Abstract
Thoracic myoepithelial tumors (MTs) are a rare group of tumors showing predominant or exclusive myoepithelial differentiation. They are poorly characterized from both a morphologic and genetic standpoint, in particular features that separate benign from malignant behavior. We examined the histologic and immunohistochemical features of 8 primary thoracic MTs and performed fluorescence in situ hybridization for EWSR1, FUS, PLAG1, and HMGA2, as well as several partner genes. Half (4/8) of the MTs occurred in large airways and 3 had infiltrative borders. All cases showed immunoreactivity for epithelial markers, in conjunction with S-100 protein or myogenic markers. MTs showed morphologic characteristics analogous to MTs at other sites, with no tumors having ductal differentiation. Necrosis and/or lymphovascular invasion was present in 5 cases, with mitotic activity ranging from 0–6 mitoses/2 mm2 (mean 1). Metastases occurred in 2 cases, and no patients died of disease. Gene rearrangements were identified in half of the cases, with EWSR1-PBX1, EWSR1-ZNF444, and FUS-KLF17 fusions identified in one case each, and one case having EWSR1 rearrangement with no partner identified. No cases were found to have HMGA2 or PLAG1 abnormalities. Compared to fusion negative tumors, fusion positive tumors tended to occur in patients who were younger (50 vs 58 yrs), female (1:3 vs 3:1 male:female ratio), and demonstrated predominantly spindle and clear cell morphology. Using a combined dataset of our case series with 16 cases from the literature, poor prognosis was significantly correlated with metastases (p=0.003), necrosis (p=0.027), and ≥ 5 mitoses per 2mm2/10 HPF (p=0.005). In summary, we identify a subset of thoracic MTs harboring rearrangements in EWSR1 or FUS, and our data suggests that necrosis and increased mitotic activity correlate with aggressive clinical behavior.
Introduction
Myoepithelial tumors (MTs) are a heterogeneous group of neoplasms showing morphologic, immunohistochemical and ultrastructural features consistent with myoepithelial differentiation. Their terminology and diagnostic criteria vary depending on location, with salivary gland and soft tissues sites being the most extensively studied to date 1-7. Our understanding of MTs in these sites has been strengthened by the identification of recurring rearrangements of EWSR1 or FUS in soft tissue MTs, and rearrangements with/or without amplification of PLAG1 or HMGA2 in salivary gland MTs 4-6,8. In the thorax, MTs are rare, poorly characterized, and their precise incidence is unclear, with fewer than 25 case reports in the literature 4,9-26,28,52. Due to their rarity, heterogeneous histomorphology, and often focal immunoreactivity, they remain a challenging diagnosis with ill-defined prognostic criteria and unclear molecular profile. In this study, we sought to investigate the histomorphologic and molecular features in a series of thoracic MTs. We also investigated clinicopathologic correlations based on our series and previously reported thoracic MTs.
Material and methods
Clinical and pathologic features
Eight cases of primary thoracic MTs from Memorial Sloan Kettering Cancer Center (MSKCC) and the personal consults of one of the authors (WDT) were included in our study. Five have been published previously, however clinical follow-up was not provided and clinicopathologic correlations were not investigated (Table 1) 4,8,10,22. The tumor location, gross anatomic features, clinical history, and clinical outcome were obtained from review of consult letters, pathology reports, clinical notes, and through conversations with pathologists and/or clinicians from the submitting institutions. Chest computed tomography (CT) scans were reviewed if available. Slides were re-reviewed in corroboration with an immunohistochemical panel in all cases. Minimum criteria for confirming the morphologic diagnosis of MT included immunoreactivity for keratins and/or epithelial membrane antigen (EMA), in conjunction with detection of S-100 protein or myogenic markers (calponin or smooth muscle actin (SMA)). Immunoreactivity for the squamous/basal cell marker p63 was recorded, when done, but was not used as a criterion for myoepithelial differentiation. Immunoreactivity for all stains was recorded as positive (>50% of tumor cells staining), focal (<50% of tumor cells staining), or negative (no tumor cells staining). The tumors were assessed morphologically for the following characteristics: borders (well circumscribed or infiltrative); architectural pattern (nests, sheets, fascicles, or reticular); stromal characteristics (myxoid, chondroid, or hyalinized); cytologic features (clear cell, epithelioid, spindle cell, or plasmacytoid); nuclear pleomorphism (nil-mild, moderate, or marked); mitotic activity (average whole number of mitotic figures/2 mm2 based on review of 3 sets of 2 mm2 (6 mm2) in areas of highest mitotic activity using an Olympus BX40 microscope with a standard 22 mm eyepiece); lymphovascular invasion (LVI) (present or absent); and necrosis (present or absent). Tumor borders were considered well circumscribed if the tumor-parenchymal interface was sharply defined or if the tumor was entirely endobronchial/tracheal with no infiltration of bronchial/tracheal wall. Infiltrative borders were defined as a lobulated and irregular tumor-parenchymal interface with extension into adjacent parenchyma. Statistical analysis was performed using SPSS v. 22.
Table 1. Clinical features of thoracic myoepithelial tumors.
| Case | Age | Sex | Presentation | Smoking | Location | Size (cm) | T stage | Treatment | Metastasis | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1* | 75 | F | Unknown | Yes | Subpleural | 10.0 | 3 | Lobectomy | Unknown | Unknown |
| 2* | 27 | F | Incidental | Yes | Endobronchial | 3.2 | 2a | Lobectomy | None | NED; 110 mo |
| 3• | 64 | F | Hemoptysis | No | Intraparenchymal | 12.5 | 3 | Wide resection | Abdominal wall, liver | AWD; 84 mo |
| 4† | 30 | M | Unknown | No | Endobronchial | 2.6 | 1b | Sublobar resection | None | NED; 72 mo |
| 5‡ | 77 | M | Unknown | Yes | Endotracheal | 1.3 | 1a | Snare | None | NED; 60 mo |
| 6 | 45 | F | Hemoptysis | No | Intraparenchymal | 2.3 | 1b | Lobectomy | None | NED; 19 mo |
| 7 | 57 | M | Cough | Yes | Intraparenchymal | 1.7 | 1a | Lobectomy + CT/RT | Ipsilateral lobe | AWD; 43 mo |
| 8 | 54 | M | Hemoptysis | Yes | Endobronchial | 0.7 | 1a | Lobectomy | None | NED; 15 mo |
Immunohistochemistry
Immunohistochemical stains submitted from outside institutions were reviewed. On in-house and outside cases in which further immunohistochemical workup was needed, the following antibodies were used as required: Calponin (Cell Marque; clone EP798Y, prediluted), S100 protein (Leica; polyclonal, 1:2000), SMA (Vector; clone ASM1, 1:50), Pancytokeratin (Dako; clone MNF 116, 1:1600), TTF-1 (Ventana; clone 8G7G3/1, prediluted), Cytokeratin Cam 5.2 (Becton Dickinson; clone Cam 5.2, 1:50), CK 5/6 (Ventana; clone D5/16B4, prediluted), EMA (Ventana; clone 43B2, prediluted), and p63 (Dako; clone 4A4, 1:200).
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) on interphase nuclei from paraffin-embedded 4-μm sections was performed applying custom probes using bacterial artificial chromosomes (BAC). All cases were screened in a stepwise fashion in the following order using probes flanking EWSR1 in 22q12, FUS in 16p11, PLAG1 in 8q12, and HMGA2 in 12q14. Cases that showed rearrangement in EWSR1 or FUS were screened for partners with probes for PBX1 in 1q23, PBX3 in 9q33, KLF17 in 1p34, ZNF444 in 19q13, POU5F1 in 6p21, CREB1 in 2q34, ATF1 in 12q13, and NR4A3 in 9q22. BAC clones were chosen according to UCSC genome browser (http://genome.ucsc.edu). The BAC clones were obtained from BACPAC sources from Children's Hospital of Oakland Research Institute (Oakland, CA; http:// bacpac.chori.org). DNA from individual BACs was isolated according to the manufacturer's instructions, labeled with different fluorochromes in a nick translation reaction, denatured, and hybridized to pretreated slides. Slides were then incubated, washed, and mounted with DAPI in an antifade solution, as previously described 4. The genomic location of each BAC set was verified by hybridizing them to normal metaphase chromosomes. Two-hundred successive nuclei were examined using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany), controlled by Isis 5 software (Metasystems). A positive score was interpreted when at least 20% of the nuclei showed a break-apart signal. Nuclei with incomplete set of signals were omitted from the score. In selective cases, two-color FISH fusion assay was applied using probe-sets centromerically flanking one gene and telomerically flanking the partner gene, to confirm the fusion between EWSR1 or FUS and the partner genes.
Results
MSKCC Cases
Patient age ranged from 27 to 77 years (mean 54 ± 18 yrs), with a male to female ratio of 1:1 (Table 1). The majority (5/8) of the patients had a history of smoking, and in cases with available clinical presentation, all except one (4/5) were symptomatic with either hemoptysis or non-bloody cough. Half (4/8) of the tumors were located in large airways (endobronchial or endotracheal), and these tended to be smaller on average than those located in intraparenchymal or subpleural locations (2.0 ± 1.2 cm vs 6.6 ± 5.4 cm). In cases with available chest CT scans (n=4), MTs occurring in large airways were well circumscribed, homogeneous, polypoid and sessile, while intraparenchymal tumors ranged from well-defined nodules to irregular masses with calcifications (Fig. 1). All cases were surgically managed, with lobectomy as the most common procedure (n=5). Adjuvant chemotherapy and radiotherapy were administered in one case. Based on gross tumor size and the current TNM staging system for tumors of the lung, primary tumor (T) stage was determined as follows: T1a (3 cases), T1b (2 cases), T2a (1 case), and T3 (2 cases)27. In cases for which clinical follow-up was available, it ranged from 15-110 months (mean 58 ± 35 months), and metastases were documented in only two cases, with no patients dying of disease.
Figure 1.
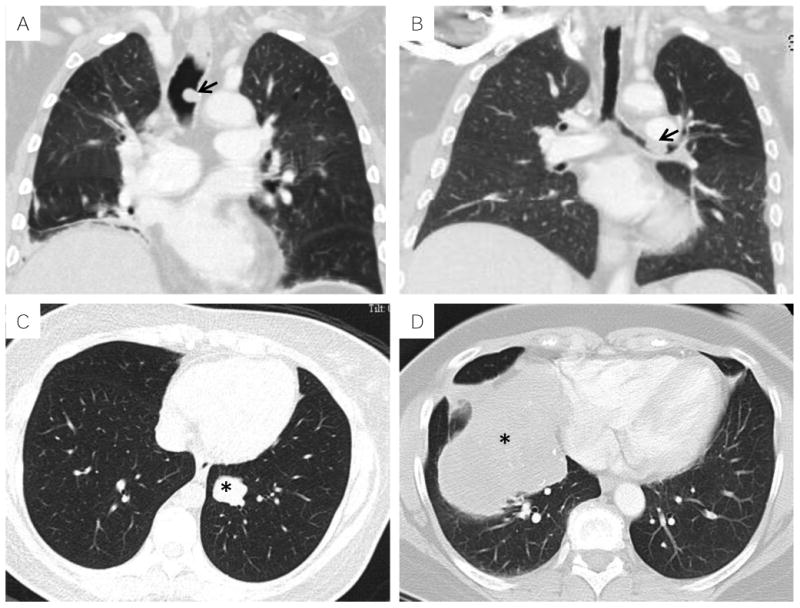
Radiologic features of thoracic MTs on computed tomography: A, sessile endotracheal polyp (arrow, Case #5); B, sessile endobronchial polyp (arrow, Case #8); C, well circumscribed intraparenchymal nodule (asterisk, Case #6); D, large intraparenchymal mass with calcifications and mass effect (asterisk, Case#3).
On gross examination, tumors within large airways ranged from sessile polyps to submucosal masses with compression of adjacent airway (Fig. 2). No capsule was identified grossly in any of the tumors. Prior to fixation, tumors were firm, grey to yellow, and generally had a homogeneous cut surface with a few having cystic and/or hemorrhagic change. Microscopically, none of the tumors were encapsulated, and the majority were well-circumscribed (n=5)(Table 2; Fig. 3). Most cases had a mixture of histologic patterns, with solid sheets or nested growth pattern, and associated hyalinized or myxoid stroma. Reticular pattern and chondroid matrix were seen in only one case each (Table 2; Fig. 4). Osteoclast-like giant cells and psammomatous calcifications were focal findings in one case each. Cytologic characteristics also varied within individual tumors, with clear cells being the most common cytomorphology (n=5), followed by epithelioid (n=4), spindle (n=4), and plasmacytoid (n=2).
Figure 2.
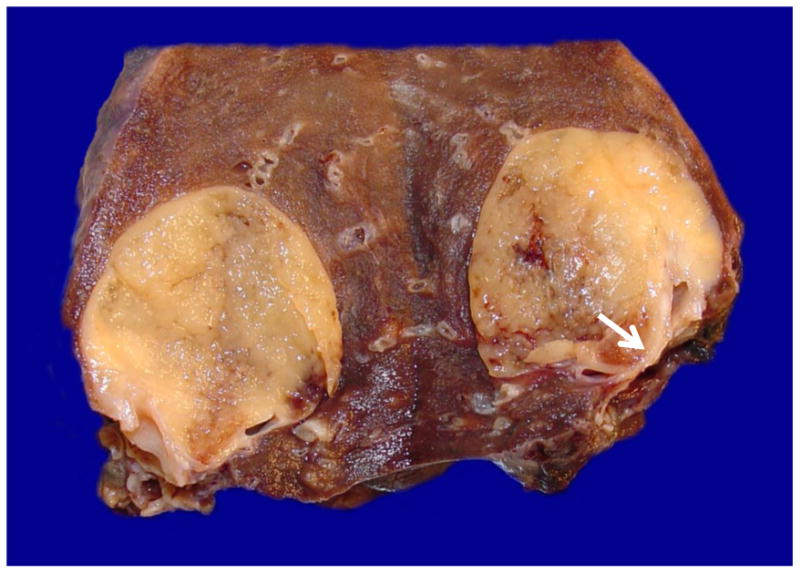
Gross image of a bisected submucosal MT with compression of adjacent bronchus (arrow, Case #2)
Table 2. Histologic features of thoracic myoepithelial tumors.
| Case | Borders | Architecture | Matrix | Cytomorphology | Atypia | Mitoses /2 mm2 | Necrosis | LVI |
|---|---|---|---|---|---|---|---|---|
| 1 | Infiltrative | Nests, fascicles | Hyalinized | Spindle, clear cell | None/mild | 6 | Present | Present |
| 2 | Infiltrative | Nests | Myxoid, hyalinized | Spindle, clear cell | Moderate | 1 | Absent | Present |
| 3 | Well circumscribed | Sheets, fascicles | Chondroid, hyalinized | Spindle, clear cell | None/mild | <1 | Present | Present |
| 4 | Well circumscribed | Sheets | Myxoid, hyalinized | Spindle, clear cell | Moderate | 0 | Present | Absent |
| 5 | Well circumscribed | Nests, sheets | Myxoid | Epithelioid, plasmacytoid | Moderate | 0 | Absent | Absent |
| 6 | Well circumscribed | Nests, sheets | Hyalinized | Epithelioid | None/mild | <1 | Absent | Absent |
| 7 | Infiltrative | Nests, sheets | Hyalinized | Epithelioid, clear cell | Moderate | 2 | Present | Absent |
| 8 | Well circumscribed | Nests, reticular, sheets | Myxoid, hyalinized | Epithelioid, plasmacytoid | None/mild | 0 | Absent | Absent |
LVI=lymphovascular invasion
Figure 3.
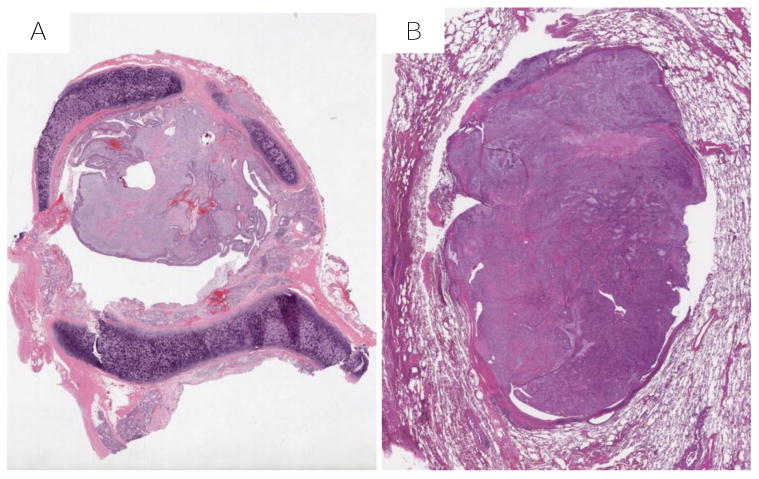
Border characteristics of thoracic MTs (50X, H & E): A, entirely endobronchial MT (Case #8); B, well circumscribed intraparenchymal MT (Case #6);
Figure 4.
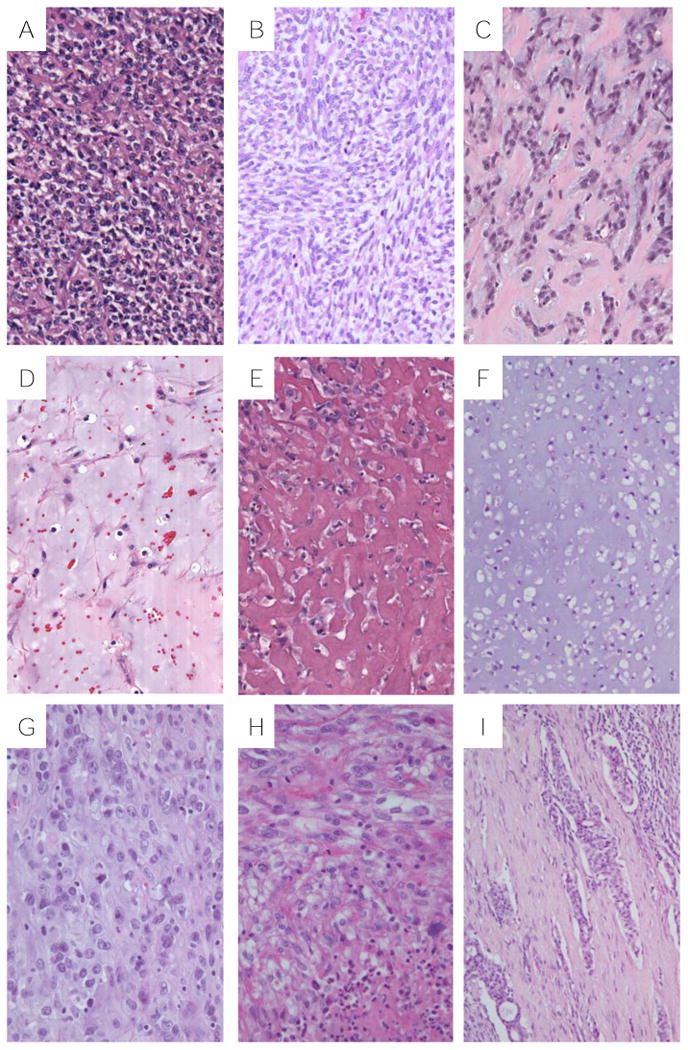
Histologic features of thoracic MTs: A, sheets of round cells with clear cytoplasm (Case #3); B, sheets of spindle cells with clear cytoplasm (Case #3); C, plasmacytoid cells in a reticular pattern (Case #8); D, spindle cells in a myxoid stroma (Case #2); E, epithelioid cells in a hyalinized matrix (Case #6); F, clear cells in a chondroid matrix (Case#3); G, epithelioid cells with moderate cytologic atypia (Case #7); H, Mitotically active spindle cells with focal necrosis (Case#1); I, Myoepithelial tumor cells within lymphovascular channels (Case #2).
Nuclear pleomorphism was typically mild and at most moderate, with no cases showing frank cytologic atypia and/or marked nuclear pleomorphism (Table 2; Figure 4). Necrosis and/or LVI were present in 5 cases, with 2 cases having both necrosis and LVI and 3 cases with either but not both (Table 2; Fig. 4). Mitotic activity ranged from 0 – 6 mitoses/ 2 mm2 (mean 1).
As shown in Table 3, all cases showed positivity for cytokeratins and/or epithelial membrane antigen (EMA), in conjunction with detection of S100 protein or myogenic markers (calponin or smooth muscle actin (SMA)). In 5 cases there was positivity for both S100 and a myogenic marker (Fig. 5). Only 1 case was negative for CK, while 2 cases were S100 protein negative. p63 was positive in only half of cases tested (3/6). When performed, calponin was positive in 6/7 cases, while SMA only in 3/6. All cases were negative for TTF-1 (data not shown).
Table 3. Immunohistochemical and molecular features of thoracic myoepithelial tumors.
| Case | CK | EMA | S100 | SMA | Calponin | p63 | Rearrangement |
|---|---|---|---|---|---|---|---|
| 1 | Focal | Focal | + | Focal | Focal | + | EWSR1-PBX1 |
| 2 | Focal | Focal | Focal | + | + | ND | EWSR1 |
| 3 | + | - | + | + | - | + | EWSR1-ZNF444 |
| 4 | + | ND | + | - | ND | - | FUS-KLF17 |
| 5 | + | - | - | - | + | - | None |
| 6 | Focal | + | - | - | + | + | None |
| 7 | - | + | Focal | ND | + | - | None |
| 8 | Focal | - | + | ND | + | ND | None |
Figure 5.
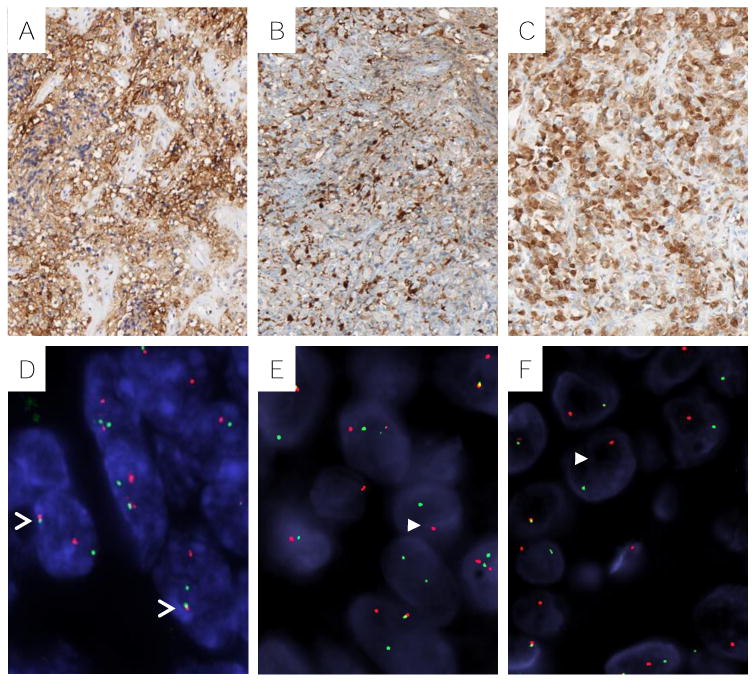
Immunohistochemical and cytogenetic features of thoracic MTs. Representative MT (Case#7) with diffuse positivity for EMA (A), focal positivity for S100 (B), and diffuse positivity for calponin (C). D, MT (Case #3) with EWSR1 (green signal) and ZNF444 (red signal) fusion (open arrowhead). FUS fused MT (Case#4) with split of telomeric (red) and centromeric (green) signals (solid arrowhead) for FUS (E) and KLF17 (F).
FISH demonstrated gene rearrangements in 4 cases, with 3 cases involving EWSR1 and one case FUS (Table 3; Fig. 5). None of the cases harbored HMGA2 or PLAG1 abnormalities. Partner genes were identified in 2 of the 3 EWSR1 rearranged cases, resulting in EWSR1-PBX1 and EWSR1-ZNF444 fusions, while a FUS-KLF17 fusion was detected in the FUS rearranged case (Fig. 5). Compared to fusion negative cases, tumors with EWSR1 or FUS rearrangements occurred in patients who were on average 8 years younger (50 vs 58 yrs), more commonly females (1:3 vs 3:1 male:female ratio), with more cases having necrosis (3 vs 1) and a slightly higher mean mitotic activity (2 vs <1) (Table 4). All fusion positive tumors had spindle and clear cell components, while an epithelioid component was present in all fusion negative tumors. Statistical analysis was not performed in this comparison due to small sample size.
Table 4. Clinicopathologic characteristics of fusion positive versus fusion negative thoracic myoepithelial tumors.
| EWS/FUS fusion positive | Fusion negative | |
|---|---|---|
| Number of cases (n) | 4 | 4 |
| Mean age (yrs) | 50 | 58 |
| Male : female ratio | 1:3 | 3:1 |
| Endobronchial/tracheal | 2 | 2 |
| Cytomorphology | Spindle and clear cells in all cases | Epithelioid component in all cases |
| Mitoses/2 mm2 (mean & range) | 2 (0-6) | <1 (0-2) |
| Necrosis (n) | 3 | 1 |
Literature Review
Of the previously reported cases of thoracic MTs, we identified 16 cases that fit our criteria for the diagnosis of primary thoracic MT, namely compatible morphology with confirmation of myoepithelial differentiation by ultrastructural studies or immunohistochemistry (Table 5). In general, the clinical characteristics of the cases in the literature were similar to our series, with no significant difference (unpaired two tailed T-test) in continuous variables such as age (50 ± 17 yrs vs 55 ± 17 yrs; p=0.59), tumor size (4.5 ± 3.2 cm vs 3.9 ± 4.3 cm; p=0.89), or mitotic count per 10 high power field (HPF)/ 2 mm2 (mean 7 vs 1; p=0.14). Similar to our series, most cases had a mixture of cytomorphologies with spindle cell being the most common (81%; 13/16 cases), and only one case was described as having marked cytologic atypia. Four reported cases had metastatic disease: one case with no evidence of disease (NED); one alive with disease (AWD); one case dead of disease (DOD); and one case dead of other causes (DOC; lung adenocarcinoma) 14,20,25,28.
Table 5. Clinicopathologic features of previously reported thoracic myoepithelial tumors.
| Case | Age | Sex | Location | Size (cm) | Cytomorphology | Mitoses/10 HPF | Necrosis | Cytologic atypia | LVI | Metastasis | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case 124 | 60 | M | Subpleural | 3.3 | Spindle, clear cell | < 1 | Absent | Mild | N/A | None | N/A |
| Case 214 | 58 | M | Endobronchial | 3.8 | Spindle, plasmacytoid | N/A | Present | N/A | N/A | Hip, forearm | DOC; 12 mos |
| Case 39 | 54 | F | Intraparenchymal | 2.5 | Spindle | N/A | N/A | Mild | N/A | None | NED; 24 mos |
| Case 420 | 46 | M | Endobronchial | 6.5 | Spindle, plasmacytoid | 13 | Present | N/A | N/A | Contralateral lung | AWD; 7 mos |
| Case 526 | 60 | F | Intraparenchymal | 2 | Spindle, plasmacytoid | 0 | Absent | Mild | N/A | None | N/A |
| Case 613 | 37 | F | Endobronchial | 7 | Spindle, plasmacytoid, clear cell | 1 | N/A | Mild | N/A | None | NED; 15 mos |
| Case718 | 48 | M | Intraparenchymal | 1.5 | Spindle | 5 | N/A | Moderate | N/A | None | NED; 15 mos |
| Case 821 | 35 | F | Endobronchial | 13 | Spindle, epithelioid | N/A | N/A | Mild | N/A | None | N/A |
| Case 921 | 44 | F | Endobronchial | 7 | Spindle, epithelioid, plasmacytoid | > 10 | Present | Moderate | N/A | None | NED; 6 mos |
| Case 1011 | 40 | F | Endobronchial | 7 | Spindle, epithelioid | N/A | N/A | Mild | N/A | None | NED; 48 mos |
| Case 1116 | 18 | F | Endobronchial | 2.5 | Plasmacytoid, clear cell | 0 | Absent | Mild | N/A | None | NED; 6 mos |
| Case 1225 | 76 | M | Parenchymal | 2.2 | Epithelioid, clear cell | 32 | Present | Mild | Present | Brain | DOD; 11 mos |
| Case 1317 | 23 | M | Endotracheal | 2.3 | Spindle, epithelioid | 8 | Present | Marked | Absent | None | NED; 6 mos |
| Case 1423 | 67 | F | Endotracheal | 2 | Spindle, epithelioid | 0 | Absent | Mild | Absent | None | NED; 15 mos |
| Case 1552 | 72 | F | Endobronchial | 1.5 | Spindle, clear cell | 4 | Absent | Mild | Absent | None | NED; 7 mos |
| Case 1628 | 66 | M | N/A | 8 | Clear cell | N/A | N/A | N/A | Present | Lymph node | NED; 14 mos |
AWD = alive with disease; NED = no evidence of disease; DOD = dead of disease; DOC = dead of other causes; HPF=high power field; LVI=lymphovascular invasion
Univariate survival analysis (Log rank (Mantel Cox) test) on a combined dataset of our series with previously published cases (n=24), using AWD and DOD as events, showed that patients with metastasis had a worse 5-year survival than those without (25% vs 100%, p=0.003), as did the presence of necrosis (33% vs 100%, p=0.027; Fig. 7). As a continuous variable, the number of mitoses showed significant correlation (Cox regression) with outcome (HR 1.113; 95% CI 1.005-1.232; p = 0.039), and patients with tumors having a mitotic count of greater than or equal to 5 mitoses per 2 mm2/10 HPF had significantly worse 5- year survival than those with less than 5 mitoses per 2mm2/10 HPF (50% vs 80% ; p=0.005; Fig. 7). There was a trend towards significance for male sex and worse survival (p=0.052). No significant correlations with were identified for other variables including age, smoking history, symptoms, location, tumor size, cytomorphology, matrix characteristics, cytologic atypia, or LVI.
Figure 7.
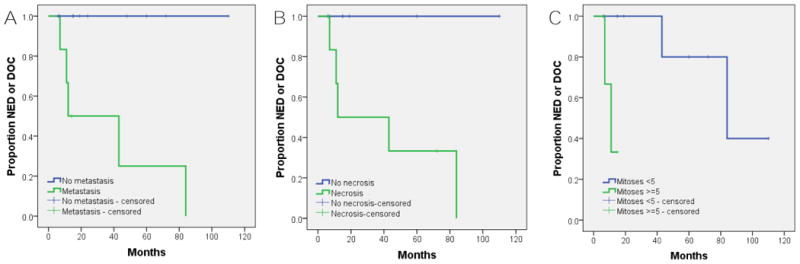
Kaplan-Meier survival analysis using alive with disease and dead of disease as events showing worse outcome associated with: A, metastasis (p=0.003); B, presence of necrosis (p=0.027); C, ≥5 mitoses per 10 high power field/2 mm2 (p=0.005). NED=No evidence of disease; DOC=Dead of other causes.
Discussion
The results of our study show that thoracic MTs display a range of morphology and clinical behavior with a subset harboring rearrangements involving EWSR1 or FUS genes. Based on our series and previously reported cases fitting our inclusion criteria, thoracic MTs are tumors of adulthood, with an age range of 18-77 yrs (mean 52) in the combined data set. This is similar to myoepithelial carcinomas of the salivary gland, which have all been reported in adults with the exception of one in a 14 year-old male 6,7,28,30,31. In contrast, approximately 10-20% of soft tissue MTs occur in the pediatric population, with several cases reported in children under 10 years of age 4,32,33.
From a radiologic point of view, MTs should be considered in the differential diagnosis of tumors typically occurring in the tracheo-bronchial tree, including carcinoid tumors, salivary-gland type tumors, papillomas, and squamous cell carcinomas 34,35. Well-circumscribed peripheral MTs would enter into the differential of primary solid nodules and masses occurring in this location including hamartoma, sclerosing pneumocytoma, inflammatory myofibroblastic tumor, as well as solitary fibrous tumor or localized malignant mesothelioma if involving the pleura 36,37. When infiltrative, peripheral and subpleural MTs can mimic adenocarcinoma, synovial sarcoma as well as a wide variety of other malignant lesions of the lung and pleura 36,37.
Thoracic MTs share the architectural, cytologic and stromal characteristics of MTs of the salivary glands and soft tissue/bone 31,32,38,39. While in soft tissue and bone, MTs are grouped with mixed tumors showing ductal differentiation, salivary gland tumors with more than occasional true luminal differentiation are classified as pleomorphic adenoma rather than MT 1,3,29. None of the cases in our series had glandular differentiation, and of the cases included in our literature review, epithelial elements were reported in only three cases 9,21. It is possible that in some of these reported cases, the epithelial components likely represent entrapped respiratory/alveolar epithelium, characterized by well-defined cytokeratin positive epithelial lined spaces 9. Indeed, interstitial growth of pulmonary MTs can cause entrapment of hyperplastic respiratory epithelium, resulting in a fibroadenoma-like growth pattern that can be confused with pulmonary hamartoma21. Our results therefore support the current World Health Organization (WHO) definition of thoracic MTs to be tumors predominantly or exclusively myoepithelial differentiation, in distinction to pleomorphic adenomas or mixed tumors that also show ductal differentiation 29.
Combined tumors consisting of both myoepithelial elements and non-small cell carcinoma have been reported 14. We encountered one such tumor, composed of lung adenocarcinoma with a myoepithelial component (Fig. 6, C), which was excluded from our dataset. A previously reported case of MT with malignant squamous elements occurred in a 58 year-old male who died with metastatic disease 5 years after initial surgery 14. Our case of MT with adenocarcinoma occurred in an 80 year-old female who also died with metastatic disease 15 months following resection. The histogenesis and classification of these combined tumors is unclear and they may fall within the spectrum of pleomorphic carcinomas of the lung, which are known to behave aggressively29.
Figure 6.
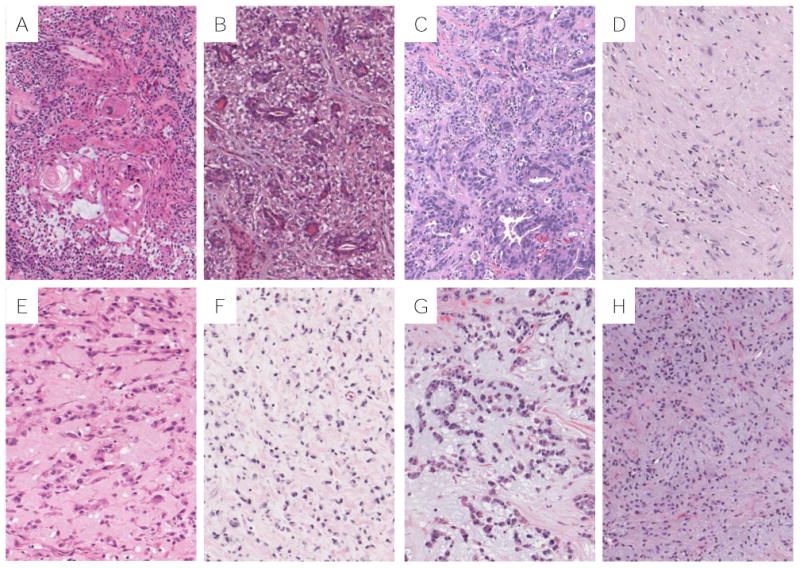
Differential diagnosis for thoracic MTs. A, Endobronchial pleomorphic adenoma with squamous component; B, pulmonary epithelial-myoepithelial carcinoma with bi-layered duct like structures; C, pleomorphic carcinoma with adenocarcinoma and malignant myoepithelial component; D, schwannoma of chest wall with myxoid matrix; E, primary pulmonary myxoid sarcoma; F, metastatic melanoma with myxoid stroma; G, metastatic matrix producing carcinoma of the breast; H, metastatic ossifying fibromyxoid tumor.
The morphology and immunostaining characteristics of thoracic MTs, while distinctive, have overlapping features with a wide variety of both primary and metastatic tumors involving the lung (Fig. 6). In particular, MTs need to be distinguished from other pulmonary salivary gland type tumors such as adenoid cystic carcinoma, pleomorphic adenoma and epithelial-myoepithelial carcinoma. All these tumors have a myoepithelial component that shares similar features with thoracic MTs, and in the absence of clearly identifiable epithelial components on biopsy, morphologic distinction would require examination of an excisional specimen. Other primary thoracic tumors to consider are schwannomas, which can occasionally have myxoid stroma, and primary pulmonary myxoid sarcoma (PPMS), which can have striking morphologic similarity to MTs. Immunohistochemistry can generally resolve this differential, and in difficult cases FISH can also be used to exclude a PPMS, which harbors a characteristic EWSR1-CREB1 fusion40. Finally, there are a number of metastatic tumors that can have overlapping morphologic and immunohistochemical features with MTs including metastatic melanoma, matrix producing carcinoma of the breast, ossifying fibromyxoid tumor (OFT), extraskeletal myxoid chondrosarcoma (EMC), clear cell sarcoma (CCS), and Ewing sarcoma. Close clinical evaluation is therefore required to exclude a metastasis, including from a salivary gland or soft tissue primary, as lung metastasis can occur several years after resection7,31,32,41. MT could also be confused with squamous cell carcinoma, particularly in small biopsies, because they can both express p63 and/or p40.
Identifying prognostically significant histologic and clinical features of MTs has been challenging in both thoracic and extrathoracic sites. In soft tissue, the only reliable criterion for malignancy is the presence of moderate to severe nuclear atypia 1,32. In the salivary glands, malignancy has been traditionally determined by infiltrative growth characterized by multinodular invasive tumor borders with destruction of adjacent salivary gland tissue 3,31,42,43. More recently, additional features such as tumor necrosis and/or mitotic activity ≥ 6/10 HPFs were shown to correlate with worse outcome in salivary gland myoepithelial carcinomas 7. In the same study, tumor size >5 cm and presence of a pleomorphic adenoma component (myoepithelial carcinoma ex-pleomorphic adenoma) also correlated significantly with overall and distant recurrences. None of the cases in our series had a component of pleomorphic adenoma and/or ductal differentiation, and tumor size did not correlate with outcome. Interestingly however, in our study, necrosis and mitotic activity were the two histologic parameters that correlated with worse prognosis, with a similar cutoff for mitoses of ≥ 5 per 10 HPFs/2mm2. Two previously reported pulmonary pleomorphic adenomas with malignant clinical behavior also had ≥ 5 per 10 HPFs. 44. It is worth noting that the standard of 10 HPF is known not to be equivalent for every microscope, and many of the reports of thoracic MTs cited in this paper do not specify which microscope and eyepiece was used. The microscope used in this study has a wider field of view than older models that were likely used in previous publications. We used 2mm2 rather than 10 HPF so mitotic counts can be standardized across different microscope models 45,46.
Our study, which is the first to have studied thoracic MTs at a molecular level, revealed that half of these harbor rearrangements involving EWSR1 or FUS. This is similar in incidence to reported rates of EWSR1 and/or FUS rearrangements in soft tissue/bone MTs 4. In our series, there appeared to be enrichment of spindle and clear cell morphology in the fusion positive tumors compared to epithelioid morphology in the fusion negative tumors. EWSR1 rearrangement has been demonstrated in one other recently reported case of lung MT, and it was described as having clear cell morphology 28. No significant correlation between morphology and fusion status could be demonstrated in our study. While this may be due to small sample size, it may also be due to tumor heterogeneity, as studies in other sites have also been unable to demonstrate robust correlation of cytomorphologic cell type or matrix characteristics with molecular status 4,6. The absence of PLAG1 or HMGA2 abnormalities in thoracic MTs suggests that they have distinct histogenesis from myoepithelial carcinomas ex-pleomorphic adenomas, of which the majority harbor abnormalities in these genes 6. While traditionally associated with soft tissue MTs, EWSR1 rearrangement has also been recently identified in a subset of salivary gland MTs28.
It is worth emphasizing that in the appropriate morphologic and immunohistochemical context, confirmation of a EWSR1 or FUS related rearrangement can be a powerful adjunct tool in confirming the diagnosis of MT. However, as the presence of EWSR1 and FUS rearrangements can be seen in tumors included in the differential diagnosis of MTs, additional molecular testing is needed to confirm their gene partner in difficult cases. These should include not only gene partners reported in MTs, but also NR4A3 (rearranged in EMC), CREB1 (rearranged in PPMS), ATF1 (rearranged in CCS), and if the clinical suspicion is high, FLI-1 and other partners associated with Ewing sarcoma.
While EWSR1-PBX1, EWSR1-ZNF444, and FUS-KLF17 fusions have only been reported in one thoracic MT each, they have been previously described in soft tissue/bone MTs 4,8,47,48. Of the previously reported EWSR1-PBX1 fusion positive MTs, 4 cases occurred in the deep soft tissues of the extremities and 1 case in the iliac bone 4,47. Of these, clinical follow-up was reported in only one case, occurring in a 59 year-old female deep to the fascia in the arch of the foot, and the patient had NED at 7 months following surgical excision 47. While we were unable to obtain clinical follow up in our case with EWSR1-PBX1 fusion, the tumor had malignant features in the form of mitotic activity >5/2 mm2 and necrosis. EWSR1-ZNF444 has been reported in one case of an occipital soft tissue MT in a 40 year-old female 48 who developed lung metastases, and died 9 years and 6 months after the initial diagnosis. Our EWSR1-ZNF444 positive MT also behaved in an aggressive manner, with abdominal wall and liver metastasis, but the patient is still alive 7 years since the original diagnosis. Of the 3 reported extrapulmonary MTs with FUS-KLF17 fusion, one occurred in the skin, one in the tibia, and one in the foot, with the latter developing metastasis to the thigh 8. A benign clinical course was observed in our single case harboring FUS-KLF17 fusion, with NED at 72 months, despite the presence of necrosis. Overall, while EWSR1-ZNF444 fusion appears to be associated with aggressive behavior, there are too few cases to draw a meaningful conclusion as to correlations between fusion status and outcome.
As with most EWSR1 and FUS-associated fusions, all 3 partner genes (PBX1, ZNF444, KLF17) encode for transcription factors that bind DNA and regulate transcription. PBX1 has been identified as a partner of TCF3 in a subset of B cell acute lymphoblastic leukemia, with the fusion product potentially acting on mRNA transport and export 49. ZNF444 appears to promote the transcription of SCARF2 which codes for a protein in lipoprotein metabolism, however the functional consequences of its fusion with EWSR1 and how this relates to oncogenesis has yet to be resolved 50. Reduced KLF17 levels have been implicated in tumor growth and progression at various sites, including lung adenocarcinoma, and it has been hypothesized that the FUS-KLF17 chimeric transcript may disrupt translation of the protein 8,51. Thus, while there is some insight into EWSR1 and FUS partner gene function, the precise mechanism by which each of these genes promote oncogenesis remains to be delineated.
In conclusion, thoracic MTs are a histologically and clinically diverse group of tumors which share morphologic findings with their counterparts at other sites. Despite characteristic features, MTs are challenging to recognize due to overlapping morphologic and immunohistochemical properties with a variety of both primary and metastatic thoracic tumors. This emphasizes the use of strict criteria for the diagnosis of thoracic MTs, including absent or limited ductal differentiation, and confirmation of myoepithelial differentiation by epithelial, S100 protein, and myogenic immunomarkers. Confirmation of EWSR1 and FUS gene rearrangements is a powerful adjunct diagnostic tool, as these are present in half of thoracic MTs. Furthermore, our study has identified prognostically significant histologic features, in particular the presence of necrosis and mitotic activity ≥5 per 2 mm2, which are significantly correlated with worse clinical outcome. Thus, comprehensive histologic assessment of thoracic MTs with thorough sampling and close attention to these features can provide important prognostic information and be a valuable contribution to patient management.
Footnotes
Conflicts of interest: none
References
- 1.Fletcher CDM, World Health Organization, International Agency for Research on Cancer . WHO classification of tumours of soft tissue and bone. 4th. Lyon: IARC Press; 2013. [Google Scholar]
- 2.Lakhani SR, International Agency for Research on Cancer., World Health Organization . WHO classification of tumours of the breast. 4th. Lyon: International Agency for Research on Cancer; 2012. [Google Scholar]
- 3.Barnes L, International Academy of Pathology., World Health Organization et al. Pathology and genetics of head and neck tumours. Lyon: IARC Press; 2005. [Google Scholar]
- 4.Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes, chromosomes & cancer. 2010 Dec;49(12):1114–1124. doi: 10.1002/gcc.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonescu CR, Zhang L, Shao SY, et al. Frequent PLAG1 gene rearrangements in skin and soft tissue myoepithelioma with ductal differentiation. Genes, chromosomes & cancer. 2013 Jul;52(7):675–682. doi: 10.1002/gcc.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katabi N, Ghossein R, Ho A, et al. Consistent PLAG1 and HMGA2 abnormalities distinguish carcinoma ex-pleomorphic adenoma from its de novo counterparts. Human pathology. 2015 Jan;46(1):26–33. doi: 10.1016/j.humpath.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong M, Drill EN, Morris L, et al. Prognostic Factors in Myoepithelial Carcinoma of Salivary Glands: A Clinicopathologic Study of 48 Cases. The American journal of surgical pathology. 2015 Apr 3; doi: 10.1097/PAS.0000000000000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang SC, Chen HW, Zhang L, et al. Novel FUS-KLF17 and EWSR1-KLF17 fusions in myoepithelial tumors. Genes, chromosomes & cancer. 2015 Feb 23; doi: 10.1002/gcc.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cagirici U, Sayiner A, Inci I, et al. Myoepithelioma of the lung. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2000 Feb;17(2):187–189. doi: 10.1016/s1010-7940(00)00338-9. [DOI] [PubMed] [Google Scholar]
- 10.Chand M, Mann JM, Sabayev V, et al. Endotracheal myoepithelioma. Chest. 2011 Jul;140(1):242–244. doi: 10.1378/chest.10-2976. [DOI] [PubMed] [Google Scholar]
- 11.Dahiya D. Endobronchial myoepithelioma - A rare pulmonary tumour. JK Science. 2007;9(2):100–101. [Google Scholar]
- 12.Damiani S, Magrini E, Farnedi A, et al. Basal cell (myoepithelial) adenocarcinoma of the lung. First case with cytogenetic findings. Histopathology. 2004 Oct;45(4):422–424. doi: 10.1111/j.1365-2559.2004.01910.x. [DOI] [PubMed] [Google Scholar]
- 13.El Mezni F, Zeddini A, Hamzaoui A, et al. [Benign myoepithelioma of the lung] Revue de pneumologie clinique. 2004 Nov;60(5 Pt 1):282–284. doi: 10.1016/s0761-8417(04)72114-8. [DOI] [PubMed] [Google Scholar]
- 14.Higashiyama M, Kodama K, Yokouchi H, et al. Myoepithelioma of the lung: report of two cases and review of the literature. Lung cancer (Amsterdam, Netherlands) 1998 Apr;20(1):47–56. doi: 10.1016/s0169-5002(98)00006-3. [DOI] [PubMed] [Google Scholar]
- 15.Hysi I, Wattez H, Benhamed L, et al. Primary pulmonary myoepithelial carcinoma. Interactive cardiovascular and thoracic surgery. 2011 Aug;13(2):226–228. doi: 10.1510/icvts.2011.270934. [DOI] [PubMed] [Google Scholar]
- 16.Kourda J, Ismail O, Smati BH, et al. Benign myoepithelioma of the lung - a case report and review of the literature. Cases journal. 2010;3(1):25. doi: 10.1186/1757-1626-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Wang J, Li H, et al. Myoepithelial carcinoma: first case reported in the trachea. Pathology international. 2012 Jan;62(1):55–59. doi: 10.1111/j.1440-1827.2011.02746.x. [DOI] [PubMed] [Google Scholar]
- 18.Masuya D, Haba R, Huang CL, et al. Myoepithelial carcinoma of the lung. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2005 Nov;28(5):775–777. doi: 10.1016/j.ejcts.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Meyer A, Delarue J, Peuteuil G, et al. [A case of myoepithelial cell tumor localized in the region near the fissure of the left lung] Journal francais de medecine et chirurgie thoraciques. 1954;8(3):310–316. [PubMed] [Google Scholar]
- 20.Miura K, Harada H, Aiba S, et al. Myoepithelial carcinoma of the lung arising from bronchial submucosa. The American journal of surgical pathology. 2000 Sep;24(9):1300–1304. doi: 10.1097/00000478-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Pelosi G, Rodriguez J, Viale G, et al. Salivary gland-type tumors with myoepithelial differentiation arising in pulmonary hamartoma: report of 2 cases of a hitherto unrecognized association. The American journal of surgical pathology. 2006 Mar;30(3):375–387. doi: 10.1097/01.pas.0000190785.95326.4b. [DOI] [PubMed] [Google Scholar]
- 22.Sarkaria IS, DeLair D, Travis WD, et al. Primary myoepithelial carcinoma of the lung: a rare entity treated with parenchymal sparing resection. Journal of cardiothoracic surgery. 2011;6:27. doi: 10.1186/1749-8090-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sekine A, Morishita Y, Okudela K, et al. Benign myoepithelioma in the intrathoracic trachea. Internal medicine. 2014;53(14):1535–1538. doi: 10.2169/internalmedicine.53.1697. [DOI] [PubMed] [Google Scholar]
- 24.Strickler JG, Hegstrom J, Thomas MJ, et al. Myoepithelioma of the lung. Archives of pathology & laboratory medicine. 1987 Nov;111(11):1082–1085. [PubMed] [Google Scholar]
- 25.Tanahashi J, Kashima K, Daa T, et al. Pulmonary myoepithelial carcinoma resembling matrix-producing carcinoma of the breast: case report and review of the literature. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica. 2010 May;118(5):401–406. doi: 10.1111/j.1600-0463.2010.02606.x. [DOI] [PubMed] [Google Scholar]
- 26.Veeramachaneni R, Gulick J, Halldorsson AO, et al. Benign myoepithelioma of the lung: a case report and review of the literature. Archives of pathology & laboratory medicine. 2001 Nov;125(11):1494–1496. doi: 10.5858/2001-125-1494-BMOTL. [DOI] [PubMed] [Google Scholar]
- 27.Compton CC, American Joint Committee on Cancer . AJCC cancer staging atlas. 2nd. New York ; London: Springer; 2012. [Google Scholar]
- 28.Skalova A, Weinreb I, Hyrcza M, et al. Clear cell myoepithelial carcinoma of salivary glands showing EWSR1 rearrangement: molecular analysis of 94 salivary gland carcinomas with prominent clear cell component. The American journal of surgical pathology. 2015 Mar;39(3):338–348. doi: 10.1097/PAS.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 29.Travis WD, World Health Organization., International Agency for Research on Cancer et al. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon Oxford: IARC Press, Oxford University Press (distributor); 2015. [Google Scholar]
- 30.Di Palma S, Guzzo M. Malignant myoepithelioma of salivary glands: clinicopathological features of ten cases. Virchows Archiv A, Pathological anatomy and histopathology. 1993;423(5):389–396. doi: 10.1007/BF01607152. [DOI] [PubMed] [Google Scholar]
- 31.Savera AT, Sloman A, Huvos AG, et al. Myoepithelial carcinoma of the salivary glands: a clinicopathologic study of 25 patients. The American journal of surgical pathology. 2000 Jun;24(6):761–774. doi: 10.1097/00000478-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Hornick JL, Fletcher CD. Myoepithelial tumors of soft tissue: a clinicopathologic and immunohistochemical study of 101 cases with evaluation of prognostic parameters. The American journal of surgical pathology. 2003 Sep;27(9):1183–1196. doi: 10.1097/00000478-200309000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Jo VY, Fletcher CD. Myoepithelial neoplasms of soft tissue: an updated review of the clinicopathologic, immunophenotypic, and genetic features. Head and neck pathology. 2015 Mar;9(1):32–38. doi: 10.1007/s12105-015-0618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko JM, Jung JI, Park SH, et al. Benign tumors of the tracheobronchial tree: CT-pathologic correlation. AJR American journal of roentgenology. 2006 May;186(5):1304–1313. doi: 10.2214/AJR.04.1893. [DOI] [PubMed] [Google Scholar]
- 35.Park CM, Goo JM, Lee HJ, et al. Tumors in the tracheobronchial tree: CT and FDG PET features. Radiographics : a review publication of the Radiological Society of North America, Inc. 2009 Jan-Feb;29(1):55–71. doi: 10.1148/rg.291085126. [DOI] [PubMed] [Google Scholar]
- 36.Furuya K, Yasumori K, Takeo S, et al. Lung CT: Part 1, Mimickers of lung cancer--spectrum of CT findings with pathologic correlation. AJR American journal of roentgenology. 2012 Oct;199(4):W454–463. doi: 10.2214/AJR.10.7262. [DOI] [PubMed] [Google Scholar]
- 37.Salahudeen HM, Hoey ET, Robertson RJ, et al. CT appearances of pleural tumours. Clinical radiology. 2009 Sep;64(9):918–930. doi: 10.1016/j.crad.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Dardick I. Myoepithelioma: definitions and diagnostic criteria. Ultrastructural pathology. 1995 Sep-Oct;19(5):335–345. doi: 10.3109/01913129509021906. [DOI] [PubMed] [Google Scholar]
- 39.Khademi B, Kazemi T, Bayat A, et al. Salivary gland myoepithelial neoplasms: a clinical and cytopathologic study of 15 cases and review of the literature. Acta cytologica. 2010 Nov-Dec;54(6):1111–1117. doi: 10.1159/000325253. [DOI] [PubMed] [Google Scholar]
- 40.Thway K, Fisher C. Myoepithelial tumor of soft tissue: histology and genetics of an evolving entity. Advances in anatomic pathology. 2014 Nov;21(6):411–419. doi: 10.1097/PAP.0000000000000039. [DOI] [PubMed] [Google Scholar]
- 41.Gleason BC, Fletcher CD. Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. The American journal of surgical pathology. 2007 Dec;31(12):1813–1824. doi: 10.1097/PAS.0b013e31805f6775. [DOI] [PubMed] [Google Scholar]
- 42.Ellis GL, Auclair PL, American Registry of Pathology et al. Tumors of the salivary glands. Washington, DC: American Registry of Pathology in collaboration with the Armed Forces Institute of Pathology; 2008. [Google Scholar]
- 43.Seethala RR. An update on grading of salivary gland carcinomas. Head and neck pathology. 2009 Mar;3(1):69–77. doi: 10.1007/s12105-009-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moran CA, Suster S, Askin FB, et al. Benign and malignant salivary gland-type mixed tumors of the lung. Clinicopathologic and immunohistochemical study of eight cases. Cancer. 1994 May 15;73(10):2481–2490. doi: 10.1002/1097-0142(19940515)73:10<2481::aid-cncr2820731006>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 45.Kirschner J. Olympus' New BX40 and BX50 Product Line. INTERNATIONAL LABMATE. 1993;18:17–17. [Google Scholar]
- 46.Travis WD, Rush W, Flieder DB, et al. Survival analysis of 200 pulmonary neuroendocrine tumors with clarification of criteria for atypical carcinoid and its separation from typical carcinoid. The American journal of surgical pathology. 1998 Aug;22(8):934–944. doi: 10.1097/00000478-199808000-00003. [DOI] [PubMed] [Google Scholar]
- 47.Brandal P, Panagopoulos I, Bjerkehagen B, et al. Detection of a t(1;22)(q23;q12) translocation leading to an EWSR1-PBX1 fusion gene in a myoepithelioma. Genes, chromosomes & cancer. 2008 Jul;47(7):558–564. doi: 10.1002/gcc.20559. [DOI] [PubMed] [Google Scholar]
- 48.Brandal P, Panagopoulos I, Bjerkehagen B, et al. t(19;22)(q13;q12) Translocation leading to the novel fusion gene EWSR1-ZNF444 in soft tissue myoepithelial carcinoma. Genes, chromosomes & cancer. 2009 Dec;48(12):1051–1056. doi: 10.1002/gcc.20706. [DOI] [PubMed] [Google Scholar]
- 49.Hajingabo LJ, Daakour S, Martin M, et al. Predicting interactome network perturbations in human cancer: application to gene fusions in acute lymphoblastic leukemia. Molecular biology of the cell. 2014 Dec 1;25(24):3973–3985. doi: 10.1091/mbc.E14-06-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adachi H, Tsujimoto M. Characterization of the human gene encoding the scavenger receptor expressed by endothelial cell and its regulation by a novel transcription factor, endothelial zinc finger protein-2. The Journal of biological chemistry. 2002 Jul 5;277(27):24014–24021. doi: 10.1074/jbc.M201854200. [DOI] [PubMed] [Google Scholar]
- 51.Cai XD, Zhou YB, Huang LX, et al. Reduced expression of Kruppel-like factor 17 is related to tumor growth and poor prognosis in lung adenocarcinoma. Biochemical and biophysical research communications. 2012 Feb 3;418(1):67–73. doi: 10.1016/j.bbrc.2011.12.129. [DOI] [PubMed] [Google Scholar]
- 52.Rosen LE, Singh RI, Vercillo M, et al. Myoepithelial carcinoma of the lung : a review. Applied immunohistochemistry and molecular morphology. 2014 Dec 16;23(6):397–401. doi: 10.1097/PAI.0000000000000113. [DOI] [PubMed] [Google Scholar]


