Abstract
Neutrophils are central players in the innate immune system. They generate neutrophil extracellular traps (NETs), which protect against invading pathogens but are also associated with the development of autoimmune and/or inflammatory diseases and thrombosis. Here, we report that lactoferrin, one of the components of NETs, translocated from the cytoplasm to the plasma membrane and markedly suppressed NETs release. Furthermore, exogenous lactoferrin shrunk the chromatin fibers found in released NETs, without affecting the generation of oxygen radicals, but this failed after chemical removal of the positive charge of lactoferrin, suggesting that charge-charge interactions between lactoferrin and NETs were required for this function. In a model of immune complex-induced NET formation in vivo, intravenous lactoferrin injection markedly reduced the extent of NET formation. These observations suggest that lactoferrin serves as an intrinsic inhibitor of NETs release into the circulation. Thus, lactoferrin may represent a therapeutic lead for controlling NETs release in autoimmune and/or inflammatory diseases.
Abbreviations: LLf, lactoferrin; NET, neutrophil extracellular trap; MPO, myeloperoxidase; TRALI, transfusion-related acute lung injury; pDC, plasmacytoid dendritic cells; ANCA, anti-neutrophil cytoplasmic antibody; SLE, systemic lupus erythematosus; PMA, phorbol 12-myristate 13-acetate; GA, glutaric anhydride; SA, succinic anhydride; HOCl, hypochlorous acid; LPO, lactoperoxidase; ROS, reactive oxygen species; RPA, reverse passive Arthus
Keywords: Neutrophil extracellular traps (NETs), Lactoferrin, Chromatin, Oxygen radicals
Highlights
-
•
We developed a real-time cell imaging system which can visualize neutrophil activation, DNA and cell membrane structures.
-
•
Lactoferrin serves as an intrinsic inhibitor of neutrophil extracellular traps (NETs) release into the circulation.
-
•
Lactoferrin may be a novel therapeutic target in pathological conditions related to NETs.
Neutrophils generate neutrophil extracellular traps (NETs), which protect against invading pathogens but are also associated with the development of autoimmune and/or inflammatory diseases and thrombosis. Controlling NETs release is one of the promising therapeutic strategies for the treatment of these diseases. Lactoferrin is a multifunctional protein which is found mainly in human maternal milk, tears, and neutrophils granules and so on. We found that lactoferrin serves an endogenous inhibitor of NETs release into the circulation in inflammatory conditions. Thus, lactoferrin may represent a therapeutic lead for controlling NETs release in autoimmune and/or inflammatory diseases.
1. Introduction
Neutrophil migration to inflammatory sites and subsequent phagocytosis and intracellular elimination of microbes is essential for host defense (Nathan, 2006). Activated neutrophils also release chromatin fibers called neutrophil extracellular traps (NETs), which trap and kill bacteria (Brinkmann et al., 2004), tuberculosis pathogens (Ramos-Kichik et al., 2009), fungi (Urban et al., 2009), and parasites (Guimarães-Costa et al., 2009). NETosis, the process of NET formation, includes plasma membrane rupture and chromatin fiber release following the collapse of the nuclear membrane (Fuchs et al., 2007).
While NETs are beneficial for host defense, their formation can also be harmful. For example, in sepsis (Clark et al., 2007) and transfusion-related acute lung injury (TRALI) (Caudrillier et al., 2012), NET formation induces damage to endothelial cells leading to massive thrombosis in target organs such as the lungs and kidneys. NETs may also serve as a source of auto-antigens that activate plasmacytoid dendritic cells (pDCs), thus triggering the activation of autoreactive B cells in the context of autoimmune diseases (Lande et al., 2011). In systemic lupus erythematosus (SLE), a reduction in NET degradation due to the impaired functioning of DNase1 results in the progression of lupus nephritis (Hakkim et al., 2010), and in autoimmune small-vessel vasculitis, anti-neutrophil cytoplasmic antibody (ANCA) directly stimulates neutrophils to drive NET formation (Kessenbrock et al., 2009). In addition, the important role of NETs in the pathogenesis of atherosclerosis has been reported (Knight et al., 2014, Döring et al., 2014).
We previously studied the mechanism of neutrophil-dependent vasculitis (Hirahashi et al., 2006) and glomerulonephritis (Hirahashi et al., 2009) and found that activation of leucocyte β2 integrin Mac-1 (CD11b/CD18, CR3) is a critical determinant of disease activity and inflammation-induced thrombosis. Because NET formation requires Mac-1 activation and is a downstream effector for neutrophil-mediated cytotoxicity (Neeli et al., 2009), we hypothesized that a substance that safely regulates NET formation may have applications in the treatment of inflammatory diseases.
Lactoferrin (Lf), which is found mainly in human exocrine fluids (such as maternal milk or tears) and specific granules (secondary granules) of human neutrophils (Broxmeyer et al., 1978), exhibits antibacterial properties (Pütsep et al., 2002) and possesses a strong positive charge. In addition to DNA and histones, NET fibers contain extranuclear proteins and proteins such as elastase, myeloperoxidase (MPO), and Lf, which are components of neutrophil granules (Urban et al., 2009). Although elastase and MPO are essential for NET formation (Papayannopoulos et al., 2010), the biological significance of Lf remains unclear.
In this study, we investigated the contribution of Lf to NET formation during inflammation. We developed a real-time cell imaging system using confocal fluorescence microscopy that can be used to visualize neutrophil activation, DNA and cell membrane structures by utilizing our original probes for reactive oxygen species in the cells. Here, we identified Lf as an intrinsic inhibitor of NETs release and a promising therapeutic target in pathological conditions related to NETs.
2. Materials and Methods
2.1. Isolation of Neutrophils and Platelets
Human peripheral blood samples (15 mL) were obtained from healthy volunteers who were not taking any regular medication. Neutrophils were isolated using Mono-Poly Resolving Medium (DS Pharma Biomedical, Japan) from whole blood collected in EDTA. Neutrophils were suspended in culture medium containing DMEM supplemented with 2% heat-inactivated human serum albumin (Sigma-Aldrich, St. Louis, MO, USA) and 4 mM l-glutamine (Sigma-Aldrich) at 8 °C. Cell purity (> 95%) was confirmed by Giemsa staining. Platelets were isolated at room temperature according to previously published methods (Müller et al., 2003). All procedures were conducted with permission from the medical ethics committee of The University of Tokyo, Japan.
2.2. Analysis of NET Formation
Neutrophils were seeded at 1 × 105 cells/well in μ-Slide 8-well plates (Ibidi, Germany) and incubated for 30 min with 2–200 μg/mL Lf from human neutrophils (Sigma-Aldrich) or bovine milk (Sigma-Aldrich), 200 μg/mL angiogenin, 200 μg/mL lactoperoxidase, 200 μg/mL G-Lf, 200 μg/mL S-Lf (generated as described for the charge-conversion of Lf), 10 μM diphenyleneiodonium chloride (DPI; Sigma-Aldrich) as an NADPH oxidase inhibitor, 1 mM 3-amino1,2,4,triazole (3AT; Wako, Japan) as a catalase inhibitor, 200 U/mL catalase (Sigma-Aldrich), 5 ng/mL recombinant human tumor necrosis factor alpha (TNFα; R&D Systems) and/or 200 μg/mL transferrin (Wako), and 10 U/mL heparin as a negatively charged molecule. Neutrophils were then stimulated with 12.5–25 nM phorbol 12-myristate 13-acetate (PMA; Sigma-Aldrich) for 3 h, 5 μg/mL monoclonal antibodies targeting MPO (MPO-ANCA, Acris Antibodies, USA, Cat# SM1475P RRID:AB_1005506) for 3 h, and 5 × 106 platelets (for 5 × 105 neutrophils) for 1 h. For live cell imaging, neutrophils were incubated with 500 nM SYTOX Green (Invitrogen/Life Technologies), 5 μM DRAQ5 (Abcam) for staining DNA, 500 nM HySOx for probing hypochlorous acid (HOCl), and/or 5 μg/mL WGA (Invitrogen/Life Technologies) for staining the cell membrane. Confocal fluorescence microscopy (Leica SP-5) was used to visualize NET formation at 37 °C. The fluorescence intensities of SYTOX Green, HySOx- and tetramethylrhodamine-labeled WGA, and DRAQ5 were determined at 500–540, 560–620, and 650–750 nm, respectively, with excitation at 490, 550, and 630 nm, respectively. For quantification of NET formation, we counted the number of cells that released SYTOX Green fluorescence into the extracellular space by reference to the report by Brinkmann et al. (2010) and divided this number by the total number of cells in the same field. Furthermore, we used Picogreen dsDNA assay reagent to quantify NET-DNA from 1 × 105 cells per 400 μL culture medium. Activated platelet-induced NET formation was evaluated as previously described (Clark et al., 2007). Briefly, 5 × 105 neutrophils were pretreated with 10 μM SQ29548 (Santa Cruz Biotechnology; a selective thromboxane receptor antagonist) for 10 min and 200 μg/mL Lf for 30 min before stimulation. Platelets were pre-activated with or without 50 μM thrombin receptor-activating peptide (TRAP, a PAR-1 agonist; Sigma-Aldrich) for 30 min and incubated with neutrophils for 1 h at 37 °C. Soluble IC-induced NETosis was evaluated as previously described (Chen et al., 2012). For preparation of soluble ICs, BSA and anti-BSA antibodies were mixed at 4–6 times antigen excess and incubated at 37 °C overnight as previously described (Stokol et al., 2004). For each experiment, the measured values were averaged from three replicates after the background was subtracted.
2.3. Analysis of NETs in Reverse Passive Arthus (RPA) Reaction In Vivo
Analysis of NET-like structures was performed as described previously (Chen et al., 2012). Briefly, male eight-week-old WT C57BL6/j mice were treated with 20 mg/kg bovine Lf or PBS alone, followed by administration of an intrascrotal injection of anti-BSA antibodies (200 μg/300 μL, Sigma-Aldrich) and an intravenous injection of BSA (300 μg/100 μL, Sigma-Aldrich) to induce RPA reaction as previously reported (Stokol et al., 2004). After 3 h, mice were injected with SYTOX Green (25 nM, Molecular Probes, USA), and the cremaster muscle was prepared for intravital microscopy. Images were obtained using an upright epifluorescence microscope (FV1000, Olympus Imaging, USA) with a 40 × water-immersion objective. Images were recorded with a CCD camera (DP72, Olympus Imaging) and analyzed using ImageJ software (NIH). SYTOX Green-positive individual NET fibers were counted in 8–10 representative images (436 μm × 328 μm). Data are presented as the average number of fibers (± standard error of the mean [s.e.m.]) per mm2. Signals from intact SYTOX Green-positive cells were excluded from this analysis, and only those exceeding 20 μm in length were considered NET-like formations. The number of neutrophils was quantified using reflected light oblique transillumination in the area 75 μm to each side of the vessel over a 100-μm vessel length (1.5 × 10− 4 mm2).
2.4. Immunofluorescence Microscopy
Neutrophils (1 × 105) were stimulated with 25 nM PMA in the presence or absence of 200 μg/mL Lf and fixed on microslide glass (Matsunami, Japan) using cytospin. Neutrophils were fixed with 4% paraformaldehyde and blocked with 3% BSA (Sigma-Aldrich) for 1 h and incubated for 1 h at room temperature with fluorescently conjugated anti-lactoferrin antibodies (1:100, HyTest Ltd., Finland, Cat# 4L2-2B8 RRID:AB_1618690) labeled with Alexa 488 SE (Invitrogen/Life Technologies), anti-elastase antibodies (1:200, Calbiochem/Merck Millipore, Germany, Cat# 481001, RRID:AB_212213) labeled with Alexa Fluor 546 (Invitrogen/Life Technologies), or DRAQ5 for staining DNA or with 5 μg/mL WGA for staining cell membranes. A fluorescence microscope (Leica SP-5) equipped with Z-axis imaging and DIC was used to collect images. We used ImageJ processing software to normalize and quantify the signals of Lf relative to those of the background.
2.5. Transfection with Lactoferrin siRNA
HL-60 cells (ATCC, USA, Cat# CCL-240, RRID:CVCL_0002) were incubated with IMDM (ATCC) containing 20% fetal bovine serum (FBS; ATCC) and antibiotics at 37 °C. HL-60 cells were then incubated with 1.25% dimethyl sulfoxide (DMSO; ATCC) for 3 days to differentiate HL-60 granulocytes. Transfection of HL-60 cells with lactoferrin siRNA was performed according to the manufacturer's instructions (Qiagen, Germany). To verify knockdown of Lf mRNA, we purified RNA using RNeasy (Qiagen) and used this RNA to synthesize cDNA with High Capacity RNA-to-cDNA Master Mix (Applied Biosystems/Life Technologies, USA). We analyzed the comparative threshold cycles (CTs) to determine gene expression with a Step One Plus Real-Time PCR system (Applied Biosystems/Life Technologies) using TaqMan Gene Expression Master Mix (TaqMan/Life Technologies) and the following 6-carboxyfluorescein (FAM)-labeled primers: LTF, TCTGGTGCCTTCAAGTGTCTGAGAG or GAPDH, CAAGAGGAAGAGAGAGACCCTCACT. Reactions were performed under the following conditions: 50 °C for 2 min, 95 °C for 10 min as a holding step, and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. To quantify NET-DNA, 1 × 105 HL-60 cells were resuspended in 400 μL culture medium and stimulated with 25 nM PMA. DNA concentrations in the culture medium were measured using Picogreen dsDNA assay reagent at 3 h after PMA stimulation.
2.6. Electron Microscopy
Neutrophils (1 × 106) pretreated with or without 200 μg/mL lactoferrin for 30 min were stimulated with 25 nM PMA and incubated for 3 h at 37 °C. Freshly purified neutrophils were allowed to adhere to glass coverslips in culture medium. Neutrophils were then fixed in 0.1 M phosphate buffer (pH 7.4) containing 2% glutaraldehyde at 4 °C and postfixed in 2% OsO4 in 0.1 M phosphate buffer (pH 7.4) for 1 h. Samples were dehydrated through a graded ethanol series, placed in propyleneoxide, and embedded in epoxy resin (Quetol 812). Ultrathin sections made with a Leica EM UCT ultramicrotome were stained with 2% uranyl acetate in distilled water for 15 min following by modified Sato's lead solution for 5 min. Grids were viewed with a JEM-1200EX transmission electron microscope (TEM; JEOL, Japan) operating at 80 kV.
For scanning electron microscope (SEM) analysis, neutrophils were fixed in 2% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4 °C and further fixed in 2% OsO4 in 0.1 M phosphate buffer at 4 °C for 1 h. Samples were then dehydrated in ethanol, placed in isoamyl acetate, critical-point dried, coated with a layer of sublimated OsO4 using an osmium plasma coater (OPC80N; Filgen, Nagoya, Japan), and examined using an SEM (JSM-6320F; JEOL).
2.7. Electrophoresis of NETs
Electrophoresis of NETs was performed as described previously (Nakazawa et al., 2012). Briefly, 1 × 107 neutrophils were stimulated with 25 nM PMA at 37 °C. At 3 h after stimulation, we centrifuged samples and extracted DNA from Netting neutrophils (NET-DNA) into TE buffer using a DNA Extractor SP Kit (Wako). The extracted NET-DNA was incubated with 200 μg/mL Lf, G-Lf, S-Lf, 10 U/mL heparin (Sigma-Aldrich), 1 mg/mL lipopolysaccharide, and 1 U/mL DNase 1 (Roche Diagnostics, Germany) for 1 h at 37 °C. Samples were separated on 1% agarose gels at 5 × 104 neutrophils/lane and stained with ethidium bromide to illuminate under UV light.
2.8. Charge-Conversion of Lactoferrin
Alkylation of ε-amine of lysine with glutaric anhydride (GA) and succinic anhydride (SA) was performed as follows. Briefly, 0.5 mg of human lactoferrin was dissolved in 1 mL PBS(−) at pH 9.5. The solution was stirred at 0 °C for 30 min, and 2.85 mg GA or 2.5 mg SA was added. After stirring on ice for 2 h, the mixture was purified with PD MidiTrap G-25 (GE Healthcare UK Ltd, UK) according to the gravity protocol (Goda and Miyahara, 2010).
2.9. Preparation of Basic and Acidic Peptides of Lactoferrin
After acidification with hydrochloric acid to pH 2.5, a 5% (w/v) solution of bovine Lf in water was incubated with pepsin (E/S = 1/50) for 5 h at 37 °C. The hydrolysis was stopped by adjusting the pH to 7.0 using sodium hydroxide. The basic peptide of Lf was isolated using an ACTA Explorer 10S chromatography system equipped with a cation-exchange resin Mono S HR 5/5 column (GE Healthcare). The elution gradient was 2 M sodium chloride in 20 mM sodium phosphate buffer (pH 7.0) to remove impurities and undigested Lf, and 2 M ammonium chloride in 20 mM sodium phosphate buffer (pH 7.0) for elution of the basic peptide of Lf. The acidic peptide of Lf was chemically synthesized by the F-moc (fluorenylmethoxycarbonyl) method using a peptide synthesizer Model 433A (Applied Biosystems). The amino acid sequence of the peptides was analyzed using a peptide sequencer PPSQ-21A (Shimadzu).
2.10. Statistics
A survival graph was created using the Kaplan-Meier method. Survival curves were compared by the log-rank test. Data are expressed as means ± s.e.m. In all cases, unpaired t tests were used to compare two groups. Differences with P values of < 0.05 were considered significant. All statistical analyses were performed using JMP 10 software (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Lactoferrin Inhibited NET Formation In Vitro
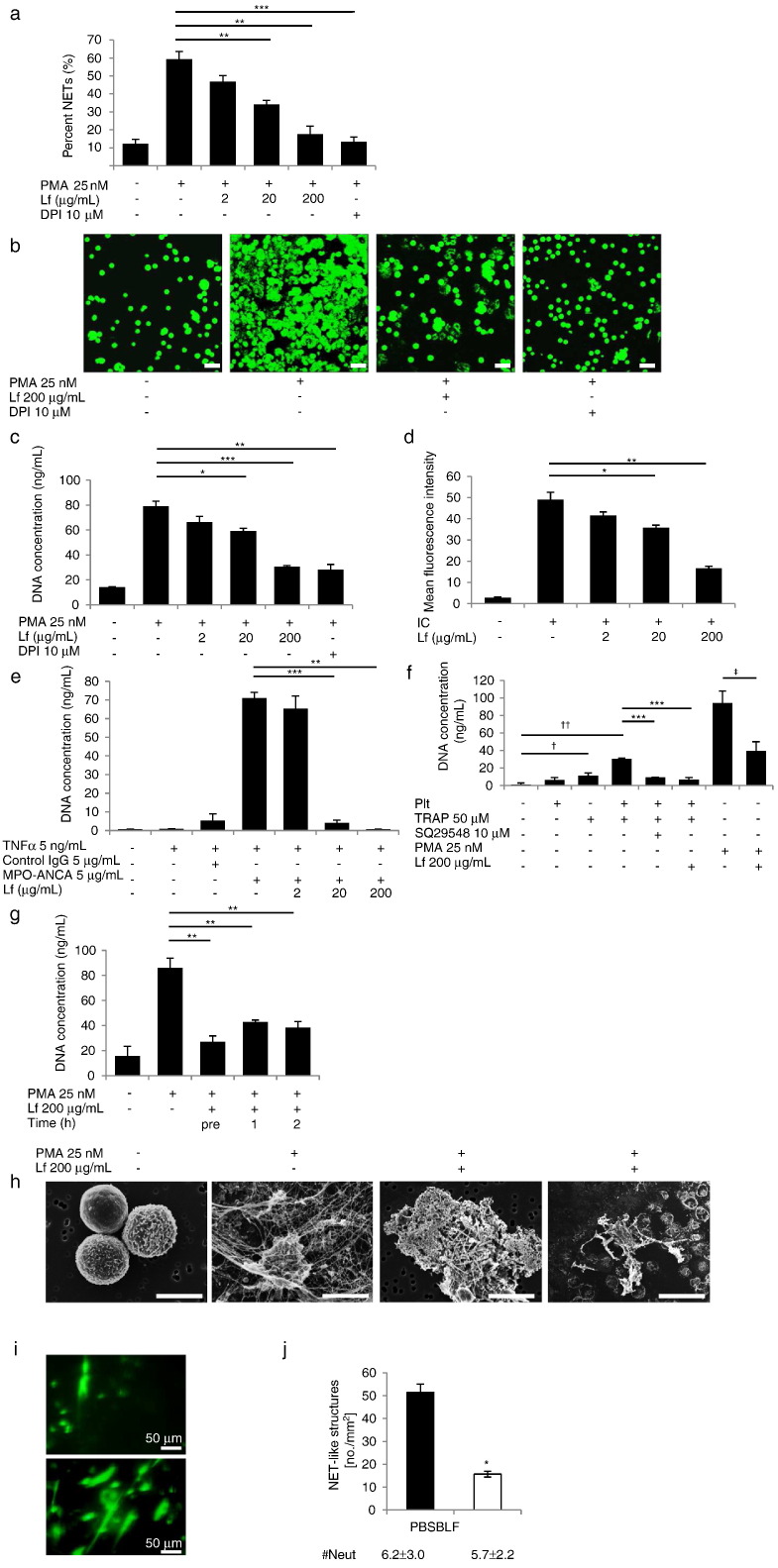
As previously described, human neutrophils were stimulated with PMA and soluble immune complexes (ICs) (Chen et al., 2012), ANCA (Kessenbrock et al., 2009), and activated platelets (Caudrillier et al., 2012) to induce NET formation. DPI, an NADPH oxidase inhibitor that inhibits NET formation by decreasing the generation of reactive oxygen species (ROS), blocked NET formation, consistent with a previous report (Fuchs et al., 2007). Lf inhibited NETs release (Movie S1A, Movie S1B) in a dose-dependent manner as assessed by microscopy (Fig. 1a and b), and blocked DNA release in the culture medium (Fig. 1c) in cells stimulated with ICs (Fig. 1d), ANCA (Fig. 1e, Movie S2A, Movie S2B), and activated platelets (Fig. 1f). In contrast, transferrin showed no effect (Fig. S1). Moreover, DNA release was significantly inhibited by Lf treatment, at even 1 and 2 h after stimulation (Fig. 1g), suggesting that Lf affected the later mechanisms of NET formation. SEM revealed that Lf dramatically changed the morphological characteristics of NETs. While individual NET fibers were observed without Lf, Lf treatment led to the formation of agglutinated fibers (Fig. 1h), suggesting that Lf prevented the spread of NETs and their release into circulation.
Fig. 1.
Lactoferrhgin inhibited NET formation both in vitro and in vivo. (a) Cells were pretreated with different concentrations of human lactoferrin, and NET formation was induced by 25 nM PMA. NET formation was measured by microscopy at 3 h after PMA stimulation. DPI (10 μM) was used as a positive control. Each group: n = 3, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. (b) Images of neutrophils pretreated with 200 μg/mL lactoferrin at 3 h after stimulation. Cells were stained with 500 nM SYTOX Green to detect DNA. Scale bars: 40 μm. NET-DNA release into supernatants induced by PMA (c), soluble IC (d), MPO-ANCA (e), or activated platelets (f) in response to different concentrations of lactoferrin. Each group: n = 3,⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001, †P < 0.05, ††P < 0.001, ‡P < 0.05. (g) NET-DNA release was measured at various times in response to 200 μg/mL lactoferrin. Each group: n = 3, **P < 0.01. (h) Scanning electron microscopy revealed dramatic morphological changes induced by lactoferrin treatment. Individual NET fibers observed without lactoferrin treatment (second left); agglutinated NET fibers in the presence of exogenous lactoferrin (right panels). Scale bars: 5 μm (left panels), 40 μm (right panels). (i, j) NET-like structures were visualized using intravital microscopy. Representative images of animals treated with PBS (lower panel) or bovine lactoferrin (upper panel) are shown (i). The number of NET-like structures per mm2 in the cremaster 3 h after induction of the RPA reaction was quantified (j). ⁎P < 0.05. The number of neutrophils (#Neuts) was not significantly different between groups. Data are representative of the means ± SEMs of three experiments.
3.2. Lactoferrin Inhibited Immune Complex-Induced NET Formation in a Model of RPA Reaction
We examined the effects of Lf on the skin RPA reaction, a model of Type III hypersensitivity elicited by the intravenous injection of BSA and the local injection of anti-BSA. FcγRIIA in human neutrophils induces NET formation independent of elastase, MPO, and oxygen radicals in vivo. Microscopic analysis showed that intravenous injection of Lf led to a reduction in SYTOX Green-positive NET fibers (Fig. 1i), and quantification of NET formation confirmed that the number of neutrophils did not differ significantly between treatment conditions (Fig. 1j).
3.3. Lactoferrin Did Not Decrease ROS Generation or Histone H3 Citrullination During NETosis
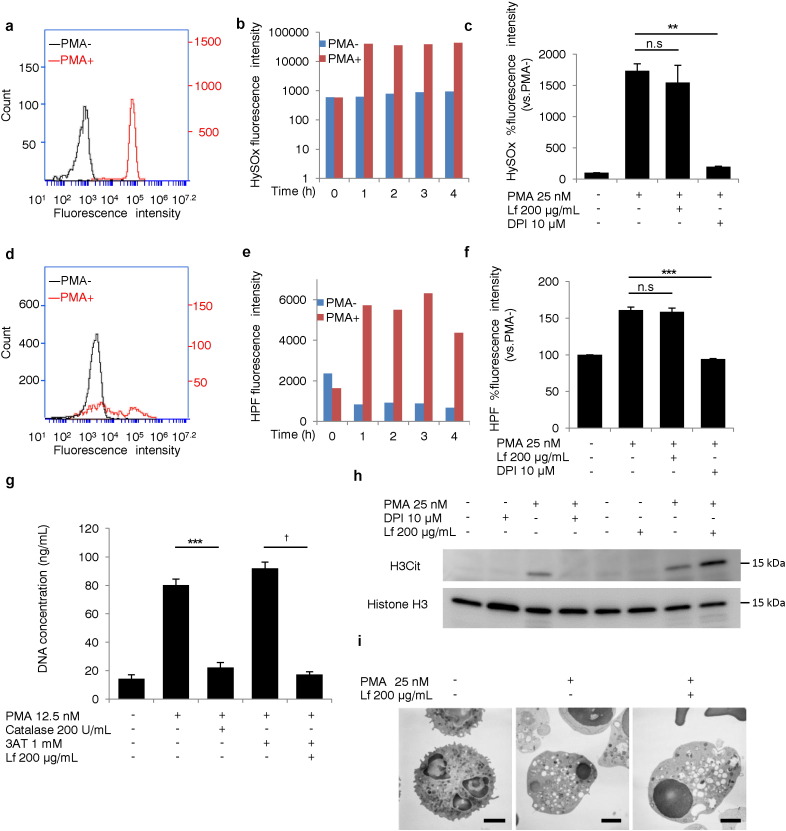
Since ROS generation plays an important role during NETosis (Fuchs et al., 2007), we evaluated whether Lf affected this process. Neutrophils were incubated with HPF (a fluorescence probe for ROS) or HySOx (a fluorescence probe for HOCl) for analysis of ROS generation in living cells (Kenmoku et al., 2007, Setsukinai et al., 2003). Fluorescence intensity of the ROS probes measured by flow cytometry increased in a time-dependent manner during NETosis (Fig. 2a, b, d, and e). Lf had no effect, while DPI significantly decreased the fluorescence intensities of these ROS probes (Fig. 2c and f). Live-cell imaging also demonstrated HOCl production in the presence of Lf (Movie S1A, Movie S1B). Catalase, which catalyzes the breakdown of hydrogen peroxide, is known to inhibit NET formation (Fuchs et al., 2007). To test whether Lf suppressed NET formation by enhancing catalase activity, neutrophils were pretreated with catalase. DNA concentration in the supernatant was significantly reduced by catalase, which was reversed by 3AT, a catalase inhibitor. Lf significantly reduced the DNA concentration, even in the presence of 3AT (Fig. 2g), indicating that Lf inhibited NET formation independently of catalase. Histone H3 citrullination, catalyzed by the enzyme peptidyl arginine deiminase 4 (PAD4), is thought to be essential for NET formation (Li et al., 2010). In our experimental system, histone H3 citrullination was completely inhibited by DPI, but was not inhibited by Lf (Fig. 2h). Autophagy may be important in NET formation, and vacuolization is one of morphological features of autophagy (Remijsen et al., 2011). In our system, massive vacuolization was observed in stimulated neutrophils, regardless of Lf treatment, as assessed by TEM (Fig. 2i), supporting that Lf inhibited NET formation independent of autophagy.
Fig. 2.
Lactoferrin did not decrease ROS generation or histone H3 citrullination during NETosis. (a–g) Effects of lactoferrin on ROS generation, (h) histone H3 citrullination, and (i) vacuolization during NETosis. The fluorescence intensities of HySOx (a–c) and HPF (d–f) added to the culture medium 30 min before PMA stimulation were measured at the indicated times (b, e) or at 1 h (a, c, d, f). Each group: n = 3, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. (g) DNA release into the supernatant was induced with 12.5 nM PMA and was measured in the presence of catalase, lactoferrin, and/or, 3AT. Each group: n = 3, ⁎⁎⁎P < 0.001, †P < 0.001. (h) Western blot analysis was used to investigate PMA-induced histone H3 citrullination in response to DPI and lactoferrin treatment. Figures are representative of two independent experiments. (i) Transmission electron microscopy analysis of vacuolization in PMA-stimulated neutrophils. Figures are representative of two independent experiments. Scale bars: 2 μm. Data represent the mean ± SEM.
3.4. The Sequences of Basic Amino Acids in Lactoferrin and Charge-Charge Interactions With NET-DNA Were Important for Inhibition of NET Formation
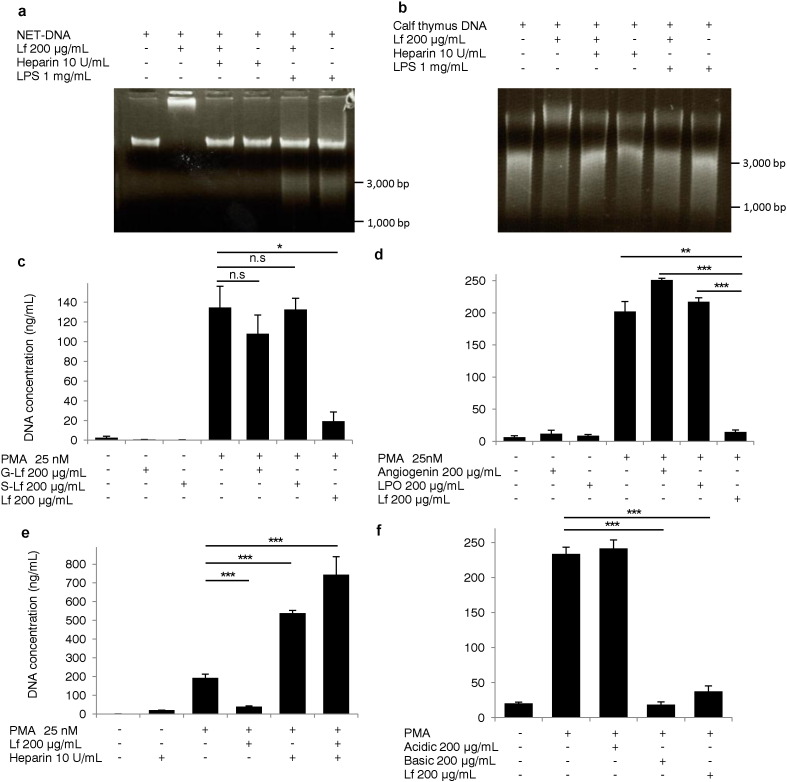
Lf is known to bind DNA via interactions of positively charged residues in the N-terminal of Lf with negatively charged DNA (He and Furmanski, 1995). We tested whether Lf directly bound to NETs via charge-charge interactions. NET-DNA (Fig. 3a) and calf thymus DNA (Fig. 3b) bands shifted upwards following treatment with Lf; this effect was negated by the addition of negatively charged molecules such as heparin or lipopolysaccharide, suggesting that Lf directly bound to NET-DNA via charge-charge interactions. We confirmed that the bands that shifted upwards were degraded by DNase1 treatment (Fig. S2A and B). To investigate whether the positive charge of Lf was a critical factor required for the inhibition of NET formation, we generated charge-converted Lf by employing site-selective chemical modifications of positive amino acid side chains using conventional acylation and glycation reactions with GA and SA (Goda and Miyahara, 2010). We successfully obtained Lf modified by GA (G-Lf) and SA (S-Lf), as assessed by Western blot analysis using anti-Lf antibodies (Fig. S2C). We confirmed that newly obtained Lf failed to bind to NET-DNA (Fig. S2D), indicating that the positive charge of Lf was lost. G-Lf and S-Lf failed to inhibit NET formation (Fig. 3c, Movie S3A, Movie S3B), suggesting that the positive charge of Lf was required to inhibit NET formation. Next, we tested angiogenin (molecular weight [MW]: 14.6 kDa; isoelectric point [pI]: 9.5), a known tumor angiogenic factor (Fett et al., 1985) and lactoperoxidase (LPO; MW: 80.6 kDa; pI: 8.0), a heme peroxidase with MW similar to Lf and abundant in milk (Sharma et al., 2013), to evaluate the effects of positively-charged molecules on NET formation. Both proteins failed to inhibit NET formation (Fig. 3d), suggesting that positive charge alone was not sufficient to suppress this process. Furthermore, we neutralized the positive charge of Lf by adding negatively charged heparin into the culture medium. Interestingly, NETs release was dramatically enhanced and widespread, even in the presence of Lf (Fig. 3e, Movie S4A, Movie S4B), confirming our hypothesis that the positive charge of Lf was vital to the inhibition of NETs release. Next, we prepared basic and acidic peptides derived from bovine Lf and analyzed effects of these peptides on NET formation. Basic peptides of Lf inhibited NET formation, while acidic peptides did not (Fig. 3f), suggesting that basic peptides, positively charged sequences of only 25 amino acids (Fig. S3), were the main molecules contributing to the inhibition of NET formation.
Fig. 3.
Lactoferrin bound NET-DNA and inhibited NET formation via charge-charge interactions. NET-DNA extracted from human neutrophils (a) and calf thymus DNA (b) treated with or without lactoferrin was analyzed by electrophoresis using 1% agarose gels. NET-DNA release into the supernatants induced by PMA in response to 200 μg/mL of lactoferrin, G-Lf, and S-Lf (c), angiogenin and LPO (d), 10 U/mL heparin (e), or acidic or basic peptides of lactoferrin (f). Each group: n = 3, ⁎P < 0.05, ⁎⁎P < 0.01, ⁎⁎⁎P < 0.001. Data are representative of three independent experiments.
3.5. Silencing Lactoferrin Enhanced NET Formation in HL-60 Cells
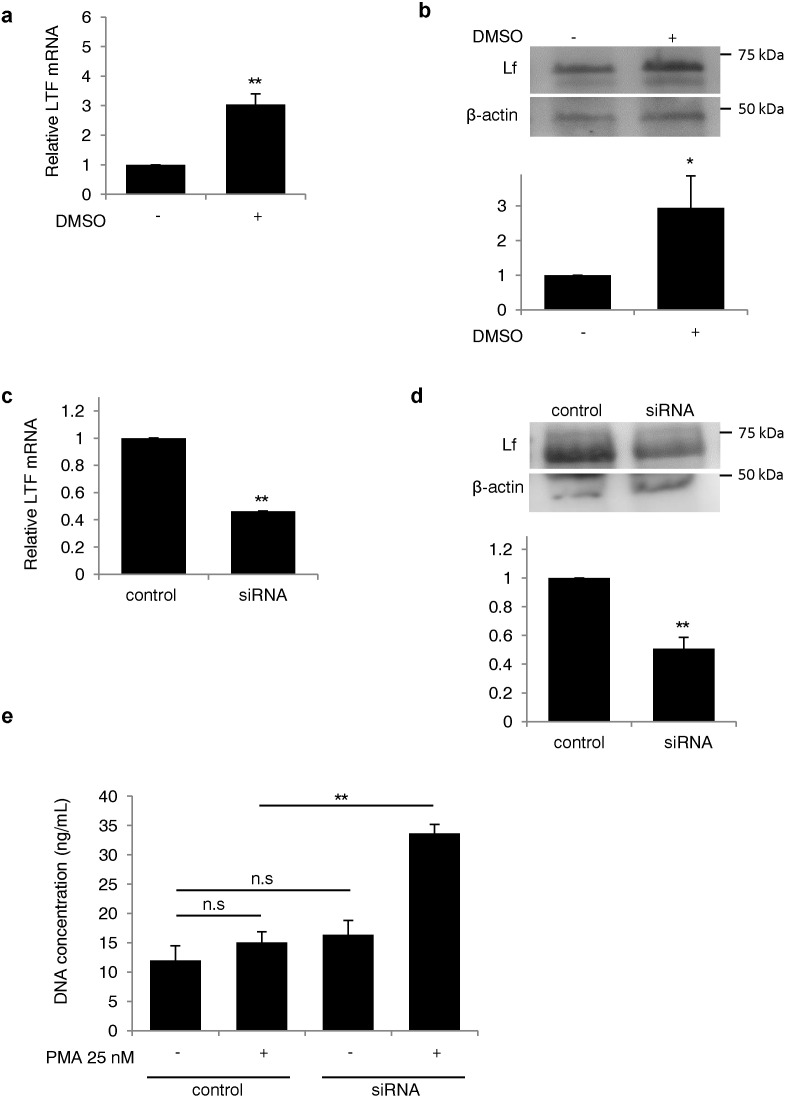
To study the role of endogenous Lf on NET formation, we knocked down Lf mRNA in the neutrophil-like human myeloid leukemia cell line, HL-60 using small-interference RNA (siRNA) and induced NET formation (Li et al., 2010). The expression of Lf mRNA and protein increased after differentiation of the cells (Fig. 4a and b) and decreased in Lf siRNA-transfected cells by 54% and 49%, respectively (Fig. 4c and d). When HL-60 granulocytes were stimulated with PMA, the concentration of extracellular DNA in the supernatant was 2.2-fold higher in cells transfected with Lf siRNA than in cells transfected with control siRNA (Fig. 4e). These data indicate that Lf serves as an endogenous inhibitor of NETs.
Fig. 4.
Silencing of lactoferrin expression enhanced NET formation in HL-60 cells. (a, b) Confirmation of lactoferrin knockdown by siRNA after differentiation of HL-60 cells. (a) Lactoferrin mRNA was measured by real-time PCR after differentiation with 1.25% DMSO. Each group: n = 6, ⁎⁎P < 0.01. (b) Lactoferrin protein levels were measured by Western blot analysis (membrane image: upper panel; quantification of lactoferrin protein normalized to β-actin expression: lower panel. Each group n = 3, ⁎P < 0.05). (c, d) Silencing of lactoferrin by siRNA transfection. (c) Lactoferrin mRNA and (d) protein expression were measured in cells transfected with lactoferrin siRNA (membrane image: upper panel; quantification of lactoferrin protein normalized to β-actin expression: lower panel). Each group: n = 5 (c), n = 3 (d), ⁎⁎P < 0.01. (e) The concentration of extracellular DNA in the supernatant was measured following transfection with lactoferrin siRNA. Each group: n = 3, ⁎⁎P < 0.01. Data are representative of the means ± SEMs of three experiments.
3.6. Lactoferrin Localized to the Plasma Cell Membrane During NETosis
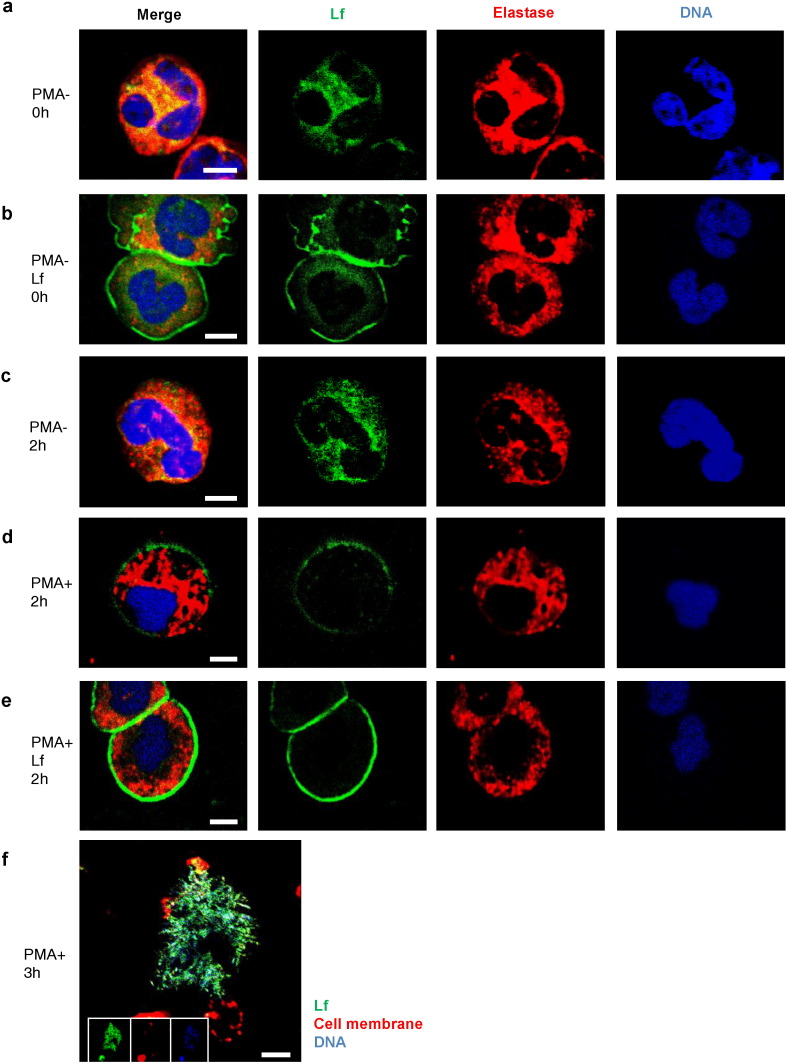
We examined the distribution of endogenous Lf, elastase, and DNA during NETosis using immunofluorescence microscopy (Fig. 5a, c, and d). Both, Lf and elastase were present in the cytoplasm of unstimulated neutrophils (Fig. 5a and c). After a 2-h PMA stimulation, Lf localized to the cell membrane (Fig. 5d). Moreover, exogenous human Lf added to the culture medium also localized to the cell membrane of unstimulated and PMA-stimulated cells (Fig. 5b and e), and protected against spreading of NETs (Movie S5A, Movie S5B, Fig. S6). Web-like DNA structures were observed in most cells after 3 h and were positive for Lf and DNA (Fig. 5f). Thus, the localization of Lf on the cell membrane during NETosis suggested that Lf interacts with NETs just before rupture of the cell membrane.
Fig. 5.
Lactoferrfebcain localized to the plasma cell membrane during NETosis. (a, c, d) Representative images acquired using immunofluorescence staining of endogenous lactoferrin (green), elastase (red), and DNA (blue) are shown. Both lactoferrin and elastase existed in the cytoplasm of unstimulated neutrophils at 0 h (a) and 2 h (c). (d) Following 2 h of PMA stimulation, lactoferrin localized to the plasma cell membrane. Exogenous lactoferrin added to the culture medium localized to the plasma cell membrane at 0 h (b) and 2 h (e). Scale bars: 5 μm. (f) Web-like DNA structures were positive for lactoferrin (green) and DNA (blue) at 3 h. Scale bar: 10 μm. Data are representative of two independent experiments.
3.7. Lactoferrin Did Not Suppress the Translocation of Elastase to the Nucleus or Elastase-Mediated Degradation of Histones
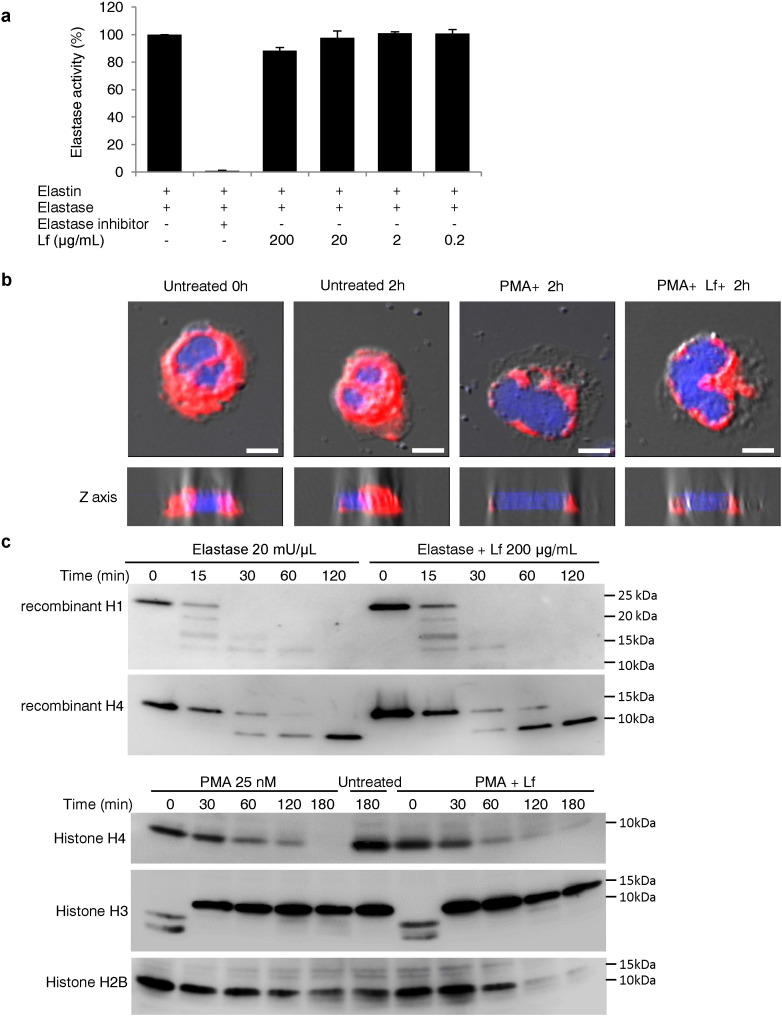
During NETosis, neutrophil elastase escapes from granules and translocates to the nucleus, where it degrades histones and promotes chromatin decondensation (Papayannopoulos et al., 2010). We evaluated whether this process was altered in the presence of Lf. We found that Lf did not directly decrease elastase activity, as measured by degradation of elastin (Fig. 6a). Translocation of elastase to the nucleus in stimulated neutrophils was also observed in the presence of Lf (Fig. 6b). Moreover, Western blot analysis revealed that Lf did not inhibit the elastase-mediated degradation of recombinant histones H1 and H4 (Fig. 6c). Furthermore, histones H2B, H3, and H4 in the neutrophils were degraded upon PMA stimulation, and these degradation events were not inhibited by Lf treatment (Fig. 6c).
Fig. 6.
Lactoferrin did not suppress the translocation of elastase into the nucleus or elastase-mediated degradation of histones. (a) The fluorescence intensity released from labeled-elastin degraded by elastase was evaluated as a measure of elastase activity. The figure is representative of three independent experiments. (b) Neutrophils pretreated with or without lactoferrin were observed by confocal microscopy stained for elastase (red) or DNA (blue), or observed as differential interference contrast (DIC) images with Z-axis imaging (lower panel) after 2 h of stimulation with PMA. Scale bars: 5 μm. Figures are representative of two independent experiments. (c) Recombinant human histone H1 and H4 pretreated with or without lactoferrin were treated with human elastase. Western blotting analysis was performed as described in the Methods (upper panels). Neutrophils were stimulated with PMA in the presence or absence of lactoferrin using anti-histone H2B, H3, and H4 antibodies (lower panels). Figures are representative of two independent experiments.
4. Discussion
In this study, we found that Lf inhibited NET formation without affecting ROS generation, PAD4-dependent histone H3 citrullination, or elastase-mediated histone degradation, which are accomplished within a few minutes, 1 h, and 2 h, respectively (Parker et al., 2012), but instead affected the later stages of NETosis.
We have previously screened the naturally derived substances that improved survival rates in Spontaneously Crescentic Glomerulonephritis/Kinjoh (SCG/kj) mice (Kinjoh et al., 1993), which are recombinant congenic mice that develop rapidly progressive renal failure with hematuria, MPO-ANCA production that resembles rapidly progressive glomerulonephritis (RPGN) in humans, and ANCA-associated vasculitis (AAV). We previously reported that one of these substances, eicosapentaenoic acid, has potential as a therapeutic agent for autoimmune small-vessel vasculitis (Hirahashi et al., 2014). In this study, we describe that Lf as another candidate. Going further, we have begun studies to investigate the mechanisms underlying the beneficial effect of Lf in AAV and hypothesize that Lf affects NET formation, which is closely associated with pathology of AAV.
Current knowledge indicates that Lf binds to DNA and contains positively charged residues at the N-terminal; this peptide is known as lactoferricin B (Britigan et al., 2001). In this study, using chemical modification methods, we showed that Lf binds to NETs via charge-charge interactions, which likely occur just before NETs release. Additionally, from our data, we propose that positively charged sequences of 25 amino acids in Lf are the key molecules inhibiting NET formation. In support of this, previous studies have demonstrated that NETs can be cleared via binding to nanomaterials (Bartneck et al., 2009), with the positive charge significantly enhancing particle trapping.
Activated platelets have been shown to induce NET formation (Clark et al., 2007) and are closely associated with the pathogenesis of deep vein thrombosis (von Brühl et al., 2012). We found that Lf inhibited activated platelet-induced NET formation in vitro, suggesting that Lf may have applications in the management of thrombosis. A recent study has defined the process of immunothrombosis as an innate immunity mechanism, to which NETs contribute (Engelmann and Massberg, 2012). The major components of NETs, i.e., DNA and histones, activate factor XII of the intrinsic coagulation pathway directly via the negatively charged surfaces of the nucleic acids and induce platelet aggregation (Chen et al., 2014, Andrews et al., 2014, Fuchs et al., 2011, Fuchs et al., 2010). Dysregulation of immunothrombosis leads to pathological vascular obstructions in inflammatory diseases. In this context, Lf may be able to properly regulate immunothrombosis by suppressing overproduction of NETs.
Several previous reports have demonstrated that heparin interferes with the formation of NET by disrupting the histone backbone of NET structure (Fuchs et al., 2010, Fuchs et al., 2011). We observed that the spread of NET structure is exaggerated in the presence of heparin, which cancels the inhibitory effect of Lf by charge-charge interaction. On the other hand, Schauer et al. demonstrated that aggregated NETs (aggNETs) suppress inflammation by degradation of inflammatory mediators in a gout model of synovium inflammation (Schauer et al., 2014). Taken together, oppositely charged heparin and Lf act differently on the NET structure, but both substances could play beneficial roles by modification of the harmful structure of NETs.
Although our data suggest that Lf is an endogenous inhibitor of NETs, neutrophils that store Lf still produce NETs. Importantly, Lf is found on NET-fibers (Urban et al., 2009, and our immunofluorescence image). Thus, excess production of NETs (providing much more Lf release) possibly downregulates further NETosis, as observed in patients of familial Mediterranean fever (Apostolidou et al., 2016).
We propose the mechanism of action of Lf during NET formation (Fig. 7). We hypothesize that endogenous Lf may inhibit NET formation but is insufficient to block excess NETs in pathological conditions. In support of this, the activity of rheumatoid arthritis correlates with anti-Lf antibody titers (Kida et al., 2011), and high levels of anti-Lf antibodies in SLE patients are associated with clinical manifestations of SLE (Caccavo et al., 2005). These reports suggest that impairment of Lf activity by anti-Lf antibodies may be the cause of inflammatory pathogenesis. In this regard, supplying exogenous Lf may have significant implications in controlling disease activities.
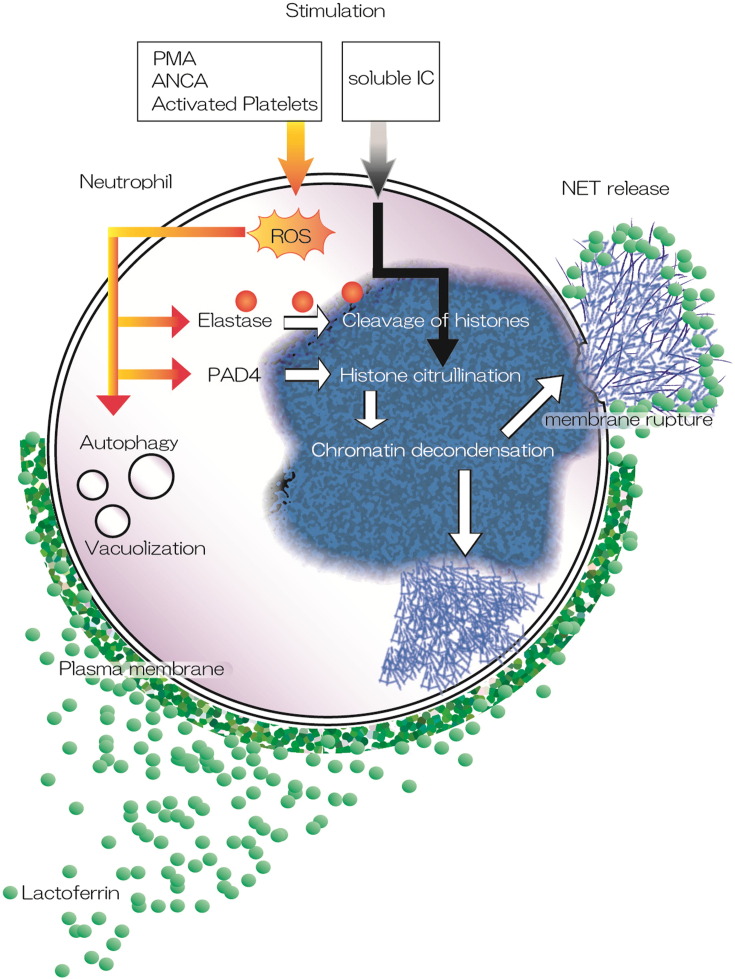
Fig. 7.
Proposed mechanism of action of lactoferrin during NET formation. We found that lactoferrin inhibited NET formation induced by various stimulants, such as PMA, MPO-ANCA, and activated platelets. Lactoferrin had no effect on intracellular pathways, such as elastase-mediated degradation of histones or histone citrullination catalyzed by PAD4 following ROS generation. Furthermore, lactoferrin also inhibited soluble IC-induced NET formation, which was independent of ROS generation. Our proposed mechanism showed that lactoferrin prevented the spread of NETs and their release by blocking membrane rupture and causing agglutination of the released chromatin fibers. This figure was produced using Illustrator CC (Adobe Systems Incorporated, USA).
NET formation can be inhibited by blocking the Raf-MEK-ERK pathway (upstream of NADPH oxidase), making this pathway a potential target of NET inhibitors (Hakkim et al., 2011). In TRALI, platelets are associated with NET formation and aspirin suppresses disease progression by decreasing platelet activation (Caudrillier et al., 2012), thereby inhibiting the production of NETs. PAD4 inhibition protects against murine lupus by inhibiting NET formation (Knight et al., 2013). However, endogenous substances that are safe and efficacious NET inhibitors have not yet been reported; our results suggest that Lf can be considered as a candidate endogenous antagonist of NETs. However, since formation of NETs is one of the important mechanisms of innate immunity in neutrophils, inhibiting NET formation may cause susceptibility to infection. However, previous reports have shown that Lf itself exhibits microbicidal activity by virtue of its iron chelating ability (Weinberg, 1984, Bellamy et al., 1992). Additionally, Lf has been shown to improve survival rates, even in septic patients (Manzoni et al., 2009).
Some limitations and caveats apply to the findings of this study. These include the lack of stability when Lf is administered in clinical settings. A lactoferrin-derived peptide with smaller molecules that is as effective as whole lactoferrin needs to be developed for clinical application.
In conclusion, we found that Lf inhibits the formation of NETs, potentially by preventing the spread of NETs via charge-charge interactions; these results have implications in the development of new and safe clinical options to control diseases associated with NETs. Further studies are required to elucidate the mechanisms of Lf-dependent regulation of NET formation.
5. Study Approval
All animal experiments were carried out following ARRIVE standard in accordance with procedures approved by the Animal Experimental Committee of the Graduate School of Medicine, The University of Tokyo, Japan.
The following are the supplementary data related to this article.
Supplementary figures.
Conflict of Interest Disclosure
We have applied for a new patent entitled “An inhibitor of leukocyte extracellular traps”.
Author Contributions
K.O. performed all experiments, analyzed the data and wrote the manuscript. M.K. and Y.U. contributed to imaging of NETs and preparation of new reagents. H.N., J.M.H., and T.M. contributed to analysis of NETs in vivo and analyzed the data. S.K. and H.K. contributed to the preparation of reagents. D.H., M.T., and M.K. contributed to the animal care and experiments. K.S., K.H., T. M., M.H., M.N., and T.F. contributed to the design of the study and critical reading of the manuscript. J.H. designed the research, conducted the experiments, wrote the manuscript and supervised the study as a corresponding author.
Acknowledgements
This study was supported by a Grant-in-aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (24591461 to J. Hirahashi), a Grant-in-Aid from the Ministry of Health, Labour and Welfare, Japan (Research on rare and intractable diseases; to J. Hirahashi), a Postdoctoral Fellowship for Research Abroad from Japan Society for the Promotion of Science (to H. Nishi), and grants from the German Research Foundation (HE-6810/1-1 to J. Herter) and from the Else Kröner-Fresenius-Stiftung (2014_A80 to J. Herter).
References
- Andrews R.K., Arthur J.F., Gardiner E. Neutrophil extracellular traps (NETs) and the role of platelets in infection. Thromb. Haemost. 2014;112:659–665. doi: 10.1160/TH-14-05-0455. [DOI] [PubMed] [Google Scholar]
- Apostolidou E., Skendros P., Kambas K., Mitroulis I., Konstantinidis T., Chrysanthopoulou A., Nakos K., Tsironidou V., Koffa M., Boumpas D.T., Ritis K. Ann. Rheum. Dis. 2016;75(1):269–277. doi: 10.1136/annrheumdis-2014-205958. (2016 Jan) [DOI] [PubMed] [Google Scholar]
- Bartneck M., Keul H.A., Zwadlo-Klarwasser G., Groll J. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Lett. 2009;10:59–63. doi: 10.1021/nl902830x. [DOI] [PubMed] [Google Scholar]
- Bellamy W., Takase M., Wakabayashi H., Kawase K., Tomita M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 1992;73:472–479. doi: 10.1111/j.1365-2672.1992.tb05007.x. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- Brinkmann V., Laube B., Abu Abed U., Goosmann C., Zychlinsky A. Neutrophil extracellular traps: how to generate and visualize them. J. Vis. Exp. 2010;36 doi: 10.3791/1724. (Feb 24) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britigan B.E., Lewis T.S., Waldschmidt M., McCormick M.L., Krieg A.M. Lactoferrin binds CpG-containing oligonucleotides and inhibits their immunostimulatory effects on human B cells. J. Immunol. 2001;167:2921–2928. doi: 10.4049/jimmunol.167.5.2921. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E., Smithyman A., Eger R.R., Meyers P.A., De Sousa M. Identification of lactoferrin as the granulocyte-derived inhibitor of colony-stimulating activity production. J. Exp. Med. 1978;148:1052–1067. doi: 10.1084/jem.148.4.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccavo D., Rigon A., Picardi A., Picardi A., Galluzzo S., Vadacca M., Ferri G.M., Amoroso A., Afeltra A. Anti-lactoferrin antibodies in systemic lupus erythematosus: isotypes and clinical correlates. Clin. Rheumatol. 2005;24:381–387. doi: 10.1007/s10067-004-1040-2. [DOI] [PubMed] [Google Scholar]
- Caudrillier A., Kessenbrock K., Gilliss B.M., Nguyen J.X., Marques M.B., Monestier M., Toy P., Werb Z., Looney M.R. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K., Nishi H., Travers R., Tsuboi N., Martinod K., Wagner D.D., Stan R., Croce K., Mayadas T.N. Endocytosis of soluble immune complexes leads to their clearance by FcγRIIIB but induces neutrophil extracellular traps via FcγRIIA in vivo. Blood. 2012;120:4421–4431. doi: 10.1182/blood-2011-12-401133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Kang R., Fan X.G., Tang D. Release and activity of histone in diseases. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S.R., Ma A.C., Tavener S.A., McDonald B., Goodarzi Z., Kelly M.M., Patel K.D., Chakrabarti S., McAvoy E., Sinclair G.D. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- Döring Y., Soehnlein O., Weber C. Neutrophils cast NETs in atherosclerosis: employing peptidylarginine deiminase as a therapeutic target. Circ. Res. 2014;14(114):931–934. doi: 10.1161/CIRCRESAHA.114.303479. [DOI] [PubMed] [Google Scholar]
- Engelmann B., Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2012;13:34–45. doi: 10.1038/nri3345. [DOI] [PubMed] [Google Scholar]
- Fett J.W., Strydom D.J., Lobb R.R., Alderman E.M., Bethune J.L., Riordan J.F., Vallee B.L. Amino acid sequence of human tumor derived angiogenin. Biochemistry. 1985;24:5486–5494. doi: 10.1021/bi00341a031. [DOI] [PubMed] [Google Scholar]
- Fuchs T.A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D., Jr., Wrobleski S.K., Wakefield T.W., Hartwig J.H., Wagner D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs T.A., Bhandari A.A., Wagner D.D. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118:3708–3714. doi: 10.1182/blood-2011-01-332676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda T., Miyahara Y. Molecularly engineered charge-conversion of proteins for sensitive biosensing. Anal. Chem. 2010;82:8946–8953. doi: 10.1021/ac1018233. [DOI] [PubMed] [Google Scholar]
- Guimarães-Costa A.B., Nascimento M.T.C., Froment G.S., Soares R.P., Morgado F.N., Conceição-Silva F., Saraiva E.M. Leishmania amazonensis promastigotes induce and are killed by neutrophil extracellular traps. Proc. Natl. Acad. Sci. U. S. A. 2009;106:6748–6753. doi: 10.1073/pnas.0900226106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkim A., Fürnrohr B.G., Amann K., Laube B., Abed U.A., Brinkmann V., Herrmann M., Voll R.E., Zychlinsky A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:9813–9818. doi: 10.1073/pnas.0909927107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakkim A., Fuchs T.A., Martinez N.E., Hess S., Prinz H., Zychlinsky A., Waldmann H. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat. Chem. Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- He J., Furmanski P. Sequence specificity and transcriptional activation in the binding of lactoferrin to DNA. Nature. 1995;373:721–724. doi: 10.1038/373721a0. [DOI] [PubMed] [Google Scholar]
- Hirahashi J., Mekala D., Van Ziffle J., Xiao L., Saffaripour S., Wagner D.D., Shapiro S.D., Lowell C., Mayadas T.N. Mac-1 signaling via Src-family and Syk kinases results in elastase-dependent thrombohemorrhagic vasculopathy. Immunity. 2006;25:271–283. doi: 10.1016/j.immuni.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Hirahashi J., Hishikawa K., Kaname S., Tsuboi N., Wang Y., Simon D.I., Stavrakis G., Shimosawa T., Xiao L., Nagahama Y., Suzuki K., Fujita T., Mayadas T.N. Mac-1 (CD11b/CD18) links inflammation and thrombosis after glomerular injury. Circulation. 2009;120:1255–1265. doi: 10.1161/CIRCULATIONAHA.109.873695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirahashi J., Kawahata K., Arita M., Iwamoto R., Hishikawa K., Honda M., Hamasaki Y., Tanaka M., Okubo K., Kurosawa M., Takase O., Nakakuki M., Saiga K., Suzuki K., Kawachi S., Tojo A., Seki G., Marumo T., Hayashi M., Fujita T. Immunomodulation with eicosapentaenoic acid supports the treatment of autoimmune small-vessel vasculitis. Sci. Rep. 2014;4:6406. doi: 10.1038/srep06406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenmoku S., Urano Y., Kojima H., Nagano T. Development of a highly specific rhodamine-based fluorescence probe for hypochlorous acid and its application to real-time imaging of phagocytosis. J. Am. Chem. Soc. 2007;129:7313–7318. doi: 10.1021/ja068740g. [DOI] [PubMed] [Google Scholar]
- Kessenbrock K., Krumbholz M., Schönermarck U., Back W., Gross W.L., Werb Z., Gröne H.J., Brinkmann V., Jenne D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat. Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida I., Kobayashi S., Takeuchi K., Tsuda H., Hashimoto H., Takasaki Y. Antineutrophil cytoplasmic antibodies against myeloperoxidase, proteinase 3, elastase, cathepsin G and lactoferrin in Japanese patients with rheumatoid arthritis. Mod. Rheumatol. 2011;21:43–50. doi: 10.1007/s10165-010-0356-9. [DOI] [PubMed] [Google Scholar]
- Kinjoh K., Kyogoku M., Good R.A. Genetic selection for crescent formation yields mouse strain with rapidly progressive glomerulonephritis and small vessel vasculitis. Proc. Natl. Acad. Sci. U. S. A. 1993;90:3413–3417. doi: 10.1073/pnas.90.8.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J.S., Zhao W., Luo W., Subramanian V., O'Dell A.A., Yalavarthi S., Hodgin J.B., Eitzman D.T., Thompson P.R., Kaplan M.J. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J. Clin. Invest. 2013;123:2981–2993. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J.S., Luo W., O'Dell A.A., Yalavarthi S., Zhao W., Subramanian V., Guo C., Grenn R.C., Thompson P.R., Eitzman D.T., Kaplan M.J. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ. Res. 2014;114:947–956. doi: 10.1161/CIRCRESAHA.114.303312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R., Ganguly D., Facchinetti V., Frasca L., Conrad C., Gregorio J., Meller S., Chamilos G., Sebasigari R., Riccieri V., Bassett R., Amuro H., Fukuhara S., Ito T., Liu Y.J., Gilliet M. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA–peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001180. (73ra19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Li M., Lindberg M.R., Kennett M.J., Xiong N., Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 2010;207:1853–1862. doi: 10.1084/jem.20100239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni P., Rinaldi M., Cattani S. Bovine lactoferrin supplementation for prevention of late-onset sepsis in very low-birth-weight neonates: a randomized trial. JAMA. 2009;302:1421–1428. doi: 10.1001/jama.2009.1403. [DOI] [PubMed] [Google Scholar]
- Müller I., Klocke A., Alex M., Kotzsch M., Luther T., Morgenstern E., Zieseniss S., Zahler S., Preissner K., Engelmann B. Intravascular tissue factor initiates coagulation via circulating microvesicles and platelets. FASEB J. 2003;17:476–478. doi: 10.1096/fj.02-0574fje. [DOI] [PubMed] [Google Scholar]
- Nakazawa D., Tomaru U., Suzuki A., Masuda S., Hasegawa R., Kobayashi T., Nishio S., Kasahara M., Ishizu A. Abnormal conformation and impaired degradation of NETs induced by propylthiouracil: implication of disordered NETs in MPO-ANCA-associated vasculitis. Arthritis Rheum. 2012;64:3779–3787. doi: 10.1002/art.34619. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Neeli I., Dwivedi N., Khan S., Radic M. Regulation of extracellular chromatin release from neutrophils. J. Innate Immun. 2009;1:194–201. doi: 10.1159/000206974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papayannopoulos V., Metzler K.D., Hakkim A., Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 2010;191:677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker H., Dragunow M., Hampton M.B., Kettle A.J., Winterbourn C.C. Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J. Leukoc. Biol. 2012;92:841–849. doi: 10.1189/jlb.1211601. [DOI] [PubMed] [Google Scholar]
- Pütsep K., Carlsson G., Boman H.G., Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360:1144–1149. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- Ramos-Kichik V., Mondragón-Flores R., Mondragón-Castelán M., Gonzalez-Pozos S., Muñiz-Hernandez S., Rojas-Espinosa O., Chacón-Salinas R., Estrada-Parra S., Estrada-García I. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis. 2009;89:29–37. doi: 10.1016/j.tube.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Remijsen Q., Kuijpers T., Wirawan E., Lippens S., Vandenabeele P., Berghe T.V. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setsukinai K.-i., Urano Y., Kakinuma K., Majima H.J., Nagano T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003;278:3170–3175. doi: 10.1074/jbc.M209264200. [DOI] [PubMed] [Google Scholar]
- Sharma S., Singh A.K., Kaushik S., Sinha M., Singh R.P., Sharma P., Sirohi H., Kaur P., Singh T.P. Lactoperoxidase: structural insights into the function, ligand binding and inhibition. Int. J. Biochem. Mol. Biol. 2013;4:108–128. [PMC free article] [PubMed] [Google Scholar]
- Stokol T., O'Donnell P., Xiao L., Knight S., Stavrakis G., Botto M., von Andrian U.H., Mayadas T.N. C1q governs deposition of circulating immune complexes and leukocyte Fcγ receptors mediate subsequent neutrophil recruitment. J. Exp. Med. 2004;200:835–846. doi: 10.1084/jem.20040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban C.F., Ermert D., Schmid M., Abu-Abed U., Goosmann C., Nacken W., Brinkmann V., Jungblut P.R., Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Path. 2009;5 doi: 10.1371/journal.ppat.1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Brühl M.L., Stark K., Steinhart A. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E.D. Iron withholding: a defense against infection and neoplasia. Physiol. Rev. 1984;64:65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures.