Abstract
Statement of the Problem
Trichomonas tenax and Entamoeba gingivalis are commensal protozoa which inhabit the human oral cavity. These parasites are found in patients with poor oral hygiene and might be a reason for progressive periodontal diseases.
Purpose
The aim of this study was to evaluate the effect of nonsurgical periodontal treatment on the frequency of these protozoa in saliva and plaque samples.
Materials and Method
In this clinical trial, samples of saliva and dental plaque were collected from 46 patients with moderate to severe chronic periodontitis before and after periodontal therapy. The samples were assessed for the frequency of parasites.
Results
The frequency of Entamoeba gingivalis was reduced in saliva (p= 0.007) and plaque (p= 0.027) three weeks after the treatment. Likewise, the frequency of Trichomonas tenax reduced in saliva (p= 0.030); however, the decrease was not significant in plaque (p= 0.913). Trichomonas tenax frequency in dental plaque directly related to the severity of periodontitis (r= 0.565, p≤ 0.000). In contrast, the number of Entamoeba gingivalis in both saliva (r= -0.405, p≤ 0.005) and plaque (r= -0.304, p= 0.040) was inversely related with the severity of the periodontal disease.
Conclusion
Nonsurgical periodontal treatment could reduce the number of Trichomonas Tenax and Entamoeba gingivalis in the oral environment of patients with chronic periodontitis.
Keywords: Trichomonas Tenax, Entamoeba Gingivalis, Periodontitis, Parasite
Introduction
Presenting in several various clinical forms, periodontitis is considered as one of the most widespread oral diseases. Approximately 5 to 20 percent of the world’s population suffers from severe generalized periodontitis.[1] Periodontal lesions contain numerous neutrophils, motile bacteria, spirillae, spinning rods, and protozoa.[2]Yet, the etiology of this multi-factorial disease is still unclear. The possible role of parasites in the development of periodontitis has been poorly studied.[3] Data from previous studies about Entamoeba gingivalis and Trichomonas is also limited[4] and have been conducted only in few countries.[5]
Trichomonas are mostly parasitic or commensal flagellates that live in low-oxygen environments.[6-8] T. tenax is currently considered as a member of the oral biofilm.[9-10] Its prevalence in the oral cavity ranges from 4 to 53% worldwide;[11] however, in patients with periodontitis; it is 3 to 4 times more than healthy individuals.[12] Investigations have been performed on the correlation between the prevalence of T. tenax and the status of periodontitis,[6, 13] which is found particularly in patients with poor oral hygiene.[13-15] It can be simply transmitted through saliva, droplet spray, kissing, using contaminated dishes, and drinking water.[4, 7, 11] Oral cavity is the common residence of Trichomonas species; although, it is occasionally found in the respiratory tract.[16-18] The significance of its presence in the respiratory tract of humans is still unclear. Pleuropulmonary trichomoniasis has been observed in bare cases.[19-21] A study in Egypt (2004) showed that the prevalence of pulmonary trichomoniasis was 8% (20 cases) in a total of 250 individuals.[22]
Entamoeba gingivalis (E. gingivalis) is found in the oropharynx, but rarely in the head and neck lesions.[23-25] This microorganism is found more common in patients with poor dentition,[24] periodontal disease, and immune suppression.[26-28] It was the first commensal found in the human oral cavity.[12, 29] E. gingivalis lives on the surface of the teeth and gum tissues.[30] Inflammatory process produces a propitious anaerobic environment for their growth.[31] The trophozoite is measured about 10-30 µm, actively motile with multiple pseudopodia, the cytoplasm contains food vacuoles with ingested bacteria, leukocytes and epithelial cells.[29] According to some studies, this amoeba is considered as an important cause of periodontal disease.[32] E. gingivalis is an opportunistic pathogen whose association with synergistic symbiotic bacteria may cause periodontal diseases in hosts with low immunity.[33-34]
Conversely, some studies have reported the general presence of E. gingivalis in disease-free individuals which was related to the amount of calculus present on the teeth.[35] As previously mentioned in several studies, these parasites exist in the oral cavity and probably play a role in development of periodontal disease. Yet, no study has investigated the patients with moderate to severe chronic periodontitis. Hence, the current study was designed to evaluate the effect of nonsurgical periodontal treatment on the level of Trichomonas tenax and Entamoeba gingivalis in dental plaques and saliva.
Materials and Method
In this randomized clinical trial (#IRCT2014071013167 N4), 46 patients with moderate to severe chronic periodontitis (according to the Periodontology Association in 1998) and the age range of 30-50 years old were selected out of those referring to the Department of Periodontics, Shiraz School of Dentistry, Iran, from March to October 2014. Informed consent was collected from the patients. The participants were screened and examined to make sure if they met the criteria for periodontal disease. The exclusion criteria were having a history of consumption of systemic antibiotics within the three preceding months, periodontal therapy during the previous six months, medications that affect the periodontium (such as immunosuppressives, antihypertensives, anticonvulsants, contraceptives), systemic diseases such as diabetes, heart disease or respiratory diseases, pregnancy, smoking or other drug abuse. A demographic form was filled out for each individual concerning age, sex, smoking habits, and medical status.
As previously mentioned, the aim of this study was to evaluate the effect of nonsurgical periodontal treatment on the frequency of T. tenax and E. gingivalis in saliva and plaque samples[5, 7, 35] before and 3 weeks after intervention. The first follow-up should be considered at least 3 weeks after treatment. This interval is needed for tissue healing.[36] To this end, the patients were randomly divided into the case (23 patients) and control (23 patients) groups by a table of random numbers. Considering the severity of periodontal disease by evaluating the mean clinical attachment loss (CAL) in the patients’ periodontium, they were also divided into one of the two subgroups of moderate (CAL=3-4mm) and severe periodontitis (CAL≥5mm).[36] Sampling was done in two sessions (baseline and three week after the treatment). After collecting the first samples from all patients, the control group received only oral hygiene instructions. Scaling and root planning was done in case group via ultrasonic set (Woodpecker; China) for 20 to 60 minutes until eliminating deposits completely. Then face-to-face oral hygiene instructions were given by using a dental model. Three weeks later, the second set of samples were prepared and delivered to the lab. The examiner who studied the saliva and plaque samples was blind to the groupings throughout the study.
Dental plaque was collected from the teeth surfaces by using sterile curettes (Gracey curette #13-14). Non-stimulated saliva (0.1 ml) was collected by using sterile pipettes (Hirschmann graduated pipette; Class AS, 10 mL volume, accuracy: 0.05 mL, Germany). The labeled smears of saliva and dental plaques were fixed with methanol and sent to the parasitology lab. Then, these Giemsa-stained smears were observed by using microscope (Nikon; Eclipse E200, Japan) at both 40X and 100X magnifications. Detection of T. tenax was established as a pear-shaped flagellated trophozoite of about 5-13µ (Figure 1). The other oral protozoan, E. gingivalis was differentiated by its size (10-20μ), presence of prominent pseudopodia and food vacuole (Figure 2).
Figure 1.
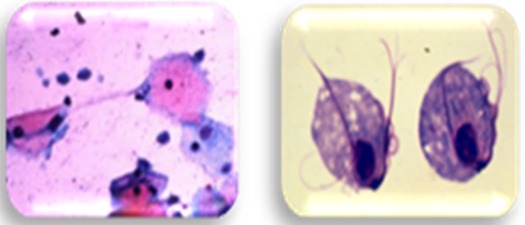
Microscopic view of Trichomonas gingivalis
Figure 2.
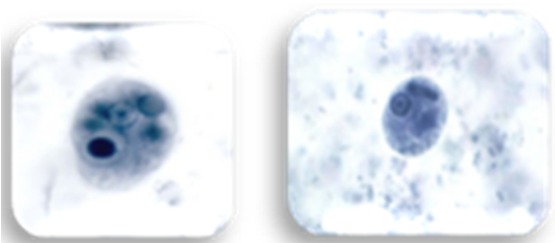
Microscopic view of Entamoeba gingivalis
Microscopic observation was repeated twice to confirm the diagnosis and distinguishing the specific pattern of each parasite family. In the microscopic study of parasites, the presence of very few number of parasites was considered as level 1 (0-1 parasites), few number as level 2 (2-4 parasites), moderate as level 3 (5-10 parasites), high as level 4 (11-15 parasites), and very high (16≤parasites) as level 5 (the results were reported qualitatively rather than quantitatively).
In this study, the probable correlation between demographic data such as age, sex, and education level and the initial abundance of parasites were analyzed in oral samples. Furthermore, the abundance of parasites after periodontal treatment was compared with the baseline values.
The statistical analysis was done by using SPSS software, version 16. The independent-samples t-test was used for variables with abnormal distribution (significance level ≤0.05). Pearson Correlation test was employed to analyze the relationship of severity of the disease (on the basis of CAL levels), age and sex with the abundance of parasites (signification level≤0.005). The changes in parasites abundance before and after the treatment were determined by using Mann-Whitney test.
Results
Out of all the 46 patients, 33 were females and 13 were males. There were 29 patients with severe periodontitis (15 cases and 14 controls), and 17 with moderate periodontitis (8 cases and 9 controls). The mean baseline CAL in the two groups was almost equal (case group= 5.74±1.711 mm, and control group= 5.64±1.677 mm).
Generally, the frequency of T. tenax was less than E. gingivalis in both saliva and dental plaque (p≤ 0.005). The severity of periodontitis was directly related with the baseline frequency of T. tenax in dental plaque (r= 0.565, p≤ 0.000); however, it had no relationship with the frequency of this parasite in saliva. The frequency of E. gingivalis in saliva (r=- 0.405, p≤ 0.05) and dental plaque (r= -0.304, p= 0.040) had a significantly reverse relationship with the severity of periodontal disease.
Performing the test of homogeneity of variances of parasites showed that the frequency of T. tenax in saliva (p=0.039) and dental plaque (p= 0.017) was statistically different in patients with different education levels. Parasites were mostly found in no-education (illiterate) group.
Post-hoc test revealed no correlation between the patient’s age and the frequency of T. tenax in dental plaque (p= 0.066) or saliva (p= 0.34). T. tenax in dental plaque was detected only in females (p≤ 0.005).
The frequency of E. gingivalis in dental plaque (p= 0.212) and saliva (p= 0.441) had no significant difference between various groups with different levels of education. It was found more frequently in males’ saliva rather than females’ (p≤ 0.005). No relationship was noticed between the age and colonization of E. gingivalis in saliva (p= 0.13) and dental plaque (p= 0.314).
Three weeks after the treatment, E. gingivalis decreased in the in case group's saliva (p= 0.007) and plaque samples (p= 0.027). T. tenax also reduced in saliva (p= 0.030); nevertheless, it did not have a significant reduction in dental plaque (p= 0.913).
Discussion
The initial treatment to eliminate pathogenic bacteria in periodontal disease is the mechanical removal of dental plaque and calculus.[37-39] Consequently, it was supposed that these methods could help reduce parasites as much as it does for perio-pathogen bacteria. The obtained results confirmed the probable efficacy of periodontal treatment in reducing the colonization of oral parasites in dental plaque except for T.tenax. No similar study was found about the effect of periodontal treatment on the management of oral parasites; however, some studies have evaluated the prevalence of these protozoans.
Ghabanchi et al.,[7] observed that E. gingivalis was more abundant than T. tenax in patients with either healthy periodontium or periodontitis, which was consistent with the results of the present study. In a study conducted by Bonner et al., 72 periodontal pockets and 33 healthy gingival sulci were sampled to evaluate the correlation between E. gingivalis colonization and periodontitis by using PCR (polymerase chain reaction).[3] Although Bonner used a much more sensitive technique (PCR) compared with this study (light microscope), both studies noted higher frequency of E. gingivalis compared with T.tenax. The only difference was that in our study, there was a significant reverse correlation between the periodontal disease and E. gingivalis frequency in saliva. Meanwhile, Bonner et al. stated that the presence of parasite was directly related to the periodontal disease in an overall view. Ibrahim and Abbas reported a direct relationship between the severity of periodontitis and abundance of E. gingivalis and T. tenax in both saliva and plaque.[26] The current study detected a direct correlation between the frequency of T. tenax in plaque and severity of periodontal disease; whereas, this direct relationship was not observed in salivary T. tenax or generally in E. gingivalis. It should be noted that the level of CAL was not considered as a determining factor in the severity of periodontal disease in Ibrahim’s study,[26] only the pocket depth was measured. Hence, the relationship between the parasites and the disease severity cannot be confirmed with certainty. They also reported a higher abundance of E. gingivalis in hypertensive patients and T. tenax in patients with increased use of antibiotics over the preceding 6 months. Unlike the present study, they did not exclude patients with systemic complications and found that both parasites increased in diabetic patients.
Ibrahim and Abbas observed that E. gingivalis was more frequent in 61-70 year-old and T. tenax in 21-30 year-old patients,[26] while; the age parameter had no significance in our study. Ghabanchi et al. detected that E. gingivalis could be found in dental plaque and saliva of healthy subjects.[7] Our study showed a reverse significant correlation between the severity of periodontitis and E. gingivalis frequency, which may confirm the abundance of this parasite even in healthy conditions. In contrast, Trim et al.[35] reported that E. gingivalis was only present in pockets deeper than 4-7 mm and this result was confirmed by Albuquerque et al.[12] and contrasted by the current study. This may be due to the differences in laboratory procedures. Hamad et al.[40] showed a positive relationship between the presence of parasite in the mouth and illiteracy or low education level which was partially confirmed by the present study. In our study, T. tenax was mostly found in illiterate patients’ saliva or dental plaque, but there was no relationship between the amount of E. gingivalis and various education levels. In a study, Ullah et al.[41] reported a positive association between the prevalence of mouth parasites and poverty. The higher incidence was found among the lower class which was to some extent in line with our study. The above-mentioned study also reported that the overall prevalence of these two parasites was higher in men than women. Our study found T. tenax only in females’ dental plaque. This might be attributed to the higher prevalence of T. vaginalis in females and the microscopic similarities of this species and T. tenax.[6] E. gingivalis was more prevalent in males’ saliva than females’. Ullah et al.[41] included smokers, snuff dippers and participants with improper brushing habits or poor oral hygiene in their study and as expected, the presence of these confounding factors was more frequent in males rather than females.
Gharavi et al.[5] found no relationship between T. tenax infection and sex or age, but E. gingivalis was related to age higher than 20 and male sex. Contrarily, Albuquerque et al. reported no correlation between age and the presence of both parasites, which was in agreement with the results of the present study.
Conclusion
Regardless of patient’s demographic characteristics, it seems that oral hygiene instructions in combination with scaling and root planning can help controlling excessive colonization of parasites, particularly E. gingivalis and T. tenax and their probable opportunistic infestation.
Acknowledgment
This study was supported by Shahid Sadoughi University of Medical Sciences and the article was derived from a doctorate thesis.
Conflict of Interest: The authors declare that there are no conflicts of interest.
References
- 1.Burt B, Science and Research, Science and Therapy Committee of the American Academy of Periodontology, authors. Position paper: epidemiology of periodontal diseases. J Periodontol. 2005; 76: 1406–1419. doi: 10.1902/jop.2005.76.8.1406. [DOI] [PubMed] [Google Scholar]
- 2.Bonner M, editor. Medical Implication of Oral Amoebiasis. 5th International Congress on Tropical Medicine and International Health. Amsterdam; Canada. Amsterdam: The Netherlands; 2007. pp. 24–28. Available at: www.calameo.com/books/ 0000884407729fb05e363. [Google Scholar]
- 3.Bonner M, Amard V, Bar-Pinatel C, Charpentier F, Chatard JM, Desmuyck Y, et al. Detection of the amoeba Entamoeba gingivalis in periodontal pockets. Parasite. 2014; 21: 30. doi: 10.1051/parasite/2014029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haghighi A, Soghandi L, Athari A, Kazemi B. Prevalence of Oral Trichomoniasis in Patients with Periodontitis and Gingivitis Using PCR and Direct Smear. Iran J Public Health. 2007; 36: 33–37. [Google Scholar]
- 5.Gharavi MJ, Hekmat S, Ebrahimi A, Jahani MR. Buccal Cavity Protozoa in Patients Referred to the Faculty of Dentistry in Tehran, Iran. Iranian Journal of Parasitology. 2006; 1: 43–46. [Google Scholar]
- 6.Kucknoor AS, Mundodi V, Alderete J. Genetic identity and differential gene expression between Trichomonas vaginalisand Trichomonas tenax. BMC Microbiol. 2009; 9: 58. doi: 10.1186/1471-2180-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghabanchi J, Zibaei M, Afkar MD, Sarbazie AH. Prevalence of oral Entamoeba gingivalis and Trichomonas tenax in patients with periodontal disease and healthy population in Shiraz, southern Iran. Indian J Dent Res. 2010; 21: 89–91. doi: 10.4103/0970-9290.62821. [DOI] [PubMed] [Google Scholar]
- 8.Al-hamiary AK, Kezar MY, AlKhafaji YA. Prevalence of Oral Protozoa in Periodontitis and Gingivitis Patients Whose Attended to Clinics Periodontics, Dentistry College\ Babylon Univ. IASJ. 2011; 3: 179–184. [Google Scholar]
- 9.Kurnatowska AJ, Dudko A, Kurnatowski P. Invasion of Trichomonas tenax in patients with periodontal diseases. Wiad Parazytol. 2004; 50: 397–403. [PubMed] [Google Scholar]
- 10.Hersh SM. Pulmonary trichomoniasis and Trichomonas tenax. J Med Microbiol. 1985; 20: 1–10. doi: 10.1099/00222615-20-1-1. [DOI] [PubMed] [Google Scholar]
- 11.Mallat H, Podglajen I, Lavarde V, Mainardi JL, Frappier J, Cornet M. Molecular characterization of Trichomonas tenax causing pulmonary infection. J Clin Microbiol. 2004; 42: 3886–3887. doi: 10.1128/JCM.42.8.3886-3887.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albuquerque RLC, Moura C, Alcântara W, Lopes A, Albuquerque F. Incidence of Entamoeba gingivalis and Trichomonas tenax in samples of dental biofilm and saliva from patients with periodontal disease. RGO. 2011; 59: 35–40. [Google Scholar]
- 13.Lewis KL, Doherty DE, Ribes J, Seabolt JP, Bensadoun ES. Empyema caused by trichomonas. Chest. 2003; 123: 291–292. doi: 10.1378/chest.123.1.291. [DOI] [PubMed] [Google Scholar]
- 14.Abualqomsaan M, Töz SO, Yolasiğmaz A, Turgay N. The investigation of Entamoeba gingivalis and Trichomonas tenax in a group of patients with periodontal disease. Turkiye Parazitol Derg. 2010; 34: 91–94. [PubMed] [Google Scholar]
- 15.Athari A, Soghandi L, Haghighi A, Kazemi B. Prevalence of oral trichomoniasis in patients with periodontitis and gingivitis using PCR and direct smear. Iranian J Public Health. 2007; 36: 33–37. [Google Scholar]
- 16.Porcheret H, Maisonneuve L, Estève V, Jagot JL, Le Pennec MP. Pleural trichomoniasis due to trichomonas tenax. Rev Mal Respir. 2002; 19: 97–99. [PubMed] [Google Scholar]
- 17.Walzer PD, Rutherford I, East R. Empyema with Trichomonas species. Am Rev Respir Dis. 1978; 118: 415–418. doi: 10.1164/arrd.1978.118.2.415. [DOI] [PubMed] [Google Scholar]
- 18.Dimasuay KG, Rivera WL. First report of Trichomonas tenax infections in the Philippines. Parasitol Int. 2014; 63: 400–402. doi: 10.1016/j.parint.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 19.Chiche L, Donati S, Corno G, Benoit S, Granier I, Chouraki M, et al. Trichomonas tenax in pulmonary and pleural diseases. Presse Med. 2005; 34(19 Pt 1): 1371–1372. doi: 10.1016/s0755-4982(05)84193-4. [DOI] [PubMed] [Google Scholar]
- 20.Wang HK, Jerng JS, Su KE, Chang SC, Yang PC. Trichomonas empyema with respiratory failure. Am J Trop Med Hyg. 2006; 75: 1234–1236. [PubMed] [Google Scholar]
- 21.Lopez-Escamilla E, Sanchez-Aguillon F, Alatorre-Fernandez CP, Aguilar-Zapata D, Arroyo-Escalante S, Arellano T, et al. New tetratrichomonas species in two patients with pleural empyema. J Clin Microbiol. 2013; 51: 3143–3146. doi: 10.1128/JCM.01056-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmoud MS, Rahman GA. Pulmonary trichomoniasis: improved diagnosis by using polymerase chain reactiontargeting Trichomonas tenax 18S rRNA gene in sputum specimens. J Egypt Soc Parasitol. 2004; 34: 197–211. [PubMed] [Google Scholar]
- 23.Perez-Jaffe L, Katz R, Gupta PK. Entamoeba gingivalis identified in a left upper neck nodule by fine-needleaspiration: a case report. Diagn Cytopathol. 1998; 18: 458–461. doi: 10.1002/(sici)1097-0339(199806)18:6<458::aid-dc15>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 24.Jian B, Kolansky AS, Baloach ZW, Gupta PK. Enta-moeba gingivalis pulmonary abscess- diagnosed by fine needle aspiration. Cytojournal. 2008; 5: 12. doi: 10.4103/1742-6413.43179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhaijee F, Bell D. Entamoe-ba gingivalis in Acute Osteomyelitis of the Mandible. Case Rep Med. 2011; 2011: 357301. doi: 10.1155/2011/357301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibrahim S, Abbas R. Evaluation of Entamoeba gingivalis and Trichomonas tenax in patients with periodontitis and gingivitis and its correlation with some risk factors. J Bagh Coll Dentistry. 2012; 24: 158–162. [Google Scholar]
- 27.Huang W, Shi JL, Li CL, Chen B, Shao LJ, Chen L, et al. Entamoeba gingivalis infection among college students in Tangshan. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2009; 27: 51–53, 61. [PubMed] [Google Scholar]
- 28.Cembranelli SB, Souto FO, Ferreira-Paim K, Richinho TT, Nunes PL, Nascentes GA, et al. First evidence of genetic intraspecific variability and occurrence of Entamoebagingivalis in HIV(+)/AIDS. PLoS One. 2013;8: e82864. doi: 10.1371/journal.pone.0082864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Saeed WM. Pathogenic effect of Entamoeba gingivalis on gingival tissues of rats. Al-Rafidain Dent J. 2003; 3: 70–73. [Google Scholar]
- 30.Onyido AE, Amadi ES, Olofin I, Onwumma AA, Okoh IC, Chikwendu CI. Prevalence of Entamoeba gingivalis and Trichomonas tenax among dental patients attending Federal School of Dental Technology and Therapy clinic, Enugu, Nigeria. Nature and Science. 2011; 9: 59–62. [Google Scholar]
- 31.Nocito-Mendoza I, Vasconi-Correas MD, Ponce de León-Horianski P, Zdero-Pandzich M. Entamoeba gingivalis and Trichomonas tenax in diabetic patients. RCOE. 2003; 8: 19–23. [Google Scholar]
- 32.Derda M, Handas E, Antczak E, Wojt JW. Incidence of Entomobea gingivalis in oral cavity of students. Jstoma. 2011; 64(10):784–795. [Google Scholar]
- 33.Liu GY, Chen JF, Wen WR, Chen WL, Lin LQ, Hong H. Experimental study on the pathogenesis of Entamoeba gingivalis. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2001; 19: 229–232. [PubMed] [Google Scholar]
- 34.El Azzouni MZ, el Badry AM. Frequency of Entamoeba gingivalis among periodontal and patients underchemotherapy. J Egypt Soc Parasitol. 1994; 24: 649–655. [PubMed] [Google Scholar]
- 35.Trim RD, Skinner MA, Farone MB, Dubois JD, Newsome AL. Use of PCR to detect Entamoeba gingivalis in diseased gingival pockets and demonstrate its absence in healthy gingival sites. Parasitol Res. 2011; 109: 857–864. doi: 10.1007/s00436-011-2312-9. [DOI] [PubMed] [Google Scholar]
- 36.Newman GM, Takei HH, Klokkevold RR, Carranza AF. Carranza`s clinical periodontology. 11th ed. Saunders: Philadelphia; 2012. p. 278. [Google Scholar]
- 37.Eberhard J, Jepsen S, Jervøe-Storm PM, Needleman I, Worthington HV. Full-mouth disinfection for the treatment of adult chronic periodontitis. Cochrane Database Syst Rev. 2008; 1: CD004622. doi: 10.1002/14651858.CD004622.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Mittal A, Nichani AS, Venugopal R, Rajani V. The effect of various ultrasonic and hand instruments on the root surfaces of human single rooted teeth: A Plani-metric and Profilometric study. J Indian Soc Periodontol. 2014; 18: 710–717. doi: 10.4103/0972-124X.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He JY, Qi GG, Huang WJ, Sun XD, Tong Y, Peng CM, et al. Short-term microbiological effects of scaling and root planing and essential-oils mouthwash in Chinese adults. J Zhejiang Univ Sci B. 2013; 14: 416–425. doi: 10.1631/jzus.B1200350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamad SS, Mohammad SH, Kader MA. Relationship between the Dental health and prevalence's Trichomonas tenax and Entamoeba gingivalis among patients attending Dental Clinics in Kirkuk. J Babylon Univ Pur Appl Scien. 2012; 20: 1441–1447. [Google Scholar]
- 41.Ullah Z, Khan M, Jan AH, Ali I. Mouth protozoa in north west frontier province of Pakistan- a study. Pakistan Oral & Dent J. 2007; 27: 245–248. [Google Scholar]


