Abstract
Background
There is little data comparing catheter-directed thrombolysis (CDT) via small saphenous veins vs. systematic thrombolysis on complications and efficacy in acute deep venous thrombosis patients. The aim of our study was to compare the efficacy and safety of CDT via the small saphenous veins with systematic thrombolysis for patients with acute deep venous thrombosis (DVT).
Material/Methods
Sixty-six patients with acute DVT admitted from June 2012 to December 2013 were divided into 2 groups: 27 patients received systemic thrombolysis (ST group) and 39 patients received CDT via the small saphenous veins (CDT group). The thrombolysis efficiency, limb circumference differences, and complications such as post-thrombotic syndrome (PTS) in the 2 groups were recorded.
Results
The angiograms demonstrated that all or part of the fresh thrombus was dissolved. There was a significant difference regarding thrombolysis efficiency between the CDT group and ST group (71.26% vs. 48.26%, P=0.001). In both groups the postoperative limb circumference changes were higher compared to the preoperative values. The differences between postoperative limb circumferences on postoperative days 7 and 14 were significantly higher in the CDT group than in the ST group (all P<0.05). The incidence of postoperative PTS in the CDT group (17.9%) was significantly lower in comparison to the ST group (51.85%) during the follow-up (P=0.007).
Conclusions
Catheter-directed thrombolysis via the small saphenous veins is an effective, safe, and feasible approach for treating acute deep venous thrombosis.
MeSH Keywords: Thrombolytic Therapy, Vascular Access Devices, Venous Thrombosis
Background
Deep venous thrombosis (DVT), which occurs primarily in the lower extremities, is prevalent in modern society. It is caused by the abnormal coagulation of the blood in deep veins in the lower extremities due to various factors, such as stasis, hypercoagulability, and endothelial injury [1]. It can result into acute pulmonary embolism (PE) and long-term complications, such as post-thrombosis syndrome (PTS). The effect of PTS may lead to chronic pain in the affected limb, edema, pruritus, or even venous claudication, stasis dermatitis, and skin ulcers, which all significantly affect quality of life. The clinical efficacy and safety of previous classic therapies, such as simple anticoagulant therapy, surgical thrombectomy, and systemic thrombolysis (ST), are not satisfactory. A previous study found that about 40% of patients with thrombosis undergoing simple anticoagulant therapy will further progress [2]. Anticoagulation does not completely eliminate the acute thrombosis. With the progression of the thrombosis, the vascular lumen is only partially recanalized and the vascular valve is damaged, which impairs the valve function of preventing blood reflux and eventually progresses into PTS. These are the main causes of PTS. A previous study reported that patients undergoing simple anticoagulant therapy have a greater than 50% risk of PTS [3].
Catheter-directed thrombolysis (CDT) has good clinical efficacy in treatment of lower-extremity deep vein thrombosis (LDVT); it can significantly reduce the incidence of PTS and improve quality of life. Currently, the popliteal vein is the most commonly used approach vein [4]. However, this approach has some limitations, such as requiring special position and failing to lyse the middle and inferior segments of thrombosis in the popliteal vein. Some studies have investigated catheter-directed thrombolysis via small saphenous veins [5,6], but there is little information on whether there are advantages of CDT via small saphenous veins in terms of complications and efficacy compared with systematic thrombolysis. The aim of this study was to compare the efficacy and safety of CDT via the small saphenous veins with systematic thrombolysis for patients with acute DVT.
Material and Methods
Patients
The study was approved by the Institutional Review Board of the Jining First People’s Hospital. Written informed consent was obtained from each subject. All uses of the medications were approved by the Chinese State Food and Drug Administration. We retrospectively collected the clinical data of patients with unilateral mixed LDVT who were treated with CDT via the small saphenous veins or systematic thrombolysis at Jining First People’s Hospital between June 2012 and December 2013. LDVT patients were diagnosed by ultrasonography and clinical classification. Inclusion criteria were: 1) unilateral mixed LDVT diagnosed by color Doppler ultrasonography or venography; 2) age ≤70 years; 3) duration of disease ≤10 days; and 4) plasma D-dimer levels >0.5 mg/L. Exclusion criteria were: 1) central or peripheral thrombus; 2) age >70 years; 3) duration of disease ≤10 days; 4) plasma D-dimer levels ≤0.5 mg/L; 5) previous history of thrombolysis treatments before being admitted to our hospital; 6) contradictions for anticoagulation, thrombolysis, or contrast medium; and 7) liver or kidney function deficiency.
The indications for CDT were: central or mixed DVT in patients in good physical condition, expected survival time longer than 1 year, lower risk of bleeding, and a duration of disease no longer than 14 days. The indications for ST were proximal DVT in patients in good physical condition, expected survival time longer than 1 year, lower risk of bleeding, no clinical presentation of phlegmasia cerulea dolens, and a duration of disease no longer than 14 days.
The patients were divided into 2 groups according to therapeutic approach: 39 cases receiving CDT via the small saphenous veins (CDT group) and 27 cases receiving systematic thrombolysis (ST group). On admission, all patients presented with pitting edema and swelling pain in the affected limb, including tenderness and tension vesicle. The clinical information on the 2 groups is detailed in Table 1.
Table 1.
Clinical characteristics of patients in two groups.
| Group | CDT group (n=39) | ST group (n=27) | P |
|---|---|---|---|
| Age | 51.5±11.7 | 51.8±11.2 | 0.913 |
| Sex | 0.621 | ||
| Male | 22 | 17 | |
| Female | 17 | 10 | |
| Duration of disease (days) | 3.79±2.31 | 4.33±2.40 | 0.363 |
| Previous DVT | 0 | 0 | |
| Predisposing factors | 0.797 | ||
| No apparent cause | 13 (33.33%) | 10 (37.04%) | |
| Surgery | 7 (17.95%) | 6 (22.22%) | |
| Long term bedridden | 5 (12.82%) | 2 (7.41%) | |
| Trauma/fractures | 7 (17.94%) | 5 (18.51%) | |
| Postpartum | 4 (10.26%) | 3 (11.11%) | |
| Malignant tumour | 3 (7.69%) | 3 (11.11%) | |
| Antipsychotic medication | 2 (5.13%) | 1 (3.70%) | |
| Long term travelling | 1 (2.56%) | 0 (0%) | |
| Two or more factors | 5 (12.82%) | 3 (11.11%) |
DVT – deep venous thrombosis.
Therapeutic method
All the patients were on bed rest. After lifting the affected limbs to a 30° angle, a subcutaneous injection of 100 IU/kg of nadroparin calcium (Glaxo Smith Kline, Suzhou, China) was administered once every 12 h. Regular unfractionated heparin therapy was continued until the International Normalized Ratio (INR) was therapeutic (INR=2–3). An intravenous drip infusion of 450 mg of Xueshuantong was administered once daily to improve microcirculation.
CDT group
We punctured the contralateral femoral vein or right internal jugular vein with Seldinger technique performed under 1% lidocaine anesthesia. An inferior vena cava angiogram was performed and an OptEase filter (Cordis Endovascular, a Johnson & Johnson Company, Warren, NJ, USA) was placed with the upper margin at 1 cm below the lower renal vein. After achieving a satisfactory location and morphology of the filter by inferior vena cava angiogram, the vessel sheath was removed and the puncture points underwent pressure bandaging. A small (1–2 cm) incision (Figure 1) was made longitudinally at the middle point between the external malleolus and Achilles tendon of affected limbs after local anaesthesia. A small saphenous vein was isolated and the 4F vascular sheath was punctured. The guide wire and catheter were advanced together and directly entered into the popliteal vein (Figure 2). Alternatively, they can first enter into a calf deep vein via the trunk branch, followed by entering the popliteal vein and proximal segments of the thrombus. Unifuse infusion catheters (Angiodynamics, Latham, NY, USA) were exchanged with effective distal thrombolysis located in the proximal thrombus. The terminal was connected with a micro-injection pump after being fixed and a total of 105 U/kg of urokinase (Pude Pharm, Shanxi, China) was micro-pumped into the terminal 4 times for 2 h each time. Deep venography of affected limbs was performed once every 48 h. After venous recanalization was obtained, the thrombolytic catheter was retracted an appropriate distance to make the side hole of the thrombolytic catheter remain in the thrombus. If there was deep venous patency or a non-obvious change in the thrombus between 2 reexaminations, thrombolysis was discontinued. The coagulation index was reexamined once every 12 h. If fibrinogen was <2.0 g/l, the doses of urokinase were halved, and thrombolysis was discontinued if fibrinogen was <1.0 g/l. The thrombolysis time did not exceed 7 days.
Figure 1.
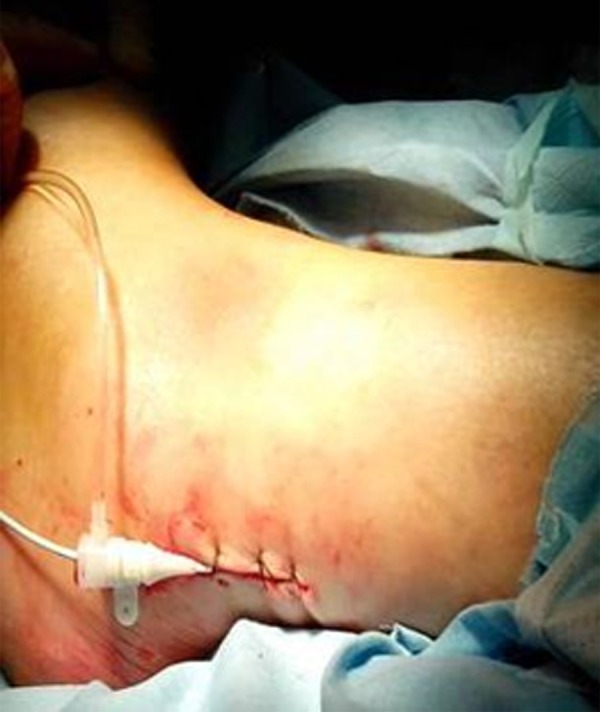
A small incision of about 1–2 cm was made longitudinally midway between the external malleolus and the Achilles tendon of affected limbs.
Figure 2.
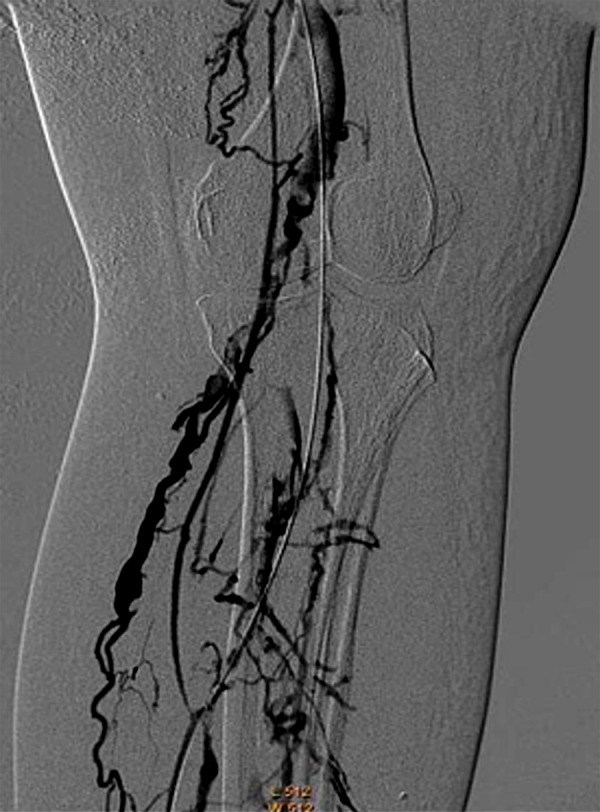
The guide wire and catheter were entered into the popliteal vein.
ST group
An inferior vena cava filter (IVCF) was not routinely placed. However, for floating thrombus and thrombus involved in the inferior vena cava confirmed by ultrasound, an IVCF was placed using the same method as in the CDT group. Urokinase was intravenously injected into the dorsal foot vein of the affected limbs and a tourniquet was applied above the ankle joint. The doses and application method of urokinase and coagulation test were the same as in the CDT group. Lower limb ultrasound was performed once every 48 h during thrombolysis. Thrombolysis was discontinued if there was deep venous patency, complete thrombolysis, or no change between 2 reexaminations.
Postoperative treatment
The IVCF were removed after thrombolysis in both groups. After thrombolysis, all the patients received oral 2.5 mg warfarin (Shanghai Xinyi Pharmaceutical Co., Ltd., Shanghai, China) anticoagulation. When the INR (an index that can monitor the anticoagulant effect) was therapeutic at between 2 and 3 on the index, nadroparin calcium was stopped. Thereafter, warfarin was subsequently used and adjusted to maintain this INR for at least 1 year along with wearing medical compression stockings (30–40 mmHg).
Observation index
All patients received Doppler ultrasound or angiography evaluations before and after thrombolysis therapy. The venograms were scored according to modified standards for venous disease by Porter and Moneta [7]. The thrombus score was calculated for 7 affected deep venous segments as detailed in a previous report [8]. Briefly, the thrombus score was classified at 0–2 according to the vein patency: 0 indicates the vein was patent and completely free of thrombus, 1 indicates partially occluded, and 2 indicates completely occluded. The total thrombus scores before and after thrombolysis was calculated by adding the thrombus scores of the 7 deep venous segments before and after thrombolysis. Thrombolysis efficiency= (total pre-lysis thrombus scores – total post-lysis thrombus scores/total pre-lysis thrombus scores) ×100%.
Limb circumference was measured at 10 cm above the superior patellar pole and 15 cm below the inferior patellar pole on both sides of the lower limb preoperatively and on postoperative days 7 and 14. Limb circumference difference (limb swelling) was calculated as the limb circumference of the affected side minus the limb circumference of the normal side of the lower limb. All the patients were followed up for 1 year and the incidence of PTS was observed. PTS is a frequent and important complication of DVT. The diagnosis of PTS is based primarily on the presence of typical symptoms and clinical signs. According to the Villalta scale, which has been demonstrated to be a reliable and valid measure of PTS in patients with confirmed DVT [9], subjects are classified as having PTS if the score is ≥5, or if a venous ulcer is present, in a leg with previous DVT.
Statistical analysis
Statistical analyses were performed using SPSS17.1 software (Chicago, IL, USA). Data are expressed as mean ± standard deviation and compared with the t test and chi-square test, as appropriate. P<0.05 was considered as statistical significance.
Results
Clinical characteristics
A total of 66 patients diagnosed with the unilateral mixed LDVT and treated with CDT via the small saphenous veins and systematic thrombolysis were included in this study. No patients had previous DVT in our series. The patients were confirmed as having iliofemoral and popliteal vein thrombosis by inferior vena cava angiogram (Figures 3, 4). There were no statistically significant differences in age, sex, duration of disease, or predisposing factors between the CDT and ST groups (all P>0.05). IVCF were successfully removed from patients in the CDT group after thrombolysis and no complications, such as perforation of the inferior vena cava or transposition of the filter, were noted. Ultrasound examination showed that 5 patients in the ST group had a floating thrombus in the inferior vena cava or iliac vein. IVCF in the ST group were successfully removed from 4 of these 5 patients and removal in 1 patient failed because the thrombus was involved with the filter.
Figure 3.
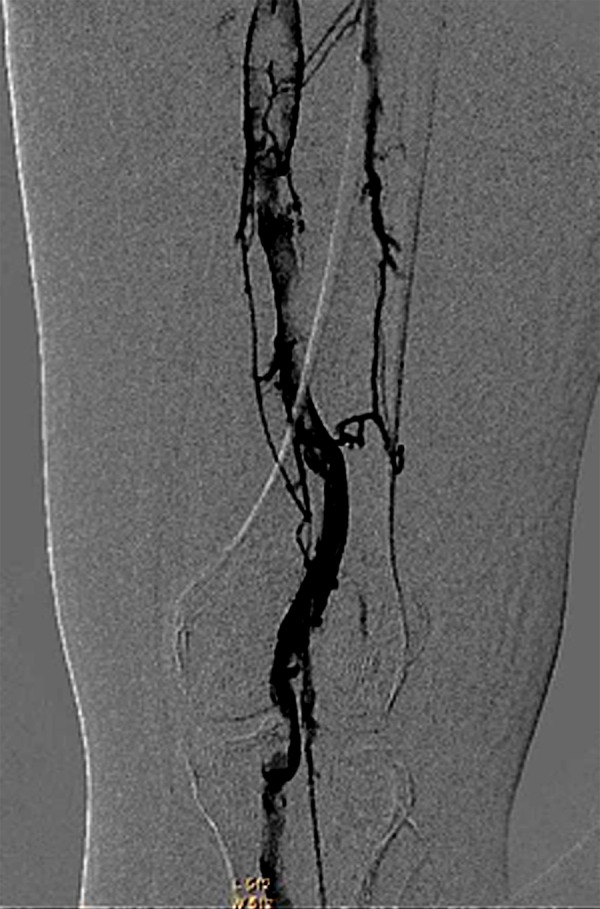
Venography shows a large amount of thrombogenesis in the femoropoplitea vein before thrombolysis.
Figure 4.
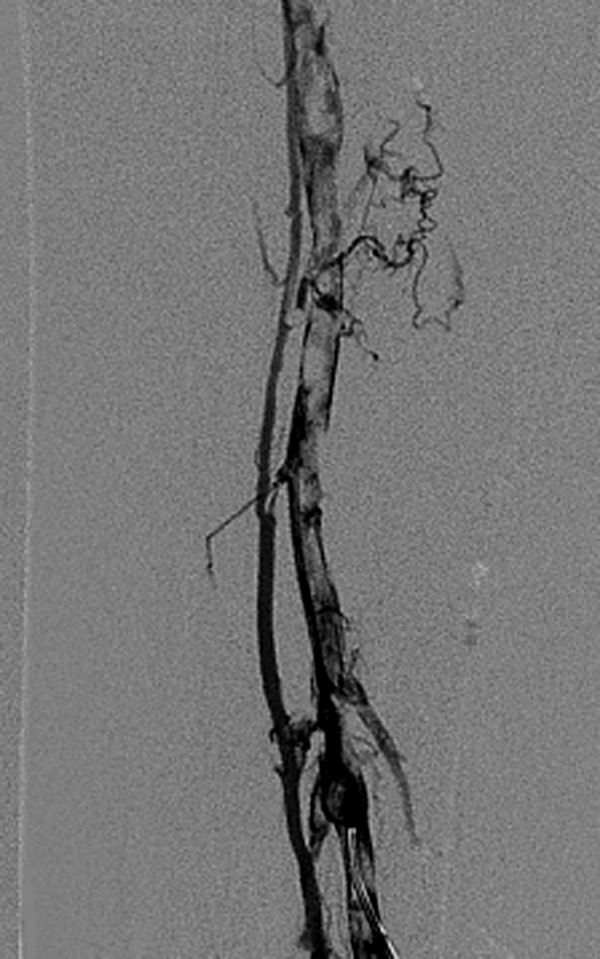
Venography shows a large amount of thrombogenesis in the femoral vein before thrombolysis.
Outcome assessment
The angiogram demonstrated that all or part of the fresh thrombus was dissolved (Figures 5, 6). The thrombolytic time was 3.95±1.49 days in the CDT group and 4.22±1.45 days in the ST group, with no statistically significant difference (P=0.461) (Table 2). There was a significant difference regarding thrombolytic efficiency between the CDT group and ST group (71.26% vs. 48.26%, P=0.001) (Table 2). The differences in limb circumferences at 10 cm above the superior patellar pole and 15 cm below the inferior patellar pole preoperatively and on the 7th and 14th days postoperatively are listed in Table 3. Preoperatively, there was no significant difference regarding the differences in limb circumferences at 10 cm above the superior patellar pole (P=0.42) and 15 cm below the inferior patellar pole (P=0.32) in the 2 groups (Table 3). In both groups the postoperative limb circumference changes were higher than the preoperative values. The differences between postoperative limb circumferences on postoperative days 7 and 14 were significantly higher in the CDT group than in the ST group (all P<0.05).
Figure 5.
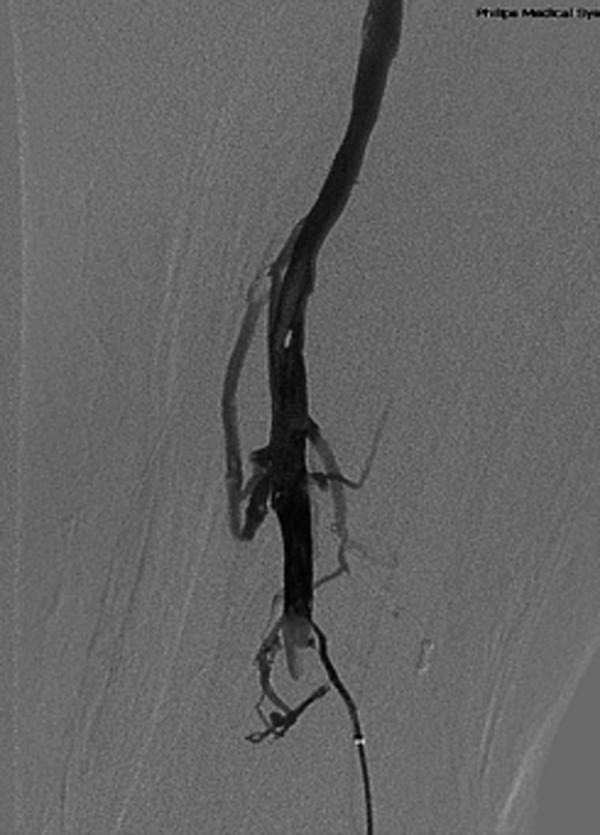
Venography shows femoral and popliteal vein thrombolysis and vessel patency 3 days after CDT via the small saphenous veins.
Figure 6.
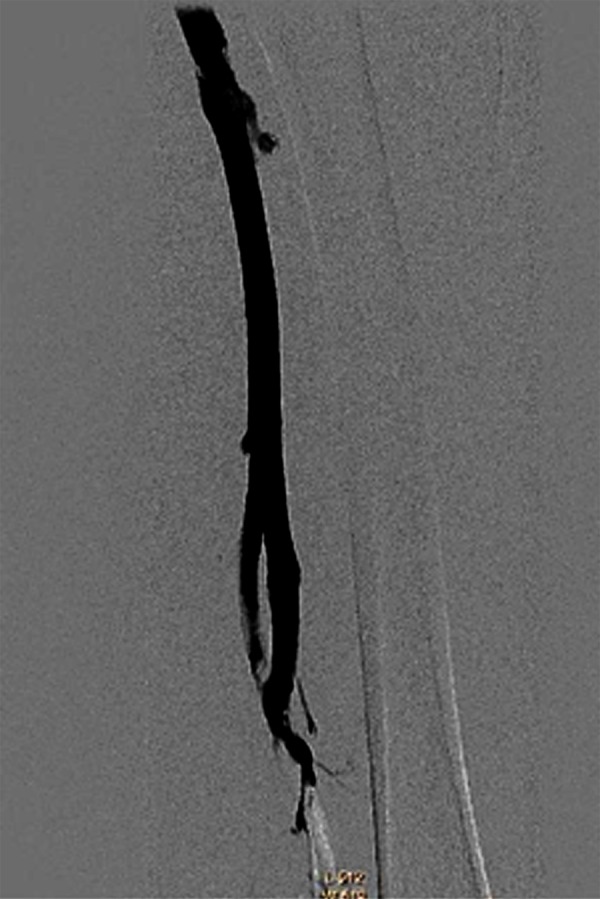
Venography shows femoral vein thrombolysis and vessel patency 4 days after thrombolysis CDT via the small saphenous veins.
Table 2.
Clinical outcomes of patients in two groups.
| Group | CDT group (n=39) | ST group (n=27) | P values |
|---|---|---|---|
| Thrombolytic efficiency | 71.26±17.66 | 48.26±15.99 | 0.001 |
| Thrombolytic time | 3.95±1.49 | 4.22±1.45 | 0.461 |
Table 3.
The differences in limb circumferences at 10 cm above the superior patellar pole and 15 cm below the inferior patellar pole preoperatively and on the 7th, 14th day postoperatively.
| Groups | Before thrombolytic therapy | 7 days after therapy | 14 days after therapy | |||
|---|---|---|---|---|---|---|
| Above knee/cm | Below knee/cm | Above knee/cm | Below knee/cm | Above knee/cm | Below knee/cm | |
| CDT group | 5.98±1.24 | 4.79±0.77 | 2.68±0.94 | 2.10±0.29 | 1.73±0.26 | 1.26±0.40 |
| ST group | 5.74±1.03 | 4.57±1.05 | 4.57±1.05 | 3.18±0.38 | 2.62±0.65 | 2.05±0.64 |
| P | 0.42 | 0.32 | 0.001 | 0.001 | 0.001 | 0.001 |
Limb circumference difference (limb swelling) was calculated as the limb circumference of the affected side minus the limb circumference of the normal side of the lower limb.
Seven patients in the CDT group (n=39) had PTS (17.9%) during the 12 months of postoperative follow-up. One out of the 7 cases had severe PTS that presented with superficial varicosis, leg eczema, foot ulceration, and venous claudication. This patient had poor thrombolytic efficiency, and only thrombosis of the popliteal vein and part of the distal superficial femoral vein were dissolved. Thrombolysis treatment was stopped at the 6th day after CDT therapy due to no obvious dissolution. Lower-limb edema was still significant at 14 weeks after the operation. During the follow-up, 14 patients in the ST group had PTS (51.85%). These 14 patients had different degrees of swelling or pain in the lower limbs during the follow-up, of which 9 patients had obvious varicose vein of the lower limbs, 2 patients had new eczema, and 1 patient had a venous ulcer in the lower limb. The incidence of postoperative PTS in the CDT group (17.9%) was significantly lower than in the ST group (51.85%) during the follow-up (P=0.007) (Table 4). During the thrombolytic therapy, 1 patient in the CDT group had bleeding of the surgical wound in the ankle and stopped bleeding after compression bandaging. One patient in the ST group developed hematuria, which was relieved after reducing the dose of urokinase. None of the 66 patients had severe bleeding or incision infection during the entire treatment.
Table 4.
Complications of patients in two groups.
| Group | CDT group (n=39) | ST group (n=27) | P |
|---|---|---|---|
| Bleeding | 1 (2.56%) | 1 (3.70%) | 1.000 |
| PTS | 7 (17.95%) | 14 (51.85%) | 0.007 |
| Thrombosis recurrence | 1 (2.56%) | 1 (3.70%) | 1.000 |
One patient in each group had recurrent thrombosis during the follow-up. The patient in the CDT group was further found to have pancreatic cancer and received surgical treatment, and the patient in the ST group developed thrombosis in the contralateral limb during oral warfarin anticoagulant, and the symptom improved after enhancing anticoagulation.
Discussion
At present, the main treatment methods for LDVT are anticoagulation, thrombolysis, surgical thrombectomy, and long-term physical therapy. Anticoagulant therapy is broadly recognized by physicians as the basic method for thrombus treatment. Anticoagulation can inhibit blood clot spread and assists with the autolysis of thrombus and the tube recanalization, which leads to relieving symptoms and reducing the incidence rate and mortality rate of PE. However, large clinical studies confirm that about 50–60% of patients have PTS after simple anticoagulant therapy [10], and this seriously affects quality of life.
Thrombolytic therapy can dissolve the thrombus effectively, recover deep vein patency, prevent thrombus spread, protect the function of the lower extremity deep vein valve, and reduce the recurrence of thrombosis and the incidence rate of PTS. Surgical thrombectomy and systematic thrombolysis confirmed by early studies can reduce the incidence rate of PE and PTS, but broad clinical use is difficult due to the complications of trauma and bleeding. However, catheter thrombolysis has received more and more attention because it causes less trauma and fewer complications and has high thrombolytic efficiency. Enden et al. demonstrated that catheter thrombolysis therapy reduced the incidence rate of PTS by 14.4% compared with simple anticoagulation [11]. The 2012 Chest Physicians of America guidelines suggested catheter thrombolysis as the preferred treatment for serious DVT [12]. In the present study, the incidence rate of PTS was 51.9% after systemic thrombolysis therapy, which was lower than the 70% incidence rate after anticoagulation. However, at present there is no report comparing the treatment effect of mixed LDVT versus systemic thrombolysis and catheter-directed thrombolysis. Results of the present study showed higher thrombolytic efficiency (71.26%) in the CDT group than in the ST group (48.26%) and lower incidence rate of PTS in the CDT group than in the ST group (P<0.01).
The popliteal vein is currently the most commonly used approach for an infusion catheter, and the success rate is high for percutaneous catheter with ultrasound. However, the popliteal vein is involved with the popliteal artery and tibial nerve; therefore, a puncture may lead to subcutaneous hematoma, arteriovenous fistula, false aneurysm, and other DVT complications. Percutaneous catheterization of the popliteal vein usually requires the patient to be in prone position, but this is difficult for patients with paralysis, obesity, or fractures, and after thoracoabdominal surgery. Contralateral femoral vein catheterization has a high failure rate because opposing the valve direction can injure the valve and it often is replaced by the femoral vein. Zhongming et al. studied catheter-directed thrombolysis treatment of LDVT via the small saphenous veins and reported a satisfactory curative effect [13]. This method is simple, with anatomical position fixed, and the small saphenous vein can be exposed after making a small 1-cm incision from the rear of the lateral malleolus. There are no important arteries or nerve tissue structure around the small saphenous veins, so it will not cause serious complications, the patient does not need to change position, and there is less obvious pain compared with use of the popliteal vein. Catheter-directed thrombolysis via the small saphenous veins can reduce injury to the popliteal vein, the thrombolytic range is greater compared with use of the popliteal vein, and it can dissolve thrombosis of the popliteal vein. Destruction and occlusion of the popliteal vein valve is often the major cause of PTS. Therefore, catheter-directed thrombolysis via the small saphenous veins can increase thrombolytic efficiency, relieve patient pain, and reduce the incidence rate of PTS. Our results also demonstrated that popliteal vein patency in the CDT group was significantly increased after thrombolytic therapy.
Avgerinos et al. reported that the incidence of PE was not significantly different when the inferior vena cava filter was placed before catheter thrombolysis [14]. Decousus et al. reported a 1.1% incidence of PE in patients who had a vena cava filter placed, which was significantly (P=0.03) lower than the 4.8% incidence in patients who received simple anticoagulation [15]. Because the thrombosis is captured on the filter in some patients [16,17], it is suggested that an inferior vena cava filter be placed in patients during thrombolytic therapy, and patients may have no clinical symptoms even if the captured thrombosis causes PE. In the present study, the 39 patients in the CDT group all had an IVCF placed before catheter thrombolysis and no PE occurred postoperatively, which might be due to the filter placement. Filters were placed in 5 out of the 27 patients in the ST group due to inferior vena cava or iliac vein floating thrombus confirmed by ultrasonography. Filter placement requires long-term anticoagulation, and due to filter fracture, inferior vena cava obstruction and perforation, and recurrent thrombosis, all patients in the present study received a retrievable filter. The filters in the CDT group were all removed after thrombolysis. In the ST group, the filters of 4 patients were removed after the thrombolysis, and we stopper filter removal in 1 patient because the thrombus was spreading to the inferior vena cava and involving the filter. The thrombosis capture rate was not higher when the filters were removed, perhaps because the fibrinolytic enzyme activated by urokinase dissolved the captured thrombosis. Decousus et al. found the incidence of DVT (20.8%) in patients who had filters placed was higher than that of the control group (11.6%) at 2 years after treatment. However, 1 patient in each group had thrombosis recurrence after 1 year of follow-up, perhaps because filter placement was not long-term. In the CDT group, 12 patients were confirmed to have iliac vein stenosis or occlusion after thrombolysis by angiography during the treatment. We consider that this stenosis is a natural barrier preventing PE. IVCF placement is not needed if the patient has obvious iliac vein stenosis or occlusion confirmed before the operation.
Recently, iliac vein stenosis has attracted attention regarding the occurrence, treatment, and recurrence of DVT, but iliac vein stenosis is difficult to effectively treat after DVT is dissolved by drugs. The incidence of DVT was over 71.7% in patients with iliac vein disease [18]. The rate of recurrent thrombosis is higher when the iliac vein is not treated in time. Meng et al. reported on 155 DVT patients receiving CDT treatment and found that 74 patients had iliac vein stenosis of over 50%; 45 of them were treated with balloon dilatation and stent implantation, and 29 of the 74 patients did not receive treatment. At 1-year follow-up, the patency rate was higher in iliac vein stenosis patients who received treatment than in those who did not receive treatment [19]. The treatment of iliac vein disease after catheter thrombolysis or surgical thrombectomy may increase the patency rate, improve the treatment effect, and reduce the incidence of PTS. If the iliac vein stenosis is over 50% after catheter thrombolysis, it should be treated with balloon dilatation or stent implantation, or even surgery, to clear the iliac vein blockage. In the present study, 12 patients in the CDT group were confirmed to have iliac vein stenosis, and 7 of them were treated with iliac vein balloon dilatation and stent implantation at the same time the filter removed after the thrombosis had resolved. The 7 patients were confirmed to have patent stents during the 1-year follow-up period after the operation. Two of the 7 patients had light PTS, but it did not affect the daily activities of the patients.
The main complication of thrombolytic therapy is bleeding, including subcutaneous hematoma, ecchymosis, hematuria, gastrointestinal, and intracranial hemorrhage. However, to date, no serious bleeding reports have been found. In the present study, each group had 1 case of postoperative hemorrhage. Thrombolytic time in the CDT group was 2–6 days, with an average of 3.95 days, and in the ST group it was 2–7 days, with an average of 4.23 days. The mean urokinase use in the CDT group and ST group were 2.37×106 U and 2.54×106 U, respectively. The low bleeding rate may be associated with the lower dosage of urokinase.
Conclusions
Our results show that catheter-directed thrombolysis is superior to systemic thrombolysis in treatment of LDVT. Catheter-directed thrombolysis via the small saphenous veins is a simpler, safer, and more effective and feasible approach compared with systemic thrombolysis for treatment of acute deep venous thrombosis. Catheter-directed thrombolysis can increase deep venous patency and decrease the incidence rate of PTS, which is of great clinical significance in treatment of LDVT.
Footnotes
Source of support: Departmental sources
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Spencer TR, Lagace RE, Waterman G. Effort thrombosis (Paget-Schroetter syndrome) in a 16-year-old male. Am J Case Rep. 2014;15:333–36. doi: 10.12659/AJCR.890726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semba CP, Dake MD. Iliofemoral deep venous thrombosis: Aggressive therapy with catheter-directed thrombolysis. Radiology. 1994;191:487–94. doi: 10.1148/radiology.191.2.8153327. [DOI] [PubMed] [Google Scholar]
- 3.Prandoni P, Lensing AW, Prins MH, et al. Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: A randomized, controlled trial. Ann Intern Med. 2004;141:249–56. doi: 10.7326/0003-4819-141-4-200408170-00004. [DOI] [PubMed] [Google Scholar]
- 4.Choi JW, Jae HJ, Kim HC, et al. CT venography for deep venous thrombosis: Can it predict catheter-directed thrombolysis prognosis in patients with iliac vein compression syndrome? Int J Cardiovasc Imaging. 2015;31:417–26. doi: 10.1007/s10554-014-0546-1. [DOI] [PubMed] [Google Scholar]
- 5.Huang CY, Hsu HL, Kuo TT, et al. Percutaneous pharmacomechanical thrombectomy offers lower risk of post-thrombotic syndrome than catheter-directed thrombolysis in patients with acute deep vein thrombosis of the lower limb. Ann Vasc Surg. 2015;29:995–1002. doi: 10.1016/j.avsg.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Du XL, Kong LS, Meng QY, et al. Safety and efficacy of low dosage of urokinase for catheter-directed thrombolysis of deep venous thrombosis. Chin Med J. 2015;128:1787–92. doi: 10.4103/0366-6999.159355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porter JM, Moneta GL. Reporting standards in venous disease: An update. International Consensus Committee on Chronic Venous Disease. J Vasc Surg. 1995;21:635–45. doi: 10.1016/s0741-5214(95)70195-8. [DOI] [PubMed] [Google Scholar]
- 8.Mewissen MW, Seabrook GR, Meissner MH, et al. Catheter-directed thrombolysis for lower extremity deep venous thrombosis: Report of a national multicenter registry. Radiology. 1999;211:39–49. doi: 10.1148/radiology.211.1.r99ap4739. [DOI] [PubMed] [Google Scholar]
- 9.Kahn SR. Measurement properties of the Villalta scale to define and classify the severity of the post-thrombotic syndrome. J Thromb Haemost. 2009;7:884–88. doi: 10.1111/j.1538-7836.2009.03339.x. [DOI] [PubMed] [Google Scholar]
- 10.Grommes J, von Trotha K, Wolf MD, et al. Catheter-directed thrombolysis in deep vein thrombosis: Which procedural measurement predicts outcome? Phlebology. 2014;29:135–39. doi: 10.1177/0268355514529394. [DOI] [PubMed] [Google Scholar]
- 11.Enden T, Haig Y, Klow NE, et al. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): A randomised controlled trial. Lancet. 2012;379:31–38. doi: 10.1016/S0140-6736(11)61753-4. [DOI] [PubMed] [Google Scholar]
- 12.Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S–94S. doi: 10.1378/chest.11-2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhongming J, Qinghua X. Transcatheter thrombolysis via the small saphenous vein for deep venous thrombosis of lower limb. Journal of Interventional Radiology. 2010;44:944–46. [Google Scholar]
- 14.Avgerinos ED, Hager ES, Jeyabalan G, et al. Inferior vena cava filter placement during thrombolysis for acute iliofemoral deep venous thrombosis. Journal of Vascular Surgery: Venous and Lymphatic Disorders. 2014;2:274–81. doi: 10.1016/j.jvsv.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Decousus H, Leizorovicz A, Parent F, et al. A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prevention du Risque d’Embolie Pulmonaire par Interruption Cave Study Group. New Eng J Med. 1998;338:409–15. doi: 10.1056/NEJM199802123380701. [DOI] [PubMed] [Google Scholar]
- 16.Offner PJ, Hawkes A, Madayag R, et al. The role of temporary inferior vena cava filters in critically ill surgical patients. Arch Surg. 2003;138:591–94. doi: 10.1001/archsurg.138.6.591. discussion 594–95. [DOI] [PubMed] [Google Scholar]
- 17.Stein PD, Kayali F, Olson RE. Twenty-one-year trends in the use of inferior vena cava filters. Arch Intern Med. 2004;164:1541–45. doi: 10.1001/archinte.164.14.1541. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, Choi D, Guk Ko Y, et al. Percutaneous treatment of deep vein thrombosis in May-Thurner syndrome. Cardiovasc Intervent Radiol. 2006;29:571–75. doi: 10.1007/s00270-004-0165-7. [DOI] [PubMed] [Google Scholar]
- 19.Meng QY, Li XQ, Jiang K, et al. Stenting of iliac vein obstruction following catheter-directed thrombolysis in lower extremity deep vein thrombosis. Chin Med J. 2013;126:3519–22. [PubMed] [Google Scholar]


