The presence of either Ki-67 antibody, OxPhos antibody, or both yields incremental ability to predict local tumor progression over and above that based on margins and lesion size alone, thereby validating previously published data and indicating that biopsy of the ablation zone immediately after radiofrequency ablation is a clinically useful tool.
Abstract
Purpose
To establish the prognostic value of biopsy of the central and marginal ablation zones for time to local tumor progression (LTP) after radiofrequency (RF) ablation of colorectal cancer liver metastasis (CLM).
Materials and Methods
A total of 47 patients with 67 CLMs were enrolled in this prospective institutional review board–approved and HIPAA-compliant study between November 2009 and August 2012. Mean tumor size was 2.1 cm (range, 0.6–4.3 cm). Biopsy of the center and margin of the ablation zone was performed immediately after RF ablation (mean number of biopsy samples per ablation zone, 1.9) and was evaluated for the presence of viable tumor cells. Samples containing tumor cells at morphologic evaluation were further interrogated with immunohistochemistry and were classified as either positive, viable tumor (V) or negative, necrotic (N). Minimal ablation margin size was evaluated in the first postablation CT study performed 4–8 weeks after ablation. Variables were evaluated as predictors of time to LTP with the competing-risks model (uni- and multivariate analyses).
Results
Technical effectiveness was evident in 66 of 67 (98%) ablated lesions on the first contrast material–enhanced CT images at 4–8-week follow-up. The cumulative incidence of LTP at 12-month follow-up was 22% (95% confidence interval [CI]: 12, 32). Samples from 16 (24%) of 67 ablation zones were classified as viable tumor. At univariate analysis, tumor size, minimal margin size, and biopsy results were significant in predicting LTP. When these variables were subsequently entered in a multivariate model, margin size of less than 5 mm (P < .001; hazard ratio [HR], 6.7) and positive biopsy results (P = .008; HR, 3.4) were significant. LTP within 12 months after RF ablation was noted in 3% (95% CI: 0, 9) of necrotic CLMs with margins of at least 5 mm.
Conclusion
Biopsy proof of complete tumor ablation and minimal ablation margins of at least 5 mm are independent predictors of LTP and yield the best oncologic outcomes.
© RSNA, 2016
Introduction
Colorectal cancer has an estimated worldwide incidence of approximately 1 360 000 cases per year, and up to half of these cancers will lead to colorectal liver metastasis (CLM) (1,2). Although hepatic resection is considered the treatment of choice, only about 20% of patients with liver metastases are surgical candidates because of excessive tumor burden, unfavorable tumor location, or comorbidities (2,3). Radiofrequency (RF) ablation has documented treatment efficacy for primary and secondary hepatic malignancies; however, incomplete tumor treatment and local tumor progression (LTP) remain important limitations to the widespread use of this treatment (1,4–10). Thus, it is crucial to develop and apply prognostic markers to identify patients at increased risk for LTP after ablation. Such markers could guide additional ablations or could indicate patients at risk who would benefit from adjuvant therapies to improve LTP-free survival and overall clinical outcome.
Prior studies have indicated that histopathologic analysis of tissue adherent to RF electrodes after ablation is feasible and possesses prognostic importance for LTP, regardless of the method used to document cell viability (11–16). However, macroscopic tissue adherent to RF electrodes after ablation is not uniformly present and is usually absent with newer RF electrodes and other ablation devices (eg, microwave, irreversible electroporation). Moreover, tissue fragments adherent to the electrodes are collected from random locations within the ablation zone, making it impossible to determine their origin (ie, center vs margin). To overcome these limitations, we designed a prospective study of pathologic examinations of tissue obtained with image-guided core-needle biopsies from the radiographic center and margin of the ablation zone immediately after RF ablation of CLM.
In the current study, we sought to establish the prognostic value of central and marginal ablation zone biopsies in relevance to LTP after CLM RF ablation.
Materials and Methods
Patient Selection and Eligibility Criteria
Patients undergoing RF ablation of CLM were assessed for enrollment in this Health Insurance Portability and Accountability Act–compliant institutional review board–approved study.
Patients eligible for enrollment were those with up to three CLMs (each < 5 cm in largest diameter) and no more than three extrahepatic sites of disease (including nodes and pulmonary nodules). Patients with uncorrectable coagulopathy (international normalized ratio >1.5 or a platelet count of <50 000/mm3) and those who were unable to undergo general anesthesia were not eligible. Tumors located less than 1 cm from a major bile duct or a major blood vessel (>7 mm in diameter) and those in proximity to the gastrointestinal tract that could not be protected with hydrodissection or other protective maneuvers also were excluded. Baseline contrast material–enhanced computed tomography (CT) was performed within 30 days of RF ablation. All patients provided written informed consent prior to RF ablation.
Treatment
Between November 2009 and August 2012, 47 consecutive patients (32 men, 15 women; age range, 34–82 years) with 67 CLMs who met the eligibility criteria underwent image-guided RF ablation. All procedures were performed with the patients under general anesthesia and with continuous hemodynamic monitoring by an anesthesiologist. RF ablation was performed with the Valleylab Cool-tip system (Covidien, Mansfield, Mass) in 42 patients, the RITA StarBurst XLi system (Angiodynamics, Queensbury, NY) in 19, the StarBurst Talon system (Angiodynamics) in two, or the LeVeen system (Boston Scientific, Natick, Mass) in four depending on tumor size, shape, and location and operator preference. RF electrode placement and accurate tumor targeting were performed with CT guidance aided by CT fluoroscopy, sonography, or both, as needed. Additional intraprocedural combined positron emission tomography (PET) and CT guidance was used for ablation of 32 (48%) of 67 tumors in 25 patients (Fig 1) (17). Electrode repositioning and overlapping ablations were performed with the intention of achieving a minimum ablation margin of 5 mm uniformly around the tumor. Fifty-one (76%) of 67 lesions required overlapping ablations (range, one to four ablations). In all cases, the manufacturer-recommended protocol was applied and completed (12,18,19). Triphasic CT was performed immediately after ablation to determine whether or not technical success was achieved.
Figure 1a:

Images in an 82-year-old female patient with one CLM who was undergoing treatment according to protocol. (a) Baseline contrast-enhanced portal phase CT image obtained 2 weeks before treatment shows a 1.5 × 1.7 cm CLM (arrow) in segment 5/6. (b) Intraprocedural PET image acquired for tumor targeting (tumor maximum standardized uptake value, 12.8) and fused with a subsequent CT image to ensure that tines are well positioned for ablation (Starburst RITA XLi; Angiodynamics) after intravenous injection of 4.4 mCi fluorine 18 (18F) fluorodeoxyglucose (FDG) and a 75-minute uptake period. (c) Core biopsies of the center and inferior margin of the ablation zone obtained with PET/CT guidance after RF ablation. Left: To sample the ablation zone where the tumor previously resided, the preablation PET image is fused with the immediate postablation CT image, overlapping the previous tumor with the ablation zone and targeting the previously PET-positive area within the ablation zone. Right: Biopsy of the presumed minimal margin of the ablation zone is performed in a similar manner, this time targeting the PET-negative area that overlaps the CT ablation zone. (d) PET/CT image of the liver obtained 60 minutes after intravenous administration of an additional 8.8 mCi 18F FDG given at the end of ablation. The FDG-avid tumor has been replaced by a photopenic area representing the ablation zone, which corresponds to treatment success. The tissue analyzed was classified as necrotic. LTP did not occur during the 9-month follow-up period.
Figure 1b:

Images in an 82-year-old female patient with one CLM who was undergoing treatment according to protocol. (a) Baseline contrast-enhanced portal phase CT image obtained 2 weeks before treatment shows a 1.5 × 1.7 cm CLM (arrow) in segment 5/6. (b) Intraprocedural PET image acquired for tumor targeting (tumor maximum standardized uptake value, 12.8) and fused with a subsequent CT image to ensure that tines are well positioned for ablation (Starburst RITA XLi; Angiodynamics) after intravenous injection of 4.4 mCi fluorine 18 (18F) fluorodeoxyglucose (FDG) and a 75-minute uptake period. (c) Core biopsies of the center and inferior margin of the ablation zone obtained with PET/CT guidance after RF ablation. Left: To sample the ablation zone where the tumor previously resided, the preablation PET image is fused with the immediate postablation CT image, overlapping the previous tumor with the ablation zone and targeting the previously PET-positive area within the ablation zone. Right: Biopsy of the presumed minimal margin of the ablation zone is performed in a similar manner, this time targeting the PET-negative area that overlaps the CT ablation zone. (d) PET/CT image of the liver obtained 60 minutes after intravenous administration of an additional 8.8 mCi 18F FDG given at the end of ablation. The FDG-avid tumor has been replaced by a photopenic area representing the ablation zone, which corresponds to treatment success. The tissue analyzed was classified as necrotic. LTP did not occur during the 9-month follow-up period.
Figure 1c:
Images in an 82-year-old female patient with one CLM who was undergoing treatment according to protocol. (a) Baseline contrast-enhanced portal phase CT image obtained 2 weeks before treatment shows a 1.5 × 1.7 cm CLM (arrow) in segment 5/6. (b) Intraprocedural PET image acquired for tumor targeting (tumor maximum standardized uptake value, 12.8) and fused with a subsequent CT image to ensure that tines are well positioned for ablation (Starburst RITA XLi; Angiodynamics) after intravenous injection of 4.4 mCi fluorine 18 (18F) fluorodeoxyglucose (FDG) and a 75-minute uptake period. (c) Core biopsies of the center and inferior margin of the ablation zone obtained with PET/CT guidance after RF ablation. Left: To sample the ablation zone where the tumor previously resided, the preablation PET image is fused with the immediate postablation CT image, overlapping the previous tumor with the ablation zone and targeting the previously PET-positive area within the ablation zone. Right: Biopsy of the presumed minimal margin of the ablation zone is performed in a similar manner, this time targeting the PET-negative area that overlaps the CT ablation zone. (d) PET/CT image of the liver obtained 60 minutes after intravenous administration of an additional 8.8 mCi 18F FDG given at the end of ablation. The FDG-avid tumor has been replaced by a photopenic area representing the ablation zone, which corresponds to treatment success. The tissue analyzed was classified as necrotic. LTP did not occur during the 9-month follow-up period.
Figure 1d:

Images in an 82-year-old female patient with one CLM who was undergoing treatment according to protocol. (a) Baseline contrast-enhanced portal phase CT image obtained 2 weeks before treatment shows a 1.5 × 1.7 cm CLM (arrow) in segment 5/6. (b) Intraprocedural PET image acquired for tumor targeting (tumor maximum standardized uptake value, 12.8) and fused with a subsequent CT image to ensure that tines are well positioned for ablation (Starburst RITA XLi; Angiodynamics) after intravenous injection of 4.4 mCi fluorine 18 (18F) fluorodeoxyglucose (FDG) and a 75-minute uptake period. (c) Core biopsies of the center and inferior margin of the ablation zone obtained with PET/CT guidance after RF ablation. Left: To sample the ablation zone where the tumor previously resided, the preablation PET image is fused with the immediate postablation CT image, overlapping the previous tumor with the ablation zone and targeting the previously PET-positive area within the ablation zone. Right: Biopsy of the presumed minimal margin of the ablation zone is performed in a similar manner, this time targeting the PET-negative area that overlaps the CT ablation zone. (d) PET/CT image of the liver obtained 60 minutes after intravenous administration of an additional 8.8 mCi 18F FDG given at the end of ablation. The FDG-avid tumor has been replaced by a photopenic area representing the ablation zone, which corresponds to treatment success. The tissue analyzed was classified as necrotic. LTP did not occur during the 9-month follow-up period.
Histopathologic and Immunohistochemical Analysis
Immediately after each RF ablation, image-guided biopsy was performed from the tumor center and the suspected minimal margin of the ablation zone by using coaxial 18–20-gauge core needles (Fig 1). In all patients, at least one core biopsy was obtained from the center of the ablation zone (n = 67). In six of 67 patients, tumors were small, and the margin was included in a 2-cm core extending from the center to the periphery of the ablation zone. An additional 61 samples were obtained from the margin of the ablation zone. The mean number of biopsy samples per ablation zone was 1.9. Any tissue fragments adherent to the RF electrodes were collected, if present.
All tissue specimens were fixed in formalin and stained with hematoxylin-eosin. A pathologist (D.S.K., 20 years of experience) then performed morphologic assessment. Specimens that displayed only characteristics of necrosis and did not contain tumor cells did not require further evaluation and were classified as necrotic. Specimens that contained any tumor cells at hematoxylin-eosin staining were further analyzed with immunohistochemistry (K.O.M., L.M.P.; 15 and 30 years of experience, respectively) for proliferative potential (with Ki-67), mitochondrial viability (with OxPhos antibody, or OXP), and ongoing apoptosis (with caspase-3) (Fig 2) (20–22).
Figure 2:
Schematic diagram of tissue analysis. Samples positive for tumor cells at hematoxylin-eosin (H&E) staining were further interrogated with immunohistochemistry. A total of 16 ablation zones were considered positive for viable tumor cells.
Positivity of Ki-67 markers, OXP markers, or both led to classification of the specimen as a viable tumor, whereas the absence of these markers and a positive apoptotic caspase-3 assay led to classification of the specimen as necrotic. In cases of coexisting positive Ki-67 or OXP and caspase-3 assays, samples were classified as viable tumors based on our hypothesis that the presence of even a single cell in the proliferative phase places the patient at increased risk for LTP (21).
Imaging Follow-up, Response, and Local Tumor Progression
To evaluate response to treatment and to define the minimal ablation margin size, liver triphasic CT was performed within 4–8 weeks after RF ablation. The minimal ablation margin size was assessed by comparing the distances of the index tumor (preablation imaging) and the ablation zone (postablation imaging) from intrahepatic landmarks on portal venous phase CT images, as previously described (23). This assessment was performed by one of four readers (R.K.D, a hepatobiliary radiologist with 6 years of experience; C.T.S, an interventional radiologist with 14 years of experience; V.S.S, a research fellow diagnostic body radiologist with 2 years of experience; and a research fellow thanked in the Acknowledgment).
The initial 4–8-week postablation CT findings were considered the new baseline for future assessments of LTP. Irregular peripheral or nodular enhancement within 1 cm of the ablated area was considered positive for residual tumor or incomplete treatment (7). Further imaging assessments were performed at 2–4-month intervals for at least 2 years after ablation and were prospectively assessed by the operating interventional radiology physicians (C.T.S., J.P.E., K.T.B., A.M.C., W.A., L.A.B., S.B.S.; 14, 6, 15, 11, 12, 15, and 12 years of experience, respectively). Evidence of tumor recurrence within 1 cm from the ablation zone seen on contrast-enhanced CT images and confirmed by a hepatobiliary radiologist (R.K.D.) was considered LTP.
Definitions
Time to LTP.—Time to LTP was defined as the time between ablation and the first radiologic evidence of LTP.
Overall survival.— Overall survival was defined as the time between initial RF ablation and patient death or most recent follow-up (24).
Central biopsy.—Central biopsy was defined as biopsy performed at the area of the ablation zone where the tumor previously resided. Central biopsies sampled the area that overlapped the target tumor without necessarily being in the geometric center of the ablation zone.
Marginal biopsy.—Marginal biopsy was defined as a biopsy that sampled the presumed minimal margin of the ablation zone on the immediate postablation CT image. Marginal biopsies were intended to sample the ablation zone between the target tumor and the surrounding normal tissue where the distance between untreated liver tissue and the edge of the target tumor was smallest. This minimal margin was estimated visually immediately after the procedure and was used only to direct the marginal biopsy, as opposed to the minimal margin calculated 4–8 weeks after ablation, which was used to measure and analyze the margin as one of the factors affecting LTP-free survival.
Complications
Periprocedural complications were defined as any complications that occurred within 30 days of RF ablation and were categorized by following the Society of Interventional Radiology guidelines (25). Major complications were those that resulted in an increased level of care and required hospitalization. All other complications were considered minor.
Statistical Analysis
Median follow-up time was determined by using the median follow-up time among the patients who were alive at the end of the study. Overall survival rates were calculated by using the Kaplan-Meier method and were stratified for tumor size, minimal ablation margin size, biopsy result, and presence of extrahepatic disease at the time of ablation by using the log-rank test.
Because patient death occurred before imaging evidence of LTP could be gathered in 23 (34%) of 67 tumors, the analysis of LTP predictors had to account for the competing event of death. The Kaplan-Meier method of LTP-free survival would censor these cases or consider them free of adverse events, potentially leading to biased estimates. To overcome this obstacle, evaluation of variable importance in predicting time to LTP was performed with the competing-risks (Fine-Gray) regression model (26). In addition, we have provided Kaplan-Meier survival curves and displayed the results of both analyses side by side for the sake of comparison.
Variables that showed statistical significance at univariate analysis were subsequently analyzed with a multivariate model. Data were analyzed with statistical software (Stata, version 12.0; Stata, College Station, Tex).
Results
Patient characteristics, tumor characteristics, and treatment details are shown in Table 1.
Table 1.
Patient, Tumor, and Treatment Characteristics

Note.—Unless otherwise indicated, data are number of patients, and data in parentheses are percentages. HAIP = hepatic arterial infusion pump, LN = lymph node.
*Data are median values, and data in parentheses are the range.
†Data are mean values, and data in parentheses are the range.
RF Ablation Efficacy
In 66 (98%) of 67 ablated lesions, there was no evidence of residual tumor at the first post-RF ablation contrast-enhanced CT scan, which was performed within 4–8 weeks after ablation. In the one patient in whom CT revealed a residual tumor 4 weeks after ablation, tissue examination from the margin of the ablation zone contained viable (Ki-67– and OXP-positive) tumor cells.
Tissue Findings
Tissue adherent to the RF electrodes was present in 10 of 42 ablations performed with the Valleylab Cool-tip probe, all 19 ablations performed with the RITA StarBurst XLi probe, all four ablations performed with the LeVeen probe, and both ablations performed with the StarBurst Talon probe. Twenty-four tissue specimens (three from the electrodes, 12 from the center, nine from margin biopsies) originating from 16 ablated CLMs contained tumor cells detected with standard hematoxylin-eosin staining. These specimens were further assessed with immunohistochemistry. Twenty-three (96%) of 24 specimens were positive for proliferation marker Ki-67. The one specimen that was negative for Ki-67 was positive for OXP and negative for caspase-3. From the same ablation zone, the tissue obtained from the margin was Ki-67 positive, leading to classification of this ablation zone as viable. Twenty (83%) of 24 lesions were positive for OXP, and two (8%) lesions were positive for caspase-3. The two lesions with coexisting Ki-67 and caspase-3 positivity were classified as viable based on the rationale explained in the Materials and Methods.
There was a correlation between margin size and presence of viable cells. Ablation zones with larger margins were less likely to have biopsies with viable cells (Fisher exact test, P = .019). However, no significant correlation existed between the biopsy site (center vs margin) and the presence of viable tumor cells. Additionally, no correlation was seen between the number of overlapping ablations and positive biopsies or minimal margin size.
LTP Findings
After a median follow-up period of 29 months, LTP occurred in 11 (69%) of 16 lesions classified as viable and in 10 (20%) of 51 lesions classified as necrotic (P < .001). The cumulative incidence of LTP was 22% (95% confidence interval [CI]: 12, 32) at 12-month follow-up and 27% (95% CI: 16, 38) at 24-month follow-up.
Univariate analysis showed that tumor size, minimal margin size, and biopsy result were significant predictors of time to LTP. In contrast, PET/CT ablation guidance was not a significant predictor (P = .54). Subsequently, significant variables were entered into a multivariate model to determine independent predictors of LTP. Positive postablation biopsy (hazard ratio [HR], 3.4; P = .008) and minimal ablation margin size (<5 mm) (HR, 6.7; P < .001) were independent predictors of shorter time to LTP (Table 2). Since this multivariate analysis is based on a regression model, we estimated the risk of LTP for a lesion with narrow margins and viable tissue at biopsy. This can be done because the regression model used is additive in log hazards (27). The HR for this subset (in reference to the group with sufficient margins and negative biopsy) is 22.8 (95% CI: 7.0, 81.1). Recurrence within the first 12 months after RF ablation occurred in one (3%; 95% CI: 0, 9) of 34 biopsy-negative ablation zones with minimal margins (≥5 mm) and in eight (73%; 95% CI: 43, 100) of 11 biopsy-positive ablation zones with inadequate margins (<5 mm) (P < .001). An example of positive central biopsy findings that recurred 6 months after ablation is shown in Figure 3. Kaplan-Meier and cumulative incidence survival curves of LTP are displayed in Figure 4.
Table 2.
Univariate and Multivariate Analyses of Factors Associated with Local Tumor Progression by Using the Competing-Risks Regression Model

Note.—All examined variables displayed significance at univariate analysis; however, only the biopsy result and the minimal margin size maintained significance at multivariate analysis. Multivariate analysis data were calculated with the competing-risks model. N = necrotic-negative biopsy, V = viable-tumor positive biopsy.
Figure 3a:

Images in a 57-year-old man who underwent RF ablation followed by immediate postablation biopsy of the ablated tumor. (a) CT image shows a 3.0 × 2.0 cm CLM in hepatic segment 6 (arrow). (b) CT image obtained 6 weeks after ablation shows the ablation zone with expected postablation findings consistent with complete ablation. (c) Photomicrograph of the tissue obtained from the ablation zone and stained with hematoxylin-eosin shows tumor cells. (d, e) Further immunhistochemical analysis was positive for proliferative nuclear marker Ki-67 (d) and mitochondrial activity (e). (f) This patient developed LTP (arrow) 6 months after ablation, which was suspected at CT (left) and was confirmed at PET (right).
Figure 4a:
(a) Cumulative incidence and (b) Kaplan-Meier survival curves for LTP. Cumulative incidence estimation (a) accounts for the competing event of death, whereas Kaplan-Meier analysis (b) treats patients who died before developing LTP as censored observations. The subgroups were created based on variables that were significant at multivariate analysis (ie, biopsy result, minimal ablation margin size). N = necrotic-negative biopsy, V = viable tumor-positive biopsy.
Figure 3b:

Images in a 57-year-old man who underwent RF ablation followed by immediate postablation biopsy of the ablated tumor. (a) CT image shows a 3.0 × 2.0 cm CLM in hepatic segment 6 (arrow). (b) CT image obtained 6 weeks after ablation shows the ablation zone with expected postablation findings consistent with complete ablation. (c) Photomicrograph of the tissue obtained from the ablation zone and stained with hematoxylin-eosin shows tumor cells. (d, e) Further immunhistochemical analysis was positive for proliferative nuclear marker Ki-67 (d) and mitochondrial activity (e). (f) This patient developed LTP (arrow) 6 months after ablation, which was suspected at CT (left) and was confirmed at PET (right).
Figure 3c:
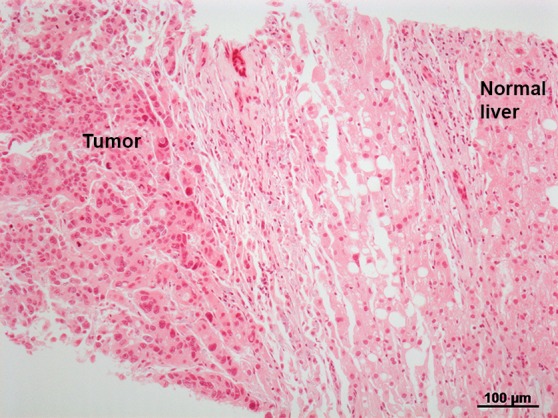
Images in a 57-year-old man who underwent RF ablation followed by immediate postablation biopsy of the ablated tumor. (a) CT image shows a 3.0 × 2.0 cm CLM in hepatic segment 6 (arrow). (b) CT image obtained 6 weeks after ablation shows the ablation zone with expected postablation findings consistent with complete ablation. (c) Photomicrograph of the tissue obtained from the ablation zone and stained with hematoxylin-eosin shows tumor cells. (d, e) Further immunhistochemical analysis was positive for proliferative nuclear marker Ki-67 (d) and mitochondrial activity (e). (f) This patient developed LTP (arrow) 6 months after ablation, which was suspected at CT (left) and was confirmed at PET (right).
Figure 3d:
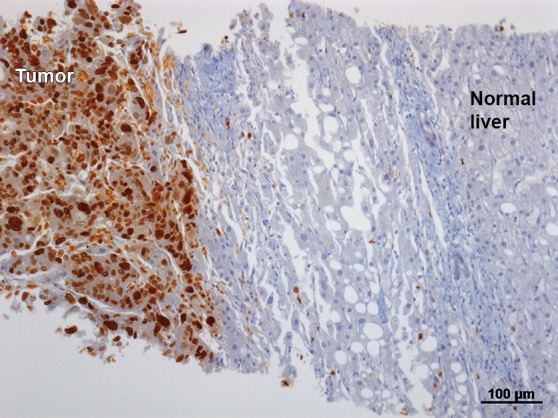
Images in a 57-year-old man who underwent RF ablation followed by immediate postablation biopsy of the ablated tumor. (a) CT image shows a 3.0 × 2.0 cm CLM in hepatic segment 6 (arrow). (b) CT image obtained 6 weeks after ablation shows the ablation zone with expected postablation findings consistent with complete ablation. (c) Photomicrograph of the tissue obtained from the ablation zone and stained with hematoxylin-eosin shows tumor cells. (d, e) Further immunhistochemical analysis was positive for proliferative nuclear marker Ki-67 (d) and mitochondrial activity (e). (f) This patient developed LTP (arrow) 6 months after ablation, which was suspected at CT (left) and was confirmed at PET (right).
Figure 3e:

Images in a 57-year-old man who underwent RF ablation followed by immediate postablation biopsy of the ablated tumor. (a) CT image shows a 3.0 × 2.0 cm CLM in hepatic segment 6 (arrow). (b) CT image obtained 6 weeks after ablation shows the ablation zone with expected postablation findings consistent with complete ablation. (c) Photomicrograph of the tissue obtained from the ablation zone and stained with hematoxylin-eosin shows tumor cells. (d, e) Further immunhistochemical analysis was positive for proliferative nuclear marker Ki-67 (d) and mitochondrial activity (e). (f) This patient developed LTP (arrow) 6 months after ablation, which was suspected at CT (left) and was confirmed at PET (right).
Figure 3f:

Images in a 57-year-old man who underwent RF ablation followed by immediate postablation biopsy of the ablated tumor. (a) CT image shows a 3.0 × 2.0 cm CLM in hepatic segment 6 (arrow). (b) CT image obtained 6 weeks after ablation shows the ablation zone with expected postablation findings consistent with complete ablation. (c) Photomicrograph of the tissue obtained from the ablation zone and stained with hematoxylin-eosin shows tumor cells. (d, e) Further immunhistochemical analysis was positive for proliferative nuclear marker Ki-67 (d) and mitochondrial activity (e). (f) This patient developed LTP (arrow) 6 months after ablation, which was suspected at CT (left) and was confirmed at PET (right).
Figure 4b:
(a) Cumulative incidence and (b) Kaplan-Meier survival curves for LTP. Cumulative incidence estimation (a) accounts for the competing event of death, whereas Kaplan-Meier analysis (b) treats patients who died before developing LTP as censored observations. The subgroups were created based on variables that were significant at multivariate analysis (ie, biopsy result, minimal ablation margin size). N = necrotic-negative biopsy, V = viable tumor-positive biopsy.
Patient Survival
Overall survival rate was 87% (95% CI: 73, 94) at 12 months, 63% (95% CI: 46, 75) at 24 months, and 46% (95% CI: 28, 63) at 36 months. Median overall survival was 36 months (95% CI: 32, 40). In all patients, the cause of death was related to cancer (ie, progression of disease). Tumor size (≤3 cm vs >3 cm), minimal ablation margin size (<5 mm vs ≥5 mm), presence of extrahepatic disease, and positive ablation zone biopsy findings were not predictors of overall survival since none of these factors were significant.
Complications
There were two occurrences of pneumothorax during the procedure that were treated with thoracostomy without further issue. These were classified as minor complications.
Major complications occurred in two patients. One patient developed fever and thrombocytopenia after RF ablation, with subsequent development of an extensive intrahepatic hematoma that required coil embolization of a right hepatic artery pseudoaneurysm 45 days after ablation. The second patient presented with progressive dyspnea on exertion and evidence of pulmonary embolism (as documented by a ventilation-perfusion scan) in the setting of underlying cardiac disease 2 weeks after ablation of two CLMs within the same session. This was treated with anticoagulation, without further sequelae.
Discussion
One limitation of percutaneous image-guided ablation when compared with surgery is the lack of pathologic evidence that the target lesion was completely ablated with sufficient tumor-negative margins. Most studies indicate that positive surgical margins have been associated with a higher risk of local recurrence and shorter overall survival (28,29), a conclusion that has been verified in recent studies performed in the context of modern preoperative chemotherapy (30,31).
Similarly, after RF ablation, the minimal ablation margin is a factor associated with local tumor control (23,32–35). Treatment effectiveness is routinely assessed with postprocedural imaging (US, CT, or MR imaging) to evaluate if the intended ablation margin has been reached, and if it has not, to direct further treatment (23,36,37). Several methods have been proposed for precise calculation of the ablation margin, including fusion imaging (32) and use of anatomic intrahepatic landmarks (23). All of these methods suffer from differences when comparing images from scans performed at different times. Despite progress in fusion and registration software, limitations of these techniques still present a challenge when attempting accurate calculation of the margin of the ablation zone around a previously treated tumor (23,32). Despite the use of these imaging modalities, incomplete tumor treatment and LTP remain common after RF ablation (7–10).
The sole use of imaging in the assessment of ablation margins presumes that the entire depicted ablation zone contains necrotic or nonviable cells; however, few studies have validated image findings with tissue characteristics after ablation. In the early radiologic-pathologic correlation study performed by Goldberg et al (38), imaging failed to depict peripheral residual untreated tumor foci, even in four of five cases in which initial tumor and ablation zone sizes were identical. Furthermore, (a) prior studies performed to evaluate tissue collected from the electrodes used for ablation and (b) our study, in which we preformed direct assessment of the ablation zone with biopsy, show that viable tumor cells may be present within the ablation zone, even when postprocedural imaging displays sufficient ablation margins and findings compatible with radiographic technical success (12,13,16). It appears that these incompletely treated viable tumor cells within the ablation zone “escape” the spatial resolution of available anatomic and functional imaging modalities. In addition, the assessment of tissue examinations in the ablation zone introduces an objective tool with which to assess ablation effectiveness. This assessment is less vulnerable to operator variability and technical limitations, such as those described in the assessment and calculation of the ablation margin (23), and it may be easier to reproduce. Nevertheless, biopsies and sampling errors can also occur, and residual tumor may not be detected in some cases. This can explain the few recurrences seen in our study even after negative biopsy results and sufficient margins were obtained.
Histopathologic examination of tumors excised immediately after RF ablation has shown regions of altered cellular morphology within the treated volume that do not correspond to coagulative necrosis or classic tumor cell appearance (38). These areas may represent either (a) cells in the early stages of irreversible apoptosis or coagulative necrosis or (b) viable cells maintaining their proliferative potential, allowing them to replicate once the cellular insult is removed (21,22). Thus, it is prudent to further interrogate such cells with available immunohistochemical staining for cytosolic and mitochondrial enzyme activity, especially in the immediate postprocedural setting. Days after RF ablation, the evolution of intracellular cascades leads to more pronounced cellular changes in the direction of irreversible apoptosis or necrosis that can be evaluated with standard hematoxylin-eosin staining (38,39). However, immunohistochemical staining has been considered superior to hematoxylin-eosin staining in the diagnosis of irreversible cellular damage up to 24 weeks after RF ablation, as noted in an earlier study by Morimoto et al (39).
An interesting and unique finding of our study is that immunohistochemistry did not alter the initial classification based on the interpretation of the morphologic (hematoxylin-eosin) stain. All 16 ablation zones containing tumor cells were also positive for Ki-67, OXP, or both. This observation differs from observations in previous studies in which ablated tissue was assessed and in which immunohistochemistry was considered necessary to determine viability and changed specimen classification in two (13%) of 15 cases (12,13). It is possible that this finding represents the acquired experience of study pathologists at our institution in the evaluation of ablated tissue, and it potentially justifies the future investigation of the utility of immediate postablation frozen sections and morphologic assessment to document complete tumor cell necrosis.
The results of our prospective study validate prior data indicating the correlation between the presence of viable tumor cells on RF electrodes after ablation and LTP-free survival. Sofocleous et al (12,13) reported that positive Ki-67 positivity was an independent predictor of shorter LTP-free survival, with a higher HR (ie, 5.1) than that in our study (HR, 3.4). Unlike the earlier study, our investigation did not show tissue viability was a predictor of overall survival, most likely due to a significantly shorter follow-up period (29 months vs 63 months) (13).
Viability of tissue adherent to electrodes was analyzed in a study by Snoeren et al (16), who used the autofluorescence method and glucose-6-phosphate diaphorase staining. They concluded that viable tissue was an independent risk factor for LTP. Despite the different methods used to document viability, the incidence of viable tissue in the Snoeren et al study (29.2%) was slightly greater than that in our study (24%) and in the prior studies by Sofocleous et al (19.1%) (12,13). This observation, in combination with the fact that viability in all studies was an independent predictor of LTP, may indicate that both methods of tissue evaluation possess similar efficacy.
Another significant finding of our study was that within 2 years, only one (3%; 95% CI: 0, 9) of 34 treated tumors with negative biopsy results and margins of at least 5 mm had LTP. Although this may represent an underestimation of the LTP rate (due to the number of tumors that may have recurred but remained undetected at follow-up imaging before patient death), this observation supports patient risk stratification using tissue and imaging data in clinical practice. The LTP rates in this patient subgroup are similar to published marginal recurrence rates after R0 resections for CLM (range, 3%–6%) (40,41). Conversely, tumor-positive biopsy of the ablation zone is equivalent to resection with tumor-positive margins. Whenever a patient has positive biopsy results, an insufficient margin after ablation, or both, adjuvant chemotherapy and a higher frequency of surveillance should be recommended.
Our study had several limitations. The most important factor limiting the weight of our conclusions was the relatively small number of enrolled patients (n = 47). In addition, pathologic evaluation with biopsies from the center and the margin of the ablation zone does not yield information about necrosis within the entire treated lesion volume, as opposed to the evaluation of resected tumors and their surgical margins. Moreover, specimens were classified as Ki-67 positive even if the evaluated tissue was only slightly positive at immunostaining. Estimation of the labeling percentage of the target CLM was not performed, thereby precluding investigation of any potential correlation between the level and grade of proliferation (Ki-67 positivity) and time to LTP. Another limitation of our study was that the minimal margin analyzed as a predictor for time to LTP was evaluated by using 4–8-week postablation CT images, while the presumed minimal margin targeted with biopsy was estimated by using CT images obtained immediately after ablation. That was a rough estimate suited to the time limitations of the procedure; however, for this study, accurate estimation and recording of the minimal margin (using CT landmarks) was performed by using CT images obtained 4–8 weeks after RF ablation, as previously described in detail (23). Software capabilities with fusion of the preablation tumor with the ablation zone are currently under development and are evolving. This will enable accurate calculation of the margin during or immediately after ablation and at subsequent time points. As indicated in our work, a positive viable tumor biopsy had a strong and significant prognostic value, as it was an early biomarker of local tumor recurrence and ablation failure. The addition of biopsy of the ablation zone introduces a more objective and reproducible significant predictive assessment of the ablation zone rather than relying on the imaging findings and margin assessment alone.
In conclusion, the presence of Ki-67, OXP, or both yields an incremental ability to predict LTP over and above that based on margins and lesion size alone, thus validating previously published data and indicating that biopsy of the ablation zone immediately after RF ablation is a clinically useful tool.
Despite advances in cross-sectional and metabolic imaging, the diagnostic and prognostic value of even a limited number of viable tumor cells within the ablation zone cannot be underestimated. Given the results of our study and the high risk for LTP in the positive biopsy group with a minimal margin size of less than 5 mm, patients with these characteristics should be considered to have residual disease, and they should be cared for accordingly. Future investigations should be directed toward the development of intraprocedural markers and corresponding surrogate image biomarkers of incomplete ablation. Such biomarkers could guide additional therapy, either by repeating or extending the ablation or by considering additional therapies (locoregional, systemic, or both), to extend time to LTP and most likely improve clinical outcomes, including patient survival.
Advances in Knowledge
■ Biopsy of the margin and center of the ablation zone immediately after radiofrequency (RF) ablation is feasible and yields clinically useful information that carries prognostic significance for local tumor progression (LTP).
■ Ablated tumors with posttreatment biopsies containing tumor cells positive for Ki-67 or OxPhos antibodies are 3.4 times more likely to recur (P = .008).
■ An ablation zone with negative tumor findings and with margins of 5 mm or more carries a cumulative incidence rate of LTP of 3% (95% confidence interval: 0, 9) at 2 years; this is comparable to reported marginal recurrence rates after R0 resections for colorectal cancer liver metastasis (CLM).
Implications for Patient Care
■ Biopsy performed immediately after ablation of CLM should be considered in the clinical practice of ablation with curative intent.
■ Ablation margins of 5 mm or larger should be created when ablation of CLM with curative intent is attempted.
■ Patients with positive postablation biopsy results and minimal ablation margins of less than 5 mm should be treated as though they have residual disease.
Acknowledgments
Acknowledgments
We acknowledge Waleed Shady, MD (research fellow in interventional radiology, Memorial Sloan-Kettering Cancer Center) and Yevgeniy Romin (digital microscopy specialist, Sloan Kettering Institute) for their contribution to this work.
Received May 1, 2015; revision requested June 23; revision received October 27; accepted December 17; final version accepted December 23.
Supported by the National Cancer Institute (grants R21 CA131763-01A1, P30 CA008748).
Disclosures of Conflicts of Interest: V.S.S. disclosed no relevant relationships. L.M.P. disclosed no relevant relationships. M.G. disclosed no relevant relationships. D.S.K. disclosed no relevant relationships. R.K.G.D. disclosed no relevant relationships. E.N.P. disclosed no relevant relationships. A.R.G. disclosed no relevant relationships. A.B. disclosed no relevant relationships. J.P.E. disclosed no relevant relationships. K.T.B. disclosed no relevant relationships. A.M.C. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: holds stock in Amgen. Other relationships: disclosed no relevant relationships. W.A. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed no relevant relationships. Other relationships: is a consultant for NeuWave Medical. L.A.B. disclosed no relevant relationships. R.P.D. disclosed no relevant relationships. N.E.K. disclosed no relevant relationships. S.B.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received grants from GE Healthcare and AngioDynamics. Other relationships: disclosed no relevant relationships. K.O.M. disclosed no relevant relationships. C.T.S. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received grants from Neuwave Medical and Perseon Medical; is a consultant for Neuwave Medical, Perseon Medical, Siemens, and Medtronic. Other relationships: disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- CLM
- cancer liver metastasis
- HR
- hazard ratio
- LTP
- local tumor progression
- RF
- radiofrequency
References
- 1.Hanna NN. Radiofrequency ablation of primary and metastatic hepatic malignancies. Clin Colorectal Cancer 2004;4(2):92–100. [DOI] [PubMed] [Google Scholar]
- 2.Alberts SR, Poston GJ. Treatment advances in liver-limited metastatic colorectal cancer. Clin Colorectal Cancer 2011;10(4):258–265. [DOI] [PubMed] [Google Scholar]
- 3.Adam R, Vinet E. Regional treatment of metastasis: surgery of colorectal liver metastases. Ann Oncol 2004;15(Suppl 4):iv103–iv106. [DOI] [PubMed] [Google Scholar]
- 4.Curley SA, Izzo F. Radiofrequency ablation of primary and metastatic liver tumors. Surg Technol Int 2002;10:99–106. [PubMed] [Google Scholar]
- 5.Chen MH, Yang W, Yan K, et al. Treatment efficacy of radiofrequency ablation of 338 patients with hepatic malignant tumor and the relevant complications. World J Gastroenterol 2005;11(40):6395–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sofocleous CT, Nascimento RG, Gonen M, et al. Radiofrequency ablation in the management of liver metastases from breast cancer. AJR Am J Roentgenol 2007;189(4):883–889. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg SN, Grassi CJ, Cardella JF, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 2005;235(3):728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology 2000;217(1):119–126. [DOI] [PubMed] [Google Scholar]
- 9.Solbiati L, Livraghi T, Goldberg SN, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 2001;221(1):159–166. [DOI] [PubMed] [Google Scholar]
- 10.White RR, Avital I, Sofocleous CT, et al. Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg 2007;11(3):256–263. [DOI] [PubMed] [Google Scholar]
- 11.Sofocleous CT, Klein KM, Hubbi B, et al. Histopathologic evaluation of tissue extracted on the radiofrequency probe after ablation of liver tumors: preliminary findings. AJR Am J Roentgenol 2004;183(1):209–213. [DOI] [PubMed] [Google Scholar]
- 12.Sofocleous CT, Nascimento RG, Petrovic LM, et al. Histopathologic and immunohistochemical features of tissue adherent to multitined electrodes after RF ablation of liver malignancies can help predict local tumor progression: initial results. Radiology 2008;249(1):364–374. [DOI] [PubMed] [Google Scholar]
- 13.Sofocleous CT, Garg S, Petrovic LM, et al. Ki-67 is a prognostic biomarker of survival after radiofrequency ablation of liver malignancies. Ann Surg Oncol 2012;19(13):4262–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sofocleous CT, Garg SK, Cohen P, et al. Ki 67 is an independent predictive biomarker of cancer specific and local recurrence-free survival after lung tumor ablation. Ann Surg Oncol 2013;20(Suppl 3):S676–S683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snoeren N, Jansen MC, Rijken AM, et al. Assessment of viable tumour tissue attached to needle applicators after local ablation of liver tumours. Dig Surg 2009;26(1):56–62. [DOI] [PubMed] [Google Scholar]
- 16.Snoeren N, Huiskens J, Rijken AM, et al. Viable tumor tissue adherent to needle applicators after local ablation: a risk factor for local tumor progression. Ann Surg Oncol 2011;18(13):3702–3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan ER, Sofocleous CT, Schöder H, et al. Split-dose technique for FDG PET/CT-guided percutaneous ablation: a method to facilitate lesion targeting and to provide immediate assessment of treatment effectiveness. Radiology 2013;268(1):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saldanha DF, Khiatani VL, Carrillo TC, et al. Current tumor ablation technologies: basic science and device review. Semin Intervent Radiol 2010;27(3):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sofocleous CT, Sideras P, Petre EN. “How we do it:” a practical approach to hepatic metastases ablation techniques. Tech Vasc Interv Radiol 2013;16(4):219–229. [DOI] [PubMed] [Google Scholar]
- 20.Weber JC, Nakano H, Bachellier P, et al. Is a proliferation index of cancer cells a reliable prognostic factor after hepatectomy in patients with colorectal liver metastases? Am J Surg 2001;182(1):81–88. [DOI] [PubMed] [Google Scholar]
- 21.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000;182(3):311–322. [DOI] [PubMed] [Google Scholar]
- 22.Fujisawa S, Romin Y, Barlas A, et al. Evaluation of YO-PRO-1 as an early marker of apoptosis following radiofrequency ablation of colon cancer liver metastases. Cytotechnology 2014;66(2):259–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol 2013;36(1):166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. Radiology 2014;273(1):241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol 2010;33(1):11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94(446):496–509. [Google Scholar]
- 27.Kalbfleisch JD, Prentice RL. The statistical analysis of failure time data. 2nd ed. Sec 8.2.3. New York, NY: Wiley, 2002. [Google Scholar]
- 28.Are C, Gonen M, Zazzali K, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg 2007;246(2):295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25(29):4575–4580. [DOI] [PubMed] [Google Scholar]
- 30.Tranchart H, Chirica M, Faron M, et al. Prognostic impact of positive surgical margins after resection of colorectal cancer liver metastases: reappraisal in the era of modern chemotherapy. World J Surg 2013;37(11):2647–2654. [DOI] [PubMed] [Google Scholar]
- 31.Andreou A, Aloia TA, Brouquet A, et al. Margin status remains an important determinant of survival after surgical resection of colorectal liver metastases in the era of modern chemotherapy. Ann Surg 2013;257(6):1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol 2010;195(3):758–765. [DOI] [PubMed] [Google Scholar]
- 33.Liu CH, Arellano RS, Uppot RN, Samir AE, Gervais DA, Mueller PR. Radiofrequency ablation of hepatic tumours: effect of post-ablation margin on local tumour progression. Eur Radiol 2010;20(4):877–885. [DOI] [PubMed] [Google Scholar]
- 34.Nakazawa T, Kokubu S, Shibuya A, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol 2007;188(2):480–488. [DOI] [PubMed] [Google Scholar]
- 35.Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology 2016;278(2):601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meloni MF, Andreano A, Zimbaro F, Lava M, Lazzaroni S, Sironi S. Contrast enhanced ultrasound: roles in immediate post-procedural and 24-h evaluation of the effectiveness of thermal ablation of liver tumors. J Ultrasound 2012;15(4):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuehl H, Antoch G, Stergar H, et al. Comparison of FDG-PET, PET/CT and MRI for follow-up of colorectal liver metastases treated with radiofrequency ablation: initial results. Eur J Radiol 2008;67(2):362–371. [DOI] [PubMed] [Google Scholar]
- 38.Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer 2000;88(11):2452–2463. [PubMed] [Google Scholar]
- 39.Morimoto M, Sugimori K, Shirato K, et al. Treatment of hepatocellular carcinoma with radiofrequency ablation: radiologic-histologic correlation during follow-up periods. Hepatology 2002;35(6):1467–1475. [DOI] [PubMed] [Google Scholar]
- 40.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241(5):715–722; discussion 722–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muratore A, Ribero D, Zimmitti G, Mellano A, Langella S, Capussotti L. Resection margin and recurrence-free survival after liver resection of colorectal metastases. Ann Surg Oncol 2010;17(5):1324–1329. [DOI] [PubMed] [Google Scholar]






