Abstract
To improve the nuclear-targeted delivery of non-viral vectors, extensive effort has been carried out on the development of smart vectors which could overcome multiple barriers. The nuclear envelope presents a major barrier to transgene delivery. Viruses are capable of crossing the nuclear envelope to efficiently deliver their genome into the nucleus through the specialized protein components. However, non-viral vectors are preferred over viral ones because of the safety concerns associated with the latter. Non-viral delivery systems have been designed to include various types of components to enable nuclear translocation at the periphery of the nucleus. This review summarizes the progress of research regarding nuclear transport mechanisms. “Smart” non-viral vectors that have been modified by peptides and other small molecules are able to facilitate the nuclear translocation and enhance the efficacy of gene expression. The resulting technology may also enhance delivery of other macromolecules to the nucleus.
Keywords: Gene delivery, non-viral vectors, nuclear translocation, targeting
Introduction
Recently, gene therapy has been developed for the treatment of debilitating human disorders, including diabetes and various cancers; these therapies can overcome the intrinsic disadvantages and serious risks of pharmacological agents. Gene therapy aims to introduce novel genes or to repair malfunctioning genes as a means to permanently treat or reverse the disorders. The exogenous “good” DNA is used to replace the defective DNA at appropriate chromosomal targets, correcting malformations. Cancer therapy is one example of a situation in which deleterious mutant alleles are replaced with functional ones [1].
The therapeutic genes encounter various extracellular and intracellular barriers after they are administered in vivo. Plasma membranes are apparent barrier to the cellular uptake of therapeutic genes. After endocytosis into the cytoplasm, genes are largely routed to the late endosomes and lysosomes and are degraded by acidic hydrolases. The remaining genes are able to escape from the hydrolysis in the endosomes and lysosomes and travel through the highly concentrated and molecularly crowded cytosol to be delivered into the nucleus. One of the major hurdles in this process is successful nuclear transport [2]. Although viral vectors can efficiently infect cells, non-viral gene delivery results in only 10–20% of the applied dose of plasmid DNA enters the targeted cells and only 1–5% of the applied dose is able to enter the nucleus [3]. Therefore, successful gene therapy requires the utilization of knowledge gained from the study of nuclear transport of viral DNA in non-viral gene delivery systems to conquer the barrier of the nuclear envelope (NE). Transportation through the cytoplasm or across the endosomal membrane is a prerequisite for gene transfer [4], making it as vital to the success of gene-based therapies as nucleus translocation. Here, we reviewed the applications of smart delivery of therapeutic DNA across the nuclear membrane. The resulting technology may also profit delivery of other macromolecules to the nucleus, such as transcription factors or enzymes, which can be used to safely regulate gene expression through alternative mechanisms.
Hindrances to nuclear delivery
Cell evolution and appearance of barriers
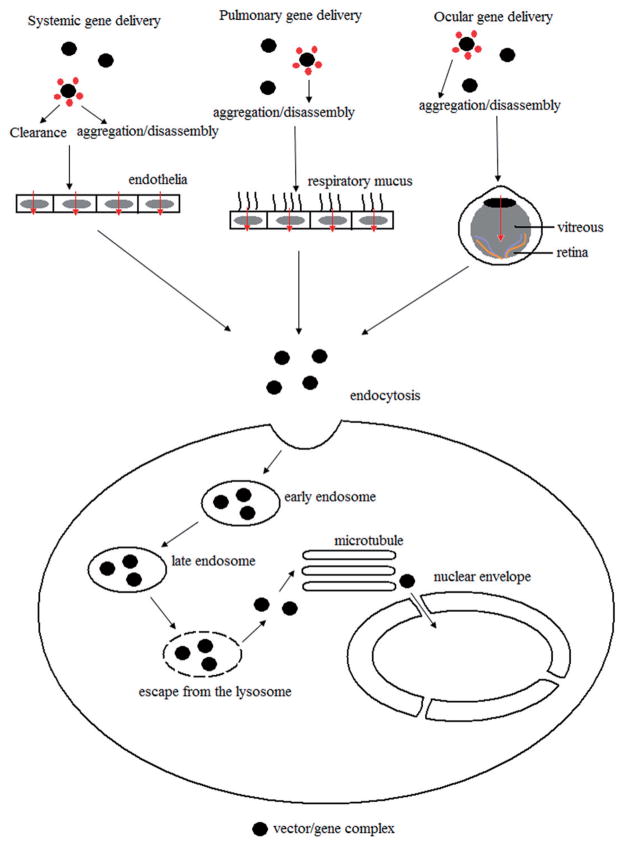
Eukaryotic cells have developed sophisticated subcellular structures to ensure compartmental functions and genetic diversity, and to protect cells from invasion of disadvantageous non-self genes [5]. Meanwhile, cells developed regulated transport mechanisms to move nutrients or other substances between the surrounding environment and the cell interior. Microbes, such as phages and bacteria, also adapted to invade these cells and avoid the barriers created by the developed complexity. Our understanding of these invasion and defense mechanisms may facilitate the delivery of therapeutic genes. This series of barriers is associated with (1) the stability of DNA and vectors in the extracellular environment, (2) cellular uptake by endocytosis, (3) escape from the endo/lysosomes, (4) transportation through the cytoplasm, and (5) importation into the nucleus (Figure 1).
Figure 1.
Extracellular and intracellular processing of non-viral vectors.
Extracellular barriers
Therapeutic genes could be delivered through different administrations, including intramuscular or intravenous (IV) injections, and pulmonary or ocular drug delivery (Figure 1). The genes may encounter many barriers dependent on the administration routes, such as blood components, endothelial cells, respiratory mucus, the blood–brain barrier, the blood–retinal barrier, extreme pH, proteases and nucleases, the immune defenses and scavenger systems [6]. Thus, the therapeutic gene must be encapsulated and delivered by a vector to the appropriate extracellular location.
Blood is one of the major barriers to an injected gene vector. Various polymers can be used to condense DNA and protect it from degradation in the blood. Usually, such polymers have an excess of positive charge to enable sufficient compaction of the negatively charged DNA. The resulting complex possesses a net positive surface-charge which might cause self-aggregation and be disadvantageous when injected into the bloodstream [7]. For instance, positively charged complexes can bind to plasma proteins and activate the complement system against exogenous material within the bloodstream. Additionally, the complex may also be cleared rapidly by the thrombocytes, leukocytes, erythrocytes or other blood components. PEGylation, including the conjugation of polyethyleneglycol (PEG) [8] before particle formation, covalent grafting of PEG [9] or N-(2-hydroxypropyl)methacrylamide (HPMA) [10] after particle formation, or the association of PEG via inclusion complex formation with the surface of β-cyclodextrin-containing polyplexes, can increase stability against self-aggregation and reduce non-self interactions.
Pulmonary delivery is also an attractive route for gene delivery. Since the cloning of the cystic fibrosis (CF) gene (CFTR) in 1989, many clinical trials have been completed, demonstrating a proof-of-principle for gene transfer to the airway. However, clinical trials suggest that targeted delivery to the lungs is rather difficult. The failure of clinical trails is attributed to respiratory mucus, alveolar fluid [11] and the secretions in the fibrotic airway.
Additionally, gene therapy could be an alternative treatment of many retinal disorders which cannot be presently remedied effectively [12,13]. Gene delivery to the posterior chamber, e.g. retinal tissues and vitreous, via systemic delivery is constrained due to the presence of the blood–retinal barrier [14]. Though the topical application may overcome the barrier, this method encounters other problems, for instant, the sclera. Intravitreal injection is less invasive than sub-retinal injection and is an alternative delivery method. This route of administration requires the complexes to travel through the vitreous, a gel-like substance containing negative charged glycosaminoglycans (GAGs). Similar to the CF mucus, this biopolymer network may cause aggregation and immobilization of the gene complex. Sanders et al. [15] reported when moderate amounts of PEG were conjugated onto the complex, aggregation in the vitreous could be partially prevented.
Plasma membranes and endocytosis
Due to the negative charge of the plasma membrane, naked DNA, a negatively charged macromolecule, does not cross membrane barriers efficiently. DNA has to rely on a vector to gain access to individual cells. The positively charged surface of a successful vector interacts with the negatively charged cell surface. However, this electrostatic interaction lacks specificity unless a specific targeting ligand is incorporated into the vector. Thus, it is necessary to preclude such non-specific interactions by reducing the charge ratio of the complex, presenting a stealth property [16] or incorporating a specific targeting ligand.
Occasionally, a vector/gene complex can fuse directly with the plasma membrane due to the properties of the vector [17] or the incorporation of a cell-penetrating peptide [18]. However, most of the time, a complex enters cells through endocytosis. Eukaryotic cells exploit various endocytic pathways [19,20], such as clathrin-dependent endocytosis via coated pits, and non-clathrin dependent endocytosis including caveolin-mediated endocytosis, clathrin and caveolinin-dependent endocytosis, and macropinocytosis. Parts of the caveolar vesicles are fused with neutral pH caveosomes, limiting delivery to the lysosomes where intracellular vesicles experience acidification and hydrolysis. The clathrin-dependent endocytosis pathway is more efficient in transfection. Several studies of different cell types demonstrate that lipoplexes are internalized through clathrin-dependent endocytosis. Polyplexes and/or lipoplexes may be internalized by other endocytic mechanisms, for example, a combination of clathrin-dependent endocytosis and clathrin-independent endocytosis [21]. Nevertheless, inhibition of transfection is seen upon treatment of cells with specific inhibitors of endocytosis or overexpression of a dominant negative mutant protein [22], which is necessary for the formation of the coated pits.
Lysosomal degradation
Once the intracellular vesicles carrying the vector are taken up via endocytosis, they usually fuse with the endocytic compartments. Initially, the vector appears within vesicles known as the early endosomes, which are located peripherally in the cell and possess a slightly acidic pH. These early endosomes are responsible for either the redistribution of material from or the return of internalized material to the cell surface. The late endosomes then traffic the vector to the lysosomes where DNA is finally broken-down. Thus, the therapeutic effect of DNA is thought to be a consequence of its escape from the endosomal vesicles.
Several mechanisms of escape have been developed, including (a) transbilayer flip-flop of anionic lipids from the cytoplasmic face of the endosomal membrane induced by cationic lipids [23], (b) charge neutralization of cationic complexing agents with the anionic macromolecules of the endo-lysosomes membrane, (c) fusion mediated by cationic lipids [24], (d) membrane destabilization [25,26], (e) osmotic swelling of the endosomes, or the “proton sponge-mediated escape” [27] and (f) the co-addition or covalent coupling of fusogenic [28] or endosome-disruptive molecules [29]. As previously mentioned, complexes are often PEGylated to pass through the extracellular compartments more effectively, yet, PEGylation may exert an inhibitory effect on endosomal escape. The PEGylation prevents close contact between the vector and the membranes of the endocytic compartment, not only inhibiting endosomal escape but also cellular uptake. Thus, reversible or exchangeable PEGylation [30], which is characterized by the ability to be removed in the endosomal compartment, is recommended.
The cytoplasmic sieve
Upon release from the endosomes, nucleic acids in naked form or as complexes must pass through the molecularly crowded cytoplasmic space toward the nucleus where transcription takes places. Observations of intracellular microinjection have demonstrated that the movement of free DNA via diffusion is slow and inefficient. Diffusion is passive and size-dependent; diffusion of large DNA (>250 bp) in cytoplasm is slowed greatly compared to that of smaller DNA. Dauty et al. found that the actin cytoskeleton is the principal determinant of size-dependent DNA mobility [31]. Free DNA can also be actively transported using the network of microtubules [32]; molecules bearing nuclear localization signals (NLSs) are able to proceed along the microtubules in a dynein-dependent manner [33]. NLSs are important not only in achieving nuclear import, but also in concentrating cargo at the perinuclear region. Since the transport by passive diffusion is usually limited, directed transport of the complex by dynein/kinesin coating could be an attractive way to transport materials to the cell periphery [34].
The nuclear transport mechanism
The nucleus is isolated from the cytoplasm by a double membrane known as the NE. The NE is punctuated by nuclear pore complexes (NPCs), through which exclusive passage into and out of the nucleus proceeds [35]. NPCs have a ring of octagonal spokes, which forms a central channel, a nuclear basket on the nucleoplasmic side and a cytoplasmic ring on the cytoplasmic side. The three rings float on top of one another while the cytoplasmic ring appear to be more weakly connected to the spoke ring than to the nuclear ring. The central channel has an inner diameter of 75 nm [36] and each clamp-like spoke is attached to the NE at two specific sites. The contact region between the membrane and the spoke ring is a porous, sponge-like structure and can be traversed through a channel that is approximately 9 nm in diameter. Integral, inner-nuclear-membrane proteins might be imported along these channels [37].
The spoke ring cavity of NPC is the main passage for nuclear translocation. Although there are no distinct structural features of the spoke ring cavity, the channel is filled by a meshwork formed by natively unfolded phenylalanine-glycine (PG) domains that form a selective, hydrophobic barrier to transport inside the NPC. This barrier is semi-permeable and prevents the diffusion of macromolecules. The physical diameter of the barrier is 9 nm, the upper limit for passive diffusion. However, during the active transport the channel can dilate to 39 nm in diameter, facilitating passage to the nucleus with assistance of NLSs [38]. The above results and the AFM image all present NPCs exhibit great plasticity [39]. The exceptional structure and mechanical flexibility of NPCs are important in fulfilling their function in translocation [40].
Mechanisms in importing of cargos through NPC into the nucleus
The precise mechanism by which the carrier–cargo complex is translocated through the NPCs remains controversial [41,42]. The affinity gradient model posits that the cargo alternate between repeating FG motifs that increase the affinity closer to the nucleus. The Brownian affinity-gate model describes the occurrence of translocation through diffusion simulated by the accumulation of macromolecules at the cytoplasmic face of NPC. The selected phase model suggests that FG repeats interact with each other to generate a tightly cross-linked gel, which only allows passage of macromolecules that can interact with FG repeats.
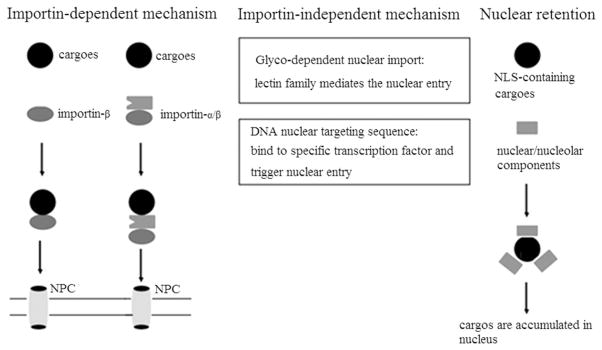
However, it is generally accepted that cargos with classic NLSs are recognized by members of the importin (also known as karyopherin) superfamily of cellular nuclear transport proteins and are then translocated into the nucleus (Figure 2). There are two main types of importin, importin-α and importin-β. The NLS containing cargo can bind either to the importin-α subunit of an importin-α/β dimmer [43,44] or directly to the importin-β [45,46] without the need of an importin-α adaptor molecule. The importin/cargo complex docks at the distal end of the NPCs cytoplasmic filaments and then transfers to the central channel of the NPC, translocating to the nucleoplasmic side. The cargo is released to the nuclear environment after the nuclear RanGTP binds to importin-β. The importin-α and/or -β subunits are then recycled back to the cytoplasm to mediate a new round of nuclear import.
Figure 2.
Mechanisms in importing of cargos into the nucleus.
During the importin-dependent transport, facilitation by cytoskeletal movement relies on microtubule and actin [47]. Using microtubule motor proteins for transport through the cytoplasm towards the nucleus, it is able to enhance conventional nuclear import [48]. Interestingly, association with dynein light chains significantly enhances the function of NLS in the cargos [49].
There are also several distinct, alternative pathways for nuclear import, which operate through the so-called importin-independent mechanisms. Glyco-dependent nuclear import is thought to mediate the nuclear translocation of glycosylated plasmids [50]. These nuclear shuttling molecules are proteins of the lectin family and appear to be specific to the type of sugar moiety. Another interesting pathway implicates DNA nuclear targeting sequences (DTS) in an active, energy-dependent and sequence-specific process. DTS has binding sites for specific transcription factors that exist in normal mammalian cells (e.g. AP1 and AP2 TEF-1). When DTS containing plasmid binds to a transcription factor, the NLS in the transcription factor triggers importin-dependent nuclear import. For instance, to enter the nucleus of smooth muscle cells, a 176 bp portion of smooth muscle gamma-action (SMGA) promoter DTS could enhance plasmid DNA expression in a controlled manner [51].
Nuclear retention, or the binding of NLS-containing cargoes to nuclear/nucleolar components, may be another mechanism through which nuclear localization is regulated [52]. Some molecules, for instance, the HIV-1 transactivator Tat, the angiogenic factor, angiogenin, and interferon-induced transcription factor IFI16, are able to accumulate in the nucleus in part due to binding to nuclear/nucleolar components.
Lessons from viruses
Much can be learned from viruses about creating an efficient complex. Viruses gain optimal intracellular access and deliver their genome (DNA or RNA) into appropriate subcellular compartment (in most cases the nucleus). Cell binding, endocytosis, endosomal escape, transport through the cytosol and nuclear import are all crucial steps in the process through which viruses infect cells. The viral journey provides insight into the cell’s trafficking machinery and can be exploited to improve non-viral gene delivery systems [53].
To infect, viruses must first attach to molecules present on the surface of host cell and internalize through an endocytic route or fusion reaction [54]. Adenovirus, a non-enveloped DNA virus (60–90 nm) with projecting fibers on the capsid, binds to the constitutive androstane receptor (CAR) and recruits integrin for internalization and is internalized through receptor-mediated endocytosis. However, herpes simplex virus-1 (HSV-1), an enveloped DNA virus, induces a fusion reaction of the viral lipid bilayer membrane with the plasma membrane, releasing the capsid in the cytoplasm of the host cells. It seems that the vector/gene complexes entering via endocytosis should be a better choice than those that fuse with the plasma membrane. Targeting of clathrin-dependent endocytosis can be achieved by coupling specific ligands to the gene complex, such as transferrin [55–57] and folate [58–60]. Preferably, the size of complex should remain smaller than 200 nm to promote clathrin-dependent endocytosis. The stability of the complex is also important to successful gene transfection [61].
Subsequently, the complex needs to escape the endosomal compartment before they merge with the destructive lysosomes. Enveloped viruses use fusion proteins to cross the membrane barrier [62], while non-enveloped viruses utilize detergent-like mechanisms (membrane disruption) [63] or barrel-stave mechanism (pores formation) [64] to escape endosomes. These processes are usually assisted by the lowering of the pH in the early and/or late endosomes. At a low pH, the predominant envelope glycoprotein in the influenza virus, hemagglutinin, is subjected to several conformational changes, leading to the protrusion of a hydrophobic spike into the endosomal membrane that initiates membrane fusion. Intrinsic protease activity of the viral capsid seems to be essential to proper maturation and endosomal escape. The adenovirus penton protein, for instance, becomes endosome-disruptive at a low pH, allowing the passage of intact virus particles through the endosomal membrane and into the cytosol [65]. Retroviruses, which are enveloped RNA viruses, have similar, pH-dependent envelope proteins [66]. To avoid rapid endosomal escape, a gene complex should possess endosomolytic properties upon the lowering of the endosomal pH. The combination of an endosomal escape moiety, including diphtheria toxin translocation domain, histidine [67], fusogenic peptides [68] and acid-transforming peptide [69], could provide various gene complexes with the ability to escape endosome [70,71]. Preferably, endosomal escape should occur close to the nuclear membrane and guarantee the therapeutic gene is delivered intact.
Once inside the cytosol, particles are actively transported to the nucleus via microtubules using the dynein motor protein. The particles then bind to the cytoplasmic face of the NPC and transfer DNA into the nucleus shortly thereafter. In response to changes in pH, reductive environment, Ca2+ concentration or enzymatic activity during cytoplasmic trafficking, viruses can experience structural changes and expose new layers like NLSs. Typically importins recognize viruses through NLSs, dock to the NPC and transport them into the nucleus. However, viruses vary considerably in their interactions with the nuclear import machinery. Adenoviruses undergo extensive disassembly prior to genome import and herpes viruses release their genome into the nucleus without immediate capsid disassembly. Parvoviruses cause damage to the NE and import through the resulting breaks [72]. Polyoma viruses and lentivirus preintegration complexes are thought to enter nucleus in intact form, whereas the corresponding complexes of onco-retroviruses delay until host cells undergo mitosis because they cannot infect interphase nuclei. Thus, to enhance intracellular transport, complexes should be equipped with ligands that are recognized by the microtubule motor protein dynein [73,74], or utilize the endosomal vesicles to release their cargo in the perinuclear region.
The coupling of NLSs to plasmid DNA has been an attractive strategy in nuclear targeting. Another method is through the incorporation of the particles into the nucleus during cell division or the trigging of the release of nucleic acids upon cell division. From this point of view, an intriguing protein, C-myc, remains attached to the microtubules when the cells are in rest but dissociates from the microtubules upon a trigger received during cell division [75].
Application of nuclear targeting
The nucleus presents a major barrier for the delivery of therapeutic genes, thus synthetic vectors are extremely inefficient in terms of the exogenous protein production per plasmid copy. A surge of research effort has been directed towards facilitating nuclear targeting of therapeutic genes or other macromolecules employing various functional peptides and small molecules including inorganic and organic molecules.
Peptide-guided nuclear transport
Non-viral vectors must overcome several barriers to achieve successful gene delivery, such as condensing and protecting DNA, targeting specific types of cells, disrupting the endosomal membrane and delivering DNA to the nucleus [76]. Peptides-based vectors are able to more easily achieve nuclear delivery, giving them an advantage over other non-viral vectors. For example, cationic peptides can interact with the negatively charged DNA through electrostatic interactions resulting in the formation of nanometer-sized particles with a net positive charge. These particles can interact with the cell membrane, internalizing into the cell to achieve gene expression [77,78].
Poly-L-lysine (PLL) is one of the first cationic peptides used to condense DNA. PLLs of a higher molecule weight have a greater net positive charge; they can better bind to DNA and form more stable complexes than PLLs of low molecule weight. However, as the length of the PLL increase, so does the cytotoxicity [79]. Additionally, although the combination of PLLs and the endosomolytic agents, such as chloroquine, will improve transfection efficiency, it also induces the cytotoxicity [80]. Many researchers have developed homogenous, polylysine-containing peptides. Oligolysine peptides can offer many advantages over PLLs, such as low toxicity, precise control of synthesis and site-specific attachment of ligands for cell targeting [81–83]. In addition, peptides must have the ability to escape the endosome to successfully deliver the cargo to the nucleus. In the endosome, some peptides (e.g. histidine-rich peptides) can serve as buffers against the proton pump to cause lysis or fusion with the endosomal membrane leading to pore formation. For example, histidine can become protonated in the acidic environment of the endosome because the imidazole group has a pKa of ~ 6.0 [79].
Fusogenic peptides
Many fusogenic peptides have been utilized to deliver DNA into the nucleus. They may also facilitate the delivery into the cytosol and promote endosomal escape. Some cell penetrating proteins (CPPs) derived from the transduction domains of proteins can interact with cell membranes. TAT peptide (TATp), melittin, transportan and penetratin peptides are some examples of CPPs that can interact in this manner. Furthermore, there are also several synthetic amphipathic peptides such as GALA (Sequence: WEAALAEALAEALAE HLAEALAEALEALAA) and KALA (Sequence: WEAKLAK ALAKALAKHLAKALAKALKACEA) that can traverse membranes [79].
TATp is one of the most frequently used CPPs and is derived from the transcriptional activator protein encoded by human immunodeficiency virus type 1 (HIV-1). Some studies have shown that the cellular penetration of TATp was via a receptor- and endocytosis-independent mechanism; however, recent studies have concluded that an endocytotic mechanism may be involved. Many TATps have been shown to translocate into the interior of various cell types [84]. Cardarelli et al. demonstrated that in the absence of competitors (i.e. intracellular cytosolic and nuclear factors) TATRRR (47YGRKKRRQRRR57) binds to importin-α and importin-β in vitro. While in live cells, the mutated TATGGG (47YGRKKRRQGGG57) is a direct target of both importin-α and importin-β. These nuclear properties of TAT can provide the basic knowledge for the rational design of localization sequence that is better tailored for nuclear import [85]. Following this rationale, de la Fuente et al. prepared stable and water-soluble, gold nanoparticles functionalized with a TAT-derived peptide sequence (GRKKRRQRRR). This functionalization has allowed the nanoparticles to penetrate the cell membrane and accumulate in the nucleus of human fibroblast cell lines in vitro [86].
GALA is a 30 amino acid synthetic peptide with a glutamic acid-alanine-leucine-alanine (EALA) repeat that also contains a histidine and tryptophan residue. Glutamic acid (Glu) was selected to provide the peptide with a pH-dependent, negatively-charged side-chain. As pH reduces from 7.0 to 5.0, GALA converts to an amphipathic α-helix from its normal state as a random coil. When the peptide is an α-helix, the EALA repeat is adjusted so that the peptide would have a hydrophobic face of sufficient hydrophobicity to interact with the lipid bilayer. Therefore, in the acidic endosomal environment, GALA is able to bind to bilayer membranes and induces leakage from phosphatidylcholine vesicles [79,87]. KALA peptide is the result of the replacement of some alanines with lysines and a reduction in glutamic acid content. KALA peptide can not only condense DNA, but can also induce membrane leakage. As pH decreased, KALA peptide can also converts into an α-helix from its usual shape as a random coil, and it is in this way that the peptide induces membrane leakage [79]. A multifunctional delivery system for recombinant genes was developed to achieve targeted gene delivery to ZR-75-1 breast cancer cells. This system used histone H1 as a condenser, KALA to destabilize the endosomal membrane, a cell targeting peptide and an NLS. This resulting recombinant vector can successfully disrupt endosomal membranes and reach cell nuclei [88].
Nuclear localization signals
To achieve nuclear targeting, many NLSs have been developed to assist the DNA to target the nucleus and allow DNA entry through the NPCs by active transport. As mentioned, DNA–NLS complexes can be recognized by specific intracellular receptor proteins as a nuclear import. Yi et al. have demonstrated that addition of NLSs increased the luciferase reporter gene expression by about 200-fold and did not induce any apparent cytotoxicity in both HeLa and Cos7 cell lines [89].
Classical NLSs are characterized by short stretches of basic amino acids. The best understood examples are those that are similar to SV40 Tag. Other classical NLSs include bipartite NLSs that resemble Xenopus protein nucleoplasmin and NLSs that are derived from yeast homeodomain contain protein MATα2 and are composed of charged/polar residues interspersed with non-polar residues. Non-classic NLSs lack the stretches of basic amino acids. The well-known example is the hydrophobic, 38-residue M9 sequence of the human mRNA-binding protein hnRNP A1. Novel classes of importin α-dependent NLSs emerge through high throughput screening of random peptide libraries [90]. Through amino acid replacement we can generate more unconventional classes of NLSs, such as redox-sensitive NLSs [91].
If the NLS have a sufficient net-positive charge, they can interact with negatively charged DNA to enhance the nuclear targeting with or without the presence of another condensing agent. However, in this way, NLSs probably dissociate from DNA before they have reached the nucleus. NLSs may also be associated with DNA with a chemical group or site-specific attachment using a peptide-nucleic acid (PNA) clamp. If the NLS peptides covalently attach to the DNA through chemical groups, NLS peptides may occur at any location of the DNA, including the gene of interest, which may lead to the inhibition of gene expression [79]. To avoid these issues, PNA clamps can be used for specific attachment of the NLS sequence to plasmid DNA. Bremner et al. increased seven-fold in gene expression by using a NLS peptide/DNA conjugate formed by site-specific linkage of an extended SV40 peptide via a PNA clamp [92].
DNA binding proteins
DNA binding proteins (DBPs) are capable of binding DNA and have been exploited as DNA carriers in gene delivery. Full-length proteins or protein fragments of significant size protect NLSs from unwanted non-specific interaction with the phosphate groups in DNA. Furthermore, these proteins keep the NLSs in appropriate, tertiary structure to promote strong binding to the relevant, nuclear transport proteins. The use of such proteins has been investigated for several years, such as transcription factors, High mobility group (HMG)-box proteins or histones.
Inducible transcription factor nuclear factor (NF)-κB, which contains an NLS sequence, could be transported into the nucleus in an importin-dependent fashion and function as a transcriptional enhancer, resulting in a 31-fold augmentation of gene expression in mammalian cells. In addition, Adi Mesika found that coupling NF-κB p50 with pDNA not only facilitated nuclear entry of the DNA but also its migration through the cytoplasm toward the nucleus [33]. HMG proteins can bind to DNA in a site-specific or non-specific fashion. HMG box 1 (HMGB1) is an abundant nuclear protein that binds to double-stranded DNA. HMGB1 is composed of HMG box A, box B and C-terminal acidic regions. Condensation of DNA by HMG-1 is sufficient to promote efficient gene transfer, while recombinant, TAT-linked HMGB1 box A (rTAT-HMGB1A) had a higher efficiency of gene transfer and no cytotoxicity to HEK293 cells [93]. The pre-requisite of being an efficient DNA carrier is that the DNA binding and importin recognition properties of these proteins are not mutually exclusive. A study regarding the ability of core histone H2B derivatives to mediate gene delivery showed histones have significant capability of importin binding and nuclear targeting. Coupled with their DNA binding abilities of histones, these capabilities make them interesting prospects for use as a gene delivery vehicle [94]. Gene expression induced by histones is tested using either H2B monomeric protein or heterodimeric form with histone H2A and found H2A/H2B/DNA complexes are able to transfect cells more efficiently than LipofectamineTM. Since the nuclear targeting and DNA binding properties of histone proteins are independent and they contain protein transduction domains (PTD), which enable them to enter intact cells in energy- and receptor-independent fashion, histones are well suited for gene transfer application [95].
Peptide/small molecule-modified pharmaceutical nanoparticles for nuclear-targeted delivery
The intracellular delivery of non-viral vectors is inefficient because they are required to overcome many barriers inside the cells. To achieve successful nuclear transport, a large number of studies have focused on introduction of various peptides or small molecules to pharmaceutical nanoparticles both in vitro and in vivo (Table 1).
Table 1.
Peptides/small molecules-modified nanoparticles for intracellular delivery.
| Peptides/small molecules | Nano-carriers | Cells or animals | Effect |
|---|---|---|---|
| TATp | Superparamagnetic iron oxide particle [96,97] | CD34+ cells | For intracellular labeling, MRI, magnetic separation of homed cells, cell imaging |
| TATp | Liposomes [98–100] | Mouse NIH/3T3 fibroblasts, human BT20 cells, and rat H9C2, U-87 MG cells | To show the potential of TAT-liposome for intracellular delivery, and the intracellular gene delivery in vitro and in vivo |
| TATp | SLN [101] | Bronchial epithelial cells and mice | To optimize gene transfer and offer the opportunity for further studies in large animal models |
| TATp | PEI-PEG conjugate [103] | A459 cells and mice | A new approach to non-viral gene carriers for lung therapy, comprising protection for plasmid DNA, low toxicity and significantly enhanced transfection efficiency under in vivo conditions |
| TATp | PEI-β-CyD conjugate [104] | Placental mesenchymal stem cells (PMSCs) | To improve the transfection efficiency to PMSCs |
| NLS | DOTAP:DOPE (1:1 w/w) liposome [105] | SKnSH mammalian neuroblastoma cells | To improve the efficiency and efficacy of non-viral methods of gene therapy |
| NLS | pH sensitive liposome [106] | Rat peritoneal macrophages | To deliver bovine serum albumin into the nucleus |
| NLS | Cobalt(II)-polybenzimidazole [107] | Various cell lines | To enhance expression of the transgenes |
| DBP (NF-κB) | PLGA/PEI nanospheres [108] | COS7 cells and human monocyte-derived dendritic cells | To enhance intracellular transport |
| DBP(HMG-1) | Linear PEI and branch PEI-based nano-particles [109,110] | Mammalian cells | To improve their gene transfer efficiency by non-viral carriers with peptides |
| DEX | PEI-based nanoparticles [112] | HepG2 cells, 293 cells | To increase the membrane perturbation and transfection efficiency |
| DEX | HA-PEI [114,115] | B16F10 cells and tumor-bearing nude mice | For double level targeted gene delivery with DNA ternary complexes |
| ATRA | PEI [118] | NIH3T3 cells, HeLa cells | To evaluate the nuclear translocation of ATRA-enriched nanoparticles |
| ATRA | Cationic liposome [119] | A mouse of metastatic lung tumor | For lung therapy |
| CaP | Asymmetric liposome with a CaP core [120–122] | H460 cells, B16F10 cells and mice | To increase the cargo delivery and release activity |
| CR8C | Cationic liposomes with a CaP core [125] | Mouse liver | To deliver pDNA to the nuclei of mouse hepatocytes |
TATp-modified pharmaceutical nanoparticles
Nanoparticles delivering different kinds of cargo, such as DNA and siRNA both in vitro and in vivo can be modified with TATp. Josephson et al. reported that TATp was conjugated to a dextran-coated, super-paramagnetic, iron oxide particle with a mean particle size of 41 nm and an average of 6.7 TATp conjugates per particle [96]. The transfection efficiency of the modified particles was enhanced over 100-fold from that of un-modified particles. NMR imaging detected that labeled cells are highly magnetic and could be retained on magnetic separation columns. Therefore, this method serves a tool for magnetic resonance imaging (MRI) or magnetic separation of homed cells in vivo. Furthermore, 4% of magnetically labeled CD34+ cells homed to bone marrow per gram of tissue and magnetically labeled cells that had homed to bone marrow could be recovered by using a magnetic separation column [97].
TATp can also be applied to the intracellular delivery of lipid-based gene carriers. Torchilin et al. have developed 200 nm liposomes that are attached by TATp and can be delivered into cells. The intracellular localization of fluorescent TATp-modified liposomes can be observed in Lewis lung carcinoma cells of mice, BT20 tumor cells of the human breast, and H9C2 cardiac myocytes of rats. Later, they designed TATp liposomes containing a cationic lipid (DOTAP) formed firm non-covalent complexes with DNA [98,99]. Following intratumoral injection of pEGFP-N1 plasmid encoding for the green fluorescent protein (GFP) formulated with TATp-liposomes, expression of GFP was observed in the tumor cells. Moreover, these liposomes enhanced the delivery of pEGFP-N1 plasmid to human brain tumor U-87 MG cells both in vivo and in vitro [100]. Rudolph et al. have discovered that incorporation of a dimeric, HIV-1 TATp into SLN gene vectors also significantly enhanced gene expression both in vitro and in vivo [101]. To improve the biocompatibility, thiocholesterol-based cationic lipids (TCL) have been designed, which can be used to package DNA and protect it from DNase digestion. When TATp (GRKKRRQRRRGYG) was incorporated onto the particle surface, the particle-cell recognition was increased and transfection efficiency was enhanced 80-fold [102].
TATp was also covalently coupled to 25 kDa polyethylenimine (PEI) through a heterobifunctional PEG spacer to form a TAT–PEG–PEI conjugate. This conjugate exhibits significantly lower toxicity in vitro and higher transfection efficiency in vivo compared to the PEI polyplex [103]. Lai et al. have discovered that PEI-β-cyclodextrin conjugated by TATp improved the transfection efficiency of PEI-β-CyD in placental mesenchymal stem cells (PMSCs) after 48 and 96 h of post-transfection incubation. The viability of PEI-β-CyD-treated PMSCs was shown to be over 80% after 5 h of treatment and 24 h of post-treatment incubation [104].
NLSs-modified pharmaceutical nanoparticles
NLSs can be used to enhance the nuclear localization of nonviral vectors. Cationic liposomes composed of DOTAP:DOPE (1:1 w/w) that are conjugated with a synthetic NLS peptide derived from the SV40 virus could be used to deliver a luciferase-encoding PGL3 plasmid into SKnSH, mammalian neuroblastoma cells. The luciferase expression of NLS-modified liposomes was enhanced three-fold compared to non-modified cationic liposomes [105]. Tachibana et al. incorporated the NLSs of the SV-40 large T-antigen in the fluorescein isothiocyanate (FITC)–bovine serum albumin, which were then encapsulated into the pH-sensitive liposomes. In the presence of NLSs, FITC-alb was successfully delivered into the nucleus, while no transport into nucleus was observed in the absence of NLSs [106]. Yin et al. conjugated NLSs (PKKKRKV) with cobalt(II)-polybenzimidazole complex to transfer genes in both normal and cancer cell lines [107]. The NLS-modified Co(II) complexes could condense more DNA than that in the absence of NLS. NLSs-bound condensates showed five-fold enhanced transfection efficacy in different cell types and lower cytotoxicity than NLS-free ones. In addition, NLSs and other functional peptides and/or small molecules can be applied simultaneously to increase the nuclear import. Moore et al. have investigated the coupling of one SV-40 peptide (a classical NLSs) or two TATp (a non-classical NLSs) to PEG-DBP vehicles to increase the transfection of PEG-DBP/DNA particles 15-fold. The coupling also resulted in efficiency similar to that of a common cationic polymer vehicle, PEI [29].
DNA-binding protein-modified pharmaceutical nanoparticles
An alternative to using DNA-binding proteins is to couple the protein to a polycation condensing agent. In combination with NF-κB analogs, the transfection of plasmid DNA by PLGA/PEI nanospheres in COS7 cells was significantly enhanced due to effective intranuclear transport [108]. Natural condensing agents may offer the opportunity to construct more organized and therefore more stable complexes. Shen et al. investigated a combined carrier that is comprised of PEI and HMG-1. They found the volume of pDNA/HMG-1/PEI complex was 104–106 times smaller than naked pDNA and the complex presented as homogeneous spheres [109]. Transfection efficiencies for pDNA/HMG-1/liner PEI complex and pDNA/HMG-1/branch PEI complex were 2.9-fold and 4.0-fold greater than that for pDNA/liner PEI and pDNA/branch PEI complexes, respectively. HMGB1/PEG-PEI combined vectors were used to deliver pDNA [110]. HMGB1 molecules could bind with the pDNA chains but does not condense pDNA well. PEG–PEI could further compact pDNA/HMGB1 complex into nanosized spherical terplex. HMGB1 in the terplex was able to assist in the transportation of pDNA into the nucleus of cells and result in transfection efficiency 2.6–4.9-fold higher than that of a common cationic polymer PEI 25 kDa.
Small molecule-modified pharmaceutical nanoparticles
Dexamethasone (DEX)
A variety of studies have been conducted on the facilitation of nuclear translocation by glucocorticoid receptor (GR). GR is constitutively expressed in the cytoplasm. Normally, GR binds to the heatshock proteins and remains in its inactive form. However, when glucocorticoid enters the cells and binds to GR, the conformation of GR changes and the receptor–ligand complex is translocated into the nucleus. Simultaneously, NPCs are dilated to approximately 140 nm and the giant pore, 300 nm in diameter, can be visualized [111].
DEX, a potent glucocorticoid, has been conjugated to polymers to improve the nuclear transport. Compared to unmodified PEI, DEX-conjugated PEI (2 kDa) of low molecule weight, increased the gene expression level by an order of magnitude in HepG2 cells and at least two orders for magnitude in 293 cells [112]. The nuclear localization and hydrophobicity of DEX contribute to its greater membrane perturbation and increases transfection efficiency. Similarly, polyamidoamine(PAMAM)-Dex showed approximately two-fold higher transfection efficiency in 293 cells than PAMAM, especially in the presence of serum [113]. In the previous studies, the Yao lab has developed a DNA ternary complex system of hyaluronic acid (HA)/PEI-DEX/DNA. In this system, PEI1800-DEX is used to compact DNA into a nanosized structure and facilitates the nuclear translocation of DNA into tumor cells. A polyanion HA was applied to improve targeted delivery to the tumor and reduce cytotoxicity. The results demonstrated that, among all complexes that were investigated, ternary complexes with ~160 nm in diameter exhibit the lowest cytotoxicity and the highest transfection efficiency in B16F10 cells. The ternary complexes can facilitate more efficient cellular uptake and nuclear transport of DNA than PEI1800-DEX/DNA binary complexes. In addition, ternary complexes of HA/PEI1800-DEX/DNA showed anti-inflammation activity and greatly suppressed tumor growth in vivo [114,115].
The relationships between structure and transfection activity were investigated using various glucocorticoid–PEI conjugates, which employed betamethasone (BET), DEX, methylprednisolone (MPL), prednisolone (PNL) and hydrocortisone (HC). The transgene expression enhanced linearly with the increasing glucocorticoid potency. The increase in transfection capability generally follows the order: HC-PEI<PNL-PEI<MPL-PEI<DEX-PEI<BET-PEI, with more pronounced enhancement in DEX-PEI and BET-PEI. The maximum transfection efficiency mediated by DEX-PEI and BET-PEI was higher than that mediated by PEI 25 kDa, even at the best weight ratio, while their cytotoxicities were lower than PEI 25 kDa.
All-trans-retinoic acid (ATRA)
Similar to the nuclear translocation of DEX-bound GRs, ATRA can be translocated into the nucleus via retinoic acid receptors (RARs), which are members of the superfamily of nuclear hormone receptors [116]. ATRA binds to specific, intracellular, lipid-binding proteins, such as retinoic acid binding proteins (CRABP-I and II) and fatty acid binding proteins (FABPs). In cells with high CRABP-II/FABP5 ratios, ATRA could function through RAR and implement nuclear import [117]. Researchers synthesized PRA in which ATRA was grafted to PEI [118]. The transfection efficiency of PRA/DNA complex was comparable to that of PEI/DNA complex in NIH3T3 cells and lower than that of PEI/DNA complex in HeLa cells. However, a mixed gene complex of PEI and PRA showed two- to four-fold enhancement of transfection efficiency as compared with PEI/DNA complex. The hydrophobicity of ATRA leads to its localization in the interior of the complex, hindering the accessibility and binding of ATRA to CRABP-II and FABP5. ATRA-incorporated, cationic liposome/IL-12 plasmid DNA complex were given intravenously in a mouse model of metastatic lung tumor. It prolonged the survival time of mice significantly, while cationic liposome/IL-12 plasmid DNA complex without ATRA only slightly improved therapeutic efficacy [119].
Calcium phosphate
Due to the fact that the calcium phosphate (CaP) is biocompatible, biodegradable and native to the body, the biomaterial is being considered for use in gene delivery to protect and transport cargo into cells, finally, into the nucleus. Moreover, CaP can escape the endosome without the assistance of additional compounds such as peptides or lysomotropic agents, because it can rapidly dissolve in the acidic environment of the endosomes. The rapid dissolution of CaP causes endosome to swell and burst, releasing the cargo into the cytoplasm. Taking advantages of this phenomenon, the Huang lab has developed several kinds of lipid coated CaP (LCP) nanoparticles formulation for efficient delivery of siRNA. The cores of the LCP were biodegradable, nano-sized, calcium-phosphate precipitate. In vitro, The LCP nanoparticles exhibit three- to four-fold higher silencing effects compared to the previously used liposome/polycation/DNA complex. When the LCP nanoparticles were modified using anisamide, a sigma receptor ligand, the gene silencing effect was approximately 70% and 50% in the cultured tumor cells and a xenograft model, respectively. On the other hand, un-targeted NPs induced very little silencing [120]. Additionally, after C57BL/6 mice received a single IV injection of antiluciferase siRNA (0.12 mg siRNA/kg) that had been formulated in targeted LCP nanoparticles, luciferase activity in metastatic B16F10 tumor-loaded lungs decreased by 78%. Targeted LCP nanoparticles prolonged the mean survival time of the mice by 27.8% while inducing no cytotoxicity at the therapeutic dose [121]. In other studies, an anionic lipid, dioleoylphosphatydic acid (DOPA) was used to coat the nano-size CaP cores so that the coated cores were soluble in organic solvents. The improvement of siRNA delivery was 40-fold in vitro and four-fold in vivo compared to that of the lipid/protamine/DNA formulation [122]. However, for this delivery system to achieve the greatest results, the delivery of therapeutic gene to the nucleus should be enhanced, as the genetic information of the cell and the transcription machinery both resides in the nucleus. Ca2+-regulated transport is one way of nucleocytoplasmic transportation. The intermediate space between the two bilayers of a NE is called the cisterna and this is where Ca2+ is stored and released to regulate the passage of molecules through NPC. When Ca2+ is present in the cisterna, the central plug lies below the cytoplasmic ring of the NPC and the pores of NPC remain open, allowing molecules to diffuse through. However, when inositol triphosphate (InsP3) diffuses into the InsP3 receptor on the outer nuclear membrane, Ca2+ will release so that NPC undergoes a conformational change, blocking the diffusion of molecules into the nucleus through the pores. CaP nanoparticles in the cytosol may have inactivated InsP3 so that the drainage of cisternal calcium ions does not occur and the plug of the NPC is “on”. In this circumstance, gene–Ca2+ complexes could readily enter into the nucleus through the pores of the NPC. Other liposomes or polymers without calcium ions cannot enter the nucleus via calcium-mediated transport [123]. Bisht et al. have prepared CaP nanoparticles that encapsulate plasmid DNA. Studies of these formulations have shown that DNA was completely encapsulated in the CaP nanoparticles resulting in protection of the DNA from external DNase. Moreover, escape from the endosome, nuclear uptake of the plasmid and subsequent expression of the genes has been observed in vitro using confocal microscopy. Thus, these CaP nanoparticles can be used as an effective non-viral vector [124]. Multifunctional membrane-core nanoparticles, composed of CaP cores, arginine-rich peptides, cationic and PEGylated lipid membranes and galactose targeting ligands have been developed by Hu et al. [125]. This synthetic vector is the most effective synthetic vectors for nuclear delivery of plasmid DNA and subsequent gene expression in hepatocytes in vivo. The inclusion of such peptides in LCP was sufficient to elicit high degrees of nuclear translocation of plasmid DNA. Comparing to linear CR8C, monocyclic CR8C significantly enhanced in vivo gene expression over 10-fold. Though 100-fold lower in activity than the hydrodynamic injection, this formulation presents as a much less invasive alternative [125].
In addition, no immune response or major organ damage was observed after treatment with siRNA formulated in targeted LCP nanoparticles [120,121], suggesting that LCP formulation was safe and weakly immunogenic for systemic targeted delivery of nucleic acids. However, Ca2+ plays an important role in cellular signaling pathway. It was also described that modulation of Ca2+ represents a major mechanism in the pathogenesis of prelethal cellular reactions to injury as well as to the mechanisms involved in both accidental and programmed cell death [126]. Therefore, it should be concerned that the accumulation of Ca2+ in cells may increase the risk of cell injury. However, unpublished results from this lab indicated that excess Ca2+ was pumped either out of the cells or into the mitochondria, keeping the intracellular Ca2+ concentration at a low level.
Application of multifunctional envelope nano device for gene delivery
Non-viral delivery systems must have various functions to enable them to overcome the barriers that arise during the delivery of cargo to the nucleus. Systems should target specific, cell-surface receptors, condense the cargo to protect it from degradation by DNase, escape from the endosomes and be able to achieve nuclear import. The multifunctional envelope nano device (MEND) has been developed to achieve all of these goals. MEND consists of a polycation that condenses with the nucleic acid and a lipid envelope that can be equipped with various functional devices (e.g. targeting ligands, PEG and functional peptides) [127].
A series of octaarginine (R8)-modified MENDs for gene delivery has been developed by Khalil et al. [128]. When negatively charged, PLL/DNA particles were coated with egg phosphatidylcholine, cholesterol (Chol) and stearyloctaarginine (STR-R8), luciferase activity increased by more than two orders of magnitude compared with that induced by PLL/DNA particles alone. When particles were coated with dioleoylphosphatidylethanolamine (DOPE)/Chol/STR-R8, luciferase activity was about four orders of magnitude higher. However, the highest luciferase activity was achieved when particles were coated with DOPE/cholesteryl hemisuccinate (CHEMS)/STR-R8. Additionally, LacZ plasmid DNA was delivered to the hair follicle cells of four-week-old, ICR mice in vivo. This advancement enabled the observation of gene expression in the treated cells. The study also elucidated the effects of the delivery of luciferase-encoding pDNA and anti-luciferase oligodeoxynucleotide (ODN) in three types of R8-MENDs that were condensed with three polycations, STR-R8, PLL and protamine. The ODN-MEND that was condensed with protamine achieved a 90% antisense effect 16 h after transfection and a persistent antisense effect of over 75% for up to 48 h [129]. Another MEND for targeted gene delivery to ZR-75-1 breast cancer cells has been developed by Soltani et al. [88]. This system consists of two tandem repeating units of truncated histone H1 to condense pDNA, a peptide ligand to target ZR-75-1 cells, KALA to disrupt endosomal membrane and NLS to facilitate pDNA to the nucleus. This vector with four functions achieved a higher gene transfection than any vector without functional motifs.
The importance of nuclear gene delivery for gene expression to gene-based therapeutic cannot be disputed. Some studies of multifunctional envelope-type nano devices are focused on the intracellular disposition rather than nuclear gene delivery. Yamada et al. have prepared novel gene delivery vectors by integrating R8-MEND and biocleavable polyrotaxanes (dimethylaminoethyl(DMAE)-SS-PRX) as a DNA condenser to improve the intranuclear DNA disposition. Surprisingly, the transfection activity of R8-MEND containing 29DMAE was almost five-fold greater than that of R8-MEND containing protamine. This finding strongly supports the theory that intra-nuclear DNA disposition plays a very important role in the gene transfection and expression induced by non-viral vectors [130]. Moreover, Akita et al. have developed a tetra-lamellar MEND (T-MEND) that is coated with two nuclear membrane-fusogenic, inner envelopes and two endosome-fusogenic, outer envelopes to overcome the endosomal and nuclear membrane barrier via a step-wise fusion process. To access the function of the nucleus-fusogenic lipid, GFP was encapsulated into rhodamine-labeled multi-lamellar liposomes and incubated with isolated nuclei. GFP was delivered to the interior of the nucleus, whereas the signal of the lipid was distributed along the nuclear membrane. T-MENDs can be used to effectively transfected into JAWSII cells (non-dividing cells) and the transfection activity significantly increased by several hundred-fold compared to that of the conventional MEND. These results suggest T-MEND efficiently delivered DNA into the nucleus through NE, resulting in great improvement of transfection activity [131].
Conclusions
The nucleus is a major barrier to the delivery of therapeutic genes. DNA or other therapeutic molecules must enter the nucleus in order to be transcribed for gene expression, integration, or replication to take place. A successful gene vector should encapsulate and protect the cargo for translocation to nucleus while overcoming the numerous intracellular and extracellular barriers present in the delivery process. Much progress in the field is modeled after the mechanism of nuclear transport employed by the viruses. The coupling of peptides or small molecules has made the non-viral systems for gene delivery more appealing and efficient. MENDs can achieve impressive nuclear transport. Hopefully, more intelligent and efficient non-viral vectors can be expected in the near future.
Acknowledgments
We thank Kelly Racette for editing the manuscript.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
This work was supported by the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. JKGQ201107), Qing Lan Project and Jiangsu Overseas Research and Training Program for University Prominent Young and Middle-aged Teachers and Presidents. It is also supported by NIH grants CA151652 and CA149363.
References
- 1.Goldstein I, Marcel V, Olivier M, et al. Understanding wild-type and mutant p53 activities in human cancer: new landmarks on the way to targeted therapies. Cancer Gene Ther. 2011;18:2–11. doi: 10.1038/cgt.2010.63. [DOI] [PubMed] [Google Scholar]
- 2.Glover DJ, Leyton DL, Moseley GW, et al. The efficiency of nuclear plasmid DNA delivery is a critical determinant of transgene expression at the single cell level. J Gene Med. 2010;12:77–85. doi: 10.1002/jgm.1406. [DOI] [PubMed] [Google Scholar]
- 3.Cohen RN, van der Aa MA, Macaraeg N, et al. Quantification of plasmid DNA copies in the nucleus after lipoplex and polyplex transfection. J Control Release. 2009;135:166–174. doi: 10.1016/j.jconrel.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Won YY, Sharma R, Konieczny SF. Missing pieces in understanding the intracellular trafficking of polycation/DNA complexes. J Control Release. 2009;139:88–93. doi: 10.1016/j.jconrel.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woese CR. On the evolution of cells. Proc Natl Acad Sci USA. 2002;99:8742–7. doi: 10.1073/pnas.132266999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wiethoff CM, Middaugh CR. Barriers to nonviral gene delivery. J Pharm Sci. 2003;92:203–17. doi: 10.1002/jps.10286. [DOI] [PubMed] [Google Scholar]
- 7.Ogris M, Wagner E. Targeting tumors with non-viral gene delivery systems. Drug Discov Today. 2002;7:479–85. doi: 10.1016/s1359-6446(02)02243-2. [DOI] [PubMed] [Google Scholar]
- 8.Collard WT, Yang Y, Kwok KY, et al. Biodistribution metabolism and in vivo gene expression of low molecular weight glycopeptide polyethylene glycol peptide DNA co-condensates. J Pharm Sci. 2000;89:499–512. doi: 10.1002/(SICI)1520-6017(200004)89:4<499::AID-JPS7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 9.Kircheis R, Blessing T, Brunner S, et al. Tumor targeting with surface-shielded ligand-polycation DNA complexes. J Control Release. 2001;72:165–70. doi: 10.1016/s0168-3659(01)00272-3. [DOI] [PubMed] [Google Scholar]
- 10.Oupicky D, Howard KA, Konák C, et al. Steric stabilization of poly-L-lysine/DNA complexes by the covalent attachment of semitelechelic poly [N-(2-hydroxypropyl)methacrylamide] Bioconjugate Chem. 2000;11:492–501. doi: 10.1021/bc990143e. [DOI] [PubMed] [Google Scholar]
- 11.Sanders N, Rudolph C, Braeckmans K, et al. Extracellular barriers in respiratory gene therapy. Adv Drug Deliv Rev. 2009;61:115–27. doi: 10.1016/j.addr.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peeters L, Sanders NN, Demeester J, et al. Challenges in non-viral ocular gene transfer. Biochem Soc Trans. 2007;35:47–9. doi: 10.1042/BST0350047. [DOI] [PubMed] [Google Scholar]
- 13.Liu C, Wong E, Miller D, et al. Lentiviral airway gene transfer in lungs of mice and sheep: successes and challenges. J Gene Med. 2010;12:647–58. doi: 10.1002/jgm.1481. [DOI] [PubMed] [Google Scholar]
- 14.Duvvuri S, Majumdar S, Mitra AK. Drug delivery to the retina: challenges and opportunities. Expert Opin Biol Ther. 2003;3:45–56. doi: 10.1517/14712598.3.1.45. [DOI] [PubMed] [Google Scholar]
- 15.Sanders NN, Peeters L, Lentacker I, et al. Wanted and unwanted properties of surface PEGylated nucleic acid nanoparticles in ocular gene transfer. J Control Release. 2007;122:226–35. doi: 10.1016/j.jconrel.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Oh EJ, Park K, Kim KS, et al. Target specific and long-acting delivery of protein peptide and nucleotide therapeutics using hyaluronic acid derivatives. J Control Release. 2010;141:2–12. doi: 10.1016/j.jconrel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Goda T, Goto Y, Ishihara K. Cell-penetrating macromolecules: direct penetration of amphipathic phospholipid polymers across plasma membrane of living cells. Biomaterials. 2010;31:2380–7. doi: 10.1016/j.biomaterials.2009.11.095. [DOI] [PubMed] [Google Scholar]
- 18.Gupta B, Levchenko TS, Torchilin VP. Intracellular delivery of large molecules and small particles by cell-penetrating proteins and peptides. Adv Drug Deliv Rev. 2005;57:637–51. doi: 10.1016/j.addr.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Midoux P, Breuzard G, Gomez JP, et al. Polymer-based gene delivery: a current review on the uptake and intracellular trafficking of polyplexes. Curr Gene Ther. 2008;8:335–52. doi: 10.2174/156652308786071014. [DOI] [PubMed] [Google Scholar]
- 20.Sahay G, Alakhova DY, Kabanov AV. Endocytosis of nanomedicines. J Control Release. 2010;145:182–95. doi: 10.1016/j.jconrel.2010.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rejman J, Conese M, Hoekstra D. Gene transfer by means of lipo- and polyplexes: role of clathrin and caveolae-mediated endocytosis. J Liposome Res. 2006;16:237–47. doi: 10.1080/08982100600848819. [DOI] [PubMed] [Google Scholar]
- 22.Cooper A, Shaul Y. Clathrin-mediated endocytosis and lysosomal cleavage of hepatitis B virus capsid-like core particles. J Biol Chem. 2006;281:16563–9. doi: 10.1074/jbc.M601418200. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Szoka FC., Jr Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35:5616–23. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 24.Lonez C, Lensink MF, Kleiren E, et al. Fusogenic activity of cationic lipids and lipid shape distribution. Cell Mol Life Sci. 2010;67:483–94. doi: 10.1007/s00018-009-0197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuhorn IS, Bakowsky U, Polushkin E, et al. Nonbilayer phase of lipoplex-membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Mol Ther. 2005;11:801–10. doi: 10.1016/j.ymthe.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 26.El-Sayed A, Masuda T, Khalil I, et al. Enhanced gene expression by a novel stearylated INF7 peptide derivative through fusion independent endosomal escape. J Control Release. 2009;138:160–7. doi: 10.1016/j.jconrel.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Yu JH, Quan JS, Kwon JT, et al. Fabrication of a novel core-shell gene delivery system based on a brush-like polycation of α β–poly (L-aspartate-graft-PEI) Pharm Res. 2009;26:2152–63. doi: 10.1007/s11095-009-9928-9. [DOI] [PubMed] [Google Scholar]
- 28.El-Sayed A, Khalil IA, Kogure K, et al. Octaarginine- and octalysine-modified nanoparticles have different modes of endosomal escape. J Biol Chem. 2008;283:23450–61. doi: 10.1074/jbc.M709387200. [DOI] [PubMed] [Google Scholar]
- 29.Moore NM, Sheppard CL, Sakiyama-Elbert SE. Characterization of a multifunctional PEG-based gene delivery system containing nuclear localization signals and endosomal escape peptides. Acta Biomater. 2009;5:854–64. doi: 10.1016/j.actbio.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 30.Auguste DT, Furman K, Wong A, et al. Triggered release of siRNA from poly(ethylene glycol)-protected pH-dependent liposomes. J Control Release. 2008;130:266–74. doi: 10.1016/j.jconrel.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dauty E, Verkman AS. Actin cytoskeleton as the principal determinant of size-dependent DNA mobility in cytoplasm: a new barrier for non-viral gene delivery. J Biol Chem. 2005;280:7823–8. doi: 10.1074/jbc.M412374200. [DOI] [PubMed] [Google Scholar]
- 32.Vaughan EE, Dean DA. Intracellular trafficking of plasmids during transfection is mediated by microtubules. Mol Ther. 2006;13:422–8. doi: 10.1016/j.ymthe.2005.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mesika A, Kiss V, Brumfeld V, et al. Enhanced intracellular mobility and nuclear accumulation of DNA plasmids associated with a karyophilic protein. Hum Gene Ther. 2005;16:200–8. doi: 10.1089/hum.2005.16.200. [DOI] [PubMed] [Google Scholar]
- 34.Yokokawa R, Tarhan MC, Kon T, et al. Simultaneous and bidirectional transport of kinesin-coated microspheres and dynein-coated microspheres on polarity-oriented microtubules. Biotechnol Bioeng. 2008;101:1–8. doi: 10.1002/bit.21874. [DOI] [PubMed] [Google Scholar]
- 35.van der Aa MA, Mastrobattista E, Oosting RS, et al. The nuclear pore complex: the gateway to successful nonviral gene delivery. Pharm Res. 2006;23:447–59. doi: 10.1007/s11095-005-9445-4. [DOI] [PubMed] [Google Scholar]
- 36.Beck M, Lucić V, Förster F, et al. Snapshots of nuclear pore complexes in action captured by cryo-electron tomography. Nature. 2007;449:611–15. doi: 10.1038/nature06170. [DOI] [PubMed] [Google Scholar]
- 37.King MC, Lusk CP, Blobel G. Karyopherin-mediated import of integral inner nuclear membrane proteins. Nature. 2006;442:1003–7. doi: 10.1038/nature05075. [DOI] [PubMed] [Google Scholar]
- 38.Panté N, Kann M. Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm. Mol Biol Cell. 2002;13:425–34. doi: 10.1091/mbc.01-06-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liashkovich I, Meyring A, Kramer A, et al. Exceptional structural and mechanical flexibility of the nuclear pore complex. J Cell Physiol. 2011;226:675–82. doi: 10.1002/jcp.22382. [DOI] [PubMed] [Google Scholar]
- 40.Terry LJ, Wente SR. Flexible gates: dynamic topologies and functions for FG nucleoporins in nucleocytoplasmic transport. Eukaryot Cell. 2009;8:1814–27. doi: 10.1128/EC.00225-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nigg EA. Nucleocytoplasmic transport: signals mechanisms and regulation. Nature. 1997;386:779–87. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 42.Burke B. Cell biology: nuclear pore complex models gel. Science. 2006;314:766–7. doi: 10.1126/science.1135739. [DOI] [PubMed] [Google Scholar]
- 43.Goldfarb DS, Corbett AH, Mason DA, et al. Importin alpha: a multipurpose nuclear-transport receptor. Trends Cell Biol. 2004;14:505–14. doi: 10.1016/j.tcb.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 44.Yang FM, Lin YC, Hu MC. Identification of two functional nuclear localization signals mediating nuclear import of liver receptor homologue-1. Cell Mol Life Sci. 2011;68:1241–53. doi: 10.1007/s00018-010-0521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harel A, Forbes DJ. Importin beta: conducting a much larger cellular symphony. Mol Cell. 2004;16:319–30. doi: 10.1016/j.molcel.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 46.Liu Q, Yu J, Zhuo X, et al. Pericentrin contains five NESs and an NLS essential for its nucleocytoplasmic trafficking during the cell cycle. Cell Res. 2010;20:948–62. doi: 10.1038/cr.2010.89. [DOI] [PubMed] [Google Scholar]
- 47.Roth DM, Moseley GW, Glover D, et al. A microtubule-facilitated nuclear import pathway for cancer regulatory proteins. Traffic. 2007;8:673–86. doi: 10.1111/j.1600-0854.2007.00564.x. [DOI] [PubMed] [Google Scholar]
- 48.Eldib M, Dean DA. Cyclic stretch of alveolar epithelial cells alters cytoskeletal micromechanics. Biotechnol Bioeng. 2011;108:446–53. doi: 10.1002/bit.22941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moseley GW, Roth DM, DeJesus MA, et al. Dynein light chain association sequences can facilitate nuclear protein import. Mol Biol Cell. 2007;18:3204–13. doi: 10.1091/mbc.E07-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Monsigny M, Rondanino C, Duverger E, et al. Glyco-dependent nuclear import of glycoproteins glycoplexes and glycosylated plasmids. Biochim Biophys Acta. 2004;1673:94–103. doi: 10.1016/j.bbagen.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 51.Miller AM, Dean DA. Cell-specific nuclear import of plasmid DNA in smooth muscle requires tissue-specific transcription factors and DNA sequences. Gene Ther. 2008;15:1107–15. doi: 10.1038/gt.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emmott E, Hiscox JA. Nucleolar targeting: the hub of the matter. EMBO Rep. 2009;10:231–8. doi: 10.1038/embor.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mudhakir D, Harashima H. Learning from the viral journey: how to enter cells and how to overcome intracellular barriers to reach the nucleus. AAPSJ. 2009;11:65–77. doi: 10.1208/s12248-009-9080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weissenhorn W, Hinz A, Gaudin Y. Virus membrane fusion. FEBS Lett. 2007;581:2150–5. doi: 10.1016/j.febslet.2007.01.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mendonca LS, Firmino F, Moreira JN, et al. Transferrin receptor-targeted liposomes encapsulating anti-BCR-ABL siRNA or asODN for chronic myeloid leukemia treatment. Bioconjug Chem. 2010;21:157–68. doi: 10.1021/bc9004365. [DOI] [PubMed] [Google Scholar]
- 56.Koppu S, Oh YJ, Edrada-Ebel R, et al. Tumor regression after systemic administration of a novel tumor-targeted gene delivery system carrying a therapeutic plasmid DNA. J Control Release. 2010;143:215–21. doi: 10.1016/j.jconrel.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 57.Zeng X, Sun YX, Qu W, et al. Biotinylated transferrin/avidin/biotinylated disulfide containing PEI bioconjugates mediated p53 gene delivery system for tumor targeted transfection. Biomaterials. 2010;31:4771–80. doi: 10.1016/j.biomaterials.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 58.Zhang C, Gao S, Jiang W, et al. Targeted minicircle DNA delivery using folate-poly(ethylene glycol)-polyethylenimine as non-viral carrier. Biomaterials. 2010;31:6075–86. doi: 10.1016/j.biomaterials.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 59.Morris VB, Sharma CP. Folate mediated in vitro targeting of depolymerized trimethylated chitosan having arginine functionality. J Colloid Interface Sci. 2010;348:360–8. doi: 10.1016/j.jcis.2010.04.090. [DOI] [PubMed] [Google Scholar]
- 60.McNeeley KM, Karathanasis E, Annapragada AV, et al. Masking and triggered unmasking of targeting ligands on nanocarriers to improve drug delivery to brain tumors. Biomaterials. 2009;30:3986–95. doi: 10.1016/j.biomaterials.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 61.Oba M, Miyata K, Osada K, et al. Polyplex micelles prepared from [omega]-cholesteryl PEG-polycation blockcopolymers for systemic gene delivery. Biomaterials. 2011;32:652–63. doi: 10.1016/j.biomaterials.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 62.Joo KI, Tai A, Lee CL, et al. Imaging multiple intermediates of single-virus membrane fusion mediated by distinct fusion proteins. Microsc Res Tech. 2010;73:886–900. doi: 10.1002/jemt.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banerjee M, Speir JA, Kwan MH, et al. Structure and function of a genetically engineered mimic of a nonenveloped virus entry intermediate. J Virol. 2010;84:4737–46. doi: 10.1128/JVI.02670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leopold PL, Crystal RG. Intracellular trafficking of adenovirus: many means to many ends. Adv Drug Deliv Rev. 2007;59:810–21. doi: 10.1016/j.addr.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 65.FitzGerald DJ, Padmanabhan R, Pastan I, et al. Adenovirus-induced release of epidermal growth factor and pseudomonas toxin into the cytosol of KB cells during receptor-mediated endocytosis. Cell. 1983;32:607–17. doi: 10.1016/0092-8674(83)90480-4. [DOI] [PubMed] [Google Scholar]
- 66.Anderson JL, Hope TJ. Intracellular trafficking of retroviral vectors: obstacles and advances. Gene Ther. 2005;12:1667–78. doi: 10.1038/sj.gt.3302591. [DOI] [PubMed] [Google Scholar]
- 67.Chang KL, Higuchi Y, Kawakami S, et al. Efficient gene transfection by histidine-modified chitosan through enhancement of endosomal escape. Bioconjug Chem. 2010;21:1087–95. doi: 10.1021/bc1000609. [DOI] [PubMed] [Google Scholar]
- 68.Nakase I, Kobayashi S, Futaki S. Endosome-disruptive peptides for improving cytosolic delivery of bioactive macromolecules. Biopolymers. 2010;94:763–70. doi: 10.1002/bip.21487. [DOI] [PubMed] [Google Scholar]
- 69.Shim MS, Kwon YJ. Acid-transforming polypeptide micelles for targeted nonviral gene delivery. Biomaterials. 2010;31:3404–13. doi: 10.1016/j.biomaterials.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 70.Kobayashi S, Nakase I, Kawabata N, et al. Cytosolic targeting of macromolecules using a pH-dependent fusogenic peptide in combination with cationic liposomes. Bioconjug Chem. 2009;20:953–9. doi: 10.1021/bc800530v. [DOI] [PubMed] [Google Scholar]
- 71.Wong SY, Sood N, Putnam D. Combinatorial evaluation of cations pH-sensitive and hydrophobic moieties for polymeric vector design. Mol Ther. 2009;17:480–90. doi: 10.1038/mt.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cohen S, Behzad AR, Carroll JB, et al. Parvoviral nuclear import: bypassing the host nuclear-transport machinery. J Gen Virol. 2006;87:3209–13. doi: 10.1099/vir.0.82232-0. [DOI] [PubMed] [Google Scholar]
- 73.Tan GS, Preuss MA, Williams JC, et al. The dynein light chain 8 binding motif of rabies virus phosphoprotein promotes efficient viral transcription. Proc Natl Acad Sci USA. 2007;104:7229–34. doi: 10.1073/pnas.0701397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bergen JM, Pun SH. Evaluation of an LC8-binding peptide for the attachment of artificial cargo to dynein. Mol Pharm. 2007;4:119–28. doi: 10.1021/mp060086o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campbell EM, Hope TJ. Role of the cytoskeleton in nuclear import. Adv Drug Deliv Rev. 2003;55:761–71. doi: 10.1016/s0169-409x(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 76.Mahato RI. Non-viral peptide-based approaches to gene delivery. J Drug Target. 1999;7:249–68. doi: 10.3109/10611869909085509. [DOI] [PubMed] [Google Scholar]
- 77.Bloomfield VA. DNA condensation. Curr Opin Struct Biol. 1996;6:334–41. doi: 10.1016/s0959-440x(96)80052-2. [DOI] [PubMed] [Google Scholar]
- 78.DeRouchey J, Hoover B, Rau DC. A comparison of DNA compaction by arginine and lysine peptides: a physical basis for arginine rich protamines. Biochemistry. 2013;52:3000–9. doi: 10.1021/bi4001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Martin ME, Rice KG. Peptide-guided gene delivery. AAPS J. 2007;9:E18–29. doi: 10.1208/aapsj0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Varga CM, Wickham TJ, Lauffenburger DA. Receptor-mediated targeting of gene delivery vectors: Insights from molecular mechanisms for improved vehicle design. Biotechnol Bioeng. 2000;70:593–605. doi: 10.1002/1097-0290(20001220)70:6<593::aid-bit1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 81.Wadhwa MS, Collard WT, Adami RC, et al. Peptide-mediated gene delivery: influence of peptide structure on gene expression. Bioconjug Chem. 1997;8:81–8. doi: 10.1021/bc960079q. [DOI] [PubMed] [Google Scholar]
- 82.McKenzie DL, Collard WT, Rice KG. Comparative gene transfer efficiency of low molecular weight polylysine DNA-condensing peptides. J Pept Res. 1999;54:311–18. doi: 10.1034/j.1399-3011.1999.00104.x. [DOI] [PubMed] [Google Scholar]
- 83.Haines AM, Irvine AS, Mountain A, et al. CL22 – a novel cationic peptide for efficient transfection of mammalian cells. Gene Ther. 2001;8:99–110. doi: 10.1038/sj.gt.3301314. [DOI] [PubMed] [Google Scholar]
- 84.Temsamani J, Vidal P. The use of cell-penetrating peptides for drug delivery. Drug Discov Today. 2004;9:1012–19. doi: 10.1016/S1359-6446(04)03279-9. [DOI] [PubMed] [Google Scholar]
- 85.Cardarelli F, Serresi M, Albanese A, et al. Quantitative analysis of Tat peptide binding to import carriers reveals unconventional nuclear transport properties. J Biol Chem. 2011;286:12292–9. doi: 10.1074/jbc.M110.203083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de la Fuente JM, Berry CC. Tat peptide as an efficient molecule to translocate gold nanoparticles into the cell nucleus. Bioconjug Chem. 2005;16:1176–80. doi: 10.1021/bc050033+. [DOI] [PubMed] [Google Scholar]
- 87.Li W, Nicol F, Szoka FC., Jr GALA: a designed synthetic pH-responsive amphipathic peptide with applications in drug and gene delivery. Adv Drug Deliv Rev. 2004;56:967–85. doi: 10.1016/j.addr.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 88.Soltani F, Sankian M, Hatefi A, et al. Development of a novel histone H1-based recombinant fusion peptide for targeted non-viral gene delivery. Int J Pharm. 2013;441:307–15. doi: 10.1016/j.ijpharm.2012.11.027. [DOI] [PubMed] [Google Scholar]
- 89.Yi WJ, Yang J, Li C, et al. Enhanced nuclear import and transfection efficiency of TAT peptide-based gene delivery systems modified by additional nuclear localization signals. Bioconjug Chem. 2012;23:125–34. doi: 10.1021/bc2005472. [DOI] [PubMed] [Google Scholar]
- 90.Lee MS, Huang YH, Huang SP, et al. Identification of a nuclear localization signal in the polo box domain of Plk1. Biochim Biophys Acta. 2009;1793:1571–8. doi: 10.1016/j.bbamcr.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 91.Kosugi S, Hasebe M, Matsumura N, et al. Six classes of nuclear localization signals specific to different binding grooves of importin α. J Biol Chem. 2009;284:478–85. doi: 10.1074/jbc.M807017200. [DOI] [PubMed] [Google Scholar]
- 92.Bremner KH, Seymour LW, Logan A, et al. Factors influencing the ability of nuclear localization sequence peptides to enhance nonviral gene delivery. Bioconjug Chem. 2004;15:152–61. doi: 10.1021/bc034140k. [DOI] [PubMed] [Google Scholar]
- 93.Kim K, Han JS, Kim HA, et al. Expression purification and characterization of TAT-high mobility group box-1A peptide as a carrier of nucleic acids. Biotechnol Lett. 2008;30:1331–7. doi: 10.1007/s10529-008-9695-4. [DOI] [PubMed] [Google Scholar]
- 94.Wagstaff KM, Glover DJ, Tremethick DJ, et al. Histone-mediated transduction as an efficient means for gene delivery. Mol Ther. 2007;15:721–31. doi: 10.1038/sj.mt.6300093. [DOI] [PubMed] [Google Scholar]
- 95.Kaouass M, Beaulieu R, Balicki D. Histonefection: novel and potent non-viral gene delivery. J Control Release. 2006;113:245–54. doi: 10.1016/j.jconrel.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 96.Josephson L, Tung CH, Moore A, et al. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug Chem. 1999;10:186–91. doi: 10.1021/bc980125h. [DOI] [PubMed] [Google Scholar]
- 97.Lewin M, Carlesso N, Tung CH, et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat Biotechnol. 2000;18:410–14. doi: 10.1038/74464. [DOI] [PubMed] [Google Scholar]
- 98.Torchilin VP, Rammohan R, Weissig V, et al. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc Natl Acad Sci U S A. 2001;98:8786–91. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Torchilin VP, Levchenko TS, Rammohan R, et al. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc Natl Acad Sci U S A. 2003;100:1972–7. doi: 10.1073/pnas.0435906100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gupta B, Levchenko TS, Torchilin VP. TAT peptide-modified liposomes provide enhanced gene delivery to intracranial human brain tumor xenografts in nude mice. Oncol Res. 2007;16:351–9. doi: 10.3727/000000006783980946. [DOI] [PubMed] [Google Scholar]
- 101.Rudolph C, Schillinger U, Ortiz A, et al. Application of novel solid lipid nanoparticle (SLN)-gene vector formulations based on a dimeric HIV-1 TAT-peptide in vitro and in vivo. Pharm Res. 2004;21:1662–9. doi: 10.1023/b:pham.0000041463.56768.ec. [DOI] [PubMed] [Google Scholar]
- 102.Huang Z, Li W, MacKay JA, et al. Thiocholesterol–basedlipids for ordered assembly of bioresponsive gene carriers. Mol Ther. 2005;11:409–17. doi: 10.1016/j.ymthe.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 103.Kleemann E, Neu M, Jekel N, et al. Nano-carriers for DNA delivery to the lung based upon a TAT-derived peptide covalently coupled to PEG-PEI. J Control Release. 2005;109:299–316. doi: 10.1016/j.jconrel.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 104.Lai WF, Tang GP, Wang X, et al. Cyclodextrin-PEI-Tat polymer as a vector for plasmid DNA delivery to placenta mesenchymal stem cells. Bionanoscience. 2011;1:89–96. doi: 10.1007/s12668-011-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aronsohn AI, Hughes JA. Nuclear localization signal peptides enhance cationic liposome-mediated gene therapy. J Drug Target. 1998;5:163–9. doi: 10.3109/10611869808995871. [DOI] [PubMed] [Google Scholar]
- 106.Tachibana R, Harashima H, Shono M, et al. Intracellular regulation of macromolecules using pH-sensitive liposomes and nuclear localization signal: qualitative and quantitative evaluation of intracellular trafficking. Biochem Biophys Res Commun. 1998;251:538–44. doi: 10.1006/bbrc.1998.9460. [DOI] [PubMed] [Google Scholar]
- 107.Yin J, Meng X, Zhang S, et al. The effect of a nuclear localization sequence on transfection efficacy of genes delivered by cobalt(II)-polybenzimidazole complexes. Biomaterials. 2012;33:7884–94. doi: 10.1016/j.biomaterials.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 108.Kanazawa T, Takashima Y, Murakoshi M, et al. Enhancement of gene transfection into human dendritic cells using cationic PLGA nanospheres with a synthesized nuclear localization signal. Int J Pharm. 2009;379:187–95. doi: 10.1016/j.ijpharm.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 109.Shen Y, Peng H, Deng J, et al. High mobility group box 1 protein enhances polyethylenimine mediated gene delivery in vitro. Int J Pharm. 2009;375:140–7. doi: 10.1016/j.ijpharm.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 110.Shen Y, Peng H, Pan S, et al. Interaction of DNA/nuclear protein/polycation and the terplexes for gene delivery. Nanotechnology. 2010;21:45–102. doi: 10.1088/0957-4484/21/4/045102. [DOI] [PubMed] [Google Scholar]
- 111.Kastrup L, Oberleithner H, Ludwig Y, et al. Nuclear envelope barrier leak induced by dexamethasone. J Cell Physiol. 2006;206:428–34. doi: 10.1002/jcp.20479. [DOI] [PubMed] [Google Scholar]
- 112.Mi Bae Y, Choi H, Lee S, et al. Dexamethasone-conjugated low molecular weight polyethylenimine as a nucleus-targeting lipopolymer gene carrier. Bioconjug Chem. 2007;18:2029–36. doi: 10.1021/bc070012a. [DOI] [PubMed] [Google Scholar]
- 113.Choi JS, Ko KS, Park JS, et al. Dexamethasone conjugated poly(amidoamine) dendrimer as a gene carrier for efficient nuclear translocation. Int J Pharm. 2006;320:171–8. doi: 10.1016/j.ijpharm.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 114.Fan Y, Yao J, Du R, et al. Ternary complexes with core-shell bilayer for double level targeted gene delivery: in vitro and in vivo evaluation. Pharm Res. 2013;30:1215–27. doi: 10.1007/s11095-012-0960-9. [DOI] [PubMed] [Google Scholar]
- 115.Fan Y, Yao J, Zhou J. Nucleus-targeting polyethyleneimine-dexamethasone conjugates for gene delivery. Chem J Chin U. 2011;32:2916–22. [Google Scholar]
- 116.Singh AT, Evens AM, Anderson RJ, et al. All trans retinoic acid nanodisks enhance retinoic acid receptor mediated apoptosis and cell cycle arrest in mantle cell lymphoma. Br J Haematol. 2010;150:158–69. doi: 10.1111/j.1365-2141.2010.08209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schug TT, Berry DC, Shaw NS, et al. Opposing effects of retinoic acid on cell growth result from alternate activation of two different nuclear receptors. Cell. 2007;129:723–33. doi: 10.1016/j.cell.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park KM, Kang HC, Cho JK, et al. All-trans-retinoic acid (ATRA)-grafted polymeric gene carriers for nuclear translocation and cell growth control. Biomaterials. 2009;30:2642–52. doi: 10.1016/j.biomaterials.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 119.Charoensit P, Kawakami S, Higuchi Y, et al. Enhanced growth inhibition of metastatic lung tumors by intravenous injection of ATRA-cationic liposome/IL-12 pDNA complexes in mice. Cancer Gene Ther. 2010;17:512–22. doi: 10.1038/cgt.2010.12. [DOI] [PubMed] [Google Scholar]
- 120.Li J, Chen YC, Tseng YC, et al. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J Control Release. 2010;142:416–21. doi: 10.1016/j.jconrel.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang Y, Li J, Liu F, et al. Systemic delivery of siRNA via LCP nanoparticle efficiently inhibits lung metastasis. Mol Ther. 2012;20:609–15. doi: 10.1038/mt.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li J, Yang Y, Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J Control Release. 2012;158:108–14. doi: 10.1016/j.jconrel.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maitra A. Calcium phosphate nanoparticles: second-generation nonviral vectors in gene therapy. Expert Rev Mol Diagn. 2005;5:893–905. doi: 10.1586/14737159.5.6.893. [DOI] [PubMed] [Google Scholar]
- 124.Bisht S, Bhakta G, Mitra S, et al. pDNA loaded calcium phosphate nanoparticles: highly efficient non-viral vector for gene delivery. Int J Pharm. 2005;288:157–68. doi: 10.1016/j.ijpharm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 125.Hu Y, Haynes MT, Wang Y, et al. A highly efficient synthetic vector: nonhydrodynamic delivery of DNA to hepatocyte nuclei in vivo. ACS Nano. 2013;7:5376–84. doi: 10.1021/nn4012384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Trump BF, Berezesky IK. Calcium-mediated cell injury and cell death. FASEB J. 1995;9:219–28. doi: 10.1096/fasebj.9.2.7781924. [DOI] [PubMed] [Google Scholar]
- 127.Kogure K, Akita H, Harashima H. Multifunctional envelope-type nano device for non-viral gene delivery: concept and application of programmed packaging. J Control Release. 2007;122:246–51. doi: 10.1016/j.jconrel.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 128.Khalil IA, Kogure K, Futaki S, et al. Octaarginine-modified multifunctional envelope-type nanoparticles for gene delivery. Gene Ther. 2007;14:682–9. doi: 10.1038/sj.gt.3302910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakamura Y, Kogure K, Yamada Y, et al. Significant and prolonged antisense effect of a multifunctional envelope-type nano device encapsulating antisense oligodeoxynucleotide. J Pharm Pharmacol. 2006;58:431–7. doi: 10.1211/jpp.58.4.0002. [DOI] [PubMed] [Google Scholar]
- 130.Yamada Y, Nomura T, Harashima H, et al. Intranuclear DNA release is a determinant of transfection activity for a non-viral vector: biocleavable polyrotaxane as a supramolecularly dissociative condenser for efficient intranuclear DNA release. Biol Pharm Bull. 2010;33:1218–22. doi: 10.1248/bpb.33.1218. [DOI] [PubMed] [Google Scholar]
- 131.Akita H, Kudo A, Minoura A, et al. Multi-layered nanoparticles for penetrating the endosome and nuclear membrane via a step-wise membrane fusion process. Biomaterials. 2009;30:2940–9. doi: 10.1016/j.biomaterials.2009.02.009. [DOI] [PubMed] [Google Scholar]