In a screening program combining annual MR imaging and mammography in women at high risk of cancer, invasive cancer was more likely to be detected at breast MR imaging, 28 of 43(65%) cancers detected at mammography were ductal carcinoma in situ, and the interval cancer rate was lower compared with that of the general population.
Abstract
Purpose
To compare the clinical, imaging, and histopathologic features of breast cancers detected at screening magnetic resonance (MR) imaging, screening mammography, and those detected between screening examinations (interval cancers) in women at high risk.
Materials and Methods
This retrospective institutional review board–approved, HIPAA-compliant review of 7519 women at high risk for breast cancer who underwent screening with MR imaging and mammography between January 2005 and December 2010 was performed to determine the number of screening-detected and interval cancers diagnosed. The need for informed consent was waived. Medical records were reviewed for age, risk factors (family or personal history of breast cancer, BRCA mutation status, history of high-risk lesion or mantle radiation), tumor histopathologic results, and time between diagnosis of interval cancer and most recent screening examination. The χ2 test and logistic regression methods were used to compare the features of screening MR imaging, screening mammography, and interval cancers. The Wilcoxon signed-rank test was used to calculate P values.
Results
A total of 18 064 screening MR imaging examinations and 26 866 screening mammographic examinations were performed. Two hundred twenty-two cancers were diagnosed in 219 women, 167 (75%) at MR imaging, 43 (19%) at mammography, and 12 (5%) interval cancers. Median age at diagnosis was 52 years. No risk factors were associated with screening MR imaging, screening mammography, or interval cancer (P > .06). Cancers found at screening MR imaging were more likely to be invasive cancer (118 of 167 [71%]; P < .0001). Of the 43 cancers found at screening mammography, 38 (88%) manifested as calcifications and 28 (65%) were ductal carcinoma in situ. Interval cancers were associated with nodal involvement (P = .005) and the triple-negative subtype (P = .03).
Conclusion
In women at high risk for breast cancer who underwent screening with mammography and MR imaging, invasive cancers were more likely to be detected at MR imaging, whereas most cancers detected at screening mammography were ductal carcinoma in situ. Interval cancers were found infrequently and were more likely to be node positive and of the triple-negative subtype.
© RSNA, 2016
Introduction
Mammography is the only imaging modality validated by multiple randomized clinical trials and meta-analyses to reduce mortality from breast cancer (1–3). However, mammography has its limitations, especially in young women with dense breasts who are at high risk for cancer. The sensitivity of mammography in young women with dense breasts is reported to be as low as 38%–50% (4). In addition, because breast cancers that develop in women at genetic risk tend to be more aggressive, approximately half of mammographic screening-detected breast cancers in these women have nodal involvement at the time of diagnosis (5–7). The rate of cancers detected between screening examinations (interval cancers) in women at high risk who undergo screening mammography is also high. For example, in one study (7), interval cancers accounted for 46% of cancers in women with BRCA mutations who underwent screening mammography.
Due to these limitations of mammography, supplemental screening with breast magnetic resonance (MR) imaging is recommended for patients at high risk for cancer. This includes women with a known BRCA1 or BRCA2 mutation and their untested first-degree relatives, women with a lifetime risk of 20%–25% or greater for breast cancer, and women with a history of chest irradiation between the ages of 10 and 30 (8,9). These recommendations are based on multiple prospective studies that showed a substantially higher sensitivity with MR imaging compared with mammography in women with a known or likely genetic mutation predisposing them to breast cancer (10–17). When data from 11 prospective studies were combined in a meta-analysis, the sensitivity was 77% for MR imaging alone, 94% for a combination of MR imaging and mammography, and 39% for mammography alone (18). The highest sensitivity was achieved by using a combination of mammography and MR imaging.
To our knowledge, few studies have been performed to examine the frequency of interval cancers in women at high risk who undergo screening with annual mammography and MR imaging or to correlate the biologic characteristics of breast cancers diagnosed with the method of detection. The purpose of this study was to compare the clinical, imaging, and histopathologic features of breast cancers detected at screening MR imaging and screening mammography and interval cancers in women at high risk.
Materials and Methods
Study Population
Our institutional review board approved this Health Insurance Portability and Accountability Act–compliant study, and the need for informed consent was waived. In a retrospective review of the radiology department database, we identified 7519 women at high risk for breast cancer who underwent screening with MR imaging and mammography between January 2005 and December 2010. A study was considered a screening MR imaging or mammographic examination if the patient did not report a clinical symptom and did not have recent abnormal results at mammography or MR imaging. If a patient had a palpable abnormality or other clinical symptom at the time of mammography or MR imaging as indicated on the clinical information sheet or in medical records, then this was not considered a screening study. This research was funded in part by the National Institute of Health/National Cancer Institute Support Grant P30 CA008748.
Breast MR imaging was performed with the patient in the prone position with a 1.5-T or 3-T commercially available system (Sigma; GE, Milwaukee, Wis) by using a dedicated surface breast coil. Imaging sequences included a localizing sequence, a sagittal fat-suppressed T2-weighted sequence, a sagittal non–fat-saturated T1-weighted sequence, and sagittal T1-weighted three-dimensional, fat-suppressed fast spoiled gradient-echo sequences before and three times after rapid bolus injection of 0.1 mmol/L of gadopentetate dimeglumine (Magnevist, Berlex, Wayne, NJ) per kilogram of body weight. All breast MR imaging examinations were interpreted by dedicated breast imaging radiologists in conjunction with the clinical history and other available breast imaging studies. All mammographic examinations at our institution during the study were performed with digital mammography units (Senographe 2000D; GE Medical Systems). For each MR imaging or mammographic examination, a final assessment result was assigned by using the American College of Radiology Breast Imaging and Reporting Data System categories from 1 to 5 (19).
Medical records were reviewed to determine the number of cancers diagnosed at screening MR imaging and mammography and the number of interval cancers diagnosed. Cancers that were diagnosed with both mammography and MR imaging performed within 30 days of one another were excluded from analysis (n = 6), because it could not be determined whether the results of one study led to biased interpretation with the other modality. Two breast imaging radiologists (J.S.S., C.E.C., with 6 and 20 years of experience, respectively) reviewed newly diagnosed cancers to see if an imaging abnormality could be seen in retrospect on images from the most recent screening examination. For those cancers diagnosed at a Breast Imaging and Reporting Data System 3 follow-up examination (n = 32), the two radiologists reviewed the MR images to determine whether the cancer was the same lesion for which follow-up was recommended; if it was, that cancer was considered a screening-detected cancer. The imaging features of the cancers, as well as the age at diagnosis, risk factors (breast density, family or personal history of breast cancer, BRCA mutation status, prior high-risk lesion, history of mantle radiation), and tumor histopathologic results were recorded after medical record review. Immunohistochemical analyses for estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) were performed by using the American Society of Clinical Oncology and College of American Pathologists guidelines (20,21). ER positivity and PR positivity were defined as greater than or equal to 1% nuclear staining. HER2 positivity was defined as having 3+ immunohistochemical results or amplification by fluorescence in situ hybridization. Tumor subtypes were categorized as luminal A (ER+ and/or PR+ and HER2−), luminal B (ER+ and/or PR+ and HER2+), triple negative (ER−, PR−, and HER2−), and HER2+ (ER−, PR−, and HER2+). The time interval between diagnosis of interval cancer and the most recent screening examination was also recorded.
Statistical Analysis
Fisher exact and χ2 tests were used to compare patient and tumor characteristics of cancers found at screening MR imaging or screening mammography and interval cancers. Analysis of variance was used to examine differences in patient age at cancer diagnosis according to mode of detection. Odds ratios and 95% confidence intervals were generated by using multinomial logistic regression with cancers found at screening MR imaging as the comparison group. Logistic regression methods were used to compare the characteristics of screening-detected versus interval cancers. The Wilcoxon signed-rank test was used to calculate P values.
Results
Two hundred twenty-two cancers were diagnosed in 219 women during the study period, including 144 (65%) invasive cancers and 78 (35%) in situ cancers. Two hundred ten (95%) were screening-detected cancers; 12 (5%) were interval cancer. Of the 210 screening-detected cancers, 167 (80%) were detected at screening MR imaging and 43 (20%) were detected at screening mammography. Of the 18 085 breast MR imaging examinations performed during the study period, 3815 were baseline MR imaging examinations and 14 250 were incidence screening MR imaging. The total number of screening mammographic examinations performed was 26 866. The mean time interval between combined screening examinations was 2.7 years (range, 1–6 years) in women with no cancer detected during the study period, 2.9 years in women with screening-detected cancer (range, 1–6 years), and 2.5 years in women with an interval cancer (range,1–5 years).
Median age at diagnosis was 52 years. No risk factor, including age, breast density, personal history of breast cancer, family history of breast cancer, BRCA1 or BRCA2 mutation status, or history of prior high-risk lesion or mantle radiation was associated with the diagnosis of cancer at screening MR imaging or mammography or the diagnosis of interval cancer (P > .06, Table 1). The number of risk factors also was not associated with mode of detection (P = .94) or lesion histologic results (P = .51) (Table 2). The number of women with a family history of breast cancer in a first- or second-degree relative is listed in Table 1. Of these, 83 of 164 (51%) women who underwent a screening MR imaging examination, 23 of 43 (53%) who underwent screening mammography, and six of 12 (50%) with interval cancer had a family history of breast cancer in a first-degree relative. Of the high-risk lesions, atypical ductal hyperplasia and lobular carcinoma in situ were most common among all three groups of women.
Table 1.
Clinical Characteristics
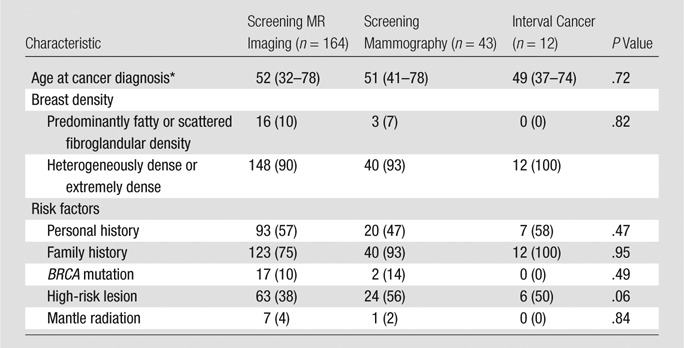
Note.—Unless otherwise indicated, data are number of patients, with percentage in parentheses.
*Data in parentheses are the range.
Table 2.
Correlation among Risk Factors, Mode of Detection, and Tumor Histologic Results
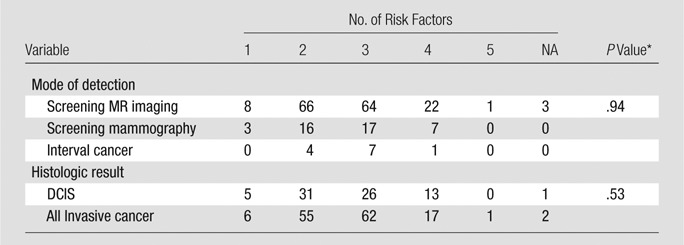
Note.—DCIS = ductal carcinoma in situ, NA = not available.
*Fisher exact test.
All interval cancers manifested as palpable abnormalities. Of the 12 interval cancers, one (8%) woman underwent mammography within the preceding 3 months; four (33%), within 4–6 months; and seven (58%) within 7–12 months. Four of 12 (33%) interval cancers were diagnosed within 6 months and eight of 12 (67%) within 7–12 months of an MR imaging examination what was negative for cancer. In these instances, no imaging abnormality could be seen on the most recent screening mammogram or MR images. Eleven of 12 (92%) interval cancers were invasive cancers (eight invasive ductal carcinoma, three invasive lobular cancer), and one of 12 (8%) was DCIS. Interval cancers showed nodal involvement at surgical excision (screening MR imaging, 19 of 118 [16%]; screening mammography, four of 15 [27%]; interval cancers, six of 11 [55%]; P = .02). Compared with all screening-detected cancers, interval cancers were also 4.5 times more likely to be triple negative than were the luminal A subtype (odds ratio, 4.5 [95% confidence interval: 1.1, 17.9]; P = .03) (Table 3). None of the interval cancers occurred in women with a BRCA mutation.
Table 3.
Association of Tumor Subtype and Screening-detected versus Interval Cancers
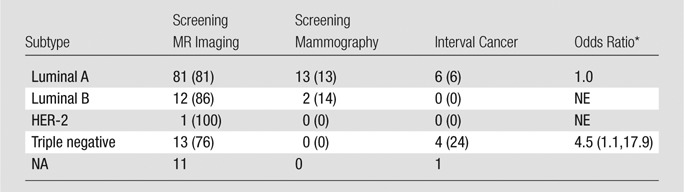
Note.—Unless otherwise indicated, data are number of cancers, with percentage in parentheses. NE = no estimate; NA = not available.
*Comparing all screen detected cancers to interval cancers. Data in parentheses are the 95% confidence interval.
Screening-detected cancers found at mammography usually were detected as calcifications (38 of 43 [88%]) rather than as masses (four of 43 [9%]) or asymmetries (one of 43 [2%]). Screening mammography was also more likely result in diagnosis of DCIS (28 of 43, 65%). In comparison, DCIS accounted for only 49 of 167 (29%) cancers detected at MR imaging and one of 12 (8%) interval cancers. Among the 43 cancers detected at screening mammography, the most recent negative screening breast MR imaging examination was performed on the same day in three (7%), within 3 months in four (9%), within 4–6 months in 14 (33%), and within 7–12 months in 22 (51%) women.
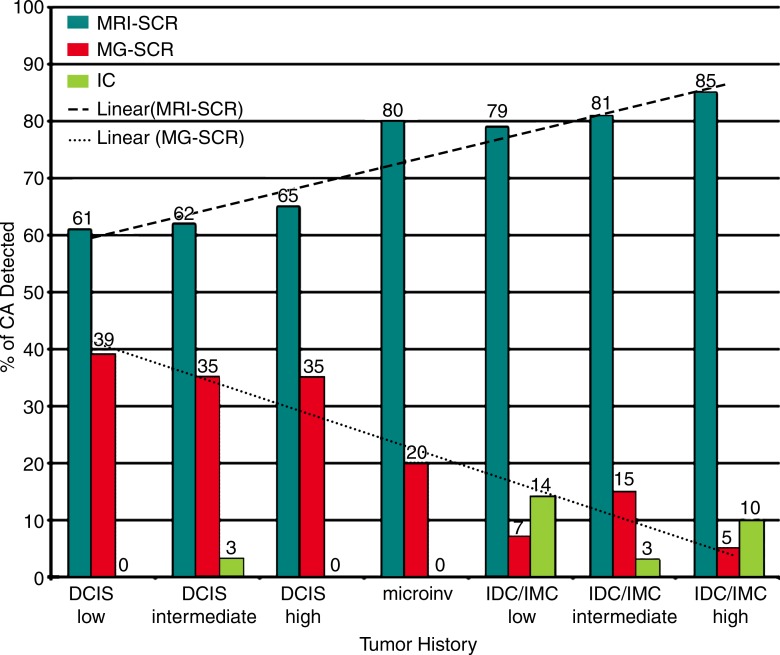
Of the 144 invasive cancers found during the study period, 118 (82%) were diagnosed at screening MR imaging, 15 (10%) at screening mammography, and 11 (8%) were interval cancers (Table 4). Screening mammography was less likely to show invasive cancers than was MR imaging (odds ratio, 0.2; 95% confidence interval: 0.1, 0.4; P < .0001). However, there was no difference in the mean size of the invasive cancers detected at MR imaging (0.8 cm) or mammography (0.7 cm). Interval cancers were slightly larger (mean size, 1.1 cm), although this difference was not statistically significant (overall, P = .12). Among the 167 patients who underwent screening MR imaging, mammography with negative results was performed on the same day in 20 (12%) women, within 3 months in 71 (43%), 4–6 months in 30 (18%), and 7–12 months in 46 (28%) women. The Figure shows a comparison of tumor histologic results and grade according to detection method. As tumor grade and histologic results increased, cancers were increasingly likely to be screening-detected at MR imaging rather than at mammography.
Table 4.
Pathologic Results for Screening-detected Tumors at MR Imaging and Mammography and Interval Cancers
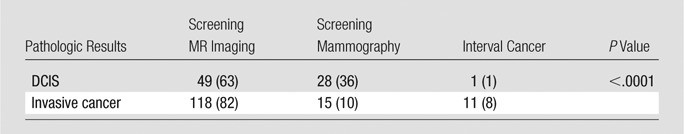
Note.—Data are number of tumors, with percentage in parentheses.
Graph shows comparison of tumor histologic results by detection method. CA =cancer, microinv = microinvasive cancer, IC = interval cancer, IDC = invasive ductal carcinoma, IMC = invasive mammary carcinoma, MG = mammography, SCR = screening detected.
Discussion
In this study, an intensive high-risk screening program incorporating both mammography and MR imaging allowed detection of predominantly node-negative, subcentimeter invasive cancers with few interval cancers. One hundred eighteen of 144 (82%) of all invasive cancers diagnosed during the study period where detected at screening breast MR imaging. In addition, 118 of 167 (71%) cancers detected at MR imaging were invasive cancers, a result similar to what has been reported previously in the literature (13,22). No clinical risk factor was associated with the diagnosis of cancer at screening MR imaging, screening mammography, or interval cancers, suggesting that there is no subgroup of women less likely to benefit from high-risk screening with a combination of mammography and MR imaging.
One highly debated criticism of breast cancer screening has been the possibility of overdiagnosis, particularly due to DCIS (23,24). DCIS represents approximately 20%–30% of mammographic screening-detected cancers in the general population (25). In comparison, DCIS was found in 28 of 43 (65%) screening mammographic examinations in women who underwent both screening mammography and MR imaging in our high-risk screening program. This difference may reflect the higher sensitivity of MR imaging for invasive cancers, depleting the reservoir of invasive cancers that could be detected at screening mammography. While the clinical importance of DCIS in the general population is controversial (23,24), results of studies (26–28) have suggested that DCIS diagnosed in women with a family history of breast cancer is associated with higher recurrence rates after breast conservation surgery. This suggests that DCIS may be more biologically aggressive in these women.
In the general population, interval cancers account for more than 20% of all breast cancers diagnosed in a mammographic screening program and tend to be larger and of a higher grade and stage compared with those seen at screening mammography (29–31). Among the women in our study, the frequency of interval cancer was reduced to 5% (12 of 222). No clinical factors were associated with the development of interval cancer, and none of the interval cancers were in women with known BRCA mutations. Our experience does not confirm those of others that MR imaging and mammography contribute equally to the detection of breast cancer in women with a history of breast irradiation (32). The small number of women (n = 8) in this group may be responsible for this difference.
For women at high risk of cancer who undergo screening with annual mammography and breast MR imaging, there are few data to support screening with both modalities synchronously or alternating mammography and MR imaging at 6-month intervals. In many studies, mammography and MR imaging were performed within 90 days of one another (10,15,33). A modeling study reported that the most efficacious screening strategy for carriers of BRCA mutation was to start screening with MR imaging annually at age 25 and to add annual mammography at age 30, staggering the two examinations at six month intervals (34). In our study, the decision to perform both studies synchronously or at 6-month intervals was determined by the patient and her clinician. Because our data show that greater than 50% of the cancers found at screening mammography were detected at 6–12 months after a negative screening MR imaging examination, there may be some benefit to alternating mammography and MR imaging every 6 months. Additional studies will be needed to determine the optimal screening strategy.
This study was limited because it was performed in a single institution and was a retrospective study. In addition, the number of screening rounds was not equivalent for all women during the study period, with a total of 18 065 screening MR imaging examinations in 7 519 women and 26 866 screening mammograms. Finally, the time interval between the screening mammogram and MR imaging studies was variable.
The results of this study suggest that annual screening with breast MR imaging and mammography in women at high risk is effective for diagnosis of invasive cancers that are predominantly smaller than a centimeter and node negative. In a high-risk screening program combining annual MR imaging and mammography, breast MR imaging is preferred for detection of invasive cancers, 65% cancers detected at mammography were DCIS, and the interval cancer rate was lower compared with that of the general population.
Advances in Knowledge
■ Breast MR imaging allowed detection of 118 of 144 (82%) of all invasive cancers in women at high risk for cancer who were screened with mammography and MR imaging.
■ In a high-risk screening program, 38 of 43 (88%) mammographic screening-detected cancers manifested as calcifications and 28 (65%) were ductal carcinoma in situ.
■ Of the 222 cancers detected in women at high risk who were screened with both MR imaging and mammography, 12 (5%) were interval cancers.
Implications for Patient Care
■ A combined breast cancer screening program with annual MR imaging and mammography reduced the interval cancer rate compared with that in the general population.
■ Because breast MR imaging allows detection of 118 or 144 (82%) invasive cancers, and most of the cancers detected at mammography were ductal carcinoma in situ, annual breast MR imaging may be the most effective single modality to screen women at high risk for biologically important invasive cancers.
Received June 28, 2015; revision requested August 25; revision received January 6; accepted January 28; final version accepted February 22.
From the 2014 RSNA Annual Meeting.
Supported by the National Cancer Institute (P30 CA008748).
Disclosures of Conflicts of Interest: J.S.S. disclosed no relevant relationships. S.S. disclosed no relevant relationships. J.B. disclosed no relevant relationships. J.K. disclosed no relevant relationships. T.H. disclosed no relevant relationships. D.D.D. disclosed no relevant relationships. C.H.L. disclosed no relevant relationships. E.A.M. disclosed no relevant relationships. C.E.C. disclosed no relevant relationships.
Abbreviations:
- DCIS
- ductal carcinoma in situ
- ER
- estrogen receptor
- HER2
- human epidermal growth factor receptor 2
- PR
- progesterone receptor
References
- 1.Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am 2004;42(5):793–806, v. [DOI] [PubMed] [Google Scholar]
- 2.Tabár L, Vitak B, Chen TH, et al. Swedish two-county trial: impact of mammographic screening on breast cancer mortality during 3 decades. Radiology 2011;260(3):658–663. [DOI] [PubMed] [Google Scholar]
- 3.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2002;137(5 Part 1):347–360. [DOI] [PubMed] [Google Scholar]
- 4.Tilanus-Linthorst M, Verhoog L, Obdeijn IM, et al. A BRCA1/2 mutation, high breast density and prominent pushing margins of a tumor independently contribute to a frequent false-negative mammography. Int J Cancer 2002;102(1):91–95. [DOI] [PubMed] [Google Scholar]
- 5.Brekelmans CT, Seynaeve C, Bartels CC, et al. Effectiveness of breast cancer surveillance in BRCA1/2 gene mutation carriers and women with high familial risk. J Clin Oncol 2001;19(4):924–930. [DOI] [PubMed] [Google Scholar]
- 6.Scheuer L, Kauff N, Robson M, et al. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol 2002;20(5):1260–1268. [DOI] [PubMed] [Google Scholar]
- 7.Komenaka IK, Ditkoff BA, Joseph KA, et al. The development of interval breast malignancies in patients with BRCA mutations. Cancer 2004;100(10):2079–2083. [DOI] [PubMed] [Google Scholar]
- 8.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
- 9.Bevers TB, Anderson BO, Bonaccio E, et al. NCCN clinical practice guidelines in oncology: breast cancer screening and diagnosis. J Natl Compr Canc Netw 2009;7(10):1060–1096. [DOI] [PubMed] [Google Scholar]
- 10.Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol 2005;23(33):8469–8476. [DOI] [PubMed] [Google Scholar]
- 11.Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med 2004;351(5):427–437. [DOI] [PubMed] [Google Scholar]
- 12.Leach MO, Boggis CR, Dixon AK, et al. Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: a prospective multicentre cohort study (MARIBS). Lancet 2005;365(9473):1769–1778. [DOI] [PubMed] [Google Scholar]
- 13.Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA 2004;292(11):1317–1325. [DOI] [PubMed] [Google Scholar]
- 14.Hartman AR, Daniel BL, Kurian AW, et al. Breast magnetic resonance image screening and ductal lavage in women at high genetic risk for breast carcinoma. Cancer 2004;100(3):479–489. [DOI] [PubMed] [Google Scholar]
- 15.Lehman CD, Isaacs C, Schnall MD, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology 2007;244(2):381–388. [DOI] [PubMed] [Google Scholar]
- 16.Sardanelli F, Podo F, D’Agnolo G, et al. Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (HIBCRIT study): interim results. Radiology 2007;242(3):698–715. [DOI] [PubMed] [Google Scholar]
- 17.Lehman CD, Blume JD, Weatherall P, et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer 2005;103(9):1898–1905. [DOI] [PubMed] [Google Scholar]
- 18.Warner E, Messersmith H, Causer P, Eisen A, Shumak R, Plewes D. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Ann Intern Med 2008;148(9):671–679. [DOI] [PubMed] [Google Scholar]
- 19.D’Orsi CJ, Bassett LW, Berg WA, et al. Breast Imaging Reporting and Data System: ACR BI-RADS-Mammography. 4th ed. Reston, Va: American College of Radiology, 2003. [Google Scholar]
- 20.Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch Pathol Lab Med 2010;134(7):e48–e72. [DOI] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31(31):3997–4013. [DOI] [PubMed] [Google Scholar]
- 22.Evans DG, Kesavan N, Lim Y, et al. MRI breast screening in high-risk women: cancer detection and survival analysis. Breast Cancer Res Treat 2014;145(3):663–672. [DOI] [PubMed] [Google Scholar]
- 23.Alvarado M, Ozanne E, Esserman L. Overdiagnosis and overtreatment of breast cancer. Am Soc Clin Oncol Educ Book 2012:e40–e45. [DOI] [PubMed] [Google Scholar]
- 24.Kuerer HM. Ductal carcinoma in situ: treatment or active surveillance? Expert Rev Anticancer Ther 2015;15(7):777–785. [DOI] [PubMed] [Google Scholar]
- 25.Ernster VL, Ballard-Barbash R, Barlow WE, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst 2002;94(20):1546–1554. [DOI] [PubMed] [Google Scholar]
- 26.Kong I, Narod SA, Taylor C, et al. Age at diagnosis predicts local recurrence in women treated with breast-conserving surgery and postoperative radiation therapy for ductal carcinoma in situ: a population-based outcomes analysis. Curr Oncol 2014;21(1):e96–e104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vicini FA, Shaitelman S, Wilkinson JB, et al. Long-term impact of young age at diagnosis on treatment outcome and patterns of failure in patients with ductal carcinoma in situ treated with breast-conserving therapy. Breast J 2013;19(4):365–373. [DOI] [PubMed] [Google Scholar]
- 28.Hiramatsu H, Bornstein BA, Recht A, et al. Local recurrence after conservative surgery and radiation therapy for ductal carcinoma in situ: Possible importance of family history. Cancer J Sci Am 1995;1(1):55–61. [PubMed] [Google Scholar]
- 29.Lowery JT, Byers T, Hokanson JE, et al. Complementary approaches to assessing risk factors for interval breast cancer. Cancer Causes Control 2011;22(1):23–31. [DOI] [PubMed] [Google Scholar]
- 30.Porter PL, El-Bastawissi AY, Mandelson MT, et al. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 1999;91(23):2020–2028. [DOI] [PubMed] [Google Scholar]
- 31.Gilliland FD, Joste N, Stauber PM, et al. Biologic characteristics of interval and screen-detected breast cancers. J Natl Cancer Inst 2000;92(9):743–749. [DOI] [PubMed] [Google Scholar]
- 32.Ng AK, Garber JE, Diller LR, et al. Prospective study of the efficacy of breast magnetic resonance imaging and mammographic screening in survivors of Hodgkin lymphoma. J Clin Oncol 2013;31(18):2282–2288. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein SP, Localio AR, Conant EF, Rosen M, Thomas KM, Schnall MD. Multimodality screening of high-risk women: a prospective cohort study. J Clin Oncol 2009;27(36):6124–6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowry KP, Lee JM, Kong CY, et al. Annual screening strategies in BRCA1 and BRCA2 gene mutation carriers: a comparative effectiveness analysis. Cancer 2012;118(8):2021–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]