This review focuses on defining the processes that regulate root system growth and the mechanisms acting during water-deficit stresses that affect growth at the cellular- and organ-wide levels.
Abstract
Water is the most limiting resource on land for plant growth, and its uptake by plants is affected by many abiotic stresses, such as salinity, cold, heat, and drought. While much research has focused on exploring the molecular mechanisms underlying the cellular signaling events governing water-stress responses, it is also important to consider the role organismal structure plays as a context for such responses. The regulation of growth in plants occurs at two spatial scales: the cell and the organ. In this review, we focus on how the regulation of growth at these different spatial scales enables plants to acclimate to water-deficit stress. The cell wall is discussed with respect to how the physical properties of this structure affect water loss and how regulatory mechanisms that affect wall extensibility maintain growth under water deficit. At a higher spatial scale, the architecture of the root system represents a highly dynamic physical network that facilitates access of the plant to a heterogeneous distribution of water in soil. We discuss the role differential growth plays in shaping the structure of this system and the physiological implications of such changes.
INTRODUCTION
An architect’s plans are drafted years in advance of construction and are discussed until every detail is decided, from the number of floors present to the color of each wall and the fixtures on every cabinet. Imagine instead if the plans for these buildings changed during the construction process. What if a new funding initiative doubled the number of floors in a research building mid-way through construction? Or perhaps the plumbing adjusted the water efficiency of the bathrooms, depending on the state of the drought? Far-fetched, perhaps, but plants do the equivalent, in biological terms, as they revise their architectural plans throughout their lives.
In plant physiology, development plays the unique role of allowing the plant to both respond to current environmental pressures and to change the structural context through which future stimuli are experienced (Dinneny, 2015). Thus, plant structures, established through the highly regulated process of growth, provide the context and the medium through which plants acclimate to environmental change. To understand the basis for the resilience and plasticity of plants to environmental pressures, a fundamental understanding of the cellular and developmental mechanisms that determine the architecture of plants is needed, along with an understanding of the functional consequences that such structures have on physiology.
Few challenges to feeding the expanding human population are as great as those associated with water availability (www.fao.org/home/en/; www.reports.weforum.org/global-risks-2015). Limited access to fresh water imposes a major restriction on the expanse of land that can be cultivated for agriculture, and major environmental damage can ensue when civil engineering is used to bring water long distances to agricultural centers (Borsa et al., 2014). Water has many roles in the plant, but most important for development is the role water plays in enabling growth (Kramer and Boyer, 1995). Through a conceptually simple process of cell wall loosening and water uptake, plant cells elongate and the pressure that builds up provides mechanical support for tissues to resist the pull of gravity or, in roots, to penetrate through hardened soil (Cosgrove, 2016a, 2016b; Cosgrove and Green, 1981). The ability of cells to take up water for growth is dependent on the availability of water in the external environment (see the “Plant-Water Relations at the Cell Scale” section for a more precise description). Under environmental conditions that cause water-deficit stress, such as drought, the total amount of water in soil becomes limiting, while under high salinity, water may be quite abundant, but the ability of cells to extract this water becomes limited due to the amount of dissolved solutes (Verslues et al., 2006). Thus, water-deficit stresses negatively affect a process that is fundamental to growth and the associated patterning mechanisms plants use to construct and support their body.
A deeper understanding of the interaction between the root with the environment requires an appreciation that such processes are highly dependent on the spatial scale considered (Passioura, 1979; Dinneny, 2015; Rellán-Álvarez et al., 2016; Robbins and Dinneny, 2015). In this review, we will focus on defining the processes that regulate growth at the two scales where this process is fundamentally controlled: the cellular and organ scales. Through this analysis, we aim to define the scale-dependent processes that are unique and how information at these two scales is ultimately integrated at the root system level.
We have specifically chosen not to cover processes that operate at the whole-plant level, as this would require coverage of a vast literature including regulation of transpiration, vascular conductivity, and movement of water across complex and poorly understood cellular paths (Christmann et al., 2013; Kramer and Boyer, 1995; Steudle and Peterson, 1998).
GROWTH CONTROL AT THE CELL SCALE
When considering how environmental cues affect the growth of the plant, a fair starting point is the cell. While the contribution of cell-scale processes to morphogenesis and organ-scale growth events is not without controversy (Kaplan, 1992; Smith et al., 1996), the flux of water into the plant is ultimately determined by cell-scale physiological parameters (Kramer and Boyer, 1995). In particular, the cell wall plays important roles in determining the mechanical properties of the cell and the resistance to water uptake and expansion (Robbins and Dinneny, 2015). Interactions between the wall and the plasma membrane, and changes in membrane tension caused by the flux of water into or out of the cell, appear to be critical in the ability of plants to sense water availability (Monshausen and Gilroy, 2009).
Plant-Water Relations at the Cell Scale
As a small, uncharged molecule, water can move into and out of a cell across the plasma membrane, but this process is facilitated by water channels, such as aquaporins (Kramer and Boyer, 1995; Maurel et al., 2008). Although aquaporins provide a mechanism to regulate water flow, its directionality is largely determined by the difference in the potential free energy of water (water potential) across the membrane. Water potential is influenced by three separable components: osmotic potential, pressure potential, and matric potential (Kramer and Boyer, 1995; Verslues et al., 2006). The concentration of solutes in a cell determines its osmotic potential, and the differential between the cytoplasm and the extracellular environment is one of the key determinants for the direction of water movement. Another important structural component of plant cells that affects the rate of water uptake is the cell wall. The degree to which the cell wall can be deformed provides mechanical resistance to water uptake as pressure within the cell (turgor pressure) rises. This potential energy, due to pressure (pressure potential), is typically positive and limits water uptake in a cell. Matric potential involves the effects of capillary forces that affect the ability of water to move freely through a physical matrix, such as soil. Stronger capillary forces cause the water to adhere more tightly to the surrounding matrix, thereby decreasing its free energy and lowering its water potential. Independent of the differences in water potential between the inside and outside of a cell, the hydraulic conductivity of the path of water movement is also critical to determine the rate of water movement in the system. A cell contacting air, even if fully humidified, will be able to take up water at only a very slow rate due to the low conductance of air for water (Robbins and Dinneny, 2015). Conversely, cells directly contacting liquid water will take up water at a much higher rate. Here, aquaporins facilitate water uptake by increasing hydraulic conductivity.
Integral for determining the shape and physical properties of a cell, the wall is a much more dynamic component of the cell than its name implies (Peaucelle et al., 2012). Different structural components of the wall interact with water and solutes in complex yet poorly understood ways that affect growth of cells and roots. In a growing cell, wall loosening allows for continued water uptake and the acquisition of a larger final volume. Thus, the growth of an organ is ultimately dependent on cell-level changes in wall extensibility, the difference in solute potential between the inside and outside of the cell, and the conductance for the path of water from the environment.
Cell Wall Properties Affect the Wall–Plasma Membrane Interface
At equilibrium, the external water potential is equal to the internal water potential of the cell. When external water potential decreases, water leaves the cell, causing a decrease in cell volume and pressure potential. As a consequence, the wall may deform and its association with the membrane can become weakened. The extent of deformation depends on the severity of water loss and rigidity of the cell wall, which can itself be affected by water availability (Verslues et al., 2006). For example, some irreversible changes can occur in the wall due to water loss, such as enhanced bonding between polymers (Moore et al., 2008). Due to the differences in elasticity between the cell wall and cell membrane, weakening of the association between the cell wall and plasma membrane may culminate in the separation of the two at very low external water potentials in a process termed plasmolysis (Figures 1B and 1C). The partial or complete loss of the interaction with the plasma membrane has a large effect on the wall and wall-associated proteins, which are anchored to the plasma membrane and are important for maintaining cell wall function. However, even under plasmolysis, when most of the protoplast separates from the cell wall, some strands of plasma membrane, termed Hechtian strands, remain attached to the wall, though the nature of these strands needs to be further studied (Figure 1C) (Oparka, 1994). Thus, the physical changes that the cell wall experiences under water-deficit stress may not be homogenous across the entire wall.
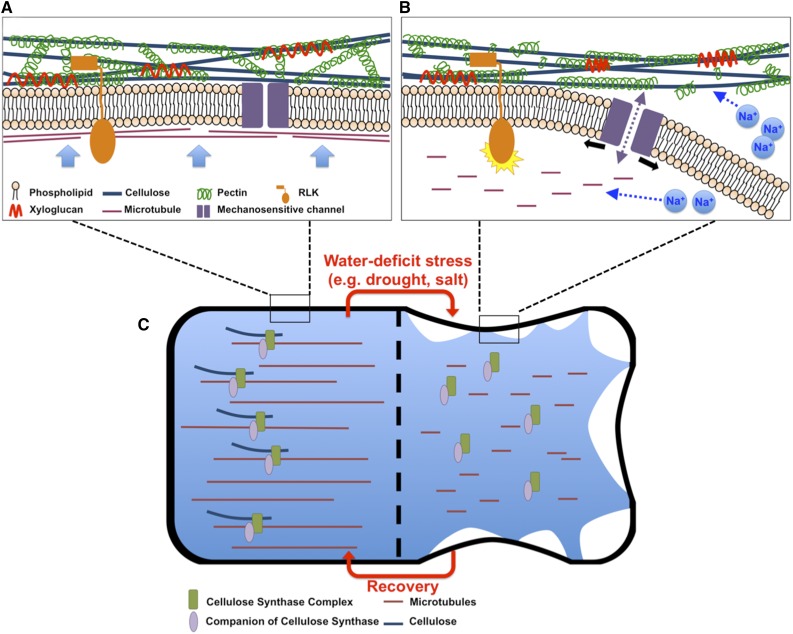
Figure 1.
Changes in the Primary Cell Wall under Water-Deficit Stress.
(A) Cell wall structure. The primary cell wall mainly consists of cellulose microfibrils, xyloglucan, and pectin. The orientation of cellulose microfibrils is largely determined by microtubule arrays. The current model suggests that xyloglucans localize to cellulose bundles, while pectins form a network that coats the cellulose. Cross-linked pectins that tether cellulose microfibrils may be load-bearing. Blue arrows indicate turgor pressure.
(B) Changes in cell wall properties under water-deficit stress. Under water-deficit stress, the cell wall deforms and dissociates from the plasma membrane. Cellulose biosynthesis is disrupted due to the depolymerization of microtubules under these conditions. Different stresses may trigger unique changes in addition to the general effect caused by water loss by the cell. For example, sodium ions (Na+) may disrupt pectin cross-links as well as microtubule arrays. Changes in cell wall integrity may be monitored directly by RLKs or indirectly by membrane-localized mechanosensitive channels.
(C) Comparison of cells under standard and salt stress conditions. With plasmolysis, most of the cell membrane dissociates from the cell wall. The cellulose synthase complex dislocates from the cell membrane as the microtubules depolymerize. CC1/2 proteins remain associated with the CSCs and aid the reassembly of the microtubule array and relocalization of CSCs under salt stress.
Water-deficit stress also affects the biosynthesis of new wall materials by regulating the localization of proteins involved in the deposition of wall components, especially in growing cells. The primary cell wall of plants comprises cellulose microfibrils embedded in a hydrated matrix of hemicellulose, pectin, and glycoproteins (Figure 1A). The relatively stiff cellulose microfibrils are considered the main load-bearing element of the cell wall and provide tensile strength (McFarlane et al., 2014). Microfibrils are deposited by cellulose synthase complexes (CSCs), which associate with the plasma membrane and track along cortical microtubules (Paredez et al., 2006; Gutierrez et al., 2009). It has been shown that microtubule arrays are sensitive to rapid changes in the osmotic potential of the environment and disassemble rapidly after plasmolysis (Figure 1) (Komis et al., 2001, 2002). The destabilization of microtubules will greatly affect wall strength, as mutants with alterations in microtubule orientation have a radially swollen cell phenotype and weakened cell wall due to reduced cellulose deposition (Burk and Ye, 2002; Zhong et al., 2002). Microtubule arrays and cellulose microfibril organization are interdependent, as the dynamics of microtubules are also dependent upon mechanical properties of the cell wall (Nick, 2013). Removal of the cell wall through enzymatic digestion destabilizes cortical microtubules in cultured tobacco (Nicotiana tabacum) cells (Akashi et al., 1990; Nick, 2013). In animal cells, integrins link microtubules to the extracellular matrix. Although transmembrane proteins homologous to animal integrins have not been identified in plants, cortical microtubules of plant cells are thought to connect with the cell wall by an analogous mechanism (Baluska et al., 2003). In future studies, it will be interesting to determine whether water deficit-induced destabilization of the microtubule array is a direct consequence of dissociation of the cell wall and plasma membrane.
Signaling Mechanisms at the Cellular Scale Utilize Mechanical Cues
Despite the importance of water for plant growth, how plant cells perceive water availability is still largely unknown. In prokaryotes and animal cells, changes in plasma membrane tension are used as a physical cue for sensing the osmotic potential of the environment (osmosensing) (Kung, 2005). Recent evidence suggests that plants may use similar mechanical cues at the plasma membrane and cell wall interface for osmosensing, although the underlying molecular machinery may not be conserved (Haswell and Verslues, 2015).
Mechanosensitive channels alter their conductivity to ions in response to changes in membrane tension and therefore can convert mechanical signals to ion flux. Recently, several mechanosensitive channels were found to be involved in osmosensing. OSCA1, a plasma membrane-localized calcium-permeable channel, was shown to regulate many aspects of osmotic responses in plants, including Ca2+ influx (Hou et al., 2014; Yuan et al., 2014). In vitro studies showed that OSCA1 Ca2+ permeability is induced by hyper-osmotic stress, which makes it a strong candidate as an osmosensor in plants, although the corresponding mutants display relatively subtle defects (Yuan et al., 2014). Interestingly, another group of putative stretch-activated Ca2+ channels, MID1-complementing activity 1 and 2 (MCA1 and MCA2), seem to mediate Ca2+ increases induced by hypo-osmotic stress (Nakagawa et al., 2007; Furuichi et al., 2012). Besides Ca2+ channels, other mechanosensitive channels may also be involved in osmosensing. Two members of another type of mechanosensitive channel, MscS-Like (MSL) 2 and 3, function in the plastid to regulate the hyper-osmotic response (Haswell and Meyerowitz, 2006; Wilson et al., 2011). Recently, MSL8, a pollen-specific ion channel, was found to be required for preventing pollen bursting under hypo-osmotic stress during rehydration (Hamilton et al., 2015). In plants, due to the close physical relationship of the plasma membrane and cell wall, the wall itself may contribute to the changes in membrane tension that occur during osmotic stress. For example, during the formation of Hechtian strands, both hyper-osmolarity and hypo-osmolarity can theoretically cause an increase in local membrane tension (Figure 1A, B) (Haswell and Verslues, 2015).
As stated above, water deficit can invoke physical changes at the interface of the plasma membrane and cell wall, leading to a potentially weakened cell wall. Therefore, proteins involved in monitoring cell wall integrity may also play a role in sensing osmotic stress (Haswell and Verslues, 2015). Plasma membrane-localized receptor-like kinases (RLKs) are a large family of proteins that play roles in early signal transduction events in many pathways. Based on their domain arrangements, two families of RLKs are likely to be involved in cell wall integrity sensing (Figures 1A and 1B). A group of RLKs, Catharanthus roseus RLK1-like kinases (CrRLK1Ls), are of particular interest. They contain a cytoplasmic serine/threonine kinase domain and an extracellular malectin-like domain that may bind to carbohydrates, as occurs in their animal counterparts (Schallus et al., 2010; Boisson-Dernier et al., 2011). The plasma membrane localization of CrRLKs has been confirmed for at least some of the members and may require the help of cell wall-localized glycoproteins, such as glycosylphosphatidylinositol-anchored proteins (Li et al., 2015). The biological functions of 6 out of 17 CrRLK subfamily members in Arabidopsis thaliana have been studied, and many of them are involved in regulating cell growth during vegetative or reproductive development, possibly by coordinating cell expansion and wall integrity (Lindner et al., 2012). THESEUS1 (THE1) was the first member of this family found to be involved in sensing cell wall integrity. Mutation in THE1 attenuates the hypocotyl elongation defect and ectopic lignin production of cellulose-deficient Arabidopsis mutants, suggesting a role in inhibiting cell expansion when cell wall defects are sensed (Hématy et al., 2007). Other members were also found to be involved in processes related to cell wall function. ANXUR1 (ANX1) and ANX2, which are specifically expressed in pollen, redundantly function in preventing the bursting of elongating pollen tubes in Arabidopsis (Boisson-Dernier et al., 2009, 2013; Miyazaki et al., 2009).
In Arabidopsis, the closest homolog of ANX1/2 is FERONIA (FER), which has a much broader expression profile (Escobar-Restrepo et al., 2007). The fer mutant displays many growth-related phenotypes, including dwarfism, abnormal root growth, root hair bursting, and reproductive defects due to pollen tube overgrowth (Huck et al., 2003; Rotman et al., 2003; Guo et al., 2009; Duan et al., 2010; Haruta et al., 2014). Recently, it was shown that FER is involved in mechanical sensing in the root through Ca2+ signaling. When the root is bent to one direction, a unique FER-dependent Ca2+ signature is detected only on the convex side of the bend (Shih et al., 2014). Interestingly, the convex side of the root experiences a mechanical stress similar to hyper-osmotic shock in that there is a reduction in the pressure potential of the cell. Therefore, one may speculate that FER could also mediate signaling under osmotic stress, although direct proof is still lacking. Despite the hypothesis that CrRLK1Ls may bind to the wall, the actual molecules that serve as ligands remain largely unknown. It is possible that the relative polymerization status of certain carbohydrates or their derivatives may be recognized by the malectin-like domain. RALF, a small peptide, is currently the only ligand identified to bind to FER (Haruta et al., 2014). RALF is found in the apoplast, and the relationship between its localization and mechanical or osmotic stress could be an interesting area of investigation.
Wall-associated kinases (WAKs) are another group of RLKs that have the potential to monitor cell wall integrity. Encoded by five closely linked genes, WAKs are considered to be pectin receptors that not only bind to cross-linked pectin, but also to pectin fragments, such as oligogalacturonides (OGs) (Kohorn and Kohorn, 2012). The release of OGs seems to be specific to biotic stress, such as pathogen infection or wounding (Ferrari et al., 2013). Binding of pectin seems to activate WAKs. Pectin activation of downstream MAP kinase activity and gene expression are blocked in wak2 mutants (Kohorn et al., 2009). Plants expressing antisense WAK RNA display a smaller cell size, suggesting WAKs are also required for normal cell elongation (Lally et al., 2001). Interestingly, wak2 mutants displayed reduced growth only in media with low osmolarity (Kohorn et al., 2006). While the function of WAKs is mostly studied in terms of defense responses, little is known about whether they are involved in abiotic stresses, such as osmotic stress (Kohorn, 2016). Besides OGs, WAKs also bind to glycine-rich proteins (Park et al., 2001). Recently, WAK1 was found to be associated with glycine-rich proteins that accumulated in cell walls during dehydration in the resurrection plant Craterostigma plantagineum, suggesting its potential role in desiccation responses (Giarola et al., 2016).
Water Deficit-Associated Stresses Directly Affect Wall Mechanics
Many water deficit-related stresses, such as salt stress and freezing stress, have additional effects independent of their impact on water availability. For example, Na+ ions present during salt stress may have a specific effect on the pectin component of the cell wall. Pectin is a complex group of large molecules whose activity is mainly regulated by two large protein families, pectin methylesterases and pectin methylesterase inhibitors, as well as Ca2+ cross-linking (Grant et al., 1973; Pelloux et al., 2007; Burton et al., 2010). Na+ ions can compete with and displace Ca2+ in pectin cross-links and thus potentially reduce wall rigidity (Figure 1B) (Yoo et al., 2003). Metal ions also directly affect pectin methylesterase activity (Nari et al., 1991; Do Amaral et al., 2005). Cold, on the other hand, reduces the fluidity of the plasma membrane and reduces the activity of cell wall enzymes and microtubule polymerization (Nick, 2013). Hence, metal ion and temperature-dependent changes in cell wall properties may modulate the plant’s response to water-deficit stress and may serve as additional signals that induce salt/freezing-specific responses.
Changes in the Cell Wall Enable Growth Recovery
At the individual-cell level, growth is regulated by turgor pressure and the rate of cell wall loosening. The growth rate of a root drops immediately after transfer to hyper-osmotic media largely due to the escape of water from the cell and the reduction in turgor pressure (Shabala and Lew, 2002; Geng et al., 2013). Interestingly, while turgor pressure can be restored rapidly through an increase in the concentration of osmolytes in the cell, a process termed osmotic adjustment (Beauzamy et al., 2014), growth rate generally recovers at a much slower pace. Depending on the strength of the applied stress, cells in the elongation zone may enter a quiescent stage before their growth rate is recovered (Geng et al., 2013). Through tissue-specific transcriptional analysis, several clusters of genes whose transcriptional regulation temporally correlate with the dynamic changes in growth were found to be enriched for genes regulating the cell wall. Brassinosteroid and gibberellic acid signaling pathways were predicted to be regulators of these genes, providing evidence for what upstream pathway may be involved in determining the timing of such events (Geng et al., 2013). Importantly, however, it is not clear how the mechanical properties and organization of the wall change during the acclimation response.
While adding newly synthesized cell wall components is not required for short-term wall expansion, cell wall biosynthetic enzymes, such as CSCs that function in cellulose biosynthesis and deposition, are required to sustain long-term growth (Cosgrove, 2014, 2016b). Disrupting the functions of CSCs by mutation or isoxaben treatment in Arabidopsis leads to growth defects, which become more obvious under salt or osmotic stress (Kang et al., 2008). Some cellulose synthase-like (CSL) genes, which are predicted to be involved in the biosynthesis of noncellulosic polysaccharides of the cell wall, are specifically induced by water-deficit stress to regulate growth (Zhu et al., 2010). For example, the csld5/sos6 (salt overly sensitive6) mutant, which appears normal under control conditions, shows root growth defects under drought, salt, or osmotic stress. Reduced pectin levels are found in the cell wall of csld5/sos6 mutants and may contribute to these wall integrity defects as well (Zhu et al., 2010). This suggests that water-deficit stress may induce changes in cell wall composition, which can be important for growth adjustment.
Microtubule arrays are sensitive to osmotic stress (>Komis et al., 2001, 2002). To restore growth during water-deficit stress, cells must reassemble their microtubule arrays and organize their distribution. Upon recovery from osmotic stress, relocalization of CSCs to the plasma membrane was observed in association with microtubule-tethered compartments (Gutierrez et al., 2009). Similarly, after prolonged salt treatment, microtubule arrays disappear after 2 h and start to reappear after 8 h, remaining stable thereafter (Endler et al., 2015). This correlates well with the membrane localization dynamics of CSCs (Endler et al., 2015). Recently, two proteins, COMPANION OF CELLULOSE SYNTHASE1 (CC1) and CC2, were found to be important in reorganizing microtubule arrays and recruiting CSCs back to the plasma membrane during salt stress. CC1 and CC2 can bind to CesA1, CesA3, and CesA6, the major components of CSCs, possibly through their C termini, and they maintain this association during salt stress after the CSCs relocate to the cytosol (Endler et al., 2015). The N termini of CC proteins bind to microtubules, which aids in the reassembly of the microtubule array (Endler et al., 2015) (Figure 1C). In the cc1 cc2 double mutant, the reassembled microtubule array is not stable under salt stress and the CSCs fail to repopulate the plasma membrane, leading to defects in cell morphology and elongation (Endler et al., 2015). The functions of CC1/CC2 proteins are specific to salt stress, and it is unclear whether similar mechanisms exist for other water deficit-related stresses.
Induction of wall-loosening proteins contributes in important ways to maintaining growth under water-deficit stress. The expression of expansins, a group of proteins implicated to act by disrupting noncovalent bonding between hemicellulose and cellulose microfibrils, is induced by water-deficit stresses across different species (Yang et al., 2004; Dai et al., 2012; Geng et al., 2013). Many expansin genes are induced within hours after transfer of maize (Zea mays) roots to low water potential medium, specifically in the growing region (Wu et al., 2001). A similar induction pattern of several expansin genes was also observed in Arabidopsis under soil water-deficit treatment (Harb et al., 2010). Consistent with these findings, overexpression of expansin genes has been reported to confer tolerance to various water-deficit stresses in different plant species, likely by maintaining root elongation, though these results should not be overinterpreted, as overexpression of expansins may lead to many pleiotropic effects on physiology that are not directly related to root growth (Guo et al., 2011; Lü et al., 2013; Xu et al., 2014).
ORGAN-SCALE GROWTH CONTROL UNDER WATER STRESS
The effect of water availability on plant physiology cannot be understood simply through extrapolating cell-scale processes to a multicellular context. Coordination of growth across an organ requires precise control of cell division and elongation rates across fields of cells with different developmental states. In the next sections, we describe classic and current work that has elucidated the mechanisms for growth control operating at the organ scale that ultimately determine the architecture of the root system and the ability of this structure to access and take up water from soil.
Restructuring of the Root Growth Zone under Water-Deficit Stress
Changing root growth in response to water-limited conditions requires a trade-off between saving metabolic resources and increasing root system area for sufficient water access and uptake. Under mild and moderate water-deficit conditions, root growth rates increase in maize compared with well-watered plants, while shoot growth is highly suppressed (Sharp and Davies, 1979; Eghball and Maranville, 1993). Under severe water deficit, however, maize roots displayed a reduction in growth (Sharp et al., 1988; Eghball and Maranville, 1993). Understanding the molecular mechanisms that control such changes in growth requires foundational knowledge of the specific growth processes that change with water deficit.
Kinematic analysis enables the determination of the spatial distribution of elemental growth rates in an organ based on the displacement of particles along the surface of that organ (Erickson, 1976; Erickson and Silk, 1980). Kinematic analyses of maize roots have been performed by making ink markings on the surface of the organ and capturing the displacement of these marks after short time intervals. Quantification of the relative displacement of these particles with respect to the root tip reveals the acceleration and deceleration of tissue strain rates moving from the apex of the root tip to the end of the growth zone. Interestingly, it was found that the rate of growth acceleration was not affected by water-deficit stress, but the positions at which elemental growth rate peaked and fell were stress sensitive (Sharp et al., 1988). These data suggest that the root enacts regulatory changes that allow continued cell expansion despite the reduction in available water. Measurements of tissue water content and osmolytes revealed that different mechanisms were used to maintain water status in specific subregions of the growth zone. In the distal region, reduction in the diameter of the root allowed tissues to acquire a lower solute potential with less solute deposition, which was primarily driven by hexose accumulation (Sharp et al., 1990). Contrastingly, in the apical region of the root tip, proline accumulation allowed these cells to maintain their capacity to grow (Voetberg and Sharp, 1991).
Abscisic acid (ABA) is an important hormonal regulator of primary root (PR) growth under water-deficit stress. Experiments in maize seedlings showed that ABA content increased in the root tip after transfer to low water-potential medium (Saab et al., 1990). The effects of ABA were tested using the carotenoid biosynthesis inhibitor fluridone, as well as the vp5 mutant, which has a defect in a similar upstream biosynthetic pathway necessary for ABA production. While exogenous ABA treatment generally causes growth inhibition, reduced ABA levels also led to reduced root growth (Saab et al., 1990). Subsequent studies revealed that the growth-promoting effects of ABA likely function through the inhibition of ethylene biosynthesis or signaling, as suppression of these pathways suppressed the effects of fluridone (Spollen et al., 2000; LeNoble et al., 2004).
More recently, studies from our group examining the suppression of PR growth by high salinity also revealed a growth-promoting role for ABA (Geng et al., 2013). Arabidopsis roots transferred to 140 mM NaCl exhibit a temporally dynamic change in growth rate, with an initial period of growth quiescence followed by growth recovery between 8 and 12 h after treatment (Geng et al., 2013). Treatment of seedlings with fluridone at different time periods during the salt stress response showed that ABA was most important for growth promotion during the recovery phase of the salt response (Geng et al., 2013). This result is intriguing, since ABA levels and associated transcriptional responses both peaked between 4 and 8 h after salt treatment, while ABA levels declined during the recovery phase (Geng et al., 2013). Fluridone-treated roots exhibit extensive radial tissue swelling, similar to ethylene treatment. Similar effects were also observed when ABA signaling was inhibited through expression of the aba insentitive1-1 mutant protein phosphatase, which inhibits downstream signaling events (Geng et al., 2013; Leung et al., 1997). Together, these data suggest that under water deficit and high salinity, growth is maintained through ABA signaling, which may act partly through ethylene antagonism. Whether ABA also has growth-inhibitory effects at earlier stages of the salt stress response is unclear.
Organ-Type-Specific Growth Responses to Water-Deficit Stress
Root systems are composed of roots with different hierarchical relationships, tissue organization, growth rates, and physiological activities (Rellán-Álvarez et al., 2016). Correlated with these structural and physiological differences, the response to abiotic stress has also been shown to distinguish the organs of the root system. Exposure to water deficit during early lateral root (LR) development revealed inhibition of postemergence growth in Arabidopsis (Xiong et al., 2006; Rellán-Álvarez et al., 2015), involving ABA and auxin signaling (Deak and Malamy, 2005; Xiong et al., 2006). Therefore, water deficit causes a clear reduction in root mass in more mature root systems (Xiong et al., 2006; Rellán-Álvarez et al., 2015). For salt stress, many genes and pathways have been identified over the past few decades that regulate the early response of roots at the cellular level (Julkowska and Testerink, 2015). However, understanding how these early responses shape root system architecture (RSA) in a soil environment remain largely elusive.
Studies from our laboratory have shown that LRs are hypersensitive to salt stress compared with the PR due to the LR-specific activation of ABA signaling in the endodermis (Duan et al., 2013; Dinneny, 2014). ABA signaling prevents postemergence LRs from growing for a period lasting several days, presumably until some unknown acclimation event occurs. Growth recovery of the PR occurs more rapidly, within hours of salt treatment, with ABA signaling being induced over a similar time scale. These organ-specific differences in ABA signaling lead to a PR-dominated root system, which may limit the number of routes by which salt enters the plant. Evaluation of PR and LR growth rates under mild and high salt concentrations over longer time periods also revealed a clear reduction in root growth (Zolla et al., 2010; Julkowska et al., 2014). However, in these studies, PR growth was more severely inhibited under long-term exposure to high salt concentrations (Julkowska et al., 2014). Differences between studies may reflect the different spatial and temporal scales at which these processes were studied and may indicate that organ-specific effects of salt stress are highly dependent on the temporal context of the experiment.
Growth Direction Is Determined Locally by Water Availability
Changing the growth direction of roots in response to environmental cues is another efficient way developmental mechanisms are used to optimize plant survival (Figure 2). Directional growth toward or away from an external stimulus is termed a positive or negative tropic response, respectively. Combinatorial effects of different tropisms shape the root system under most environmental conditions, which makes the identification of the specific pathways involved often challenging.
Figure 2.
Changes in Root Growth Direction in Response to Environmental Stimuli.
(A) Root system architecture of a taproot system under non-stress conditions. The PR grows toward the gravity vector (g) and the LRs overcome this gravity response and grow at a certain gravity set-point angle (GSA) to enable expanded soil exploration.
(B) Hydrotropism: growth of PR and LR tips toward environments with higher water potential.
(C) Xerotropism: increased gravitropic response of the PR and LRs upon water deficit.
(D) Halotropism: growth of the PR away from high salt concentrations.
Gravitropism is possibly the best understood tropism in plant roots, and since gravity is constantly present, it is the predominant tropism that all other tropisms antagonize (Figure 2). Soon after seed germination, the PR orients itself along the gravity vector for plant anchoring, as well as water and nutrient uptake (Bailey et al., 2002). In the Arabidopsis PR, gravity is mainly perceived in the two innermost S1 and S2 layers of columella cells in the root cap (Blancaflor et al., 1998). A major sensing mechanism involves the sedimentation of the starch-enriched amyloplasts or statoliths at the site of the gravity stimulus (Haberlandt, 1900; Nèmec, 1900). Research over the last several decades has shown that auxin is the key signal that links perception of the gravity vector in the root cap to differential growth in the elongation zone (Swarup et al., 2005; Sato et al., 2015). In contrast to the gravitropic response of the PR, LRs have been shown to overcome the gravity response by reducing asymmetric auxin signaling in LR columella cells to promote horizontal soil exploration (Figure 2A) (Rosquete et al., 2013).
Two tropisms related to water availability are hydrotropism and xerotropism (see below). Hydrotropism is the growth of the root tip toward environments with higher water potential (Figure 2B). Hydrotropism is often difficult to study, as the gravitropic response is typically dominant. However, agravitropic mutants offer a tool to study the effects of moisture gradients in isolation. Investigations of such mutants in pea (Pisum sativum) have shown that two independent sensing and response mechanisms are involved in hydrotropism and gravitropism (Jaffe et al., 1985), although the two seem to interact in wild-type plants. Ca2+ is likely an important transducer of both the gravitropic (Plieth and Trewavas, 2002) and hydrotropic responses (Takano et al., 1997).
While the identification of genes involved in hydrotropism could be an important step in crop improvement, few genes have been cloned and no studies have yet determined the field relevance of this trait. Three mutants with impaired hydrotropism have been identified in Arabidopsis: no hydrotropic response1 (nhr1), where the underlying gene remains to be determined (Eapen et al., 2003); mizu-kussei1 (miz1), encoding a protein of unknown function (Kobayashi et al., 2007); and miz2, a weak mutant allele of GNOM (Miyazawa et al., 2009), encoding a guanine nucleotide exchange factor involved in membrane trafficking and localization of the auxin transporter PIN1 (Steinmann et al., 1999; Geldner et al., 2003). The mutant altered hydrotropic response1 (ahr1) displays an enhanced hydrotropic response, but the causal gene remains unknown (Saucedo et al., 2012).
It was shown that root caps of wild-type plants display amyloplast degradation upon exposure to a hydrotropic stimulus, which presumably causes a decrease in gravity responsiveness (Takahashi et al., 2003; Nakayama et al., 2012). In line with this hypothesis, water-stressed roots also contain degraded amyloplasts (Takahashi et al., 2003; Nakayama et al., 2012; Cassab et al., 2013), which may be important for osmotic adjustment and presumably also makes them less responsive to gravity.
Several studies described steep root growth in plants exposed to water stress, which may enable better exploration of deeper soil strata with higher water availability (see below) (Nord and Lynch, 2009; Lynch, 2013). Here, we define xerotropism as the enhanced gravitropism of roots occurring under water deficit (Figure 2C). In a recent study published by our group, we observed this phenomenon using the GLO-Roots system (Rellán-Álvarez et al., 2015), in which a luciferase reporter enables the visualization of changes in root growth upon water-deficit treatment in a soil-like environment. We observed that LR growth directionality changed from shallow to steeper angles when water was only available in deeper layers of the soil. This avoidance mechanism was shown to be independent of hydrotropism, as the miz1 mutant showed a similar response to that of the wild type. The auxin response is necessary for a normal xerotropic response, since the auxin receptor mutant tir1 did not show a change in LR growth direction upon water-deficit treatment (Rellán-Álvarez et al., 2015). Xerotropism may involve enhancement of the gravity response, perhaps through changes in auxin biosynthesis or signaling capacity. Interestingly, similar responses are observed at elevated temperature, suggesting that various environmental cues associated with drought may affect the root system in similar ways to promote deep-soil exploration. Measurement of LR growth trajectory together with local soil moisture indicated that root angle is likely influenced by both local and systemic signaling. It will be interesting to determine whether xerotropism operates through systemic signaling while hydrotropism provides a local mechanism for growth direction control.
Besides hydrotropism and xerotropism, both of which interact with gravitropism, salt stress seems to induce a unique tropism of its own. Negative halotropism describes the growth of the root system away from salt (Figure 2D). This phenomenon has been described for PRs of Arabidopsis, tomato (Solanum lycopersicum), and sorghum (Sorghum bicolor) (Galvan-Ampudia et al., 2013). This growth pattern is caused by targeted endocytosis of the auxin efflux carrier PIN2 at the site of higher salt concentration in the root tip, leading to auxin redistribution and root bending (Galvan-Ampudia et al., 2013). One study suggested that degradation of starch in the amyloplasts of columella cells may promote salt avoidance (Sun et al., 2008). However, the sos mutants, which display impaired amyloplast degradation, retain the ability to avoid salinity. Salinity also negatively affects gravitropism, even when no gradient exists, suggesting that the effect on growth direction may be highly dependent upon the environmental context (Dinneny et al., 2008). These data suggest a more complex relationship between gravitropism and halotropism that requires further investigation.
The effect of changes in root growth directionality on drought stress avoidance has been demonstrated in field studies. In rice (Oryza sativa), a large-effect quantitative trait locus (QTL) linked to deeper root growth was shown to be involved in increased water uptake and grain yield improvement under severe water-limiting conditions (Bernier et al., 2009). Introgressing the responsible QTL, DEEPER ROOTING1 (DRO1), into a shallow-growing rice cultivar achieved increased drought avoidance and yield improvement (Uga et al., 2013). Higher DRO1 expression leads to steeper root angles due to an increase in the gravitropic response (Uga et al., 2013). This is achieved by an asymmetric accumulation of DRO1 transcripts in the outer cells of the elongation zone. There, DRO1 positively regulates cell elongation on the upper side, whereas auxin represses DRO1 expression on the lower side, causing asymmetric growth and downward bending (Uga et al., 2013). A follow-up study demonstrated that having the deep-rooting allele at the DRO1 locus enhanced tolerance to drought stress in the field (Arai-Sanoh et al., 2014). Recently, another QTL was identified (DRO3) that affects root growth angle in a DRO1-dependent manner (Uga et al., 2015). Additional QTL and genome-wide association studies in rice revealed further QTLs for deeper root growth under water-deficit conditions (Courtois et al., 2009; Lou et al., 2015; Wade et al., 2015).
Water Acts as a Potent Inducer of Branching in Root Systems
An essential mechanism to expand the size of the root system is through the patterning and induction of branches. There are two main types of root systems in angiosperms, which differ in their origin of development and branching pattern (Figure 3A). In the taproot system, the PR becomes the central axis from which LRs branch off postembryonically in a periodic manner (Lavenus et al., 2013; Van Norman et al., 2013). In contrast, some eudicots and most monocots, including all cereal crops, possess a fibrous root system, which is multiaxial and lacks a single dominant root (Mauseth, 2008; Rogers and Benfey, 2015). Recent work has highlighted the role that water can play as a local environmental cue to promote the patterning and postemergence development of root branches.
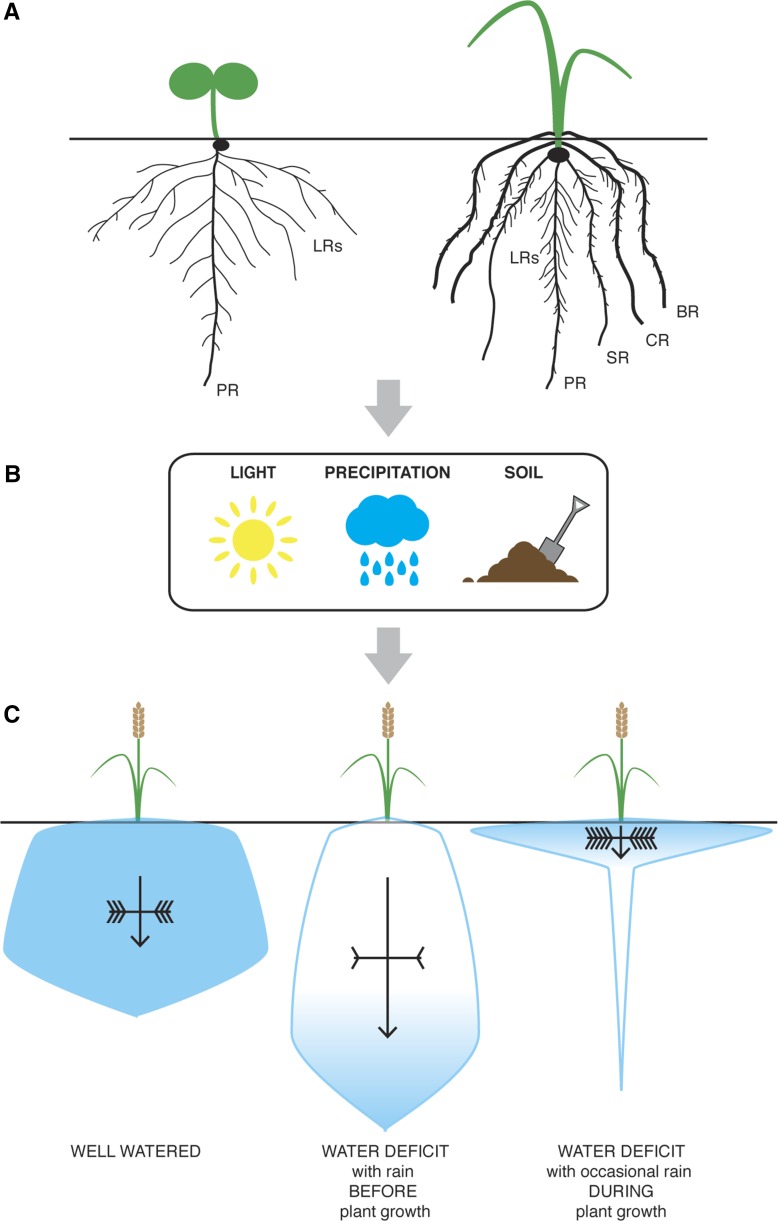
Figure 3.
Changes in RSA upon Water-Deficit Stress.
(A) RSA of a taproot system (left) and a fibrous root system (right). Taproot systems consist of a PR and LRs. Fibrous root systems consist of embryonically derived primary and seminal roots (SR), postembryonic LRs, belowground crown roots (CR), and aboveground brace roots (BR).
(B) Different environmental factors such as light, precipitation, and soil influence RSA during plant development and growth.
(C) RSA ideotypes for well-watered and water-deficit conditions. Water is shown in blue and is equally distributed in the soil under well-watered conditions. If the primary water source is rainfall before the growing season, the “steep, deep, and cheap” ideotype ensures water uptake in deep soil strata. If occasional rainfall is the primary water source, water uptake is highest in upper soil strata.
Deep root growth correlates with arrow length and branching frequency with the number of inverted arrowheads.
LR patterning is affected at the earliest stages of founder cell specification by the local distribution of water surrounding the root in a process termed hydropatterning (Bao et al., 2014). This adaptive root branching response has been observed in grasses (maize and rice), as well as eudicots (Arabidopsis) (Bao et al., 2014). Hence, roots of different species appear able to distinguish between wet surfaces and air environments to induce LRs only along the wet surface. Roots appear to respond specifically to the availability of water in the medium; reducing the water potential of the medium lowers its LR-inducing effects, while contacting roots with materials that do not conduct water such as glass or rubber did not induce LR patterning. In contrast to classic water-stress responses, hydropatterning is not dependent upon endogenous ABA signaling but is highly dependent on auxin biosynthesis and signaling (Bao et al., 2014). How roots are able to sense variation in the availability of water is not clear but may involve processes specific to the growing regions of roots, where responsiveness to the hydropatterning signal appears to be located (Robbins and Dinneny, 2015).
While hydropatterning of LRs reveals how water can regulate branching through evolutionarily conserved processes, the fibrous root system that forms in cereal crops such as maize, rice, and wheat (Triticum aestivum) has a very different developmental origin than that in Arabidopsis. In members of the Poaceae family, the majority of the root system originates postembryonically from the base of the shoot, termed the crown (Orman-Ligeza et al., 2013). These crown roots form at or below the soil surface and play important roles in water and nutrient uptake, as well as anchorage and protection against lodging. Grass species are incapable of expanding their vascular system through secondary growth (Esau, 1953); thus, the multiaxial nature of the crown root system provides a unique developmental mechanism to increase the flux of materials from the soil to the shoot.
Recent work from our group has shown that water-deficit stress causes a severe inhibition of postemergence crown root growth (Sebastian et al., 2016). Using the model grass species Setaria viridis, which facilitates the use of molecular physiology approaches due to its rapid lifecycle, limited infrastructure required for cultivation, and sequenced genome (Brutnell et al., 2015), we found that the transition from a PR-dominated system to a crown root dominated system is completely environment dependent. Under water deficit, the crown roots become arrested after emergence, whereas the PR system expands in size. Crown root arrest is irreversible at the individual root level; however, after rewatering of water deficit-treated plants, new crown root emergence is rapidly induced. Within 8 h after rewatering, crown root primordia are visible, and within 6 d, these roots take over as the dominant part of the root system.
Similar responses of crown roots were observed under water-deficit stress in other grass species including sorghum, miscanthus, and Brachypodium distachyon (Sebastian et al., 2016). Interestingly, the response was less severe in maize, where some crown root growth still occurred. The development of crown roots under water deficit in maize allowed us to test their physiological contribution to plant water status using the rootless concerning crown and seminal roots (rtcs) mutant, which completely lacks crown root development (Hetz et al., 1996; Taramino et al., 2007). Interestingly, the rtcs mutant was better able to maintain the water status of the shoot under water deficit compared with the reference inbred and was accompanied by the preservation of water in the soil in the pot (Sebastian et al., 2016). These data suggest that reduced crown root growth allows the plant to slow the extraction of water from the soil and to bank these reserves for the future. This phenomenon, known as water banking, may allow grasses to survive irregularity in precipitation patterns. Maize inbreds show tremendous variation in the degree to which crown root growth is suppressed under water deficit. These data suggest that during domestication, there may have been selection for reduced drought responsiveness, perhaps as a way of generating a larger root system. It will be interesting for future studies to identify the molecular targets of water-deficit signaling that cause crown root arrest and to generate varieties with different sensitivities to stress to identify a response that best suits soil and water management conditions in the field.
Selecting Root System-Level Traits for Better Water Usage
The dynamic responses to limited water availability on the organ scale described above lead to complex spatial arrangements of roots in soil, and the emergent properties of this system ultimately determine the expanse of soil where water can be accessed. To date, in the field of plant breeding, rather static, idealized root phenotypes, namely ideotypes, have been targeted to optimize plant growth under a particular environment or stress condition. Such ideotypes can be theoretical or based on modeling data. The “steep, deep and cheap” ideotype has been proposed for water- and nitrogen-limited conditions (Nord and Lynch, 2009; Lynch, 2013). This ideotype is defined by a root system that has roots oriented more vertically to capture deep water resources and high rates of growth, which are enabled by several anatomical features that reduce the metabolic cost per unit length of root (Figure 3C) (Lynch et al., 2014). This ideotype was designed for maize roots based on the greater availability of water and nitrogen in deeper soil strata throughout the growing season in most agricultural soils.
The steep, deep, and cheap ideotype is partly reflected in the patterns of root system growth observed in nature. While a wide range of interspecific strategies to cope with drought were observed in grassland communities (Zwicke et al., 2015), species that survive and recover best from drought combine a large root mass for water acquisition and dehydration avoidance with deeper roots with higher cell membrane stability and carbohydrate accumulation for dehydration tolerance (Zwicke et al., 2015). Importantly, however, other strategies are also observed in xerophytes. For example, perennial cactus species growing in the desert have very shallow root systems that are adapted to rapidly capture water from seasonal rains (Figure 3C) (Rundel et al., 1991). In addition, different desert species increase water uptake from seasonal rain by producing very fine roots from laterals that become highly suberized after a long dry period (Figure 3C) (Nobel and Sanderson, 1984; Salguero-Gómez and Casper, 2011).
A recent large-scale modeling approach combined RSA modeling with soil-hydrological modeling to investigate if there is one optimal RSA ideotype for enhanced drought tolerance (Tron et al., 2015). The authors conclude that the “ideal” root architecture for efficient water uptake always has to be considered with respect to the hydrological environment. In their model, deep-rooted systems provide ideal water uptake if there is sufficient rainfall before the growing season in fine soil textures (Figure 3C). However, dense root systems close to the soil surface are essential for water uptake if rainfalls are the main water source during plant development (Figure 3C).
CONCLUSIONS AND PERSPECTIVES
Biological systems are multiscale in organization, from molecules, cells, organs, and organisms to communities (Passioura, 1979). Fully understanding a given phenomenon requires studies at all levels; however, it is essential that the observations made at a specific scale be understood in the context of higher scales. One can always find patterns and rules at lower scales. However, to ensure the principles discovered are not trivial, it is essential to understand the context in which these phenomena occur. As the population of the Earth approaches over 9 billion in 2050 and we attempt to feed the massively increasing number of humans, emphasis must be placed on studies of plant–environment interactions that are of broad importance. Clearly, this is essential to ensure that studies in plant model systems have the best chance of improving agriculture. However, this is not the only goal of such a holistic approach. A clearer understanding of plant biology requires appreciation of the ecological contexts in which organisms have evolved to function. Thus, broadly impactful research on plant–environment interactions will naturally influence our ideas of how plants function in natural and agricultural field contexts.
The cell- and organ-scale processes described here serve as a foundation for our understanding of how changes in water availability affect the form and function of the plant. Current research on the mechanisms that allow cells to sense such environmental stimuli have emphasized mechanisms that operate at the cell scale. However, additional research is needed to understand how these processes act at the organ and organism scale. Where do plants sense changes in water availability and how do processes such as cell expansion affect water sensing? At the scale of the organ, how does water sensing at the single cell scale get integrated into higher order patterning processes such as hydropatterning, which likely require communication between tissue layers? Answers to these questions will allow us to understand not only how plants sense water, but also why it matters.
Acknowledgments
We thank Michael T. Raissig, Malcolm Bennett, members of the Dinneny lab, and anonymous reviewers for helpful comments during the preparation of this work. Funding was provided by the U.S. Department of Energy’s Biological and Environmental Research program (Grant DE-SC0008769 to J.R.D.), the National Science Foundation’s Plant Genome Research Program (Grant IOS-PGRP 420-40-45A to J.R.D.), the DFG German Research Foundation (research fellowship to H.L.), and the National Science Foundation Graduate Research Fellowship (Grant DGE-1147470 to N.E.R.).
AUTHOR CONTRIBUTIONS
All authors contributed to writing the article.
Footnotes
Articles can be viewed without a subscription.
References
- Akashi T., Kawasaki S., Shibaoka H. (1990). Stabilization of cortical microtubules by the cell wall in cultured tobacco cells: Effects of extensin on the cold-stability of cortical microtubules. Planta 182: 363–369. [DOI] [PubMed] [Google Scholar]
- Arai-Sanoh Y., Takai T., Yoshinaga S., Nakano H., Kojima M., Sakakibara H., Kondo M., Uga Y. (2014). Deep rooting conferred by DEEPER ROOTING 1 enhances rice yield in paddy fields. Sci. Rep. 4: 5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey P.H.J., Currey J.D., Fitter A.H. (2002). The role of root system architecture and root hairs in promoting anchorage against uprooting forces in Allium cepa and root mutants of Arabidopsis thaliana. J. Exp. Bot. 53: 333–340. [DOI] [PubMed] [Google Scholar]
- Baluska F., Samaj J., Wojtaszek P., Volkmann D., Menzel D. (2003). Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiol. 133: 482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y., et al. (2014). Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc. Natl. Acad. Sci. USA 111: 9319–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauzamy L., Nakayama N., Boudaoud A. (2014). Flowers under pressure: ins and outs of turgor regulation in development. Ann. Bot. (Lond.) 114: 1517–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier J., Serraj R., Kumar A., Venuprasad R., Impa S., Veeresh Gowda R.P., Oane R., Atlin G. (2009). The large-effect drought-resistance QTL qtl12.1 increases water uptake in upland rice. Field Crops Res. 110: 139–146. [Google Scholar]
- Blancaflor E.B., Fasano J.M., Gilroy S. (1998). Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 116: 213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A., Kessler S.A., Grossniklaus U. (2011). The walls have ears: the role of plant CrRLK1Ls in sensing and transducing extracellular signals. J. Exp. Bot. 62: 1581–1591. [DOI] [PubMed] [Google Scholar]
- Boisson-Dernier A., Lituiev D.S., Nestorova A., Franck C.M., Thirugnanarajah S., Grossniklaus U. (2013). ANXUR receptor-like kinases coordinate cell wall integrity with growth at the pollen tube tip via NADPH oxidases. PLoS Biol. 11: e1001719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisson-Dernier A., Roy S., Kritsas K., Grobei M.A., Jaciubek M., Schroeder J.I., Grossniklaus U. (2009). Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development 136: 3279–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsa A.A., Agnew D.C., Cayan D.R. (2014). Remote hydrology. Ongoing drought-induced uplift in the western United States. Science 345: 1587–1590. [DOI] [PubMed] [Google Scholar]
- Brutnell T.P., Bennetzen J.L., Vogel J.P. (2015). Brachypodium distachyon and Setaria viridis: Model genetic systems for the grasses. Annu. Rev. Plant Biol. 66: 465–485. [DOI] [PubMed] [Google Scholar]
- Burk D.H., Ye Z.-H. (2002). Alteration of oriented deposition of cellulose microfibrils by mutation of a katanin-like microtubule-severing protein. Plant Cell 14: 2145–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton R.A., Gidley M.J., Fincher G.B. (2010). Heterogeneity in the chemistry, structure and function of plant cell walls. Nat. Chem. Biol. 6: 724–732. [DOI] [PubMed] [Google Scholar]
- Cassab G.I., Eapen D., Campos M.E. (2013). Root hydrotropism: an update. Am. J. Bot. 100: 14–24. [DOI] [PubMed] [Google Scholar]
- Christmann A., Grill E., Huang J. (2013). Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 16: 293–300. [DOI] [PubMed] [Google Scholar]
- Cosgrove D.J. (2016a). Catalysts of plant cell wall loosening. F1000 Res. 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D.J. (2016b). Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 67: 463–476. [DOI] [PubMed] [Google Scholar]
- Cosgrove D.J. (2014). Re-constructing our models of cellulose and primary cell wall assembly. Curr. Opin. Plant Biol. 22: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D.J., Green P.B. (1981). Rapid suppression of growth by blue light: Biophysical mechanism of action. Plant Physiol. 68: 1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois B., Ahmadi N., Khowaja F., Price A.H., Rami J.-F., Frouin J., Hamelin C., Ruiz M. (2009). Rice root genetic architecture: Meta-analysis from a drought QTL database. Rice (N.Y.) 2: 115–128. [Google Scholar]
- Dai F., Zhang C., Jiang X., Kang M., Yin X., Lü P., Zhang X., Zheng Y., Gao J. (2012). RhNAC2 and RhEXPA4 are involved in the regulation of dehydration tolerance during the expansion of rose petals. Plant Physiol. 160: 2064–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak K.I., Malamy J. (2005). Osmotic regulation of root system architecture. Plant J. 43: 17–28. [DOI] [PubMed] [Google Scholar]
- Dinneny J.R. (2014). A gateway with a guard: how the endodermis regulates growth through hormone signaling. Plant Sci. 214: 14–19. [DOI] [PubMed] [Google Scholar]
- Dinneny J.R. (2015). A developmental biologist’s journey to rediscover the Zen of plant physiology. F1000Research 2015 4: 264. [Google Scholar]
- Dinneny J.R., Long T.A., Wang J.Y., Jung J.W., Mace D., Pointer S., Barron C., Brady S.M., Schiefelbein J., Benfey P.N. (2008). Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320: 942–945. [DOI] [PubMed] [Google Scholar]
- Do Amaral S.H., De Assis S.A., de Faria Oliveira O., Mascarenhas M. (2005). Partial purification and characterization of pectin methylesterase from orange (Citrus sinensis) cv. Pera-rio. J. Food Biochem. 29: 367–380. [Google Scholar]
- Duan L., Dietrich D., Ng C.H., Chan P.M.Y., Bhalerao R., Bennett M.J., Dinneny J.R. (2013). Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. Plant Cell 25: 324–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Q., Kita D., Li C., Cheung A.Y., Wu H.-M. (2010). FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc. Natl. Acad. Sci. USA 107: 17821–17826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eapen D., Barroso M.L., Campos M.E., Ponce G., Corkidi G., Dubrovsky J.G., Cassab G.I. (2003). A no hydrotropic response root mutant that responds positively to gravitropism in Arabidopsis. Plant Physiol. 131: 536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghball B., Maranville J.W. (1993). Root development and nitrogen influx of corn genotypes grown under combined drought and nitrogen stresses. Agron. J. 85: 147–152. [Google Scholar]
- Endler A., Kesten C., Schneider R., Zhang Y., Ivakov A., Froehlich A., Funke N., Persson S. (2015). A mechanism for sustained cellulose synthesis during salt stress. Cell 162: 1353–1364. [DOI] [PubMed] [Google Scholar]
- Erickson R.O. (1976). Modeling of plant growth. Annu. Rev. Plant Physiol. 27: 407–434. [Google Scholar]
- Erickson R.O., Silk W.K. (1980). The kinematics of plant growth. Sci. Am. 242: 134–151. [Google Scholar]
- Esau K. (1953). Plant Anatomy, 2nd ed. (New York: John Wiley & Sons; ). [Google Scholar]
- Escobar-Restrepo J.-M., Huck N., Kessler S., Gagliardini V., Gheyselinck J., Yang W.-C., Grossniklaus U. (2007). The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science 317: 656–660. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Savatin D.V., Sicilia F., Gramegna G., Cervone F., Lorenzo G.D. (2013). Oligogalacturonides: plant damage-associated molecular patterns and regulators of growth and development. Front. Plant Sci. 4: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuichi T., Iida H., Sokabe M., Tatsumi H. (2012). Expression of Arabidopsis MCA1 enhanced mechanosensitive channel activity in the Xenopus laevis oocyte plasma membrane. Plant Signal. Behav. 7: 1022–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan-Ampudia C.S., Julkowska M.M., Darwish E., Gandullo J., Korver R.A., Brunoud G., Haring M.A., Munnik T., Vernoux T., Testerink C. (2013). Halotropism is a response of plant roots to avoid a saline environment. Curr. Biol. 23: 2044–2050. [DOI] [PubMed] [Google Scholar]
- Geldner N., Anders N., Wolters H., Keicher J., Kornberger W., Muller P., Delbarre A., Ueda T., Nakano A., Jürgens G. (2003). The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230. [DOI] [PubMed] [Google Scholar]
- Geng Y., Wu R., Wee C.W., Xie F., Wei X., Chan P.M.Y., Tham C., Duan L., Dinneny J.R. (2013). A spatio-temporal understanding of growth regulation during the salt stress response in Arabidopsis. Plant Cell 25: 2132–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giarola V., Krey S., von den Driesch B., Bartels D. (2016). The Craterostigma plantagineum glycine-rich protein CpGRP1 interacts with a cell wall-associated protein kinase 1 (CpWAK1) and accumulates in leaf cell walls during dehydration. New Phytol. 210: 535–550. [DOI] [PubMed] [Google Scholar]
- Grant G.T., Morris E.R., Rees D.A., Smith P.J.C., Thom D. (1973). Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 32: 195–198. [Google Scholar]
- Guo H., Li L., Ye H., Yu X., Algreen A., Yin Y. (2009). Three related receptor-like kinases are required for optimal cell elongation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 106: 7648–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W., Zhao J., Li X., Qin L., Yan X., Liao H. (2011). A soybean β-expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. Plant J. 66: 541–552. [DOI] [PubMed] [Google Scholar]
- Gutierrez R., Lindeboom J.J., Paredez A.R., Emons A.M.C., Ehrhardt D.W. (2009). Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat. Cell Biol. 11: 797–806. [DOI] [PubMed] [Google Scholar]
- Haberlandt G. (1900). Über die Perception des geotropischen Reizes. Berichte der Deutschen Botanischen Gesellschaft 18: 261–272.
- Hamilton E.S., Jensen G.S., Maksaev G., Katims A., Sherp A.M., Haswell E.S. (2015). Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 350: 438–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harb A., Krishnan A., Ambavaram M.M.R., Pereira A. (2010). Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 154: 1254–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M., Sabat G., Stecker K., Minkoff B.B., Sussman M.R. (2014). A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343: 408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haswell E.S., Meyerowitz E.M. (2006). MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr. Biol. 16: 1–11. [DOI] [PubMed] [Google Scholar]
- Haswell E.S., Verslues P.E. (2015). The ongoing search for the molecular basis of plant osmosensing. J. Gen. Physiol. 145: 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hématy K., Sado P.-E., Van Tuinen A., Rochange S., Desnos T., Balzergue S., Pelletier S., Renou J.-P., Höfte H. (2007). A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr. Biol. 17: 922–931. [DOI] [PubMed] [Google Scholar]
- Hetz W., Hochholdinger F., Schwall M., Feix G. (1996). Isolation and characterization of rtcs, a maize mutant deficient in the formation of nodal roots. Plant J. 10: 845–857. [Google Scholar]
- Hou C., Tian W., Kleist T., He K., Garcia V., Bai F., Hao Y., Luan S., Li L. (2014). DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 24: 632–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck N., Moore J.M., Federer M., Grossniklaus U. (2003). The Arabidopsis mutant feronia disrupts the female gametophytic control of pollen tube reception. Development 130: 2149–2159. [DOI] [PubMed] [Google Scholar]
- Jaffe M.J., Takahashi H., Biro R.L. (1985). A pea mutant for the study of hydrotropism in roots. Science 230: 445–447. [DOI] [PubMed] [Google Scholar]
- Julkowska M.M., Hoefsloot H.C.J., Mol S., Feron R., de Boer G.-J., Haring M.A., Testerink C. (2014). Capturing Arabidopsis root architecture dynamics with ROOT-FIT reveals diversity in responses to salinity. Plant Physiol. 166: 1387–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julkowska M.M., Testerink C. (2015). Tuning plant signaling and growth to survive salt. Trends Plant Sci. 20: 586–594. [DOI] [PubMed] [Google Scholar]
- Kang J.S., et al. (2008). Salt tolerance of Arabidopsis thaliana requires maturation of N-glycosylated proteins in the Golgi apparatus. Proc. Natl. Acad. Sci. USA 105: 5933–5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D.R. (1992). The relationship of cells to organisms in plants: Problem and implications of an organismal perspective. Int. J. Plant Sci. 153: S28–S37. [Google Scholar]
- Kobayashi A., Takahashi A., Kakimoto Y., Miyazawa Y., Fujii N., Higashitani A., Takahashi H. (2007). A gene essential for hydrotropism in roots. Proc. Natl. Acad. Sci. USA 104: 4724–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn B.D. (2016). Cell wall-associated kinases and pectin perception. J. Exp. Bot. 67: 489–494. [DOI] [PubMed] [Google Scholar]
- Kohorn B.D., Johansen S., Shishido A., Todorova T., Martinez R., Defeo E., Obregon P. (2009). Pectin activation of MAP kinase and gene expression is WAK2 dependent. Plant J. 60: 974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn B.D., Kobayashi M., Johansen S., Riese J., Huang L.-F., Koch K., Fu S., Dotson A., Byers N. (2006). An Arabidopsis cell wall-associated kinase required for invertase activity and cell growth. Plant J. 46: 307–316. [DOI] [PubMed] [Google Scholar]
- Kohorn B.D., Kohorn S.L. (2012). The cell wall-associated kinases, WAKs, as pectin receptors. Front. Plant Sci. 3: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komis G., Apostolakos P., Galatis B. (2001). Altered patterns of tubulin polymerization in dividing leaf cells of Chlorophyton comosum after a hyperosmotic treatment. New Phytol. 149: 193–207. [DOI] [PubMed] [Google Scholar]
- Komis G., Apostolakos P., Galatis B. (2002). Hyperosmotic stress induces formation of tubulin macrotubules in root-tip cells of Triticum turgidum: their probable involvement in protoplast volume control. Plant Cell Physiol. 43: 911–922. [DOI] [PubMed] [Google Scholar]
- Kramer P.J., Boyer J.S. (1995). Water Relations of Plants and Soils. (San Diego, CA: Elsevier Science; ). [Google Scholar]
- Kung C. (2005). A possible unifying principle for mechanosensation. Nature 436: 647–654. [DOI] [PubMed] [Google Scholar]
- Lally D., Ingmire P., Tong H.Y., He Z.H. (2001). Antisense expression of a cell wall-associated protein kinase, WAK4, inhibits cell elongation and alters morphology. Plant Cell 13: 1317–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenus J., Goh T., Roberts I., Guyomarc’h S., Lucas M., De Smet I., Fukaki H., Beeckman T., Bennett M., Laplaze L. (2013). Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci. 18: 450–458. [DOI] [PubMed] [Google Scholar]
- LeNoble M.E., Spollen W.G., Sharp R.E. (2004). Maintenance of shoot growth by endogenous ABA: genetic assessment of the involvement of ethylene suppression. J. Exp. Bot. 55: 237–245. [DOI] [PubMed] [Google Scholar]
- Leung J., Merlot S., Giraudat J. (1997). The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9: 759–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., et al. (2015). Glycosylphosphatidylinositol-anchored proteins as chaperones and co-receptors for FERONIA receptor kinase signaling in Arabidopsis. eLife 4: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner H., Müller L.M., Boisson-Dernier A., Grossniklaus U. (2012). CrRLK1L receptor-like kinases: not just another brick in the wall. Curr. Opin. Plant Biol. 15: 659–669. [DOI] [PubMed] [Google Scholar]
- Lou Q., Chen L., Mei H., Wei H., Feng F., Wang P., Xia H., Li T., Luo L. (2015). Quantitative trait locus mapping of deep rooting by linkage and association analysis in rice. J. Exp. Bot. 66: 4749–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü P., Kang M., Jiang X., Dai F., Gao J., Zhang C. (2013). RhEXPA4, a rose expansin gene, modulates leaf growth and confers drought and salt tolerance to Arabidopsis. Planta 237: 1547–1559. [DOI] [PubMed] [Google Scholar]
- Lynch J.P. (2013). Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Ann. Bot. (Lond.) 112: 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J.P., Chimungu J.G., Brown K.M. (2014). Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. J. Exp. Bot. 65: 6155–6166. [DOI] [PubMed] [Google Scholar]
- Maurel C., Verdoucq L., Luu D.-T., Santoni V. (2008). Plant aquaporins: membrane channels with multiple integrated functions. Annu. Rev. Plant Biol. 59: 595–624. [DOI] [PubMed] [Google Scholar]
- Mauseth J.D. (2008). An Introduction to Plant Biology, 4th ed. (Burlington, MA: Jones and Bartlett Learning; ). [Google Scholar]
- McFarlane H.E., Döring A., Persson S. (2014). The cell biology of cellulose synthesis. Annu. Rev. Plant Biol. 65: 69–94. [DOI] [PubMed] [Google Scholar]
- Miyazaki S., Murata T., Sakurai-Ozato N., Kubo M., Demura T., Fukuda H., Hasebe M. (2009). ANXUR1 and 2, sister genes to FERONIA/SIRENE, are male factors for coordinated fertilization. Curr. Biol. 19: 1327–1331. [DOI] [PubMed] [Google Scholar]
- Miyazawa Y., Takahashi A., Kobayashi A., Kaneyasu T., Fujii N., Takahashi H. (2009). GNOM-mediated vesicular trafficking plays an essential role in hydrotropism of Arabidopsis roots. Plant Physiol. 149: 835–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen G.B., Gilroy S. (2009). Feeling green: mechanosensing in plants. Trends Cell Biol. 19: 228–235. [DOI] [PubMed] [Google Scholar]
- Moore J.P., Vicré-Gibouin M., Farrant J.M., Driouich A. (2008). Adaptations of higher plant cell walls to water loss: drought vs desiccation. Physiol. Plant. 134: 237–245. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., et al. (2007). Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc. Natl. Acad. Sci. USA 104: 3639–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M., Kaneko Y., Miyazawa Y., Fujii N., Higashitani N., Wada S., Ishida H., Yoshimoto K., Shirasu K., Yamada K., Nishimura M., Takahashi H. (2012). A possible involvement of autophagy in amyloplast degradation in columella cells during hydrotropic response of Arabidopsis roots. Planta 236: 999–1012. [DOI] [PubMed] [Google Scholar]
- Nari J., Noat G., Ricard J. (1991). Pectin methylesterase, metal ions and plant cell-wall extension. Hydrolysis of pectin by plant cell-wall pectin methylesterase. Biochem. J. 279: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nèmec B. (1900). Über die Art der Wahrnehmung des Schwerkraftreizes bei den Pflanzen. Berichte der Deutschen Botanischen Gesellschaft 18: 241.
- Nick P. (2013). Microtubules, signalling and abiotic stress. Plant J. 75: 309–323. [DOI] [PubMed] [Google Scholar]
- Nobel P.S., Sanderson J. (1984). Rectifier-like activities of roots of two desert succulents. J. Exp. Bot. 35: 727–737. [Google Scholar]
- Nord E.A., Lynch J.P. (2009). Plant phenology: a critical controller of soil resource acquisition. J. Exp. Bot. 60: 1927–1937. [DOI] [PubMed] [Google Scholar]
- Oparka K.J. (1994). Plasmolysis: new insights into an old process. New Phytol. 126: 571–591. [Google Scholar]
- Orman-Ligeza B., Parizot B., Gantet P.P., Beeckman T., Bennett M.J., Draye X. (2013). Post-embryonic root organogenesis in cereals: branching out from model plants. Trends Plant Sci. 18: 459–467. [DOI] [PubMed] [Google Scholar]
- Paredez A.R., Somerville C.R., Ehrhardt D.W. (2006). Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312: 1491–1495. [DOI] [PubMed] [Google Scholar]
- Park A.R., Cho S.K., Yun U.J., Jin M.Y., Lee S.H., Sachetto-Martins G., Park O.K. (2001). Interaction of the Arabidopsis receptor protein kinase Wak1 with a glycine-rich protein, AtGRP-3. J. Biol. Chem. 276: 26688–26693. [DOI] [PubMed] [Google Scholar]
- Passioura J.B. (1979). Accountability, philosophy and plant physiology. Search 10: 347–350. [Google Scholar]
- Peaucelle A., Braybrook S., Höfte H. (2012). Cell wall mechanics and growth control in plants: the role of pectins revisited. Front. Plant Sci. 3: 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux J., Rustérucci C., Mellerowicz E.J. (2007). New insights into pectin methylesterase structure and function. Trends Plant Sci. 12: 267–277. [DOI] [PubMed] [Google Scholar]
- Plieth C., Trewavas A.J. (2002). Reorientation of seedlings in the earth’s gravitational field induces cytosolic calcium transients. Plant Physiol. 129: 786–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellán-Álvarez R., et al. (2015). GLO-Roots: an imaging platform enabling multidimensional characterization of soil-grown root systems. eLife 4: 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellán-Álvarez R., Lobet G., Dinneny J.R. (2016). Environmental control of root system biology. Annu. Rev. Plant Biol. 67: 619–642. [DOI] [PubMed] [Google Scholar]
- Robbins N.E. II, Dinneny J.R. (2015). The divining root: moisture-driven responses of roots at the micro- and macro-scale. J. Exp. Bot. 66: 2145–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers E.D., Benfey P.N. (2015). Regulation of plant root system architecture: implications for crop advancement. Curr. Opin. Biotechnol. 32: 93–98. [DOI] [PubMed] [Google Scholar]
- Rosquete M.R., von Wangenheim D., Marhavý P., Barbez E., Stelzer E.H.K., Benková E., Maizel A., Kleine-Vehn J. (2013). An auxin transport mechanism restricts positive orthogravitropism in lateral roots. Curr. Biol. 23: 817–822. [DOI] [PubMed] [Google Scholar]
- Rotman N., Rozier F., Boavida L., Dumas C., Berger F., Faure J.-E. (2003). Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr. Biol. 13: 432–436. [DOI] [PubMed] [Google Scholar]
- Rundel P.W., Nobel, P.S., Atkinson D. (1991). Structure and function in desert root systems. In Plant Root Growth: An Ecological Perspective, D. Atkinson, ed (Oxford, UK: Blackwell Scientific Publications), pp. 349-378. [Google Scholar]
- Saab I.N., Sharp R.E., Pritchard J., Voetberg G.S. (1990). Increased endogenous abscisic acid maintains primary root growth and inhibits shoot growth of maize seedlings at low water potentials. Plant Physiol. 93: 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salguero-Gómez R., Casper B.B. (2011). Introducing short roots in a desert perennial: anatomy and spatiotemporal foraging responses to increased precipitation. New Phytol. 191: 173–183. [DOI] [PubMed] [Google Scholar]
- Sato E.M., Hijazi H., Bennett M.J., Vissenberg K., Swarup R. (2015). New insights into root gravitropic signalling. J. Exp. Bot. 66: 2155–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo M., Ponce G., Campos M.E., Eapen D., García E., Luján R., Sánchez Y., Cassab G.I. (2012). An altered hydrotropic response (ahr1) mutant of Arabidopsis recovers root hydrotropism with cytokinin. J. Exp. Bot. 63: 3587–3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallus T., Fehér K., Sternberg U., Rybin V., Muhle-Goll C. (2010). Analysis of the specific interactions between the lectin domain of malectin and diglucosides. Glycobiology 20: 1010–1020. [DOI] [PubMed] [Google Scholar]
- Sebastian J., et al. (2016). Grasses suppress shoot-borne roots to conserve water during drought. Proc. Natl. Acad. Sci. USA 113: 8861–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabala S.N., Lew R.R. (2002). Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol. 129: 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp R.E., Davies W.J. (1979). Solute regulation and growth by roots and shoots of water-stressed maize plants. Planta 147: 43–49. [DOI] [PubMed] [Google Scholar]
- Sharp R.E., Hsiao T.C., Silk W.K. (1990). Growth of the maize primary root at low water potentials : II. Role of growth and deposition of hexose and potassium in osmotic adjustment. Plant Physiol. 93: 1337–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp R.E., Silk W.K., Hsiao T.C. (1988). Growth of the maize primary root at low water potentials : I. Spatial distribution of expansive growth. Plant Physiol. 87: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih H.-W., Miller N.D., Dai C., Spalding E.P., Monshausen G.B. (2014). The receptor-like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Curr. Biol. 24: 1887–1892. [DOI] [PubMed] [Google Scholar]
- Smith L.G., Hake S., Sylvester A.W. (1996). The tangled-1 mutation alters cell division orientations throughout maize leaf development without altering leaf shape. Development 122: 481–489. [DOI] [PubMed] [Google Scholar]
- Spollen W.G., LeNoble M.E., Samuels T.D., Bernstein N., Sharp R.E. (2000). Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiol. 122: 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T., Geldner N., Grebe M., Mangold S., Jackson C.L., Paris S., Gälweiler L., Palme K., Jürgens G. (1999). Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 316–318. [DOI] [PubMed] [Google Scholar]
- Steudle E., Peterson C.a. (1998). How does water get through roots? J. Exp. Bot. 49: 775–788. [Google Scholar]
- Sun F., Zhang W., Hu H., Li B., Wang Y., Zhao Y., Li K., Liu M., Li X. (2008). Salt modulates gravity signaling pathway to regulate growth direction of primary roots in Arabidopsis. Plant Physiol. 146: 178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup R., Kramer E.M., Perry P., Knox K., Leyser H.M.O., Haseloff J., Beemster G.T.S., Bhalerao R., Bennett M.J. (2005). Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nat. Cell Biol. 7: 1057–1065. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Yamazaki Y., Kobayashi A., Higashitani A., Takahashi H. (2003). Hydrotropism interacts with gravitropism by degrading amyloplasts in seedling roots of Arabidopsis and radish. Plant Physiol. 132: 805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M., Takahashi H., Suge H. (1997). Calcium requirement for the induction of hydrotropism and enhancement of calcium-induced curvature by water stress in primary roots of pea, Pisum sativum L. Plant Cell Physiol. 38: 385–391. [Google Scholar]
- Taramino G., Sauer M., Stauffer J.L. Jr., Multani D., Niu X., Sakai H., Hochholdinger F. (2007). The maize (Zea mays L.) RTCS gene encodes a LOB domain protein that is a key regulator of embryonic seminal and post-embryonic shoot-borne root initiation. Plant J. 50: 649–659. [DOI] [PubMed] [Google Scholar]
- Tron S., Bodner G., Laio F., Ridolfi L., Leitner D. (2015). Can diversity in root architecture explain plant water use efficiency? A modeling study. Ecol. Modell. 312: 200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga Y., et al. (2013). Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 45: 1097–1102. [DOI] [PubMed] [Google Scholar]
- Uga Y., Kitomi Y., Yamamoto E., Kanno N., Kawai S., Mizubayashi T., Fukuoka S. (2015). A QTL for root growth angle on rice chromosome 7 is involved in the genetic pathway of DEEPER ROOTING 1. Rice (N. Y.) 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Norman J.M., Xuan W., Beeckman T., Benfey P.N. (2013). To branch or not to branch: the role of pre-patterning in lateral root formation. Development 140: 4301–4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues P.E., Agarwal M., Katiyar-Agarwal S., Zhu J., Zhu J.-K. (2006). Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 45: 523–539. [DOI] [PubMed] [Google Scholar]
- Voetberg G.S., Sharp R.E. (1991). Growth of the maize primary root at low water potentials: III. Role of increased proline deposition in osmotic adjustment. Plant Physiol. 96: 1125–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade L.J., et al. (2015). Environmental response and genomic regions correlated with rice root growth and yield under drought in the OryzaSNP panel across multiple study systems. PLoS One 10: e0124127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.E., Jensen G.S., Haswell E.S. (2011). Two mechanosensitive channel homologs influence division ring placement in Arabidopsis chloroplasts. Plant Cell 23: 2939–2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Thorne E.T., Sharp R.E., Cosgrove D.J. (2001). Modification of expansin transcript levels in the maize primary root at low water potentials. Plant Physiol. 126: 1471–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Wang R.-G., Mao G., Koczan J.M. (2006). Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiol. 142: 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Cai X.-T., Wang Y., Xing L., Chen Q., Xiang C.-B. (2014). HDG11 upregulates cell-wall-loosening protein genes to promote root elongation in Arabidopsis. J. Exp. Bot. 65: 4285–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Zheng B., Mao C., Qi X., Liu F., Wu P. (2004). Analysis of transcripts that are differentially expressed in three sectors of the rice root system under water deficit. Mol. Genet. Genomics 272: 433–442. [DOI] [PubMed] [Google Scholar]
- Yoo S.-H., Fishman M.L., Savary B.J., Hotchkiss A.T. Jr (2003). Monovalent salt-induced gelation of enzymatically deesterified pectin. J. Agric. Food Chem. 51: 7410–7417. [DOI] [PubMed] [Google Scholar]
- Yuan F., et al. (2014). OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514: 367–371. [DOI] [PubMed] [Google Scholar]
- Zhong R., Burk D.H., Morrison W.H. III, Ye Z.-H. (2002). A kinesin-like protein is essential for oriented deposition of cellulose microfibrils and cell wall strength. Plant Cell 14: 3101–3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Lee B.-H., Dellinger M., Cui X., Zhang C., Wu S., Nothnagel E.A., Zhu J.-K. (2010). A cellulose synthase-like protein is required for osmotic stress tolerance in Arabidopsis. Plant J. 63: 128–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla G., Heimer Y.M., Barak S. (2010). Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J. Exp. Bot. 61: 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicke M., Picon-Cochard C., Morvan-Bertrand A., Prud’homme M.-P., Volaire F. (2015). What functional strategies drive drought survival and recovery of perennial species from upland grassland? Ann. Bot. (Lond.) 116: 1001–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]