Abstract
Background and Purpose
The American Heart Association (AHA) developed the Ideal-Cardiovascular Health (CVH) index as a simple tool to promote cardiovascular health, yet its association with brain atrophy and dementia remains unexamined.
Methods
Our aim was to investigate the prospective association of Ideal-CVH with vascular brain injury, including the 10-year risks of incident stroke and dementia, as well as cognitive decline and brain atrophy on magnetic resonance imaging, measured over approximately 7 years. We studied 2,750 stroke- and dementia-free Framingham Heart Study Offspring Cohort participants (mean age 62±9 years; 45% men). Ideal-CVH was quantified on a 7-point scale with 1 point awarded for each of the following; non-smoking status, ideal body mass index, regular physical activity, healthy diet, as well as optimum blood pressure, cholesterol, and fasting blood glucose. Both recent (baseline) and remote (6.9 years earlier) Ideal-CVH scores were examined.
Results
Recent Ideal-CVH was associated with stroke (Hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.67-0.95), vascular dementia (HR, 0.49; 95% CI, 0.30-0.81), frontal brain atrophy (p=.003), and cognitive decline on tasks measuring visual memory and reasoning (p < .05). In addition to predicting stroke, vascular dementia, whole brain atrophy, and cognitive decline, remote Ideal-CVH was associated with the incidence of all-cause dementia (HR, 0.80; 95% CI, 0.67-0.97) and Alzheimer's disease (HR, 0.79; 95% CI, 0.64-0.98).
Conclusions
Adherence to the AHA's Ideal-CVH factors and behaviours, particularly in midlife, may protect against cerebrovascular disease and dementia.
Keywords: American Heart Association, brain, cerebrovascular disorders, epidemiology, risk factors
Introduction
The global prevalence of dementia is expected to double nearly every 20 years, reaching 115 million by 2050.1 In addition to the toll taken on individuals and families, the health care costs relating to dementia have the potential to devastate economies, with yearly costs estimated to exceed 1 trillion by 2050 in the US alone.2 Even in the absence of overt dementia, cognitive impairment and cerebrovascular disease are common among the elderly.3, 4 Considering the rapid increase in life expectancies, there is a pressing need to protect the brain from disease.
Modifiable vascular risk factors are established predictors of stroke and cerebrovascular disease.5 There is now growing consensus that vascular risk factors such as physical inactivity, smoking, hypertension, and obesity are also associated with the risk of cognitive decline and dementia, including its most common form, Alzheimer's disease (AD).6 Recent estimates suggest that a third of all AD cases might be due to modifiable risk factors.6 Therefore, further advocating for vascular health may, in turn, promote healthy brain aging, helping to ease the burden of AD and all forms of vascular cognitive impairment.
The American Heart Association (AHA) has proposed and advocated for the use of a simple Ideal-Cardiovascular Health (CVH) metric.7 The metric pools together 7 CVH behaviours and factors, assigning 1 point for each factor at its ideal status; with higher scores predictive of less heart disease8, 9 and stroke.9, 10 Given that the brain is vulnerable to damage in the face of vascular risk factors,11 adhering to these simple Ideal-CVH guidelines may confer protection against vascular brain injury and consequently cognitive decline and dementia. However, to our knowledge, the Ideal-CVH score is yet to be examined with respect to incident dementia. Developing a single set of guidelines to protect against heart disease, stroke and dementia may have considerable public health implications. The aim of the present study was to examine whether greater adherence to AHA Ideal-CVH guidelines was associated with a lower risk of incident stroke, incident dementia, cognitive decline and brain atrophy among participants of the Framingham Heart Study Offspring cohort.
MATERIALS AND METHODS
Study cohort
The community-based Framingham Heart Study Offspring cohort commenced in 1971 with the recruitment of 5124 individuals.12 The Offspring cohort has since been examined 9 times, with the latest examination cycle ending in 2014. The study was originally established to define risk factors for heart disease and stroke but, more recently, a major aim of the study has been to define risk factors for neurodegenerative disease.13
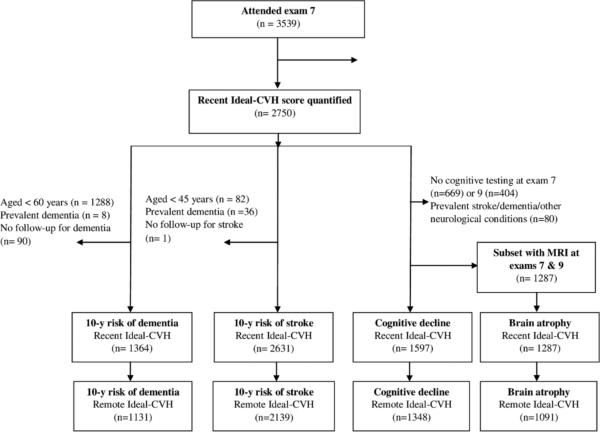
As depicted in Figure 1, we created overlapping samples enabling investigation of the 10-year risk of incident stroke and dementia (including dementia sub-type), beginning from examination cycle 7 (1998-2001) onwards. For the analysis of incident stroke, we excluded participants under the age of 45 years, those with prevalent stroke at exam 7, and those with no follow-up for stroke leaving a sample of 2,631. For the analysis of incident dementia, we excluded participants under the age of 60 years, those with prevalent dementia at exam 7, and those with no follow-up for dementia leaving 1,364 participants. We also created samples to examine the association of Ideal-CVH with the progression of cognitive decline (n= 1597) as well as brain atrophy and white matter injury on MRI of the brain (n= 1287). Changes in the neuropsychological and MRI outcomes were examined over approximately 7 years, from exam 7 onwards. All participants provided written informed consent and the study was approved by the Institutional Review Board and Boston University Medical Center.
Figure 1.
Study flow diagram
Primary exposure – the Ideal-CVH score
Ideal-CVH scores were calculated according to AHA guidelines7 by summing together 7 dichotomous variables with zero reflecting poor and 7 Ideal-CVH status. One point was awarded for each of the following; current self-reported non-smoker, body mass index < 25 and > 18.5Kg/m2, adequate physical activity, a healthy diet, untreated total cholesterol <200mg/dL, untreated resting blood pressure <120/<80 mmHg, and fasting blood glucose <100 mg/dL. All factors were obtained from the Framingham Heart Study clinic except diet and physical activity, which were measured with the use of a validated food frequency and physical activity index questionnaire, respectively. Blood pressures were measured twice by a physician, with the average systolic and diastolic value used in the Ideal-CVH score. See Supplemental Table I in the online-only Data Supplement for complete details of how Ideal-CVH scores were calculated, including quantification of diet and physical activity. Given that certain vascular risk factors have an age-dependent association with dementia and cognitive function,14 we calculated both recent (exam 7; 1998-2001) and remote (exam 5; 1991-1995) Ideal-CVH scores.
Assessment of incident stroke and dementia
We examined whether recent and remote Ideal-CVH were associated with the 10-year risk of stroke, all-cause dementia, clinically characterized AD and vascular dementia (VaD), beginning from exam 7. Follow-up occurred over a maximum of 10 years from the baseline exam to the time of incident event. Follow-up time for participants with no incident events was censored at the last time they were known to be event free, again through to a maximum of 10 years. There were 27, 944 person-years for stroke and 11, 386 person-years for dementia.
We assessed the incidence of stroke by monitoring hospital admissions in Framingham and by reviewing all available medical records and results. Stroke was defined as focal neurological symptoms of rapid onset and presumed vascular origin, lasting more than 24 hours or resulting in death within 24 hours. A diagnosis of dementia was made in accordance with the Diagnostic and Statistical Manual of Mental Disorders, 4th edition.15 Type of dementia was also recorded (please see the Supplemental Methods online for details on the assessment of incident stroke and incident dementia).
Assessment of cognitive decline
Cognitive decline was assessed as annualized change over a mean follow-up of 7 years, beginning from exam 7. We examined a battery of validated neuropsychological testssensitive to vascular brain injury as well as 1 verbal memory task, sensitive to deficits typically observed in AD.16 Our battery included Visual Reproductions Delayed and Logical Memory Delayed from the Wechsler Memory Scale, Similarities from the Wechsler Adult Intelligence Scale, Trail Making A and B as well as a global measure of cognitive decline derived from principal component analysis (please see Supplemental Methods and Supplemental Table II online). All cognitive tests were treated such that higher scores reflected less decline in performance.
Assessment of brain atrophy and white matter injury
We examined annualized change in brain atrophy and white matter injury on MRI, also over a mean follow-up of 7 years, from exam 7 onwards. We examined measures of total brain volume, frontal brain volume, lateral ventricular volume, and white matter hyperintensity volume (WMHV). Total brain volume was calculated as the total brain parenchymal volume. Lateral ventricular volumes were calculated by analyzing central cerebrospinal fluid spaces, excluding the temporal horn. We used a Siemens 1T or 1.5T field strength machine with a T2-weighted double spin-echo coronal imaging sequence in contiguous slices of 4mm. Full details of the imaging methodology are published elsewhere.17 Analysis of MRI images was completed by a Neurologist (CD), blind to subject demographics as well as Ideal-CVH scores.
Statistical analysis
Results were analysed using SAS Software (SAS Institute, Cary, N.C.). The assumption of proportionality of hazards was examined and confirmed by including terms for interaction with time. We used multivariable Cox proportional hazards regression models to examine the association between Ideal-CVH and incident stroke, all-cause dementia, AD, and VaD. For these analyses, Hazard Ratios (HR) are presented accompanied by 95% CIs. The associations between Ideal-CVH and the annualized change in each MRI and neuropsychological outcome were examined using linear regression. All models were adjusted for age at exam 7 and sex. Models involving dementia and Alzheimer's disease were additionally adjusted for education. Models involving the MRI outcomes were additionally adjusted for age squared and baseline (exam 7) scores on the respective measure while neuropsychological outcomes were further adjusted for baseline scores and education. All analyses were performed separately, using recent (Exam 7) and remote (Exam 5) Ideal-CVH scores as the exposure. Results were considered statistically significant if p < 0.05.
Results
Sample demographics
The characteristics of the cohorts are summarized in Table 1. Across the samples, the mean Ideal-CVH score ranged from 3.1-3.3, with less than 1% of the sample having either a maximum or minimum Ideal-CVH score. The elapsed time between the quantification of recent and remote Ideal-CVH was 6.9 years (SD 0.9).
Table 1.
Characteristics of the study samples
| Stroke (n=2631) | Dementia (n=1364) | Neuropsychology/MRI* (n=1597) | |
|---|---|---|---|
| Age, y | 62 (9) | 69 (6) | 61 (9) |
| Men, n (%) | 1188 (45) | 637 (47) | 709 (44) |
| Education, n (%) | |||
| No high school degree | 92 (4) | 67 (5) | 42 (3) |
| High school degree | 769 (30) | 454 (34) | 418 (26) |
| Some college | 750 (29) | 374 (28) | 480 (30) |
| College graduate | 939 (37) | 428 (32) | 659 (41) |
| Body mass index, kg/m2 | 28 (5) | 28 (5) | 28 (5) |
| SBP, mmHg | 127 (19) | 133 (19) | 125 (18) |
| DBP, mmHg | 74 (10) | 73 (10) | 74 (9) |
| HTN, n (%) | 1200 (46) | 784 (57) | 635 (40) |
| HTN treatment, n (%) | 887 (34) | 589 (43) | 469 (29) |
| Total cholesterol, mg/dL | 201.19 (36.64) | 199.67 (36.08) | 201.49 (35.71) |
| HDL cholesterol, mg/dL | 54.04 (16.97) | 53.15 (16.79) | 54.06 (16.74) |
| Diabetes mellitus, n (%) | 312 (12) | 208 (15) | 161 (10) |
| Prevalent CVD, n (%) | 286 (11) | 237 (17) | 145 (9) |
| Current smoker, n (%) | 308 (12) | 102 (7) | 156 (10) |
| Apolipoprotein ε4 +, % | 576 (22) | 302 (22) | 360 (23) |
| Ideal-CVH, score | 3.1 (1.4) | 3.1 (1.3) | 3.3 (1.4) |
| Ideal-CVH frequencies, n (%) | |||
| 0 | 22 (0.8) | 10 (0.7) | 9 (0.6) |
| 1 | 245 (9.3) | 110 (8.1) | 133 (8.3) |
| 2 | 638 (24.3) | 348 (25.5) | 352 (22.0) |
| 3 | 734 (27.9) | 418 (30.7) | 443 (27.7) |
| 4 | 569 (21.6) | 305 (22.4) | 366 (22.9) |
| 5 | 275 (10.5) | 118 (8.7) | 188 (11.8) |
| 6 | 124 (4.7) | 48 (3.5) | 93 (5.8) |
| 7 | 24 (0.9) | 7 (0.5) | 13 (0.8) |
CVH = ideal-cardiovascular health, DBP = diastolic blood pressure, HDL = high density lipoprotein, HS = high school, HTN = hypertension, MRI = magnetic resonance imaging. SBP = systolic blood pressure. Means and standard deviations are reported, unless stated otherwise. MRI measures are available on a subset of the participants with neuropsychological measures (n=1287).
The number of events and characteristics of the outcome measures can be seen in Table 2. Of those participants studied for incident dementia, 25 (3%) had prevalent stroke at baseline. Of the 87 subjects who developed stroke during the study, 7 (8%) subjects also developed incident dementia during follow-up. Four (5%) of these cases were consistent with clinical AD, and 5 (6%) were consistent with VaD. The mean time from baseline to incident all-cause dementia onset was 5.6 years (SD= 2.7 years).
Table 2.
Characteristics of the outcome measures
| Measure | |
|---|---|
| 10-year incidence | |
| Stroke, n (%) | 87 (3) |
| All-cause dementia, n (%) | 84 (6) |
| Alzheimer's disease, n (%) | 64 (5) |
| Vascular Dementia, n (%) | 14 (1) |
| Neuropsychological outcomes | |
| Global cognition baseline/annualized change | 0.11 (0.94) / −0.02 (0.09) |
| VRD baseline/annualized change | 8.4 (3.3) / −0.1 (0.5) |
| Similarities baseline/annualized change | 17.1 (3.4) / −0.04 (0.5) |
| Trails A baseline*/annualized change | 0.5 (0.2) / −1.1 (0.2) |
| Trails B baseline*/annualized change | 1.3 (0.9) / 0.1 (0.2) |
| LMD baseline/annualized change | 10.7 (3.5) / 0.1 (0.6) |
| MRI outcomes | |
| TBV baseline/annualized change, cm3 | 1002.6 (111.0) / −5.8 (4.2) |
| FBV baseline/annualized change, cm3 | 459.4 (59.0) / −2.9 (5.4) |
| LVV baseline/annualized change, cm3 | 21.8 (14.0) / 1.0 (0.9) |
| WMHV baseline*/annualized change | 1.0 (2.7) / 0.2 (0.4) |
FBV = frontal brain volume LMD = logical memory delayed, LVV = lateral ventricular volumes, TBV= total brain volume, MRI = magnetic resonance imaging, WMHV = white matter hyperintensity volume, VRD = visual reproductions delayed.
indicates that values have been log transformed, The sample sizes are 2631 for incident stroke, 1364 for incident dementia and 1597 for neuropsychology. MRI measures are available on a subset of the participants with neuropsychological measures (n=1287).
Ideal-CVH and the 10-year risk of stroke and dementia
Higher recent Ideal-CVH scores were associated with a lower 10-year risk of incident stroke and VaD (Table 3). There was no association between recent Ideal-CVH and incident all-cause dementia or clinically apparent AD. Higher remote Ideal-CVH was associated with a lower 10-year risk of incident stroke, all-cause dementia, AD, and VaD.
Table 3.
Ideal-Cardiovascular Health and the age- and sex-adjusted 10-year risk of incident stroke and dementia
| Event | Recent Ideal-CVH |
Remote Ideal-CVH |
||||
|---|---|---|---|---|---|---|
| n cases / subjects | HR (95% CI) | P | n cases / subjects | HR (95% CI) | P | |
| Stroke | 87/2631 | 0.80 (0.67, 0.95) | .01 | 70/2139 | 0.79 (0.66, 0.94) | .01 |
| All-cause dementia | 84/1364 | 0.91 (0.76, 1.09) | .30 | 69/1131 | 0.80 (0.67, 0.97) | .02 |
| Alzheimer's disease | 64/1364 | 0.94 (0·77, 1.15) | .53 | 51/1131 | 0.79 (0.64, 0.98) | .006 |
| Vascular dementia | 14/1364 | 0.49 (0.30, 0.81) | .006 | 13/1131 | 0.61 (0.39, 0.95) | .03 |
CI = Confidence Interval, CVH = Ideal-Cardiovascular Health, HR = Hazard Ratio. Results are adjusted for age, sex.
Ideal-CVH and the risk of subclinical brain injury and cognitive decline
Both higher recent and remote Ideal-CVH scores were associated with less cognitive decline on the tasks of Visual Reproductions Delayed and Similarities (Table 4), indicating less decline in the domains of visual memory, reasoning, and verbal comprehension. Higher remote Ideal-CVH was also associated with less global cognitive decline, as measured by the principal component score. Higher recent Ideal-CVH was associated with less frontal brain atrophy whereas higher remote Ideal-CVH was associated with less whole brain atrophy.
Table 4.
Ideal-Cardiovascular Health and Annualized Change in Cognition and Markers of Brain Atrophy
| Measures | Recent Ideal-CVH |
Remote Ideal-CVH |
||
|---|---|---|---|---|
| β±SE | P | β±SE | P | |
| Cognitive decline | ||||
| Global decline | 0.003±0.002 | .07 | 0.006±0.002 | .002 |
| Visual reproductions delayed | 0.02±0.01 | .01 | 0.02±0.01 | .01 |
| Similarities | 0.02±0.01 | .04 | 0.04±0.01 | <.001 |
| Trails A | 0.001±0.002 | .46 | −0.002±0.002 | .43 |
| Trails B | −0.01±0.004 | .13 | −0.01±0.004 | .08 |
| Logical Memory Delayed | −0.01±0.01 | .51 | 0.01±0.01 | .44 |
| Brain atrophy and white matter injury | ||||
| Total brain volume | 0.09±0.08 | .26 | 0·19±0.08 | .02 |
| Frontal brain volume | 0.31±0.10 | .003 | 0.15±0.11 | .16 |
| Lateral Ventricular Volume | 0.02±0.01 | .10 | 0.002±0.02 | .88 |
| WMHV | −0.0002±0.01 | .98 | 0.0003±0.01 | .97 |
CVH = Cardiovascular health; WMHV = White matter hyperintensity volume. Analyses are adjusted for age, sex, age squared, baseline (exam 7) scores on each respective measure and education (in the case of cognitive decline).
Secondary analysis
An additional 14-point Ideal-CVH score was created using 3-levels for each variable (0=poor status, 1=intermediate status and 2=ideal status). Analyses were repeated using both the recent and remote 14-point Ideal CVH score. Results were similar to the 7-point Ideal-CVH scores, with the exception that the remote 14-point Ideal CVH score was neither associated with the incidence of clinical AD nor VAD (please see Supplemental Tables III-IV online).
DISCUSSION
There is a pressing need to develop effective strategies to prevent stroke and dementia. This community-based prospective cohort study revealed that adherence to the AHA's Ideal-CVH guidelines was associated with a lower risk of vascular brain injury, including a lower risk of stroke, dementia, cognitive decline, and brain atrophy. To our knowledge, this is the first study to demonstrate an association between the Ideal-CVH score and the risk of incident dementia. These data support the hypothesis that maintaining CVH protects against all forms of vascular brain injury, including AD. The concept of Ideal-CVH should thus be further promoted to protect the brain, as well as the heart, from vascular risk factors.
The present results are consistent with that of the Northern Manhattan9 and Kailuan10 studies both of which found that Ideal-CVH was associated with a lower risk of stroke. Similarly consistent with our findings, both the Coronary Artery Risk Development in Young Adults10 and the Maine-Syracuse Longitudinal18 Studies reported that Ideal-CVH was associated with superior neuropsychological performance, across multiple cognitive domains. The present study extends this previous body of research by demonstrating that higher Ideal-CVH was associated with a vascular pattern of subclinical brain injury and cognitive decline. Although Ideal-CVH was not associated with WMHV in the present study, we have previously found total brain volume to be more sensitive to vascular risk factors in the Framingham Offspring Cohort,19, 20 perhaps because WMHs are an uncommon outcome on T2-weighted images in young adults.21 A principal finding of the current study was that remote Ideal-CVH scores (reflecting midlife CVH) were associated with the future risk of dementia, including AD, VaD, and all-cause dementia.
Many experts conceptualize AD as stemming from the abnormal accumulation of amyloid beta within the brain,22 yet drug discovery has targeted this single mechanism with disappointing results.23 An alternative approach is to conceptualize dementia as a syndrome involving the convergence of multiple pathways and causes.24 Autopsy studies suggest that cerebrovascular disease often coexists with the neurodegenerative hallmarks of AD, even when individuals are diagnosed with one form of dementia during life.25 There is emerging evidence to suggest that, although the worldwide number of dementia cases is increasing, the age-specific risk may be declining in high-income countries.24, 26 This decline appears to coincide with higher educational attainment and improved management of cardiovascular risk factors.24, 26 Such findings complement the present results by suggesting that further promoting CVH may help ease the growing burden of dementia.
The association between Ideal-CVH and stroke was similar for both recent and remote variables. In contrast, remote Ideal-CVH was a better predictor of all-cause dementia, AD and global cognitive decline as compared to recent Ideal-CVH. This discrepancy may reflect the fact that a stroke is an acute event that can develop quickly in response to poor CVH whereas clinical dementia develops insidiously over decades. The calculation of remote Ideal-CVH captures a longer length of exposure, allowing more time for dementia to develop in those with poor CVH. Our findings are in line with other studies suggesting that vascular risk factors are most strongly associated with the risk of later-life cognitive impairment and dementia when measured closer to midlife.14
The focus of the Ideal-CVH score is on modifiable health behavior and factors.7 Assuming causality, this implies that, with education, guidance, and motivation, individuals can increase their Ideal-CVH score and potentially decrease their risk of vascular brain injury and dementia. In particular, middle-aged adults should be informed of the importance of adhering to Ideal-CVH guidelines. It is necessary to consider that the 7 Ideal-CVH variables are not mutually exclusive. Advocating for regular physical activity, quitting or never smoking, and adhering to a healthy diet are the likely cornerstone to maintaining many of the CVH factors at their ideal statuses, such as normal body weight, blood pressure, cholesterol, and blood glucose.
Limitations of the present study include the fact that ethnic minorities were not well represented in our cohort limiting the generalizability of our findings to these populations. Second, diet and physical activity were measured using self-report techniques, which may be subject to recall bias or social desirability. Scores for diet and physical activity were also calculated using a formula that, while consistent with other publications8, varied from the exact AHA definition. Lastly, we did not examine the individual components of the Ideal-CVH score, given the potential overlap with other Framingham Heart Study publications, which have previously reported on the association of single vascular risk factors with neurological outcomes.
Conclusions
Preventing dementia is one of the most profound challenges facing our aging society. Cardiovascular disease risk factors appears to increase the risk of dementia suggesting that maintaining optimal cardiovascular health may be useful in lowering dementia risk.11, 25 Thus, there is cause for optimism, with emerging evidence suggesting that society can ‘both grow older and lower dementia burden.'27 In line with this notion, our data suggests that adhering to CVH guidelines protects against all forms of vascular brain injury, lessening the burden of cognitive decline, stroke, brain atrophy, and dementia, including AD. Further promoting Ideal-CVH, particularly to middle-aged adults, may improve neurological outcomes for our aging citizens.
Supplementary Material
Acknowledgments
Funding Sources
Dr Pase is funded by an Australian National Health and Medical Research Council (NHMRC) Early Career Fellowship (APP1089698) and his work on stroke is funded by a Rebecca L Cooper Medical Research Foundation grant. The Framingham Heart Study is supported by the National Heart, Lung and Blood Institute (NHLBI) Framingham Heart Study (contracts N01-HC-25195 and HHSN268201500001I) and grants from the National Institute of Neurological Disorders and Stroke (NS17950), and the National Institute of Aging (AG08122, AG033193, and AG049607). Prof DeCarli directs the UC Davis Alzheimer's Disease Center with funding from the NIH (P30 AG010182).
Footnotes
Disclosures
Dr. DeCarli is a consultant to Novartis on a clinical trial of LCZ696 for heart failure. All other authors report no disclosures.
References
- 1.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2013;9:63–75. e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Alzhiemer's Associataion 2015 Alzheimer's disease facts and figures. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida D, Ninomiya T, Doi Y, Hata J, Fukuhara M, Ikeda F, et al. Prevalence and Causes of Functional Disability in an Elderly General Population of Japanese: The Hisayama Study. Journal of Epidemiology. 2012;22:222–229. doi: 10.2188/jea.JE20110083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men: The Mayo Clinic Study of Aging. Neurology. 2010;75:889–897. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke. 1991;22:312–8. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 6.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: An analysis of population-based data. The Lancet Neurology. 2014;13:788–794. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction: The American Heart Association's Strategic Impact Goal Through 2020 and Beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 8.Xanthakis V, Enserro DM, Murabito JM, Polak JF, Wollert KC, Januzzi JL, et al. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation. 2014;130:1676–83. doi: 10.1161/CIRCULATIONAHA.114.009273. [DOI] [PubMed] [Google Scholar]
- 9.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MS, Sacco RL. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and hispanics: the northern Manhattan study. Circulation. 2012;125:2975–84. doi: 10.1161/CIRCULATIONAHA.111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, et al. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013;44:2451–6. doi: 10.1161/STROKEAHA.113.678839. [DOI] [PubMed] [Google Scholar]
- 11.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The framingham offspring study. Design and preliminary data. Preventive Medicine. 1975;4:518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 13.Wolf PA. Contributions of the Framingham Heart Study to stroke and dementia epidemiologic research at 60 years. Archives of Neurology. 2012;69:567–71. doi: 10.1001/archneurol.2011.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurology. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 15.American Psychatric Association . Diagnostic and Statistical Manual of Mental Disorders 4th ed. Text Revision. 4th ed. American Psychatric Assocaition; Washington DC: 2000. [Google Scholar]
- 16.Graham NL, Emery T, Hodges JR. Distinctive cognitive profiles in Alzheimer's disease and subcortical vascular dementia. Journal of Neurology, Neurosurgery & Psychiatry. 2004;75:61–71. [PMC free article] [PubMed] [Google Scholar]
- 17.DeCarli C, Massaro J, Harvey D, Hald J, Tullberg M, Au R, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiology of Aging. 2005;26:491–510. doi: 10.1016/j.neurobiolaging.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 18.Crichton GE, Elias MF, Davey A, Alkerwi A. Cardiovascular health and cognitive function: the Maine-Syracuse Longitudinal Study. PLoS One. 2014;9:e89317. doi: 10.1371/journal.pone.0089317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seshadri S, Wolf PA, Beiser AS, Selhub J, Au R, Jacques PF, et al. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Archives of Neurology. 2008;65:642–9. doi: 10.1001/archneur.65.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debette S, Beiser A, Hoffmann U, Decarli C, O'Donnell CJ, Massaro JM, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Annals of Neurology. 2010;68:136–44. doi: 10.1002/ana.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hopkins RO, Beck CJ, Burnett DL, Weaver LK, Victoroff J, Bigler ED. Prevalence of white matter hyperintensities in a young healthy population. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2006;16:243–51. doi: 10.1111/j.1552-6569.2006.00047.x. [DOI] [PubMed] [Google Scholar]
- 22.Jack CR, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Update on hypothetical model of Alzheimer's disease biomarkers. Lancet neurology. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg RN, Petersen RC. The human alzheimer disease project: A new call to arms. JAMA Neurology. 2015;72:626–628. doi: 10.1001/jamaneurol.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. The New England Journal of Medicine. 2013;369:2275–7. doi: 10.1056/NEJMp1311405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kling MA, Trojanowski JQ, Wolk DA, Lee VMY, Arnold SE. Vascular disease and dementias: Paradigm shifts to drive research in new directions. Alzheimer's and Dementia. 2013;9:76–92. doi: 10.1016/j.jalz.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langa KM. Is the risk of Alzheimer's disease and dementia declining? Alzheimer's Research & Therapy. 2015;7:34. doi: 10.1186/s13195-015-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The Lancet Neurology. Societies can both grow old and lower dementia burden. The Lancet Neurology. 2015;14:967. doi: 10.1016/S1474-4422(15)00223-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.