Abstract
Colorectal cancer (CRC) is a major public health problem. Early CRC detection, pretherapeutic responsiveness prediction, and postoperative micrometastasis monitoring are the hallmarks for successful CRC treatment. Here, the methodologies used for detecting circulating tumor cells (CTCs) from CRC are reviewed. In addition to the traditional CRC biomarkers, the persistent presence of posttherapeutic CTCs indicates resistance to adjuvant chemotherapy and/or radiotherapy; hence, CTCs also play a decisive role in the subsequent relapse of CRC. Moreover, the genetic and phenotypic profiling of CTCs often differs from that of the primary tumor; this difference can be used to select the most effective targeted therapy. Consequently, studying CTCs can potentially individualize treatment strategies for patients with CRC. Therefore, CTC detection and characterization may be valuable tools for refining prognosis, and CTCs can be used in a real-time tumor biopsy for designing individually tailored therapy against CRC.
Introduction
Colorectal cancer (CRC) is the second and third most commonly diagnosed cancer among women and men worldwide, respectively, with more than 1.2 million new cases and 608,700 deaths being estimated in 2008 [1]. Early CRC detection is the hallmark of successful treatment. Fecal occult blood tests show high false-positive rates, and other diagnostic methods such as double-contrast barium enemas, flexible sigmoidoscopy, and colonoscopy are highly invasive; therefore, these are not preferable for broad screening [2], [3], [4], [5]. With recent advances in imaging technology and other diagnostic modalities, including computed tomography and magnetic resonance imaging, sufficient sensitivity (e.g., detection of tumor nodules of ≥1 cm in diameter) has been achieved; however, high costs and radiation exposure restrict their use for screening [6], [7]. Therefore, more reliable, relatively inexpensive, and noninvasive methods are required for early diagnosis of CRC.
Curative surgery remains the mainstay of CRC therapy; however, approximately half of the patients receiving surgery alone ultimately relapse and die of metastatic CRC (mCRC) [8]. Although the treatment of advanced CRC using a multidisciplinary approach has improved considerably, patients with postoperative relapse or mCRC still have poor prognosis [9]. The recurrence rate was 33.6% in stage III colon cancer patients receiving a postoperative FOLFOX4 [(oxaliplatin + leucovorin + fluorouracil (FU)] regimen during a 5-year MOSAIC follow-up period [10] and 27.8% in stage II and III colon cancer patients using the same adjuvant regimen in a 4-year National Surgical Adjuvant Breast and Bowel Project (NSABP) protocol C-07 study [11]. For several decades, efforts have been expended on the early detection of recurrent tumors to ensure adequate and effective treatment and improve patients' prognoses [12]. Undetected micrometastatic tumor cells with reduced response to chemotherapeutic regimens contribute to the failure of primary curative surgery with subsequent adjuvant chemotherapy in patients with advanced CRC [13].
Although cancer biomarker discovery has rapidly proliferated and numerous biomarkers have been reported, relatively few of these are in clinical use. Some biomarkers do not translate into clinical practice, probably because of inherent technical challenges in their testing; in most cases, this failure is engendered by overlaps in the ranges of normal individuals and cancer patients, hindering an accurate distinction. Identifying specific colon tumor-associated molecular markers and developing accurate assays for effective disease monitoring would considerably improve the early diagnosis of recurrence, leading to more effective treatment [14]. Tumor relapse after curative resection of CRC and adjuvant chemotherapy is attributed to tumor cell dissemination and resistance to adjuvant chemotherapy. Heterogeneous tumor behaviors and individual patient responses to chemotherapeutic agents lead to variable outcomes. Therefore, the detection of tumor-shed cells in the bloodstream is highly critical for early identification of postoperative and/or adjuvant chemotherapeutic patients with CRC requiring further optimal therapy [15].
Primary tumors begin shedding neoplastic cells into the circulation at an early stage [16], [17], and approximately 106 cells are shed daily per gram of tumor [18]. The presence of circulating tumor cells (CTCs) was confirmed with Engell's documentation of cancer cells in the circulation in 1955 [19]. CTCs constitute a heterogeneous population of cells with different biological characteristics and are often different from their respective primary counterparts. Because early detection is one of the most effective means of reducing cancer mortality, CTCs can potentially aid in achieving an early noninvasive early diagnosis of cancer [20], [21]. The genetic and phenotypic profiling of CTCs often differs from that of primary tumors, and it can be used to select the most effective targeted therapy [22]. CTC characterization at different time points during the course of disease may provide useful predictive information for selecting the most appropriate treatment. Moreover, CTC detection is correlated with disease stage, relapse rate, and survival.
Traditional Clinical Outcome Indicators among Patients with CRC
Clinicopathological Indicators
The anatomic extent of tumor (pTNM staging) and the residual tumor status following treatment (residual tumor or R classification) are the strongest predictors of outcome for patients with CRC. A careful pTNM classification enables an accurate estimation of prognosis; therefore, it can be considered the gold standard for analyzing the results of any treatment [23]. The 5-year survival rates observed after R0 resection of CRC were 55% to 60%, but for R1 and R2 resections, they were approximately 5% each [23].
Similar data were reported by a prospective multicenter observational study conducted by the German Study Group Colo-Rectal Carcinoma: The most critical tumor-related prognostic factors following surgical treatment were residual tumor status and anatomic extent, as described through the Union for International Cancer Control (UICC) pTNM stage grouping [24]. The locoregional recurrence rate is influenced by tumor-related (e.g., stage and tumor site) and treatment-related factors [24]. The observed 5-year survival rates [95% confidence intervals (CIs)] were 55% (52%-58%) for R0 and only 7% (3%-11%) for R1 and R2 resections. Following R0 resection, the 5-year survival rates varied according to the disease stages: pT, 24%-74%, and pN, 33%-68% [24]. Moreover, according to the stages, the 5-year survival rates (95% CIs) were 74% (68%-80%) for stage I, 62% (56%-68%) for stage II, 40% (35%-45%) for stage III, and 9% (0%-21%) for stage IV CRC. Based on the 5-year survival rates (95% CIs), stage III CRC was prognostically inhomogeneous: pN1, 47% (39%-55%), pN2 to 3, 34% (27%-41%; P < .01) [24].
The conventional pathological variables used for predicting postoperative relapse in stage II CRC patients who have undergone curative resection are as follows: the depth of tumor invasion (P < .001), presence of vascular invasion (P < .001), presence of perineural invasion (P = .048), and number of examined lymph nodes (P = .031) [25]. Perineural invasion, a distinct pathological entity, is less commonly observed among patients with CRC compared with lymphovascular invasion [26]. Zorzos et al. reported a significant association of perineural invasion with overexpression of P-glycoprotein, a multidrug-resistant protein [27], in patients with colon cancer; this partially clarified the significant role of perineural invasion in CRC patients with systemic chemotherapy resistance [27]. Poeschl et al. reported that perineural invasion in postoperative specimens of CRC patients was significantly associated with several histopathological variables indicating aggressive tumor behavior, such as lymphatic invasion, venous invasion, tumor budding, an infiltrative tumor growth pattern, and an incomplete tumor-free resection margin [28]. The 5-year disease-free survival rates of patients with perineural invasion (11%) were significantly poorer than those of patients without perineural invasion (68%) [28]. Similarly, Lu et al. reported that perineural invasion is a significant independent predictor of postchemotherapeutic relapse in patients with stage III colon cancer [29].
Tumor Markers
The carcinoembryonic antigen (CEA) test measures the amount of CEA—a protein that may appear in the blood of patients with certain types of cancer, particularly CRC; it may also be present in patients with pancreatic, breast, ovarian, or lung cancer. Pretherapeutic and posttherapeutic increases in serum CEA levels among patients with CRC can predict deeper local invasion of tumors, higher occult metastasis risks, and higher posttherapeutic relapse rates [30], [31]. Although CEA is a tumor marker widely used for following patients with CRC, its lack of sensitivity has yet to be resolved. Sorbye and Dahl [32] reported a transient CEA surge (15%; 4/27) in mCRC patients receiving oxaliplatin-based chemotherapy; detection of this inappropriate CEA elevation could inaccurately direct the therapeutic protocol of a patient with CRC toward further disease progression. The 2006 update of The American Society of Clinical Oncology recommendations states that caution should be used when interpreting an increased CEA levels during the first 4 to 6 weeks of a new therapy because spurious early increases may occur, particularly after oxaliplatin administration [33]. A retrospective study identified that low serum albumin levels (P = .011), advanced UICC stage (P < .001), and high serum CEA levels (P < .001) were independent prognostic factors for cancer-specific survival. Furthermore, a multivariate analysis showed that an age of ≥65 years, advanced UICC stage, and high CEA levels (all P < .001) were independent prognostic factors for overall survival [34]. Preoperative serum CEA and albumin levels as well as age are supplementary to UICC staging systems for predicting survival among stage II and III CRC patients undergoing surgical treatment. In addition to the conventional UICC staging system, considering the additional characteristics of the prognostic factors might be imperative in patients with CRC before surgical treatment.
Other Relevant Novel Pathological Factors
The collaborative study (RASCAL study) was to clarify the association between KRAS mutations, patient outcome, and tumor characteristics by use of data from CRC patients worldwide [35]. KRAS gene mutations were associated with increased risk of relapse and death in the RASCAL study [35]. The epidermal growth factor receptor (EGFR) is a major therapeutic target in CRCs [36]. Activating mutations of the KRAS gene stimulates the RAS/RAF/MAPK pathway independent of EGFR activation; therefore, CRCs with KRAS mutations are resistant to EGFR inhibitors [37]. CRC patients with the BRAF mutation tend to have a poor prognosis [38], [39].
Vascular endothelial growth factor (VEGF), a crucial predictor of early postoperative relapse in patients with stage I to III CRC, may facilitate identifying patients who would benefit from intensive follow-up and therapeutic programs. VEGF overexpression can predict not only early postoperative relapse but also disease-free survival (P < .001) and overall survival (P = .002) [40]. However, the coexistence of cyclin D1 and VEGF expression might be a poor prognostic factor for stage I to III CRC patients after curative resection [41]. Reduced peritherapeutic VEGF expression could be a predictor of responsiveness to first-line FOLFIRI + bevacizumab therapy in patients with mCRC [42].
Patients with mCRC expressing high EGFR levels are more likely to exhibit higher progression-free and overall survival rates when treated with cetuximab + chemotherapy (all P < .05) [43]. In 2013, Huang et al. demonstrated that EGFR expression is of prognostic value for patients with metachronous mCRC [44]. Positive EGFR expression was a significant independent prognostic factor for disease-free survival (hazard rate = 4.012; 95% CI, 1.130-8.445; P = .006) and overall survival (hazard rate = 3.090; 95% CI, 1.477-10.900; P = .028) in patients with metachronous mCRC [44].
The genetic polymorphisms of excision repair cross‐complementation group 1 and X‐ray repair cross‐complementing protein 1 may be useful in predicting clinical outcomes among Taiwanese mCRC patients treated with FOLFOX-4 [45]. Furthermore, excision repair cross‐complementation group 1 overexpression is a predictor of not only early failure but also disease-free survival (P < .001) and overall survival (P < .001) in stage III CRC patients undergoing FOLFOX-4 adjuvant chemotherapy [46].
Microsatellite instability (MSI) is a clonal change in the number of repeated DNA nucleotide units in microsatellites. Although most CRCs develop through a chromosomal instability pathway, approximately 12% to 15% have deficient DNA mismatch repair (dMMR) that is characterized in the tumor by MSI. Immunohistochemistry for MMR proteins can be applied as a screening test or a supportive test for MSI analysis [47]. 5-FU–based adjuvant chemotherapy does not improve the outcome of stage II or III MSI tumors [48]. Available data indicated that patients with stage II dMMR CRCs have an excellent prognosis and do not benefit from 5-FU–based adjuvant chemotherapy, which supports their recommended management through surgery alone. By contrast, the benefit of the standard adjuvant chemotherapy by using the FOLFOX regimen in patients with stage III dMMR CRC requires further research; therefore, all patients should be treated with standard adjuvant FOLFOX [49].
Cell-free DNA fragments are shed into the bloodstream by cells undergoing apoptosis or necrosis, and the load of circulating cell-free DNA (cfDNA) correlates with tumor staging and prognosis. Moreover, recent advances in the sensitivity and accuracy of DNA analyses have enabled genotyping of cfDNA for somatic genomic alterations in tumors. The ability to detect and quantify tumor mutations has proven effective in tracking tumor dynamics in real time and as a liquid biopsy that can be used for various previously unfeasible clinical and investigational applications [50]. In recent years, cfDNA analysis as a potential screening test for CRC has been the prime focus [51]. The detection of epigenetic and genetic alterations in cfDNA, such as DNA methylation and mutations, and related RNAs was reported to improve cancer detection on the basis of unique, CRC-specific patterns. CRC-specific nucleic acid biomarkers in peripheral blood can be potential screening markers [51].
Comparison of Traditional Method and Array Technique for Detecting CTCs
Immunocytochemistry, Immunohistochemistry, and Reverse-Transcriptase Polymerase Chain Reaction
CTCs are present in the peripheral blood and possess antigenic and genetic characteristics of a specific tumor type [52]. Active angiogenesis may occur in cancer tissues growing to 2 mm in diameter [53]. CTCs are often detected in the blood of patients with cancer [54], [55], [56]. The 2000s, the commonly used techniques for detecting nucleic acid material in disseminated tumor cells, were antibody-based assays (e.g., immunohistochemistry) [57], [58] and polymerase chain reaction (PCR), reverse-transcriptase PCR (RT-PCR), and real-time quantitative PCR (Q-PCR) assays; these enabled the sensitive detection of CTCs [55], [59], [60], [61] and minute quantities of tumor-related molecular markers in the peripheral blood of patients with various early or advanced cancer type.
The diagnosis and therapy of early-stage CRC tumors can potentially reduce the morbidity and mortality among patients with CRC [24]. Membrane arrays can also considerably improve the diagnosis rate of early-stage CRC [62]. In 2006, for CTC detection, Wang et al. used RT-PCR to detect human telomerase reverse transcriptase (hTERT), cytokeratin (CK)-19, CK-20, and CEA mRNA in the peripheral blood of 72 patients with CRC and 30 healthy individuals [63]; their results revealed that RT-PCR is feasible for detecting CEA mRNA and that it may be a promising tool for early prediction of micrometastatic CTCs in patients with CRC [63]. Moreover, this postoperative CTC detection is helpful in the earlier prediction of postoperative relapse in CRC patients with normal perioperative serum CEA levels, with a median lead time of 6 months before detection of actual elevated CEA levels [64]. These data suggest that the development of a more effective marker than CEA is required for monitoring the response of mCRC patients to systemic therapy.
Because CTCs are usually found at very low frequencies in normal peripheral blood mononuclear cells, tumor cell enrichment techniques including density gradient centrifugation (Ficoll-Hypaque separation) and immunomagnetic or size filtration procedures are often used to enrich tumor cells before their detection [65], [66]. Although immunocytochemistry enables the morphological assessment of stained cells, molecular assays are generally more sensitive [67], [68]. The use of different methodologies for CTC detection has shown conflicting results, and the lack of a standardized technology impedes the implementation of CTC measurement in routine clinical practice. In addition to the obvious and significant differences in CTC detection rates among the molecular methods, such methods entail analyzing only one molecular target per test [55], [59], [60], [61]. Because of the heterogeneity of gene marker expression in peripheral blood, multimarker assays are considered more reliable and sensitive than single-marker assays are. [52], [54], [56], [69]. A panel of molecular markers analyzed using the gene chip technique would be necessary to increase the sensitivity, specificity, and accuracy of CTC detection [52]. Therefore, well-standardized detection methods for multiple CTC-related markers are highly required.
Veridex's Cell Search Assay
CTCs were enumerated with immunomagnetic separation from 7.5 ml of blood by the CellSearch System (Veridex LLC, Raritan, NJ), and the detection rate of no less than two CTCs occurred at 30% (99 of 333) in CRCs [70]. CTCs were defined as epithelial cell adhesion molecule isolated intact cells staining positive for cytokeratin and negative for CD45, and the number of CTCs before and during treatment was an independent predictor of progression-free survival and overall survival in patients with mCRC [71]. Baseline CTC count (> or =3 or <3 CTCs/7.5 ml of blood) was an important prognostic factor within specific subgroups defined by treatment or patient characteristics [72].
Membrane Arrays
Although we previously showed that real-time Q-PCR for CEA mRNA could be a promising tool for early detection of micrometastatic CTCs [63], using it for detecting multiple markers can be time- and labor-intensive procedures in clinical practice. In 2004, Wang et al. suggested that identifying CTC DNA through molecular detection of mutations in APC, KRAS, and TP53 may facilitate early detection of postoperative recurrence or metastases in patients with CRC [73]. In 2005, cDNA microarray technology was applied to identify colorectal tumor–related functional genes, which were overexpressed continuously from colorectal adenoma to adenocarcinoma [74]; in this robust biochip assay, using a panel of informative mRNA markers was imperative for simultaneous high-quality detection of CTC. Among the 23 genes used, 22 were involved in cell motility, cell adhesion, chemokine activity, signal transduction, cytoskeleton organization, proteolysis, apoptosis, and cell proliferation [74]; the identified genes provided valuable information regarding CRC carcinogenesis and metastasis and represented potential novel targets for new strategies of CRC diagnosis [74].
In 2006, our study group evaluated the simultaneous detection of multiple mRNA markers by using a colorimetric membrane array approach in the peripheral blood of CRC patients for early diagnosis [62], [75]. The identified CRC-related oligonucleotides were synthesized and then spotted on a nylon membrane to construct a CRC diagnostic genechip. Digoxigenin-labeled cDNA was amplified by using RT-PCR from the peripheral blood of the patients with CRC and hybridized to the gene chip. Consequently, hybridization signals were detected through color development. Furthermore, Yeh et al. constructed a CRC diagnostic gene chip including six markers (CK-19, CK-20, CEA, REG4, uPA, and TIAM1 mRNA) for further clinical evaluation; the sensitivity (88.8%), specificity (87.8%), and accuracy (88.2%) of this membrane array-based diagnostic method with multiple CRC markers were much higher than those of methods entailing the use of single markers [62]. We demonstrated a significantly high correlation between real-time Q-PCR and the membrane array method in the detection of CTCs [75]; the membrane array results were strongly associated with those of real-time Q-PCR (P < .001), and the sensitivity and specificity of the colorimetric membrane array method for CTC detection were 94.3% and 94%, respectively [75]. The membrane array for detecting CTC-related multiple mRNA markers from peripheral blood could be used not only for early CRC diagnosis but also for postoperative surveillance of patients with CRC [20], [62], [64], [75].
Weighted Enzymatic Chip Array
Although the colorimetric membrane array method can be a promising approach for the clinical detection of CTCs in patients with CRC, its sensitivity, specificity, and accuracy must still be improved. In the membrane array method, each gene is calculated using the same value, and the corresponding outcome is interpreted; however, this prevents the differentiation of the importance of genes in the prognosis of specific diseases — a major limitation impeding the clinical application of this technique [76]. In addition, digoxigenin used on colorimetric membrane array platform is expensive for laboratory diagnosis, and their operating technique requires skill. Therefore, our research team developed a cheaper new-generation gene chip operation platform that uses the biotin-avidin enzyme system to replace the conventional digoxigenin system. Furthermore, we weighted multiple target genes, those involved in cancer development, on the same gene chip for improving the accuracy of CTC detection and successfully established the weighted enzymatic chip array (WEnCA) platform [77]. WEnCA has great potential clinical applications for CTC detection: In Taiwan, the use of this innovative, high-throughput technique with multiple mRNA markers significantly improved the clinical diagnosis of early CRC and distinguished patients with CRC from individuals with sufficient accuracy and in shorter time and lower cost [76], [77].
CTCs as Surrogate Markers for Determining Clinical Outcomes of CRC
Chemotherapy Predictive Markers
A recurrence rate of approximately 30% has been reported among colon cancer patients receiving the postoperative FOLFOX4 adjuvant regimen [10], [11]. Relapse in stage III CRC patients receiving postoperative adjuvant chemotherapy is attributed mainly to reduced response to the chemotherapeutic regimen. Therefore, detecting CTCs can be valuable for identifying potential metastases earlier and selecting chemotherapy-resistant patients who would benefit from other therapeutic regimens.
The response of CRC patients to cetuximab can be determined using the KRAS mutational status of the tumor [78]. We previously developed membrane arrays as a promising tool to detect CTCs with KRAS in patients with malignancies [20]. The detection of KRAS mutations has been clinically applied to mCRC patients being treated with cetuximab and FOLFOX4 or FOLFIRI (leucovorin +5-FU + irinotecan) [79]. Patients with CTC carrying wild-type KRAS show longer progression-free survival and overall survival when treated with cetuximab + chemotherapy (P < .0001) [79]. The detection of KRAS in peripheral blood may predict the response to cetuximab plus chemotherapy in patients with mCRC [79]. These findings indicate that the detection of KRAS mutational status in CTCs by using gene expression array has potential clinical applications for selecting mCRC patients who may benefit from cetuximab therapy.
The persistent presence of postchemotherapeutic CTCs is a potential powerful surrogate marker for determining clinical outcomes in stage III colon cancer patients receiving adjuvant mFOLFOX chemotherapy [29].
Prognostic Marker
CRC is a frequently lethal disease with heterogeneous outcomes and drug responses. To resolve inconsistencies among the reported gene expression–based CRC classifications and facilitate clinical translation, Guinney et al. formed an international consortium dedicated to large-scale data sharing and analytics across expert groups [80]. They showed marked interconnectivity between six independent classification systems coalescing into four consensus molecular subtypes (CMSs) with distinguishing features: CMS1 (microsatellite instability immune, 14%), hypermutated, microsatellite unstable and strong immune activation; CMS2 (canonical, 37%), epithelial, marked WNT and MYC signaling activation; CMS3 (metabolic, 13%), epithelial and evident metabolic dysregulation; and CMS4 (mesenchymal, 23%), prominent transforming growth factor–beta activation, stromal invasion, and angiogenesis. Samples with mixed features (13%) possibly represent transition phenotype or intratumoral heterogeneity [80].
In advanced-stage CRC, the availability of CTCs may enable more efficient disease monitoring, particularly in patients with mCRC showing no measurable increase in the levels of CEA or other markers. Our recent investigations have demonstrated that the persistent presence of postoperative CTCs is a poor prognostic factor for patients with CRC after curative resection [81], [82].
The colorimetric membrane array method has been evaluated as a potential diagnostic and postoperative surveillance tool for detecting CTCs by using four mRNA markers (hTERT, CK-19, CK-20, and CEA mRNA) in the peripheral blood of CRC patients with normal perioperative serum CEA levels [64]. CRC patients expressing all four mRNA markers showed significantly poorer survival rates than did those expressing fewer than four markers. Therefore, membrane array is helpful for early prediction of postoperative relapse in CRC patients with normal CEA levels [64].
Adjuvant chemotherapy is not routinely recommended in patients diagnosed with UICC stage II CRC. However, up to 30% of patients with stage II CRC relapse within 5 years of surgery because of recurrent CRC or mCRC. Identifying reliable prognostic factors in high-risk patients with stage II CRC patients is imperative. In 2007, a colorimetric membrane array comprising a panel of mRNA markers was used to detect CTCs in the peripheral blood of 194 patients with stage II CRC for identifying a subgroup of patients at high risk for relapse [25]; the study reported that the assay is a potential auxiliary tool to conventional clinicopathological variables used for predicting of postoperative relapse in stage II CRC patients who have undergone curative resection [25]. In addition, Uen et al. and Lu et al. have demonstrated that the persistent presence of postoperative CTCs is a poor prognostic factor for patients with CRC after curative resection [81], [82]. In 2008, among 438 stage I to III CRC patients who underwent curative resection, the persistent presence of CTCs was strongly correlated with poorer relapse-free survival rates (all P < .001) [81]. Similarly, the presence of persistent postoperative CTCs was strongly correlated with poorer disease-free and overall survival rates (both P < .001) in patients with UICC stage II/III colon cancer patients [82].
Conclusions
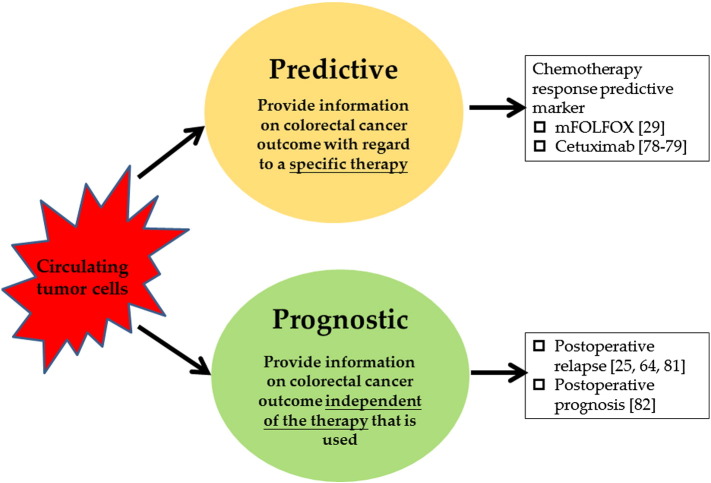
CTC detection and characterization may be valuable tools to refine prognosis, and CTCs can be predictive biomarkers in a real-time tumor biopsy for designing individually tailored cancer therapy (Figure 1). Through the detection of CTC-related mRNA markers, in addition to Veridex's CellSearch assay, the gene chip techniques, including membrane array and WEnCA, are reliable for early diagnosis and prognosis prediction in patients with CRC. In addition to the assessments of pathological marker (e.g., perineural invasion and lymphovascular invasion) or traditional tumor marker (e.g., serum CEA) levels, the persistent presence of posttherapeutic CTCs in CRC patients is a potentially valuable tool for predicting relapse. These tools might gender therapeutic considerations and options such as curative surgery, prolonged adjuvant chemotherapy and radiotherapy durations, as well as change in therapeutic agents. However, additional validation studies are warranted for applying CTCs as prognostic factors or therapeutic strategies and for developing new biomarkers for CRC.
Figure 1.
CTC detection and characterization may be a valuable tool to refine prognosis, and CTCs can be predictive biomarkers.
Competing Interest
The authors declare no potential conflicts of interest.
Authors' Contributions
M.-Y. H. designed the study, reviewed the literature, wrote the first draft, and prepared the schemes. H.-L. T. assisted in editing. J.-J. H. and J.-Y. W. critically reviewed and revised the paper.
Footnotes
Funding: The work was supported by the Ministry of Science and Technology (MOST103-2325-B-037-005, MOST 103-2314-B-037-010-MY3); Kaohsiung Medical University Hospital (KMUH103-3M58, KMUH104-4M46, KMUH104-4M51); Center for Biomarkers and Biotech Drugs, Kaohsiung Medical University, Aim for the Top Universities Grant (KMU-TP104C00, KMU-TP104C01, KMU-TP104C02); the Grant of Biosignature in Colorectal Cancers, Academia Sinica, Taiwan; Excellence for Cancer Research Center Grant, funded by the Ministry of Health and Welfare (MOHW105-TDU-B-212-134007), Health and welfare surcharge of tobacco products, Taiwan, Republic of China.
Contributor Information
Joh-Jong Huang, Email: jjhua511227@gmail.com.
Jaw-Yuan Wang, Email: cy612112@ms14.hinet.net.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284(15):1954–1961. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 3.Jumba GE. Diagnostic challenges in detection of colorectal cancer. East Afr Med J. 2008;85(6):257–258. [PubMed] [Google Scholar]
- 4.Ahlquist DA, Shuber AP. Stool screening for colorectal cancer: evolution from occult blood to molecular markers. Clin Chim Acta. 2002;315(1–2):157–168. doi: 10.1016/s0009-8981(01)00712-4. [DOI] [PubMed] [Google Scholar]
- 5.Solomon MJ, McLeod RS. Periodic health examination, 1994 update: 2. Screening strategies for colorectal cancer. Canadian Task Force on the Periodic Health Examination. CMAJ. 1994;150(12):1961–1970. [PMC free article] [PubMed] [Google Scholar]
- 6.Lautenbach E, Forde KA, Neugut AI. Benefits of colonoscopic surveillance after curative resection of colorectal cancer. Ann Surg. 1994;220(2):206–211. doi: 10.1097/00000658-199408000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoury DA, Opelka FG, Beck DE, Hicks TC, Timmcke AE, Gathright JB., Jr. Colon surveillance after colorectal cancer surgery. Dis Colon rectum. 1996;39(3):252–256. doi: 10.1007/BF02049461. [DOI] [PubMed] [Google Scholar]
- 8.Obrand DI, Gordon PH. Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon rectum. 1997;40(1):15–24. doi: 10.1007/BF02055676. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher DJ, Kemeny N. Metastatic colorectal cancer: from improved survival to potential cure. Oncology. 2010;78(3–4):237–248. doi: 10.1159/000315730. [DOI] [PubMed] [Google Scholar]
- 10.Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009. 2009;27(19):3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 11.Kuebler JP, Wieand HS, O'Connell MJ, Smith RE, Colangelo LH, Yothers G, Petrelli NJ, Findlay MP, Seay TE, Atkins JN. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25(16):2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez-Moranta F, Salo J, Arcusa A, Boadas J, Pinol V, Bessa X, Batiste-Alentorn E, Lacy AM, Delgado S, Maurel J. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol. 2006;24(3):386–393. doi: 10.1200/JCO.2005.02.0826. [DOI] [PubMed] [Google Scholar]
- 13.Steinert R, Hantschick M, Vieth M, Gastinger I, Kuhnel F, Lippert H, Reymond MA. Influence of subclinical tumor spreading on survival after curative surgery for colorectal cancer. Arch Surg. 2008;143(2):122–128. doi: 10.1001/archsurg.2007.49. [DOI] [PubMed] [Google Scholar]
- 14.Nannini M, Pantaleo MA, Maleddu A, Astolfi A, Formica S, Biasco G. Gene expression profiling in colorectal cancer using microarray technologies: results and perspectives. Cancer Treat Rev. 2009;35(3):201–209. doi: 10.1016/j.ctrv.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Rahbari NN, Aigner M, Thorlund K, Mollberg N, Motschall E, Jensen K, Diener MK, Buchler MW, Koch M, Weitz J. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology. 2010;138(5):1714–1726. doi: 10.1053/j.gastro.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PW, Burchill SA, Selby PJ. The molecular detection of circulating tumour cells. Br J Cancer. 1995;72(2):268–276. doi: 10.1038/bjc.1995.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelkey TJ, Frierson HF, Jr., Bruns DE. Molecular and immunological detection of circulating tumor cells and micrometastases from solid tumors. Clin Chem. 1996;42(9):1369–1381. [PubMed] [Google Scholar]
- 18.Chang YS, di Tomaso E, McDonald DM, Jones R, Jain RK, Munn LL. Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc Natl Acad Sci U S A. 2000;97(26):14608–14613. doi: 10.1073/pnas.97.26.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engell HC. Cancer cells in the circulating blood; a clinical study on the occurrence of cancer cells in the peripheral blood and in venous blood draining the tumour area at operation. Acta Chir Scand Suppl. 1955;201:1–70. [PubMed] [Google Scholar]
- 20.Chen YF, Wang JY, Wu CH, Chen FM, Cheng TL, Lin SR. Detection of circulating cancer cells with K-ras oncogene using membrane array. Cancer Lett. 2005;229(1):115–122. doi: 10.1016/j.canlet.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Hardingham JE, Kotasek D, Sage RE, Eaton MC, Pascoe VH, Dobrovic A. Detection of circulating tumor cells in colorectal cancer by immunobead-PCR is a sensitive prognostic marker for relapse of disease. Mol Med. 1995;1(7):789–794. [PMC free article] [PubMed] [Google Scholar]
- 22.Wang HM, Lin SR, Uen YH, Wang JY. Molecular detection of circulating tumor cells in colorectal cancer patients: from laboratory investigation to clinical implication. Fooyin J Health Sci. 2009;1(1):2–10. [Google Scholar]
- 23.Hermanek P. pTNM and residual tumor classifications: problems of assessment and prognostic significance. World J Surg. 1995;19(2):184–190. doi: 10.1007/BF00308624. [DOI] [PubMed] [Google Scholar]
- 24.Hermanek P, Wiebelt H, Staimmer D, Riedl S. Prognostic factors of rectum carcinoma—experience of the German multicentre study SGCRC. German Study Group Colo-Rectal Carcinoma. Tumori. 1995;81(3 Suppl):60–64. [PubMed] [Google Scholar]
- 25.Uen YH, Lin SR, Wu DC, Su YC, Wu JY, Cheng TL, Chi CW, Wang JY. Prognostic significance of multiple molecular markers for patients with stage II colorectal cancer undergoing curative resection. Ann Surg. 2007;246(6):1040–1046. doi: 10.1097/SLA.0b013e318142d918. [DOI] [PubMed] [Google Scholar]
- 26.Washington MK. Colorectal carcinoma: selected issues in pathologic examination and staging and determination of prognostic factors. Arch Pathol Lab Med. 2008;132(10):1600–1607. doi: 10.5858/2008-132-1600-CCSIIP. [DOI] [PubMed] [Google Scholar]
- 27.Zorzos HS, Lazaris AC, Korkolopoulou PA, Kavantzas NG, Tseleni-Balafouta S, Patsouris ES, Tsavaris NV, Davaris PS. Multidrug resistance proteins and topoisomerase IIalpha expression in colon cancer: association with metastatic potential. Pathology. 2003;35(4):315–318. doi: 10.1080/0031302031000150524. [DOI] [PubMed] [Google Scholar]
- 28.Poeschl E.M., Pollheimer M.J., Kornprat P., Lindtner R.A., Schlemmer A., Rehak P., Vieth M., Langner C. Perineural invasion: correlation with aggressive phenotype and independent prognostic variable in both colon and rectum cancer. J Clin Oncol. 2010;28(21):e358–e360. doi: 10.1200/JCO.2009.27.3581. [author reply e361-352]. [DOI] [PubMed] [Google Scholar]
- 29.Lu CY, Tsai HL, Uen YH, Hu HM, Chen CW, Cheng TL, Lin SR, Wang JY. Circulating tumor cells as a surrogate marker for determining clinical outcome to mFOLFOX chemotherapy in patients with stage III colon cancer. Br J Cancer. 2013;108(4):791–797. doi: 10.1038/bjc.2012.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCall JL, Black RB, Rich CA, Harvey JR, Baker RA, Watts JM, Toouli J. The value of serum carcinoembryonic antigen in predicting recurrent disease following curative resection of colorectal cancer. Dis Colon rectum. 1994;37(9):875–881. doi: 10.1007/BF02052591. [DOI] [PubMed] [Google Scholar]
- 31.Wiratkapun S, Kraemer M, Seow-Choen F, Ho YH, Eu KW. High preoperative serum carcinoembryonic antigen predicts metastatic recurrence in potentially curative colonic cancer: results of a five-year study. Dis Colon rectum. 2001;44(2):231–235. doi: 10.1007/BF02234298. [DOI] [PubMed] [Google Scholar]
- 32.Sorbye H, Dahl O. Carcinoembryonic antigen surge in metastatic colorectal cancer patients responding to oxaliplatin combination chemotherapy: implications for tumor marker monitoring and guidelines. J Clin Oncol. 2003;21(23):4466–4467. doi: 10.1200/JCO.2003.99.200. [DOI] [PubMed] [Google Scholar]
- 33.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, Somerfield MR, Hayes DF, Bast RC., Jr. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24(33):5313–5327. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 34.Sun LC, Chu KS, Cheng SC, Lu CY, Kuo CH, Hsieh JS, Shih YL, Chang SJ, Wang JY. Preoperative serum carcinoembryonic antigen, albumin and age are supplementary to UICC staging systems in predicting survival for colorectal cancer patients undergoing surgical treatment. BMC Cancer. 2009;9:288. doi: 10.1186/1471-2407-9-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andreyev HJ, Norman AR, Cunningham D, Oates JR, Clarke PA. Kirsten ras mutations in patients with colorectal cancer: the multicenter "RASCAL" study. J Natl Cancer Inst. 1998;90(9):675–684. doi: 10.1093/jnci/90.9.675. [DOI] [PubMed] [Google Scholar]
- 36.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 37.Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(10):1626–1634. doi: 10.1200/JCO.2007.14.7116. [DOI] [PubMed] [Google Scholar]
- 38.Sclafani F, Gullo G, Sheahan K, Crown J. BRAF mutations in melanoma and colorectal cancer: a single oncogenic mutation with different tumour phenotypes and clinical implications. Crit Rev Oncol Hematol. 2013;87(1):55–68. doi: 10.1016/j.critrevonc.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 39.Xu Q, Xu AT, Zhu MM, Tong JL, Xu XT, Ran ZH. Predictive and prognostic roles of BRAF mutation in patients with metastatic colorectal cancer treated with anti-epidermal growth factor receptor monoclonal antibodies: a meta-analysis. J Dig Dis. 2013;14(8):409–416. doi: 10.1111/1751-2980.12063. [DOI] [PubMed] [Google Scholar]
- 40.Tsai HL, Yang IP, Lin CH, Chai CY, Huang YH, Chen CF, Hou MF, Kuo CH, Juo SH, Wang JY. Predictive value of vascular endothelial growth factor overexpression in early relapse of colorectal cancer patients after curative resection. Int J Colorectal Dis. 2013;28(3):415–424. doi: 10.1007/s00384-012-1570-z. [DOI] [PubMed] [Google Scholar]
- 41.Tsai HL, Yeh YS, Chang YT, Yang IP, Lin CH, Kuo CH, Juo SH, Wang JY. Co-existence of cyclin D1 and vascular endothelial growth factor protein expression is a poor prognostic factor for UICC stage I-III colorectal cancer patients after curative resection. J Surg Oncol. 2013;107(2):148–154. doi: 10.1002/jso.23243. [DOI] [PubMed] [Google Scholar]
- 42.Tsai HL, Lin CH, Huang CW, Yang IP, Yeh YS, Hsu WH, Wu JY, Kuo CH, Tseng FY, Wang JY. Decreased peritherapeutic VEGF expression could be a predictor of responsiveness to first-line FOLFIRI plus bevacizumab in mCRC patients. Int J Clin Exp Pathol. 2015;8(2):1900–1910. [PMC free article] [PubMed] [Google Scholar]
- 43.Yen LC, Uen YH, Wu DC, Lu CY, Yu FJ, Wu IC, Lin SR, Wang JY. Activating KRAS mutations and overexpression of epidermal growth factor receptor as independent predictors in metastatic colorectal cancer patients treated with cetuximab. Ann Surg. 2010;251(2):254–260. doi: 10.1097/SLA.0b013e3181bc9d96. [DOI] [PubMed] [Google Scholar]
- 44.Huang CW, Tsai HL, Chen YT, Huang CM, Ma CJ, Lu CY, Kuo CH, Wu DC, Chai CY, Wang JY. The prognostic values of EGFR expression and KRAS mutation in patients with synchronous or metachronous metastatic colorectal cancer. BMC Cancer. 2013;13:599. doi: 10.1186/1471-2407-13-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang MY, Huang ML, Chen MJ, Lu CY, Chen CF, Tsai PC, Chuang SC, Hou MF, Lin SR, Wang JY. Multiple genetic polymorphisms in the prediction of clinical outcome of metastatic colorectal cancer patients treated with first-line FOLFOX-4 chemotherapy. Pharmacogenet Genomics. 2011;21(1):18–25. doi: 10.1097/FPC.0b013e3283415124. [DOI] [PubMed] [Google Scholar]
- 46.Huang MY, Tsai HL, Lin CH, Huang CW, Ma CJ, Huang CM, Chai CY, Wang JY. Predictive value of ERCC1, ERCC2, and XRCC1 overexpression for stage III colorectal cancer patients receiving FOLFOX-4 adjuvant chemotherapy. J Surg Oncol. 2013;108(7):457–464. doi: 10.1002/jso.23422. [DOI] [PubMed] [Google Scholar]
- 47.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, Meltzer SJ, Rodriguez-Bigas MA, Fodde R, Ranzani GN. A National Cancer Institute workshop on microsatellite instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58(22):5248–5257. [PubMed] [Google Scholar]
- 48.de la Chapelle A, Hampel H. Clinical relevance of microsatellite instability in colorectal cancer. J Clin Oncol. 2010;28(20):3380–3387. doi: 10.1200/JCO.2009.27.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawakami H, Zaanan A, Sinicrope FA. Microsatellite instability testing and its role in the management of colorectal cancer. Curr Treat Options Oncol. 2015;16(7):30. doi: 10.1007/s11864-015-0348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diaz LA, Jr., Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579–586. doi: 10.1200/JCO.2012.45.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Toth K, Bartak BK, Tulassay Z, Molnar B. Circulating cell-free nucleic acids as biomarkers in colorectal cancer screening and diagnosis. Expert Rev Mol Diagn. 2016;16(2):239–252. doi: 10.1586/14737159.2016.1132164. [DOI] [PubMed] [Google Scholar]
- 52.Racila E, Euhus D, Weiss AJ, Rao C, McConnell J, Terstappen LW, Uhr JW. Detection and characterization of carcinoma cells in the blood. Proc Natl Acad Sci U S A. 1998;95(8):4589–4594. doi: 10.1073/pnas.95.8.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143(2):401–409. [PMC free article] [PubMed] [Google Scholar]
- 54.Baker MK, Mikhitarian K, Osta W, Callahan K, Hoda R, Brescia F, Kneuper-Hall R, Mitas M, Cole DJ, Gillanders WE. Molecular detection of breast cancer cells in the peripheral blood of advanced-stage breast cancer patients using multimarker real-time reverse transcription-polymerase chain reaction and a novel porous barrier density gradient centrifugation technology. Clin Cancer Res. 2003;9(13):4865–4871. [PubMed] [Google Scholar]
- 55.Schuster R, Max N, Mann B, Heufelder K, Thilo F, Grone J, Rokos F, Buhr HJ, Thiel E, Keilholz U. Quantitative real-time RT-PCR for detection of disseminated tumor cells in peripheral blood of patients with colorectal cancer using different mRNA markers. Int J Cancer. 2004;108(2):219–227. doi: 10.1002/ijc.11547. [DOI] [PubMed] [Google Scholar]
- 56.Sher YP, Shih JY, Yang PC, Roffler SR, Chu YW, Wu CW, Yu CL, Peck K. Prognosis of non-small cell lung cancer patients by detecting circulating cancer cells in the peripheral blood with multiple marker genes. Clin Cancer Res. 2005;11(1):173–179. [PubMed] [Google Scholar]
- 57.Wahlberg SS, Schmeits J, Thomas G, Loda M, Garber J, Syngal S, Kolodner RD, Fox E. Evaluation of microsatellite instability and immunohistochemistry for the prediction of germ-line MSH2 and MLH1 mutations in hereditary nonpolyposis colon cancer families. Cancer Res. 2002;62(12):3485–3492. [PubMed] [Google Scholar]
- 58.Giuliani L, Ciotti M, Stoppacciaro A, Pasquini A, Silvestri I, De Matteis A, Frati L, Agliano AM. UDP-glucuronosyltransferases 1A expression in human urinary bladder and colon cancer by immunohistochemistry. Oncol Rep. 2005;13(2):185–191. [PubMed] [Google Scholar]
- 59.Oberg AN, Lindmark GE, Israelsson AC, Hammarstrom SG, Hammarstrom ML. Detection of occult tumour cells in lymph nodes of colorectal cancer patients using real-time quantitative RT-PCR for CEA and CK20 mRNAS. Int J Cancer. 2004;111(1):101–110. doi: 10.1002/ijc.20231. [DOI] [PubMed] [Google Scholar]
- 60.Taback B, Hoon DS. Circulating nucleic acids and proteomics of plasma/serum: clinical utility. Ann N Y Acad Sci. 2004;1022:1–8. doi: 10.1196/annals.1318.002. [DOI] [PubMed] [Google Scholar]
- 61.Chiu ST, Hsieh FJ, Chen SW, Chen CL, Shu HF, Li H. Clinicopathologic correlation of up-regulated genes identified using cDNA microarray and real-time reverse transcription-PCR in human colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14(2):437–443. doi: 10.1158/1055-9965.EPI-04-0396. [DOI] [PubMed] [Google Scholar]
- 62.Yeh CS, Wang JY, Wu CH, Chong IW, Chung FY, Wang YH, Yu YP, Lin SR. Molecular detection of circulating cancer cells in the peripheral blood of patients with colorectal cancer by using membrane array with a multiple mRNA marker panel. Int J Oncol. 2006;28(2):411–420. [PubMed] [Google Scholar]
- 63.Wang JY, Wu CH, Lu CY, Hsieh JS, Wu DC, Huang SY, Lin SR. Molecular detection of circulating tumor cells in the peripheral blood of patients with colorectal cancer using RT-PCR: significance of the prediction of postoperative metastasis. World J Surg. 2006;30(6):1007–1013. doi: 10.1007/s00268-005-0485-z. [DOI] [PubMed] [Google Scholar]
- 64.Wang JY, Lin SR, Wu DC, Lu CY, Yu FJ, Hsieh JS, Cheng TL, Koay LB, Uen YH. Multiple molecular markers as predictors of colorectal cancer in patients with normal perioperative serum carcinoembryonic antigen levels. Clin Cancer Res. 2007;13(8):2406–2413. doi: 10.1158/1078-0432.CCR-06-2054. [DOI] [PubMed] [Google Scholar]
- 65.Alunni-Fabbroni M, Sandri MT. Circulating tumour cells in clinical practice: methods of detection and possible characterization. Methods. 2010;50(4):289–297. doi: 10.1016/j.ymeth.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 66.Zheng S, Lin H, Liu JQ, Balic M, Datar R, Cote RJ, Tai YC. Membrane microfilter device for selective capture, electrolysis and genomic analysis of human circulating tumor cells. J Chromatogr A. 2007;1162(2):154–161. doi: 10.1016/j.chroma.2007.05.064. [DOI] [PubMed] [Google Scholar]
- 67.Daniele L, Annaratone L, Allia E, Mariani S, Armando E, Bosco M, Macri L, Cassoni P, D'Armento G, Bussolati G. Technical limits of comparison of step-sectioning,immunohistochemistry and RT-PCR on breast cancer sentinel nodes: a study on methacarn-fixed tissue. J Cell Mol Med. 2009;13(9B):4042–4050. doi: 10.1111/j.1582-4934.2008.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang HJ, Yang MJ, Yang YH, Hou MF, Hsueh EJ, Lin SR. MMP13 is potentially a new tumor marker for breast cancer diagnosis. Oncol Rep. 2009;22(5):1119–1127. doi: 10.3892/or_00000544. [DOI] [PubMed] [Google Scholar]
- 69.Hoon DS, Wang Y, Dale PS, Conrad AJ, Schmid P, Garrison D, Kuo C, Foshag LJ, Nizze AJ, Morton DL. Detection of occult melanoma cells in blood with a multiple-marker polymerase chain reaction assay. J Clin Oncol. 1995;13(8):2109–2116. doi: 10.1200/JCO.1995.13.8.2109. [DOI] [PubMed] [Google Scholar]
- 70.Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 71.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse M, Mitchell E, Miller MC. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26(19):3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 72.Cohen SJ, Punt CJ, Iannotti N, Saidman BH, Sabbath KD, Gabrail NY, Picus J, Morse MA, Mitchell E, Miller MC. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol. 2009;20(7):1223–1229. doi: 10.1093/annonc/mdn786. [DOI] [PubMed] [Google Scholar]
- 73.Wang JY, Hsieh JS, Chang MY, Huang TJ, Chen FM, Cheng TL, Alexandersen K, Huang YS, Tzou WS, Lin SR. Molecular detection of APC, K- ras, and p53 mutations in the serum of colorectal cancer patients as circulating biomarkers. World J Surg. 2004;28(7):721–726. doi: 10.1007/s00268-004-7366-8. [DOI] [PubMed] [Google Scholar]
- 74.Wang JY, Yeh CS, Tzou WS, Hsieh JS, Chen FM, Lu CY, Yu FJ, Cheng TL, Huang TJ, Lin SR. Analysis of progressively overexpressed genes in tumorigenesis of colorectal cancers using cDNA microarray. Oncol Rep. 2005;14(1):65–72. [PubMed] [Google Scholar]
- 75.Wang JY, Yeh CS, Chen YF, Wu CH, Hsieh JS, Huang TJ, Huang SY, Lin SR. Development and evaluation of a colorimetric membrane-array method for the detection of circulating tumor cells in the peripheral blood of Taiwanese patients with colorectal cancer. Int J Mol Med. 2006;17(5):737–747. [PubMed] [Google Scholar]
- 76.Tsao DA, Yang MJ, Chang HJ, Yen LC, Chiu HH, Hsueh EJ, Chen YF, Lin SR. A fast and convenient new technique to detect the therapeutic target, K-ras mutant, from peripheral blood in non-small cell lung cancer patients. Lung Cancer. 2010;68(1):51–57. doi: 10.1016/j.lungcan.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 77.Yang MJ, Chiu HH, Wang HM, Yen LC, Tsao DA, Hsiao CP, Chen YF, Wang JY, Lin SR. Enhancing detection of circulating tumor cells with activating KRAS oncogene in patients with colorectal cancer by weighted chemiluminescent membrane array method. Ann Surg Oncol. 2010;17(2):624–633. doi: 10.1245/s10434-009-0831-8. [DOI] [PubMed] [Google Scholar]
- 78.Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66(8):3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 79.Yen LC, Yeh YS, Chen CW, Wang HM, Tsai HL, Lu CY, Chang YT, Chu KS, Lin SR, Wang JY. Detection of KRAS oncogene in peripheral blood as a predictor of the response to cetuximab plus chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res. 2009;15(13):4508–4513. doi: 10.1158/1078-0432.CCR-08-3179. [DOI] [PubMed] [Google Scholar]
- 80.Guinney J, Dienstmann R, Wang X, de Reynies A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uen YH, Lu CY, Tsai HL, Yu FJ, Huang MY, Cheng TL, Lin SR, Wang JY. Persistent presence of postoperative circulating tumor cells is a poor prognostic factor for patients with stage I-III colorectal cancer after curative resection. Ann Surg Oncol. 2008;15(8):2120–2128. doi: 10.1245/s10434-008-9961-7. [DOI] [PubMed] [Google Scholar]
- 82.Lu CY, Uen YH, Tsai HL, Chuang SC, Hou MF, Wu DC, Juo SH, Lin SR, Wang JY. Molecular detection of persistent postoperative circulating tumour cells in stages II and III colon cancer patients via multiple blood sampling: prognostic significance of detection for early relapse. Br J Cancer. 2011;104(7):1178–1184. doi: 10.1038/bjc.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]