Abstract
siRNA therapeutics has developed rapidly and already there are clinical trials ongoing or planned; however, the delivery of siRNA into cells, tissues or organs remains to be a major obstacle. Lipid-based vectors hold the most promising position among non-viral vectors, as they have a similar structure to cell or organelle membranes. But when used in the form of liposomes, these vectors have shown some problems. Therefore, either the nature of lipids themselves or forms used should be improved. As a novel class of lipid like materials, lipidoids have the advantages of easy synthesis and the ability for delivering siRNA to obtain excellent silencing activity. However, the toxicities of lipidoids have not been thoroughly studied. pH responsive lipids have also gained great attention recently, though some of the amine-based lipids are not novel in terms of chemical structures. More complex self-assembly structures, such as LPD (LPH) and LCP, may provide a good solution to siRNA delivery. They have demonstrated controlled particle morphology and size and siRNA delivery activity for both in vitro and in vivo.
Keywords: Lipids, siRNA delivery, RNA interference, gene therapy
Introduction
RNA interference (RNAi) was discovered by Fire et al. (1998) who demonstrated that double-stranded RNA is much more effective at producing interference than either strand individually and that interference occurs at the post-transcriptional level. It has been recognized as ‘one of the most exciting discoveries in biology in the last couple of years’ (Jana, 2004). The potent gene silencing in a sequence specific manner has caused it to attract much attention for applications to biosciences and medicines (John, 2007; Karagiannis, 2005; Geisbert, 2010; Pan, 2011). RNAi is a naturally occurring process that mediates sequence specific inhibition of gene expression through the activation of a protein complex called RNA-induced silencing complex (RISC). RNAs in RISC are small duplex molecules termed siRNAs (21–23nt) produced through the cleavage of long dsRNAs (Zamore, 2000). The RISC cleaves the target mRNA at a sequence-specific position, guided by the antisense strand of the siRNA. It has been recognized that the siRNA strand incorporated into RISC is recycled, thus repeatedly down-regulating gene expression with only a small amount of siRNA. The entire process occurs within the cytoplasm of the cell, thus circumventing the need for nuclear delivery and alleviating concerns over the direct modification of the host genome. Therefore, researchers have paid considerable attention for its potential to down-regulate genes for therapies (Tan, 2011). This is especially the case for the ‘undrugable targets’.
Though siRNAs have advantages over the long dsRNAs, such as being easily prepared by chemical methods and easily handled during biological assays, they still exhibit the problems of poor membrane permeability and nuclease resistance which limit their applicability to therapeutic use. Therefore, RNAi therapeutics requires suitable delivery vehicles for both in vitro and in vivo applications (Perkel, 2009). The success of RNAi critically depends on suitable delivery vectors that have the high efficiency transfer of siRNA to target cells, as well as a favourable safety profile. An ideal vehicle for cancer therapy should meet at least four major criteria: the evasion of the mononuclear phagocytic system (MPS), extravasation from the blood circulation into the tumor, diffusion through the extracellular matrix to bind with tumor cells, and escape from the endosome to release the cargo siRNA into the cytoplasm (Whitehead, 2009; Wang, 2012).
Broadly, the vectors are classified mainly into two categories: viral and non-viral (Liu, 2002). The successful application of siRNA, is largely dependent on the development of a delivery vehicle which should be administered efficiently, safely, and repeatedly, if needed. Viral systems usually give high transfection efficiencies, but safety concerns from potential mutation, recombination, oncogenic effect and high cost greatly limit their therapeutic applications. In contrast, non-viral vectors are believed to cause fewer safety problems due to their relative simplicity. Lipids have long been known to be the most promising vectors, as they are amphiphilic molecules that spontaneously assemble into micelles or bilayers. An extensive range of lipids for the delivery of siRNA have been developed, though nonspecific cytotoxicity associated with cationic liposomes has been observed (Farhood, 1992; Romoren, 2004; Scales, 2006).
Since the first description of successful in vitro transfection with a cationic lipid by Felgner et al in 1987 (Felgner, 1987), numerous cationic lipids have been synthesized and used for delivery of nucleic acids into cells during the last 25 years (Adrian, 2010; Mével, 2010; Tao, 2010; Guo, 2011; Sparks, 2012). Cationic lipids were first used in the form of liposomes, as they could improve the gene delivery efficacy owing to their typical bilayer structure when forming lipoplexes with nucleic acids. Some helper lipids (co-lipids) such as cholesterol, dioleylphosphatidyl choline (DOPC) or dioleylphosphatidyl ethanolamine (DOPE), typically neutral lipids (Zuhorn, 2005), are often employed with cationic lipids. They play a very important role during the formation of lipoplexes by combining cationic liposomes and siRNA, as they could determine the morphology of lipoplex. Many reviews (Zabner, 1997; Woodle, 2001; Zabner, 2002; Zhang, 2004) discussing cationic liposomes for plasmid DNA delivery are available. It seems that cationic lipids combined with co-lipids could not meet the requirements of siRNA delivery in spite of the fact that a large amount of compounds have been explored.
A brief overview of lipid based liposomes related to siRNA delivery
Since the pioneer work in the late 1980s, a large amount of papers have been published on the delivery of genetic materials via liposomes (Malone, 1989). There are a number of commercially available cationic liposome/lipid based systems, such as DOTAP, Lipofectin, RNAifect, Oligofectamine, Lipofectamine and TransIT TKO (Omidi, 2003; Gilmore, 2004; Khan, 2004; Judge, 2005; Morrissey, 2005; Pirollo, 2007). One of the earliest lipoplexes developed for nucleic acid delivery is the commercially-available Lipofectin (Felgner, 1987). This formulation of cationic liposomes, assembled from a mixture of N -[1-(2,3-dioleyloxy)propyl]-N,N,N-trimethylammonium chloride (DOTMA) and DOPE, demonstrated successful transfection with mRNA for a broad range of cell lines (Malone, 1989). The formulation has also shown successful transfection with siRNA for human cell lines (Beale, 2003). DOTAP (N-[1-(2,3-dioleoyloxy)]-N,N,N -trimethyl ammonium propane) and Oligofectamine were some of the first lipid formulations to be used for the in vivo delivery of siRNA and effective gene silencing of tumor necrosis factor α (TNF-α) and β-catenin in mice (Sorensen, 2003; Verma, 2003). Fluorescein-labeled siRNA was injected into adult mice to investigate cationic liposome-mediated intravenous and intraperitoneal delivery. The results showed that DOTAP containing liposomes can deliver siRNA into various cell types. Unlike in mouse cells, these siRNA can activate the nonspecific pathway in human freshly isolated monocytes to produce TNF-α and IL-6 (Sioud, 2003). Sorensen et al. (2003) also used cationic DOTAP liposomes to inject siRNA against TNF-α resulting in a suppression of the lethal reaction to lipopolysaccharide (LPS) injections in a mouse model of sepsis. Additionally, successful silencing of a marker gene (GFP) in liver cells after intravenous injection of liposomes was reported. Flynn et al. (2004) used Lipofectamine to deliver IL12-p40siRNA to target the expression of IL12-p40 in a model of LPS-induced inflammation. Significant reduction of immune reaction in treated animals was obtained, presumably via reduced IL12 production in peritoneal macrophages.
Many other non-commercial cationic lipids are being investigated for promising uses both in vitro and in vivo. For example, Khoury et al. (2006) demonstrated cationic lipid 2-(3-[Bis-(3-amino-propyl)-amino]-propylamino)-N-ditetradecylcarbamoylme-thylacetamide (RPR209120) combined with DOPE can efficiently deliver siRNA designed to silence TNF-α in collagen-induced arthritis. Similarly, Sato et al. (2007) indicated that a galactosylated liposome/siRNA complex could induce silencing of endogenous hepatic gene expression with no observed liver toxicity. Grinstaff and co-workers connected nucleosides with alkyl chains to create nucleoside lipids for gene (Chabaud, 2006) and siRNA (Ceballos, 2009) delivery in a human liver cell line. The unique feature of this concept is that the system has been designed to be charge-reversible (Tan, 2011). Akinc et al. (2008) designed and synthesized a large amount of compounds called lipidoids which could show promising applications in the future. One method for improving the efficiency of transfection is to link lipophilic siRNA to lipid moieties (e.g. derivatives of cholesterol, lithocholic acid or lauric acid). The lipid moieties are covalently linked to the 5′-ends of the RNAs using phosphoramidite chemistry. It was found that siRNA with a modified sense strand down-regulated β-galactosidase expression to a higher extent than either siRNA with a modified antisense strand or two modified strands (Lorenz, 2004).
PEGylation is a successful way to improve pharmacokinetics of siRNA in vivo and to form ‘stealth’ liposomes (SL). PEGylated liposomes are a clinically approved delivery system for doxorubicin, and therefore represent a viable option for delivering siRNA in humans (Zimmermann, 2006). When dipalmitoylphosphatidylcholine (DPPC), dipalmitoylphosphatidylglycerol (DPPG) and dipalmitoylphosphatidylethanolamine-polyethyleneglycol2000 (DPPE-PEG2000) were combined to prepare DPPG:DPPC:DPPE-PEG2000 SL, entrapped siRNA targeting enhanced green fluorescent protein (EGFP) did not silence gene expression of HeLa-cells stably expressing EGFP. However, preliminary flow cytometry and confocal microscopy data showed that the SL siRNA formulation increased uptake of siRNA into vesicular compartments of HeLa cells in a concentration-dependent manner that could be augmented by exogenous sPLA2. SL can also be used to apply target siRNA to inflamed tissue for silencing cytokine expression in rheumatoid arthritis (Foged, 2007).
Even after many years of research, lipoplex still needs to be improved in terms of both stability and toxicity to cells. Though the stability and delivery efficiency of lipoplex can be enhanced by increasing the ratio of cationic lipid to siRNA, it can also lead to an increase in cytotoxicity (Ozpolat, 2010). Some solutions have been suggested. For example, pentavalent cationic lipoplexes have been used for siRNA delivery. These multivalent lipids exhibited not only a higher silencing efficiency than monovalent lipids, but also a lower cytotoxicity by minimizing the amount of lipids required for complex formation (Bouxsein, 2007).
Though cationic liposome-mediated RNAi has become popular in recent years, not all responses are positive. The large proportion of ‘unused’ RNA molecules could become toxic for cells or trigger a response that could change cell metabolism (Barreau, 2006). Clearly, our understanding of liposome delivery of siRNA is still evolving, and more research is needed. Given more research, especially regarding experiments done in vivo, liposome delivery may be developed into a promising tool for therapeutic application of siRNA (Li, 2006a). For this reason, we will focus on two promising classes of lipids: lipidoids and pH responsive lipids. To overcome the drawbacks of lipoplex, researchers have assembled different nanostructures such as lipid-protamine-DNA (LPD), lipid-calcium-phosphate (LCP), solid lipid nanoparticles (SLN) and stable nucleic acid-lipid particles (SNALP) based on lipids. We will illustrate examples of these self-assembled structures. The aim is to build a bridge between lipids and controlled assemblies for facilitating the use of lipids for siRNA delivery.
pH-responsive cationic lipids
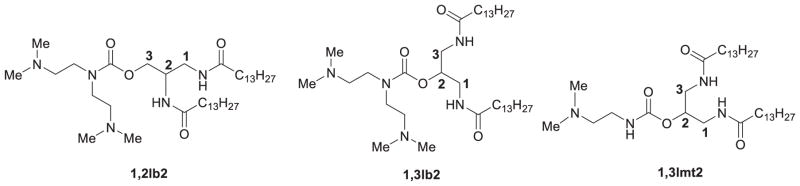
pH-responsive lipids for efficient drug/gene delivery have attracted increasing attention in the past decade. Tertiary amines have long been used for the delivery of genes, but have been found to have more uses regarding response to the pH of cells. For example, Spelios (2007) synthesized cationic lipids by using bis-(2-dimethylaminoethane) and mono-(2-dimethylaminoethane) as head groups through the linkage of a carbamate bond (Figure 1). The bis-heads lipids were more ionizable than mono-head ones; the pH-expandable polar head-groups and greater intramolecular distance between the hydrophobic chains formed assemblies that resulted in high transfection activity. Efficient binding and compaction of pDNA, increased acyl chain fluidity, and high molecular elasticity all contributed to the high transfection activity. However, the authors did not mention any effects of carbamate on the physicochemical properties and transfection efficiency, as carbamate itself is a pH dependent group. When incorporating a carbamate group into the linker, it may be assumed that the pH decrease will act as a trigger, disconnecting the hydrophobic and hydrophilic portions of the lipoplex, releasing DNA after entering endosomes within the cell (Liu, 2005a). We synthesized carbamate-linked cationic lipids for liposome-mediated gene delivery, which proved to have good gene transfection properties in different cell lines. As they are chemically stable and biodegradable, many cationic lipids of this kind have been synthesized (Liu, 2005a; Liu, 2005b; Liu, 2008; Zhao, 2011). To maintain the balance between serum stability and transfection efficiency, Chan et al. (2012a, 2012b) have designed and synthesized a hydrolysable acid-labile PEG-lipid (HPEG-lipid, PEG MW 2000). This PEG-lipid was stable at physiological pH, but was cleaved at a low pH; the HPEG-lipid was stable at a neutral pH for more than 24 h, but degraded completely within 1 h at pH 4, leading to particle aggregation. HPEG-lipoplexes showed lower toxicity and enhanced transfection in comparison to lipoplexes stabilized with pH-stable PEG-lipids. Live-cell images showed that both pH-sensitive and pH-stable PEG-lipoplexes were internalized to quantitatively similar particle distributions within the first 2 h of incubation. Thus, the increased transfection of the HPEG-lipoplexes can be attributed to efficient endosomal escape, enabled by the novel HPEG-lipid.
Figure 1.
Chemical structures of lipids with bis-(2-dimethylaminoethane) and mono-(2-dimethylaminoethane) as head groups.
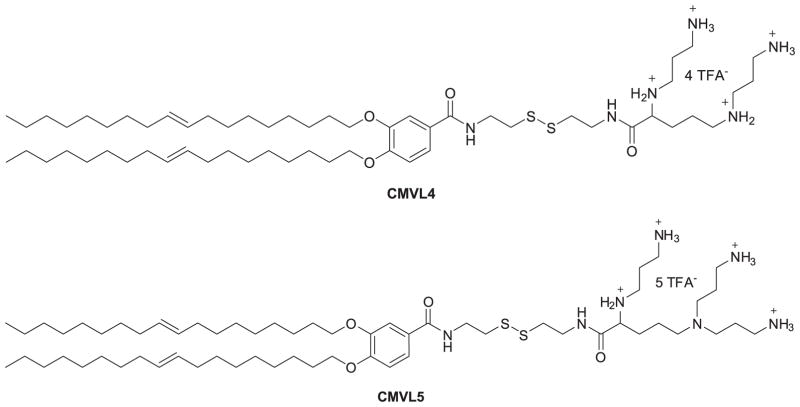
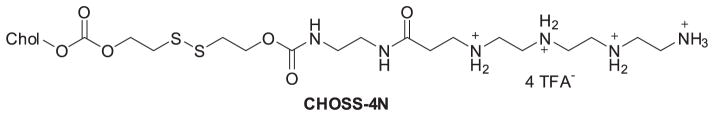
Based on their previous study of multivalent cationic lipids (MVLn, n = 2 to 5) (Ewert, 2002; Ahmad, 2005; Bouxsein, 2007), Shirazi et al. (2011) put a disulfide bond spacer between the headgroup and lipophilic tails of MVLn lipids to give a series of degradable vectors (CMVLn) for gene delivery. This spacer would respond to the reducing milieu of the cytoplasm and be cleaved to decrease lipid toxicity. Among these degradable lipids, CMVL4 and CMVL5 (Figure 2) showed transfection efficiency comparable to MVL5 and some commercial transfection reagents while also having lower toxicity to cells. These results demonstrated that degradable disulfide spacers may be used to reduce the cytotoxicity of synthetic nonviral gene delivery carriers without compromising their transfection efficiency. Although the disulfide bond incorporated into cationic lipids for pH-based response is not a novel idea (Jiang, 2010), there is still a relatively positive impact on the decreased toxicity elicited. This research therefore appears to be very promising opportunity for further lipid design utilizing disulfide bonds for enhancing the performance of siRNA delivery vectors. For example, a new series of cholesterol-disulfide lipids bearing cholesterol and a variety of head groups via disulfide and carbonate bond linkages have been synthesized. The results demonstrated low cytotoxicity, strong pDNA binding affinity, high transfection, particularly high intracellular uptake capability, and specific cellular localization of pDNA at the periphery of cell nuclei for newly prepared CHOSS lipids (Figure 3) (Sheng, 2011).
Figure 2.
Chemical structures of CMVL4 and CMVL5.
Figure 3.
Chemical structures of CHOSS-4N.
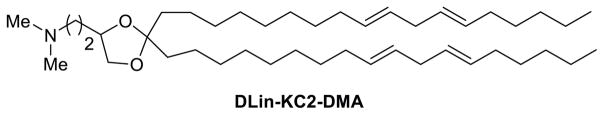
Based on the work of pH-responsive lipids for gene delivery Semple et al. (2010) synthesized a series of pH-responsive lipid molecules that contain ionizable amine-based head groups, which could efficiently formulate nucleic acids at a low pH and maintain a neutral or low cationic surface charge density at pH 7.4. By maintaining this surface charge density, the amine head group provides a longer half-life in circulation and reduces nonspecific cytotoxicity. Once reaching the acidic environment of the endosome, the head group should become protonated and positively charged so as to be available for ion pairing with the negatively charged endosomal lipids. The best-performing lipid, DLin-KC2-DMA (Figure 4), containing two cis double bonds per hydrocarbon chain, a tertiary amine head group and a ketal-ring linker, was formulated into SNALP. This lipid was shown to be well-tolerated in both rodents and non-human primates, exhibiting in vivo activity at siRNA doses as low as 0.01 mg/kg in rodents, as well as the silencing of a therapeutically significant gene (TTR) in nonhuman primates. Lipids showing pH-responsive ability are successful both in vitro and in vivo; their low toxicity and low dose of siRNA in the formulations may lead them to clinical success.
Figure 4.
Chemical structure of DLin-KC2-DMA.
Lipidoids
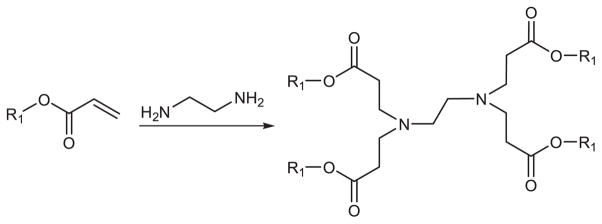
Akinc (2008) synthesized a library of over 1200 lipid-like materials through the conjugate addition of an amine to an acrylate or acrylamide, termed lipidoids (Figure 5). Depending on the number of addition sites in the amino monomer, lipidoids can be formed 1 to 7 tails. The advantage of this method is the one-step synthetic scheme which enables the straightforward parallel generation of large libraries of delivery materials. The safety and efficacy of lipidoids were evaluated for siRNA delivery performance in three animal models: mice, rats and nonhuman primates. Therapeutic efficacy was observed in vivo in liver, lung and peritoneal macrophages. The author concluded that certain design criteria were necessary for creating future intracellular delivery agents, including (i) amide linkages, (ii) more than two alkyl tails, (iii) tail length in the range of 8–12 carbons and, (iv) a secondary amine. The study suggests that these materials may have broad utility for both local and systemic delivery of RNA therapeutics.
Figure 5.
Synthesis schematic of lipidoids by the conjugate addition of amine to α,β-unsaturated carbonyl compounds.
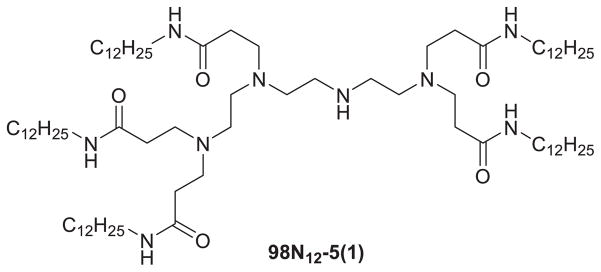
Based on one of the novel lipid-like materials, 98N12-5(1) (Figure 6), the group obtained a final optimized formulation (LNP01). LNP01 has a lipid composition of 98N12-5(1):cholesterol:PEG lipid = 42:48:10 (mol:mol:mol), total lipid:siRNA = ~7.5:1 (wt:wt), C14 alkyl chain length on the PEG lipid, and a particle size of roughly 50–60 nm. It has been used successfully to silence multiple (>10) genes in several species (i.e. mouse, rat, hamster, and monkey). The lead formulation developed was liver targeted (>90% injected dose distributes to liver) and can induce fully reversible, long-duration gene silencing without a loss of activity following repeat administration (Akinc, 2009). Huang et al. (2009) used these lipidoids to deliver CLDN3 siRNA in 3 different ovarian cancer models. Their results suggested intratumoral injection of lipidoid/CLDN3 siRNA into OVCAR-3 xenografts resulted in dramatic silencing of CLDN3, significant reduction in cell proliferation, reduction in tumor growth, and a significant increase in the number of apoptotic cells. Furthermore, intraperitoneal injection of lipidoid-formulated CLDN3 siRNA resulted in a substantial reduction in tumor burden in MISIIR/Tag transgenic mice and mice bearing tumors derived from mouse ovarian surface epithelial cells. Toxicity was not observed after multiple i.p. injections and treatment of mice with nonimmunostimulatory 2′-OMe modified CLDN3 siRNA was as effective in suppressing tumor growth as unmodified siRNA. This method may provide a therapeutic solution to ovarian cancer.
Figure 6.
Chemical structure of 98N12-5(1).
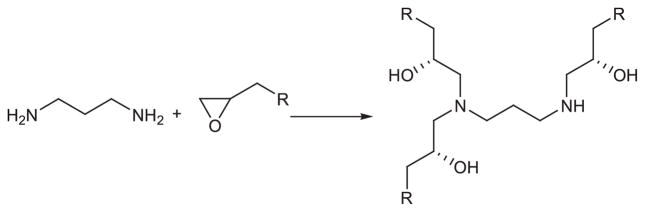
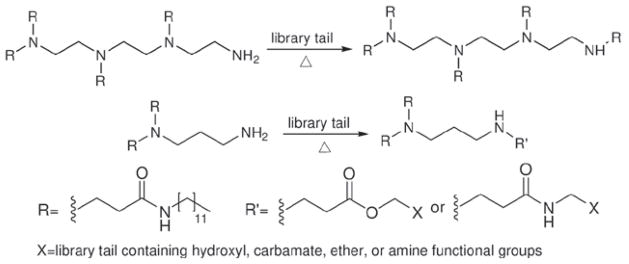
After the first library of lipidoids was proved effective for delivery of siRNA, Love (2010) made an epoxide-derived lipidoid library through combinatorial synthesis (Figure 7). A formulation has been identified that enabled siRNA-directed liver gene silencing in mice at doses below 0.01 mg/kg. After a single injection, this formulation was shown to specifically inhibit expression of five hepatic genes simultaneously. The potential of this formulation was further validated in nonhuman primates, where high levels of knockdown of the clinically relevant gene transthyretin were observed at doses as low as 0.03 mg/kg. Further, they synthesized materials of lipid-like tails and feature appendages containing hydroxyl, carbamate, ether, or amine functional groups as well as variations in alkyl chain length and branching (Figure 8). The relationship between lipid chemical modification and delivery performance in vitro was studied using a luciferase reporter system in HeLa cells to show the impact of the functional group depending on the overall amine content and tail number of the delivery vectors (Mahon, 2010).
Figure 7.
Synthesis schematic of lipidoids by the conjugate addition of amine to epoxides.
Figure 8.
Synthesis schematic of lipidoids by the conjugate addition of amine to lipid-like tails containing hydroxyl, carbamate, ether, or amine functional groups.
More recently, binary combinations of these ionizable, lipid-like materials have been used to synergistically achieve gene silencing (Whitehead, 2011). They found that ineffective, individual lipid-like materials could be formulated together in a single delivery vehicle to induce near-complete knockdown of firefly luciferase and factor VII in HeLa cells and in mice, respectively. Among the 3,780 formulations that were made through 630 binary pairs of 36 synthetic lipidoids, the combinations of 86N15–98O13 were chosen as representatives to demonstrate the synergistic action by mediated cellular uptake and endosomal escape. The data indicated that formulating lipid-like materials in combination can significantly improve siRNA delivery outcomes while also increasing the material space available for therapeutic development. Research demonstrates the binary formulation strategy could be an important technique for siRNA or other nucleic acid delivery.
Hybrid siRNA delivery systems based on lipids
Since cationic lipids were successfully used for the delivery of nucleic acids, researchers have been designing and synthesizing a large amount of new compounds (Arpicco, 2004; Bianco, 2005). However, they do not function well in terms of transfection efficiency, leading to the development of hybrid delivery systems based on lipids. At first, helper lipids were incorporated in the systems to yield high transfection efficiency through controlling the morphology (Ma, 2007) of lipoplex and destabilizing the endosome membrane (Farhood, 1995). Later, various lipid-based nano-assemblies including LPD (Li, 2006b), LCP (Li, 2010), SNALP (Heyes, 2005; Morrissey, 2005), and SLN, were produced through the combined use of lipids, proteins, polymers, inorganic particles and other materials. These nano-assemblies are believed to be much more uniform in size than lipoplex and have the ability to escape from clearance to enter into targeted cells. Hence, more of these assemblies are entering the clinical trial phase.
LPD (LPH)
Gao (1996) discovered that several cationic polymers with high molecular weights, such as poly(L-lysine) and protamine, can enhance the transfection efficiency of some cationic liposomes by 2–28-fold in a number of cell lines in vitro. This discovery was based on a report that some polycations, such as polylysine, histone, and protamine, can effectively condense DNA to about 30–100 nm in diameter (Wagner, 1991). It was then found that the condensation agent protamine sulfate USP was superior to poly-L-lysine and other types of protamine (Sorgi, 1997). At the same, LPD was used to transfer DNA in vivo. The optimal dose was approximately 50 mg per mouse at which a concentration of approximately 20 ng luciferase protein per milligram extracted tissue protein could be detected in the lung (Li, 1997).
Based on the study of DNA delivery by using LPD, the formulation was extended for the delivery of siRNA. AS-ODN or siRNA against human survivin was mixed with a carrier DNA (calf thymus DNA) before complexing with protamine. The resulting particles were coated with cationic liposomes, consisting of DOTAP and cholesterol, to obtain LPD nanoparticles. Finally, ligand targeting and steric stabilizing components were incorporated into the preformed LPD nanoparticles using DSPE-PEG-anisamide. It was found that tumor cell delivery and antisense activity of PEGylated nanoparticles were sequence dependent and rely on the presence of the anisamide ligand. The uptake of oligonucleotide in targeted, PEGylated nanoparticles could be completed by excess free ligand. The results suggest that the ligand-targeted and sterically-stabilized nanoparticles can provide a selective delivery of AS-ODN and siRNA into lung cancer cells, down-regulate survivin, and sensitize the cells to anticancer drugs (cisplatin) (Li, 2006b; Li, 2006c).
The systemic in vivo study using the ligand targeted, PEGylated LPD formulation showed significant increase in cellular uptake via the specific receptor-mediated pathway. It was estimated that both LPD-PEG and LPD-PEG-AA could deliver a large fraction of the injected dose per gram of organ weight. Nonspecific reticuloendothelial system (RES) uptake causes the majority of losses of the administered dose. This targeted formulation also demonstrated a strong gene-silencing effect mediated by RNAi. Data showed that the surface-modified LPD delivered siRNA predominantly to the tumor after intravenous administration. The formulation provided an advantage of high tumor targeting and low RES uptake, which implied its potential for RNAi-based tumor therapy (Li, 2006c).
Further, LPD formulation was used to target metastasis model tumors with sigma receptor–expressing murine melanoma cells, B16F10. The lung metastasis model was established by intravenous (IV) injection of the B16F10 cells into C57BL/6 mice. In B16F10 melanoma cells targeted nanoparticles (NP) showed a 4-fold increase in delivery efficiency compared to non-targeted NP. Simultaneous silencing of three oncogenes in the metastatic nodules was obtained using siRNA against MDM2, c-myc, and vascular endothelial growth factor (VEGF) co-formulated in the targeted LPD. Two consecutive IV injections of siRNA in the targeted NP significantly reduced the lung metastasis (~70–80%) at a relatively low dose (0.45 mg/kg), whereas free siRNA and the non-targeted nanoparticles showed little effect. The targeted NP formulation prolonged the mean survival time of the animals by 30%, compared to the untreated controls. At the therapeutic dose, the targeted NP showed little local and systemic immunotoxicity and did not decrease the body weight or damage the major organs (Li, 2008a). In the subsequent study (Li, 2008b), the dose was decreased to 0.15 mg/kg, and resulted in 70–80% gene silencing in the whole lung metastasis. The toxicity of the NP formulations was evaluated by their induction of the proinflammatory cytokines (IL6, IL12, TNF, IFN-α). None of the NP formulations were immunotoxic. Additionally, no body weight decrease was observed for any of the mice treated with the formulations.
The formulations were improved by the replacement of calf thymus DNA with hyaluronic acid. In this procedure, protamine and a mixture of siRNA and hyaluronic acid were mixed to prepare a negatively charged complex. Then, cationic liposomes were added to coat the complex with lipids via charge-charge interaction to prepare the LPH NP. LPH was further modified by DSPE-PEG or DSPE-PEG-anisamide using the postinsertion method. The particle size, zeta potential, and siRNA encapsulation efficiency of the formulation were approximately 115 nm, +25 mV and 90%, respectively. LPH silenced 80% of luciferase activity in the metastatic B16F10 tumor in the lung after a single IV injection (0.15 mg siRNA/kg), nearly the same amount as the corresponding LPD formulation. However, the targeted LPH showed very little immunotoxicity in a wide dose range (0.15–1.2 mg siRNA/kg), while the LPD (liposome-protamine-DNA nanoparticle) had a relatively narrow therapeutic window (0.15–0.45 mg/kg). The ED50 for the luciferase silencing in the B16F10 melanoma model was 75 μg/kg in siRNA, but the induction of both IL-6 and IL-12 cytokines by LPH was significantly lower than that of the corresponding LPD formulation (Chono, 2008; Gao, 2009). Later, the LPH formulation was modified with tumor-targeting, single-chain antibody fragment (scFv) for systemic delivery of siRNA and microRNA (miRNA) into experimental lung metastasis of murine B16F10 melanoma. The siRNAs delivered by the scFv targeted nanoparticles efficiently down-regulated the target genes (c-Myc/MDM2/VEGF) in the lung metastasis. Two daily IV injections of the combined siRNAs in the scFv-targeted nanoparticles significantly reduced the tumor load in the lung. In this study, it was first reported that miR-34a and siRNA were co-formulated in scFv-targeted nanoparticles to obtain an enhanced anticancer effect (Chen, 2010c).
The replacement of DOTAP with DSGLA (a nonglycerol based cationic lipid which contains both a guanidinium and a lysine residue as the cationic headgroup) in LPD caused an increase in efficiency of the down-regulation of pERK in H460 cells. A synergistic cell-killing effect in promoting cellular apoptosis was also observed with DSGLA in the formulation. The fluorescently labeled siRNA was efficiently delivered into the cytoplasm of H460 xenograft tumor by the LPD-PEG-AA containing either DOTAP or DSGLA 4 h after IV injection. Three daily injections (0.6 mg/kg each) of siRNA could effectively silence the epidermal growth factor receptor (EGFR) in the tumor, but the formulation containing DSGLA induced more cellular apoptosis. Hence, a significant improvement in tumor growth inhibition has been observed after dosing with LPD-PEG-AA containing DSGLA (Chen, 2009).
Another formulation is the use of N,N-distearyl-N-methyl-N-2-(N′-arginyl) aminoethyl ammonium chloride (DSAA) liposome to coat the polyplex cores for delivering a c-Myc siRNA into the cytoplasm of B16F10 murine melanoma cells. Significant tumor growth inhibition was observed with nanoparticles composed of DSAA with DOTAP. Three daily injections of c-Myc siRNA formulated in this way could impair tumor growth, with an ED50 of about 0.55 mg/kg. Additionally, it was found that the targeted DSAA nanoparticles containing c-Myc siRNA sensitized B16F10 cells to paclitaxel (Taxol), caused a complete inhibition of tumor growth for 1 week. Treatments of c-Myc siRNA in the targeted nanoparticles containing DSAA also showed significant inhibition on the growth of MDA-MB-435 tumor (Chen, 2010b).
Two formulations, LPD and LPD-II (anionic liposome formulation), were used for systemic co-delivery of doxorubicin (Dox) and a therapeutic siRNA to multiple drug resistance (MDR) tumors. The results showed that both multifunctional nanoparticle formulations could deliver Dox and siRNA to MDR tumors simultaneously. Though siRNA and Dox delivered by targeted LPD and LPD-II showed similar apoptosis inductions and therapeutic efficacies, LPD nanoparticles containing DSAA induced more toxicity compared with LPD-II nanoparticles. Compared with the LPD nanoparticles, the LPD-II nanoparticles could also carry more Dox in the formulation. Therefore, LPD-II nanoparticles with higher entrapment efficiency of Dox and a lower toxicity profile may show a larger therapeutic window and a greater potential for clinical application for cancer therapy (Chen, 2010a).
The LPD (including LPH) formulations are more successful than other formulations (e.g. lipoplexes). The differences in the components of the delivery systems highlight this success. Cationic lipids and protamine can interact with negatively charged siRNA to form liposome-coated nanoparticles. The introduction of PEG provides surface steric stabilization to prevent the aggregation of the resulting complex with serum components. A large amount of PEG could be grafted to the NP surface as the result of the improved stability of the lipid bilayer due to charge-charge interaction with the core. The thick PEG layer at the NP surface gives the ability of the NP to evade RES (Li, 2009). The anisamide ligands are attached to the distal end of the PEG chain to increase cellular target. Cationic lipids are also necessary for endosome lysis and intracellular release of siRNA. The mechanism of the endosome membrane destabilization is most likely due to the formation of ion pair complex between the cationic lipids in the nanoparticles and the negatively charged anionic lipids in the endosome membrane. The versatile choice of lipids and polymers (such as protamine and hyaluronic acid), the co-delivery of siRNA, and chemodrugs are attractive features of this type of formulation to create solutions for the delivery of siRNA.
LCP
Although LPD showed success in delivering siRNA after IV injection, improvements were needed to address the low siRNA release efficiency and moderate toxicity. It has been shown that the release of siRNA into the cytoplasm is variable depending on the cell. To improve the cargo release in the target cells, the core of the nanoparticle of LPD was replaced with a biodegradable and acid-sensitive calcium phosphate (CaP) nanoprecipitate to give a new delivery formulation, LCP. The calcium phosphate core is acid sensitive; therefore, LCP disassembles at a low pH in the endosome, causing an osmotic pressure increase, endosome swelling and rupture to release the entrapped siRNA (Li, 2010). The increase of intracellular Ca2+ concentration shown by using a calcium specific dye, Fura-2, demonstrates the mechanism. The anisamide modified LCP silenced about 70% and 50% of luciferase activity for H-460 tumor cells in culture and those grown in a xenograft model, respectively. The new LCP formulation improved the in vitro silencing effect 40-fold compared to the previous LPD formulation, while maintaining a negligible immunotoxicity. After a single IV injection of anti-luciferase siRNA (0.12 mg siRNA/kg) formulated in targeted a LCP formulation (exhibiting a 40 nm particle size, a +25 mV zeta-potential, and 91% siRNA encapsulation efficiency), luciferase activity in metastatic B16F10 tumor-loaded lungs was decreased by 78% in C57BL/6 mice.
Later, the first generation of LCP was improved by using an anionic lipid, dioleoyl phosphatydic acid (DOPA), as the inner leaflet lipid to coat the nano-size CaP cores. A suitable neutral or cationic lipid was used as the outer leaflet lipid to form an asymmetric lipid bilayer structure which was verified by the measurement of NP zeta potential. PEGylation of NP was accomplished by including a PEG-phospholipid conjugate, with or without a targeting ligand, anisamide, in the outer leaflet lipid mixture. The resulting LCP-II had a size of about 25–30 nm in diameter and contained a hollow core, as revealed by TEM imaging. The sub-cellular distribution studied in the sigma receptor positive human H460 lung cancer cells indicated that LCP-II could release more cargo to the cytoplasm than the previous LPD formulation, leading to a significant (~40 fold in vitro and ~4 fold in vivo) improvement in siRNA delivery. However, a bio-distribution study showed that LCP-II required more PEGylation for RES evasion than the previous LPD, probably due to increased surface curvature in LCP-II (Li, 2012). In a therapeutic experiment, siRNA against MDM2, c-myc, and VEGF co-formulated in the targeted LCP-II resulted in simultaneous silencing of the respective oncogenes in metastatic nodules. Treatment with siRNA in the targeted NP significantly reduced lung metastases (~70–80%) at a relatively low dose (0.36 mg/kg). Moreover, this targeted LCP-II NP significantly prolonged the mean survival time of the animals by 27.8% compared to the control group without showing any toxicity at the therapeutic dose (Yang, 2012).
Conclusion
Lipids are promising and versatile carriers because they can be custom-designed with specifically funtional properties which allow for protection of the siRNA, steric stabilization, targeting, membrane destabilization and triggered drug release (Foged, 2012). Although the cationic lipid-based delivery systems have demonstrated the potential of siRNA as future human medicines, they still need more development effort (Oh, 2009). Lipid-based liposomes used to deliver siRNA do not have optimal encapsulation efficiency; that is, many siRNA molecules stay freely in solutions. If we want to decrease the amount of free siRNA, the number of liposomes will need to be increased to cause additional toxicity both in vitro and in vivo. The other challenge that remains is the control of morphologies and sizes of lipoplexes. Therefore, though as low as 0.01 mg/kg of siRNA dose could silence genes efficiently, it was very difficult to advance these formulations into clinical trials. Perhaps the controlled assembly of lipids into biodegradable cores with well defined morphology and size, such as in the cases of LPD and LCP, could provide a real solution to the problem, though relatively high siRNA dose (0.12 or 0.15 mg/kg) is required in mice. To date, most studies address proof-of-concept but do not investigate any possible toxicity of the applied siRNA formulations. Lipidic carriers are not only able to increase immune reactions, but can also possess intrinsic immunostimulatory activity (Foged, 2012). Several recent papers have described an unspecific interferon response after administration of lipid-formulated siRNA molecules to mice (Hornung, 2005; Juliano, 2008). With the progress of lipids based systems for delivering siRNA, more and more biological side-effects may reveal; the safety profiles of cationic lipids and lipid-based delivery systems must be further investigated. This is especially true for the lipidoids.
The structure of cationic lipids is known to affect transfection efficiency and toxicity of cationic lipid-based delivery systems (Lv, 2006). Therefore, novel lipids with high transfection efficiency and low toxicity are highly pursued in research. Although lipids with pH sensitive groups are believed to improve nucleic acid delivery (Schroeder, 2009), over 20 years of research has been completed, and a large amount of compounds has been designed and synthesized, barriers still remain. From our point of view, lipids (such as lipidoids), newly developed and with very low toxicity, should be incorporated into controlled assemblies with polymer (e.g. protamine), inorganic (e.g. calcium phosphate), any other available cores to impart specific performance functionality.
Acknowledgments
We would like to express thanks to Kelly Racette for editing the manuscript.
Footnotes
Declaration of interest
The original work was supported by NIH grants (CA129835, CA129421, CA149363 and CA151652), the National Natural Science Foundation of China (21176046) and the Fundamental Research Funds for the Central Universities (DC12010104).
References
- Adrian JE, Morselt HW, Süss R, Barnert S, Kok JW, Asgeirsdóttir SA, Ruiters MH, Molema G, Kamps JA. Targeted SAINT-O-Somes for improved intracellular delivery of siRNA and cytotoxic drugs into endothelial cells. J Control Release. 2010;144:341–349. doi: 10.1016/j.jconrel.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Evans HM, Ewert K, George CX, Samuel CE, Safinya CR. New multivalent cationic lipids reveal bell curve for transfection efficiency versus membrane charge density: lipid-DNA complexes for gene delivery. J Gene Med. 2005;7:739–748. doi: 10.1002/jgm.717. [DOI] [PubMed] [Google Scholar]
- Akinc A, Goldberg M, Qin J, Dorkin JR, Gamba-Vitalo C, Maier M, Jayaprakash KN, Jayaraman M, Rajeev KG, Manoharan M, Koteliansky V, Röhl I, Leshchiner ES, Langer R, Anderson DG. Development of Lipidoid–siRNA Formulations for Systemic Delivery to the Liver. Mol Ther. 2009;17:872–879. doi: 10.1038/mt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinc A, Zumbuehl A, Goldberg M, Leshchiner ES, Busini V, Hossain N, Bacallado SA, Nguyen DN, Fuller J, Alvarez R, Borodovsky A, Borland T, Constien R, de Fougerolles A, Dorkin JR, Narayanannair Jayaprakash K, Jayaraman M, John M, Koteliansky V, Manoharan M, Nechev L, Qin J, Racie T, Raitcheva D, Rajeev KG, Sah DW, Soutschek J, Toudjarska I, Vornlocher HP, Zimmermann TS, Langer R, Anderson DG. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nat Biotechnol. 2008;26:561–569. doi: 10.1038/nbt1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpicco S, Canevari S, Ceruti M, Galmozzi E, Rocco F, Cattel L. Synthesis characterization and transfection activity of new saturated and unsaturated cationic lipids. IL FARMACO. 2004;59:869–878. doi: 10.1016/j.farmac.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Barreau C, Dutertre S, Paillard L, Osborne HB. Liposome-mediated RNA transfection should be used with caution. RNA. 2006;12:1790–1793. doi: 10.1261/rna.191706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale G, Hollins AJ, Benboubetra M, Sohail M, Fox SP, Benter I, Akhtar S. Gene silencing nucleic acids designed by scanning arrays: anti-EGFR activity of siRNA, ribozyme and DNA enzymes targeting a single hybridization-accessible region using the same delivery system. J Drug Target. 2003;11:449–456. doi: 10.1080/1061186042000207039. [DOI] [PubMed] [Google Scholar]
- Bianco A, Bonadies F, Napolitano R, Ortaggi G. A simple approach to DC-cholesterol, its analogues and vitamin D-based cationic lipids for gene therapy. Lett Org Chem. 2005;2:79–82. [Google Scholar]
- Bouxsein NF, McAllister CS, Ewert KK, Samuel CE, Safinya CR. Structure and gene silencing activities of monovalent and pentavalent cationic lipid vectors complexed with siRNA. Biochemistry. 2007;46:4785–4792. doi: 10.1021/bi062138l. [DOI] [PubMed] [Google Scholar]
- Bouxsein NF, McAllister CS, Ewert KK, Samuel CE, Safinya CR. Structure and gene silencing activities of monovalent and pentavalent cationic lipid vectors complexed with siRNA. Biochemistry. 2007;46:4785–4792. doi: 10.1021/bi062138l. [DOI] [PubMed] [Google Scholar]
- Ceballos C, Prata CA, Giorgio S, Garzino F, Payet D, Barthélémy P, Grinstaff MW, Camplo M. Cationic nucleoside lipids based on a 3-nitropyrrole universal base for siRNA delivery. Bioconjug Chem. 2009;20:193–196. doi: 10.1021/bc800432n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevc G, Richardsen H. Lipid vesicles and membrane fusion. Adv Drug Deliv Rev. 1999;38:207–232. doi: 10.1016/s0169-409x(99)00030-7. [DOI] [PubMed] [Google Scholar]
- Chabaud P, Camplo M, Payet D, Serin G, Moreau L, Barthélémy P, Grinstaff MW. Cationic nucleoside lipids for gene delivery. Bioconjug Chem. 2006;17:466–472. doi: 10.1021/bc050162q. [DOI] [PubMed] [Google Scholar]
- Chan CL, Majzoub R, Shirazi RS, Liang KS, Ewert KK, Safinya CR. pH-Sensitive pegylated cationic lipid-DNA complexes for gene delivery: transfection efficiency and live cell imaging studies. Biophys J. 2012a;102:501–502a. [Google Scholar]
- Chan CL, Majzoub RN, Shirazi RS, Ewert KK, Chen YJ, Liang KS, Safinya CR. Endosomal escape and transfection efficiency of PEGylated cationic liposome-DNA complexes prepared with an acid-labile PEG-lipid. Biomaterials. 2012;33:4928–4935. doi: 10.1016/j.biomaterials.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bathula SR, Li J, Huang L. Multifunctional nanoparticles delivering small interfering RNA and doxorubicin overcome drug resistance in cancer. J Biol Chem. 2010;285:22639–22650. doi: 10.1074/jbc.M110.125906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bathula SR, Yang Q, Huang L. Targeted nanoparticles deliver siRNA to melanoma. J Invest Dermatol. 2010;130:2790–2798. doi: 10.1038/jid.2010.222. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhu X, Zhang X, Liu B, Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol Ther. 2010;18:1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sen J, Bathula SR, Yang Q, Fittipaldi R, Huang L. Novel cationic lipid that delivers siRNA and enhances therapeutic effect in lung cancer cells. Mol Pharm. 2009;6:696–705. doi: 10.1021/mp800136v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chono S, Li SD, Conwell CC, Huang L. An efficient and low immunostimulatory nanoparticle formulation for systemic siRNA delivery to the tumor. J Control Release. 2008;131:64–69. doi: 10.1016/j.jconrel.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wolf HK, de Raad M, Snel C, van Steenbergen MJ, Fens MH, Storm G, Hennink WE. Biodegradable poly(2-dimethylamino ethylamino)phosphazene for in vivo gene delivery to tumor cells. Effect of polymer molecular weight. Pharm Res. 2007;24:1572–1580. doi: 10.1007/s11095-007-9299-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elouahabi A, Ruysschaert JM. Formation and intracellular trafficking of lipoplexes and polyplexes. Mol Ther. 2005;11:336–347. doi: 10.1016/j.ymthe.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Ewert K, Ahmad A, Evans HM, Schmidt HW, Safinya CR. Efficient synthesis and cell-transfection properties of a new multivalent cationic lipid for nonviral gene delivery. J Med Chem. 2002;45:5023–5029. doi: 10.1021/jm020233w. [DOI] [PubMed] [Google Scholar]
- Farhood H, Bottega R, Epand RM, Huang L. Effect of cationic cholesterol derivatives on gene transfer and protein kinase C activity. Biochim Biophys Acta. 1992;1111:239–246. doi: 10.1016/0005-2736(92)90316-e. [DOI] [PubMed] [Google Scholar]
- Farhood H, Serbina N, Huang L. The role of dioleoyl phosphatidylethanolamine in cationic liposome mediated gene transfer. Biochim Biophys Acta. 1995;1235:289–295. doi: 10.1016/0005-2736(95)80016-9. [DOI] [PubMed] [Google Scholar]
- Felgner PL, Gadek TR, Holm M, Roman R, Chan HS, Wenz M, Northrop JP, Ringold GM, Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Flynn MA, Casey DG, Todryk SM, Mahon BP. Efficient delivery of small interfering RNA for inhibition of IL-12p40 expression in vivo. J Inflamm (Lond) 2004;1:4. doi: 10.1186/1476-9255-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foged C, Nielsen HM, Frokjaer S. Liposomes for phospholipase A2 triggered siRNA release: preparation and in vitro test. Int J Pharm. 2007;331:160–166. doi: 10.1016/j.ijpharm.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Foged C. siRNA delivery with lipid-based systems: promises and pitfalls. Curr Top Med Chem. 2012;12:97–107. doi: 10.2174/156802612798919141. [DOI] [PubMed] [Google Scholar]
- Gao K, Huang L. Nonviral methods for siRNA delivery. Mol Pharm. 2009;6:651–658. doi: 10.1021/mp800134q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Huang L. Potentiation of cationic liposome-mediated gene delivery by polycations. Biochemistry. 1996;35:1027–1036. doi: 10.1021/bi952436a. [DOI] [PubMed] [Google Scholar]
- Geisbert TW, Lee AC, Robbins M, Geisbert JB, Honko AN, Sood V, Johnson JC, de Jong S, Tavakoli I, Judge A, Hensley LE, Maclachlan I. Postexposure protection of non-human primates against a lethal Ebola virus challenge with RNA interference: a proof-of-concept study. Lancet. 2010;375:1896–1905. doi: 10.1016/S0140-6736(10)60357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore IR, Fox SP, Hollins AJ, Sohail M, Akhtar S. The design and exogenous delivery of siRNA for post-transcriptional gene silencing. J Drug Target. 2004;12:315–340. doi: 10.1080/10611860400006257. [DOI] [PubMed] [Google Scholar]
- Guo J, Bourre L, Soden DM, O’Sullivan GC, O’Driscoll C. Can non-viral technologies knockdown the barriers to siRNA delivery and achieve the next generation of cancer therapeutics? Biotechnol Adv. 2011;29:402–417. doi: 10.1016/j.biotechadv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Heyes J, Palmer L, Bremner K, MacLachlan I. Cationic lipid saturation influences intracellular delivery of encapsulated nucleic acids. J Control Release. 2005;107:276–287. doi: 10.1016/j.jconrel.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, Noronha A, Manoharan M, Akira S, de Fougerolles A, Endres S, Hartmann G. Sequence-specific potent induction of IFN-alpha by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bao Y, Peng W, Goldberg M, Love K, Bumcrot DA, Cole G, Langer R, Anderson DG, Sawicki JA. Claudin-3 gene silencing with siRNA suppresses ovarian tumor growth and metastasis. Proc Natl Acad Sci USA. 2009;106:3426–3430. doi: 10.1073/pnas.0813348106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jana S, Chakraborty C, Nandi S, Deb JK. RNA interference: potential therapeutic targets. Appl Microbiol Biotechnol. 2004;65:649–657. doi: 10.1007/s00253-004-1732-1. [DOI] [PubMed] [Google Scholar]
- Jiang S, Zhang Y. Upconversion nanoparticle-based FRET system for study of siRNA in live cells. Langmuir. 2010;26:6689–6694. doi: 10.1021/la904011q. [DOI] [PubMed] [Google Scholar]
- John M, Constien R, Akinc A, Goldberg M, Moon YA, Spranger M, Hadwiger P, Soutschek J, Vornlocher HP, Manoharan M, Stoffel M, Langer R, Anderson DG, Horton JD, Koteliansky V, Bumcrot D. Effective RNAi-mediated gene silencing without interruption of the endogenous microRNA pathway. Nature. 2007;449:745–747. doi: 10.1038/nature06179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Juliano R, Alam MR, Dixit V, Kang H. Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res. 2008;36:4158–4171. doi: 10.1093/nar/gkn342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis TC, El-Osta A. RNA interference and potential therapeutic applications of short interfering RNAs. Cancer Gene Ther. 2005;12:787–795. doi: 10.1038/sj.cgt.7700857. [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Guo S. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell. 1995;81:611–620. doi: 10.1016/0092-8674(95)90082-9. [DOI] [PubMed] [Google Scholar]
- Khan A, Benboubetra M, Sayyed PZ, Ng KW, Fox S, Beck G, Benter IF, Akhtar S. Sustained polymeric delivery of gene silencing antisense ODNs, siRNA, DNAzymes and ribozymes: in vitro and in vivo studies. J Drug Target. 2004;12:393–404. doi: 10.1080/10611860400003858. [DOI] [PubMed] [Google Scholar]
- Khoury M, Louis-Plence P, Escriou V, Noel D, Largeau C, Cantos C, Scherman D, Jorgensen C, Apparailly F. Efficient new cationic liposome formulation for systemic delivery of small interfering RNA silencing tumor necrosis factor α in experimental arthritis. Arthritis Rheum. 2006;54:1867–1877. doi: 10.1002/art.21876. [DOI] [PubMed] [Google Scholar]
- Li CX, Parker A, Menocal E, Xiang S, Borodyansky L, Fruehauf JH. Delivery of RNA interference. Cell Cycle. 2006;5:2103–2109. doi: 10.4161/cc.5.18.3192. [DOI] [PubMed] [Google Scholar]
- Li J, Chen YC, Tseng YC, Mozumdar S, Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J Control Release. 2010;142:416–421. doi: 10.1016/j.jconrel.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yang Y, Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J Control Release. 2012;158:108–114. doi: 10.1016/j.jconrel.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Huang L. In vivo gene transfer via intravenous administration of cationic lipid–protamine–DNA (LPD) complexes. Gene Ther. 1997;4:891–900. doi: 10.1038/sj.gt.3300482. [DOI] [PubMed] [Google Scholar]
- Li SD, Chono S, Huang L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol Ther. 2008;16:942–946. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SD, Chono S, Huang L. Efficient gene silencing in metastatic tumor by siRNA formulated in surface-modified nanoparticles. J Control Release. 2008;126:77–84. doi: 10.1016/j.jconrel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SD, Huang L. Targeted delivery of antisense oligodeoxynucleotide and small interference RNA into lung cancer cells. Mol Pharm. 2006;3:579–588. doi: 10.1021/mp060039w. [DOI] [PubMed] [Google Scholar]
- Li SD, Huang L. Surface-modified LPD nanoparticles for tumor targeting. Ann N Y Acad Sci. 2006;1082:1–8. doi: 10.1196/annals.1348.001. [DOI] [PubMed] [Google Scholar]
- Li SD, Huang L. Nanoparticles evading the reticuloendothelial system: role of the supported bilayer. Biochim Biophys Acta. 2009;1788:2259–2266. doi: 10.1016/j.bbamem.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Hu J, Qiao W, Li Z, Zhang S, Cheng L. Synthesis of carbamate-linked lipids for gene delivery. Bioorg Med Chem Lett. 2005;15:3147–3150. doi: 10.1016/j.bmcl.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Liu D, Hu J, Qiao W, Li Z, Zhan S, Cheng L. Synthesis and characterization of a series of carbamate-linked cationic lipids for gene delivery. Lipids. 2005;40:839–848. doi: 10.1007/s11745-005-1446-5. [DOI] [PubMed] [Google Scholar]
- Liu D, Qiao W, Li Z, Chen Y, Cui X, Li K, Yu L, Yan K, Zhu L, Guo Y, Cheng L. Structure-function relationship research of glycerol backbone-based cationic lipids for gene delivery. Chem Biol Drug Des. 2008;71:336–344. doi: 10.1111/j.1747-0285.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Huang L. Development of non-viral vectors for systemic gene delivery. J Control Release. 2002;78:259–266. doi: 10.1016/s0168-3659(01)00494-1. [DOI] [PubMed] [Google Scholar]
- Lorenz C, Hadwiger P, John M, Vornlocher HP, Unverzagt C. Steroid and lipid conjugates of siRNAs to enhance cellular uptake and gene silencing in liver cells. Bioorg Med Chem Lett. 2004;14:4975–4977. doi: 10.1016/j.bmcl.2004.07.018. [DOI] [PubMed] [Google Scholar]
- Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, Qin J, Cantley W, Qin LL, Racie T, Frank-Kamenetsky M, Yip KN, Alvarez R, Sah DW, de Fougerolles A, Fitzgerald K, Koteliansky V, Akinc A, Langer R, Anderson DG. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107:1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Ma B, Zhang S, Jiang H, Zhao B, Lv H. Lipoplex morphologies and their influences on transfection efficiency in gene delivery. J Control Release. 2007;123:184–194. doi: 10.1016/j.jconrel.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Mahon KP, Love KT, Whitehead KA, Qin J, Akinc A, Leshchiner E, Leshchiner I, Langer R, Anderson DG. Combinatorial approach to determine functional group effects on lipidoid-mediated siRNA delivery. Bioconjug Chem. 2010;21:1448–1454. doi: 10.1021/bc100041r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone RW, Felgner PL, Verma IM. Cationic liposome-mediated RNA transfection. Proc Natl Acad Sci USA. 1989;86:6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamot C, Drummond DC, Noble CO, Kallab V, Guo Z, Hong K, Kirpotin DB, Park JW. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65:11631–11638. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- Mével M, Kamaly N, Carmona S, Oliver MH, Jorgensen MR, Crowther C, Salazar FH, Marion PL, Fujino M, Natori Y, Thanou M, Arbuthnot P, Yaouanc JJ, Jaffrès PA, Miller AD. DODAG; a versatile new cationic lipid that mediates efficient delivery of pDNA and siRNA. J Control Release. 2010;143:222–232. doi: 10.1016/j.jconrel.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Midoux P, Pichon C, Yaouanc JJ, Jaffrès PA. Chemical vectors for gene delivery: a current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br J Pharmacol. 2009;157:166–178. doi: 10.1111/j.1476-5381.2009.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morille M, Passirani C, Vonarbourg A, Clavreul A, Benoit JP. Progress in developing cationic vectors for non-viral systemic gene therapy against cancer. Biomaterials. 2008;29:3477–3496. doi: 10.1016/j.biomaterials.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, Hartsough K, Machemer L, Radka S, Jadhav V, Vaish N, Zinnen S, Vargeese C, Bowman K, Shaffer CS, Jeffs LB, Judge A, MacLachlan I, Polisky B. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- Oh YK, Park TG. siRNA delivery systems for cancer treatment. Adv Drug Deliv Rev. 2009;61:850–862. doi: 10.1016/j.addr.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Hollins AJ, Benboubetra M, Drayton R, Benter IF, Akhtar S. Toxicogenomics of non-viral vectors for gene therapy: a microarray study of lipofectin- and oligofectamine-induced gene expression changes in human epithelial cells. J Drug Target. 2003;11:311–323. doi: 10.1080/10611860310001636908. [DOI] [PubMed] [Google Scholar]
- Ozpolat B, Sood AK, Lopez-Berestein G. Nanomedicine based approaches for the delivery of siRNA in cancer. J Intern Med. 2010;267:44–53. doi: 10.1111/j.1365-2796.2009.02191.x. [DOI] [PubMed] [Google Scholar]
- Pan X, Thompson R, Meng X, Wu D, Xu L. Tumor-targeted RNA-interference: functional non-viral nanovectors. Am J Cancer Res. 2011;1:25–42. [PMC free article] [PubMed] [Google Scholar]
- Park K. Systemic siRNA delivery using biocompatible calcium phosphate nanoparticles. J Control Release. 2010;142:295. doi: 10.1016/j.jconrel.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Perkel JM. RNAi therapeutics: a two-year update. Science. 2009;326:454–456. [Google Scholar]
- Pirollo KF, Rait A, Zhou Q, Hwang SH, Dagata JA, Zon G, Hogrefe RI, Palchik G, Chang EH. Materializing the potential of small interfering RNA via a tumor-targeting nanodelivery system. Cancer Res. 2007;67:2938–2943. doi: 10.1158/0008-5472.CAN-06-4535. [DOI] [PubMed] [Google Scholar]
- Romøren K, Thu BJ, Bols NC, Evensen Ø. Transfection efficiency and cytotoxicity of cationic liposomes in salmonid cell lines of hepatocyte and macrophage origin. Biochim Biophys Acta. 2004;1663:127–134. doi: 10.1016/j.bbamem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Rossin R, Pan D, Qi K, Turner JL, Sun X, Wooley KL, Welch MJ. 64Cu-labeled folate-conjugated shell cross-linked nanoparticles for tumor imaging and radiotherapy: synthesis, radiolabeling, and biologic evaluation. J Nucl Med. 2005;46:1210–1218. [PubMed] [Google Scholar]
- Sato A, Takagi M, Shimamoto A, Kawakami S, Hashida M. Small interfering RNA delivery to the liver by intravenous administration of galactosylated cationic liposomes in mice. Biomaterials. 2007;28:1434–1442. doi: 10.1016/j.biomaterials.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Scales CW, Huang F, Li N, Vasilieva YA, Ray J, Convertine AJ, McCormick CL. Corona-stabilized interpolyelectrolyte complexes of siRNA with non-immunogenic, hydrophilic/cationic block copolymers prepared by aqueous RAFT polymerization. Macromolecules. 2006;39:6871–6881. [Google Scholar]
- Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2010;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- Sheng R, Luo T, Zhu Y, Li H, Sun J, Chen S, Sun W, Cao A. The intracellular plasmid DNA localization of cationic reducible cholesterol-disulfide lipids. Biomaterials. 2011;32:3507–3519. doi: 10.1016/j.biomaterials.2011.01.055. [DOI] [PubMed] [Google Scholar]
- Shirazi RS, Ewert KK, Leal C, Majzoub RN, Bouxsein NF, Safinya CR. Synthesis and characterization of degradable multivalent cationic lipids with disulfide-bond spacers for gene delivery. Biochim Biophys Acta. 2011;1808:2156–2166. doi: 10.1016/j.bbamem.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioud M, Sørensen DR. Cationic liposome-mediated delivery of siRNAs in adult mice. Biochem Biophys Res Commun. 2003;312:1220–1225. doi: 10.1016/j.bbrc.2003.11.057. [DOI] [PubMed] [Google Scholar]
- Sorgi FL, Bhattacharya S, Huang L. Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther. 1997;4:961–968. doi: 10.1038/sj.gt.3300484. [DOI] [PubMed] [Google Scholar]
- Sparks J, Slobodkin G, Matar M, Congo R, Ulkoski D, Rea-Ramsey A, Pence C, Rice J, McClure D, Polach KJ, Brunhoeber E, Wilkinson L, Wallace K, Anwer K, Fewell JG. Versatile cationic lipids for siRNA delivery. J Control Release. 2012;158:269–276. doi: 10.1016/j.jconrel.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Spelios M, Nedd S, Matsunaga N, Savva M. Effect of spacer attachment sites and pH-sensitive headgroup expansion on cationic lipid-mediated gene delivery of three novel myristoyl derivatives. Biophys Chem. 2007;129:137–147. doi: 10.1016/j.bpc.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SJ, Kiatwuthinon P, Roh YH, Kahn JS, Luo D. Engineering Nanocarriers for siRNA Delivery. Small. 2011;7:841–856. doi: 10.1002/smll.201001389. [DOI] [PubMed] [Google Scholar]
- Tao W, Davide JP, Cai M, Zhang GJ, South VJ, Matter A, Ng B, Zhang Y, Sepp-Lorenzino L. Noninvasive imaging of lipid nanoparticle-mediated systemic delivery of small-interfering RNA to the liver. Mol Ther. 2010;18:1657–1666. doi: 10.1038/mt.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma UN, Surabhi RM, Schmaltieg A, Becerra C, Gaynor RB. RNAs directed against betacatenin inhibit the in vitro and in vivo growth of colon cancer cells. Clin Cancer Res. 2003;9:1291–1300. [PubMed] [Google Scholar]
- Sørensen DR, Leirdal M, Sioud M. Gene silencing by systemic delivery of synthetic siRNAs in adult mice. J Mol Biol. 2003;327:761–766. doi: 10.1016/s0022-2836(03)00181-5. [DOI] [PubMed] [Google Scholar]
- Wagner E, Cotten M, Foisner R, Birnstiel ML. Transferrin-polycation-DNA complexes: the effect of polycations on the structure of the complex and DNA delivery to cells. Proc Natl Acad Sci USA. 1991;88:4255–4259. doi: 10.1073/pnas.88.10.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead KA, Sahay G, Li GZ, Love KT, Alabi CA, Ma M, Zurenko C, Querbes W, Langer RS, Anderson DG. Synergistic silencing: combinations of lipid-like materials for efficacious siRNA delivery. Mol Ther. 2011;19:1688–1694. doi: 10.1038/mt.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodle MC, Scaria P. Cationic liposomes and nucleic acids. Curr Opin Colloid Interface Sci. 2001;6:78–84. [Google Scholar]
- Yang Y, Li J, Liu F, Huang L. Systemic delivery of siRNA via LCP nanoparticle efficiently inhibits lung metastasis. Mol Ther. 2012;20:609–615. doi: 10.1038/mt.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J. Cationic lipids used in gene transfer. Adv Drug Deliv Rev. 1997;27:17–28. doi: 10.1016/s0169-409x(97)00019-7. [DOI] [PubMed] [Google Scholar]
- Zamore PD, Tuschl T, Sharp PA, Bartel DP. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- Zhang S, Xu Y, Wang B, Qiao W, Liu D, Li Z. Cationic compounds used in lipoplexes and polyplexes for gene delivery. J Control Release. 2004;100:165–180. doi: 10.1016/j.jconrel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Zhao YN, Zhi DF, Zhang SB. Cationic liposomes in different structural levels for gene delivery. In: Yuan XB, editor. Non-viral Gene Therapy. Rijeka, Croatia: InTech; 2011. pp. 293–318. [Google Scholar]
- Zhdanov RI, Podobed OV, Vlassov VV. Cationic lipid-DNA complexes-lipoplexes-for gene transfer and therapy. Bioelectrochemistry. 2002;58:53–64. doi: 10.1016/s1567-5394(02)00132-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann TS, Lee AC, Akinc A, Bramlage B, Bumcrot D, Fedoruk MN, Harborth J, Heyes JA, Jeffs LB, John M, Judge AD, Lam K, McClintock K, Nechev LV, Palmer LR, Racie T, Röhl I, Seiffert S, Shanmugam S, Sood V, Soutschek J, Toudjarska I, Wheat AJ, Yaworski E, Zedalis W, Koteliansky V, Manoharan M, Vornlocher HP, MacLachlan I. RNAi-mediated gene silencing in non-human primates. Nature. 2006;441:111–114. doi: 10.1038/nature04688. [DOI] [PubMed] [Google Scholar]
- Zuhorn IS, Bakowsky U, Polushkin E, Visser WH, Stuart MC, Engberts JB, Hoekstra D. Nonbilayer phase of lipoplex-membrane mixture determines endosomal escape of genetic cargo and transfection efficiency. Mol Ther. 2005;11:801–810. doi: 10.1016/j.ymthe.2004.12.018. [DOI] [PubMed] [Google Scholar]