Abstract
Prenatal alcohol exposure can produce permanent alterations in brain structure and profound behavioral deficits. Mouse models can help discover mechanisms and identify potentially useful interventions. This study examined long-term influences of either a single or repeated alcohol exposure during the third-trimester equivalent on survival of new neurons in the hippocampus, behavioral performance on the Passive avoidance and Rotarod tasks, and the potential role of exercise as a therapeutic intervention. C57BL/6J male mice received either saline or 5 g/kg ethanol split into two s.c. injections, two hours apart, on postnatal day (PD)7 (Experiment 1) or on PD5, 7 and 9 (Experiment 2). All mice were weaned on PD21 and received either a running wheel or remained sedentary from PD35-PD80/81. From PD36-45, mice received i.p. injections of 50 mg/kg bromodeoxyuridine (BrdU) to label dividing cells. Behavioral testing occurred between PD72-79. Number of surviving BrdU+ cells and immature neurons (doublecortin; DCX+) were measured at PD80-81. Alcohol did not affect number of BrdU+ or DCX+ cells in either experiment. Running significantly increased number of BrdU+ and DCX+ cells in both treatment groups. Alcohol-induced deficits on Rotarod performance and acquisition of the Passive avoidance task (Day 1) were evident only in Experiment 2 and running rescued these deficits. These data suggest neonatal alcohol exposure does not result in long-term impairments in adult hippocampal neurogenesis in the mouse model. Three doses of ethanol were necessary to induce behavioral deficits. Finally, the mechanisms by which exercise ameliorated the neonatal alcohol induced behavioral deficits remain unknown.
Keywords: bromodeoxyuridine, Passive avoidance, running, hippocampus, fetal alcohol spectrum disorders
1. INTRODUCTION
Approximately 2–5% of all live births in the US are diagnosed as cases of Fetal Alcohol Spectrum Disorders (FASD), an unchanging number despite clear knowledge and the preventable nature of FASD (May et al., 2009). In humans, prenatal alcohol exposure produces a wide range of long-lasting neurobehavioral deficits, including physical, cognitive, learning and behavioral disabilities (Calhoun et al., 2006). For example, FASD patients exhibit long-lasting deficits in working memory and behavioral flexibility (Connor et al., 2000; Rasmussen, 2005), behaviors that require the proper functioning of multiple brain regions at once, many of which are still developing during and appear particularly sensitive to alcohol exposure during the third-trimester.
The third trimester is comparable to the first two weeks of postnatal life in mice and rats in terms of brain development (Dobbing and Sands, 1979). A large literature has established that rodents neonatally exposed to alcohol exhibit impaired performance on a variety of behavioral tasks as adults (for review see Patten et al., 2014). Of note, only a few studies have demonstrated a significant influence of neonatal alcohol exposure on Passive avoidance performance (Abel, 1982; Barron and Riley, 1990; Becker and Randall, 1989; Riley et al., 1979), a task in which mice must learn and remember to inhibit their natural response to move to the dark side of the chamber and instead stay in the lit side of the chamber. This task involves multiple brain regions (Kemble and Tapp, 1968; Walsh et al., 1984), including, but not limited to, the prefrontal cortex and the hippocampus, two brain regions well-known for being particularly sensitive to third-trimester equivalent alcohol exposure. In fact, Barron and Riley (1990), using an artificial rearing model, demonstrated that third-trimester equivalent alcohol exposure impaired both acquisition and retention of this task in PD23 rats. Whether a similar result is evident in mice and whether this effect persists into adulthood remains unknown. However, given the large number of adult FASD patients suffering from working memory and behavioral flexibility deficits, this is a well-suited behavioral task to use when developing interventions.
Neonatal alcohol exposure produces long-lasting neuroanatomical deficits in the hippocampus of rodents. This is particularly apparent following a third-trimester equivalent exposure (Gil-Mohapel et al., 2010; Livy et al., 2003). Of all the brain regions impacted from alcohol, the hippocampus is arguably the most capable of extensive regeneration and repair (Brown et al., 2003; Magarinos et al., 2006; Olson et al., 2006). Still, it is possible that neonatal exposure can produce a persistent reduction in hippocampal plasticity. Some studies show reductions in adult hippocampal neurogenesis and suggest the loss contributes to behavioral deficits (Gil-Mohapel et al., 2014; Hamilton et al., 2011; Ieraci and Herrera, 2007; Klintsova et al., 2007), while, other studies fail to find an effect of neonatal ethanol exposure on adult neurogenesis (Choi et al., 2005; Gil-Mohapel et al., 2014; Helfer et al., 2009; Wozniak et al., 2004). These studies used both rats and mice of different strains. The lack of consensus warrants additional research specifically examining the impact of different timing and duration of alcohol exposures on adult hippocampal neurogenesis in a single mouse strain.
Regardless of whether or not neonatal alcohol exposure results in long-lasting deficits in adult neurogenesis in rodents, exercise is known to increase levels of adult neurogenesis and improve behavioral performance across a broad range of tasks in alcohol-exposed rats (Christie et al., 2005; Gil-Mohapel et al., 2011; Hamilton et al., 2012; Hamilton et al., 2014; Redila et al., 2006). Therefore, increasing levels of adult neurogenesis in alcohol-exposed mice could rescue hippocampal function either through compensation or repair. The goal of this study was to determine the extent to which a single (PD7) or repeated (PD5, 7 and 9) binge ethanol exposure reduces adult hippocampal neurogenesis, and impairs behavioral performance on the Rotarod and Passive avoidance tasks, and whether exercise can mitigate any deficits.
2. MATERIALS AND METHODS
Seventy-one (Experiment 1:36; Experiment 2:35) male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) as adults were used plus an additional 37 pups euthanized at PD7 for blood-ethanol concentration measurements. The 71 mice were generated from 20 pairs (10 pairs per Experiment), and the 37 pups from 7 pairs. The 71 mice were randomized into four groups: saline-sedentary, saline-runner, alcohol-sedentary, alcohol-runner (Experiment 1: n=9 per group; Experiment 2, n=7, 8, 10, and 10, respectively). No more than two animals per litter were assigned to a given treatment condition. Mice were housed in standard polycarbonate shoebox cages (29 cm × 19 cm × 13 cm; L × W × H) with corncob bedding (Teklad 7012; Harlan Teklad, Madison, WI, USA). Rooms were controlled for temperature (21°C ± 1°C) and photo-period (12:12 D:L; lights off at 10:00am and on at 10:00pm). Food and water was provided ad libitum. The Beckman Institute Animal Facility is AAALAC approved. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines. All efforts were made to minimize the number of mice used and their suffering.
2.1. Neonatal Treatment
Experiment 1
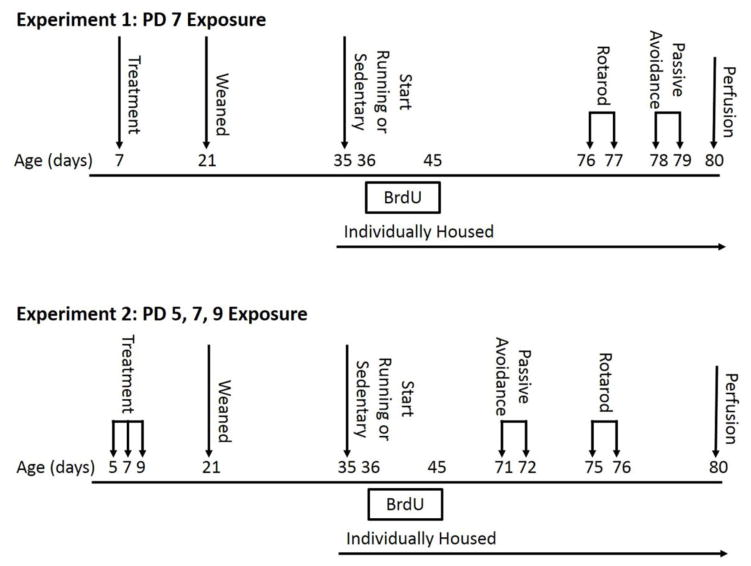
We followed established methods to deliver ethanol to neonatal mice (Ieraci and Herrera, 2007; Lantz et al., 2012; Wozniak et al., 2004). Pups from ten litters were injected subcutaneously (s.c.) over the shoulders, into the loose skin over the neck, with either a sterile saline solution or a dose of 5 g/kg of ethanol (between 0.03 – 0.08 ml of a 20% ethanol solution per injection; Decon Laboratories, Inc., King of Prussia, PA, USA; 20% ethanol in sterile saline solution; 15.8 ml/kg), which was divided into two injections, two hours apart. To avoid leakage of the solution, the liquid was released slowly into the animal and the needle remained in the animal for 3–5 seconds following before slowly being removed in order to allow the liquid time to be absorbed. Whole litters were injected with either saline or ethanol. The males from each litter were then assigned to the 2 treatments (runner or sedentary). Litters had 2–4 males, hence there were 1 or 2 males from the same litter per treatment combination. Dose was chosen based on the literature (Ieraci and Herrera, 2007; Ikonomidou et al., 2000; Lantz et al., 2012; Wagner et al., 2014; Wozniak et al., 2004). On PD21, all mice were weaned into groups of four by sex (Fig. 1).
Figure 1. Experimental Timeline.
For Experiment 1, mice were exposed to a single day of treatment, of either sterile saline or 5 g/kg ethanol (split into two doses, two hours apart), on PD 7. For Experiment 2, mice received three days of treatment (sterile saline or 5 g/kg ethanol) on PD 5, 7, and 9. All mice were weaned on PD 21 and singly housed on PD 35, the onset of intervention, which consisted of either voluntary access to a running wheel or sedentary condition. Newly dividing cells were labeled with bromodeoxyuridine (BrdU) for the first ten days of intervention. Mice underwent Passive avoidance and Rotarod testing during adulthood, after being exposed to the assigned intervention for at least thirty days. Differences in the order of the testing procedures were due to the availability of testing equipment.
Experiment 2
The same procedure as described above was used with the exception that mice received treatments on PD5, 7, and 9.
2.2 Blood Ethanol Concentration Analysis
Trunk blood samples were collected from an additional cohort of 37 mice in order to verify that alcohol concentrations in the blood (BECs) reached a toxic level, above 200 mg/dl for four consecutive hours or more, as BECs at this level have been shown to produce widespread apoptotic neurodegeneration (Ikonomidou et al., 2000). Mice were assigned to one of six groups at PD7: control (no alcohol administered; n=3) or alcohol administered then trunk blood collected at the following time points after the first injection of alcohol: 30 min (n=8), 1 hr (n=x8), 3 hr (n=7), 5 hr (n=5), and 9 hr (n=6). Note that for all time points greater than or equal to 3 hr, the mice would have received a second injection at 2 hr for a total of 5 g/kg. Immediately following collection in heparinized tubes, trunk blood was centrifuged at 1000 × g at 4 °C for 10 minutes after which plasma was collected and immediately stored at −80 °C.
Plasma samples were analyzed using the Colorimetric Alcohol Assay Kit (STA-620; Cell Biolabs, Inc, San Diego, CA, USA). Briefly, the stock Ethanol Standard was diluted using deionized water in order to prepare an ethanol standard (200 mM). Said ethanol standard was then further diluted in a 1X Assay Buffer to produce standards in the concentration range of 0–200 μm. Next, all samples were diluted in 1X Assay Buffer (1:80). Following this, 10 μL of the diluted ethanol standards or samples were added to a 96-well microtiter plate (all standards and samples were run in duplicate). The microtiter plate was then covered and placed to the side while the Reaction Mixture was prepared. The Reaction Mixture consisted of 8.7 mL of deionized water, 1 mL of 10X Assay Buffer, 100μL of 100X Enzyme Mixture and 200μL of the 50X Colorimetric Probe. The Reaction Mixture was mixed and then 90μL was added to each well in the microtiter plate. The plate was covered and subsequently incubated at 37 °C for 30 minutes. Finally, absorbances were read at 570 nm on a microplate reader (EPOCH; Biotek Instruments Inc, Winooski, VT, USA) and the ethanol concentration of samples was calculated.
2.3 Exercise or Sedentary Intervention
On PD35, all mice (Experiment 1 and Experiment 2) were singly housed with or without running wheels in order to obtain individual running levels. The intervention was counterbalanced within the treatments. Sedentary mice were housed in standard polycarbonate shoebox cages, as previously described. Running mice were housed in cages (36 cm × 20 cm × 14cm; L × W × H) with a 23 cm diameter wheel mounted to the cage top (Respironics, Bend, OR, USA). Wheel rotations were recorded continuously in one minute increments via magnetic switches interfaced to a computer throughout the experiment. Mice assigned to the sedentary condition were not housed in cages with locked wheels because mice climb in locked wheels and we intended to keep physical activity to a minimum in the sedentary group. Similar to previous studies from our lab, mice were given at least 30 days access to the exercise intervention prior to any behavioral intervention.
2.4 5′bromo-5′-deoxyuridine (BrdU) Injections
Following established procedures, from PD36-45, all mice received a daily intraperitoneal (i.p.) injection of BrdU (50 mg/kg) to label newly dividing cells in the dentate gyrus of the hippocampus (Choi et al., 2005; Helfer et al., 2009; Ieraci and Herrera, 2007; Klintsova et al., 2007)
2.5 Rotarod
Given that children with FASD typically have poor motor coordination, we wanted to determine first whether a PD7 or a PD5,7 and 9 alcohol exposure could impair motor coordination, and second, whether exercise could ameliorate any alcohol-induced deficit. Mice underwent Rotarod testing beginning one hour after lights off either on PD76-77 (Experiment 1) or PD75-76 (Experiment 2). Mice were placed on a stationary Rotarod (AccuRotor Rota Rod Tall Unit, 63 cm fall height, 30 mm diameter rotating dowel; Accuscan, Columbus, OH, USA), the dowel was accelerated at 60 rpm/min, and latency to fall recorded. Mice were tested for two consecutive days with four consecutive trials per day.
2.6 Passive avoidance
Experiment 1
Mice underwent Passive avoidance testing on PD78-79 beginning one hour after lights off. On PD78, mice were trained in the Passive avoidance chamber (San Diego Instruments, San Diego, CA, USA). The chamber consisted of two compartments, one lit and one unlit, separated by a guillotine door. The bottom of the chamber contained a metal grid through which a mild foot shock could be delivered. Each mouse was placed in the lit side of a chamber and the guillotine door was raised. Latency to cross over into the unlit side was recorded. When the mouse crossed over into the unlit compartment, the guillotine door closed and a mild foot shock at 0.5 mV was delivered. The mouse then remained in the dark chamber for ten seconds, a post conditioned stimulus period. Following this, the unlit compartment, was illuminated with the house light and the guillotine door was opened. This ensured animals were conditioned to the light/dark cues and not a particular side of the apparatus. Mice were trained to remain in the lit chamber for 120 seconds. The total number of trials it took to reach criterion was determined for each mouse. On Day 2, mice were placed into the lit compartment and their latency to enter the dark side was recorded.
Experiment 2
The same procedure as stated above was repeated for Experiment 2 except mice were tested on PD72-73, due to equipment availability.
2.7 Perfusions
Mice were euthanized on either PD80 (Experiment 1) or PD81 (Experiment 2). First, they were anesthetized with 100 mg/kg sodium pentobarbital via i.p. injection and then perfused transcardially with 4% paraformaldehyde in phosphate buffer solution (PBS; 0.287% sodium phosphate monobasic anhydrous, 1.102% sodium phosphate dibasic anhydrous, 0.9% sodium chloride in water). Brains were postfixed overnight and transferred to 30% sucrose in PBS for long-term storage. Brains were sectioned using a cryostat into 40 μm thick coronal sections and stored in cryoprotectant (30% ethylene glycol, 25% glycerin, 45% PBS) at −20°C.
2.7.1 BrdU- diaminobenzidine (DAB; Experiment 1 and 2)
To detect levels of survival in BrdU-positive (newly divided) cells in the dentate gyrus BrdU-DAB staining was performed. For each mouse, a one-in-six series of sections (i.e., series of sections throughout the rostro-caudal extent of the brain with 240 μm increments separating each section) was stained. Free-floating sections were washed in tris-buffer solution (TBS; 1.3 % Trizma hydrochloride, 0.19% Trizma base, 0.9% sodium chloride) and then treated with 0.6% hydrogen peroxide in TBS. To denature DNA, sections were treated with 50% deionized formamide, 10% 20x saline-sodium citrate (SCC) buffer, 2N hydrochloric acid, and 0.1M boric acid. Sections were then treated with 0.1% Triton-X and 3% goat serum in TBS (TBS-X plus) and incubated in primary antibody against BrdU made in rat (OBT0030; Accurate, Westbury, NY, USA) at a dilution of 1:200 in TBS-X plus for 72 hours at 4 °C. Sections were then washed in TBS, blocked with TBS-X plus for 30 minutes, and incubated in secondary antibody against rat made in goat (BA-9400; Vector Laboratories, Burlingame CA, USA) at a dilution of 1:250 in TBS-X plus for 100 minutes at room temperature. Sections were treated using the Vectastain Elite ABC Kit (PK-6200; Vector, Burlingame, CA, USA) and stained using a DAB kit (D4418-505ET; Sigma, St. Louis, MO, USA).
2.7.2 Doublecortin (DCX)-DAB (Experiment 2)
To estimate total numbers of immature neurons in the granule cell layer of the dentate gyrus at the time when the mice were euthanized, DCX-DAB staining was conducted. The immunohistochemistry proceeded similar to BrdU-DAB except omitting the DNA denaturing steps. The primary antibody was goat anti-DCX (1:1000; sc-8066; Santa Cruz Biotechnology, Santa Cruz, CA); secondary antibody was biotinylated donkey anti-goat (1:200; sc-2042; Santa Cruz Biotechnology, Santa Cruz, CA).
2.8 Image Analysis
Following Clark et al. (2008), the entire bilateral granule cell layer, represented in the one-in-six series, was photographed by systematically advancing the field of view of the Zeiss brightfield light microscope and taking multiple photographs, via AxioCam interfaced to computer, under 10x (total 100x) magnification. A large depth of field was used so that all particles within the section were visible in each photograph. For BrdU-DAB, these photographs were then analyzed using ImageJ software (NIH, Bethesda, MD, USA) to generate estimates of total number of BrdU-labeled cells per cubic micrometer dentate gyrus sampled and area of the granule cell layer within the sections. Specifically, in each image, the granule cell layer was traced and BrdU-positive nuclei were counted within the traced region automatically by setting a fixed threshold to remove background, and adjusted for double counting at the plane of the section. A subset of animals were also hand counted, and the hand counts regressed against the automated values to establish accurate counts of total numbers of labeled neurons. The R2 value for these relationships were greater than 90%. The equation was generated from individual sections from multiple individuals representing the full range in cell counts from among the smallest number to the largest number. Hence the equation describes the relationship for all groups and all individuals. Volume of the granule cell layer was estimated by tracing the 2-dimensional area of the granule cell layer in the bilateral 1-in-6 series, and multiplying by the distance between sections (240 microns). For DCX-DAB, the protocol mirrored that of BrdU-DAB image analysis except that DCX-positive cells were hand-counted.
2.9 Statistical Analysis
Data were analyzed using SPSS (version 21) statistical software. In all analyses, p < 0.05 was considered statistically significant. Given that whole litters were injected with either saline or ethanol, data were first analyzed with litter included as a random effect. Litter was not found to be significant on any measure, therefore we removed it for all reported statistics. Thus, the following variables were analyzed using a repeated measure analysis with Day as the within measure and Treatment (alcohol vs. saline) and Intervention (runner vs. sedentary) as the between factors: body weights (g), latency to fall from the Rotarod (s). The following variables were analyzed using a two-way analysis of variance (ANOVA), with Treatment (alcohol vs. saline) and Intervention (runner vs. sedentary) as the two factors: brain weights (g), total number of BrdU+ and DCX+ cells, average distance run on wheels, number of trials to criterion, latency to cross in Passive avoidance (s). Post-hoc analyzes (LSD) were used identify significant pairwise differences between means (p < 0.05).
3. RESULTS
3.1 Body Weights
All mice continued to gain weight throughout treatments (Table 1). Data from the neonatal period (PD5, 7, 9) versus adolescence and adulthood (PD35, 44, 80, 81) were analyzed separately. In Experiment 1, despite litters being randomly assigned to a Treatment, there was a significant main effect of Treatment (F1,69 = 55.8, p < 0.001), wherein alcohol-exposed mice were heavier than saline-exposed mice prior to neonatal treatment. This main effect of Treatment was still evident on PD35 (F1,34 = 4.2, p < 0.05). However, by PD44, alcohol and saline mice had similar body weights and remained similar at PD80. These data suggest that the single alcohol exposure on PD7 had no long-term impact on body weights.
Table 1.
Effect of postnatal alcohol exposure on body weight (g)
| PD 5 | PD 7 | PD 9 | PD 35 | PD 44 | PD 80/81 | ||
|---|---|---|---|---|---|---|---|
| Experiment 1 | Saline | N/A | 3.02 ± 0.08 | N/A | 17.37 ± 0.44 | 18.41 ± 0.37 | 22.48 ± 0.44 |
| Alcohol | N/A | 3.79 ± 0.07** | N/A | 18.42 ± 0.26* | 19.06 ± 0.29 | 23.31 ± 0.42 | |
| Experiment 2 | Saline | 3.02 ± 0.06 | 4.20 ± 0.10 | 5.34 ± 0.15 | 18.20 ± 0.42 | 18.69 ± 0.49 | 23.58 ± 0.41 |
| Alcohol | 3.07 ± 0.10 | 3.58 ± 0.11** | 4.55 ± 0.14** | 16.20 ± 0.27** | 16.77 ± 0.36* | 21.80 ± 0.33** |
PD, postnatal day. The weights are reported as group means ± SEM.
p < 0.05;
p<0.001
In Experiment 2, neonatal body weights were collected on PD5, PD7 and PD9. Overall, there was a main effect of Day (F1,59 = 416.0, p < 0.001), a main effect of Treatment (F1,59 = 11.2, p < 0.001) and a Day x Treatment interaction (F1,59 = 22.0, p < 0.001). No differences in body weights were evident at the onset of the experiment. However, by PD7, alcohol-exposed mice weighed significantly less than saline-exposed mice (p < 0.001). This was still evident on PD9 (p < 0.001). A main effect of Treatment remained throughout adolescence (PD35: F1,33 = 17.4, p < 0.001; PD44: F1,33 = 10.7) and adulthood (p < 0.01; PD81: F1,33 = 11.8, p < 0.001), wherein alcohol-exposed mice weighed less than saline-exposed mice. These results suggest that PD5, 7 and 9 produced a long-lasting suppression of growth that persisted to adulthood.
3.2 Wheel Running
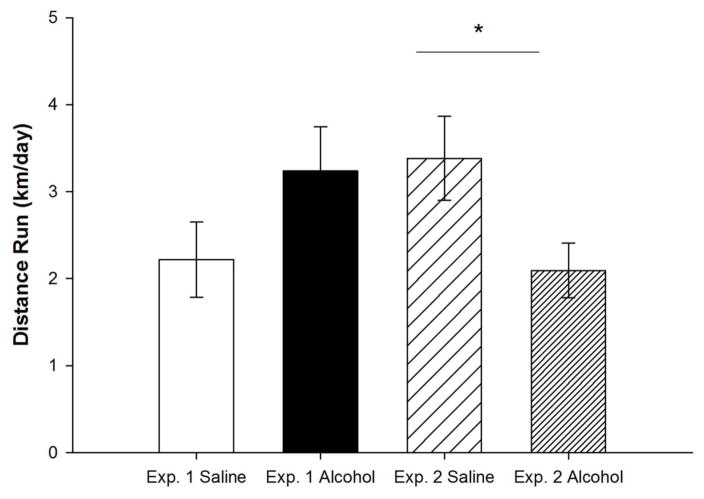
The effects of neonatal ethanol exposure on wheel running behavior depended on the experiment, as indicated by a significant Treatment by Experiment interaction (F1,29 = 6.6, p < 0.01; Fig. 2). In Experiment 1, 5 g/kg ethanol administered on PD7 had no significant influence on wheel running relative to saline controls (F1,13 = 2.0, p = 0.183), whereas in Experiment 2, 5 g/kg administered on PD5, 7 and 9 significantly decreased running (F1,16 = 5.4, p < 0.05). It is likely that the different alcohol administrations produce different FASD profiles. Whereas the single binge produces no significant effect, the repeated-binge produces a long-lasting suppression of motivation for physical activity, as suggested by the decreased running levels.
Figure 2. Wheel Running Levels.
Average daily running distance (km) of alcohol-exposed and saline-exposed mice for Experiment 1 and 2. In Experiment 2, saline-exposed mice ran significantly more than alcohol-exposed mice. All values represent mean ± SEM. *p < 0.05.
3.3 Blood Ethanol Concentrations
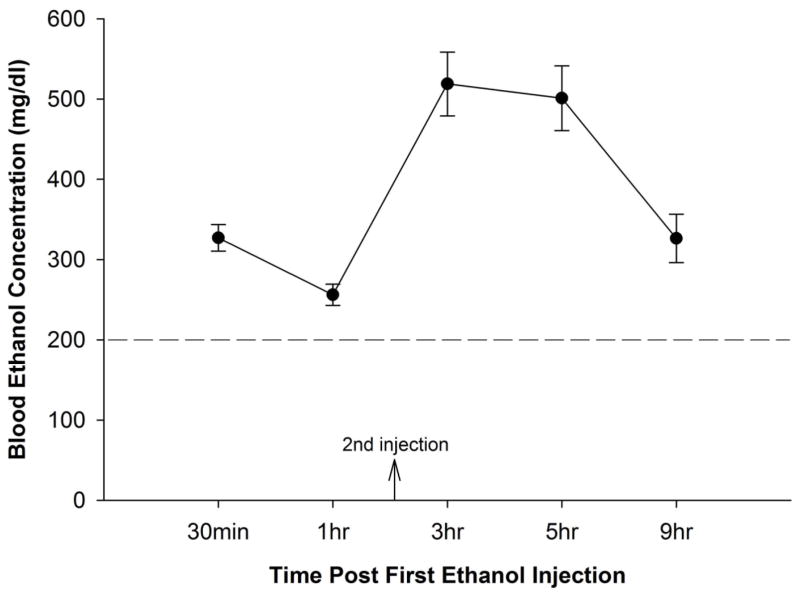
A dose of 5 g/kg of ethanol, administered by two injections two hours apart, produced significantly high and long-lasting blood ethanol concentration levels that ranged from 300–500 mg/dl at time points 3 hr, 5 hr, and 9 hr (Fig. 3). Results of the ANOVA indicated a significant effect of time point. All pairwise comparisons were different (p < 0.05) except 9 hr vs 30 min, and 5 hr vs 3 hr. These results are consistent with those of Ikonomidou and colleagues (2000).
Figure 3. Blood Ethanol Concentrations.
On PD 7, mice were administered 5 g/kg ethanol (split into two doses, two hours apart). Data points refer to the time after the first of the two injections. The blood ethanol curve illustrates that this dose is sufficient to produce blood ethanol concentrations that exceed the toxic threshold limit (200 mg/dl) for over eight hours.
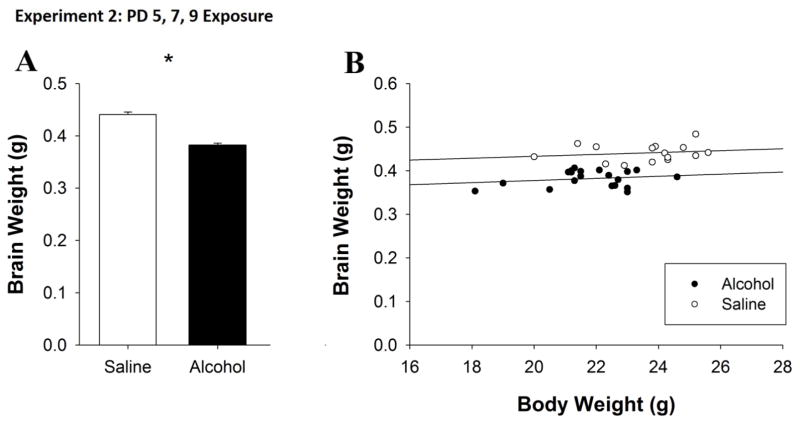
3.4 Brain Weights
In Experiment 2, following perfusion, the brains of mice were weighed in order to determine whether alcohol exposure had long-term effects on brain weight. A t-test revealed a main effect of Treatment (t33 = 9.1, p < 0.001). The brains of alcohol-exposed mice weighed significantly less than those of saline-exposed mice (Fig. 4A). This decrease was also evident in the calculated brain/body weights ratios for both Treatment groups (Saline: 0.0188g ± 0.0004g, Alcohol: 0.0176g ± 0.0003g). Further, when PD81 body mass was included as a covariate in an ANCOVA, body mass was significantly correlated with brain weight (F1,31 = 30.6, p < 0.001) and the effect of Treatment was also significant (F1,31 = 51.8, p < 0.001). These results suggest that body mass was correlated with brain weight within each group but that matched for body size, alcohol-exposed mice had reduced brain weight as compared to saline-exposed mice (Fig. 4B).
Figure 4. PD 5, 7 and 9 alcohol exposure produced long-term effects on brain weight as adults.
A. The brains of alcohol-exposed mice were significantly smaller than those of saline-exposed mice. B. When matched for body size, alcohol-exposed mice had reduced brain weight as compared to saline-exposed mice. All values represent mean ± SEM. *p < 0.05.
3.5 BrdU+ Cell Counts
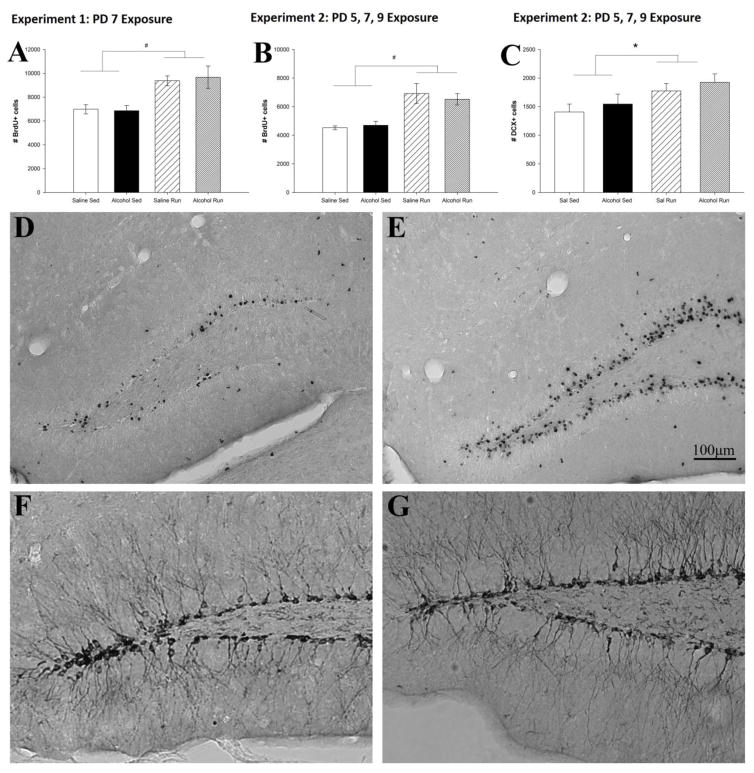
Exercise increased total number of BrdU+ cells (Fig. 5A, B). ANOVAs revealed a main effect of Intervention in Experiments 1 (F1,30 = 18.1, p < 0.001) and 2 (F1,30 = 18.1, p < 0.001) (Fig. 5D, E). Posthoc comparisons indicated the effect occurred in both alcohol (p < 0.01) and saline (p < 0.01) mice. No differences in the number of surviving BrdU+ cells between alcohol-exposed and saline-exposed mice was evident in either the running or sedentary mice.
Figure 5. Running increases levels of adult neurogenesis.
Total number of bromodeoxyuridine (BrdU) positive cells is used as an estimate of hippocampal neurogenesis. Running significantly increased the number of BrdU+ cells in alcohol-exposed and saline-exposed mice in both Experiment 1 (A) and Experiment 2 (B) as well as the number of DCX+ cells in Experiment 2 (C) Representative image of a BrdU/DAB stained coronal section from a saline-sedentary (D) and saline-runner (E). Representative image of a DCX/DAB stained coronal section of a saline-sedentary (F) and saline-runner (G). Note: differences between experiments in the number of BrdU+ cells are expected due to variation in the BrdU immunohistochemical signal to noise between batches. All values represent mean ± SEM. #p < 0.001, *p < 0.05.
Doublecortin (DCX)+ Cell Counts
Experiment 2
Exercise increased the number of immature neurons (DCX+ cells) in the dentate gyrus granule cell layer (Fig. 5C), while the PD5, 7 and 9 alcohol exposure had no impact. This was indicated by a main effect of Intervention (F1,20 = 6.4, p < 0.05), but no significant effect of Treatment or Treatment x Intervention interaction (Fig. 5F, G).
3.6 Rotarod
Experiment 1
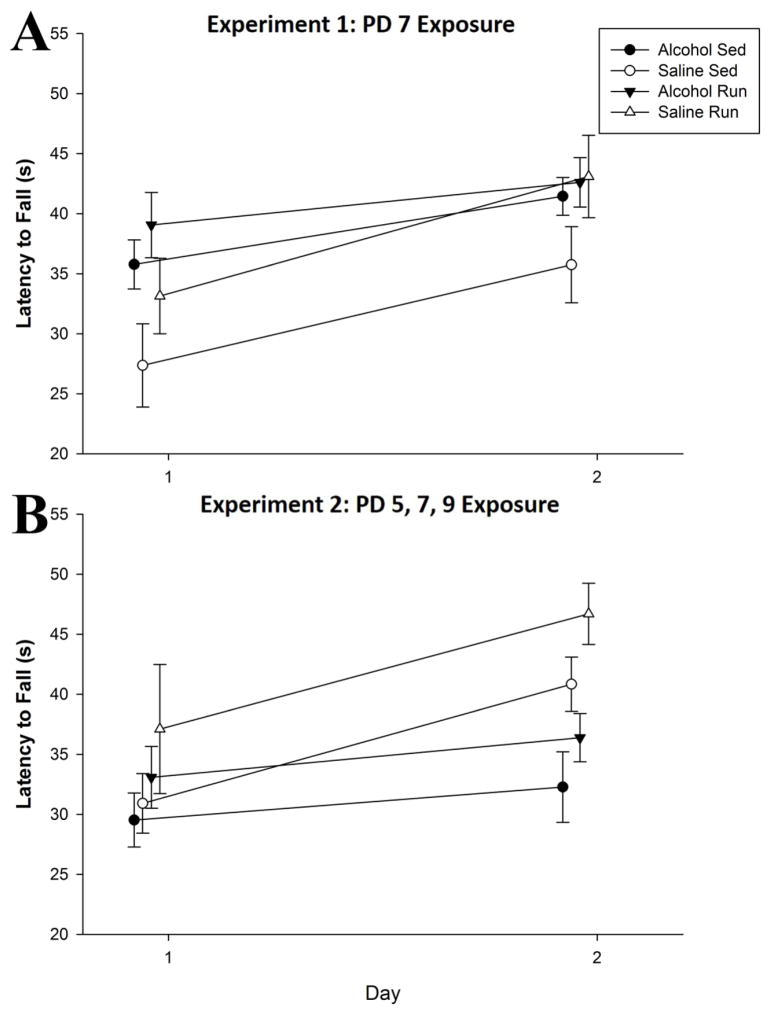
A repeated-measures ANOVA revealed a main effect of Day (F1,32 = 30.8, p < 0.01), signifying improved performance in all mice as the days progressed (Fig. 6A). There was a trend for a main effect of Intervention (p = 0.059) and Treatment (p = 0.087), but no significant Interaction (F<1).
Figure 6. Impact of neonatal alcohol exposure on Rotarod performance is dependent on pattern of administration.
A. PD 7 alcohol exposure fails to impact Rotarod performance in adulthood on both Days of Rotarod testing. Running wheel access did not improve Rotarod performance in either saline or alcohol-exposed mice. All groups displayed enhanced performance on Day 2 as compared to Day 1. B. PD 5, 7 and 9 alcohol exposure leads to long-term deficits on Rotarod performance, as alcohol-sedentary mice failed to improve across days. Access to a running wheel enhanced Rotarod performance. All values represent mean ± SEM. *p < 0.05.
Experiment 2
A repeated-measures ANOVA revealed, a main effect of Day (F1,32 = 15.3, p < 0.001) along with a Day x Treatment interaction (F1,32 = 4.3, p = 0.047), suggesting saline mice showed greater improvement in performance across days compared to their alcohol counterparts Fig. 6B). In addition, both a main effect of Treatment (F1,32 = 6.4, p = 0.017), and a main effect of Intervention (F1,32 = 4.2, p < 0.049) were observed, indicating that overall, alcohol impaired performance on the Rotarod, while runners exhibited an enhanced performance. LSD post hoc tests indicate alcohol-sedentary mice displayed worse performance than both saline-sedentary (p < 0.05) and saline-runners (p < 0.001) but not alcohol-runners. Moreover, alcohol-runners displayed worse performance than saline-runners (p < 0.01) but not saline-sedentary mice. These data suggest that a PD5, 7 and 9 alcohol exposure impairs performance on the Rotarod in adulthood, and that running can recover some of the deficit.
3.7 Passive avoidance
Experiment 1
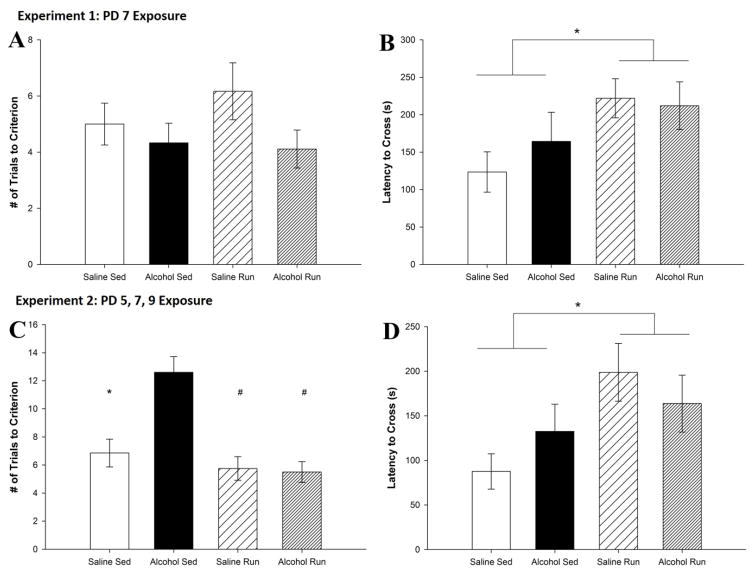
On Day 1, no significant differences between groups were observed (Fig. 7A). On Day 2, a main effect of Intervention (F1,29 = 5.0, p < 0.05) was evident (Fig. 7B), wherein all runners remembered the Passive avoidance task better as they had a greater latency to enter the dark chamber than did sedentary mice. However, neither a Treatment by Intervention nor a main effect of Treatment was found. Together, these data indicate that a single PD7 binge exposure to alcohol does not impact performance on the Passive avoidance task in adulthood, while running enhances performance regardless of neonatal treatment.
Figure 7. PD 5, 7 and 9 administration but not PD 7 alone impairs Passive avoidance performance.
A. Number of trials to reach criterion on Day 1 for Experiment 1. There were no differences between alcohol-exposed and saline-exposed mice as well as no differences between running and sedentary mice. B. Latency to cross over into the dark chamber on Day 2 for Experiment 1. Exercise significantly increased the latency to cross over for both the alcohol-exposed and the saline exposed-mice. C. Number of trials to learn the Passive avoidance task on Day 1 for Experiment 2. Alcohol exposure significantly increased the number of trials it took to reach criterion for sedentary mice. Running significantly decreased the trials to criterion for alcohol-exposed mice. D. Latency to cross over into the dark chamber on Day 2 for Experiment 2. Exercise significantly increased the latency to cross over in both the alcohol-exposed and the saline-exposed mice. All values represent mean ± SEM. *p < 0.05, #p < 0.001.
Experiment 2
On Day 1, a main effect of Treatment (F1,33 = 90.0, p < 0.01), Intervention (F1,32 = 67.0, p < 0.001) and a Treatment by Intervention interaction (F1,31 = 60.4, p < 0.01) were observed (Fig. 7C). Alcohol-sedentary mice showed a profound deficit in task acquisition, requiring a significantly higher number of trials to avoid entering the dark chamber compared to alcohol-runner (p < 0.01), saline-runner (p < 0.01) and saline-sedentary mice (p < 0.05). On Day 2, a main effect of Intervention (F1,31 = 5.4, p < 0.05) was found wherein runners remembered the Passive avoidance task better as they had a greater latency to enter the dark chamber than did sedentary mice (Fig. 7D). Neither a Treatment x Intervention nor a main effect of Treatment was evident. Overall, these data indicate that PD5, 7, and 9 alcohol exposure is sufficient to impact acquisition but not retention of the Passive avoidance task in adulthood, while running enhances retention of the task regardless of neonatal treatment.
4. DISCUSSION
These results demonstrate that a third-trimester equivalent exposure to either a single or multiple binge of alcohol has no long-lasting influence on the number of surviving BrdU+ cells in the C57BL/6J mouse model (Fig. 5). This is important given that previous literature was mixed on the issue. It is possible that the effect of neonatal alcohol on the number of surviving BrdU+ cells is strain dependent. Further, a single binge was not sufficient, while multiple binges were sufficient, to cause observable behavioral deficits in Passive avoidance and Rotarod performance (Figs. 6, 7). These data support the use of a three-binge model in C57BL/6J mice for inducing reliable behavioral deficits on these tasks. Finally, the data support the idea that exercise can ameliorate alcohol-induced cognitive deficits, and that this occurs in concert with increased adult hippocampal neurogenesis. However, because neonatal alcohol exposure did not impair adult hippocampal neurogenesis, these results suggest that effects of exercise provide global compensation rather than repair specific damages induced from neonatal alcohol exposure.
Results of our study suggest that effects of neonatal alcohol exposure on the number of surviving BrdU+ cells and immature neurons (DCX+ cells) may be much smaller and dependent on methodological differences than originally hypothesized. The vast majority of surviving cells in the hippocampal dentate gyrus are neurons (Clark et al., 2009; Helfer et al., 2009; Holmes et al., 2004; van Praag et al., 1999; van Praag et al., 2005), while less than 10% are glial cells (Colcombe et al., 2004; Crews et al., 2006; Hamilton et al., 2015). Research suggests that these percentages are not influenced by developmental alcohol exposure (Choi et al., 2005; Helfer et al., 2009). Therefore, we conclude that neonatal alcohol exposure (both single and multiple binges) did not impact levels of adult hippocampal neurogenesis (Fig. 5). These data are consistent with a growing number of studies in rodents (Gil-Mohapel et al., 2011; Helfer et al., 2009; Klintsova et al., 2007; Patten et al., 2013; Wozniak et al., 2004). However, other studies implicate neonatal alcohol exposure as the cause of reduced cell survival (Hamilton et al., 2012; Hamilton et al., 2014; Hamilton et al., 2011; Ieraci and Herrera, 2007; Klintsova et al., 2007). We now interpret these differences as likely related to strain, age or method of measuring neurogenesis. No effect was observed in C57BL/6J mice (Wozniak et al., 2004), while CD1 mice exhibited a deficit (Ieraci and Herrera, 2007). Prenatal ethanol significantly decreased levels of adult hippocampal neurogenesis in adult but not adolescent rats (Gil-Mohapel et al., 2014). In studies from the same lab, multiple injections (Helfer et al., 2009) versus a single injection of BrdU (Hamilton et al., 2012; Hamilton et al., 2014; Hamilton et al., 2011) produce differing results. Together, these data suggest that the impact of neonatal alcohol exposure on levels of adult hippocampal neurogenesis may not be as robust as originally hypothesized and the differences in the literature could simply be due to idiosyncratic methodological differences.
In rodent models of FASD, different binge schedules influence behavioral outcome and we wanted to determine the appropriate binge schedule for inducing reliable behavioral deficits on the Passive avoidance and Rotarod tasks, because each of these tasks involves a different subset of vulnerable neuronal substrates. What we discovered was that a multiple-binge produced behavioral deficits in both tasks whereas the single binge had no effect (Fig. 6B; 7A, C). Other multiple-binge paradigms have been shown to impair performance on Trace conditioning (Murawski et al., 2013), Morris water maze (Goodlett and Johnson, 1997) and Rotarod (Klintsova et al., 1998) in rodents. Wagner and colleagues (2014) demonstrated a single PD7 binge alcohol exposure produced learning deficits in PD70-79 using the Morris water maze task in C57BL/6J mice. However, a previous study found this only to be true in adolescents not adults (Wozniak et al., 2004). It is possible that the Morris water maze involves a different subset of neuronal substrates than either the Passive avoidance or Rotarod tasks do individually. In any case, results from our study suggest that the three-binge model is better suited for detecting behavioral deficits on the Passive avoidance and Rotarod tasks.
The Passive avoidance task is useful in its design: to successfully perform the task, the animal must learn to not move. It does not require the animal to be able to swim successfully or run smoothly, but rather the animal just needs to stay in the same place. Therefore, it could be hypothesized that animals with impaired motor coordination would be, by default, good at this task. Yet, our results show that despite an impaired performance on the Rotarod task, PD 5, 7 and 9 alcohol-exposed mice require a higher number of trials to reach criterion on the Passive avoidance task (i.e. exhibit increased motor activity). This suggests, that the behavioral deficits exhibited on the Passive avoidance task are not the result of any motor impairment but rather are consistent with the hallmark FASD features of increased hyperactivity and attention deficits.
Voluntary wheel running was an effective intervention. Not only did it increase adult neurogenesis (Fig. 5) and improve motor coordination on the Rotarod (Fig. 6B), but it also enhanced performance on the Passive avoidance task (Fig. 7). The beneficial influence of voluntary exercise has been well established in the healthy brain. Motor coordination and learning on the Rotarod are likely related to exercise-induced changes in cerebellum (Cotman and Berchtold, 2002; Isaacs et al., 1992). Passive avoidance requires an animal to learn and remember an association between a behavioral action, moving to the dark side of the chamber, with an aversive stimulus, the electric shock. This type of spatial and associative learning and memory involves the hippocampus, and potentially hippocampal neurogenesis (van Praag et al., 1999). However, if increased neurogenesis was involved, our data suggest the effect was through compensation rather than repair because the alcohol-exposed mice did not show impaired neurogenesis.
Alternatively, behavioral improvements from exercise in our study may have nothing to do with neurogenesis. Exercise upregulates a number of trophic factors throughout the brain (Cotman and Berchtold, 2002; Rasmussen et al., 2009; Vaynman and Gomez-Pinilla, 2005). These trophic factors promote the differentiation, neurite extension and survival of a variety of neuronal populations in many different brain regions, not only the hippocampus (Colcombe et al., 2004; Cotman and Berchtold, 2002; Rasmussen et al., 2009). Exercise promotes cerebellar plasticity. In mice raised in an environment with continual access to free running the dendritic trees of cerebellar Purkinje cells are significantly more complex and contain a greater number of spines when compared to those of their control littermates (Pysh and Weiss, 1979). Further, complex motor skill learning has been associated with increased numbers of parallel fiber to Purkinje cell synapses (Kleim et al., 1998) as well as increased BDNF expression in the cerebellum (Klintsova et al., 2004). Therefore, it is likely that exercise ameliorated deficits in the Rotarod task by affecting plasticity in the cerebellum. Similarly, Passive avoidance performance could have been improved via changes in the prefrontal cortex (Kemble and Tapp, 1968; Walsh et al., 1984). In addition to learning and remembering the association, Passive avoidance requires the mouse to suppress the innate desire to cross over to the dark side, and a prominent brain region involved in behavioral inhibition is the prefrontal cortex. Third-trimester equivalent alcohol exposure is known to impact prefrontal anatomy (Hamilton et al., 2015; Hamilton et al., 2010; Lawrence et al., 2012). Prenatal alcohol exposure induced both significant cell loss in the prefrontal cortex and impaired reversal learning on a go/no-go procedure in rats (Mihalick et al., 2001). Additionally, heavy prenatal alcohol exposure impaired performance on response-inhibition tasks in humans (Fryer et al., 2007; Ware et al., 2015). More recently, exercise has been shown to partially reverse neonatal alcohol-induced deficits in the morphology of prefrontal Layer II/III pyramidal neurons (Hamilton et al., 2015). Therefore, it is possible that exercise rescued Passive avoidance deficits in our study by repairing morphological deficits in multiple brain regions.
It is important to acknowledge certain limitations within this study. First, the model itself brings with it certain caveats. Undoubtedly, there is a mild form of neonatal stress through the alcohol administration. However, to our knowledge, this is true of all the models. In the commonly used pair-feeding method, alcohol-treated dams eat ad libatum, while their pair-fed counterparts are calorie restricted, thus introducing a stress component (Hellemans et al., 2010). Further gastric intubation of pups is inherently stressful, given the inclusion of the sham-intubated group, which serves as a stress control. Moreover, the gastric intubation is typically done in rats, it is exceedingly challenging in mice given the small size of the pups. Thus, there does not appear to be one model that is one hundred percent stress free. Second, given the need to record individual running levels, mice were singly housed. This brings with it certain caveats as studies have shown that single housing can induce stress responses in both rats (Chappell et al., 2013) and mice (Berry et al., 2012). For example, single housing during adolescence not only reduced the explorative behavior of CD-1 female mice but also their locomotor activity was increased (Haupt and Schaefers, 2010). Further, single housing in C57BL/6J adult male mice increased anxiety and depressive-like behaviors and also reduced BDNF levels, notably in the frontal cortex (Berry et al., 2012). However, a growing literature suggests that single housing may not be stressful for mice. Studies demonstrate that single housing does not increase stress markers in male mice compared with group housing (Arndt et al., 2009; Hunt and Hambly, 2006). In fact, one study found that group-housed male mice actually exhibited significantly higher corticosterone levels than did their single-housed counterparts (Kamakura et al., 2016). Overall, the literature suggests that the impact of single housing is not ideal, but important nonetheless in this animal model, given the need to record individual running levels. Third, clearly the PD 5, 7 and 9 alcohol-exposed pups suffered some undernutrition during the alcohol exposure procedure given the short-term reduction in body weight, which in this case extended all the way into adulthood. It is possible that the short-term under nourishment contributed to the long lasting effects observed for body mass, brain mass and behavior. Under nourishment would have occurred for both temporary loss of macro and micronutrients during a period of massive growth. One well-studied essential nutrient during development is choline. Choline-deficient pups exhibit impaired learning and memory when tested later in life (Fernandes de Abreu et al., 2010; Moreno et al., 2013; Peak et al., 2015). Interestingly, choline supplementation consistently has been shown to attenuate behavioral deficits in rat pups exposed to fetal alcohol (Ryan et al., 2008; Schneider and Thomas, 2016; Thomas and Tran, 2012; Zhu et al., 2016). Therefore, some of the deficits we observed from our neonatal alcohol treatment could have exerted their influence through under nourishment rather than pharmacological effects directly related to ethanol. This is not necessarily a limitation of the model but an important consideration since many fetal alcohol babies are born underweight (Larroque et al., 1993; May et al., 2009; May et al., 2014), and the low birth weights may be attributed in part to the moms getting most of their calories from alcohol (May et al., 2014).
5. CONCLUSION
Together, these results illustrate that PD 5, 7 and 9 alcohol exposure causes behavioral deficits on both the Passive avoidance and the Rotarod task while having no impact on adult hippocampal neurogenesis levels in the C57BL/6J mouse model. Passive avoidance is a unique behavioral task that can assess multiple neural substrates, while requiring minimum strenuous activity from the animal to ensure the results do not simply reflect general motor impairments. In the current study, despite performing poorly on the Rotarod task, the alcohol-exposed animals required significantly more trials to learn the Passive Avoidance task, suggesting this was a learning not a motor deficit. In addition, these data illustrate the potential utility of exercise as a therapeutic intervention for both fetal alcohol-induced motor and cognitive deficits. It is possible that the beneficial effects of exercise on behavioral performance could be partially mediated by increased neurogenesis via compensation but not repair because neurogenesis was not impaired from alcohol. However, it is well established that exercise has many effects on the healthy and diseased brain. Hence, future research should explore alternative mechanisms (i.e. potential repair of prefrontal cortex and cerebellar deficits) as mechanisms underlying behavioral recovery from early postnatal exposure to multiple alcohol binges.
Research Highlights.
PD5, 7 and 9 but not PD5 alcohol exposure impairs passive avoidance acquisition.
PD5, 7 and 9 but not PD5 alcohol exposure impairs rotarod performance.
Neonatal alcohol exposure does not impact adult neurogenesis levels.
Acknowledgments
Funding: This research was supported by Justin Rhodes’ lab at the University of Illinois at Urbana-Champaign and funding from NIH 1 R01 MH083807 (JSR) and NIH 1 RO1 DA027487 (JSR). Additionally, support was provided by the Arnold O. and Mabel M. Beckman Foundation through the Beckman Institute Postdoctoral Fellowship Program at the University of Illinois at Urbana-Champaign (GFH).
This research was supported by Justin Rhodes’ lab at the University of Illinois at Urbana-Champaign and funding from NIH 1 R01 MH083807 (JSR) and NIH 1 RO1 DA027487 (JSR). Additionally, support was provided by the Arnold O. and Mabel M. Beckman Foundation through the Beckman Institute Postdoctoral Fellowship Program at the University of Illinois at Urbana-Champaign (GFH). The authors would like to extend their sincere gratitude to the Beckman Institute Animal Facility staff for excellent animal care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel EL. In utero alcohol exposure and developmental delay of response inhibition. Alcohol Clin Exp Res. 1982;6(3):369–76. doi: 10.1111/j.1530-0277.1982.tb04993.x. [DOI] [PubMed] [Google Scholar]
- Arndt SS, Laarakker MC, van Lith HA, van der Staay FJ, Gieling E, Salomons AR, van’t Klooster J, Ohl F. Individual housing of mice--impact on behaviour and stress responses. Physiol Behav. 2009;97(3–4):385–93. doi: 10.1016/j.physbeh.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Barron S, Riley EP. Passive avoidance performance following neonatal alcohol exposure. Neurotoxicol Teratol. 1990;12(2):135–8. doi: 10.1016/0892-0362(90)90125-v. [DOI] [PubMed] [Google Scholar]
- Becker HC, Randall CL. Effects of prenatal ethanol exposure in C57BL mice on locomotor activity and passive avoidance behavior. Psychopharmacology (Berl) 1989;97(1):40–4. doi: 10.1007/BF00443410. [DOI] [PubMed] [Google Scholar]
- Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, Cirulli F. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology. 2012;37(6):762–72. doi: 10.1016/j.psyneuen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17(10):2042–6. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Calhoun F, Attilia ML, Spagnolo PA, Rotondo C, Mancinelli R, Ceccanti M. National Institute on Alcohol Abuse and Alcoholism and the study of fetal alcohol spectrum disorders. The International Consortium. Ann Ist Super Sanita. 2006;42(1):4–7. [PubMed] [Google Scholar]
- Chappell AM, Carter E, McCool BA, Weiner JL. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcohol Clin Exp Res. 2013;37(Suppl 1):E394–403. doi: 10.1111/j.1530-0277.2012.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi IY, Allan AM, Cunningham LA. Moderate fetal alcohol exposure impairs the neurogenic response to an enriched environment in adult mice. Alcohol Clin Exp Res. 2005;29(11):2053–62. doi: 10.1097/01.alc.0000187037.02670.59. [DOI] [PubMed] [Google Scholar]
- Christie BR, Swann SE, Fox CJ, Froc D, Lieblich SE, Redila V, Webber A. Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur J Neurosci. 2005;21(6):1719–26. doi: 10.1111/j.1460-9568.2005.04004.x. [DOI] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19(10):937–50. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155(4):1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci U S A. 2004;101(9):3316–21. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Dev Neuropsychol. 2000;18(3):331–54. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137(2):437–45. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Fernandes de Abreu DA, Nivet E, Baril N, Khrestchatisky M, Roman F, Feron F. Developmental vitamin D deficiency alters learning in C57Bl/6J mice. Behav Brain Res. 2010;208(2):603–8. doi: 10.1016/j.bbr.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Fryer SL, Tapert SF, Mattson SN, Paulus MP, Spadoni AD, Riley EP. Prenatal alcohol exposure affects frontal-striatal BOLD response during inhibitory control. Alcohol Clin Exp Res. 2007;31(8):1415–24. doi: 10.1111/j.1530-0277.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: insights from different rodent models. Brain Res Rev. 2010;64(2):283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Patten A, Cox A, Kainer L, Giles E, Brocardo PS, Christie BR. Altered adult hippocampal neuronal maturation in a rat model of fetal alcohol syndrome. Brain Res. 2011;1384:29–41. doi: 10.1016/j.brainres.2011.01.116. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Titterness AK, Patten AR, Taylor S, Ratzlaff A, Ratzlaff T, Helfer J, Christie BR. Prenatal ethanol exposure differentially affects hippocampal neurogenesis in the adolescent and aged brain. Neuroscience. 2014;273:174–88. doi: 10.1016/j.neuroscience.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19(6):435–46. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Hamilton GF, Boschen KE, Goodlett CR, Greenough WT, Klintsova AY. Housing in environmental complexity following wheel running augments survival of newly generated hippocampal neurons in a rat model of binge alcohol exposure during the third trimester equivalent. Alcohol Clin Exp Res. 2012;36(7):1196–204. doi: 10.1111/j.1530-0277.2011.01726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Criss KJ, Klintsova AY. Voluntary exercise partially reverses neonatal alcohol-induced deficits in mPFC layer II/III dendritic morphology of male adolescent rats. Synapse. 2015;69(8):405–15. doi: 10.1002/syn.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Jablonski SA, Schiffino FL, St Cyr SA, Stanton ME, Klintsova AY. Exercise and environment as an intervention for neonatal alcohol effects on hippocampal adult neurogenesis and learning. Neuroscience. 2014;265:274–90. doi: 10.1016/j.neuroscience.2014.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Murawski NJ, St Cyr SA, Jablonski SA, Schiffino FL, Stanton ME, Klintsova AY. Neonatal alcohol exposure disrupts hippocampal neurogenesis and contextual fear conditioning in adult rats. Brain Res. 2011;1412:88–101. doi: 10.1016/j.brainres.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure decreases dendritic complexity while increasing the density of mature spines in mPFC Layer II/III pyramidal neurons. Synapse. 2010;64(2):127–35. doi: 10.1002/syn.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haupt M, Schaefers AT. Effects of postweaning social and physical deprivation on locomotor activity patterns and explorative behavior in female CD-1 mice. Dev Psychobiol. 2010;52(4):383–93. doi: 10.1002/dev.20439. [DOI] [PubMed] [Google Scholar]
- Helfer JL, Goodlett CR, Greenough WT, Klintsova AY. The effects of exercise on adolescent hippocampal neurogenesis in a rat model of binge alcohol exposure during the brain growth spurt. Brain Res. 2009;1294:1–11. doi: 10.1016/j.brainres.2009.07.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellemans KG, Sliwowska JH, Verma P, Weinberg J. Prenatal alcohol exposure: fetal programming and later life vulnerability to stress, depression and anxiety disorders. Neurosci Biobehav Rev. 2010;34(6):791–807. doi: 10.1016/j.neubiorev.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res. 2004;76(2):216–22. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- Hunt C, Hambly C. Faecal corticosterone concentrations indicate that separately housed male mice are not more stressed than group housed males. Physiol Behav. 2006;87(3):519–26. doi: 10.1016/j.physbeh.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Single alcohol exposure in early life damages hippocampal stem/progenitor cells and reduces adult neurogenesis. Neurobiol Dis. 2007;26(3):597–605. doi: 10.1016/j.nbd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287(5455):1056–60. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12(1):110–9. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- Kamakura R, Kovalainen M, Leppaluoto J, Herzig KH, Makela KA. The effects of group and single housing and automated animal monitoring on urinary corticosterone levels in male C57BL/6 mice. Physiol Rep. 2016;4(3) doi: 10.14814/phy2.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemble ED, Tapp JT. Passive and Active Avoidance Performance Following Small Amygdaloid Lesions in Rats. Physiology & Behavior. 1968;3(5):713-&. [Google Scholar]
- Kleim JA, Swain RA, Armstrong KA, Napper RM, Jones TA, Greenough WT. Selective synaptic plasticity within the cerebellar cortex following complex motor skill learning. Neurobiol Learn Mem. 1998;69(3):274–89. doi: 10.1006/nlme.1998.3827. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Cowell RM, Swain RA, Napper RM, Goodlett CR, Greenough WT. Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge-like alcohol exposure in rats. I. Behavioral results. Brain Res. 1998;800(1):48–61. doi: 10.1016/s0006-8993(98)00495-8. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028(1):92–104. doi: 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Klintsova AY, Helfer JL, Calizo LH, Dong WK, Goodlett CR, Greenough WT. Persistent impairment of hippocampal neurogenesis in young adult rats following early postnatal alcohol exposure. Alcohol Clin Exp Res. 2007;31(12):2073–82. doi: 10.1111/j.1530-0277.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- Lantz CL, Wang W, Medina AE. Early alcohol exposure disrupts visual cortex plasticity in mice. Int J Dev Neurosci. 2012;30(5):351–7. doi: 10.1016/j.ijdevneu.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larroque B, Kaminski M, Lelong N, Subtil D, Dehaene P. Effects of birth weight of alcohol and caffeine consumption during pregnancy. Am J Epidemiol. 1993;137(9):941–50. doi: 10.1093/oxfordjournals.aje.a116764. [DOI] [PubMed] [Google Scholar]
- Lawrence RC, Otero NK, Kelly SJ. Selective effects of perinatal ethanol exposure in medial prefrontal cortex and nucleus accumbens. Neurotoxicol Teratol. 2012;34(1):128–35. doi: 10.1016/j.ntt.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25(4):447–58. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Saboureau M, Pevet P. Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proc Natl Acad Sci U S A. 2006;103(49):18775–80. doi: 10.1073/pnas.0608785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15(3):176–92. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- May PA, Hamrick KJ, Corbin KD, Hasken JM, Marais AS, Brooke LE, Blankenship J, Hoyme HE, Gossage JP. Dietary intake, nutrition, and fetal alcohol spectrum disorders in the Western Cape Province of South Africa. Reprod Toxicol. 2014;46:31–9. doi: 10.1016/j.reprotox.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalick SM, Crandall JE, Langlois JC, Krienke JD, Dube WV. Prenatal ethanol exposure, generalized learning impairment, and medial prefrontal cortical deficits in rats. Neurotoxicol Teratol. 2001;23(5):453–62. doi: 10.1016/s0892-0362(01)00168-4. [DOI] [PubMed] [Google Scholar]
- Moreno HC, de Brugada I, Carias D, Gallo M. Long-lasting effects of prenatal dietary choline availability on object recognition memory ability in adult rats. Nutr Neurosci. 2013;16(6):269–74. doi: 10.1179/1476830513Y.0000000055. [DOI] [PubMed] [Google Scholar]
- Murawski NJ, Jablonski SA, Brown KL, Stanton ME. Effects of neonatal alcohol dose and exposure window on long delay and trace eyeblink conditioning in juvenile rats. Behav Brain Res. 2013;236(1):307–18. doi: 10.1016/j.bbr.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16(3):250–60. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- Patten AR, Fontaine CJ, Christie BR. A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front Pediatr. 2014;2:93. doi: 10.3389/fped.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten AR, Moller DJ, Graham J, Gil-Mohapel J, Christie BR. Liquid diets reduce cell proliferation but not neurogenesis in the adult rat hippocampus. Neuroscience. 2013;254:173–84. doi: 10.1016/j.neuroscience.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Peak JN, Turner KM, Burne TH. The effect of developmental vitamin D deficiency in male and female Sprague-Dawley rats on decision-making using a rodent gambling task. Physiol Behav. 2015;138:319–24. doi: 10.1016/j.physbeh.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Pysh JJ, Weiss GM. Exercise during development induces an increase in Purkinje cell dendritic tree size. Science. 1979;206(4415):230–2. doi: 10.1126/science.482938. [DOI] [PubMed] [Google Scholar]
- Rasmussen C. Executive functioning and working memory in fetal alcohol spectrum disorder. Alcohol Clin Exp Res. 2005;29(8):1359–67. doi: 10.1097/01.alc.0000175040.91007.d0. [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94(10):1062–9. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- Redila VA, Olson AK, Swann SE, Mohades G, Webber AJ, Weinberg J, Christie BR. Hippocampal cell proliferation is reduced following prenatal ethanol exposure but can be rescued with voluntary exercise. Hippocampus. 2006;16(3):305–11. doi: 10.1002/hipo.20164. [DOI] [PubMed] [Google Scholar]
- Riley EP, Lochry EA, Shapiro NR. Lack of response inhibition in rats prenatally exposed to alcohol. Psychopharmacology (Berl) 1979;62(1):47–52. doi: 10.1007/BF00426034. [DOI] [PubMed] [Google Scholar]
- Ryan SH, Williams JK, Thomas JD. Choline supplementation attenuates learning deficits associated with neonatal alcohol exposure in the rat: effects of varying the timing of choline administration. Brain Res. 2008;1237:91–100. doi: 10.1016/j.brainres.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RD, Thomas JD. Adolescent Choline Supplementation Attenuates Working Memory Deficits in Rats Exposed to Alcohol During the Third Trimester Equivalent. Alcohol Clin Exp Res. 2016;40(4):897–905. doi: 10.1111/acer.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JD, Tran TD. Choline supplementation mitigates trace, but not delay, eyeblink conditioning deficits in rats exposed to alcohol during development. Hippocampus. 2012;22(3):619–30. doi: 10.1002/hipo.20925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25(38):8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Gomez-Pinilla F. License to run: exercise impacts functional plasticity in the intact and injured central nervous system by using neurotrophins. Neurorehabil Neural Repair. 2005;19(4):283–95. doi: 10.1177/1545968305280753. [DOI] [PubMed] [Google Scholar]
- Wagner JL, Zhou FC, Goodlett CR. Effects of one- and three-day binge alcohol exposure in neonatal C57BL/6 mice on spatial learning and memory in adolescence and adulthood. Alcohol. 2014;48(2):99–111. doi: 10.1016/j.alcohol.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TJ, Tilson HA, DeHaven DL, Mailman RB, Fisher A, Hanin I. AF64A, a cholinergic neurotoxin, selectively depletes acetylcholine in hippocampus and cortex, and produces long-term passive avoidance and radial-arm maze deficits in the rat. Brain Res. 1984;321(1):91–102. doi: 10.1016/0006-8993(84)90684-x. [DOI] [PubMed] [Google Scholar]
- Ware AL, Infante MA, O’Brien JW, Tapert SF, Jones KL, Riley EP, Mattson SN. An fMRI study of behavioral response inhibition in adolescents with and without histories of heavy prenatal alcohol exposure. Behav Brain Res. 2015;278:137–46. doi: 10.1016/j.bbr.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, Young C, Olney JW, Muglia LJ. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol Dis. 2004;17(3):403–14. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Zhu CH, Wu T, Jin Y, Huang BX, Zhou RF, Wang YQ, Luo XL, Zhu HL. Prenatal choline supplementation attenuates spatial learning deficits of offspring rats exposed to low-protein diet during fetal period. J Nutr Biochem. 2016;32:163–70. doi: 10.1016/j.jnutbio.2015.09.003. [DOI] [PubMed] [Google Scholar]