Abstract
Background
Although it has been reported that hypoxic exposure can attenuate hypertension, heart disease, diabetes, and some other diseases, effects of hypoxia on osteoporosis are still unknown.
Material/Methods
The current study investigated whether short-term hypoxic exposure (in comparison with normoxic conditions) affects bone metabolism in normal or ovariectomized (OVX) adult female rats in an vivo study. Micro-computed tomography bone volume/structural analyses, histological examination, and serum bone turnover biochemical assays were used. In addition, the expressions of some associated major regulatory molecules were measured in osteoblastic cultures.
Results
While the 14-day hypoxic exposure did not change the bone-remodeling process in normal adult female rats, it decreased bone volume, osteoclast density, and serum bone formation marker (alkaline phosphatase) level, but increased osteoclast density and serum bone resorption marker (C-telopeptide of collagen) level in OVX rats. The bone marrow adipocyte number and serum fatty acid binding protein-4 level were increased in OVX-hypoxic rats compared with OVX-normoxic rats. Consistently, in human MG-63 osteoblastic cultures, the hypoxic condition suppressed protein expression of osteogenic transcriptional factors Runx2 and osterix, elevated protein expression of osteoclastogenic cytokine receptor activator of nuclear factor kappa-B ligand, but reduced that of osteoclastogenic inhibitor osteoprotegerin.
Conclusions
Our results suggest that, although no change occurred in the bone-remodeling process in normal adult female rats after hypoxic exposure, under the estrogen-deficient osteoporotic condition, the hypoxic condition can alter the bone microenvironment so that it may further impair osteoblastic differentiation and enhance osteoclastic formation, and thus reduce bone formation, enhance bone resorption, and accelerate bone loss.
MeSH Keywords: Cell Dedifferentiation, Cell Hypoxia, Osteoblasts, Osteoclasts, Osteoporosis
Background
The hypoxic condition was considered as the primary component of a high-altitude environment in past decades, which is known to lead physical and pathological changes in mammals [1]. It has been widely reported that hypoxic exposure can attenuate hypertension, heart disease, diabetes, and respiratory diseases, as well as central nervous system diseases [2,3]. However, despite these potential beneficial effects of the hypoxic condition, no studies to date have investigated the effects of hypoxia on osteoporosis.
Osteoporosis is the most common metabolic skeletal disease worldwide and is characterized by a reduction in bone mass and bone strength, and an increase in fracture risk with great morbidity [4,5]. Although multiple mechanisms have been implicated to be involved in osteoporosis, menopause and age-related bone loss have been the major causes of primary osteoporosis [6,7]. Estrogen deficiency is considered to be the most important contributing factor in the pathological process of osteoporosis [8], and more than 90% of the osteoporosis-related morbidity worldwide is observed in postmenopausal women more than 60 years old [9]. After menopause, bone resorption (carried out by resorptive cell-osteoclasts) outpaces bone formation (carried out by bone-forming cell-osteoblasts) in the bone-remodeling process.
Recent in vitro studies reported that the hypoxic condition reduced osteoblastogenesis via inhibition of phosphatidylinositol 3-kinase (PI3K)/Akt signal pathway [10,11]. However, no evidence has been presented so far indicating whether hypoxic exposure impairs the differentiation of osteoblasts and affects bone metabolism in vivo either with or without the postmenopausal state. The current study investigated whether hypoxic exposure (in comparison to normoxic conditions) affects bone metabolism in normal adult rats or in ovariectomized (OVX) rats and expression of some associated major regulatory molecules in osteoblastic cultures.
Material and Methods
Animals and OVX/hypoxic models
Thirty-seven female Sprague Dawley (SD) rats (12 weeks old) were purchased from the Experimental Animal Center of Sun Yat-Sen University (Guangzhou, China) and were housed under specific pathogen-free (SPF) conditions with day and night cycle of 12 h each. The temperature and humidity were maintained at 25±3°C and 65±5%, respectively. Thirteen rats were randomly separated into the hypoxic group (n=7) and the control normoxic group (n=6) without any operation. Following the separation, the hypoxic conditioning was initiated in the hypoxic group. Meanwhile, the other 24 female rats underwent an ovariectomy after being anesthetized via intraperitoneal injection of pentobarbital sodium-phosphate-buffered saline (PBS) solution with the dose of 30 mg/kg body weight at 12 weeks of age according to the classic protocol [12]. Four weeks after the operation, the OVX rats were randomly separated into hypoxic (n=12) or normoxic (n=12) groups. Exposure of rats to the hypoxic and normoxic conditions was performed according to the classic protocol for experimental high-altitude imitation [13]. No animal died in all the experimental processes.
After hypoxic or normoxic treatment for 14 days, rats were anesthetized via intraperitoneal injection of pentobarbital sodium-PBS solution with the dose of 30 mg/kg body weight and were sacrificed for collecting blood samples and harvesting femur samples for various analyses described below. Blood samples were collected from the dorsal aorta and were centrifuged at 4000 r/min for 6 min (4°C) to obtain serum. After centrifugation, serum samples were stored at −80°C.
Serum CTX, FABP-4, and ALP measurements
Serum bone turnover markers were analyzed by using rat enzyme-linked immunosorbent assay (ELISA) kits of C-telopeptide of collagen (CTX, a marker for bone resorption), alkaline phosphatase (ALP, a marker for bone formation), and fatty acid binding protein-4 (FABP-4, a marker for fatty acid metabolism and metabolic syndrome) (all purchased from BoHua Biotech, Shanghai, China) to examine treatment systemic effects on bone remodeling. Assays were carried out as instructed with absorbance being measured at 450 nm using an EL800 automated microplate reader (Bio-Tek Instruments, Winooski, Vermont, USA). The resulting concentrations of CTX (ng/mL), FABP-4 (ng/mL), and ALP (U/L) were measured using the respective standard curves of the assays.
Micro-computed tomography
To examine treatment effects on bone structure and volume, proximal femoral metaphyses (a region known to be most active in bone remodeling in rats) were analyzed by micro-computed tomography (μ-CT), using the ZKKS-MCT-Sharp-III scanner (Caskaisheng, Guangzhou, China). The femoral trabecular region was measured from 30 spongiosa slices (30 μm thick), and the growth plate was treated as the label to identify a consistent location to start the region of interest for analysis. The 3D-MED 3.0 software (Institute of Automation, Chinese Academy of Science, Xi’an, China) was used to measured the bone mineral density (BMD) and bone volume/tissue volume (BV/TV).
Histological analyses
For histological analyses, the right femurs were decalcified by glycerinum-EDTA (10% quality) for 12 weeks after fixation, dehydrated with concentrated ethanol, and embedded in paraffin wax after being washed with xylene; they then were cut into 5 μm sections. Femurs were cut sagittally at the distal femoral region and cut transversally at the mid-diaphyseal level. For examining general bone morphology and measuring densities of osteoblasts and bone marrow adipocytes, sections were dewaxed and rehydrated, and then were subjected to routine hematoxylin and eosin (H&E) staining. According to the classic method [14], the trabecular bone surface-adhered cuboidal cells whose nuclei were co-stained with hematoxylin were counted as osteoblasts (cells/mm2 trabecular perimeter) in the secondary spongiosa. Meanwhile, the bone marrow adipose stacks whose nuclei were co-stained with hematoxylin were counted as adipose cells in the lower secondary spongiosa (cells/mm2 bone marrow area). For identification of osteoclasts, one set of sections were stained for tartrate-resistant acid phosphatase (TRAP, a marker of osteoclasts) using the TRAP staining kit (Sigma-Aldrich, California, USA) and counter-stained with methyl green. In TRAP-stained sections, the irregular red cells whose nuclei were co-stained in green were counted in the secondary spongiosa as osteoclasts (cells/mm2 trabecular perimeter). Numbers of the above types of cells were counted by three volunteers in four fields randomly selected (400×) under blinding using ImagePro 4.5 software (Leeds Precision Instruments, Minneapolis, Minnesota, USA).
Hypoxic exposure of MG-63 osteoblastic cells and cell viability
To examine the effects of hypoxic exposure on osteoblasts in vitro, human osteosarcoma-derived osteoblastic MG-63 cells were cultured in 75 cm2 flasks for 3 days in Dulbecco’s Modified Eagle Medium (DMEM, high glucose) supplemented with 10% fetal calf serum, penicillin 100 U/mL, and streptomycin 100 mg/mL. Confluent cultures were trypsinized, passaged, and sub-cultured prior to being subjected to hypoxic/normoxic treatment. Hypoxic/normoxic conditions were applied after detached MG-63 cells were transplanted into six-well plates (2×105 cells/well) and cultured under normal conditions for 12 hours. For the hypoxic condition, MG-63 cells were gassed with 95% N2 and 5% CO2 at 37°C for 1 h. Then cells were transferred into a normoxic incubator (95% air, 5% CO2) for an additional 12 h for re-oxygenation.
To examine potential hypoxic treatment effects on cell viability, a MTT assay was carried out. Following exposure to hypoxic or normoxic condition in vitro, 1×104 MG-63 cells were plated in a 96-well plate (150 μL of medium/well), then incubated at 37°C for 12 hours. Then, 20 μL of 5 mg/mL MTT solution in PBS was added to each well and mixed for 5 minutes prior to being incubated (37°C, 5% CO2), allowing MTT incorporation for 3 to 4 hours. Following incubation, the medium was discarded and the wells were set to dry prior to formazan (MTT metabolic product) being thoroughly mixed and resuspended in 200 μL of DMSO. The optical density was measured at 560 nm after subtracting the background measurement at 670 nm.
Western blot analyses
To examine the treatment effects in osteoblasts on protein expression of major regulatory genes of bone formation and resorption, MG-63 osteoblastic cells were washed with cold PBS, then lysed on ice for 30 min with radio immunoprecipitation assay (RIPA) buffer. Then, protein samples (30 mg respectively) were separated on 8% sodium dodecyl sulfate (SDS) gels and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were incubated with rabbit-derived antibodies against receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegerin (OPG), Runt-related transcription factor 2 (RUNX-2), osterix, osteocalcin (1:1000; all from Santa Cruz Biotech, California, USA), and β-actin (1:1000; Bioworld Technology, St. Louis, Missouri, USA) after being blocked with dry skim milk-infused PBS buffer (4%) for 1 h. Following these steps, the membranes were incubated with peroxidase-conjugated secondary antibody (goat anti-rabbit or a goat anti-mouse; 1:1000; Cell Signaling Technology, Shanghai, China) for 1 h and then were visualized by enhanced chemiluminescence (ECL; Santa Cruz Biotech) using Kodak X-OMAT LS film (Eastman Kodak, Rochester, New York, USA) post PBS washing three times. All Western blot analyses in this study were repeated at least three times
Statistical analysis
Data were expressed as means ±SD, and one-way analysis of variance (ANOVA) was performed for the analysis of statistical significance. Representative experiments were presented in the result section and figures of this study. Statistical significance was achieved when P<0.05.
Results
Effects of hypoxic exposure on trabecular bone volume and microarchitecture
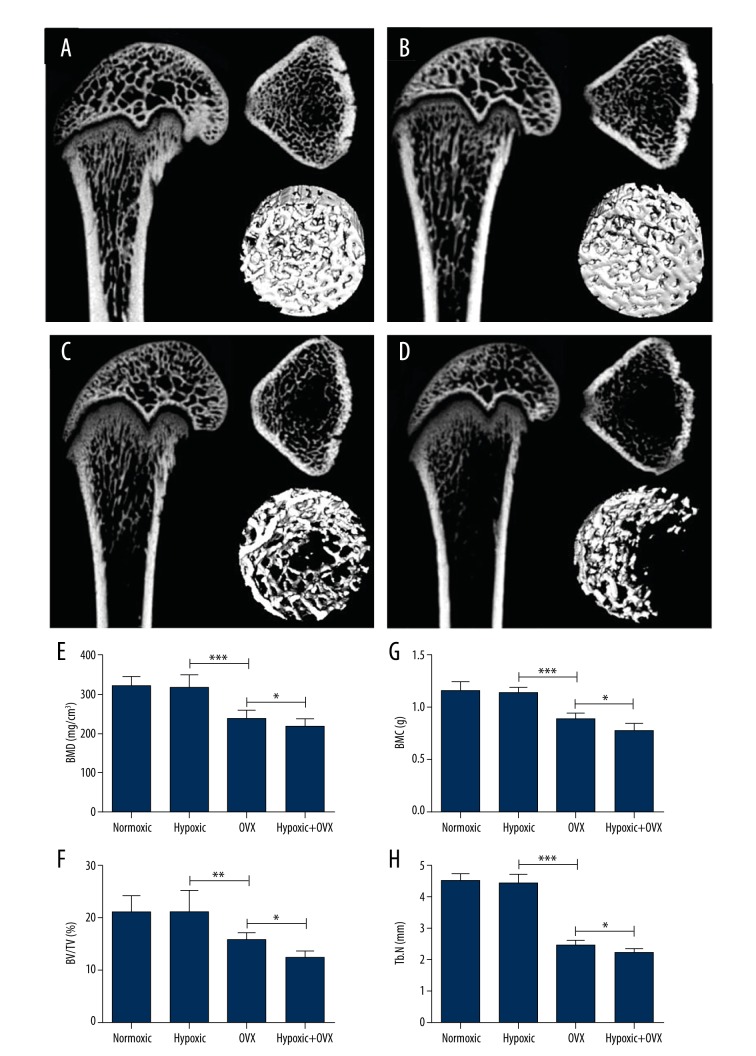
Short-term hypoxic exposure did not appear to affect trabecular bone structure and volume in the normal rats. After 14 days of hypoxia exposure, as analyzed by μ-CT, no statistical differences were observed in BMD, bone mineral content (BMC), BV/TV%, and trabecular number (Tb.N) between the hypoxic and normoxic groups (Figure 1A, 1B, 1E–1H).
Figure 1.
Micro-CT analyses of effects of 14-day hypoxic or normoxic exposure on femoral distal trabecular bone mineral density (BMD), bone mineral content (BMC), bone volume/tissue volume (BV/TV%), and trabecular number (Tb.N) in normal (A, B, E–H) or ovariectomized (OVX) rats (C–H). A single asterisk (*) represents P<0.05; a double asterisk (**) represents P<0.01; a triple asterisk (***) represents P<0.001.
However, hypoxic exposure for 14 days at 4 weeks after the ovariectomy procedure caused significant bone loss, although it did not affect the rate of body weight gain in OVX rats over 3 months following the ovariectomy procedure. Following the hypoxic exposure in OVX rats, as examined by μ-CT scanning, there was a significant enhanced bone loss based on measurements in BMD (P<0.05), BMC (P<0.05), BV/TV (P<0.05), and Tb.N (P<0.05) when compared with the normoxic OVX group (Figure 1C–1H). Meanwhile, compared with the normal hypoxic group, the significant reduction of BMD (P<0.001), BMC (P<0.001), BV/TV (P<0.001), and Tb.N (P<0.001) in the OVX group demonstrated the success of OVX-induced bone loss.
Treatment effects on osteoblast and osteoclast densities on trabecular bone surface as well as bone marrow adipocyte number
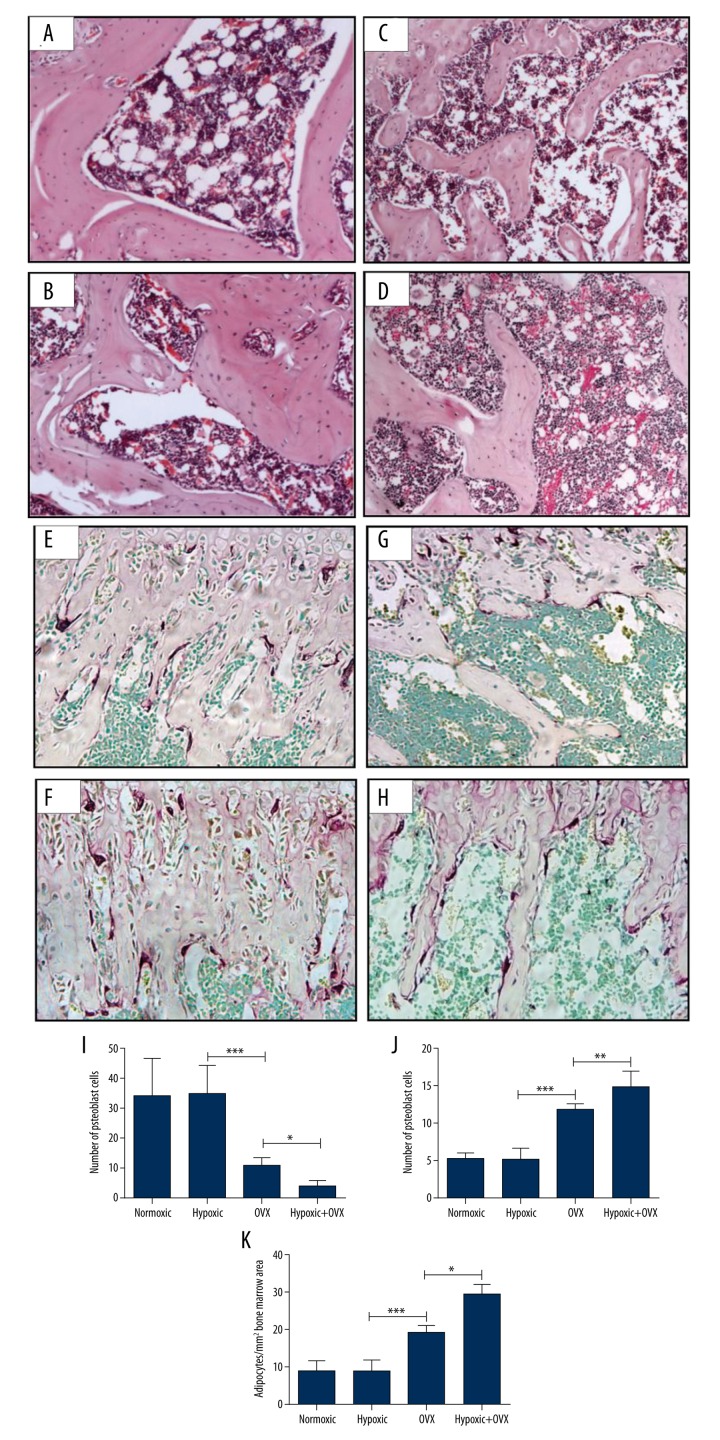
As shown in Figure 2, in normal rats, 14-day hypoxic treatment did not affect the osteoblast numbers (Figure 2A, 2B, 2I; P>0.05) and the osteoclast numbers (Figure 2E, 2F, 2J; P>0.05). However, in the OVX rats, the osteoblast number in the hypoxic group was statistically significantly lower than that in the normoxic group (Figure 2C, 2D, 2L; P<0.05), and the osteoclast number was significantly higher in the hypoxic OVX rats when compared with the normoxic OVX rats (Figure 2G, 2H, 2J; P<0.01). On the other hand, compared with the normal-hypoxic group, the significant enhancement of osteoclast number and reduction of osteoblast number in the OVX group demonstrated the success of OVX-induced bone loss.
Figure 2.
Effects of 14-day hypoxic or normoxic exposure on densities of osteoblasts and adipocytes (normoxic: A, C; hypoxic: B, D; statistical analysis: I, K; on H&E-stained sections, 400× magnification) and osteoclasts (normoxic: E, G; hypoxic: F, H; statistical analysis: J; on TRAP-stained sections, 400× magnification) on femoral distal trabecular bone surfaces in normal or ovariectomized (OVX) rats. A single asterisk (*) represents P<0.05; a double asterisk (**) represents P<0.01; a triple asterisk (***) represents P<0.001.
The numbers of adipose cells were counted on the H&E-stained sections at the femur bone marrow (at the lower secondary spongiosa region). While there were no significant differences in bone marrow adipocyte density in the normal rats between the hypoxic group and the normoxic group (Figure 2A, 2B, 2K), there was a higher density of bone marrow adipocytes in hypoxic OVX rats compared with normoxic OVX rats (Figure 2C, 2D, 2K; P<0.001). Therefore, the short-term hypoxic condition could influence the osteogenesis-related adipose metabolism in OVX rats, and this influence may be an estrogen-dependent process. As expected, the adipocyte density in the OVX normoxic rats was higher than that in the normal normoxic rats.
Serum biochemical analyses of bone and adipose metabolism-related biomarkers
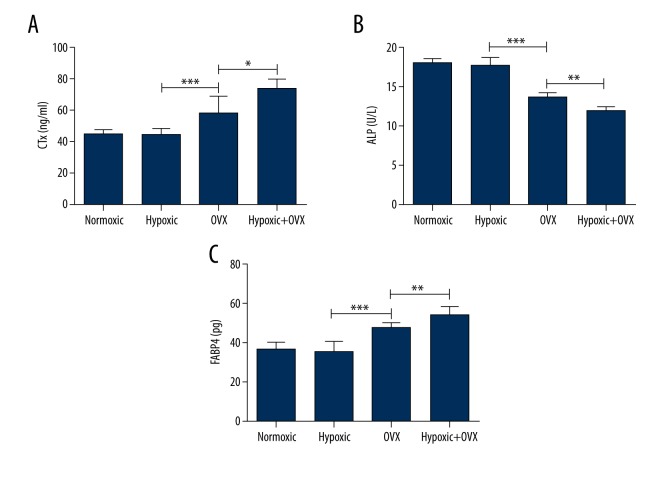
To examine treatment systemic effects on bone turnover and fat metabolism, serum levels of bone resorption marker CTX, bone formation marker ALP, and fatty acid metabolism and metabolic syndrome marker FABP-4 were analyzed. In the normal rats, no differences in serum levels of CTX, ALP, and FABP-4 were observed between the hypoxic and normoxic groups (Figure 3A–3C). However, in OVX rats, hypoxic exposure significantly increased serum levels of CTX and FABP-4 and decreased serum ALP level (Figure 3A–3C). Simultaneously, compared with the normal hypoxic group, the significant enhancements of CTX and FABP-4 levels and reduction of ALP level in the OVX group demonstrated the success of OVX-induced bone loss. These data indicated that a short-term hypoxic exposure did not alter the skeletal metabolism in normal rats but could accelerate bone loss in OVX rats.
Figure 3.
Effects of 14-day hypoxic or normoxic exposure on serum levels of C-telopeptide of collagen (CTX), alkaline phosphatase (ALP), and fatty acid binding protein-4 (FABP-4) in normal or ovariectomized (OVX) (A–C) rats. A single asterisk (*) represents P<0.05; a double asterisk (**) represents P<0.01; a triple asterisk (***) represents P<0.001.
Effects of hypoxic exposure in osteoblastic cultures on expression of proteins regulating osteoblast and osteoclast formation
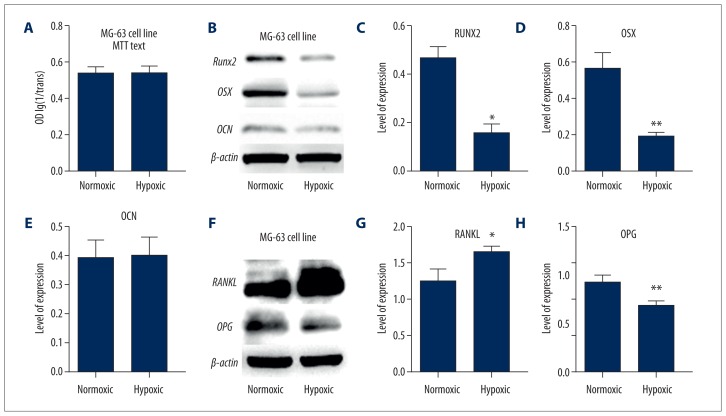
To investigate some potential mechanisms for hypoxia-induced changes in bone/fat metabolism, treatment effects of hypoxia on cultured MG-63 osteoblastic cells were examined. A MTT cell viability test showed that there were no significant differences in cell viability between the hypoxic and normoxic groups of cells (Figure 4A). Through Western blotting analyses, treatment effects were examined on the expression levels of osteoblast differentiation markers (Runx2, OSX, and OCN) in MG-63 cells following hypoxic exposure (Figure 4B). While OCN expression in MG-63 cells was non-significantly different between the hypoxic condition and the normal condition, the levels of Runx2 and OSX expression were lower in the cells exposed to the hypoxic condition compared with the normoxic condition (P<0.05 and 0.01, respectively). These data suggest that osteoblastic differentiation may be inhibited by hypoxic exposure. Furthermore, the expression of RANKL in MG-63 cells was significantly higher and the OPG level significantly lower after hypoxia exposure when compared with normal culture (Figure 4C).
Figure 4.
Effects of hypoxic or normoxic exposure on cell viability as examined by MTT assays (A) and protein expression of osteogenesis-related molecules (Runx2, OSX, and OCN) (B–E), as well as osteoclastogenesis-regulatory molecules (RANKL and OPG) (F–H) as analyzed by Western blot analyses (with the internal loading control β-actin) in cultured osteoblastic MG-63 cells. Statistical analyses of Western blot test results. A single asterisk (*) represents P<0.05; a double asterisk (**) represents P<0.01; a triple asterisk (***) represents P<0.001.
Discussion
Currently, potential effects of high-altitude living (e.g., highland residents) and hypoxia exposure on the morbidity of osteoporosis are not exactly understood. Recently, Bozzini et al. reported the effects of different simulated high altitudes (1850, 2900, 4100, and 5450m) on femur biomechanical properties in female growing rats that were exposed to the simulated high altitudes for more than 22 hours daily for 42 days [15]. It was found that, due to a smaller bone mass, the long bones of the hypoxia-exposed rats were weaker than those of normoxia controls [15]. In the present study, we investigated the effects of short-term hypoxia, representative of a transient or non-sustained high-altitude environment, on bone metabolism in both normal and OVX rats and in vitro MG-63 osteoblastic cells. This study showed that the short-term hypoxic exposure did not change the bone-remodeling process in normal adult female rats; however, it decreased bone formation and increased bone resorption, and thus increased bone loss and enhanced bone marrow adipose tissue accumulation, in estrogen-deficient OVX rats. Furthermore, the transient hypoxic culture impaired the osteoblastogenesis and enhanced expression of the osteoclastogenic signal (increased RANKL but OPG levels) of MG-63 osteoblasts in vitro. The post-hypoxic condition osteoclastogenic enhancement in our study is consistent with the past study of human osteoclasts that underwent hypoxia exposure [16]. Therefore, these data indicate that short-term hypoxic exposure may accelerate bone loss in OVX rats or in postmenopausal women with osteoporosis.
In this study, bone loss was shown to be enhanced in OVX rats by short-term hypoxic exposure, which was accompanied by an increase in the number of bone-resorptive osteoclasts and a decrease in the number of bone-forming osteoblasts on trabecular bone surfaces. Although OVX models were performed for more than 8 weeks in most studies, the hypoxic exposure in our study was measured at 4 weeks post OVX to avoid severe and irreversible bone loss of rats [17]. In addition, there was an increased serum level of the bone resorption marker CTX but a decreased serum level of the bone formation marker ALP, as well as an increase in adipocyte density in the bone marrow, following hypoxic exposure in OVX rats. While these changes were not observed in normal rats after hypoxic exposure, this suggests that imbalanced bone remodeling (decreased bone formation and increased bone resorption) caused by hypoxia occurs in female rats when estrogen is deficient. This can imply that short-term hypoxic exposure in postmenopausal women may cause accelerated bone loss.
As multiple signal pathways are involved in the bone remodeling system, the factors contributing to the changes in bone metabolism as a result of hypoxia still need to be investigated [10]. The hypoxic microenvironment has been considered as a vital factor for the hypoxia-induced factor (HIF-1)-dependent angiogenesis that is critical for bone formation during bone development and regeneration after trauma [18,19]. Consistently, bone marrow stromal cell differentiation and osteoblastogenesis can be inhibited by hypoxic culture in vitro [20]. However, potential molecular mechanisms for hypoxic exposure-induced bone loss in OVX rats remain to be clarified in future studies. In addition, although it has been reported in the past decades that hypoxia can increase adipogenesis, no clear evidence has indicated that the adipogenesis in bone marrow can be modulated by hypoxic exposure in vivo [21].
As a means to investigate potential mechanisms of the impact of hypoxia on bone remodeling, the current study also examined hypoxic-exposure effects on osteoblast viability and expression of genes regulating osteoblastogenesis and osteoclastogenesis in treated MG-63 osteoblastic culture. While recent studies have shown that the hypoxic condition could damage the cell cycle of osteoblasts and block their proliferation, the current study showed that the hypoxic exposure did not affect osteoblast viability as measured by MTT assays [11]; this contradiction may be because the vitro hypoxia imitation in our study was a mild and short-term exposure (5% O2 for 1 hour). Although the terminal biomarker of osteogenesis, osteocalcin, showed low expression with both hypoxic exposure and normal culture in vitro due to the limited culture time, our study demonstrated that the hypoxic condition suppressed protein expression of osteogenic biomarkers including transcriptional factors Runx2 and OSX [22], suggesting that hypoxic treatment of osteoblastic culture can reduce osteoblast differentiation. This finding is consistent with the recent study where osteoblastic differentiation was shown to be inhibited by the hypoxic condition [20].
In addition, as osteoclastogenesis is modulated by osteoblasts through the RANK/RANKL/OPG signal pathway [8], this study measured the expression of the key osteoclastogenic cytokine RANKL and the major osteoclastogenic inhibitor OPG in osteoblasts exposed to hypoxic conditions in vitro. We showed that the treated osteoblastic culture had elevated protein expression of RANKL but a reduced level of OPG, suggesting that hypoxia-treated osteoblasts have a higher ability to induce osteoclastic differentiation and formation and osteoclastic activity. These results suggest that a high-altitude environment may impair osteoblastic differentiation and enhance osteoclastic formation. In addition, while it has been demonstrated that estrogen can induce the apoptosis of osteoclasts and estrogen deficiency could stimulate osteoclastogenesis in OVX rats [23,24], potential interaction of estrogen action and the RANK/RANKL/OPG system in osteoclastic over-formation under the hypoxic condition remains to be investigated. Further studies are required to verify whether the hypoxia-induced accelerated bone loss in OVX rats may be due to the reduced differentiation of osteoblasts and over-formation and/or activation of osteoclasts resulting from higher level RANKL but lower level OPG in osteoblasts.
On the other hand, the imbalance of BMSC differentiation is considered to be a major factor in menopausal osteoporosis; the increased fat mass in the bone marrow has been shown to be associated with a reduction in bone mass, and this bone loss and bone marrow adiposity inverse relationship has been demonstrated [25,26]. In our results, consistent with recent investigations, the fat mass was elevated in OVX rats; furthermore, the hypoxic exposure also enhanced the adipogenesis of bone marrow significantly in OVX rats. Although these results suggest that the hypoxic condition may have altered the balance of adipose/osteoblast differentiation of bone marrow MSCs and resulted in bone loss and adipogenesis, deeper elaboration of a mechanism is still needed. Simultaneously, consistent with our results, a recent report suggested that bone marrow adipocytes influenced the coupling of osteoblast and osteoclast differentiation, and may be relevant to bone-loss disorders [27].
Conclusions
This study observed an enhanced bone loss in OVX rats (accompanied by reduced bone formation, increased resorption, and bone marrow adiposity) following hypoxic exposure. These findings suggest that high-altitude environments may play a harmful role in bone metabolism and contribute to the development/progression of postmenopausal osteoporosis. Although our in vitro studies suggest that the hypoxic condition can suppress osteogenic differentiation and enhance osteoclastic formation, further studies are required to investigate whether it is so under an estrogen-deficient culture condition, and future work is required to study potential mechanisms for the enhanced bone loss in rats with estrogen deficiency.
Footnotes
Source of support: This study was partly supported by The Science Foundation of Guangdong No. 2 Provincial People’s Hospital (YQ2015-006). LW is supported by a NHMRC Postgraduate Research Scholarship grant. CJX is supported by an Australia NHMRC Senior Research Fellowship
Declaration of interests
The authors declare that there are no conflict of interests regarding the publication of this paper.
References
- 1.Xu C, Dong C, Xu C, et al. Effect of iron supplementation on the expression of hypoxia-inducible factor and antioxidant status in rats exposed to high-altitude hypoxia environment. Biol Trace Elem Res. 2014;162(1–3):142–52. doi: 10.1007/s12011-014-0166-6. [DOI] [PubMed] [Google Scholar]
- 2.Czerniczyniec A, La Padula P, Bustamante J, et al. Mitochondrial function in rat cerebral cortex and hippocampus after short- and long-term hypobaric hypoxia. Brain Res. 2015;1598:66–75. doi: 10.1016/j.brainres.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 3.Qiao Y, Liu Z, Yan X, et al. Effect of intermittent hypoxia on neuro-functional recovery post brain ischemia in mice. J Mol Neurosci. 2015;55(4):923–30. doi: 10.1007/s12031-014-0447-8. [DOI] [PubMed] [Google Scholar]
- 4.Cao J, Ou G, Yang N, et al. Impact of targeted PPARgamma disruption on bone remodeling. Mol Cell Endocrinol. 2015;410:27–34. doi: 10.1016/j.mce.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abrahamsen B, Osmond C, Cooper C. Life expectancy in patients treated for osteoporosis: observational cohort study using national danish prescription data. J Bone Miner Res. 2015;30(9):1553–59. doi: 10.1002/jbmr.2478. [DOI] [PubMed] [Google Scholar]
- 6.Godfrin-Valnet M, Khan KA, Guillot X, et al. Sirtuin 1 activity in peripheral blood mononuclear cells of patients with osteoporosis. Med Sci Monit Basic Res. 2014;20:142–45. doi: 10.12659/MSMBR.891372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien KR, Karsenty G. Longevity and lineages: toward the integrative biology of degenerative diseases in heart, muscle, and bone. Cell. 2005;120(4):533–44. doi: 10.1016/j.cell.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–42. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 9.Harada S, Rodan GA. Control of osteoblast function and regulation of bone mass. Nature. 2003;423(6937):349–55. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 10.Zou W, Yang S, Zhang T, et al. Hypoxia enhances glucocorticoid-induced apoptosis and cell cycle arrest via the PI3K/Akt signaling pathway in osteoblastic cells. J Bone Miner Metab. 2015;33(6):615–24. doi: 10.1007/s00774-014-0627-1. [DOI] [PubMed] [Google Scholar]
- 11.Ma HP, Ma XN, Ge BF, et al. Icariin attenuates hypoxia-induced oxidative stress and apoptosis in osteoblasts and preserves their osteogenic differentiation potential in vitro. Cell Prolif. 2014;47(6):527–39. doi: 10.1111/cpr.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun D, Zheng X, Chen Y, et al. Enhancement of osteogenesis post-splenectomy does not attenuate bone loss in ovariectomized rats. J Orthop Res. 2015;33(9):1356–63. doi: 10.1002/jor.22825. [DOI] [PubMed] [Google Scholar]
- 13.Zhu XH, Yan HC, Zhang J, et al. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci. 2010;30(38):12653–63. doi: 10.1523/JNEUROSCI.6414-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King TJ, Shandala T, Lee AM, et al. Potential effects of phytoestrogen genistein in modulating acute methotrexate chemotherapy-induced osteoclastogenesis and bone damage in rats. Int J Mol Sci. 2015;16(8):18293–311. doi: 10.3390/ijms160818293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozzini C, Champin GM, Alippi RM, et al. Static biomechanics in bone from growing rats exposed chronically to simulated high altitudes. High Alt Med Biol. 2013;14(4):367–74. doi: 10.1089/ham.2013.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Utting JC, Flanagan AM, Brandao-Burch A, et al. Hypoxia stimulates osteoclast formation from human peripheral blood. Cell Biochem Funct. 2010;28(5):374–80. doi: 10.1002/cbf.1660. [DOI] [PubMed] [Google Scholar]
- 17.Govindarajan P, Schlewitz G, Schliefke N, et al. Implications of combined ovariectomy/multi-deficiency diet on rat bone with age-related variation in bone parameters and bone loss at multiple skeletal sites by DEXA. Med Sci Monit Basic Res. 2013;19:76–86. doi: 10.12659/MSMBR.883815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drager J, Harvey EJ, Barralet J. Hypoxia signalling manipulation for bone regeneration. Exp Rev Mol Med. 2015;17:e6. doi: 10.1017/erm.2015.4. [DOI] [PubMed] [Google Scholar]
- 19.Zhou N, Hu N, Liao JY, et al. HIF-1alpha as a regulator of BMP2-induced chondrogenic differentiation, osteogenic differentiation, and endochondral ossification in stem cells. Cell Physiol Biochem. 2015;36(1):44–60. doi: 10.1159/000374052. [DOI] [PubMed] [Google Scholar]
- 20.Sun G, Peng H. HIF-1alpha-induced microRNA-210 reduces hypoxia-induced osteoblast MG-63 cell apoptosis. Biosc Biotechnol Biochem. 2015;79(8):1232–39. doi: 10.1080/09168451.2014.1003128. [DOI] [PubMed] [Google Scholar]
- 21.Trayhurn P. Hypoxia and adipocyte physiology: Implications for adipose tissue dysfunction in obesity. Ann Rev Nutr. 2014;34:207–36. doi: 10.1146/annurev-nutr-071812-161156. [DOI] [PubMed] [Google Scholar]
- 22.Abdallah BM, Kassem M. New factors controlling the balance between osteoblastogenesis and adipogenesis. Bone. 2012;50(2):540–45. doi: 10.1016/j.bone.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 23.Marie PJ, Kassem M. Osteoblasts in osteoporosis: past, emerging, and future anabolic targets. Eur J Endocrinol. 2011;165(1):1–10. doi: 10.1530/EJE-11-0132. [DOI] [PubMed] [Google Scholar]
- 24.Yan X, Ye TW. Early molecular responses of bone to estrogen deficiency induced by ovariectomy in rats. Int J Clin Exp Med. 2015;8(4):5470–77. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Fan CM, Foster BK, Hui SK, et al. Prevention of bone growth defects, increased bone resorption and marrow adiposity with folinic acid in rats receiving long-term methotrexate. PloS One. 2012;7(10):e46915. doi: 10.1371/journal.pone.0046915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hui SK, Sharkey L, Kidder LS, et al. The influence of therapeutic radiation on the patterns of bone marrow in ovary-intact and ovariectomized mice. PloS One. 2012;7(8):e42668. doi: 10.1371/journal.pone.0042668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muruganandan S, Sinal CJ. The impact of bone marrow adipocytes on osteoblast and osteoclast differentiation. IUBMB Life. 2014 doi: 10.1002/iub.1254. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]