Abstract
OBJECTIVE: To evaluate the value of anatomic and volumetric functional magnetic resonance imaging (MRI) in early assessment of response to trans-arterial chemoembolization (TACE) in hypovascular liver metastases. METHODS: This retrospective study included 52 metastatic lesions (42 targeted and 10 non-targeted) in 17 patients who underwent MRI before and early after TACE. Two reviewers reported response by anatomic criteria (Response Evaluation Criteria in Solid Tumor [RECIST], modified RECIST [mRECIST], and European Association for the Study of Liver Disease [EASL]) and functional criteria (volumetric apparent diffusion coefficient and contrast enhancement). Treatment endpoint was RECIST at 6 months. A 2-sample paired t test was used to compare the mean changes after intra-arterial therapy. P < .05 was considered statistically significant. RESULTS: Reduction in mRECIST and EASL at 1 month was significant in the whole cohort as well as in responders by RECIST at 6 months, and the changes fulfilled partial response criteria for both metrics in responders. Responders also had significant changes in volumetric apparent diffusion coefficient (P = .01 and P = .03) and contrast enhancement (P < .0001 and P < .0001) at 1 month for both readers, respectively. CONCLUSION: At 1 month post treatment, responders did not fulfill RECIST criteria but fulfilled mRECIST and EASL criteria. In addition, volumetric contrast-enhanced and diffusion-weighted MRI may be helpful in evaluating early treatment response after TACE in hypovascular liver metastases in patients who have failed to respond to initial chemotherapy.
Introduction
The liver is the second most common site of metastatic disease and metastases account for majority of malignant liver lesions since their incidence is 18 to 40 times more common than primary liver tumors [1]. Based on the vascularity, metastatic lesions are categorized as hyper-vascular and hypo-vascular. Hyper-vascular liver metastases, most commonly caused by neuroendocrine tumors, renal cell carcinoma, melanoma and thyroid carcinoma, have early enhancement in hepatic arterial phase (HAP), while hypo-vascular metastases mostly originate from gastrointestinal malignancies, breast and lung cancer, and they demonstrate slower and less intense enhancement in portal venous phase (PVP) [2]. Most of the patients with liver metastasis are not eligible for surgery due to large tumor burden and inadequate remaining liver tissue. These patients may also become resistant to systemic chemotherapy at this advanced stage of the disease. In such cases, loco-regional therapy like intra-arterial therapy (IAT) may be the only treatment modality [3]. Trans-arterial chemoembolization (TACE) is a combination of two tumor treatment techniques: delivering a high concentration of chemotherapy drugs directly to tumor vascular bed and selective embolization of the tumor feeding arteries. Besides having the benefit of lower systemic side effects, it also preserves normal hepatic cells from toxic exposure [4].
Early identification of non-responders helps clinicians avoid futile cost and side effects of un-necessary treatment [5], [6]. The Response Evaluation Criteria in Solid Tumor (RECIST), modified RECIST (mRECIST) and European Association for the Study of Liver Disease (EASL) are well established metrics that are widely used to determine treatment response in malignant liver tumors, especially the hyper-vascular type. However, these metrics have limitations in assessing early response to IAT, since they are measured in a single axial plane and are reader dependent [6], [7], [8]. Besides, tumor necrosis induced by TACE, possible paradoxical enlargement of the lesion early after treatment and heterogeneity of tumor boundaries make these metrics less reliable in assessment of tumor response [6], [9]. To assess treatment response to loco-regional therapies like TACE, assessment of physiologic, functional and metabolic changes in hepatic lesions is beneficial. Functional imaging biomarkers including dynamic contrast enhanced magnetic resonance imaging (MRI), diffusion weighted MRI, MR spectroscopy, and positron emission tomography scan have been recently utilized [5], [6], [9], [10], [11], [12]. IAT-induced cellular necrosis and cellular viability of tumor remnants are reflected by vascularity. Non-viable and viable parts of tumor can be distinguished by differences in contrast enhancement, as necrotic parts of the tumor show decreased or no enhancement, compared to viable tissue which shows increased enhancement [5], [6], [9], [11]. Apparent diffusion coefficient (ADC) acquired by diffusion-weighted MR imaging (DWI), represents cellular integrity and motion of water molecules. Viable tumors are highly cellular with intact cell membrane that restricts water motion and results in lower ADC values, whereas cellular necrosis causes increased permeability of cell membrane and free water movement beyond cells, resulting in a higher ADC value [10], [12]. Previous studies have shown both decreased enhancement and increased ADC values early after IAT in different primary and metastatic liver tumors [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. Most of these studies assessed hypervascular liver tumors like hepatocellular carcinoma and metastases from neuroendocrine tumors, and very few have assessed hypovascular lesions. Therefore, our objective was to determine if the volumetric changes of contrast-enhancement and ADC value could help to detect early treatment response following TACE in hypo-vascular liver metastases in patients who have failed to respond to initial chemotherapy.
Materials and Methods
Study Population
This Health Insurance Portability and Accountability Act compliant retrospective study was approved by our institutional review board and waiver for informed consent was obtained. Medical records of patients with liver metastases who have failed to respond to conventional chemotherapy and undergone TACE at our institution from January 2008 to September 2014 were reviewed. Patients between 18 and 90 years old, with hypo-vascular metastatic liver tumors with absent or limited extra hepatic disease, who had MRI study including contrast enhanced MRI and DWI before and 4 to 6 weeks after treatment were included in the study. Of 27 patients who had hypo-vascular liver metastases, 10 patients excluded; two patients for not having follow-up MRI, 2 patients for not having baseline contrast enhanced MRI and 6 patients for having the MR study done on a different vendor not compatible with the software used for image analysis. The remaining 17 patients were included in the study and their pre- and post-treatment MR images were analyzed by 2 readers.
IAT Technique
TACE, using a combination of Cisplatin 100 mg (Bristol Myers Squibb, Princeton, NJ), Doxorubicin 50 mg (Adriamycin; Pharmacia-Upjohn, Kalamazoo, MI) and Mitomycin C 10 mg (Bedford Laboratories, Bedford, OH) mixed in 10 ml of water-soluble contrast medium (Omnipaque; Winthrop Pharmaceuticals, New York, NY) was performed according to our standard institution protocol [25].
MR Imaging Technique and Parameters
All 17 patients had two MR imaging studies each, the first as a baseline study and the second as early follow-up, 1 month post-treatment after IAT. Both studies were performed using a 1.5 T magnet (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany) with a phased-array torso coil, using our standard clinical protocol including (a) T2-weighted turbo spin echo sequence (matrix size, 256 × 256; slice thickness, 8 mm; inter-slice gap, 2 mm; 25 to 37 slices; repetition time/echo time 4500/92 ms and receiver bandwidth, 32 kHz), (b) breath-hold unenhanced and contrast-enhanced (after injection of 0.1 mmol gadopentetate dimeglumine [Magnevist; Bayer, Wayne, NJ] per kilogram of body weight) T1-weighted three-dimensional fat-suppressed spoiled gradient-echo images (field of view, 320 to 400 mm; matrix, 192 × 160; slice thickness, 2.5 mm; 96 to 112 slices per phase; repetition time/echo time 5.77/2.77 ms, receiver bandwidth 64 kHz and flip angle 10°) in the hepatic arterial phase (HAP; 20 seconds), portal venous phase (PVP; 70 seconds) and delayed phase (3 minutes), and (c) a breath-hold diffusion-weighted echo-planar sequence (matrix size, 128 × 128; section thickness, 8 mm; intersection gap, 2 mm; 48 sections; b value = 0 and 750 s/mm2; repetition time/echo time 3000/69 ms and receiver bandwidth 64 kHz). A third imaging evaluation at 6-month post-treatment (either an MRI or CT scan) was performed to determine RECIST response to IAT. Patients were stratified into responders (complete or partial response) and non-responders (stable or progressive disease) as defined in Table 1.
Table 1.
Current Response Evaluation Criteria
| RECIST | mRECIST | EASL | |
|---|---|---|---|
| Complete Response (CR) | 100% decrease in maximum diameter of target lesion | 100% decrease in maximum enhancing diameter | 100% decrease of enhancing tissue of target lesion |
| Partial Response (PR) | ≥ 30% decrease of longest diameter | ≥ 30% decrease of longest enhancing diameter | ≥ 50% decrease bi-dimensional enhancing are of tumor |
| Stable Disease (SD) | < 30% decrease or ≤ 20% increase in lesion size | < 30% decrease to ≤ 20% increase in enhancing tissue | < 50% decrease to ≤ 25% increase in enhancing tissue |
| Progression of Disease (PD) | Increase of > 20% in lesion size or new lesions | > 20% increase of maximum enhancing diameter or new enhancing lesion | > 25% increase of enhancing tissue or new enhancing lesion |
MR Imaging Analysis
Lesion Identification
One radiologist (IRK) with 17 years experience in abdominal imaging reviewed all the images and selected up to 5 hypo-vascular metastatic lesions > 1 cm diameter per patient in both targeted and non-targeted lobes. Targeted and non-targeted lobes were identified by reviewing images and procedure reports. Hypo-vascular liver lesions showed lower intensity compared to normal liver parenchyma on T1 weighted images, had ring enhancement in the HAP and were more distinct in the PVP. A total of 42 targeted and 10 non-targeted lesions were chosen from all patients. Subsequently, 2 readers (CX and FS with 6 years and one year experience in abdominal MR imaging, respectively) who were blinded to clinical data, independently performed image analysis.
Conventional and volumetric metrics assessment
Baseline and early (1 month) post-treatment MR images were transferred to a picture archiving and communication system (Advanced Visualization, Emageon and Enterprise Visual Medical system, Merge Healthcare, Chicago, IL). Three conventional anatomical metrics and volumetric functional metrics were measured for each targeted and non-targeted lesion at baseline and at 1 month post therapy. Conventional metrics, including RECIST, mRECIST and EASL were measured in portal venous phase, using Emageon Ultravisual software. Volumetric metrics including HAP, PVP enhancement and ADC values were acquired by semi-automatic segmentation of the entire tumor volume using MR OncoTreat Software (Siemens Healthcare, Erlangen, Germany) as previously described [20], [26]. The software automatically calculated volumetric percent enhancement using [(AE − PC)/AE] * 100 and [(VE − PC)/VE] * 100, where PC represents signal intensity in pre-contrast phase and AE and VE represent signal intensity in subsequent arterial and venous contrast-enhanced phases.
Study endpoint
The endpoint for assessment of each lesion was RECIST response at 6 months. Measurements were performed by the expert radiologist (IRK) who was aware of the location of the lesion but was blinded to all clinical data as well as the results of the conventional and volumetric metrics at 1 month post therapy obtained by the 2 other readers.
Statistical Analysis
Comparison between conventional and volumetric metrics before and 1 month after TACE for all targeted and non-targeted lesions was made using a two-sample paired t-test. Similar comparisons were also made for targeted lesions after stratification into responders and non-responders by RECIST at 6 months. Inter-observer agreement was performed using intraclass correlation coefficient (ICC) with ANOVA. ICC results were interpreted according to the following criteria: poor (ICC < 0.50), moderate (0.50 < ICC < 0.75), good (0.75 < ICC < 0.90), and excellent (ICC > 0.90). Statistical analysis was performed with a software package (SPSS 16.0; SPSS, Chicago, IL). P value of less than .05 was considered statistically significant.
Results
Demographic Data
Table 2 summarizes the demographic data of recruited patients. A total of 42 targeted and 10 non targeted lesions were analyzed. Patients had MR study 21±13 days before and 32±23 days after TACE session. The number of TACE sessions that the patients received within 6 months of baseline MRI was 1 session for 11 patients, 2 sessions for 4 patients, 3 sessions for 1 patient and 4 sessions for 1 patient. Twenty-seven (64%) targeted lesions were in the right lobe and 15 (36%) were in the left lobe. Primary diagnosis was made by biopsy in all patients.
Table 2.
Demographic Information
| Age(y)⁎ | |
|---|---|
| All patients | 60.6±11.9 (43–83) |
| Male | 61.9±12.6 (46–83) |
| Female | 58.7±12.6 (43–76) |
| Gender | |
| Male | 10 (59) |
| Female | 7 (41) |
| Ethnicity | |
| Caucasian | 13 (76) |
| African American | 1 (6) |
| Asian | 2 (12) |
| Hispanic | 1 (6) |
| Diagnosis | Patients(N=17) | Lesions(N=42) |
|---|---|---|
| Colorectal cancer | 9 (53) | 25 (59) |
| Breast cancer | 5 (29) | 10 (24) |
| Leiomyosarcoma | 1 (6) | 5 (12) |
| Nasopharyngeal carcinoma | 2 (12) | 2 (5) |
Note— unless otherwise indicated, data is displayed as number of patients with percentages in parentheses.
Data are mean ± standard deviation with range in parenthesis.
MR Imaging Findings
Conventional and volumetric metrics in the targeted lesions at 1 month
Table 4 shows anatomic and volumetric measurements for the targeted group before and early (1 month) after treatment for both readers.
Table 4.
Changes in Anatomical and Volumetric Functional Metrics Early (1 month) after IAT for the 2 Readers for Targeted Lesions (N = 42)
| Reader 1 |
Reader 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Before Treatment | After Treatment | Mean percentage change (%) | P value* | Before Treatment | After Treatment | Mean percentage change (%) | P value* |
| Anatomical metrics | ||||||||
| RECIST (cm) | 4.3±3.4 | 4.5±3.6 | 4.2±6.3 | 0.4 | 4.2±3.4 | 4.4±3.6 | 4.3±7.7 | 0.3 |
| mRECIST(cm)⁎⁎ | 3.3±3.3 | 2.8±3.4 | − 16.9±2.2 | 0.01 | 3.1±3.2 | 2.4±3.3 | − 22.8±3.8 | 0.005 |
| EASL (cm2)⁎⁎ | 14.8±33.3 | 12.3±32.5 | − 16.8±2.5 | 0.02 | 13.5±32.7 | 10.8±32.2 | − 20.6±1.7 | 0.02 |
| Volumetric metrics | ||||||||
| Hepatic Arterial (%) | 31.4±25.1 | 23.9±24.5 | − 23.9±2.3 | 0.14 | 31.5±25.2 | 23.9±24.5 | − 24.2±2.9 | 0.14 |
| Portal Venous (%) | 63.8±33.8 | 43.1±28.7 | − 32.4±15.3 | 0.0001 | 63.6±34.2 | 42.6±28.3 | − 33.0±17.0 | < 0.0001 |
| ADC (× 10− 3 mm2/s)⁎⁎⁎ | 1.408±.0353 | 1.592±0.383 | 13.1±8.5 | 0.0007 | 1.413±0.358 | 1.571±0.392 | 11.2±9.3 | 0.003 |
Note. Unless otherwise indicated, data are means ± standard deviations. *P value is obtained from independent-samples t test.
mRECIST and EASL could not be measured in 7 (17%) lesions by Reader 1 and 9 (21%) lesions by Reader 2.
ADC map was not available in 5 targeted lesions.
As reported by Reader 1, anatomic enhancement showed statistically significant changes in mRECIST (P = .01) and EASL (P = .02), but no significant change was seen in RECIST (P = .4). In 7 out of 42 lesions (17%), neither mRECIST, nor EASL could be measured by Reader 1. Volumetric measurements of the tumor in PVP (P = .0001) and ADC value (P = .0007) showed statistically significant changes 1 month after treatment; however arterial enhancement did not show any significant changes (P = .14).
As reported by Reader 2, anatomic enhancement showed statistically significant changes in mRECIST (P = .005) and EASL (P = .02), but no significant change was seen in RECIST (P = .3). In 9 out of 42 lesions (21%), neither mRECIST, nor EASL could be measured by Reader 2. Volumetric measurements of the tumor in PVP (P < .0001) and ADC value (P = .003) showed statistically significant changes 1 month after treatment; however arterial enhancement did not show any significant changes (P = .14).
Conventional and volumetric metrics in the non-targeted lesions at 1 month
Table 5 shows anatomic and volumetric changes in non-targeted lesions for both readers. None of the anatomic and volumetric measurements by either reader showed a statistically significant difference after treatment in non-targeted lesions.
Table 5.
Changes in Anatomical and Volumetric Functional Metrics Early (1 month) after IAT for the 2 Readers for Non-Targeted Lesions (N = 10)
| Reader 1 |
Reader 2 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Before treatment | After treatment | Mean percentage change (%) | P value⁎ | Before treatment | After treatment | Mean percentage change (%) | P value⁎ |
| Anatomical metrics | ||||||||
| RECIST (cm) | 4.2±1.4 | 4.3±1.2 | 2.4±17.2 | 0.7 | 4.1±1.5 | 4.0±1.3 | − 1.3±17.7 | 0.8 |
| mRECIST (cm) | 3.8±1.4 | 3.4±1.5 | − 11.1±9.3 | 0.5 | 3.4±1.3 | 3.1±1.7 | − 7.7±28.0 | 0.5 |
| EASL (cm2) | 12.3±8.4 | 9.9±6.0 | − 19.7±28.3 | 0.4 | 9.0±6.6 | 8.9±6.9 | − 0.8±4.7 | 0.97 |
| Volumetric metrics | ||||||||
| Hepatic arterial (%) | 39.2±32.2 | 54.0±41.0 | 37.9±27.3 | 0.09 | 38.7±31.5 | 55.1±42.0 | 42.1±33.2 | 0.07 |
| Portal venous (%) | 51.2±31.0 | 84.3±54.1 | 64.7±74.7 | 0.05 | 51.6±32.1 | 84.9±55.4 | 64.6±73 | 0.05 |
| ADC (× 10− 3 mm2/s)⁎ | 1.308±0.535 | 1.475±0.227 | 12.7±57.5 | 0.4 | 1.266±0.509 | 1.425±0.243 | 12.6±52.1 | 0.5 |
Note. Unless otherwise indicated, data are means ± standard deviations. *P value is obtained from independent-samples t test.
ADC map was not available in 4 non-targeted lesions.
Conventional and volumetric metrics in responders at 1 month
All 42 lesions were stratified into responders (n = 15) and non-responders (n = 27) based on RECIST response at 6 months. Table 6, Table 7 show the pre-treatment and early post-treatment (1 month) changes in anatomic and volumetric metrics for the 2 readers.
Table 6.
Changes in Anatomical and Volumetric Functional Metrics before and Early (1 month) Follow-Up after IAT Treatment According to Response by RECIST 6 Months after IAT for Reader 1
| Responders(N = 15) |
Non-responders (N = 27) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Before Treatment | After Treatment | Mean percentage change (%) | P value* | Before Treatment | After Treatment | Mean Percentage change (%) | P value* |
| Anatomical metrics | ||||||||
| RECIST (cm) | 3.0±1.8 | 2.8±2.8 | − 9.6±53.3a | 0.4 | 5.0±3.9 | 5.5±3.7 | 8.8±4.6 | 0.06 |
| mRECIST (cm)⁎⁎ | 2.9±1.9 | 1.4±1.9 | − 51.1±1.5b | 0.0001 | 3.6±3.9 | 3.5±3.8 | − 1.1±2.2 | 0.9 |
| EASL (cm2)⁎⁎ | 8.6±9.5 | 3.8±6.9 | − 55.4±27.1c | 0.0009 | 18.2±40.9 | 17.0±39.7 | − 6.7±2.8 | 0.4 |
| Volumetric metrics | ||||||||
| Hepatic Arterial (%) | 35.0±27.9 | 20.9±15.6 | − 40.2±44.3 | 0.07 | 29.4±23.7 | 25.5±28.4 | − 13.2±20.1 | 0.6 |
| Portal Venous (%) | 82.9±28.3 | 40.5±25.9 | − 51.2±8.3 | 0.0001 | 53.2±32.4 | 44.6±30.5 | − 16.2±5.9 | 0.05 |
| ADC(× 10− 3 mm2/s)⁎⁎⁎ | 1.236±0.260 | 1.543±0.294 | 24.8±12.8 | 0.01 | 1.490±0.366 | 1.616±0.423 | 8.4±15.4 | 0.03 |
Note. unless otherwise indicated, data are means ± standard deviations. * P value is obtained from independent-samples t test.
mRECIST and EASL could not be measured in 6 (40%) of responder lesions and 1 (4%) of non-responder lesions.
ADC map was not available in 3 responder and 2 nonresponder lesions.
The reduction in size did not fulfill partial response by RECIST.
The reduction in enhancement fulfilled partial response by mRECIST.
The reduction in enhancement fulfilled partial response by EASL.
Table 7.
Changes in Anatomical and Volumetric Functional Metrics before and Early (1 month) Follow-Up after IAT Treatment According to Response by RECIST 6 Months after IAT for Reader 2
| Responders (N = 15) |
Non-responders (N = 27) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Before treatment | After treatment | Mean percentage change (%) | P value* | Before treatment | After treatment | Mean percentage change (%) | P value* |
| Anatomical metrics | ||||||||
| RECIST (cm) | 2.9±1.7 | 2.7±2.7 | − 8.1±54.9 | 0.5 | 4.9±3.8 | 5.3±3.8 | 8.4±1.8 | 0.07 |
| mRECIST (cm)⁎⁎ | 2.8±1.9 | 1.1±1.6 | − 60.3±14.3b | < 0.0001 | 3.2±3.8 | 3.1±3.9 | − 4.5±1.5 | 0.6 |
| EASL (cm2)⁎⁎ | 8.3±9.9 | 2.4±5.3 | − 71.1±46.6c | 0.01 | 16.4±40.1 | 15.4±39.4 | − 6.4±1.8 | 0.4 |
| Volumetric metrics | ||||||||
| Hepatic Arterial (%) | 35.8±28.8 | 20.6±16.0 | − 42.6±44.5 | 0.07 | 29.0±23.2 | 25.7±28.2 | − 11.5±21.6 | 0.6 |
| Portal Venous (%) | 82.7±28.4 | 39.8±26.4 | − 51.9±7.2 | 0.0001 | 53.0±32.8 | 44.2±29.8 | − 16.6±9.3 | 0.05 |
| ADC (× 10− 3 mm2/s)⁎⁎⁎ | 1.240±0.252 | 1.500±0.307 | 20.9±21.6 | 0.03 | 1.496±0.376 | 1.605±0.428 | 7.3±13.8 | 0.05 |
Note. Unless otherwise indicated, data are means ± standard deviations. * P value is obtained from independent-samples t test.
a The reduction in size did not fulfill response by RECIST.
mRECIST and EASL could not be measured in 7 (47%) of responder lesions and 2 (7%) of non-responder lesions.
ADC map was not available in 3 responder and 2 non responder lesions.
The reduction in enhancement fulfilled partial response by mRECIST.
The reduction in enhancement fulfilled partial response by EASL.
For Reader 1, responders by RECIST at 6 months showed significant changes in anatomic enhancement by mRECIST (P = .0001) and EASL (P = .0009) as well as volumetric enhancement in PVP (P = .0001) and ADC value (P = .01), but no significant changes in RECIST at 1 month (P = .39) and volumetric enhancement in HAP (P = .07) (Figure 1). In 6 out of 15 responder lesions (40%), neither mRECIST nor EASL could be measured by Reader 1.
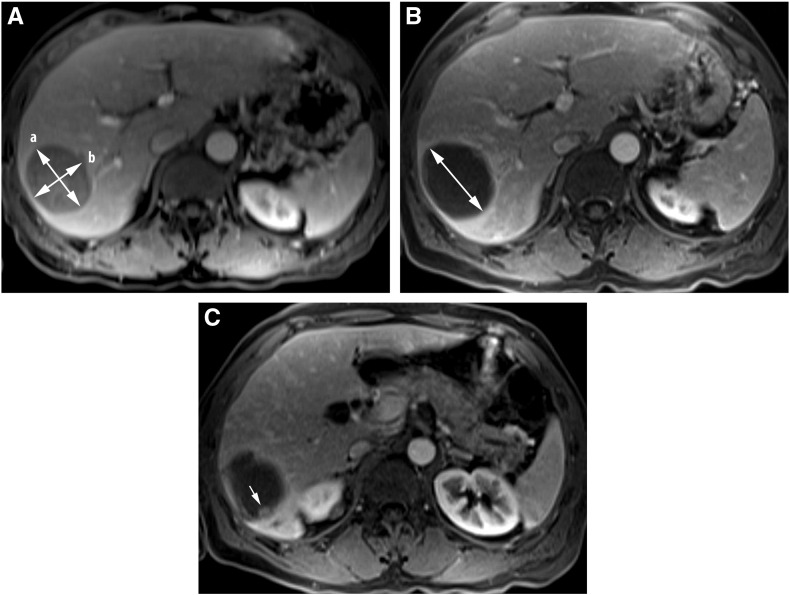
Figure 1.
Anatomic and volumetric functional analyses of colorectal cancer liver metastases in a 61-year-old male patient. RECIST, mRECIST and EASL measured in PVP at baseline (A) and early (one month) post treatment (B, C). RECIST increased from 5.6 cm at baseline (double sided arrow “a” in Figure A) to 6.3 cm (double sided arrow in Figure B). mRECIST (double sided arrow “a” in Figure A) and EASL (a× b in Figure A) both decreased significantly on early (one month) post treatment (B, C). mRECIST decreased from 6.3 cm to 1.4 cm, and EASL decreased from 26.3 cm2 to 1.1 cm2.
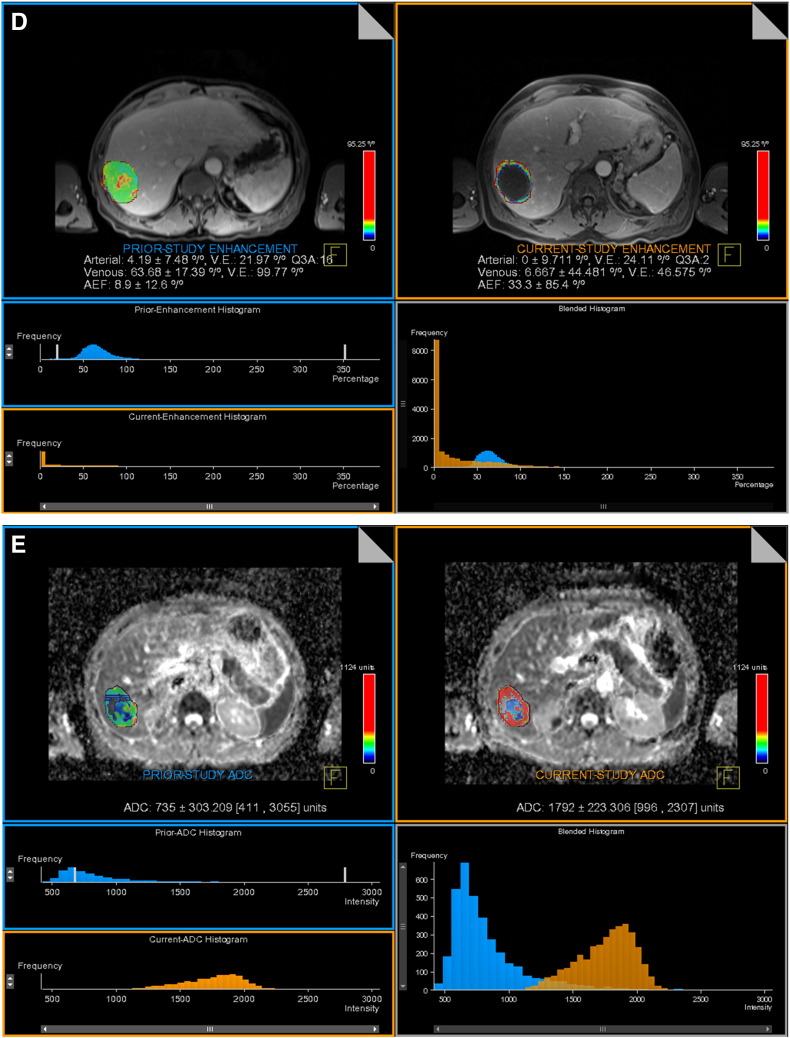
Volumetric enhancement map (D) in the HAP and PVP shows significant decrease in enhancement from 4.2% and 63.7% pre-treatment (top left) to 0% and 6.7% post treatment (top right). Pre-treatment histogram is depicted in blue and post treatment is depicted in orange (bottom left). Leftward shift in the blended histogram (bottom right) indicates favorable response to therapy.
Volumetric ADC map (E) shows significant increase from 0.73×10− 3 mm2/s (top left) to 1.79×10− 3 mm2/s (top right) after TACE. Pre-treatment histogram is depicted in blue and post treatment is depicted in orange (bottom left). Rightward shift in the blended histogram (bottom right) indicates favorable response to therapy.
For Reader 2, responders by RECIST at 6 months showed significant changes in anatomic enhancement by mRECIST (P < .0001) and EASL (P = .01) as well as volumetric enhancement in PVP (P = .0001) and ADC value (P = .03), but no significant changes in RECIST at 1 month (P = .47) and volumetric enhancement in HAP (P = .07). In 7 out of 15 responder lesions (47%), neither mRECIST nor EASL could be measured by Reader 2.
Conventional and volumetric metrics in nonresponders at 1 month
None of the anatomic metrics showed significant changes in non-responder group, measured by Reader 1. Volumetric enhancement in PVP showed borderline significance (P = .05); but mean percentage change was small (− 16.2±5.9). Also volumetric ADC value showed a significant increase in non-responder group (P = .03); however, mean percentage change was small (8.4±15.4).
None of the anatomic metrics showed significant changes in non-responder group, measured by Reader 2. Volumetric enhancement in PVP and ADC value showed changes with borderline significance (P = .05); however, the mean percentage change was small for both of them (− 16.6%±9.3 and 7.3%±13.8, respectively).
Inter-reader agreement
Inter observer agreement was excellent for both anatomic and volumetric measurements for 2 readers (ICC > 0.90) (Table 3).
Table 3.
Intraclass Correlation Coefficient and P value for Anatomical and Volumetric Functional Metrics before and Early Follow-Up after IAT Treatment between 2 Readers
| Parameter | Before Treatment |
After Treatment |
||
|---|---|---|---|---|
| ICC value (95% CI) P value⁎ | ICC value (95% CI) P value⁎ | |||
| Anatomical metrics | ||||
| RECIST (cm) | 0.998 (0.996-0.999) | < .0001 | 0.997 (0.993-0.998) | < .0001 |
| mRECIST (cm) | 0.988 (0.973-0.994) | < .0001 | 0.980 (0.955-0.990) | < .0001 |
| EASL (cm2) | 0.996 (0.991-0.998) | < .0001 | 0.996 (0.992-0.998) | < .0001 |
| RECIST at 6 month | 0.999 (0.999-1.000) | < .0001 | ||
| Volumetric metrics | ||||
| Hepatic arterial (%) | 0.999 (0.998-0.999) | < .0001 | 0.999 (0.999-1.000) | < .0001 |
| Portal Venous (%) | 0.999 (0.998-0.999) | < .0001 | 0.999 (0.998-0.999) | < .0001 |
| ADC (mm2/s) | 0.996 (0.992-0.998) | < .0001 | 0.994 (0.986-0.997) | < .0001 |
ICC values were interpreted in accordance with the following criteria: poor (ICC < 0.50), moderate (0.50 < ICC < 0.75), good (0.75 < ICC < 0.90), and excellent (ICC > 0.90).
P value obtained from ANOVA.
Discussion
This retrospective study reported the value of volumetric enhancement and ADC value changes in addition to conventional anatomic metrics in assessment of early response to TACE in hypo-vascular liver metastases. Conventional anatomic criteria have been widely used for primary and metastatic liver malignancies; however, their reliability has been questioned given the heterogeneous changes after loco-regional therapies which make these uni- and bi-dimensional metrics limited in prediction of early treatment response [13], [14], [15], [16], [18], [19], [20], [21]. Besides, loco-regional therapies are more cytostatic, compared to conventional chemotherapy, and they will primarily affect the functional and physiologic behavior of the tumor more than its size, hence the necessity for introducing new reproducible and quantifiable biomarkers as surrogates of tumor response is apparent [6], [7], [8], [13], [14], [15], [16], [18], [19], [20].
Former studies have shown promising role for volumetric enhancement and ADC changes in evaluating treatment response of different primary and hyper-vascular metastatic liver lesions [13], [14], [15], [16], [17], [18], [19], [20], [21], [23], [24], [27]. Quantitative and functional evaluation of hepatic lesions provide a more comprehensive assessment of tumor response, as these metrics consider changes in the whole tumor as opposed to conventional criteria which assess the tumor in a single axial plane. In addition, former studies have shown a higher correlation between these volumetric metrics with overall survival and histopathological evaluation compared with anatomic metrics [13], [14], [16], [22], [28], [29]. Yet, to date, only a few studies have assessed similar application of both volumetric and anatomic metrics for hypo-vascular liver metastases.
Anatomic and Volumetric changes in Targeted lesions
Our results showed statistically significant reduction in anatomic enhancement measured by mRECIST and EASL in the targeted cohort early after therapy, without a significant change in RECIST as measured by tumor size (Table 4). These metrics did not show any significant changes in the non-targeted group. In spite of its statistical significance, reduction in mRECIST and EASL was small (16.9% and 16.8%, respectively for Reader 1) and did not meet partial response criteria. Volumetric arterial phase enhancement in the targeted group decreased by 23.9% for Reader 1 after therapy. However, this reduction was not statistically significant (P = .14) likely due to the hypo-vascular nature of these tumors. Reduction in volumetric venous enhancement in the targeted group (32.4% for Reader 1) was higher than that of the arterial phase and was statistically significant (P = .0001). Targeted lesions also demonstrated increase in ADC values (13.1% for Reader 1) and the change post therapy was statistically significant (P = .0001). These findings were similar for Reader 2.
Anatomic and Volumetric changes in Responder Lesions
Targeted lesions were stratified into responders and non-responders based on RECIST at 6 months. Fifteen lesions were considered responders and 27 were non-responders. As reported in prior studies, responders by RECIST at 6 months did not show statistically significant reduction in tumor size at 1 month (9.6%, P = .4 for Reader 1), limiting the application of RECIST in assessing tumor response after IAT [19], [21] (Table 6). Responders had > 50% reduction in enhancement at 1 month post therapy, and fulfilled partial response criteria for both mRECIST and EASL (Table 6, Table 7). However, in a subset of patients (40% of responders and 4% of non-responders for Reader 1) these measurements could not be obtained, due to the difficulty in identifying residual small nodular or linear enhancement in tumors that are hypo-vascular even before treatment. While this could be a major limitation of using these metrics in routine assessment of response to therapy, our results suggest that if measurable, mRECIST and EASL which are typically used to assess response in hyper-vascular lesions could also be useful in assessing response in hypo-vascular lesions. Our results are similar to those reported in a recent study, where quantitative EASL measurement of colorectal cancer liver metastases was a good predictor of IAT response and patient survival; although the study design and endpoints was different from our study [29].
Volumetric assessment of PVP enhancement in responders showed 51.2% reduction (P = .0001) as reported by Reader 1, which is in line with results of previous studies [13], [18]. Although mean HAP enhancement in responders showed reduction of 40.2% for Reader 1 the difference was of borderline significance (P = .07). This could be explained by the hypo-vascular nature of the tumors resulting in obscurity of the lesion in the arterial phase.
Diffusion weighted imaging has been widely used as a noninvasive tool for response evaluation in different tumors. ADC values reflect the integrity of the cell membranes by depicting the distribution of water molecules within the tumor and hence can represent the viability of tumor cells after treatment [10], [11], [12]. Former studies have reported increased ADC values in responders compared to non-responders [13], [14], [15], [16], [17], [18], [19], [20], [21], [23], [24]. Our data is consistent with previous reports and showed a significant increase in volumetric ADC in the targeted cohort and in the responders group, but not in non-targeted group. Volumetric ADC increased in responders by 24.8%, 1 month after IAT for Reader 1. According to our observations 67% of lesions with > 20% increase in ADC value showed response according to RECIST at 6 months, which is in line with earlier reports [15]. Taking both ADC value and enhancement in PVP in to account, 80% of responder lesions showed either > 20% increase in ADC value and/or > 50% decrease in venous enhancement, as opposed to 26% of non-responder lesions which showed the same trend.
Baseline Findings in Responder Lesions
In the current study it was noted that responders had a smaller tumor size at baseline, compared to non-responders (3.0 cm vs. 5.0 cm, respectively for Reader 1).
Volumetric pre-treatment enhancement in the PVP was significantly higher for responders compared to non-responders (82.9% vs. 53.2%, respectively for Reader 1). Also volumetric pretreatment ADC value was lower for responders compared to non-responders (1.236 × 10− 3 vs. 1.490 × 10− 3 mm2/s, respectively for Reader 1). These results suggest that targeted lesions that are smaller and less vascular with more cellularity (lower ADC) at baseline are more likely to respond to therapy compared to the larger, more vascular and less cellular tumors.
Inter-Reader Agreement
In the current study, the inter-observer agreement was excellent for both volumetric and anatomic metrics, compared to a former study which reported excellent variability for volumetric metrics and moderate to good variability for anatomic metrics [30]. The former study included infiltrative, ill-defined hepatocellular carcinoma lesions; whereas we have evaluated non-infiltrative, better-defined metastatic lesions which can explain the discrepancy between the two studies.
Study Limitations
Our study had some limitations. First, the sample size was relatively small. Larger studies are required to confirm our findings. Second, we had no histopathological proof for our results. However, since most of these patients were unresectable it was unlikely that pathological confirmation of tumor necrosis would have been obtained. The heterogeneity of the tumors we included may preclude drawing conclusions about individual tumor’s response to therapy. However, our objective was to determine if imaging biomarkers used in this study can predict future response by conventional criteria, regardless of the tumor pathology and survival.
Conclusion
In conclusion, according to our findings RECIST criteria are limited in assessing early response to TACE. Volumetric assessment of enhancement and ADC value changes could be helpful to identify early response to TACE in liver malignancies. These non-invasive methods provide an insight into functional and physiologic changes of the tumor. Since these biomarkers can provide information regarding to non-responding lesions, they may be helpful in assessing treatment response in future clinical trials.
Footnotes
None of the authors listed (Fatemeh Sobhani, Chunmiao Xu, Emi Murano, Li Pan, Neda Rastegar, Ihab R. Kamel) has conflict of interest.
References
- 1.Imam K, Bluemke DA. MR imaging in the evaluation of hepatic metastases. Magn Reson Imaging Clin N Am. 2000;8(4):741–756. [PubMed] [Google Scholar]
- 2.Danet IM, Semelka RC, Leonardou P, Braga L, Vaidean G, Woosley JT, Kanematsu M. Spectrum of MRI appearances of untreated metastases of the liver. AJR Am J Roentgenol. 2003;181(3):809–817. doi: 10.2214/ajr.181.3.1810809. [DOI] [PubMed] [Google Scholar]
- 3.Adam A. Interventional radiology in the treatment of hepatic metastases. Cancer Treat Rev. 2002;28(2):93–99. doi: 10.1053/ctrv.2002.9258. [DOI] [PubMed] [Google Scholar]
- 4.Memon K, Lewandowski RJ, Riaz A, Salem R. Chemoembolization and radioembolization for metastatic disease to the liver: available data and future studies. Curr Treat Options in Oncol. 2012;13(3):403–415. doi: 10.1007/s11864-012-0200-x. [DOI] [PubMed] [Google Scholar]
- 5.Harry VN, Semple SI, Parkin DE, Gilbert FJ. Use of new imaging techniques to predict tumour response to therapy. Lancet Oncol. 2010;11(1):92–102. doi: 10.1016/S1470-2045(09)70190-1. [DOI] [PubMed] [Google Scholar]
- 6.Assumpcao L, Choti M, Pawlik TM, Gecshwind JF, Kamel IR. Functional MR imaging as a new paradigm for image guidance. Abdom Imaging. 2009;34(6):675–685. doi: 10.1007/s00261-008-9481-8. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 8.Therasse P, Eisenhauer EA, Verweij J. RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer. 2006;42(8):1031–1039. doi: 10.1016/j.ejca.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Guindalini FD, Botelho MP, Harmath CB, Sandrasegaran K, Miller FH, Salem R, Yaghmai V. Assessment of liver tumor response to therapy: role of quantitative imaging. Radiographics. 2013;33(6):1781–1800. doi: 10.1148/rg.336135511. [DOI] [PubMed] [Google Scholar]
- 10.Malayeri AA, El Khouli RH, Zaheer A, Jacobs MA, Corona-Villalobos CP, Kamel IR, Macura KJ. Principles and applications of diffusion-weighted imaging in cancer detection, staging, and treatment follow-up. Radiographics. 2011;31(6):1773–1791. doi: 10.1148/rg.316115515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vossen JA, Buijs M, Kamel IR. Assessment of tumor response on MR imaging after locoregional therapy. Tech Vasc Interv Radiol. 2006;9(3):125–132. doi: 10.1053/j.tvir.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Thoeny HC, Ross BD. Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J Magn Reson Imaging. 2010;32(1):2–16. doi: 10.1002/jmri.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gowdra Halappa V, Corona-Villalobos CP, Bonekamp S, Li Z, Reyes D, Cosgrove D, Pawlik TM, Diaz LA, Bhagat N, Eng J. Neuroendocrine liver metastasis treated by using intraarterial therapy: volumetric functional imaging biomarkers of early tumor response and survival. Radiology. 2013;266(2):502–513. doi: 10.1148/radiol.12120495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Z, Bonekamp S, Halappa VG, Corona-Villalobos CP, Pawlik T, Bhagat N, Reyes D, Lai H, Geschwind JF, Kamel IR. Islet cell liver metastases: assessment of volumetric early response with functional MR imaging after transarterial chemoembolization. Radiology. 2012;264(1):97–109. doi: 10.1148/radiol.12112161. [DOI] [PubMed] [Google Scholar]
- 15.Bonekamp S, Shen J, Salibi N, Lai HC, Geschwind J, Kamel IR. Early response of hepatic malignancies to locoregional therapy-value of diffusion-weighted magnetic resonance imaging and proton magnetic resonance spectroscopy. J Comput Assist Tomogr. 2011;35(2):167–173. doi: 10.1097/RCT.0b013e3182004bfb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buijs M, Kamel IR, Vossen JA, Georgiades CS, Hong K, Geschwind JF. Assessment of metastatic breast cancer response to chemoembolization with contrast agent enhanced and diffusion-weighted MR imaging. J Vasc Interv Radiol. 2007;18(8):957–963. doi: 10.1016/j.jvir.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 17.Tam HH, Collins DJ, Brown G, Chau I, Cunningham D, Leach MO, Koh DM. The role of pre-treatment diffusion-weighted MRI in predicting long-term outcome of colorectal liver metastasis. Br J Radiol. 2013;86(1030):20130281. doi: 10.1259/bjr.20130281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonekamp S, Li Z, Geschwind JF, Halappa VG, Corona-Villalobos CP, Reyes D, Pawlik TM, Bonekamp D, Eng J, Kamel IR. Unresectable hepatocellular carcinoma: MR imaging after intraarterial therapy. Part I. Identification and validation of volumetric functional response criteria. Radiology. 2013;268(2):420–430. doi: 10.1148/radiol.13122307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halappa VG, Bonekamp S, Corona-Villalobos CP, Li Z, Mensa M, Reyes D, Eng J, Bhagat N, Pawlik TM, Geschwind JF. Intrahepatic cholangiocarcinoma treated with local-regional therapy: quantitative volumetric apparent diffusion coefficient maps for assessment of tumor response. Radiology. 2012;264(1):285–294. doi: 10.1148/radiol.12112142. [DOI] [PubMed] [Google Scholar]
- 20.Bonekamp S, Jolepalem P, Lazo M, Gulsun MA, Kiraly AP, Kamel IR. Hepatocellular carcinoma: response to TACE assessed with semiautomated volumetric and functional analysis of diffusion-weighted and contrast-enhanced MR imaging data. Radiology. 2011;260(3):752–761. doi: 10.1148/radiol.11102330. [DOI] [PubMed] [Google Scholar]
- 21.Kamel IR, Liapi E, Reyes DK, Zahurak M, Bluemke DA, Geschwind JF. Unresectable hepatocellular carcinoma: serial early vascular and cellular changes after transarterial chemoembolization as detected with MR imaging. Radiology. 2009;250(2):466–473. doi: 10.1148/radiol.2502072222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chapiro J, Wood LD, Lin M, Duran R, Cornish T, Lesage D, Charu V, Schernthaner R, Wang Z, Tacher V. Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology. 2014;273(3):746–758. doi: 10.1148/radiol.14140033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koh DM, Scurr E, Collins D, Kanber B, Norman A, Leach MO, Husband JE. Predicting response of colorectal hepatic metastasis: value of pretreatment apparent diffusion coefficients. AJR Am J Roentgenol. 2007;188(4):1001–1008. doi: 10.2214/AJR.06.0601. [DOI] [PubMed] [Google Scholar]
- 24.Cui Y, Zhang XP, Sun YS, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases. Radiology. 2008;248(3):894–900. doi: 10.1148/radiol.2483071407. [DOI] [PubMed] [Google Scholar]
- 25.Geschwind JF, Ramsey DE, Cleffken B, van der Wal BC, Kobeiter H, Juluru K, Hartnell GG, Choti MA. Transcatheter arterial chemoembolization of liver tumors: effects of embolization protocol on injectable volume of chemotherapy and subsequent arterial patency. Cardiovasc Intervent Radiol. 2003;26(2):111–117. doi: 10.1007/s00270-002-2524-6. [DOI] [PubMed] [Google Scholar]
- 26.Gulsun MA WC, Strecker R, Meredith G, Kamel IR. Seventheenth Meeting of the International Society for Magnetic Resonance in Medicine. ISMRM; Berkley, CA: 2009. A new tool for volumetric and functional analysis of hepatic tumors monitored with multi-modal MRI [abstr] p. 2876. [Google Scholar]
- 27.Duran R, Chapiro J, Frangakis C, Lin M, Schlachter TR, Schernthaner RE, Wang Z, Savic LJ, Tacher V, Kamel IR. Uveal Melanoma Metastatic to the Liver: The Role of Quantitative Volumetric Contrast-Enhanced MR Imaging in the Assessment of Early Tumor Response after Transarterial Chemoembolization. Transl Oncol. 2014;7(4):447–455. doi: 10.1016/j.tranon.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapiro J, Duran R, Lin M, Mungo B, Schlachter T, Schernthaner R, Gorodetski B, Wang Z, Geschwind JF. Transarterial chemoembolization in soft-tissue sarcoma metastases to the liver - The use of imaging biomarkers as predictors of patient survival. Eur J Radiol. 2015;84(3):424–430. doi: 10.1016/j.ejrad.2014.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapiro J, Duran R, Lin M, Schernthaner R, Lesage D, Wang Z, Savic LJ, Geschwind JF. Early survival prediction after intra-arterial therapies: a 3D quantitative MRI assessment of tumour response after TACE or radioembolization of colorectal cancer metastases to the liver. Eur Radiol. 2015;25(7):1993–2003. doi: 10.1007/s00330-015-3595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonekamp D, Bonekamp S, Halappa VG, Geschwind JF, Eng J, Corona-Villalobos CP, Pawlik TM, Kamel IR. Interobserver agreement of semi-automated and manual measurements of functional MRI metrics of treatment response in hepatocellular carcinoma. Eur J Radiol. 2014;83(3):487–496. doi: 10.1016/j.ejrad.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]