Abstract
Prostate cancer (PCa) is the most frequently diagnosed cancer and the second leading cause of cancer death among men in Western countries. Current screening techniques are based on the measurement of serum prostate specific antigen (PSA) levels and digital rectal examination. A decisive diagnosis of PCa is based on prostate biopsies; however, this approach can lead to false-positive and false-negative results. Therefore, it is important to discover new biomarkers for the diagnosis of PCa, preferably noninvasive ones. Metabolomics is an approach that allows the analysis of the entire metabolic profile of a biological system. As neoplastic cells have a unique metabolic phenotype related to cancer development and progression, the identification of dysfunctional metabolic pathways using metabolomics can be used to discover cancer biomarkers and therapeutic targets. In this study, we review several metabolomics studies performed in prostatic fluid, blood plasma/serum, urine, tissues and immortalized cultured cell lines with the objective of discovering alterations in the metabolic phenotype of PCa and thus discovering new biomarkers for the diagnosis of PCa. Encouraging results using metabolomics have been reported for PCa, with sarcosine being one of the most promising biomarkers identified to date. However, the use of sarcosine as a PCa biomarker in the clinic remains a controversial issue within the scientific community. Beyond sarcosine, other metabolites are considered to be biomarkers for PCa, but they still need clinical validation. Despite the lack of metabolomics biomarkers reaching clinical practice, metabolomics proved to be a powerful tool in the discovery of new biomarkers for PCa detection.
Introduction
Systems biology applied to cancer research encompasses the “omics” tools, including genomics, transcriptomics, proteomics, and metabolomics, which complement each other and are capable of measuring changes in several entities (genes, transcripts, proteins, or metabolites, respectively) simultaneously, providing an overview of various physiological or pathological conditions [1], [2], [3].
Metabolomics can provide an idea of the physiological status of a biological system, and therefore, alterations in the “normal” metabolome may be indicative of disease. These alterations in the “normal” metabolome have the potential to deliver new diagnostic markers for the detection and prognosis of diseases and to monitor the response to therapeutic interventions [4]. Metabolomics also has the potential to give new understanding of the phenotypic changes resultant from genetic alterations, environmental influence, and toxicological influence [5].
The term metabolomics includes the assessment of all the endogenous metabolites produced by the organism including small molecule intermediates and end products of biochemical reactions in a cell (approximately ≤1500 Da), as well as exogenous metabolites, such as drugs, products from the body flora, and food. Instead of genes and proteins that participate in these biological processes, the metabolites produced are indicators of what is happening in the metabolism of a cell in physiological or pathophysiological conditions. Thus, metabolites can be altered in such diseases as cancer [1], [6], [7], [8], [9].
As neoplastic cells have a unique metabolic phenotype related to cancer development and progression, the identification of dysfunctional metabolic pathways through metabolomics can be used to identify cancer biomarkers and discover therapeutic targets [5], [6], [10].
Prostate Cancer
Prostate cancer (PCa) is the second most diagnosed cancer in men [11], principally affecting men over 50 years old [12], and is the fifth leading cause of cancer-related deaths in men worldwide [11]. Statistically, in 25% of men worldwide with PCa that develop metastatic disease [13], the bones are the principal targets of PCa metastasis [14]. Given that PCa has a long latency period and is potentially curable, it is essential to develop efficient and precocious screening methods for its early detection and characterization [12].
The quantification of prostate serum antigen (PSA) and the digital rectal examination are the most common screening techniques used for PCa diagnosis. However, performing a prostate biopsy is mandatory for a final diagnosis [12]. Serum PSA levels higher than 4.0 ng/ml are a sign of PCa [14], although PSA is not able to differentiate patients with aggressive PCa from those with indolent disease [15]. The value of PSA screening is also controversial because of its limited sensitivity and specificity [1], [16]. Recent studies suggested that certain PCa patients may present with PSA levels below 4.0 ng/ml [14]. This fact leads to false negatives, as no reliable cutoff values exist to demonstrate the unequivocal presence of PCa [16], [17]. Furthermore, PSA levels may be affected by several other factors, such as age, prostatitis, urinary tract infection, and benign prostate hyperplasia, leading inevitably to false-positive results [14], [16], [18], [19].
The biopsy analysis can also provide false-negative results when the tumor is small; when the cancer cells are distributed heterogeneously; and in early PCa stage when, histologically, the tumor appears benign [20], [21], [22]. Thus, samples obtained during the biopsy for histopathologic analysis may not be representative of the cancer [23].
The lack of a consistent biomarker for PCa diagnosis and monitoring highlights the need for novel, specific, sensitive, and cost-effective biomarkers to implement the best treatment approach in a precocious state of the disease [14].
Altered Metabolism of PCa Cells
In 1920, Otto Warburg discovered that cancer cells, unlike nonmalignant cells, preferentially produce ATP through the glycolytic pathway (anaerobic pathway) instead of the Krebs cycle, even in the presence of oxygen. This capability of cancer cells to sustain high rates of glycolysis for ATP generation is known as the Warburg effect [24], [25], [26].
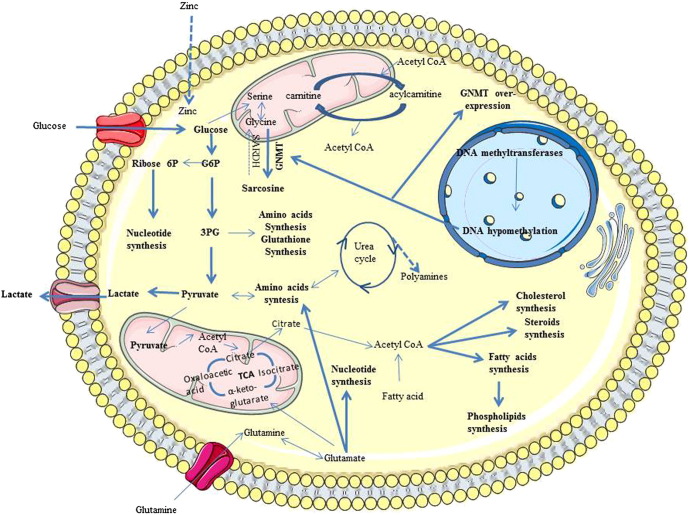
Despite the relevance of the Warburg effect in cancer cells, the Krebs cycle and oxidative phosphorylation also play an important role in many types of cancer, including PCa. Recent evidence suggests that increased citrate oxidation is an important metabolic characteristic in PCa that supports the high cellular energy demand [27]. One of the major functions of prostate cells is the production of citrate, PSA, and polyamines, such as spermine, which are the major components of prostatic fluid. Therefore, prostate cells have a distinct metabolic profile as they produce specific compounds [1], [16]. The production of citrate by prostate cells is very high in comparison with other organs [28]. Unlike other cells in the organism, prostate cell metabolism significantly favors citrate synthesis over citrate utilization, which makes the prostate peripheral zone epithelium unique among human cells [29]. Usually, cells degrade citrate in aerobic ATP production, with citrate being oxidized during the Krebs cycle as part of the intermediary metabolism of glucose. However, nonmalignant prostate cells accumulate and secrete citrate. The oxidation of citrate is catalyzed by mitochondrial aconitase (m-aconitase). In normal prostate cells, m-aconitase is inhibited by the high intracellular concentrations of zinc, leading to citrate accumulation (Figure 1) [28], [29]. Extensive metabolic alterations occur when prostate cells experience neoplastic transformation. One of the most relevant alterations is citrate oxidation, because cancer cells are unable to accumulate zinc, and without elevated levels of zinc, m-aconitase is no longer inhibited and can catalyze citrate oxidation [2], [4], [27], [28], [30]. This transformation of citrate accumulation in healthy prostate cells to oxidized citrate in malignant prostate cells results in more efficient energy production. This is probably an early event in the progression to malignancy and precedes the histopathological identification of malignant cells [27], [31], [32].
Figure 1.
Schematic illustration of the most significantly altered metabolic pathways in PCa cells. Dashed lines = downregulated pathway; continuous line = upregulated pathway. Metabolites overexpressed in PCa cells are shown in bold. TCA, tricarboxylic acid (cycle); AAs, amino acids; DNA, deoxyribonucleic acid; GNMT, glycine N-methyltransferase; SARDH, sarcosine dehydrogenase; G6P, glucose-6-phosphate; 6P, 6-phosphate; 3PG, 3-phosphoglycerate; CoA, coenzyme A.
For citrate synthesis, oxaloacetate and acetyl-coenzyme A (acetyl-CoA) are essential, but whereas oxaloacetate is regenerated in the Krebs cycle, acetyl-CoA is consumed. To ensure that cancer cells have the needed energy for rapid proliferation, it is necessary to maintain elevated rates of citrate oxidation, and thus, the availability of acetyl-CoA is required. Some studies suggested that to maintain this accelerated citrate oxidation, alterations in fatty acid metabolism are needed to provide both ATP and acetyl-CoA [27], [33], [34] (Figure 1).
Beyond the Krebs cycle and glycolysis, glucose also can be degraded by the pentose phosphate pathway. This metabolic pathway provides NADPH and ribose-5-phosphate (important for the synthesis of nucleic acids and nucleotides), thus promoting anabolic reactions and redox homeostasis. In a recent study, Tsouko et al. (2014) demonstrated that androgen receptor (AR) signaling augmented the levels of glucose-6-phosphate dehydrogenase (G6PD) (key enzyme for pentose phosphate pathway), NADPH, and ribose synthesis in hormone-sensitive PCa cells and castrate-resistant PCa (CRPC) cells. After inhibition of mammalian target of rapamycin with rapamycin, the upregulation of G6PD is abolished. Hence, these studies revealed a relationship between the upregulation of G6PD via AR and mammalian target of rapamycin. These results suggested the importance of pentose phosphate pathway for PCa growth [35].
Cell proliferation and intercellular signaling are dependent on increased lipid biosynthesis. Acetyl-CoA also plays an important role in this metabolic alteration because it is a precursor for lipogenesis and cholesterogenesis and can be produced by transformation of citrate in the cytosol [1]. Sterol regulatory element-binding protein–1, an essential transcription factor for lipogenesis, is also implicated in AR transcriptional regulation. Beyond increased lipogenesis, sterol regulatory element-binding protein–1 also increased reactive oxygen species production and the expression of NADPH oxidase, which leads to proliferation, migration, and invasion of PCa cells [36], [37], [38]. In PCa cells, the levels of choline and creatine are increased because there is an augmentation of membrane synthesis for cell proliferation [16].
Glutamine also has an important role in the maintenance of lipogenesis, as well as to provide intermediates for the Krebs cycle through glutaminolysis (where glutamine is transformed into glutamate by glutaminase and then glutamate is transformed into α-ketoglutarate). The observation that the glutamine transporter and glutaminase are both overexpressed in PCa cells was proof of the importance of this mechanism in PCa [38], [39], [40]. The α-ketoglutarate derivate from glutamine can contribute to the formation of citrate when incorporated into the Krebs cycle (oxidation pathway); however, α-ketoglutarate can also be transformed into citrate by the reversal of the tricarboxylic acid cycle through reductive carboxylation. This alteration of oxidation to reductive carboxylation is promoted by hypoxia and leads to lipid synthesis and tumor growth [41]. The tumor-stromal interactions also have an important role in PCa development. The myofibroblastic microenvironment, formed from the interaction of cancer cells with “cancer-associated fibroblasts”, is important for the reverse Warburg effect. Cancer-associated fibroblasts in the myofibroblastic microenvironment undergo the Warburg effect, induced by epithelial cancer cells, and secrete lactate and pyruvate. The lactate and pyruvate are taken up by the PCa cells and used for the Krebs cycle, anabolic metabolism, and cell proliferation [38], [42], [43].
Metabolomics Studies in PCa Model Systems and Biological Fluids
The most common models and biological fluids used to perform metabolomics studies are tissue and cultured cell lines and human urine, serum/plasma, prostatic fluid/seminal fluid, respectively.
The use of urine as a sample for metabolomics studies has many advantages compared with serum: urine is easier to obtain and handle, needs less sample preparation, and has higher amounts of metabolites and a lower protein content [12], [44], [45]. Blood plasma/serum has some advantages compared with urine, as the diurnal variation and the intra- and intervariability are lower. However, serum and plasma are more complex matrices than urine, having a higher concentration of proteins, and sample collection is more invasive [5].
Seminal fluids, obtained by ejaculation, come from the seminal vesicles, prostate, and epididymis. Prostatic fluid is collected after prostate massage, and the composition of this biofluid is simpler than seminal fluid [46], [47]. The use of seminal/prostatic fluids has some advantages compared with the use of other biofluids, as these samples are richer in prostatic metabolites because the metabolites do not need to cross blood-tissue barriers once they are naturally secreted into the seminal/prostatic fluid. Thus, seminal/prostatic fluids are less affected by confounding factors. However, these biofluids may be difficult to collect in men with erectile dysfunction, and a portion of men may have personal or ethical problems with giving these types of samples [48].
The collection of tissue samples is more invasive than the collection of other matrices; however, the use of matched malignant and normal adjacent prostate tissue is a good strategy to reduce intraindividual variability in metabolomics studies [49].
In vitro models are increasingly used because of interindividual variability, and difficulties enrolling patients in these models are nonexistent. In addition, cell lines have a perfectly defined cell state which allows the analysis of a targeted metabolic status [2], [50], [51]. However, they do not efficiently simulate the complex cell-cell and cell-matrix interactions occurring within an organism [2], [52].
There are two different metabolomics approaches to discover biomarkers for cancer: the top-down approach and the bottom-up approach. Both approaches have advantages and disadvantages. The top-down approach has the advantage of starting the metabolome evaluation using a real sample (urine or plasma) that could be used in a clinical practice. However, urine and plasma are complex biological matrices and have metabolites from different origins, and the metabolites may be more diluted. The advantage of the bottom-up approach (starts with the metabolic analyses of cell lines) is that cultured cell lines are a simpler biological system with less interference factors. In fact, studies with immortalized cultured cells are important to eliminate confounding factors, such as the age of patients, smoking habits, diet, and other diseases that influence the intervariability in plasma and urine. Nevertheless, the findings in cultured cell lines may not be directly extrapolated to the real disease as it is practically impossible to simulate complex cell–cell and cell–matrix interactions in cell cultures of PCa. Both approaches used together can be useful tools to obtain metabolic information that can discriminate the metabolic pathways in PCa cells.
Studies with Human Fluids and Model Systems
Urine Studies
Table 1 summarizes the major metabolites and metabolic pathways that have been found to be dysregulated in metabolomics studies performed on urine samples from PCa patients.
Table 1.
Metabolomic Studies Performed in Urines from PCa Patients
| PCa Subject Group | Control Group | Analytical Platform | Statistical Methods | Total Metabolites Found/Discriminative Metabolites Found | Discriminatory Metabolites/Biomarkers | Metabolic Pathways Dysregulated | Ref. |
|---|---|---|---|---|---|---|---|
| n= 13 | n= 24 | GS-MS | Binary strings, Similarity coefficients | 91/21 | Butyrolactone, methyl vinyl ketone, methylamine, N-ethylformamide, acetonitrile dimethylamino, pyridine, N-methyl-formamide, acetaldehyde, acetamide, 1-methyl-piperidine, 1-piperidineacetonitrile, dimethylamine, pyrrole, methacrolein, N-N-dimethylamine, 3-methyl-pyridine, 2-methyl-1H-pyrrole, 2-octanone, 1-ethyl-1H-pyrrole, 2-n-butylacrolein and methyl propyl disulfide | NS | [65] |
| n= 59 | n= 51 | LC-MS GS-MS |
Wilcoxon P test, hierarchical clustering, nonparametric tests | 583/34 | Sarcosine (+) | Alterations in glycine synthesis and degradation | [53] |
| n= 106 |
n= 57 (33 patients with no evidence of malignancy plus 24 HC) |
GC-MS | Nonparametric statistical tests and ROC | NS/0 | No relevant differences in sarcosine levels between patients with and without PCa | [61] | |
| n= 3 | n= 5 | LC-MS | NS | NS/5 | 1.Sarcosine 2.Proline 3.Kynurenine (+) 4.Uracil (+) 5.Glycerol 3-phosphate (+) |
1. Alterations in glycine synthesis and degradation Sarcosine is an intermediate compound in the metabolism of choline. 2. Alteration in amino acids metabolism 3. Alteration in kynurenine pathway 4. Alteration in pyrimidine metabolism 5. Alteration in energetic metabolism |
[55] |
| n= 25 PCa patients developing biochemical recurrence | n= 29 PCa patients who remained recurrence-free | GC-MS | ROC | 8/2 | Sarcosine (+) Cysteine (+) (in the group developing biochemical recurrence) |
Alterations in glycine synthesis and degradation | [56] |
| n= 33 |
n= 23 (13 HC plus 10 patients with BPH) |
GC-MS | Nonparametric statistical tests and ROC | NS/1 | Sarcosine (+) | Alterations in glycine synthesis and degradation | [57] |
| n= 86 | n= 45 | LC-MS | ROC | NS/1 | Diagnostic value of sarcosine was modest; relationship with clinicopathologic parameters was not found | Alterations in glycine synthesis and degradation | [58] |
| n= 20 | n= 28 (8 patients with BPH plus 20 HC) | GC-MS | PCA ROC |
81/5 | Dihydroxybutanoic acid (+), xylonic acid (+), pyrimidine (−), ribofuranoside(−), and xylopyranose(−) | Alterations in carbohydrate and energy metabolism | [60] |
| n= 211 | n= 134 | GC-MS | ROC | NS/1 | Sarcosine (+) | Alterations in glycine synthesis and degradation Sarcosine is an intermediate compound in the metabolism of choline. |
[59] |
| n= 32 | n= 32 | LC-MS GC-MS |
PCA PLS-DA |
1132/15 | 1. Glycine (−), serine(−), threonine (−), alanine (−) 2. Glutamine (−), citrate (−), aconitate (−), succinate (−) 3. Sucrose (−), sorbose (−), arabinose (−), arabitol (−), inositol (−), galactaric acid (−) 4.Carnitines (−) |
1. Alteration in amino acids metabolism 2. Disturbance in energy metabolism 3. Dysregulation in carbohydrates degradation 4. Alteration in long-chain fatty acids metabolism |
[63] |
| n= 59 | n= 43 | GC-MS | RF LDA |
196/4 | 1. 2,6-dimethyl-7-octen-2-ol (−), 3-octanone (−), 2-octanone (−) 2. Pentanal (+) |
1. Increase of utilization of these metabolites for increased energy consumption 2. Inflammatory conditions via the excessive production of reactive oxygen species, known to induce lipid peroxidation |
[64] |
BPH, benign prostatic hypertrophy; GS-MS, Gas chromatography–mass spectrometry; HC, healthy controls; LDA, linear discriminant analysis; LC-MS, Liquid chromatography–mass spectrometry; NS, not specified; PCA, Principal component analysis; PLS-DA,Partial least squares discriminant analysis RF, random forest; ROC, receiver-operator characteristic.
(+): levels increased in PCa; (−): levels decreased in PCa.
One of the most relevant metabolomics studies was performed by Seekumar et al. (2009), where sarcosine (N-methylglycine) was discovered as possible biomarker in urine for PCa. Sarcosine, an intermediate product in the synthesis and degradation of glycine, was found to be highly elevated during PCa progression to metastasis and was not detected or was presented at very low concentrations in the urine of healthy individuals [53]. Carcinogenesis alters the biosynthesis of sarcosine, although the importance of sarcosine in carcinogenesis remains unknown. It is known that glycine N-methyltransferase (GNMT) has a significant role in the metabolism of PCa tissues. This enzyme catalyzes the conversion of glycine to sarcosine and also participates both in the metabolism of methionine and in gluconeogenesis [54]. Similar results were achieved in other studies performed with different analytical platforms using urine as the matrix (Table 1) [55], [56], [57], [58], [59]. However, other studies concluded that urinary sarcosine levels were not significantly different between PCa patients and healthy controls (Table 1) [60], [61]. According to Issaq and Veenstra (2011), several causes for such divergences can be associated to different study design and methods, which can have diverse specificities and sensitivities, and also to interindividual differences [62].
Others common metabolic alterations detected in urine from PCa patients are alterations in amino acids, organic acids, sphingolipids, fatty acids, and carbohydrates. Dysregulation of carbohydrate degradation occurs because carbohydrates can be used by cancer cells for energy production. Alterations in carnitine profiles were also detected; carnitines and their derivatives are important for conservation of regular mitochondrial function as well as the transport of activated long-chain fatty acids from the cytoplasm to the mitochondrial compartment. The results also suggested disturbances in energy metabolism, including the Krebs cycle, which were expected given the Warburg effect and the alteration of the activity of m-aconitase, as previously explained (Table 1) [60], [63].
Potentiality of urinary volatile organic compounds to discriminate between PCa samples and controls was also evaluated in several different studies. In these studies, volatile organic compounds were able to differentiate urine from PCa patients and from control individuals (Table 1) [64], [65].
Plasma and Serum Studies
A summary of metabolomics studies performed on serum and plasma samples can be found in Table 2.
Table 2.
Metabolic Studies in Serum/Plasma from PCa patients
| PCa Subject Group | Control Group | Analytical Platform | Statistical Methods | Total Metabolites Found/Discriminative Metabolites Found | Discriminatory Metabolites/Biomarkers | Metabolic Pathways Dysregulated | Ref. |
|---|---|---|---|---|---|---|---|
| n= 962 | n= 1061 HC | GC-MS | Conditional logistic regression | NS/5 | Palmitic acid (+), stearic acid (−), myristic acid (+), linolenic acid (+), eicosapentaenoic acids (+) |
Alteration in lipid metabolism | [70] |
| n= 85 HG plus n= 120 LG | n= 114 HC | FIA-MS/MS LC-MS/MS | ROC and logistic regression model | 112/5 | 1. Lysophosphatidyl-choline (C16:0 and C18:0) (−) 2. Serotonin (−) 3. Aspartic acid (+) 4. Ornithine (−) |
1. Alteration in lipid metabolism 2. Alteration in growth inhibition and induction of apoptosis 3. Alteration in protein biosynthesis 4. Ornithine decarboxylase overexpression |
[75] |
| n= 561 | n= 1034 HC | GC-MS LC-MS |
Wilcoxon signed rank tests and χ2 tests | 7/3 | Choline (+), vitamin B2 (+), methylmalonic acid (−) | Alteration in membrane phospholipidic metabolism | [71] |
| n= 134 | n= 666 HC | LC-MS | ROC | 19/7 | Glutamine (−), alanine (+), valine (−), isoleucine (+), tryptophan (−), ornithine (+), lysine (+) | Alteration in free amino acid metabolism | [73] |
|
n= 28 Serum from patients developing biochemical recurrence |
n= 30 Serum from patients with recurrence free 5 years after prostatectomy |
LC-MS GC-MS |
ROC | 9/3 | Cystathionine (+), homocysteine (+), cysteine (+) | Alteration in methionine metabolism | [56] |
|
n= 36 Fasting plasma from PCa patients 3 months after the therapy initiation |
n= 36 Fasting plasma from PCa before starting androgen deprivation therapy |
LC-MS GC-MS |
t tests | 504/56 | 1. DHEAS (−), epiandrosterone sulfate (−), androsterone sulfate (−), cortisol (−), 4-androsten-3β (−),17β-diol disulfates 1 & 2 (−), 5α-androstan-3β (−) 17β-diol disulfate (−), pregnen-diol disulfate (−), pregn steroid monosulfate (−) and andro steroid monosulfates 1 & 2 (−). 2. Cholate (+), glycocholate (+), taurocholate (+), chenodeoxycholate (+), taurochenodeoxycholate (+), ursodeoxycholate (+), hyodeoxycholate (+), deoxycholate (+), taurodeoxycholate glycodeoxycholate (+), glycochenodeoxycholate (+), 7-ketodeoxycholate (+) glycochenodeoxycholate (+), glycolithocholate sulfate (+), taurolithocholate 3-sulfate (+), glycocholenate sulfate (−), and taurocholenate sulfate (−) 3. Carnitines (−), ketone bodies (−),dicarboxylic acids 4. 2-hydroxybutyrate (−) and branched-chain keto-acid dehydrogenase complex products (−) |
1. Steroids metabolism 2. Bile acids and intermediates of bile acid metabolism 3. Lipid oxidation 4. Markers of insulin resistance |
[78] |
| n= 290 | n= 312 | Fluorometric assay | ROC | 1/1 | Sarcosine (+) | Alterations in glycine synthesis and degradation | [66] |
| n= 105 | n= 36 | ESI-MS/MS | PCA and HCA | 390/35 | Phosphatidylethano-lamine (+), ether-linked phosphatidylethanola mine (+), ether-linked phosphatidylcholine (+) | Alteration in lipid metabolism | [72] |
|
n= 1122 (813 serum from patients with nonaggressive PCa plus 309 serum from patients with aggressive PCa (Gleason score ˃ 8)) |
n= 1112 | LC-MS | ROC | NS/1 | Sarcosine (+) | Alterations in glycine synthesis and degradation | [67] |
| n= 25 | n= 100 HC | Immunoassay | NS | 4/1 | Insulin (+) | Alteration in energetic metabolism | [76] |
| n= 64 | n= 50 HC | LC-MS | PCA PLS |
480/49 | Azelaic acid, uric acid, tryptophan, lysoPC (18:0/0:0), 3-oxo-9,11-tridecadienoic acid, 3-hydroxy-tetradecanedioic acid, 6-hydroxy-pentadecanedioic acid, 5-(2-methylpropyl)-2-oxooxolane-3-carboxylic acid, 5-butyl-2-oxooxolane-3-carboxylic acid, lysoPE (0:0/18:2), LysoPE (18:2/0:0), lysoPC (18:2/0:0), cortolone-3-glucuronide, pregnanetriol glucuronide, androstenedione, decanoic acid, menthol glucuronide, citronellol glucuronide, l-α-amino-1H-pyrrole-1-hexanoic acid, lysoPC (0:0/18:2), phenylalanyl phenylalanine 3β,16α-dihydroxyandrostenone sulfate, 2-tert-butyl-1,4-benzenediol sulfate, indoxyl sulfuric acid,10-dihydroxy-12Z,15Z-octadecadienoic acid,12,13-dihydroxy-9Z,15Z-octadecadienoic acid, 5,16-dihydroxy-9Z,12Z-octadecadienoic acid, 27-nor-5β-cholestane-3α,7α,12α,24,25-pentol glucuronide, hexadecanedioic acid phenylacetylglutamine, heptadecanoic acid, n-[(3α,5β,7β)-7-hydroxy-24-oxo-3-(sulfooxy)cholan-24-yl]-glycine, n-[(3α,5β,7α)-3-hydroxy-24-oxo-7-(sulfooxy)cholan-24-yl]-glycine, glycochenodeoxycholate-3-sulfate, 5-isopropyl-2-methylphenol, sulfate, 5-carboxy-α-chromanol glucuronide, indole-3-carboxaldehyde, androsterone sulfate, 5α-dihydrotestosterone sulfate and etiocholanolone sulfate |
Alteration in fatty acids metabolism, amino acids metabolism, lysophospholipids metabolism, and bile acids metabolism and alteration in steroid hormone biosynthesis pathway | [69] |
|
n= 70 40 LG PCa plus 30 HG PCa |
n= 32 HC | 1H-NMR | PCA, OPLS-DA and ROC | NS/4 | 1.Alanine (+), pyruvate(+) 2.Sarcosine (+) glycine (−) |
1. Alteration in energetic metabolism and lipogenesis 2. Alterations in glycine synthesis and degradation |
[68] |
| n= 29 | n= 21 BPH | NMR LC-MS GC-MS |
PCA and ROC | 348/53 | 1. Acylcarnitines 2. Choline (glycerol-phospholipids) 3. Arginine |
1. Alteration in fatty acids metabolism 2. Alteration in membrane phospholipidic metabolism 3. Alteration in amino acids metabolism |
[74] |
BPH, Benign prostatic hypertrophy; DHEAS, dehydroepiandrosterone sulfate; HC, Healthy Controls; HCA, hierarchical clustering analysis; GS-MS, Gas chromatography–mass spectrometry; HG, high grade; LC-MS, Liquid chromatography–mass spectrometry; LG, low grade; NS, Not specified; OPLS-DA, orthogonal partial least squares discriminant analysis; PCA, principal component analysis; PLS, Partial least squares; ROC, Receiver-Operator Characteristic.
(+): levels increased in PCa; (−): levels decreased in PCa.
Studies evaluating sarcosine as a biomarker for PCa were performed in serum samples using different analytical platforms [fluorometric assay, liquid chromatography–mass spectrometry (LC-MS), and 1H-nuclear magnetic resonance (NMR)]. The results showed that PCa samples had elevated levels of sarcosine and could distinguish low-grade from high-grade PCa, suggesting plasmatic level of sarcosine as a good biomarker for PCa (Table 2) [66], [67], [68].
Beyond the alteration in sarcosine levels, metabolomics studies of serum/plasma from PCa patients also revealed alterations in fatty acids, amino acids, lysophospholipids, bile acids, and metabolites related to the steroid hormone biosynthesis pathway. The alteration in fatty acids is related to changes in lipid β-oxidation necessary to provide energy for abnormal cell proliferation (Table 2) [69], [68], [70], [71], [72], [73], [74], [75]. Alterations in energetic metabolism are also common [68], [76] (Table 2), and increased levels of glucose in serum samples at the time of PCa diagnosis were associated with an increased risk of recurrences after therapy with radical prostatectomy or radiation therapy [77].
Serum and plasma metabolomics studies can also be used to assess alterations in the metabolic profile caused by medical treatment. The metabolic profile of fasting plasma from PCa patients before starting androgen deprivation therapy and 3 months after the therapy initiation was analyzed [78]. As expected, steroid levels decreased during androgen deprivation therapy, whereas the levels of most bile acids and their metabolites increased with therapy. Bile acids have an important role in the control of serum lipids, glycemic regulation, and energy homeostasis. Lower levels of metabolites related to lipid metabolism after 3 months of treatment were also observed. Carnitines, ketones, dicarboxylic acids, and the levels of 2-hydroxybutyrate and branched-chain keto-acid dehydrogenase complex products (biomarkers of insulin resistance) were also present in lower levels after the therapy, indicating a reduction in the catabolic state [78].
Prostatic Fluid and Seminal Fluid Studies
Table 3 presents a summary of metabolomics studies performed in prostatic fluid and seminal fluid from PCa patients.
Table 3.
Metabolomic Studies in Prostatic and Seminal Fluid from PCa Patients
| PCa Subject Group | Control Group | Analytical Platform | Statistical Methods | Total Metabolites Found/Discriminative Metabolites Found | Discriminatory Metabolites/Biomarkers | Metabolic Pathways Dysregulated | Ref. |
|---|---|---|---|---|---|---|---|
| n= 13 (prostatic fluid) | n= 28 chronic prostatitis, n= 28 adenoma n= 22 HC | Fluorescence | Student’s t test and Kolmogorov-Smirnov test | 1/1 | Zinc (−) | Lose capability to accumulated zinc | [79] |
| n= 4 (prostatic fluid) |
n= 10 BPH n= 12 |
NMR | Multiple regression | NS/3 | 1. Citrate (−) 2. Spermine (−) and myo-inositol (−) |
1. Alteration in energetic metabolism 2. Alteration in polyamines synthesis |
[80] |
| n= 3 (seminal fluid) |
n= 3 n= 1 BPH |
NMR | NS | NS/1 | Citrate (−) | Alteration in energetic metabolism | [46] |
|
n= 21 (seminal fluid) n= 7 (prostatic fluid) |
n= 16 (seminal fluid) n= 17 (prostatic fluid) |
NMR | ROC | NS/1 | Citrate (−) | Alteration in energetic metabolism | [81] |
| n= 52 (prostatic fluid) | n= 26 | NMR | LR and ROC | 9/3 | 1. Citrate(−) 2. Myo-inositol (−) and spermine (−) |
1. Alteration in energetic metabolism 2. Alteration in polyamines synthesis |
[82] |
BPH, Benign prostatic hypertrophy; LR, logistic regression; NMR, Nuclear magnetic resonance; NS, Not specified; ROC, Receiver-operator characteristic.
(+): levels increased in PCa; (−): levels decreased in PCa.
Prostatic and seminal fluids are other biofluids that may be used to perform PCa metabolomics studies to discover alterations in cancer cell metabolism and noninvasive biomarkers for PCa detection.
As explained previously, normal prostate cells have the ability to accumulate zinc and consequently accumulate citrate. However, PCa cells lose this ability. Several metabolomics studies support this theory. The analysis of prostatic and seminal fluid using different analytical platforms (fluorescence technique and NMR) revealed reduced levels of zinc and citrate in PCa groups when compared with the controls (Table 3) [46], [79], [80], [81], [82]. Kline et al. (2006) also concluded that citrate level tests outperform the PSA test in PCa detection. Additionally, the analysis of citrate in semen has the same efficacy as the analysis of citrate in prostatic secretion for detecting PCa [81].
Another important function of normal prostate cells is the synthesis of polyamines, such as spermine and myo-inositol. The analysis of prostatic and seminal fluid from PCa patients showed significantly decreased levels of spermine and myo-inositol (Table 3) [80], [82]. Serkova et al. (2008) also demonstrated that the reduction in citrate, spermine, and myo-inositol levels is independent of the patient’s age [82].
Ex Vivo Tissue Studies
Table 4 presents a summary of metabolomics studies performed in PCa tissues.
Table 4.
Metabolomic Studies in Prostate Cancer Tissue
| PCa Subject Group | Control Group | Analytical Platform | Statistical Methods | Total Metabolites Found/Discriminative Metabolites Found | Discriminatory Metabolites/Biomarkers | Metabolic Pathways Dysregulated | Ref. |
|---|---|---|---|---|---|---|---|
| n= 21 |
n= 66 Benign prostate tissue |
MRS | LDA | NS/6 | 1. Citrate (−) 2. Taurine (+), glutamate (+) |
1. Reduced citrate synthesis (Krebs cycle) 2. Alteration in energy metabolism |
[85] |
|
n= 10 Malignant human prostate tissue |
n= 10 BPH |
1H-NMR | Nonparametric test of Kruskal-Wallis | NS/3 | 1. Citrate (−) 2. Choline (+) 3. Myo-inositol (+) |
1. Reduced citrate synthesis (Krebs cycle) 2. Increased membrane turnover 3. Altered membrane metabolism |
[86] |
|
n= 15 Adenocarcinoma |
n= 1 HC |
1H-NMR | Linear regression analysis | NS/2 | 1. Citrate (−) 2. Spermine (−) |
1. Reduced citrate synthesis 2. Reduced spermine synthesis (amino acid synthesis) |
[87] |
|
n= 27 Adenocarcinoma |
n= 44 BPH |
MRS | NS | 22/3 | 1. Citrate (−) 2. Choline (+) 3. Lipid/lysine ratio (+) |
1. Reduced citrate synthesis 2. Increased membrane turnover 3. Increased cellular proliferation |
[88] |
| n= 20 |
n= 33 Benign tissue |
1H-NMR | Nonparametric Spearman correlation coefficients | NS/8 | 1. Choline (+), phosphocholine (+), glycerophospho-choline (+) 2. Taurine (+) 3. Myo-inositol (+), scyllo-inositol (+) 4. Citrate (−), polyamines (−) |
1. Alteration in phospholipid membrane synthesis and hydrolysis 2. Alteration in energy metabolism 3. Altered membrane metabolism 4. Reduced citrate and polyamines synthesis |
[89] |
| n= 15 |
n= 32 Benign prostatic tissue |
1H-NMR | Z statistics | NS/5 | Phosphocholine (+), glycerol-phosphocholine (+), phosphor-ethanolamine (+), glycerophospho-ethanolamine (+), ethanolamine (−) | Alterations on phospholipid membrane assembly and catabolism | [92] |
| n= 16 |
n= 82 Benign prostate biopsies |
1H-NMR | NS | NS/2 | 1. Lactate (+) 2. Alanine (+) |
1. “Warburg effect” 2. Intensification in glycolytic flux and increased protein synthesis in cancer cells |
[90] |
| n= 18 | n= 30 | 1H-NMR | LR | NS/7 | tCho/Cit (+), Cho/Cr (+), GPC + PC)/Cr (+), Lac/Al (+) Cit/Cr (−) | Alterations in citrate synthesis (Krebs cycle), in membrane turnover, and in energetic metabolism | [84] |
| n= 12 Localized PCa n= 14 metastatic PCa | n= 16 Benign tissue adjacent to PCa | LC-MS GC-MS |
Wilcoxon P test, hierarchical clustering, nonparametric tests |
626/60 | 1. Sarcosine (+) 2. Uracil (+) 3.Kynurenine (+) 4. Glycerol-3-phosphate (+), 5. Leucine (+), proline (+) |
1. Alterations in glycine synthesis and degradation Sarcosine is an intermediate compound in the metabolism of choline. 2. Alterartion in pyrimidine metabolism 3.Alteration in Kynurenine pathway 4. Alteration in energetic metabolism 5. Alteration in amino acids metabolism |
[53] |
| n= 27 | n= 54 | NMR | NS | NS/NS | Omega-6 PUFA (+) | Alteration in lipid metabolism | [95] |
|
n= 16 patients with chemical failure |
n= 32 Patients without recurrence after prostatectomy |
1H-NMR | PCA | NS/6 | 1. Spermine 2. Myo-inositol, phosphoryl, scyllo-inositol 3. Choline 4. Glutamate, glutamine |
1. Reduced spermine synthesis (amino acid synthesis) 2. Altered membrane metabolism 3. Alteration in phospholipid membrane synthesis and hydrolysis 4.Alteration in energy metabolism |
[93] |
| n= 41 |
n= 108 Benign adjacent prostate tissue |
1H-NMR | Binary logistic regression and multivariate linear regression | 13/6 | 1. Choline compounds (+) 2. Myo-inositol (−), scyllo-inosito (+) |
1. Alteration in phospholipid membrane synthesis and hydrolysis 2. Altered membrane metabolism |
[94] |
| n= 92 | n= 92 | GC-MS | Nonparametric statistical tests and ROC | NS/1 | Sarcosine (+) (sarcosine levels were not related with tumor stage, grade or biochemical recurrence) |
Alterations in glycine synthesis and degradation. Sarcosine is an intermediate compound in the metabolism of choline. | [83] |
| n= 331 | n= 178 | GC-MS LC-MS |
ROC | 469/200 | 1. Sarcosine (+) 2. Kynurenine (+) 3. Proline (+) 4. Uracil (+) 5.Glycerol-3-phosphate (+), threonine (+) citrate (−) ADP-ribose (−) 15-hydroxyeicosatetraenoic acid(−) 6.Polyanines (−) |
1. Alterations in glycine synthesis and degradation Sarcosine is an intermediate compound in the metabolism of choline. 2. Alteration in kynurenine pathway 3.Alteration in amino acids metabolism 4.Alterartion in pyrimidine metabolism 5.Alteration in energetic metabolism 6.Reduced polyamines synthesis |
[15] |
|
n= 11 Localized PCa plus n= 10 metastatic PCa |
n= 11 Benign adjacent prostate samples |
GC-MS | ROC | NS/1 | Sarcosine (+) (progressive elevation from benign tissue to localized tumors and metastatic disease) | Alterations in glycine synthesis and degradation Sarcosine is an intermediate compound in the metabolism of choline. |
[59] |
| n= 95 |
n= 95 Normal adjacent prostate tissue |
GC-MS LC-MS |
ROC, Univariate Cox regression, Kaplan-Meier analyses, and multivariate Cox regression analyses |
820/9 | 1. Gluconic acid (−), maltotriose (−) 2. Aminoadipic acid (+) 3. Cerebronic acid (+), glycerophosphoethanol-amine (+), 2-hydroxybehenic acid (+), tricosanoic acid (+) 4. Isopentenyl pyrophosphate (+) 5. 7-methylguanine (+) |
1. Alteration in carbohydrate metabolism 2. Increased fatty acid synthesis 3. Alteration in lipid metabolism 4. Intermediate in the steroid synthesis pathway, indicates an increase of cholesterol levels 5. DNA damage |
[49] |
|
n= 30 LG PCa plus n= 81 HG PCa |
n= 47 Normal adjacent tissue |
MRS | PCA, PLS and PLS-DA | 23/2 | 1. Citrate (−) 2. Spermine (−) |
1. Reduced citrate synthesis 2. Reduced spermine synthesis |
[91] |
BPH, Benign prostatic hypertrophy; Cho/Cr, choline/creatinine; Cit/Cr, citrate/creatinine; GS-MS, Gas chromatography–mass spectrometry; (GPC + PC)/Cr, (glycerol-phosphocholine + phosphoryl-choline)/creatinine; HC, Healthy Controls; HG, High grade; Lac/Al, lactate/alanine; LC-MS, Liquid chromatography–mass spectrometry; LDA, Linear discriminant analysis; LG, Low grade; LR, Linear regression MRS, magnetic resonance spectroscopy; NMR, Nuclear magnetic resonance; NS, Not specified; PCA, Principal component analysis; PLS, partial least squares; PLS-DA, partial least squares discriminant analysis; tCho/Cit, total choline/citrate.
(+): levels increased in PCa; (−): levels decreased in PCa.
The value of sarcosine as a PCa biomarker was also evaluated in prostate tissue samples using different analytical platforms. The levels of sarcosine were increased in PCa samples (Table 4) [15], [53], [59], [83]. Results also revealed that sarcosine levels were significantly elevated in metastatic PCa and clinically localized PCa tissue samples, whereas in benign samples, sarcosine was not detected. These results indicate that sarcosine may be a good biomarker for monitoring disease progression and aggressiveness [53].
Other metabolites, namely citrate, lactate, and alanine, were frequently altered in PCa tissue samples. These results suggest alterations in citrate synthesis (Krebs cycle) and in energetic metabolism. As previously explained, the PCa cells switch from citrate accumulation to citrate oxidation when becoming malignant. PCa cells also undergo the Warburg effect, all of which explain these alterations (Table 4) [15], [84], [85], [86], [87], [88], [89], [90], [91].
It is also well established that cancer cells have elevated proliferation rates, and this is reflected in alterations in membrane metabolism. Several metabolomics studies performed in PCa tissue revealed an increase of choline levels in PCa samples, which indicates alterations in phospholipid membrane synthesis and hydrolysis (Table 4) [84], [86], [88], [89], [92], [93], [94]. Because this elevated proliferation rate also increases cell energy requirements, PCa samples also have alterations in lipid metabolism (as lipids may be used by the cells to produce energy) (Table 4) [49], [95].
Beyond the study of the metabolic pathways involved in cancer development, it is also important to assess which metabolic pathways are involved in the growth of bone metastases. The results obtained from a study [78] revealed a significant increase in cholesterol levels in bone metastases tissues from PCa patients. The metabolic profile from PCa bone metastases indicates high energy metabolism, which may be related to highly proliferating cells. This conclusion was based on the elevated levels of certain metabolites, such as threonine, glutamate, phenylalanine, citrate, fumarate, glycerol-3-phosphate, and fatty acids. Another relevant metabolic alteration in PCa bone metastases tissue was the elevated levels of myo-inositol-1-phosphate, which may indicate active cell signaling involving inositol-based compounds as second messengers. Inositol-based molecules are related to the activation of protein kinase C, and the activation of this molecule is important for cell proliferation, apoptosis, differentiation, invasion, and angiogenesis. The concentration of sarcosine was increased in bone metastases from PCa and from other cancers, which reveals that sarcosine may not be specific to PCa. Metabolites such as threonine, asparagine, fumarate, and linoleic acid are present in high levels in samples from bone metastases. The levels of these metabolites were also increased in samples of primary prostate tissues from patients with confirmed bone metastases. Linoleic acid, an essential fatty acid, was associated with PCa progression. Furthermore, linoleic acid may also be associated with the inflammatory response because linoleic acid is transformed into arachidonic acid. Arachidonic acid is a precursor for prostaglandins, which have an important role in inflammation [96].
In Vitro Studies
Table 5 summarizes general information on in vitro metabolomics studies in PCa-derived cell lines.
Table 5.
Metabolomic Studies Performed in Human PCa-Derived Cell Lines
| Cancer Cell Lines | Control Group | Analytical Platform | Statistical Methods | Total Metabolites Found/Discriminative Metabolites Found | Discriminatory Metabolites/Biomarkers | Metabolic Pathways Dysregulated | Ref. |
|---|---|---|---|---|---|---|---|
| AD prostate carcinoma LNCaP cell line | Androgen-independent prostate carcinoma PC-3 cell line | MRS | NS | 3/2 | Uptake of ethanolamine and N,N′-dimethylethanolamine (+) (principally in AD cells in presence of androgens) | Alteration in membrane lipid synthesis | [101] |
| VCaP, DU145, 22RV1, and LNCaP | PrEC and RWPE | LC-MS GC-MS |
Wilcoxon P test, hierarchical clustering, nonparametric tests | 1/1 | Sarcosine (+) | Alterations in glycine synthesis and degradation | [53] |
| Androgen-nonresponsive PC3 and DU145 cell lines Androgen-responsive VCaP (treated with synthetic androgen) and LNCaP cell lines |
RWPE | LC-MS | HCA | 1553/674 |
Malignant cell lines 1. Sarcosine (+) 2. Threonine (+), phenylalanine (+), alanine (+), creatine (+),creatinine (+), citrulline (+), tryptophan (−), 1-methyl tryptophan (−) and kyneuric acid (−) Androgen-responsive cell lines 3. Serine (+), threonine (+), lysine (+), homocysteine (+), asparagine (+), alanine (+), glutamic acid (+) 4. S-adenosylmethionine (−), homocysteine (+) Androgen treatment resulted in further perturbations in amino acid metabolism and increased methylation. |
1. Alterations in glycine synthesis and degradation Sarcosine is an intermediate compound in the metabolism of choline. 2. Alteration in amino acids metabolism 3. Alteration in amino acids metabolism 4. Elevated methylation activity |
[97] |
| PC3 and LNCaP treated with LY294002 (inhibitor of the PI3K signaling pathway) or 17AAG (inhibitor of the HSP90 protein chaperone) | PC3 and LNCaP untreated | MRS | PCA | NS/24 |
After both treatments: lactate (−), alanine (−), fumarate (−) LY294002 treatment: phosphocholine (−), glutathione (−), glutamine (+), valine (+), leucine (+), isoleucine (+) 17AAG treatment: phosphocholine (+), citrate (+), glutamine (−), valine (+), leucine (+), isoleucine (+), myo-inositol (+), taurine (+) |
PI3K and HSP90 inhibition | [102] |
| Low-invasiveness WPE1-NB14 and high-invasiveness WPE1-NB11 cell lines | RWPE-1 | 1H-NMR | PLS-DA | NS/10 | 1. Leucine (−), valine (−), isoleucine (−), glutamine (−), glutamate (−), β-hydroxyisovalerate (−) 2. Glycine (−) 3. Lactate, alanine 4. Phosphocholine (+) |
1. Increased protein synthesis and amino acid catabolism 2. Alterations in methylation and synthesis and degradation of sarcosine 3. Alteration in energetic metabolism; 4. Alteration in choline metabolism |
[98] |
| Androgen-nonresponsive PC3 and androgen-responsive LNCaP cell lines | PNT1A | 1H-NMR | Two-way analysis of variance followed by Bonferroni posttest | NS/3 | Glucose consumption (+) PC3 cells: Lactate (+) Alanine (+) Lactate/alanine ratio (+) |
Increased levels of oxidative stress in PC3 cells. Androgen-responsive and -nonresponsive PCa cells showed different glycolytic metabolism profiles. | [100] |
| DU145, PC3, and LNCaP (knockdown of GNMT, SARDH, or PIPOX and overexpression of GNMT, SARDH, or PIPOX (convert sarcosine back to glycine) | RWPE | GC-MS | ROC | NS/1 |
Overexpression of GNMT: sarcosine (+) (increase in invasion). Knockdown of GNMT: sarcosine (−) (reduction in cell proliferation, invasion, and greater percentage of cell death) Overexpression of SARDH or PIPOX: sarcosine (−) (reduced invasion) Knockdown of SARDH: sarcosine (+) (increase proliferation and invasion) Knockdown of PIPOX: sarcosine (+) (increased invasion) |
Alteration in glycine synthesis and degradation | [59] |
| CRPC cell C4-2, 22Rv1 and LNCaP-abl | Androgen receptor positive LNCaP and MDA-PCa-2a and MDA-PCa-2b |
LC-MS | HCA | 150/38 |
CRPC cells: 1. Sugars and intermediates associated with energy metabolism and signaling (+) AD cells 2. Carnitines, amino acids, and their methylated derivatives (+) |
1. Alteration in energy metabolism and signaling 2. Alteration in amino acid metabolism |
[99] |
| Highly metastatic LNCaP-LN3 treated with DCA and poorly metastatic LNCaP treated with DCA | LNCaP-LN3 and LNCaP untreated | NMR | Nonparametric test | NS/3 |
LNCaP-LN3 treated with DCA: Lac/Cr (−), Lac/Cho (−), Lac/Al (−), and Lac/(Cho + Cr + Al) (−) LNCaP (poorly metastatic) treated with DCA: no change in lactate/metabolite ratios |
Reversion of Warburg effect (LNCaP-LN3 cells respond better to DCA) | [103] |
AD, Androgen dependent; CRPC, castrate-resistant prostate cancer; DCA, Dichloroacetate; GC-MS, Gas chromatography–mass spectrometry; GNMT, Glycine-N-methyl transferase; HCA, Hierarchical clustering analysis; Lac/Al, lactate/ alanine; Lac/Cr, lactate/creatine; Lac/Cho, lactate/choline; Lac/(Cho + Cr + Ala), lactate/(choline + creatine + alanine); LC-MS, Liquid chromatography–mass spectrometry; NMR, Nuclear magnetic resonance; MRS, Magnetic resonance spectroscopy; NS, Not specified; PCA, Principal component analysis; PIPOX, Pipecolic acid oxidase; PLS-DA, Partial least squares discriminant analysis; ROC, Receiver-operating characteristics analysis; SARD, Sarcosine dehydrogenase.
(+), Levels increased in PCa; (-), Levels decreased in PCa.
The biological relevance of sarcosine was evaluated in four immortalized PCa cell lines, in primary benign prostate epithelial cells, and in an immortalized benign prostate epithelial cell line. Sarcosine levels were increased in malignant cell lines when compared with benign cell lines [53], [97]. Furthermore, alterations in the expression of the enzymes involved in sarcosine metabolism influence cell proliferation, invasion, and cell death, which suggest the importance of sarcosine in PCa metabolism (Table 5) [53], [59]. In an effort to understand the role of sarcosine in PCa progression, Sreekumar et al. (2009) evaluated the role of androgen signaling and the genes ERG and ETV1 (important mediators of PCa progression). The results showed that after treatment with androgens, cell lines that were ERG positive and ETV1 positive had increased GNMT expression and decreased sarcosine dehydrogenase (SARDH) expression [53].
In agreement with the results from metabolomics studies performed in other matrices (urine, plasma/serum, and tissues), presented here previously, alterations in amino acid metabolism were also observed in the studies performed in PCa cell lines. PCa cells lines revealed alterations in the levels of certain amino acids, such as leucine, valine, or isoleucine (Table 5) [97], [98], [99].
The increase of lactate and alanine levels in PCa cell lines is frequently observed in metabolomics studies. These alterations suggest changes in cellular energy metabolism (Table 5) [97], [98], [99], [100].
As reported for other matrices, metabolomics studies in PCa also revealed that PCa cells experience alterations in membrane metabolism with changes, for example, in choline metabolite levels (Table 5) [98], [101]. Androgen signaling has an important role in the development and progression of PCa. In fact, one current therapy for metastatic PCa is the use of antiandrogen agents; however, with the progression of the disease, patients normally develop resistance to this therapy, and it is currently impossible to predict if the cancer will progress into a castration-resistant state. The androgen-responsive cell lines can be characterized by increased levels of spermine, N-acetylspermine, serine, threonine, lysine, homocysteine, asparagine, alanine, and glutamic acid, as well as decreased levels of S-adenosylmethionine, with a simultaneous increase in levels of its breakdown product, homocysteine. These findings indicate that androgen-responsive cell lines have an elevated methylation activity. Androgen treatment resulted in further perturbations in amino acid metabolism and in a shift toward increased methylation. Androgen-nonresponsive cell lines and androgen-responsive cell lines also have differences in their glycolytic metabolism profiles. Androgen-dependent PCa cells and androgen-independent PCa cells also show differences in membrane lipid synthesis (Table 5) [97], [100], [101].
Metabolomics studies in cell lines may also be used to evaluate the alterations that occur after a pharmacological therapy. The treatment of PCa cells appears to lead to changes in energetic metabolism and choline metabolism (Table 5) [102]. Dichloroacetate (DCA) is an inhibitor of pyruvate dehydrogenase kinase, and inhibition of this enzyme has the potential to reverse the Warburg effect due to the increased pyruvate uptake into mitochondria. After treatment with this drug, the highly metastatic cells showed significantly lower levels of lactate/metabolite ratios [Lac/Cr, Lac/Cho, Lac/Al, and Lac/(Cho + Cr + Al)], whereas in poorly metastatic cells, no changes in lactate/metabolite ratios were found after the treatment. These findings suggest that highly metastatic cells are more dependent on lactate production (Table 5) [103].
Conclusions and Future Directions
The introduction of PSA testing has radically altered how PCa is diagnosed and managed. However, this test may lead to a false-positive or false-negative diagnosis. This drawback has given rise to serious efforts toward the discovery of new biomarkers, preferentially noninvasive, which have better specificity and sensitivity.
Because metabolic alterations are the last step in the cellular response to diseases, metabolomics can be successfully used to discover new biomarkers for cancer. In this regard, several studies have been conducted to characterize the metabolic profile of PCa.
One of the major obstacles in data interpretation is that the metabolic profile is influenced by various factors, such as age, diet, drugs, and chronobiological variations, among others. Additional problems include sample preparation, the analytical procedures, and the statistical platforms used. Major differences in these conditions can compromise the comparison of results among different studies and consequently compromise the discovery of new biomarkers. Another intrinsic difficulty with metabolomics studies is the massive amount of data produced that is difficult statistically to analyze. Despite these difficulties, metabolomics is a powerful tool for the discovery of new biomarkers for PCa detection, biomarkers indicative of cancer prognosis, disease progression, and therapeutic response, as well as identifying new therapeutic targets.
The metabolomics studies described in this review revealed different results, but almost all studies in urine, plasma/serum, prostatic fluids, tissues, and cell lines associated PCa with decreased levels of citrate and polyamines and increased levels of choline, lactate, and amino acids.
Further studies are still needed to confirm the results of these studies and identify an inexpensive, noninvasive, sensible, and specific biomarker for PCa.
Acknowledgements
This work received financial support from the European Union (FEDER funds POCI/01/0145/FEDER/007728) and National Funds (FCT/MEC, Fundação para a Ciência e Tecnologia and Ministério da Educação e Ciência) under the Partnership Agreement PT2020 UID/MULTI/04378/2013.
Contributor Information
Ana Rita Lima, Email: ritacmlima@hotmail.com.
Paula Guedes de Pinho, Email: pguedes@ff.up.pt.
References
- 1.Trock BJ. Application of metabolomics to prostate cancer. Urol Oncol. 2011;29:572–581. doi: 10.1016/j.urolonc.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halama A. Metabolomics in cell culture—a strategy to study crucial metabolic pathways in cancer development and the response to treatment. Arch Biochem Biophys. 2014;564:100–109. doi: 10.1016/j.abb.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 3.He JC, Chuang PY, Ma'ayan A, Iyengar R. Systems biology of kidney diseases. Kidney Int. 2012;81:22–39. doi: 10.1038/ki.2011.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramautar R, Berger R, van der Greef J, Hankemeier T. Human metabolomics: strategies to understand biology. Curr Opin Chem Biol. 2013;17:841–846. doi: 10.1016/j.cbpa.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Monteiro M, Carvalho M, de Lourdes Bastos M, de Pinho P. Biomarkers in renal cell carcinoma: a metabolomics approach. Metabolomics. 2014;10:1210–1222. [Google Scholar]
- 6.Aboud OA, Weiss RH. New opportunities from the cancer metabolome. Clin Chem. 2013;59:138–146. doi: 10.1373/clinchem.2012.184598. [DOI] [PubMed] [Google Scholar]
- 7.Monteiro MS, Carvalho M, Bastos ML, de Pinho PG. Metabolomics analysis for biomarker discovery: advances and challenges. Curr Med Chem. 2013;20:257–271. doi: 10.2174/092986713804806621. [DOI] [PubMed] [Google Scholar]
- 8.Weiss RH, Kim K. Metabolomics in the study of kidney diseases. Nat Rev Nephrol. 2012;8:22–33. doi: 10.1038/nrneph.2011.152. [DOI] [PubMed] [Google Scholar]
- 9.Monteiro M, Carvalho M, Henrique R, Jeronimo C, Moreira N, de Lourdes Bastos M, de Pinho PG. Analysis of volatile human urinary metabolome by solid-phase microextraction in combination with gas chromatography–mass spectrometry for biomarker discovery: application in a pilot study to discriminate patients with renal cell carcinoma. Eur J Cancer. 2014;50:1993–2002. doi: 10.1016/j.ejca.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Monteiro MS, Carvalho M, Bastos MdL, Pinho PG. Identification and data processing methods in metabolomics: Future Science Ltd. 2015. Potentiality of volatile organic compounds to discriminate patients with cancer by using chemometric tools; pp. 166–184. [Google Scholar]
- 11.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 12.Rigau M, Olivan M, Garcia M, Sequeiros T, Montes M, Colas E, Llaurado M, Planas J, Torres Id, Morote J. The present and future of prostate cancer urine biomarkers. Int J Mol Sci. 2013;14:12620–12649. doi: 10.3390/ijms140612620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoun Fouad, Peltier Alexandre, van Velthoven Roland. A Comprehensive Review of Contemporary Role of Local Treatment of the Primary Tumor and/or the Metastases in Metastatic Prostate Cancer. BioMed Research International, vol. 2014, Article ID 501213, 12 pages, 2014. 2014;2014:501213. doi: 10.1155/2014/501213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimakakos Andreas, Armakolas Athanasios, Koutsilieris Michael. Novel Tools for Prostate Cancer Prognosis, Diagnosis, and Follow-Up. BioMed Research International, vol. 2014, Article ID 890697, 9 pages, 2014. 2014;2014:890697. doi: 10.1155/2014/890697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDunn JE, Li Z, Adam KP, Neri BP, Wolfert RL, Milburn MV, Lotan Y, Wheeler TM. Metabolomic signatures of aggressive prostate cancer. Prostate. 2013;73:1547–1560. doi: 10.1002/pros.22704. [DOI] [PubMed] [Google Scholar]
- 16.Roberts MJ, Schirra HJ, Lavin MF, Gardiner RA. Metabolomics: a novel approach to early and noninvasive prostate cancer detection. Korean J Urol. 2011;52:79–89. doi: 10.4111/kju.2011.52.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barry MJ. Screening for prostate cancer—the controversy that refuses to die. N Engl J Med. 2009;360:1351–1354. doi: 10.1056/NEJMe0901166. [DOI] [PubMed] [Google Scholar]
- 18.Abrate A, Lughezzani G, Gadda GM, Lista G, Kinzikeeva E, Fossati N, Larcher A, Dell'Oglio P, Mistretta F, Buffi N. Clinical use of [− 2]proPSA (p2PSA) and its derivatives (%p2PSA and Prostate Health Index) for the detection of prostate cancer: a review of the literature. Korean J Urol. 2014;55:436–445. doi: 10.4111/kju.2014.55.7.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Link RE, Shariat SF, Nguyen CV, Farr A, Weinberg AD, Morton RA, Richardson B, Bernard D, Slawin KM. Variation in prostate specific antigen results from 2 different assay platforms: clinical impact on 2304 patients undergoing prostate cancer screening. J Urol. 2004;171:2234–2238. doi: 10.1097/01.ju.0000127736.86597.e7. [DOI] [PubMed] [Google Scholar]
- 20.Wu CL, Jordan KW, Ratai EM, Sheng J, Adkins CB, Defeo EM, Jenkins BG, Ying L, McDougal WS, Cheng LL. Metabolomic imaging for human prostate cancer detection. Sci Transl Med. 2010;2:16ra18. doi: 10.1126/scitranslmed.3000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andriole GL, Crawford ED, Grubb RL, III, Buys SS, Chia D, Church TR, Fouad MN, Isaacs C, Kvale PA, Reding DJ. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spur EM, Decelle EA, Cheng LL. Metabolomic imaging of prostate cancer with magnetic resonance spectroscopy and mass spectrometry. Eur J Nucl Med Mol Imaging. 2013;40(Suppl. 1):S60–S71. doi: 10.1007/s00259-013-2379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ukimura O, Coleman JA, de la Taille A, Emberton M, Epstein JI, Freedland SJ, Giannarini G, Kibel AS, Montironi R, Ploussard G. Contemporary role of systematic prostate biopsies: indications, techniques, and implications for patient care. Eur Urol. 2013;63:214–230. doi: 10.1016/j.eururo.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 24.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 25.Warburg OPK, Negelein F. Über den Stoffwechsel der Carcinom-Zelle. Biochem Z. 1924;152:319–344. [Google Scholar]
- 26.Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 2013;104:275–281. doi: 10.1111/cas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pertega-Gomes N, Baltazar F. Lactate transporters in the context of prostate cancer metabolism: what do we know? Int J Mol Sci. 2014;15:18333–18348. doi: 10.3390/ijms151018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costello LC, Franklin RB. Concepts of citrate production and secretion by prostate. 1. Metabolic relationships. Prostate. 1991;18:25–46. doi: 10.1002/pros.2990180104. [DOI] [PubMed] [Google Scholar]
- 29.Costello LC, Franklin RB. Prostatic fluid electrolyte composition for the screening of prostate cancer: a potential solution to a major problem. Prostate Cancer Prostatic Dis. 2009;12:17–24. doi: 10.1038/pcan.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dakubo GD, Parr RL, Costello LC, Franklin RB, Thayer RE. Altered metabolism and mitochondrial genome in prostate cancer. J Clin Pathol. 2006;59:10–16. doi: 10.1136/jcp.2005.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costello LC, Liu Y, Franklin RB, Kennedy MC. Zinc inhibition of mitochondrial aconitase and its importance in citrate metabolism of prostate epithelial cells. J Biol Chem. 1997;272:28875–28881. doi: 10.1074/jbc.272.46.28875. [DOI] [PubMed] [Google Scholar]
- 32.Costello LC, Franklin RB. The intermediary metabolism of the prostate: a key to understanding the pathogenesis and progression of prostate malignancy. Oncology (Williston Park) 2000;59:269–282. doi: 10.1159/000012183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zha S, Ferdinandusse S, Hicks JL, Denis S, Dunn TA, Wanders RJ, Luo J, De Marzo AM, Isaacs WB. Peroxisomal branched chain fatty acid beta-oxidation pathway is upregulated in prostate cancer. Prostate. 2005;63:316–323. doi: 10.1002/pros.20177. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Zuckier LS, Ghesani NV. Dominant uptake of fatty acid over glucose by prostate cells: a potential new diagnostic and therapeutic approach. Anticancer Res. 2010;30:369–374. [PubMed] [Google Scholar]
- 35.Tsouko E, Khan AS, White MA, Han JJ, Shi Y, Merchant FA, Sharpe MA, Xin L, Frigo DE. Regulation of the pentose phosphate pathway by an androgen receptor-mTOR–mediated mechanism and its role in prostate cancer cell growth. Oncogenesis. 2014;3:e103. doi: 10.1038/oncsis.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang WC, Zhau HE, Chung LW. Androgen receptor survival signaling is blocked by anti-beta2-microglobulin monoclonal antibody via a MAPK/lipogenic pathway in human prostate cancer cells. J Biol Chem. 2010;285:7947–7956. doi: 10.1074/jbc.M109.092759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang WC, Li X, Liu J, Lin J, Chung LW. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol Cancer Res. 2012;10:133–142. doi: 10.1158/1541-7786.MCR-11-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lucarelli G, Rutigliano M, Galleggiante V, Giglio A, Palazzo S, Ferro M, Simone C, Bettocchi C, Battaglia M, Ditonno P. Metabolomic profiling for the identification of novel diagnostic markers in prostate cancer. Expert Rev Mol Diagn. 2015;15:1211–1224. doi: 10.1586/14737159.2015.1069711. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q, Hardie RA, Hoy AJ, van Geldermalsen M, Gao D, Fazli L, Sadowski MC, Balaban S, Schreuder M, Nagarajah R. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J Pathol. 2015;236:278–289. doi: 10.1002/path.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan T, Gao L, Wu G, Shen G, Xie S, Wen H, Yang J, Zhou Y, Tu Z, Qian W. Elevated expression of glutaminase confers glucose utilization via glutaminolysis in prostate cancer. Biochem Biophys Res Commun. 2015;456:452–458. doi: 10.1016/j.bbrc.2014.11.105. [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2015;73(2):377–392. doi: 10.1007/s00018-015-2070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- 43.Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, Lanciotti M, Serni S, Cirri P, Chiarugi P. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72:5130–5140. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- 44.Wilkosz J, Brys M, Rozanski W. Urine markers and prostate cancer. Cent Eur J Urol. 2011;64:9–14. doi: 10.5173/ceju.2011.01.art2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang T, Watson DG, Wang L, Abbas M, Murdoch L, Bashford L, Ahmad I, Lam NY, Ng AC, Leung HY. Application of holistic liquid chromatography–high resolution mass spectrometry based urinary metabolomics for prostate cancer detection and biomarker discovery. PLoS One. 2013;8:e65880. doi: 10.1371/journal.pone.0065880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Averna TA, Kline EE, Smith AY, Sillerud LO. A decrease in 1H nuclear magnetic resonance spectroscopically determined citrate in human seminal fluid accompanies the development of prostate adenocarcinoma. J Urol. 2005;173:433–438. doi: 10.1097/01.ju.0000148949.72314.d7. [DOI] [PubMed] [Google Scholar]
- 47.Kumar V, Dwivedi DK, Jagannathan NR. High-resolution NMR spectroscopy of human body fluids and tissues in relation to prostate cancer. NMR Biomed. 2014;27:80–89. doi: 10.1002/nbm.2979. [DOI] [PubMed] [Google Scholar]
- 48.Roberts MJ, Richards RS, Gardiner RA, Selth LA. Seminal fluid: a useful source of prostate cancer biomarkers? Biomark Med. 2015;9:77–80. doi: 10.2217/bmm.14.110. [DOI] [PubMed] [Google Scholar]
- 49.Jung K, Reszka R, Kamlage B, Bethan B, Stephan C, Lein M, Kristiansen G. Tissue metabolite profiling identifies differentiating and prognostic biomarkers for prostate carcinoma. Int J Cancer. 2013;133:2914–2924. doi: 10.1002/ijc.28303. [DOI] [PubMed] [Google Scholar]
- 50.Zhang A, Sun H, Xu H, Qiu S, Wang X. Cell metabolomics. OMICS. 2013;17:495–501. doi: 10.1089/omi.2012.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cuperlovic-Culf M, Barnett DA, Culf AS, Chute I. Cell culture metabolomics: applications and future directions. Drug Discov Today. 2010;15:610–621. doi: 10.1016/j.drudis.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Keshari KR, Sriram R, Van Criekinge M, Wilson DM, Wang ZJ, Vigneron DB, Peehl DM, Kurhanewicz J. Metabolic reprogramming and validation of hyperpolarized 13C lactate as a prostate cancer biomarker using a human prostate tissue slice culture bioreactor. Prostate. 2013;73:1171–1181. doi: 10.1002/pros.22665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 54.Cernei N, Heger Z, Gumulec J, Zitka O, Masarik M, Babula P, Eckschlager T, Stiborova M, Kizek R, Adam V. Sarcosine as a potential prostate cancer biomarker—a review. Int J Mol Sci. 2013;14:13893–13908. doi: 10.3390/ijms140713893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang Y, Cheng X, Wang C, Ma Y. Quantitative determination of sarcosine and related compounds in urinary samples by liquid chromatography with tandem mass spectrometry. Anal Chem. 2010;82:9022–9027. doi: 10.1021/ac1019914. [DOI] [PubMed] [Google Scholar]
- 56.Stabler S, Koyama T, Zhao Z, Martinez-Ferrer M, Allen RH, Luka Z, Loukachevitch LV, Clark PE, Wagner C, Bhowmick NA. Serum methionine metabolites are risk factors for metastatic prostate cancer progression. PLoS One. 2011;6:e22486. doi: 10.1371/journal.pone.0022486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bianchi F, Dugheri S, Musci M, Bonacchi A, Salvadori E, Arcangeli G, Cupelli V, Lanciotti M, Masieri L, Serni S. Fully automated solid-phase microextraction-fast gas chromatography–mass spectrometry method using a new ionic liquid column for high-throughput analysis of sarcosine and N-ethylglycine in human urine and urinary sediments. Anal Chim Acta. 2011;707:197–203. doi: 10.1016/j.aca.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 58.Cao DL, Ye DW, Zhang HL, Zhu Y, Wang YX, Yao XD. A multiplex model of combining gene-based, protein-based, and metabolite-based with positive and negative markers in urine for the early diagnosis of prostate cancer. Prostate. 2011;71:700–710. doi: 10.1002/pros.21286. [DOI] [PubMed] [Google Scholar]
- 59.Khan AP, Rajendiran TM, Ateeq B, Asangani IA, Athanikar JN, Yocum AK, Mehra R, Siddiqui J, Palapattu G, Wei JT. The role of sarcosine metabolism in prostate cancer progression. Neoplasia. 2013;15:491–501. doi: 10.1593/neo.13314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu H, Liu T, Ma C, Xue R, Deng C, Zeng H, Shen X. GC/MS-based metabolomic approach to validate the role of urinary sarcosine and target biomarkers for human prostate cancer by microwave-assisted derivatization. Anal Bioanal Chem. 2011;401:635–646. doi: 10.1007/s00216-011-5098-9. [DOI] [PubMed] [Google Scholar]
- 61.Jentzmik F, Stephan C, Miller K, Schrader M, Erbersdobler A, Kristiansen G, Lein M, Jung K. Sarcosine in urine after digital rectal examination fails as a marker in prostate cancer detection and identification of aggressive tumours. Eur Urol. 2010;58:12–18. doi: 10.1016/j.eururo.2010.01.035. [discussion 20–11] [DOI] [PubMed] [Google Scholar]
- 62.Issaq HJ, Veenstra TD. Is sarcosine a biomarker for prostate cancer? J Sep Sci. 2011;34:3619–3621. doi: 10.1002/jssc.201100572. [DOI] [PubMed] [Google Scholar]
- 63.Struck-Lewicka W, Kordalewska M, Bujak R, Yumba Mpanga A, Markuszewski M, Jacyna J, Matuszewski M, Kaliszan R, Markuszewski MJ. Urine metabolic fingerprinting using LC-MS and GC-MS reveals metabolite changes in prostate cancer: A pilot study. J Pharm Biomed Anal. 2015;111:351–361. doi: 10.1016/j.jpba.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 64.Khalid T, Aggio R, White P, De Lacy Costello B, Persad R, Al-Kateb H, Jones P, Probert CS, Ratcliffe N. Urinary volatile organic compounds for the detection of prostate cancer. PLoS One. 2015;10:e0143283. doi: 10.1371/journal.pone.0143283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Steve Smith PW, Redding Jemma, Ratcliffe Norman M, Probert Chris S J. Application of similarity coefficients to predict disease using volatile organic compounds. IEEE Sens J. 2010;10:92–96. [Google Scholar]
- 66.Lucarelli G, Fanelli M, Larocca AM, Germinario CA, Rutigliano M, Vavallo A, Selvaggi FP, Bettocchi C, Battaglia M, Ditonno P. Serum sarcosine increases the accuracy of prostate cancer detection in patients with total serum PSA less than 4.0 ng/ml. Prostate. 2012;72:1611–1621. doi: 10.1002/pros.22514. [DOI] [PubMed] [Google Scholar]
- 67.Koutros S, Meyer TE, Fox SD, Issaq HJ, Veenstra TD, Huang WY, Yu K, Albanes D, Chu LW, Andriole G. Prospective evaluation of serum sarcosine and risk of prostate cancer in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Carcinogenesis. 2013;34:2281–2285. doi: 10.1093/carcin/bgt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar D, Gupta A, Mandhani A, Sankhwar SN. Metabolomics-derived prostate cancer biomarkers: fact or fiction? J Proteome Res. 2015;14:1455–1464. doi: 10.1021/pr5011108. [DOI] [PubMed] [Google Scholar]
- 69.Zang X, Jones CM, Long TQ, Monge ME, Zhou M, Walker LD, Mezencev R, Gray A, McDonald JF, Fernandez FM. Feasibility of detecting prostate cancer by ultraperformance liquid chromatography–mass spectrometry serum metabolomics. J Proteome Res. 2014;13:3444–3454. doi: 10.1021/pr500409q. [DOI] [PubMed] [Google Scholar]
- 70.Crowe FL, Allen NE, Appleby PN. Fatty acid composition of plasma phospholipids and risk of prostate cancer in a case–control analysis nested within the European Prospective Investigation into Cancer and Nutrition. Am J Clin Nutr. 2008;88:1353–1363. doi: 10.3945/ajcn.2008.26369. [DOI] [PubMed] [Google Scholar]
- 71.Johansson M, Van Guelpen B, Vollset SE, Hultdin J, Bergh A, Key T, Midttun O, Hallmans G, Ueland PM, Stattin P. One-carbon metabolism and prostate cancer risk: prospective investigation of seven circulating B vitamins and metabolites. Cancer Epidemiol Biomarkers Prev. 2009;18:1538–1543. doi: 10.1158/1055-9965.EPI-08-1193. [DOI] [PubMed] [Google Scholar]
- 72.Zhou X, Mao J, Ai J, Deng Y, Roth MR, Pound C, Henegar J, Welti R, Bigler SA. Identification of plasma lipid biomarkers for prostate cancer by lipidomics and bioinformatics. PLoS One. 2012;7:e48889. doi: 10.1371/journal.pone.0048889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Miyagi Y, Higashiyama M, Gochi A, Akaike M, Ishikawa T, Miura T, Saruki N, Bando E, Kimura H, Imamur F. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS One. 2011;6:e24143. doi: 10.1371/journal.pone.0024143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Giskeodegard GF, Hansen AF, Bertilsson H, Gonzalez SV, Kristiansen KA, Bruheim P, Mjos SA, Angelsen A, Bathen TF, Tessem MB. Metabolic markers in blood can separate prostate cancer from benign prostatic hyperplasia. Br J Cancer. 2015;113:1712–1719. doi: 10.1038/bjc.2015.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Osl M, Dreiseitl S, Pfeifer B, Weinberger K, Klocker H, Bartsch G, Schafer G, Tilg B, Graber A, Baumgartner C. A new rule-based algorithm for identifying metabolic markers in prostate cancer using tandem mass spectrometry. Bioinformatics. 2008;24:2908–2914. doi: 10.1093/bioinformatics/btn506. [DOI] [PubMed] [Google Scholar]
- 76.Pandeya DR, Mittal A, Sathian B, Bhatta B. Role of hyperinsulinemia in increased risk of prostate cancer: a case control study from Kathmandu Valley. Asian Pac J Cancer Prev. 2014;15:1031–1033. doi: 10.7314/apjcp.2014.15.2.1031. [DOI] [PubMed] [Google Scholar]
- 77.Wright JL, Plymate SR, Porter MP, Gore JL, Lin DW, Hu E, Zeliadt SB. Hyperglycemia and prostate cancer recurrence in men treated for localized prostate cancer. Prostate Cancer Prostatic Dis. 2013;16:204–208. doi: 10.1038/pcan.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saylor PJ, Karoly ED, Smith MR. Prospective study of changes in the metabolomic profiles of men during their first three months of androgen deprivation therapy for prostate cancer. Clin Cancer Res. 2012;18:3677–3685. doi: 10.1158/1078-0432.CCR-11-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zaichick VY, Sviridova TV, Zaichick SV. Zinc concentration in human prostatic fluid: normal, chronic prostatitis, adenoma and cancer. Int Urol Nephrol. 1996;28:687–694. doi: 10.1007/BF02552165. [DOI] [PubMed] [Google Scholar]
- 80.Lynch MJ, Nicholson JK. Proton MRS of human prostatic fluid: correlations between citrate, spermine, and myo-inositol levels and changes with disease. Prostate. 1997;30:248–255. doi: 10.1002/(sici)1097-0045(19970301)30:4<248::aid-pros4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 81.Kline EE, Treat EG, Averna TA, Davis MS, Smith AY, Sillerud LO. Citrate concentrations in human seminal fluid and expressed prostatic fluid determined via 1H nuclear magnetic resonance spectroscopy outperform prostate specific antigen in prostate cancer detection. J Urol. 2006;176:2274–2279. doi: 10.1016/j.juro.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 82.Serkova NJ, Gamito EJ, Jones RH, O'Donnell C, Brown JL, Green S, Sullivan H, Hedlund T, Crawford ED. The metabolites citrate, myo-inositol, and spermine are potential age-independent markers of prostate cancer in human expressed prostatic secretions. Prostate. 2008;68:620–628. doi: 10.1002/pros.20727. [DOI] [PubMed] [Google Scholar]
- 83.Jentzmik F, Stephan C, Lein M, Miller K, Kamlage B, Bethan B, Kristiansen G, Jung K. Sarcosine in prostate cancer tissue is not a differential metabolite for prostate cancer aggressiveness and biochemical progression. J Urol. 2011;185:706–711. doi: 10.1016/j.juro.2010.09.077. [DOI] [PubMed] [Google Scholar]
- 84.van Asten JJ, Cuijpers V, Hulsbergen-van de Kaa C, Soede-Huijbregts C, Witjes JA, Verhofstad A, Heerschap A. High resolution magic angle spinning NMR spectroscopy for metabolic assessment of cancer presence and Gleason score in human prostate needle biopsies. MAGMA. 2008;21:435–442. doi: 10.1007/s10334-008-0156-9. [DOI] [PubMed] [Google Scholar]
- 85.Hahn P, Smith IC, Leboldus L, Littman C, Somorjai RL, Bezabeh T. The classification of benign and malignant human prostate tissue by multivariate analysis of 1H magnetic resonance spectra. Cancer Res. 1997;57:3398–3401. [PubMed] [Google Scholar]
- 86.Garcia-Segura JM, Sanchez-Chapado M, Ibarburen C, Viano J, Angulo JC, Gonzalez J, Rodriguez-Vallejo JM. In vivo proton magnetic resonance spectroscopy of diseased prostate: spectroscopic features of malignant versus benign pathology. Magn Reson Imaging. 1999;17:755–765. doi: 10.1016/s0730-725x(99)00006-5. [DOI] [PubMed] [Google Scholar]
- 87.Cheng LL, Wu C, Smith MR, Gonzalez RG. Non-destructive quantitation of spermine in human prostate tissue samples using HRMAS 1H NMR spectroscopy at 9.4 T. FEBS Lett. 2001;494:112–116. doi: 10.1016/s0014-5793(01)02329-8. [DOI] [PubMed] [Google Scholar]
- 88.Swindle P, McCredie S, Russell P, Himmelreich U, Khadra M, Lean C, Mountford C. Pathologic characterization of human prostate tissue with proton MR spectroscopy. Radiology. 2003;228:144–151. doi: 10.1148/radiol.2281011808. [DOI] [PubMed] [Google Scholar]
- 89.Swanson MG, Vigneron DB, Tabatabai ZL, Males RG, Schmitt L, Carroll PR, James JK, Hurd RE, Kurhanewicz J. Proton HR-MAS spectroscopy and quantitative pathologic analysis of MRI/3D-MRSI-targeted postsurgical prostate tissues. Magn Reson Med. 2003;50:944–954. doi: 10.1002/mrm.10614. [DOI] [PubMed] [Google Scholar]
- 90.Tessem MB, Swanson MG, Keshari KR, Albers MJ, Joun D, Tabatabai ZL, Simko JP, Shinohara K, Nelson SJ, Vigneron DB. Evaluation of lactate and alanine as metabolic biomarkers of prostate cancer using 1H HR-MAS spectroscopy of biopsy tissues. Magn Reson Med. 2008;60:510–516. doi: 10.1002/mrm.21694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Giskeodegard GF, Bertilsson H, Selnaes KM, Wright AJ, Bathen TF, Viset T, Halgunset J, Angelsen A, Gribbestad IS, Tessem MB. Spermine and citrate as metabolic biomarkers for assessing prostate cancer aggressiveness. PLoS One. 2013;8:e62375. doi: 10.1371/journal.pone.0062375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Swanson MG, Keshari KR, Tabatabai ZL, Simko JP, Shinohara K, Carroll PR, Zektzer AS, Kurhanewicz J. Quantification of choline- and ethanolamine-containing metabolites in human prostate tissues using 1H HR-MAS total correlation spectroscopy. Magn Reson Med. 2008;60:33–40. doi: 10.1002/mrm.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maxeiner A, Adkins CB, Zhang Y, Taupitz M, Halpern EF, McDougal WS, Wu CL, Cheng LL. Retrospective analysis of prostate cancer recurrence potential with tissue metabolomic profiles. Prostate. 2010;70:710–717. doi: 10.1002/pros.21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stenman K, Stattin P, Stenlund H, Riklund K, Grobner G, Bergh A. H HRMAS NMR derived bio-markers related to tumor grade, tumor cell fraction, and cell proliferation in prostate tissue samples. Biomark Insights. 2011;6:39–47. doi: 10.4137/BMI.S6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Stenman K, Hauksson JB, Grobner G, Stattin P, Bergh A, Riklund K. Detection of polyunsaturated omega-6 fatty acid in human malignant prostate tissue by 1D and 2D high-resolution magic angle spinning NMR spectroscopy. MAGMA. 2009;22:327–331. doi: 10.1007/s10334-009-0187-x. [DOI] [PubMed] [Google Scholar]
- 96.Thysell E, Surowiec I, Hornberg E, Crnalic S, Widmark A, Johansson AI, Stattin P, Bergh A, Moritz T, Antti H. Metabolomic characterization of human prostate cancer bone metastases reveals increased levels of cholesterol. PLoS One. 2010;5:e14175. doi: 10.1371/journal.pone.0014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Putluri N, Shojaie A, Vasu VT, Nalluri S, Vareed SK, Putluri V, Vivekanandan-Giri A, Byun J, Pennathur S, Sana TR. Metabolomic profiling reveals a role for androgen in activating amino acid metabolism and methylation in prostate cancer cells. PLoS One. 2011;6:e21417. doi: 10.1371/journal.pone.0021417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Teahan O, Bevan CL, Waxman J, Keun HC. Metabolic signatures of malignant progression in prostate epithelial cells. Int J Biochem Cell Biol. 2011;43:1002–1009. doi: 10.1016/j.biocel.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 99.Kaushik AK, Vareed SK, Basu S, Putluri V, Putluri N, Panzitt K, Brennan CA, Chinnaiyan AM, Vergara IA, Erho N. Metabolomic profiling identifies biochemical pathways associated with castration-resistant prostate cancer. J Proteome Res. 2014;13:1088–1100. doi: 10.1021/pr401106h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vaz CV, Alves MG, Marques R, Moreira PI, Oliveira PF, Maia CJ, Socorro S. Androgen-responsive and nonresponsive prostate cancer cells present a distinct glycolytic metabolism profile. Int J Biochem Cell Biol. 2012;44:2077–2084. doi: 10.1016/j.biocel.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 101.Mintz A, Wang L, Ponde DE. Comparison of radiolabeled choline and ethanolamine as probe for cancer detection. Cancer Biol Ther. 2008;7:742–747. doi: 10.4161/cbt.7.5.5746. [DOI] [PubMed] [Google Scholar]
- 102.Lodi A, Ronen SM. Magnetic resonance spectroscopy detectable metabolomic fingerprint of response to antineoplastic treatment. PLoS One. 2011;6:e26155. doi: 10.1371/journal.pone.0026155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kailavasan M, Rehman I, Reynolds S, Bucur A, Tozer G, Paley M. NMR-based evaluation of the metabolic profile and response to dichloroacetate of human prostate cancer cells. NMR Biomed. 2014;27:610–616. doi: 10.1002/nbm.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]