment for Leber's hereditary optic neuropathy.
There are currently no effective treatments for Leber's hereditary optic neuropathy (LHON). Short-term studies using virus-based gene therapy have yielded promising results. We performed systemic examinations and visual function tests to evaluate the long-term safety and efficacy of gene therapy for LHON. Over a 3-year follow-up period, five out of nine patients had visual function improvement, and no serious adverse effects were noted.
Leber's hereditary optic neuropathy (LHON) is one of the most common causes of blindness in young adults. Unfortunately, there is currently no effective treatment. The most common point mutation that leads to the development of LHON is the mitochondrial DNA 11778 G-to-A point mutation (Mackey et al., 1996). In China, the G11778A point mutation is present in 90% of LHON patients (Cui et al., 2013). Therefore, we selected this mutation as the target for gene therapy. After a series of successful animal experiments (Shi et al., 2012, Pei et al., 2013, Gao et al., 2013), a total of 9 patients were administered an intravitreal injection of rAAV2-ND4 (recombinant adeno-associated virus carrying the NADH–ubiquinone oxidoreductase subunit 4 gene) in 2011 and 2012. Early therapy outcomes for these patients have been previously reported (Wan et al., 2016), but the patients were only monitored for 9 months in that study.
After examining the effects of unilateral intravitreal rAAV2-ND4 injection on the injected eye, we noticed some effects of the gene therapy in the uninjected eye. Following the completion of our animal experiments, Patient 1 from the unilateral injection study chose to have gene therapy also administered in the fellow eye. Additionally, several patients who had received gene therapy in only 1 eye began to show visual acuity improvements in the uninjected eye. This led us to wonder if the gene therapy administered to 1 eye had affected the other uninjected eye or if visual acuity improvements had resulted from spontaneous recovery.
Here, we report the long-term (36 months) clinical outcomes of the 9 patients who received gene therapy for LHON. Patient 1, who received gene therapy in both eyes, is examined and described separately. The clinical results of the other 8 patients who received unilateral therapy are reported together.
1. Methods
1.1. Recombinant adeno-associated virus
Construction of a vector containing the target gene is the key in gene therapy. However, the ND4 gene is found in mitochondrial DNA, and exogenous gene transfection is not suitable for the mutation of mitochondrial DNA. Instead, the mitochondrial DNA sequence of ND4 was modified to a nuclear DNA sequence, which makes the translation of ND4 protein consistent with the normal translation of the protein in the mitochondria. We constructed recombinant adenovirus 2-ND4 by inserting a mitochondrial targeting sequence (MTS). The original vector was produced and purified at Beijing FivePlus Molecular Medicine Institute Co. Ltd. (Beijing, China). The recombinant plasmid was examined by restriction enzyme digestion and named pAAV2neo-COX10-ND4-COX103′ UTR.
1.2. Patients and the molecular analysis
Nine patients were enrolled in this study. They were all diagnosed by a polymerase chain reaction (PCR)-based test using an amplification-refractory mutation system to detect the presence or absence of 3 nucleotide substitutions known to cause LHON (3460 G > A, 11,778 G > A, and 14,484 T > C). The molecular genetic analyses were performed at Tongji Hospital Genetic Diagnosis Center (Wuhan, China).
1.3. Study design and oversight
This was an open-label study on LHON patients with a confirmed G11778 A mutation.
Patients who did not experience spontaneous visual recovery after 12 months of clinical observation were considered for enrollment. The study protocol was reviewed and approved by the ethics committee of Ezhou Central Hospital. All patients and their guardians provided written informed consent to participate in the study. This study is registered at ClinicalTrials.gov (registration number: NCT01267422; registration date: December 2010). All study conduct adhered to the tenets of the Declaration of Helsinki.
1.4. Intravitreal injection and dose
In this study, we selected a relatively lower dose than that used for congenital amaurosis gene therapy. The dose was 5 × 109 vg/0.05 mL for patients younger than 12 years, and 1 × 1010 vg/0.05 mL for patients older than 12 years. Children younger than 12 years received half the dose for safety reasons. An intravitreal injection of rAAV2-ND4 was administered to one eye. In order to improve study safety, the eye with poorer visual acuity was chosen as the injection eye. A similar methodology was adopted in previous studies (Maguire et al., 2008, Bainbridge et al., 2008). If both eyes had the same visual acuity, the right eye was designated as the injection eye. Because of safety considerations, patients underwent the gene therapy in two batches (3 patients were injected in 2011, and 6 patients were injected in 2012). Details of the experimental design and methodology have been previously published (Wan et al., 2016) and are briefly described below.
1.5. Ophthalmologic examinations
The primary endpoint of this study was the change in best-corrected visual acuity (BCVA) after gene therapy administration. Visual acuity measured just prior to treatment was used as the baseline value. The BCVA was measured using a 2.5-m standard logMAR chart (Star Kang Medical Technology Co., Ltd., Wen Zhou, China). A change in logMAR BCVA greater than or equal to 0.3 was considered clinically significant (Yu-Wai-Man et al., 2011, Klopstock et al., 2011). A cutoff of 0.3 was chosen because it is equivalent to 15 letters on the Early Treatment for Diabetic Retinopathy Study (ETDRS) chart (Beck et al., 2003).
Visual field testing (Humphrey field analyzer, Carl Zeiss 740i, Carl Zeiss, Shanghai, China) was performed using the 30–2 central threshold test and SITA fast algorithm. The primary endpoints were the visual field index (VFI) and mean deviation (MD).
The Spectralis HRA + OCT system (Heidelberg Engineering, Heidelberg, Germany) was used to measure average overall and quadrant (superior, inferior, temporal, and nasal) retinal nerve fiber layer (RNFL) thicknesses.
The pattern-reversal visual evoked potential stimulus (PR-VEP; DV-100, Shanghai Dikon Medical, Shanghai, China) was an alternating black-and-white checkerboard pattern with pattern reversal frequencies of 1.0 and 100.0 Hz. The stimulation duration was 500 ms and 100 PR-VEPs were averaged to obtain the waveforms used in the analyses. The average screen brightness was 5 cd/m2; the pattern had a spatial frequency of 25 ms/s and a contrast ratio of 97%. The primary endpoint was the P100 waveform amplitude and latency.
Other ophthalmologic examinations included anterior segment slit-lamp examination, intraocular pressure measurement (TOPCON-CT-80 Computerized Auto Tonometer, TOPCON, Tokyo, Japan), and fundus examination (NIDEK Autofocus Fundus Camera, AFC-230, Nidek, Tokyo, Japan). The specifics of each type of examination have been reported previously (Wan et al., 2016). All examinations were performed by the same experienced ophthalmologist or optometrist.
1.6. Effect of gene therapy on uninjected eyes
1.6.1. Animal experiments
A total of 60 randomly selected, 4-week-old C57bl mice were used in this study. All mice were bred and provided by the Animal Center of Tongji Hospital (Wuhan, China). The experimental procedures and approaches were similar to those of previously published reports (Gao et al., 2013).
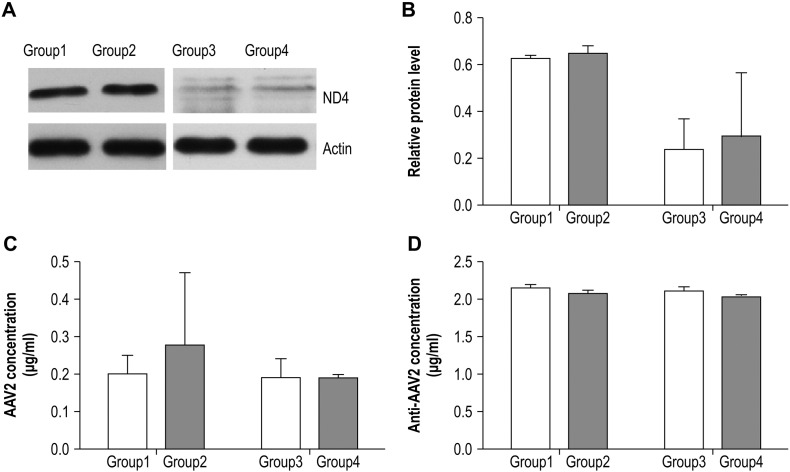
The mice were divided into 4 groups. Groups 1 and 2 received a unilateral intravitreal phosphate-buffered saline (PBS) injection and rAAV2-ND4 injection, respectively, followed by a contralateral rAAV2-ND4 intravitreal injection in both groups 1 month later. Groups 3 and 4 received a unilateral intravitreal PBS injection and rAAV2-ND4 injection, respectively, followed by a contralateral intravitreal rAAV2-ND4 injection in both groups 3 months later. All intravitreal injections of rAAV2-ND4 were 5 μL (1 × 1011 μg/mL) in volume.
The mice were sacrificed 1 month after the second intravitreal injection and the retinas of all mice were isolated. Western blotting was used to evaluate the levels of ND4 expression. Blood samples collected from the heart were used in an enzyme-linked immunosorbent assay (ELISA) to determine AAV2 and AAV2 antibody concentrations. All experimental methods have already been described in our previous reports (Gao et al., 2013, Wan et al., 2016). ND4 expression and concentrations of AAV2 and AAV2 antibody were compared between Groups 1 and 2 and between Groups 3 and 4 using paired t-tests.
1.6.2. Bilateral Gene therapy (patient 1)
Because Patient 1 experienced a large improvement in visual acuity after the first treatment, gene therapy treatment of the contralateral eye was strongly requested. As the safety of the therapy had been verified in the animal experiments, after a 12-month interval, we performed gene therapy on the fellow eye, which was approved by the hospital's Ethics Committee. Patient 1 thus received gene therapy in both eyes, and the 2 eyes were injected 12 months apart using the same methods.
One, 3, and 6 months after the second injection, the patient underwent thorough systemic and ophthalmologic examinations, including physical examinations with routine blood, urine, liver, kidney, and immune function tests. All laboratory analyses were performed by the Laboratory Department of Tongji Hospital. Tests for human T lymphocyte subsets CD3 +, CD3 +/CD4 +, and CD3 +/CD8 + were conducted by the Central Laboratory of Tongji Hospital.
The third part of the physical examination was a neutralizing antibody assay performed using flow cytometry. Serum concentrations of ND4, AAV, and interferon (IFN)-γ were measured via ELISA. For the detailed methodology, please refer to our previous publication (Wan et al., 2016).
The patient was closely monitored for 36 months following treatment of the fellow eye.
1.7. Statistical analyses
Because of the small number of samples in our study, we adopted previously used protocols (Maguire et al., 2008, Bainbridge et al., 2008) regarding BCVA analysis for small sample sizes. The outcomes of visual acuity for efficacy were descriptive in nature and defined as any improvement in visual function rather than using statistical methods. Using a non-parametric test (Mann–Whitney test), we compared the BCVA changes between patients with a disease duration ≥ 2 years (n = 4) and < 2 years (n = 4). We also compared the mean age at onset, duration, and RNFL between the patients who experienced an improvement after the therapy (BCVA change ≥ 0.3 logMAR [n = 4]) and those who did not (BCVA change < 0.3 logMAR [n = 4]). In the animal studies, data were statistically analyzed with the independent two-sample t-test. All values are presented as the mean ± standard deviation. Statistical significance was defined as p < 0.05.
2. Results
2.1. Safety evaluation and adverse events
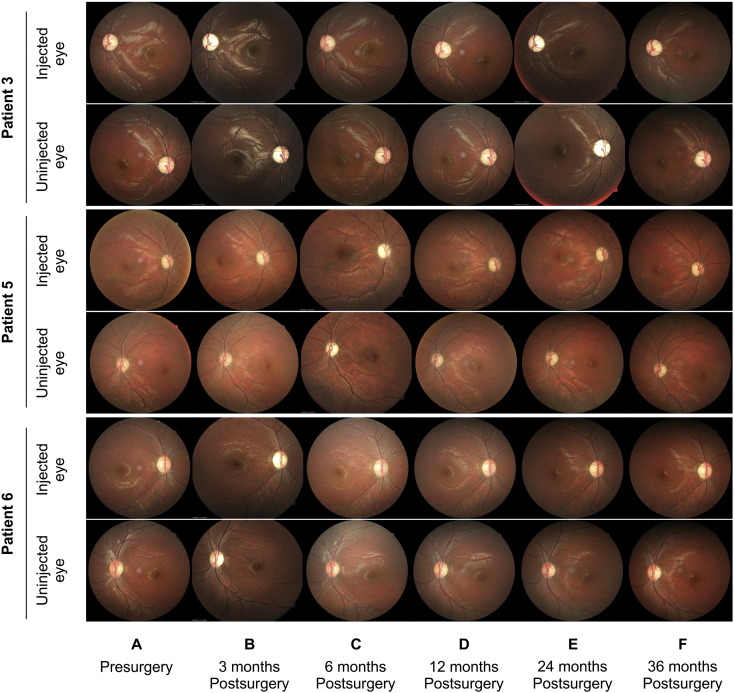
Ocular adverse events, including cataracts, retinal detachment, and endophthalmitis were not observed in any patient during the 36-month follow-up period. No retinal (Fig. 1) or other ocular tissue damage was detected. Additionally, systemic examinations did not reveal any abnormal change during the 36-month observation period. Systemic immunity examination results have been reported previously (Wan et al., 2016). None of the patients had any obvious immune abnormalities or events.
Fig. 1.
Fundus photography obtained before and after the intravitreal injection of rAAV2-ND4 (n = 8 patients). A representative 30° fundus photograph showing the disc and macula of injected and uninjected eyes before (A) and at 3 months (B), 6 months (C), 12 months (D), 24 months (E), and 36 months (F) after intravitreal injection. The retinal structure appears normal in all photographs with no apparent abnormalities.
2.2. Best-corrected visual acuity
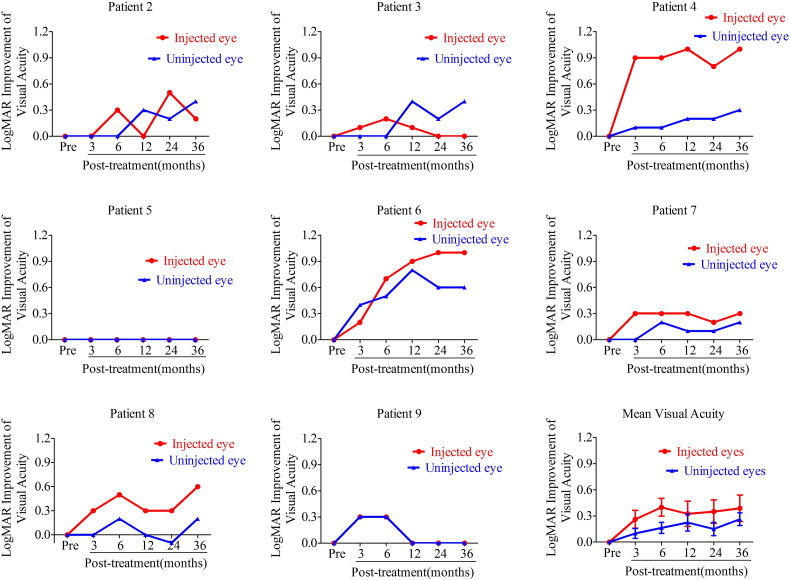
Compared with baseline values, the BCVA improved in both the injected and uninjected eyes in Patients 4, 6, 7, and 8 over the 36-month follow-up period. The logMAR BCVA of only the uninjected eye improved by 0.2 in Patients 7 and 8. The BCVAs of the injected eyes in Patients 2 and 3 were improved at 3 and 6 months after treatment compared to baseline. However, visual acuity began to decrease after 6 months. In contrast, BCVA in the uninjected eye increased for 12 months after treatment and improvements were maintained for 36 months. In Patient 9, bilateral BCVA improved initially, but decreased back to baseline after 12 months. The visual acuity of Patient 5 did not change significantly during the 36-month follow-up period (Table 1, Table 2, Fig. 2).
Table 1.
Clinical data of 9 LHON patients.
| Patients | Sex | Age at onset of LHON | Age at gene therapy | LogMAR BCVA before gene therapy |
LogMAR BCVA 36 months after gene therapy |
||
|---|---|---|---|---|---|---|---|
| IE | UIE | IE | UIE | ||||
| *1 | Male | 14 | 18 | 2.0 (R) | 2.0 (L) | 1.7 (R) | 1.7 (L) |
| 2 | Male | 8 | 10 | 1.7 (R) | 0.9 (L) | 1.5 (R) | 0.5 (L) |
| 3 | Male | 7 | 9 | 1.2 (L) | 1.0 (R) | 1.2 (L) | 0.6 (R) |
| 4 | Male | 13 | 21 | 2.0 (L) | 1.1 (R) | 1.0 (L) | 0.8 (R) |
| 5 | Male | 16 | 17 | 2.3 (R) | 2.3 (L) | 2.3 (R) | 2.3 (L) |
| 6 | Female | 8 | 9 | 1.1 (R) | 1.0 (L) | 0.1 (R) | 0.4 (L) |
| 7 | Female | 9 | 26 | 1.2 (L) | 0.9 (R) | 0.9 (L) | 0.7 (R) |
| 8 | Male | 13 | 17 | 1.4 (L) | 1.7 (R) | 1.1 (L) | 1.2 (R) |
| 9 | Male | 43 | 46 | 2.0 (R) | 2.0 (L) | 2.0 (R) | 2.0 (L) |
| *Average | 14.56 | 19.22 | 1.61 | 1.36 | 1.26 | 1.06 | |
Note (*): patient 1 was the only one who received bilateral gene therapy. *Mean has excluded the patient 1. LogMAR, logarithm of the minimum angle of resolution; BCVA, best-corrected visual acuity; IE, injected eye; UIE, uninjected eye; R, Right eye; L, Left eye.
Table 2.
LogMAR vision acuity of injected and uninjected eyes of Case 2 to Case 9.
| Patient 2 |
Patient 3 |
Patient 4 |
Patient 5 |
Patient 6 |
Patient 7 |
Patient 8 |
Patient 9 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | U | I | U | I | U | I | U | I | U | I | U | I | U | I | U | |
| Presurgery | 1.7 | 0.9 | 1.2 | 1 | 2 | 1.1 | 2.3 | 2.3 | 1.1 | 1 | 1.2 | 0.9 | 1.7 | 1.4 | 2 | 2 |
| 3 months postsurgery | 1.7 | 0.9 | 1.1 | 1 | 1.1 | 1 | 2.3 | 2.3 | 0.9 | 0.6 | 0.9 | 0.9 | 1.4 | 1.4 | 1.7 | 1.7 |
| 6 months postsurgery | 1.4 | 0.9 | 1 | 1 | 1.1 | 1 | 2.3 | 2.3 | 0.5 | 0.5 | 0.9 | 0.7 | 1.2 | 1.2 | 1.7 | 1.7 |
| 12 months postsurgery | 1.7 | 0.6 | 1.1 | 0.6 | 1 | 0.9 | 2.3 | 2.3 | 0.2 | 0.2 | 0.9 | 0.8 | 1.4 | 1.4 | 2 | 2 |
| 24 months postsurgery | 1.2 | 0.7 | 1.2 | 0.8 | 1.2 | 0.9 | 2.3 | 2.3 | 0.1 | 0.4 | 1 | 0.8 | 1.4 | 1.5 | 2 | 2 |
| 36 months postsurger | 1.5 | 0.5 | 1.2 | 0.6 | 1 | 0.8 | 2.3 | 2.3 | 0.1 | 0.4 | 0.9 | 0.7 | 1.1 | 1.2 | 2 | 2 |
I, injected; U, uninjected.
Fig. 2.
Improvement in logMAR visual acuity from baseline after the intravitreal injection of rAAV2-ND4 (n = 8 patients). Visual acuity improvements from baseline in injected and uninjected eyes at 3, 6, 12, 24, and 36 months after the intravitreal injection of rAAV2-ND4. Visual acuity improved in the injected eyes of Patients 2, 4, 6, 7, 8, and 9 and in the uninjected eyes of Patients 2, 3, 4, 6, and 9. However, visual acuity decreased in some patients until 36 months after gene therapy, but the mean visual acuity of the injected and uninjected eyes improved in all 8 patients. Mean logMAR BCVA in the injected eyes of all 8 patients who received unilateral gene therapy improved by 0.26 ± 0.29, 0.40 ± 0.29, 0.33 ± 0.41, 0.35 ± 0.39, and 0.39 ± 0.43 at 3, 6, 12, 24, and 36 months, respectively. Overall, patients had the best BCVA 6 months after treatment. Mean logMAR BCVA of the uninjected eyes in these same 8 patients improved by 0.10 ± 0.16, 0.16 ± 0.18, 0.23 ± 0.28, 0.15 ± 0.21, and 0.26 ± 0.21 at 3, 6, 12, 24, and 36 months, respectively.
At 36 months post-injection, in patients with a disease duration < 2 years, an improvement of 0.3 logMAR was observed in the treated eye and 0.35 logMAR in the untreated eye. In patients with a disease duration ≥ 2 years, an improvement of 0.4 logMAR was observed in the treated eye and 0.25 logMAR in the untreated eye. There was no significant difference between these two groups (Table 3). There was no significant difference in the mean age at onset, duration, and RNFL between the patients who experienced an improvement after the therapy (BCVA change ≥ 0.3 logMAR [n = 4]) and those who did not (BCVA change < 0.3 logMAR [n = 4]) (Table 4).
Table 3.
Comparison of BCVA changes according to disease duration.
| BCVA (logMAR) change from baseline to 36 months | IE mean change (logMAR) | UIE mean change (logMAR) |
|---|---|---|
| Patients with ≤ 2 y duration (n = 4) | − 0.30 logMAR | − 0.35 logMAR |
| Patients with > 2 y duration (n = 4*) | − 0.40 logMAR | − 0.25 logMAR |
| P | 0.65 | 0.47 |
Note (*): Excludes patient 1 who received bilateral gene therapy.
LogMAR, logarithm of the minimum angle of resolution; BCVA, best-corrected visual acuity; IE, injected eye; UIE, uninjected eye.
Table 4.
Comparison of mean age at onset, disease duration, and RNFL according to therapeutic outcome.
| BCVA (logMAR) change from baseline to 36 months (Injected eye)* | BCVA change ≥ 0.3 logMAR (n = 4) | BCVA change < 0.3 logMAR (n = 4) | p |
|---|---|---|---|
| Mean age at onset (y) | 10.75 ± 1.32 | 18.50 ± 8.41 | 0.88 |
| Mean duration (y) | 7.50 ± 3.48 | 2.00 ± 0.41 | 0.19 |
| Mean RNFL (pre) (μm) | 50.38 ± 4.90 | 44.25 ± 2.42 | 0.39 |
| Mean RNFL (post) (μm) | 46.13 ± 2.79 | 46.25 ± 6.01 | 1.00 |
Note (*): Excludes patient 1 who received bilateral gene therapy.
LogMAR, logarithm of the minimum angle of resolution; BCVA, best-corrected visual acuity; RNFL, thickness of the retinal nerve fiber layer; pre, pre-treatment; post, post-treatment.
2.3. Visual field
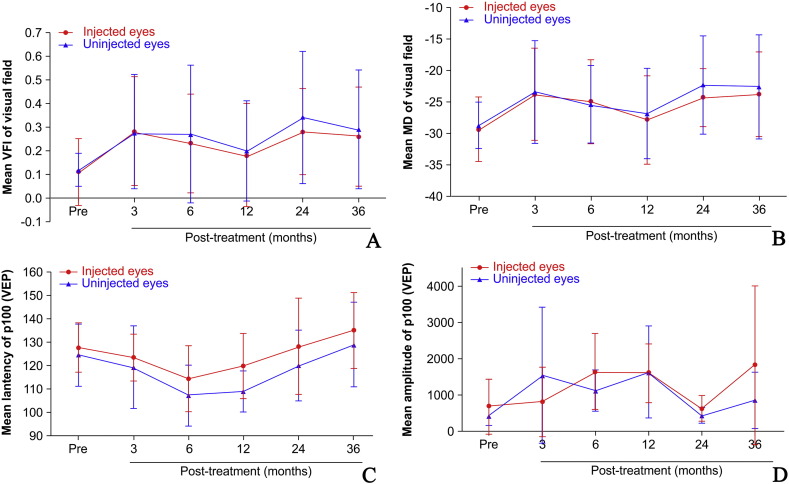
The visual field (VF) improved simultaneously with visual acuity. The VFIs in the injected and uninjected eyes were significantly better 36 months after treatment than at baseline in 5 patients (Patients 2, 3, 4, 6, and 7; Table 5, Table 6, Fig. 3A, B). Patient 2 refused VF testing because of poor central visual acuity, but agreed to it 12 months after receiving gene therapy when visual acuity had improved. The results showed that in the injected and uninjected eyes, the VFI gradually increased, MD decreased, and VF improved. The injected and uninjected eyes of Patients 3, 4, 8, and 9 had maximal VF improvements between 3 and 6 months after treatment, which decreased thereafter. VF parameters improved progressively in Patients 6 and 7.
Table 5.
Ophthalmologic examination results of injected eyes of 8 patients before and 36 months after intravitreal injection.
| P100 amplitudes(uV) |
RNFL thickness (average) |
MD of visual field (dB) |
VFI of visual field (dB) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Eye | Before | After | Before | After | Before | After | Before | After |
| 2 | Right | 86.1 | 189 | 48.5 | 45.75 | – | − 21.83 | – | 28% |
| 3 | Left | 303 | 265 | 48 | 62 | − 19.47 | − 15.53 | 42% | 54% |
| 4 | Left | 1090 | 1270 | 43.25 | 42.25 | − 30.02 | − 25.18 | 8% | 21% |
| 5 | Right | 2320 | 227 | 38.5 | 32.75 | − 33.01 | − 33.82 | 2% | 1% |
| 6 | Right | 152 | 6620 | 61.25 | 48.25 | − 34.92 | − 14.66 | 0% | 60% |
| 7 | Left | 146 | 2980 | 56 | 53 | − 32.41 | − 21.30 | 3% | 32% |
| 8 | Right | 763 | 2050 | 41 | 41 | − 26.45 | − 29.49 | 16% | 8% |
| 9 | Left | 618 | 916 | 42 | 44.5 | − 29.36 | − 28.44 | 9% | 8% |
| Average | 684.76 | 1814.6 | 46.19 | 45.69 | − 29.38 | − 23.78 | 11% | 27% | |
P100 amplitudes: amplitudes P100 values increase means the visual function improvement; RNFL: retinal nerve fiber layer; MD: mean deviation, value close to 0 considered as normal; VFI: visual field index, value close to 100% considered as normal.
Table 6.
Ophthalmologic examination results of uninjected eyes of 8 patients before and 36 months after intravitreal injection.
| P100 amplitudes(uV) |
Mean RNFL thickness (um) |
MD of visual field (dB) |
VFI of visual field (dB) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Eye | Before | After | Before | After | Before | After | Before | After |
| 2 | Left | 347 | 234 | 54.75 | 53 | – | − 15.93 | – | 47% |
| 3 | Right | 158 | 364 | 50.75 | 42.75 | − 23.51 | − 16.33 | 24% | 51% |
| 4 | Right | 849 | 230 | 46 | 46.75 | − 25.81 | − 21.22 | 18% | 27% |
| 5 | Left | 456 | 1530 | 43.5 | 36 | − 32.42 | − 33.67 | 3% | 1% |
| 6 | Left | 113 | 768 | 81 | 54.25 | − 31.71 | − 9.31 | 9% | 74% |
| 7 | Right | 291 | 944 | 42 | 42 | − 32.9 | − 28.54 | 4% | 17% |
| 8 | Left | 681 | 2420 | 39 | 43.25 | − 26.87 | − 28.93 | 13% | 11% |
| 9 | Right | 512 | 406 | 44.5 | 47.5 | − 27.69 | − 26.53 | 13% | 11% |
| Average | 425.88 | 862 | 50.19 | 45.69 | − 28.70 | − 22.56 | 12% | 30% | |
P100 amplitudes: amplitudes P100 values increase means the visual function improvement; RNFL: retinal nerve fiber layer; MD: mean deviation, value close to 0 considered as normal; VFI: visual field index, value close to 100% considered as normal.
Fig. 3.
The visual field index (VFI), mean defect (MD), and pattern-reversal visual evoked potential P100 waveform in the injected and uninjected eyes of 8 patients who received unilateral gene therapy. The mean VFI (A) and MD (B) values of the injected and uninjected eyes before and 3, 6, 12, 24, and 36 months after intravitreal injection. Five patients had VFI and MD improvements after treatment, but Patient 5 had no improvement. Error bars represent one standard deviation. The latency (C) and amplitude (D) of the P100 waveform in the injected and uninjected eyes of the 8 patients who received unilateral gene therapy are shown. After gene therapy, P100 latency decreased and P100 amplitude increased in both the injected and uninjected eyes within 6 months. This indicated an improvement in optic nerve function after gene therapy. Error bars represent one standard deviation. Mean VFI in the 8 patients who received unilateral gene therapy was 11 ± 0.15%, 28 ± 0.23%, 23 ± 0.22%, 18 ± 0.23%, 28 ± 0.18%, and 27 ± 0.22% before and 3, 6, 12, 24, and 36 months after treatment, respectively. The mean VFIs of the uninjected eyes were 12 ± 0.07%, 29 ± 0.25%, 27 ± 0.29%, 20 ± 0.21%, 34 ± 0.28%, and 30 ± 0.25% before and 3, 6, 12, 24, and 36 months after treatment, respectively (Table 3, Table 4, Fig. 3A, B).
2.4. Visual evoked potentials
Visual evoked potentials (VEPs) in the 8 patients who received unilateral intravitreal rAAV2-ND4 showed a significant decrease in P100 latency for 6 months after treatment, which suggested an improvement in optic nerve function, but returned to baseline levels thereafter. Additionally, gene therapy increased the mean P100 amplitude in the injected eye from 684.76 ± 749.00 nV before treatment to 1814.60 ± 2176.95 nV 36 months after treatment, but this change was not statistically significant (p = 0.250). The uninjected eye also showed an increase in amplitude from 425.88 ± 252.56 nV before treatment to 862 ± 768.94 nV after 36 months (Table 5, Table 6; Fig. 3C,D), but this change was also not statistically significant (p = 0.145). These changes, even though not significant, suggest limited optic nerve function improvement in both eyes, particularly between 6 and 12 months after gene therapy.
2.5. Optical coherence tomography
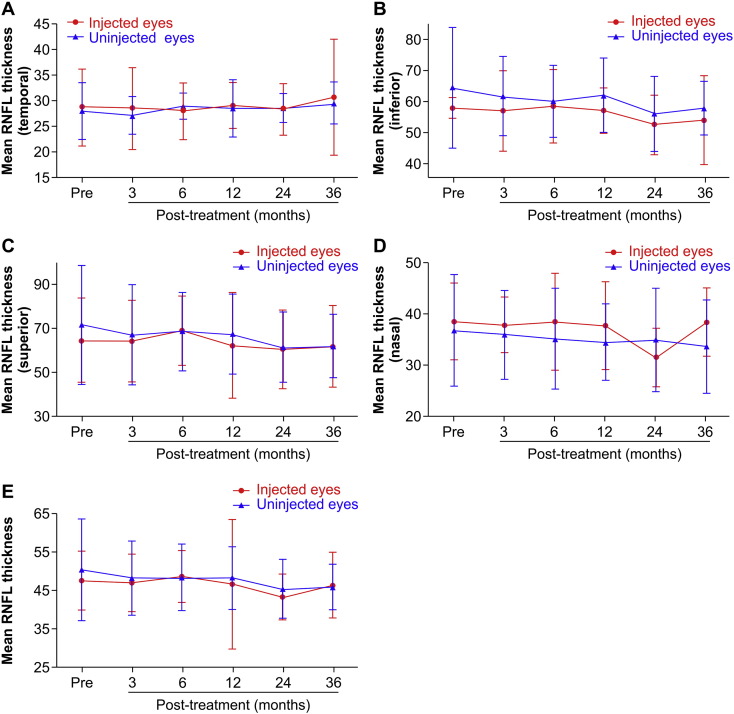
Compared with other examination methods, optical coherence tomography (OCT) measurements remained the most stable over time. The retinal nerve fiber layer (RNFL) thickness in the injected eyes did not change significantly over the 36-month follow-up period. However, RNFL thicknesses in the uninjected eyes were significantly lower at 24 months after treatment than at baseline over the entire retina and in each quadrant (superior, inferior, temporal, and nasal; Table 5, Table 6, Fig. 4).
Fig. 4.
Optical coherence tomography (OCT) measurements of retinal nerve fiber layer (RNFL) thicknesses in 8 patients who received unilateral gene therapy. A. Temporal RNFL thickness. B. Inferior RNFL thickness. C. Superior RNFL thickness. D. Nasal RNFL thickness. E. Mean RNFL thickness. Average RNFL thicknesses before and 3, 6, 12, 24, and 36 months after treatment were 47.31 ± 7.87, 46.78 ± 7.54, 48.47 ± 7.79, 46.46 ± 7.79, 43.13 ± 6.07, and 46.19 ± 8.67 μm, respectively. RNFL thicknesses of the uninjected eyes before and 3, 6, 12, 24, and 36 months after treatment were 50.19 ± 13.40, 47.97 ± 9.70, 48.19 ± 8.78, 48.08 ± 8.11, 45.25 ± 7.69, and 45.69 ± 6.01 μm, respectively. Specific measurements revealed a decreasing trend in RNFL thickness over time. There were no significant changes in RNFL thickness in the injected eyes over time (baseline: 47.31 ± 7.87 μm; at 36 months: 46.19 ± 8.67 μm, p = 0.691). However, RNFL thickness in the uninjected eyes began to decline 24 months after treatment (baseline: 50.19 ± 13.40 μm; at 36 months: 45.69 ± 6.01 μm, p = 0.245).
2.6. Bilateral Gene therapy
2.6.1. Animal experiment results
Our animal experiments indicated that successive bilateral intravitreal injections of rAAV-ND4 resulted in the long-term, relatively stable expression of retinal ND4 in the second eye injected. Serum concentrations of AAV2 and the AAV2 antibody were not abnormal at any time point examined (Fig. 5).
Fig. 5.
Effects of gene therapy on the uninjected eyes of mice. A–B. The retinal ND4 protein level, measured using Western blot analysis, after injection of the second eye. There was no significant difference between Groups 1 and 2 (p = 0.22) or between Groups 3 and 4 (p = 0.78). C. Serum AAV2 concentration as measured using ELISA. There was no significant difference between Groups 1 and 2 (p = 0.86) or between Groups 3 and 4 (p = 0.51). D. Serum AAV2 antibody concentration as measured using ELISA. There was no significant difference between Groups 1 and 2 (p = 0.20) or between Groups 3 and 4 (p = 0.20). Error bars represent one standard deviation.
2.7. Bilateral gene therapy (patient 1)
2.7.1. Systemic findings
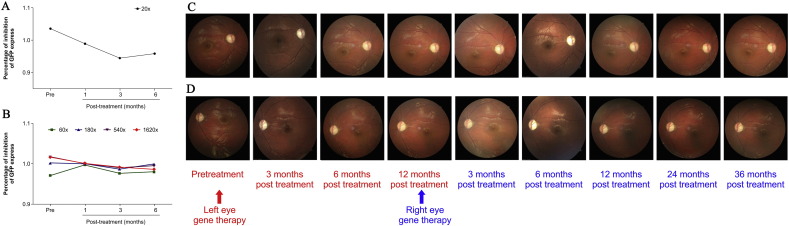
All of Patient 1's blood, urine, liver, kidney, and immune function tests were normal. The measured CD3 +/CD4 + and CD3 +/CD8 + values were also normal. However, the AAV2-neutralizing antibody assay results differed between baseline and at 3 months after treatment. In Patient 1 before and at 3 months after treatment, the AAV2-neutralizing antibody assay showed a difference between the 1:20 serum AAV2 and serum-free AAV2 concentrations. However, in Patient 1 at 1 and 6 months after treatment, the results of the neutralizing antibody assay were not different between the 1:20 serum AAV2 and serum-free AAV2 concentrations (Fig. 6A, B).
Fig. 6.
Clinical anti-AAV2-neutralizing antibody assay results following gene therapy administration in the fellow eye and fundus photography of patient 1 in both eyes 12 months apart. A. The percentage of green fluorescent protein (GFP) expression inhibition (1:20). B. The percentage of GFP expression inhibition (1:60, 1:180, 1:540, and 1:1620). Three months after receiving gene therapy, GFP expression inhibition increased when a 1:20 serum concentration was compared with serum-free medium. The inhibition of GFP expression returned to pre-treatment levels 6 months after gene therapy. Photographs of the right (C) and left (D) fundi are shown. The photographs were taken 3, 6, and 12 months after the administration of an intravitreal rAAV2-ND4 injection in the left eye. At 12 months, an intravitreal rAAV2-ND4 injection was also administered in the right eye. Photographs were also taken at 3, 6, 12, 24, and 36 months after treatment of the right eye. No apparent retinal abnormalities were identified.
The optical densities of AAV2 before and at 1, 3, and 6 months after treatment were 0.64, 0.75, 0.34, and 0.39, respectively. The optical densities of ND4 before and at 1, 3, and 6 months after treatment were 1.17, 1.19, 1.26, and 1.38, respectively. The optical densities of IFN-γ before and at 1, 3, and 6 months after treatment were 1.58, 1.42, 1.56, and 1.70, respectively.
2.7.2. Ophthalmologic findings
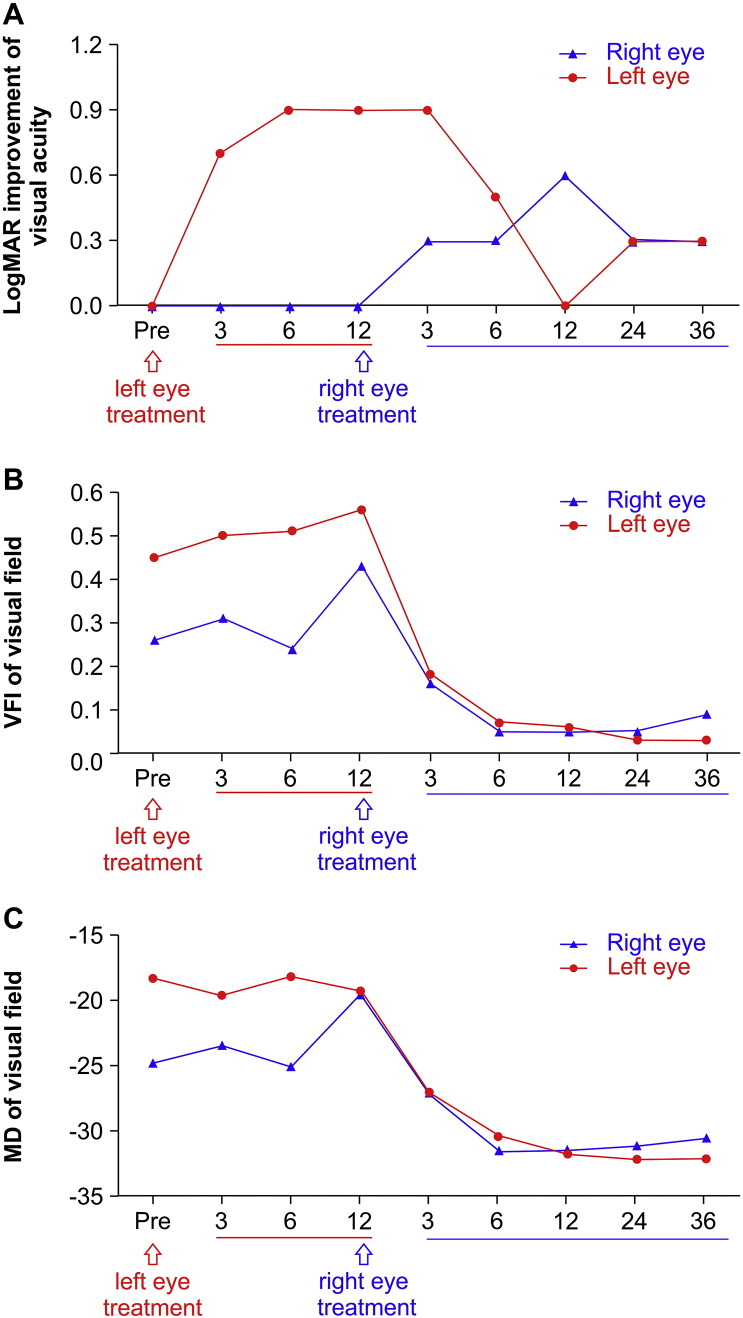
Color fundus photography did not reveal any serious complications, and the retinas and optic nerves in both eyes showed no significant changes from baseline (Fig. 6C,D). The maximum visual acuity in the injected eye occurred 6 months after treatment. One year after administering gene therapy in the left eye, the right eye was administered with gene therapy using the same methods. Three months later, logMAR BCVA in the left eye decreased by 0.6 (from 1.1 to 1.7). Twelve months later, logMAR BCVA in the right eye also began to decrease. Fortunately, logMAR BCVA stabilized at 24 months and was 1.7 at 36 months, a 0.3 improvement over baseline (1.7 vs. 2.0, Fig. 7A).
Fig. 7.
Improvement in logMAR visual acuity and changes in visual field parameters in a patient who received gene therapy in both eyes 12 months apart (Patient 1). A. Visual acuity improved from baseline in the first eye following gene therapy in that eye, but decreased 6 months after gene therapy in the second eye. B. Serial visual field index (VFI) measurements showed that bilateral VFI decreased after the second eye received gene therapy. C. Mean defect (MD) changes showed that bilateral MD worsened after gene therapy administration in the second eye, indicating greater visual field damage.
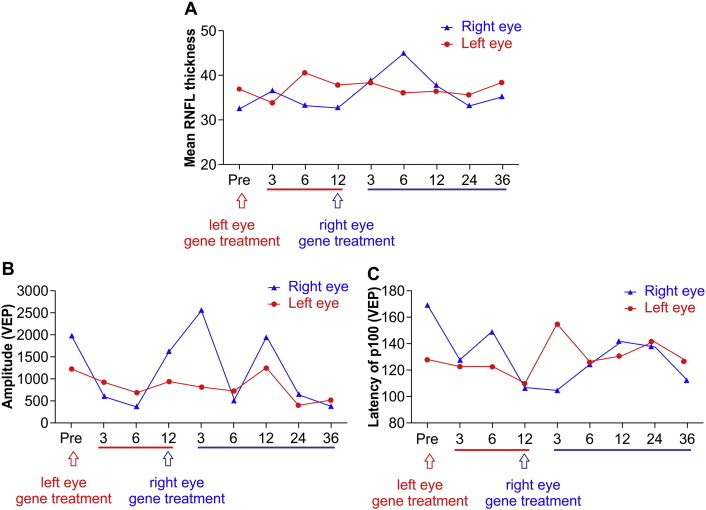
Visual field testing results showed similar trends as those observed for visual acuity. After gene therapy was administered in the left eye (first eye), the bilateral VFI and MD both improved. However, after treatment was administered in the right eye (second eye), the bilateral VFI and MD deteriorated (Fig. 7B, C). Additionally, OCT revealed that the RNFL thickness remained stable and unchanged (Fig. 8A).
Fig. 8.
Retinal nerve fiber layer thickness (RNFL) and visual evoked potentials (VEPs) after bilateral administration of gene therapy (Patient 1). Injections were administered 12 months apart. A. The RNFL thickness did not change in either eye during the 48-month observation period. B. The amplitude of the VEP P100 component decreased in both eyes following the administration of gene therapy in the second eye. C. The VEP latency increased after the administration of gene therapy in the second eye. Both VEP changes are indicative of reduced optic nerve function.
An examination of PR-VEPs revealed fluctuations in P100 latency and amplitude during the follow-up period. Twelve months after gene therapy was administered in the first eye, P100 latency was lower than at baseline, and the amplitude was greater than at baseline. These results suggest an improvement in optic nerve function. In contrast, 36 months after gene therapy was administered in the second eye, P100 latency was longer than at baseline, and the amplitude was lower than at baseline. This suggested a deterioration in optic nerve function (Fig. 8B,C).
3. Discussion
In 2008, gene therapy for Leber's congenital amaurosis was administered for the first time (Maguire et al., 2008, Bainbridge et al., 2008). Gene therapy offers new hope for the treatment of numerous hereditary ocular diseases and is extremely promising for treating LHON (Cwerman-Thibault et al., 2014, Meyerson et al., 2015). We previously reported results obtained 9 months after treating LHON patients with an intravitreal injection of rAAV2-ND4 (Wan et al., 2016). Feuer et al. also reported short-term (3–6 months) preliminary results of 5 LHON patients treated with gene therapy (Feuer et al., 2016).
There were 4 patients (Patients 2, 3, 5, and 9) with no significant improvement in visual acuity 36 months after gene therapy (< 0.3 logMAR). However, it should be stressed that > 2 years after therapy administration, the visual acuities of Patients 2 and 9 increased by > 0.3 logMAR and were maintained for a certain period. No patient demonstrated a more serious decline in visual acuity from baseline. This finding further supports the safety of gene therapy with the AAV vector. Regarding the timing of the gene therapy, patients underwent it after an LHON clinical history of 1 year (Patients 5 and 6), 2 years (Patients 2 and 3), 3 years (Patient 9), 4 years (Patients 1 and 8), 8 years (Patient 4), and 17 years (Patient 7). In the cases where the therapy was ineffective, RNFL thicknesses ranged from 42 to 48.5 μm before treatment and the duration of the LHON-associated visual acuity decrease ranged between 2 and 3 years. These patients did not have any identifiable common characteristics (Table 3, Table 4).
The VEP findings suggest that gene therapy can improve optic nerve function in LHON patients. The RNFL thicknesses in the injected eyes did not change, but those in uninjected eyes decreased from baseline to 24 months after gene therapy administration. Previous studies have shown that RNFL thickness continuously declines in LHON patients (Barboni et al., 2012, Zhang et al., 2014). Therefore, our results may indicate that gene therapy provides better RNFL protection for the injected eye, but does not have protective effects in the uninjected eye 24 months after gene therapy. However, the number of participants in our study was small and the difference in proportions is only suggestive and not definitive.
Patient 1 was the only patient who received bilateral treatment, so we only reported our clinical observations. After the second eye (right eye) received gene therapy, visual acuity decreased and VF defect severity increased in the first eye (left eye) for unknown reasons. These changes could have been a coincidence or could have been caused by a humoral immune response to AAV2 after gene therapy was administered in the first eye (Bainbridge et al., 2015, Tseng and Agbandje-McKenna, 2014). However, systemic examinations prior to administering gene therapy in the second eye showed normal humoral immune responses. Because we cannot know for certain why VF and visual acuity changes occurred in the first eye, further investigation is needed. For now, we do not recommend that gene therapy be performed in the fellow eyes of patients with LHON.
It is worth noting that in the 3-year LHON gene therapy follow-up, we found 2 unexpected phenomena. First, patients had visual acuity improvements over baseline after treatment, but some patients had BCVA fluctuations during the 3-year follow-up period. This was particularly evident in Patients 2 and 9. Second, visual acuity of the contralateral eye appeared to improve in some patients. The results of a previous study by Feuer et al. (2016) are consistent with those found here.
Regarding the first point, BCVA was our primary endpoint for LHON gene therapy, but this measure is somewhat subjective. An improvement in logMAR visual acuity was defined as an improvement of 0.3 or more. This definition minimizes the influence of subjective factors and changes in vision greater than or equal to 0.3 logMAR during the 36-month follow-up period were also defined as relative visual fluctuations. Based on this standard, visual regression fluctuations only occurred in Patients 2 and 9 and were not obvious in other patients. Visual acuity fluctuations may have been related to protein expression stability after transfection with ND4, other persistent disease factors, and/or patient-specific factors. Regarding the second point, according to the 0.3 logMAR standard, Patients 2, 3, 4, and 6 demonstrated visual acuity improvements in uninjected eyes. The following two reasons may explain this phenomenon: spontaneous visual recovery and influence of the contralateral eye. Despite the fact that 4–33% of LHON patients with the G11778 A mutation have spontaneous visual recovery (Lam et al., 2014), these patients had an LHON onset history (visual acuity decline) of > 12 months prior to the gene therapy clinical trial. In addition, we excluded some patients who had shown spontaneous visual recovery before the final gene therapy to reduce the effect of these factors on our study. Feuer et al. (2016) also suggested that spontaneous visual recovery was unlikely to be responsible for visual acuity improvements following gene therapy (Feuer et al., 2016).
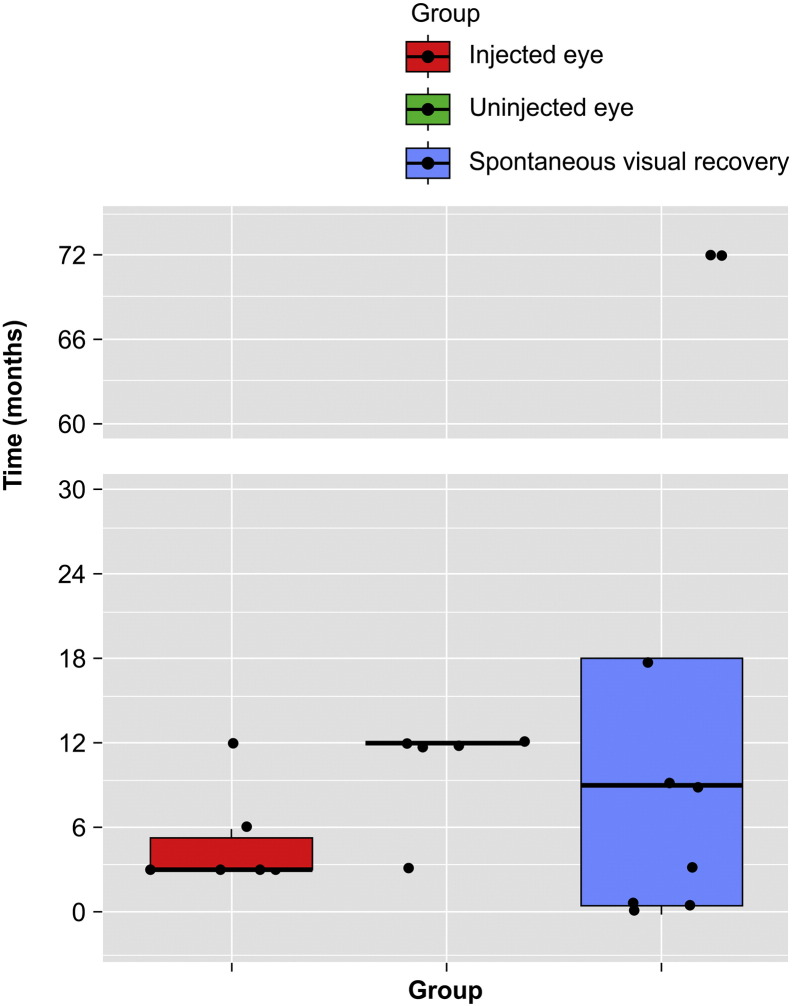
The effect of spontaneous visual recovery was further ruled out by our comparative analysis of visual recovery times in patients who received gene therapy and those who had a spontaneous visual recovery (Supplementary material, Fig. S1). Some striking differences were noted. Visual acuity mainly improved between 3 and 6 months after gene therapy in the injected eye and between 3 and 12 months in the uninjected eye. In contrast, the spontaneous visual recovery time was widely variable and ranged between 1 h and 72 months from the onset of vision loss (Fig. S2). These findings suggest that spontaneous visual recovery occurs at random, but visual recovery following an intravitreal injection of rAAV2-ND4 occurs within a fixed time frame. It should be noted that this comparative analysis had some unavoidable limitations, including the possible introduction of biases resulting from the study population race and sample size, testing time, physician's behavioral differences, and other uncontrolled variables. In addition, the sample size was small and patients who had spontaneous visual recovery may have failed to seek timely treatment. These factors would impact the general applicability of our comparative analysis results. However, improvements in the uninjected eyes never occurred before improvements in the injected eyes. Visual acuity improvements in both injected and uninjected eyes occurred within 6 months after injection. All these facts reduce the likelihood that a spontaneous visual recovery resulted in visual acuity improvements following gene therapy.
Fig. S1.
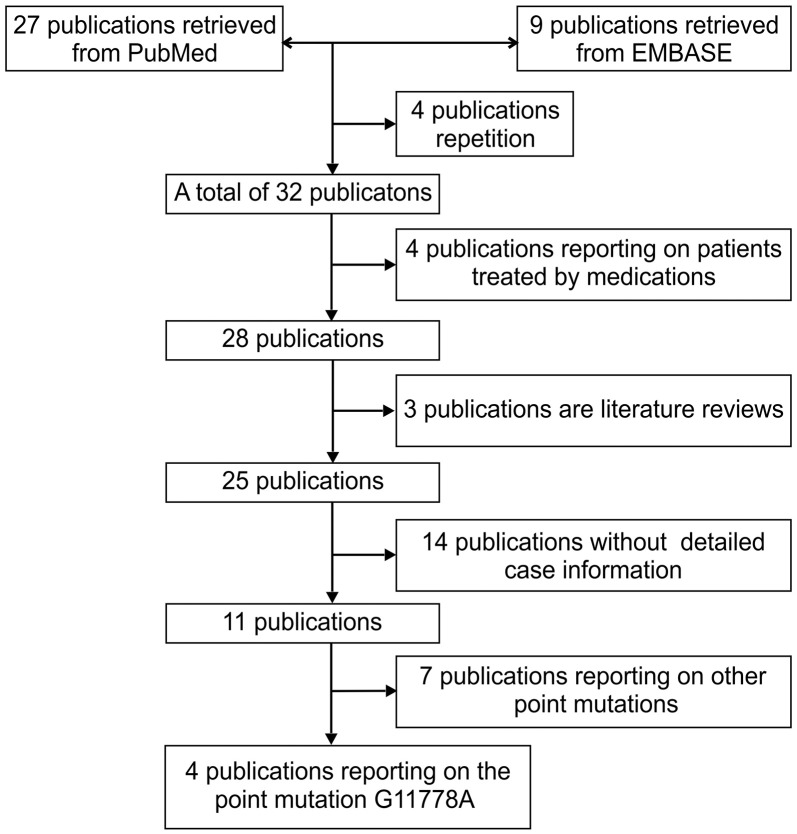
Study flow diagram showing the article selection process for the meta-analysis performed on the literature.
Fig. S2.
Visual function recovery time in injected eyes, uninjected eyes, and spontaneous visual recovery eyes. Visual acuity improvements in injected eyes mainly occurred between 3 and 6 months after gene therapy, while improvements in uninjected eyes mainly occurred between 3 and 12 months. In contrast, visual acuity improvements in spontaneous visual recovery eyes was highly variable and occurred between 1 h and 72 months. The timing between the injected and uninjected eyes was not significantly different (Mood's median test, Z = − 0.913, p = 0.361), but the timing between injected and spontaneous visual recovery eyes (Z = − 2.79, p = 0.009) and between uninjected and spontaneous visual recovery eyes (Z = − 2.21, p = 0.026) was significantly different.
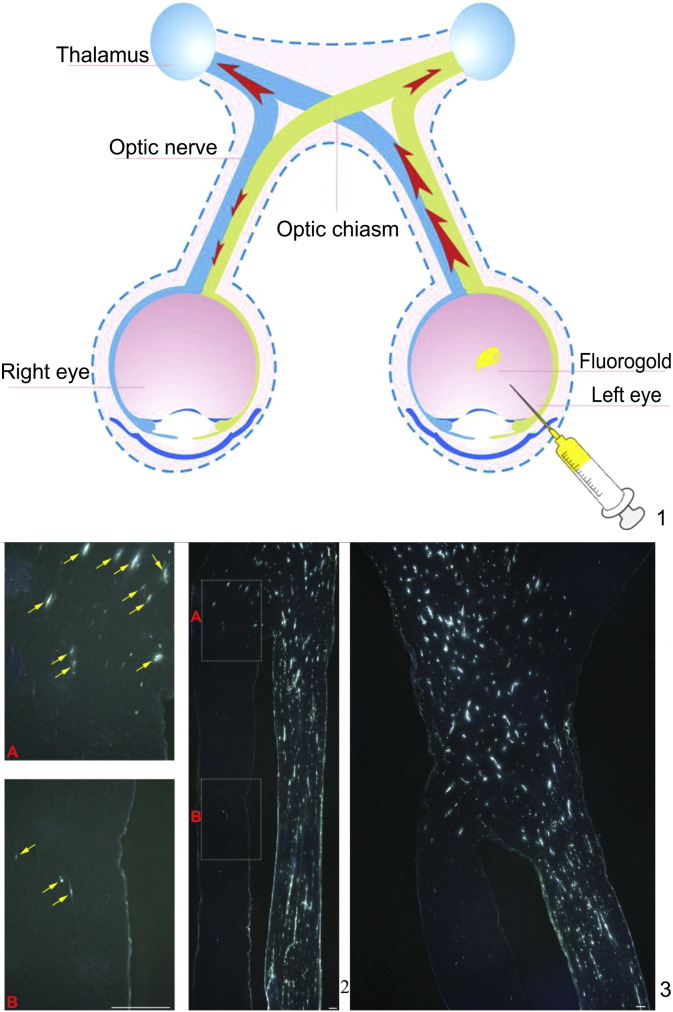
The mechanism by which gene therapy affected the contralateral eye was investigated by Yang et al. (2015). In animal experiments, a fluorescent gold tracer was used to verify the link between the eyes (Fig. S3). Their results suggested that some physical communication between the eyes may have occurred via the optic chiasm (Yang et al., 2015). Luo et al. (2013) and Pernet et al. (2013) showed that a few regenerating nerve axons crossed the optic chiasm into the contralateral optic nerve and grew toward the contralateral retina. These studies also suggest the possibility of direct communication between the optic nerves. At the same time, this might play a certain role in exploring the unknown field of binocular optic nerves. The visual recovery curve of Patient 1 was particularly useful for determining the timing of drug action because both eyes received treatment.
Fig. S3.
Direct material exchange between optic nerve axons. A. Sprague-Dawley rats administered a unilateral intravitreal injection of fluorogold (FG). B. A small proportion of FG did diffuse to the contralateral optic nerve anterior to the optic chiasm (2A, 2B). C. The FG reached the optic chiasm via optic nerve axons of the injected eye. Most of the FG that diffused to the contralateral optic nerve remained posterior to the optic chiasm. Only a small proportion of FG diffused to the ipsilateral optic nerve posterior to the optic chiasm. Therefore, direct material exchange occurred between the optic nerves (A, C). This mechanism may play a role in visual function improvements in the uninjected eye in LHON patients who receive gene therapy. Scale bar = 100 μm.
In conclusion, our long-term (3-year) results suggested that intravitreal injection of rAAV2-ND4 for LHON is a safe and promising treatment, as affirmed in a small sample of clinical trials. Treatment efficacy also suggests treatment feasibility. Additional enrollment and follow-up is underway and is expected to be completed in coming years.
The following are the supplementary data related to this article.
Supplementary materials.
Contributors
BL is the guarantor of this work and designed the study and performed the surgeries, anterior segment examination, and direct ophthalmoscopy of the fundus. BL and SY prepared the manuscript. SY and SQM performed the animal experiments. SY, SQM, XW, HH, and HP analyzed the anti-AAV2 neutralizing antibody assay data and performed the CD3 +, CD3 +/CD4 +, CD3 +/CD8 +, BCVA, intraocular pressure, ocular fundus photography, visual field, IOP, and VEP tests, anterior segment examination, and direct ophthalmoscopy of the fundus. MJZ followed the patients in the intensive care unit. JJY performed BCVA and intraocular pressure tests. XYD cloned the rAAV2-ND4 construct. CC and DWW performed the genetic diagnosis. All authors have approved publication of the manuscript.
Declaration of interests
BL received funding from the National Nature Science Foundation of China (Grants #81271015, #30872823, and #30801260) and by the LHON Special Research Foundation of the Wuhan Phoebus Biological Technology Limited Company. The other authors declare no competing interests.
Acknowledgements
We would like to express our gratitude to the 9 patients and their family members for choosing to receive gene therapy and for participating in this research. This research was supported by the National Nature Science Foundation of China (Grants #81271015, #30872823, and #30801260) and by the LHON Special Research Foundation of the Wuhan Phoebus Biological Technology Limited Company.
References
- Bainbridge J.W., Smith A.J., Barker S.S. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Bainbridge J.W., Mehat M.S., Sundaram V. Long-term effect of gene therapy on Leber's congenital amaurosis. N. Engl. J. Med. 2015;372:1887–1897. doi: 10.1056/NEJMoa1414221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barboni P., Savini G., Feuer W.J. Retinal nerve fiber layer thickness variability in Leber hereditary optic neuropathy carriers. Eur. J. Ophthalmol. 2012;22:985–991. doi: 10.5301/ejo.5000154. [DOI] [PubMed] [Google Scholar]
- Beck R.W., Moke P.S., Turpin A.H. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J. Ophthalmol. 2003;135:194–205. doi: 10.1016/s0002-9394(02)01825-1. [DOI] [PubMed] [Google Scholar]
- Cui G., Ding H., Xu Y., Li B., Wang D.W. Applications of the method of high resolution melting analysis for diagnosis of Leber's disease and the three primary mutation spectrum of LHON in the Han Chinese population. Gene. 2013;512:108–112. doi: 10.1016/j.gene.2012.09.110. [DOI] [PubMed] [Google Scholar]
- Cwerman-Thibault H., Augustin S., Ellouze S., Sahel J.A., Corral-Debrinski M. Gene therapy for mitochondrial diseases: Leber hereditary optic neuropathy as the first candidate for a clinical trial. C. R. Biol. 2014;337:193–206. doi: 10.1016/j.crvi.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Feuer W.J., Schiffman J.C., Davis J.L. Gene therapy for Leber hereditary optic neuropathy: initial results. Ophthalmology. 2016;123:558–570. doi: 10.1016/j.ophtha.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., Shi H., Pei Z. Comparison of immunosuppressive effects and ND4 expression among different immunosuppressive strategies following AAV2-ND4 gene treatment for Leber hereditary optic neuropathy. J. Huazhong Univ. Sci. Technol. Med. Sci. 2013;42:187–191. [Google Scholar]
- Klopstock T., Yu-Wai-Man P., Dimitriadis K. A randomized placebo-controlled trial of idebenone in Leber's hereditary optic neuropathy. Brain. 2011;134:2677–2686. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam B.L., Feuer W.J., Schiffman J.C. Trial end points and natural history in patients with G11778A Leber hereditary optic neuropathy: preparation for gene therapy clinical trial. JAMA Ophthalmol. 2014;132:428–436. doi: 10.1001/jamaophthalmol.2013.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X., Salgueiro Y., Beckerman S.R., Lemmon V.P., Tsoulfas P., Park K.K. Three-dimensional evaluation of retinal ganglion cell axon regeneration and pathfinding in whole mouse tissue after injury. Exp. Neurol. 2013;247:653–662. doi: 10.1016/j.expneurol.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey D.A., Oostra R.J., Rosenberg T. Primary pathogenic mtDNA mutations in multigeneration pedigrees with Leber hereditary optic neuropathy. Am. J. Hum. Genet. 1996;59:481–485. [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M., Simonelli F., Pierce E.A. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson C., Van Stavern G., McClelland C. Leber hereditary optic neuropathy: current perspectives. Clin. Ophthalmol. 2015;9:1165–1176. doi: 10.2147/OPTH.S62021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei H., Wan X., Hu W. Constructing and detecting a new rAAV2/2-ND4. Eye Sci. 2013;28:55–59. [PubMed] [Google Scholar]
- Pernet V., Joly S., Dalkara D. Long-distance axonal regeneration induced by CNTF gene transfer is impaired by axonal misguidance in the injured adult optic nerve. Neurobiol. Dis. 2013;51:202–213. doi: 10.1016/j.nbd.2012.11.011. [DOI] [PubMed] [Google Scholar]
- Shi H., Gao J., Pei H. Adeno-associated virus-mediated gene delivery of the human ND4 complex I subunit in rabbit eyes. Clin. Exp. Ophthalmol. 2012;40:888–894. doi: 10.1111/j.1442-9071.2012.02815.x. [DOI] [PubMed] [Google Scholar]
- Tseng Y.S., Agbandje-McKenna M. Mapping the AAV capsid host antibody response toward the development of second generation Gene delivery vectors. Front. Immunol. 2014;5:9. doi: 10.3389/fimmu.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan X., Pei H., Zhao M.J. Efficacy and safety of rAAV2-ND4 treatment for Leber's hereditary optic neuropathy. Sci. Rep. 2016;6:21587. doi: 10.1038/srep21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., He H., Zhu Y. Chemical and material communication between the optic nerves in rats. Clin. Exp. Ophthalmol. 2015;43:742–748. doi: 10.1111/ceo.12547. [DOI] [PubMed] [Google Scholar]
- Yu-Wai-Man P., Griffiths P.G., Chinnery P.F. Mitochondrial optic neuropathies — disease mechanisms and therapeutic strategies. Prog. Retin. Eye Res. 2011;30:81–114. doi: 10.1016/j.preteyeres.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Huang H., Wei S. Characterization of macular thickness changes in Leber's hereditary optic neuropathy by optical coherence tomography. BMC Ophthalmol. 2014;14:105. doi: 10.1186/1471-2415-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.