Abstract
Cytomegalovirus accelerates transplant vascular sclerosis (TVS) and chronic rejection (CR) in solid organ transplants; however, the mechanisms involved are unclear. We determined the efficacy of a CMV vaccine in preventing CMV‐accelerated rat cardiac allograft rejection in naïve recipients of CMV+ donor hearts. F344 donor rats were infected with RCMV 5 days prior to heterotopic cardiac transplantation into CMV‐naïve or H2O2‐inactivated RCMV‐vaccinated Lewis recipients. Recipients of RCMV‐infected donor hearts rejected at POD59, whereas vaccinated recipients exhibited a significantly prolonged time to rejection‐POD97, similar to recipients of uninfected donor hearts (POD108). Although all of the donor hearts were preinfected, the vaccinated recipients had lower graft and PBMC viral loads at POD 7 compared to unvaccinated controls. Adoptive T cell and passive antibody transfers from vaccinated Lewis rats into naïve recipients demonstrate that both T‐cell and B‐cell arms of the adaptive immune response provide protection against CMV‐accelerated rejection. Similar findings were obtained when testing three different adjuvants in passive transfer experiments. We have determined that the timing of the vaccine prior to transplantation and the specific adjuvant play critical roles in mediating anti‐viral responses and promoting graft survival. CMV vaccination prior to transplantation may effectively increase graft survival.
Keywords: basic (laboratory) research / science, heart transplantation / cardiology, infectious disease, animal models, vaccine, infection and infectious agents, viral: Cytomegalovirus (CMV), graft survival
Short abstract
A rat cytomegalovirus vaccine induces therapeutic immunity preventing virus‐accelerated chronic cardiac allograft rejection.
Abbreviations
- ADCC
antibody‐dependent cellular cytotoxicity
- CR
chronic rejection
- CsA
cyclosporin A
- ECL
enhanced chemiluminescence
- ELISA
enzyme‐linked immunoabsorbent assay
- gB
glycoprotein B
- HCMV
human cytomegalovirus
- H2O2
hydrogen peroxide
- HRP
horseradish peroxidase
- Ig
immunoglobulin
- IVIG
intraveneous immunoglobulin
- MCMV
mouse cytomegalovirus
- MPL
monophosphoryl lipid A
- NI
neointimal index
- OPD
O‐phenylenediamine dihydrochloride
- PBS
phosphate buffered saline
- PCR
polymerase chain reaction
- PFU
plaque forming unit
- POD
postoperative day
- RCMV
rat cytomegalovirus
- SMG
submandibular gland
- SOT
solid organ transplantation
- TBS
tris‐buffered saline
- TVS
transplant vascular sclerosis
Introduction
In human and animal models of solid organ transplantation (SOT), cytomegalovirus (CMV) infection accelerates transplant vascular sclerosis (TVS) resulting in graft failure 1, 2, 3, 4, 5. In a rat model of heart, kidney and small bowel transplants, we have demonstrated that acute ratCMV (RCMV) infection dramatically decreases mean time to develop TVS and subsequent graft failure, and increases the severity of vascular disease in graft vessels 6, 7. Ganciclovir prevented TVS and delayed rejection in cardiac transplant recipients acutely infected with CMV but not in recipients of allografts from CMV latently infected donors 8. The most common scenario in SOT is latent CMV infection in either organ donor or recipient, or both, underscoring the importance of preventing latent virus reactivation impacting downstream consequences of CMV disease and graft survival.
Prophylactic CMV treatment in transplant recipients is a common CMV prevention strategy, although some transplant centers use a preemptive approach. The utility of antiviral agents for HCMV treatment is often limited by side effects, noncompliance and costs 9, 10, 11, 12, 13. Development of a vaccine to prevent CMV reactivation with high efficacy, low toxicity and low cost would avoid these significant issues. Thus far, endeavors to achieve this end have been unsuccessful. Live‐attenuated vaccines utilizing the laboratory‐adapted HCMV strain AD169 or clinical strains Towne and Towne/Toledo chimeras elicited antibody responses in seronegative individuals 14, 15. However, the Towne vaccine failed to prevent HCMV infection following renal transplantation but did reduce CMV disease 16, 17. The addition of immune enhancing agents such as IL‐12 potentiated the efficacy of immune responses 18, 19. A disadvantage of live‐attenuated vaccines is their ability to establish latent infection and reactivate in immune compromised transplant recipients. Subunit vaccines avoid introduction of infectious virus; however, these vaccines are generally limited to one viral protein or a few of the greater than 150 CMV‐encoded proteins. For example, a vaccine containing recombinant glycoprotein B has undergone clinical trials (clinicaltrials.gov # NCT00133497), and generated substantial neutralizing antibody titers in CMV‐naïve subjects and boosting Ab and T cell responses in CMV+ subjects 20, 21. In bone marrow transplant patients, a gB DNA plasmid vaccine reduced CMV occurrence and need for antiviral therapy 22. The protective value in targeting a single viral protein is unclear and the use of other viral proteins such as immediate early protein 1 and pp65 may garner greater protection 23.
Anti‐CMV T cell and antibody responses in infected individuals are robust. CMV‐specific T cells can account for >10% of total circulating memory T cells 24. However, this vigorous CMV‐specific immune response does not offer protection from re‐infection 25, 26 due to the immune evasion characteristics of the virus 27. Development of a CMV vaccine that boosts immunity in immunosuppressed transplant recipients would protect the patient from infection and/or reactivation, and prevent potentially life‐threatening accelerated allograft rejection. The goal of this study was to test the immunogenicity and efficacy of a novel inactivated CMV vaccine in a rat model of cardiac transplant. We created a vaccine by inactivating CMV using hydrogen peroxide, a method that preserves antigenic structure and immunogenicity while completely inactivating viruses 28. CMV‐naïve rats were vaccinated prior to receiving CMV+ donor cardiac allografts. We then determined the vaccine effects on chronic allograft rejection and viral dissemination. This RCMV vaccine provides a springboard for developing a multivalent HCMV vaccine.
Materials and Methods
RCMV vaccination
Maastricht strain RCMV was prepared from rat fibroblasts as previously described 29, 30, 31. Equal volumes of RCMV (5 × 106 pfu/mL) were mixed with 6% hydrogen peroxide diluted in water for 4 h 28. The virus: H2O2 solution was dialyzed overnight against PBS using a Spectra/PorCE Float‐a‐lyzer (25 000 dalton cutoff; Spectrum Labs, Rancho Dominguez, CA). Aliquots of vaccine (0.1 μg/μL) were stored at −80°C. CMV‐naïve Lewis rats (recipients) were untreated or vaccinated with inactivated RCMV either 35 or 70 days prior to transplantation (Figure 1). At vaccination, 10 μg inactivated RCMV was mixed with an equal volume of Complete Freund's adjuvant (FCA) and injected intramuscularly. Rats were boosted at 28 days with 10 μg of vaccine in Incomplete Freund's adjuvant (FIA).
Figure 1.
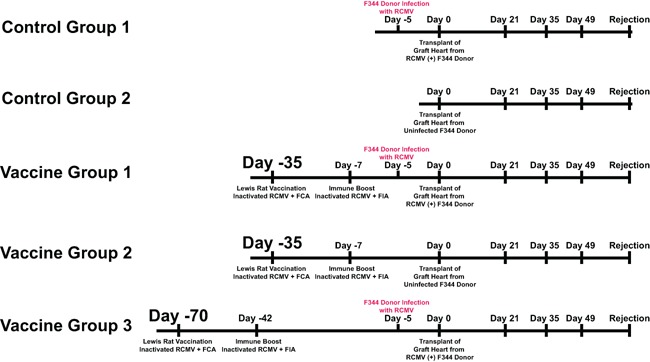
Vaccine experimental design. Depicted is the vaccine experimental strategy and study time line. A total of five groups were included in this study: (1) nonvaccinated recipient of an infected donor heart; (2) nonvaccinated recipient of uninfected donor heart; (3) vaccinated recipient at 35 days before transplantation that was boosted at 28 days later received an infected donor heart; (4) vaccinated recipient at 35 days before transplantation that was boosted 28 days later received an uninfected donor heart; (5) vaccinated recipient at 70 days before transplantation that was boosted 28 days later received an infected donor heart. Donors were infected at 5 days pretransplant to ensure that each donor heart was infected and that infection was not affected by pre‐existing immunity in the vaccinated groups, which would skew the results. Rejection was monitored and the animals were euthanized at the time of CR at which time blood, salivary glands, graft heart, and other major organs were harvested for analysis. A second cohort of Nonvaccinated, Day 35, and Day 70 vaccinated allograft recipients was harvested at postoperative day (POD) 7 to monitor the effect of vaccination on virus levels at early times posttransplant.
Heart transplantation
Animals were housed in the Portland Veterans Administration Medical Center animal facility, which is AAALAC accredited and complies with USDA and HHS animal care requirements. F344 rats (donors) were infected with 1 × 105 pfu salivary gland‐derived RCMV 30, 32, 33 5 days prior to heterotopic cardiac transplantation into Lewis recipients 5, 33. Recipients were treated with CsA (10 mg/kg/day; Novartis, East Hanover, NJ) for 10 days to prevent acute rejection. Animals were examined daily for CR by manual monitoring of graft heartbeat grade and harvested upon graft failure 5. Blood was drawn on postoperative days (POD) 21, 35, 49, 63 and at CR. TVS was assessed in paraffin embedded tissue sections stained with H&E and elastic von Gieson. The extent of TVS formation was determined by the neointimal index 33, 34. Allograft TVS severity and time to rejection were compared between groups using Student's t‐test. A second cohort of transplanted rats was harvested at POD 7 to assess early effects of vaccination on virus dissemination.
Adoptive and passive transfer experiments
Lewis rats were vaccinated, boosted (70 day protocol) and harvested one day pretransplantation. Total blood was clotted and serum pooled. Total splenic lymphocytes were isolated using Ficoll‐Paque Plus (GE Healthcare, Piscataway, NJ) 8. T cells were isolated using a BD FACSAria II cell sorter by staining 8 × 108 lymphocytes with antibodies to CD4 (APC‐Cy7, Biolegend, San Diego, CA), CD8 (PerCP‐Cy5, BD Biosciences, San Jose, CA), CD44h (FITC, BD Biosciences) and CD62L (PE, BD Biosciences), which delineate T cell subtypes (naïve, central and effector memory phenotypes) 8. T cells were pelleted by centrifugation and resuspended in RPMI‐1640. That day Lewis recipient rats (n = 6) received approximately 8 × 106 effector/central memory T cells (CD4–2 × 106, CD8–6 × 106; purity 98.7%) i.v. (500 μL) or 1 mL of pooled serum i.p. (n = 6/group). Adoptive transfer rats were transplanted with RCMV infected F344 hearts and harvested at CR.
Prime/boost of Lewis rats with 10 μg of inactivated RCMV prepared with Alum (0.1%) and/or monophosporyl lipid A (MPL; 5 μg) was performed to compare adjuvant efficacy. Control groups consisted of a) unvaccinated group; b) vaccinated with inactivated virus alone with no adjuvant; and c) inactivated virus prepared in Freund's adjuvant. At 70 days postprime, serum was collected from vaccinated animals and 1 mL pooled serum was injected i.p. into Lewis recipients. An additional group received a rat anti‐RCMV gB neutralizing mAb (10 mg/kg) i.p. Lewis rats were transplanted the following day with RCMV‐infected F344 rat donor hearts.
PCR detection of RCMV
To determine viral DNA load, total DNA was isolated from graft heart, salivary glands and PBMC using DNazol 8. DNA (0.5 μg) was analyzed by TaqMan PCR using a probe/primer set recognizing RCMV DNA polymerase sequence (sensitivity <100 copies) 33, 35.
RCMV‐specific enzyme‐linked immunoabsorbent assay (ELISA)
Antibody responses were assessed by RCMV‐specific ELISA as described 8. High binding ELISA plates (Corning, Tewksbury, MA) were coated with RCMV‐infected cellular lysates (10 μg/mL). Plates were blocked with 2% milk in 0.05% Tween‐PBS. Triplicate serial serum dilutions were incubated for 2 h at room temperature then washed with 0.05%Tween‐PBS. Plates were incubated 1 h with horseradish peroxidase‐conjugated anti‐rat IgG (Life Technologies, Grand Island, NY), anti‐rat‐IgG1, ‐IgG2a, ‐IgG2b, ‐IgG2c or ‐IgM and washed. Chromogen OPD substrate (Life Technologies) was added to allow detection and quantification of bound antibody molecules. Endpoint antibody titers were calculated using log‐log transformation of linear portion of the curve.
Plaque reduction neutralization assay
Virus‐neutralizing capacity was assessed by plaque reduction assays. 1:20 dilutions of heat inactivated serum (56°C, 30 min) were incubated with 100 PFU RCMV 1 h at 37°C. Mixtures were applied to confluent rat fibroblasts and incubated 2 h at 37°C. A 1 mL overlay of carboyxmethyl cellulose (Sigma‐Aldrich, St. Louis, MO) was applied. At 5 days, plates were fixed with 3.7% formalin and stained with methylene blue. Average plaque number (three replicates) was compared to control serum using Student's t‐test.
SDS‐PAGE and western blot analysis
RCMV‐infected cell lysates were loaded onto NuPAGE 4–12% gradient gels (Life Technologies) and transferred to Immobilon‐P blotting membrane for immunostaining. Membranes were blocked in 2% Milk in 0.02% Tween‐20 (Block) for 15 min. Rat sera (1:1000 in Block) was added for 1 h, and membranes washed in TBS containing 0.2% Tween (TBST). Secondary goat anti‐rat‐HRP conjugate was added at 1:40,000 in Block for 20 min and washed in TBST. Membranes were exposed to ECL Advance Lumigen‐TNA (GE Healthcare) and exposed to Biomax Light Film (Kodak, Rochester, NY).
Results
Inactivated RCMV vaccine prevents CMV‐accelerated rejection
We used a CMV polyvalent vaccine in a rat heart transplant CR model with RCMV D + /R‐ and assessed protection against CMV‐accelerated CR/TVS. Inactivated RCMV vaccine was produced using hydrogen peroxide inactivation 28. H2O2 treatment resulted in complete inactivation of RCMV as determined by plaque assay (data not shown). Lewis recipients were vaccinated with 10 μg of inactivated RCMV with FCA (Figure 1). At 28 days post–initial vaccination, animals were boosted with 10 μg of inactive RCMV plus FIA. The vaccinated rats were divided into two groups. Vaccine group 1 was transplanted at 7 days following booster (35‐day vaccine), while vaccine group 3 was transplanted at 42 days following booster (70‐day vaccine). Both groups received RCMV‐infected F344 donor hearts to mimic the highest risk group (D + /R‐) for CMV infection and disease in human transplantation. Vaccine group 2 (35‐day vaccine) received an uninfected donor heart to control for effects on allograft survival or TVS. Non‐vaccinated Lewis recipients transplanted with either infected or uninfected F344 donor hearts served as additional controls (Control groups 1&2).
Chronic allograft rejection was accelerated from a mean of 108 days post transplantation to 59 days when CMV‐infected donor hearts were transplanted (Figure 2A and B). The 35‐day vaccination strategy did not prolong average time to CR in either infected or uninfected heart groups (Figure 2B, terminal rejection POD 61 and POD 104, respectively). However, recipients of infected hearts that received the 70‐day vaccine had a significant delay in time to terminal graft rejection (POD 97). TVS results paralleled the graft rejection data (Figure 2C) with significantly greater degree of vasculopathy in infected donor hearts of unvaccinated controls and the 35‐day vaccine group (NI = 90 and 87) than in animals receiving the 70‐day regimen (NI = 59; p < 0.05) or uninfected controls (NI = 54; p < 0.05). Of note, in parallel with the rejection data, the 35‐day vaccinated recipients of uninfected hearts had similar NI as the no vaccine‐no infection group and day 70 vaccine with RCMV infection (NI = 58; p = NS). These data demonstrate that 70‐day vaccination regimen with inactivated RCMV delays the time to rejection and decreases NI severity. Importantly, pretransplant vaccination timing is critical. In order to confirm the presence of posttransplantation CMV infection, we quantified viral DNA load in salivary glands of recipients at time of CR (Figure 3A). While the 35‐day vaccinated animals had an eightfold reduction in viral load compared to unvaccinated controls, the 70‐day regimen had a > 100‐fold reduction compared to unvaccinated controls (p < 0.01).
Figure 2.
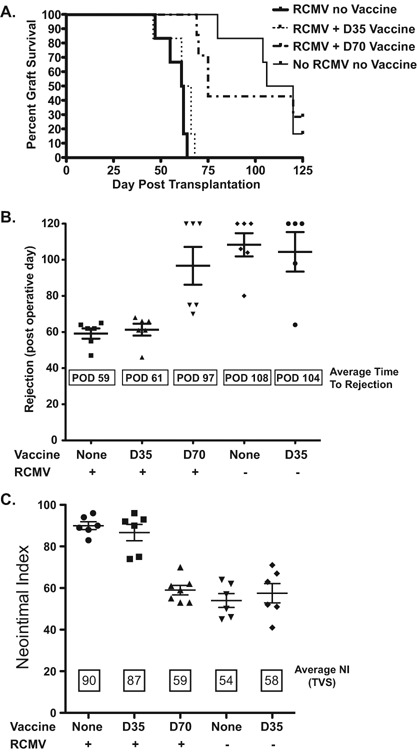
Vaccine promotes allograft survival of RCMV infected donor hearts. Vaccination of recipients with H2O2‐inactivated RCMV at 70 pretransplantation promotes survival of RCMV‐infected donor hearts compared to unvaccinated controls and recipients vaccinated at 35 days pretransplant. Heterotopic heart allografts (RCMV + / −) were transplanted into RCMV‐naïve recipients or animals vaccinated at 35 or 70 pretransplant (n = 6). Heart graft recipients were monitored over a 120‐day period for clinical graft rejection. (A) Graft survival curves were produced using GraphPad Prism 5 software. (B) Graph depicts the mean time of rejection for each group as well as the time to rejection for each replicate. (C) Sections of graft hearts were stained with H&E and elastic von Gieson in order to determine the degree of vascular disease in the coronary arteries. The average neointimal index (vascular disease, TVS) for each group as well as the NI for each individual heart graft were graphed (means and SEM) using Prism 5 software. Standard deviation is indicated with error bars. The severity of TVS (NI scores) between the vaccine groups was analyzed and compared using Student's t‐test (p‐values < 0.05 were considered statistically significant).
Figure 3.
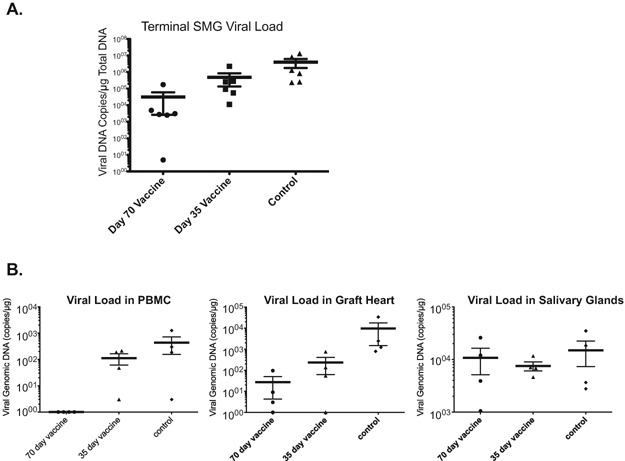
Day 70 vaccine reduced viral load in allograft recipients at CR. Total DNA was prepared using the DNAzol method. Viral DNA loads were determined by quantitative PCR using virus specific primers and probe; and then the results were compared between the groups using Student's t‐test (p‐values < 0.05 were considered statistically significant). (A) Vaccination of recipients with H2O2‐inactivated RCMV at 70 days pretransplant reduces viral load in salivary glands at the time of graft rejection compared to unvaccinated controls and recipients vaccinated with 35‐day regimen (*p < 0.01). (B) Vaccination of recipients with H2O2‐inactivated RCMV at POD −70 significantly reduces viral load in recipient PBMC and allograft heart but not salivary glands at 7 days posttransplantation compared to unvaccinated controls and recipients that were vaccinated at POD −35. SMG: submandibular gland.
To determine the effect of vaccine on early viral load and tissue specificity, we harvested PBMC, heart allografts, and salivary glands at POD 7 and assessed viral load by quantitative PCR. We found differential levels of RCMV in the various tissues (Figure 3B). The 70‐day vaccinated group had nearly undetectable viral load in the PBMC's, detectable but very low levels in the heart graft, and similar levels in the salivary glands compared to the other groups. This finding supports the concept that CMV‐accelerated graft rejection can be thwarted by a vaccine that reduces viral load in the graft tissue but doesn't prevent virus reactivation/dissemination.
RCMV vaccine elicits antibody responses
To address the protective functional difference in immune response elicited by the 70‐day vaccination compared to the 35‐day vaccine strategy, we analyzed anti‐RCMV antibody (Ab) responses in recipients at 0 (pretransplantation), POD21, 35, 49 and at terminal CR by RCMV‐specific ELISA. As expected, all naïve recipients (i.e. at the pre‐vaccination time point) were anti‐RCMV Ab negative (Figure 4A). Vaccinated animals had detectable anti‐RCMV Ab levels at time of transplantation. However, the 70‐day vaccine group had approximately threefold higher levels of anti‐RCMV Ab, compared to the 35‐day vaccine group (p = 0.24). Vaccine‐induced Ab responses were durable, lasting >100 days, even in the face of receiving an allograft from an uninfected donor (green line). Interestingly, Ab levels at POD21 in the 70‐day vaccine group experienced a 2‐log increase in anti‐RCMV IgG whereas no increase was seen at this time point in the 35‐day vaccine group. Ab responses for these two groups were similar by POD35, however, at POD49 and at CR, there was an additional increase in Ab levels in the 70‐day vaccine group not seen in the 35‐day group. To determine isotype specificities of antibody responses, we profiled pretransplant anti‐RCMV responses for the 70‐day and 35‐day vaccination strategies by ELISA. Both vaccination strategies elicited comparable IgG1, IgG2a, and IgG2b responses (Figure 4B). However, the 70‐day vaccine group had substantially increased levels of IgM and IgG2c anti‐RCMV specific antibodies compared to the 35‐day group. In addition, western blotting analysis of sera from both groups showed that 70‐day vaccination elicits a broader array of anti‐RCMV responses (Figure 4C).
Figure 4.
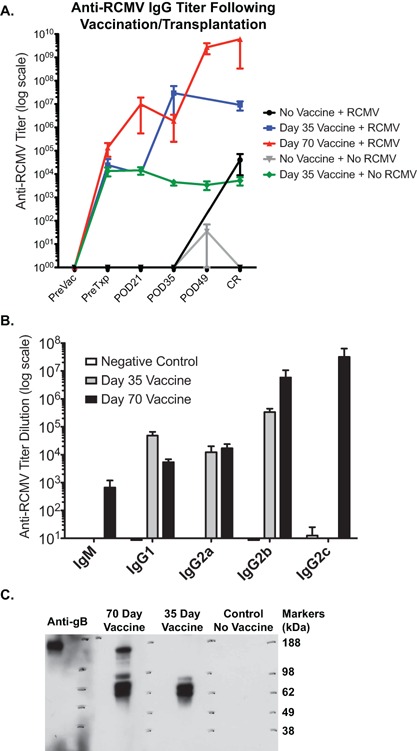
Vaccine elicits increased antibody responses to RCMV. The production of anti‐RCMV antibodies in the sera from vaccinated recipients at various times pre‐ and posttransplantation was measured using standard ELISA detecting RCMV‐infected cell lysates as the antigenic source. (A) Total IgG antibody binding was detected using a pan‐anti rat IgG antibody coupled to horseradish peroxidase. CR = chronic rejection. (B) Primary antibody binding was detected using anti‐rat isotype‐specific secondary antibodies (IgG1, IgG2a, IgG2b, IgG2c, and IgM). Animals vaccinated against inactivated RCMV with Freund's adjuvant using the 70‐day vaccine regimen were higher for IgM, IgG2b and IgG2c compared to animals vaccinated using the 35‐day vaccine protocol. N = 6 per group. (C) Western blot analysis was used to measure the presence and breadth of the antibody response in serum from uninfected control rats, the 35 day and 70 day vaccine groups compared to a rat monoclonal antibody that recognizes gB. RCMV infected cell lysates were separated by SDS‐PAGE and transferred to PVDF membrane. Blots were blocked and primary antibodies were bound for 1 h at room temperature. Primary antibody binding was detected using anti‐rat total Ig conjugated to horseradish peroxidase and developed by ECL and autoradiography.
To assess functionality of Ab responses, we determined anti‐RCMV neutralizing potential using plaque reduction neutralization assays. No detectable neutralizing antibodies were seen at time of transplantation (PreTxp day −1) (Figure 5). The 70‐day vaccine group developed 50% virus neutralizing activity by POD21, and the levels increased with time. By contrast, the 35‐day vaccine group and unvaccinated controls lagged significantly behind the 70‐day group. Analyses of pretransplant anti‐RCMV antibody responses revealed a longer period between vaccination and transplantation promotes a more robust and diverse immune response, translating into improved protection against rejection, less allograft TVS and improved overall graft survival.
Figure 5.
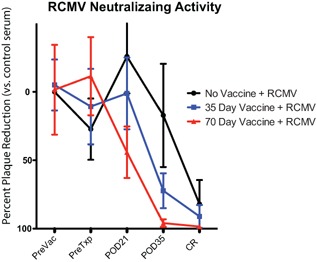
Day 70 vaccine is more efficacious at promoting neutralizing antibody development in transplanted rats. Plaque reduction neutralizing assays were performed on serum from nonvaccinated controls, Day 35 vaccine and Day 70 vaccine groups; (n = 6). A 1:20 dilution of serum was added to 100 pfu of RCMV for 1 h at 37°C and then added to rat fibroblasts for plaque assay. Data is presented as percent plaque reduction compared to control sera.
Vaccine serum and T cells confer protection against CMV‐accelerated CR
To identify immune correlates of protection by H2O2‐inactivated RCMV vaccine, an additional cohort of Lewis rats were vaccinated as described (70‐day vaccine; Figure 6A). One day before transplantation, naïve rats received either immune serum from the vaccinated animals (1 mL i.p.) or a mixture of memory T cells (2 × 106 CD4+ plus 6 × 106 CD8+ EM/CM; i.v. purity = 98.7%). The next day, recipients were transplanted with hearts from RCMV‐infected F344 rats and time to rejection and TVS severity were determined. As shown in Figure 6 B and C, the mean time to rejection of RMCV‐infected allografts for passive transfer recipients was 108 days and paralleled that of uninfected controls, and resulted in a significantly longer survival than untreated RCMV‐infected animals (59 days; p < 0.0001). The severity of disease (NI) mirrored time to rejection. Scores for NI were 90, 55 and 54 for untreated RCMV infection, passive transfer and uninfected groups, respectively (Figure 6D). T cell adoptive transfer recipients similarly demonstrated a significant clinical benefit with respect to time to rejection (92 vs. 59 days; p < 0.0001) and severity of vasculopathy (NI = 57 vs. 90; p < 0.0001). Adoptive T cell transfer results were less robust than for the passive transfer group (Figure 6B,C&D). Importantly, the viral load in the submandibular glands at CR was significantly reduced in both passive and adoptive transfer recipients compared to untreated RCMV infected controls (Figure 7). Both treatment groups demonstrated a > 100‐fold reduction in salivary gland viral load (p < 0.0002). Indeed, 2/4 animals that received immune serum demonstrated >5 Log10 reduction in virus titer. These data support the importance of reduction in viral load for impacting CMV accelerated TVS and CR.
Figure 6.
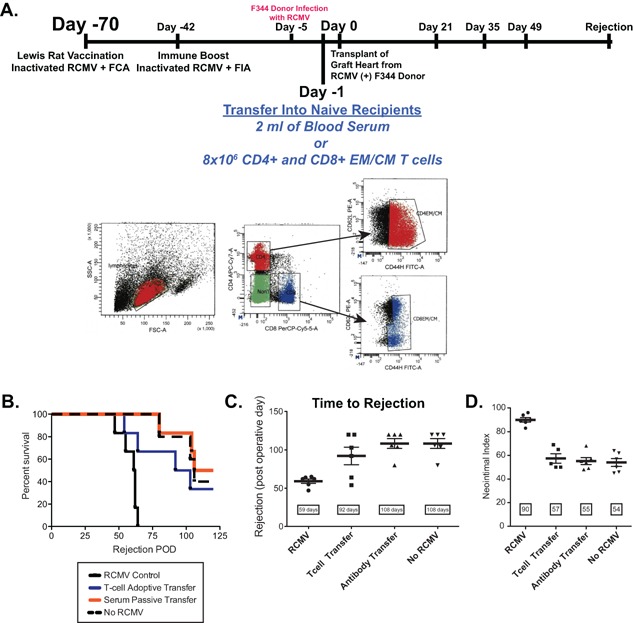
Identifying correlates of protection for anti‐RCMV vaccine. To determine the mechanisms that mediate protection from RCMV infection/accelerated rejection in the vaccinated animals, we performed passive transfer of either serum or splenic T cells from vaccinated rats into RCMV‐naïve recipients prior to transplantation of an infected donor heart. (A) Depicted is the vaccine/transfer experimental strategy. Naïve Lewis rats were vaccinated with hydrogen peroxide inactivated RCMV using the 70‐day regimen. At 1 day pretransplant, CMV‐naïve Lewis recipient rats were injected with 1 mL of serum or a mixture of 6 × 106 CD4+ and CD8+ central memory or effector memory T cells isolated from total splenocytes by flow cytometry activated cell sorting FACS (n = 6). Shown is the analysis of the rat CD4/CD8 effector and central memory cells. The recipient rats were transplanted with an infected F344 donor heart. Recipients of uninfected rats served as negative controls and recipients of infected hearts (untreated) served as positive controls for RCMV‐accelerated CR. Time to rejection was determined for each group and graphed in a survival plot (B) or graphed as the average time to rejection (C). Shown in panel D is the average neointimal index of allograft vessels at the time of chronic rejection. Transfer of immune serum prevented RCMV‐accelerated rejection and TVS formation in infected donor hearts compared to controls.
Figure 7.
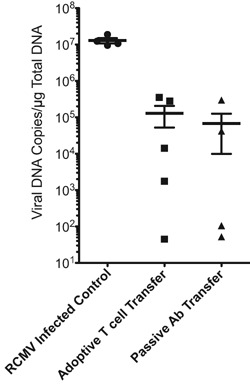
Transfer of serum or T cells from vaccinated rats to naïve recipients reduced viral load in allograft recipient salivary glands at CR. Transfer of vaccine immune serum or T cells reduces viral load in salivary glands at the time of graft rejection compared to unvaccinated controls (p < 0.02). Total DNA was prepared using DNAzol and viral genomic DNA levels were measured by quantitative PCR. The results were compared between the groups using Student's t‐test.
Since passive antibody transfer from vaccinated animals into CMV‐naïve recipients was most effective in increasing graft survival of RCMV‐infected donor hearts with Freund's adjuvant, we next compared the efficacy of antibody responses induced by several human adjuvants (Alum, MPL, or Alum/MPL combination). At 1 day pretransplantation, Group 1 received immune sera (1 mL i.p.) from a Lewis rat vaccinated with H2O2‐inactivated RCMV (no adjuvant control); Group 2 vaccine was prepared with Alum; Group 3 with MPL; Group 4 with Alum plus MPL; Group 5 with Complete Freund's; and Group 6 contained unvaccinated controls. Group 7 received 10 mg/kg of a rat anti‐RCMV gB mAb (IgG2b isotype) i.p., which neutralized RCMV in the presence of complement (Figure S1). All passive antibody recipients were transplanted with hearts from RCMV‐infected F344 rats (n = 6/group). The mean time to allograft rejection in Groups 4 and 5 was significantly increased compared to all other groups (93.8 and 97.7 days vs. 60.8, 60.3, 68.3, 57.3, and 54.8 days, respectively; p < 0.002) (Figure 8 A and B). Similarly, TVS was significantly reduced in grafts from Groups 4 and 5 compared to Groups 1, 2, 6, and 7 (NI = 63 and 62 vs. 85, 86, 89 and 90, respectively; p < 0.001) (Figure 8C); and also versus Group 3 (NI = 79; p < 0.01). To determine whether differences in graft survival correlated with antiviral antibody titers, we used ELISA to measure anti‐RCMV antibody dilution titers for total IgG, IgM and subsets of IgG (Figure S2). The antiviral dilution levels for specific rat IgG isotypes are shown in Figure S2. The salivary gland infection status in animals of Groups 4 and 5 was substantially lower than Groups 1, 2, 3, 6 (data not shown). Combined, our data indicate an additional immune correlate provided by inactivated viral vaccine arms the host with a multifaceted protection against CMV‐accelerated allograft rejection.
Figure 8.
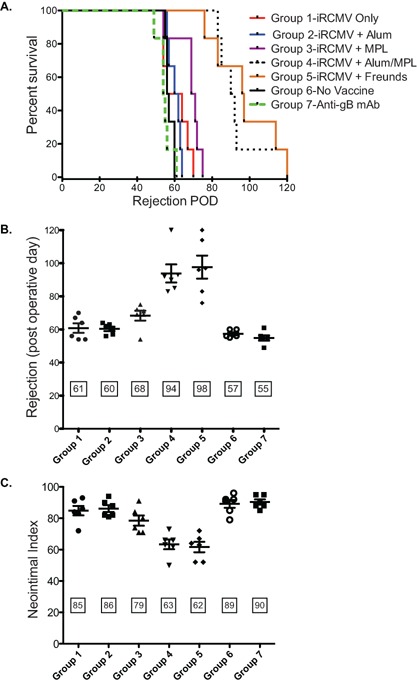
Anti‐RCMV vaccine efficacy is adjuvant‐dependent. Three adjuvants were compared for their effect on efficacy of the anti‐RCMV vaccine in recipients of CMV‐infected donor allografts. CMV‐naïve Lewis rats were vaccinated with hydrogen peroxide inactivated RCMV adjuvanted with Alum, MPL, Alum + MPL, or Freund's using the 70‐day vaccination regimen. At 1 day pretransplant, Lewis recipient rats were injected with 1 mL of blood serum derived from the vaccinated animals (n = 6). The recipient rats were transplanted with a RCMV‐infected F344 donor heart. Group 1 received serum from vaccinated animals with H202‐inactivated virus alone with no added adjuvant; Group 2 vaccine was adjuvanted with Alum; Group 3 with MPL; Group 4 with Alum + MPL; Group 5 with Freund's adjuvant; Group 6 were untreated recipients of infected hearts to serve as positive controls for RCMV‐accelerated CR; and Group 7 were treated with a rat monoclonal antibody directed against RCMV‐gB that neutralizes in the presence of complement. (A) Time to rejection was determined for each group and graphed in a survival plot (B) or graphed as the average time to rejection. Shown in panel C is the average neointimal index of allograft vessels at the time of chronic rejection. Transfer of immune serum from rats vaccinated using the adjuvant Alum + MPL prevented RCMV‐accelerated rejection and TVS formation in infected donor hearts to levels observed for those animals receiving Freund's adjuvant.
Discussion
Cytomegalovirus continues to negatively impact outcomes in solid organ transplantation, especially during reactivation following cessation of antiviral prophylaxis. In addition, CMV infection in the transplant recipient directly and indirectly enhances graft vascular disease and accelerates acute and chronic rejection. Historically, the development of a diverse spectrum of anti‐viral agents, such as ganciclovir, and/or Hepatitis A, B, and influenza vaccines have impacted graft outcomes and patient survival post transplantation 36. While a CMV vaccine potentially represents a cost‐effective method for inducing and/or enhancing protective immunity against CMV in transplant patients, the implementation of a highly efficacious vaccine has remained elusive due to critical factors including: (1) lifelong CMV persistence, making vaccine testing in immunocompetent populations unfeasible; (2) CMV encodes a number of immune modulatory genes that reduce the potency of the antiviral immune response; (3) while natural immunity prevents outward disease in otherwise healthy individuals it does not prevent re‐infection, suggesting that sterilizing immunity may be very difficult, if not impossible. In this study, we determined the efficacy of a novel vaccine in preventing CMV disease and accelerated TVS/CR in rat heart allografts. We inactivated RCMV using hydrogen peroxide, a method that generates highly efficacious inactivated vaccines 28. Our results demonstrate that a prime/boost approach followed by a period of immune maturation between vaccination and transplantation evokes a protective response by preventing CMV‐accelerated TVS and extending graft survival to control levels. The vaccine effectively reduced viral loads in PBMC and graft hearts at POD 7 but failed to elicit sterilizing immunity. Our current findings support the concept that control of virus replication early in the post transplant period contributes to the reduction of CMV‐accelerated rejection.
Both humoral and cellular mediated immunity are potentially activated during vaccination. Since transfer of T cells or antiserum from vaccinated animals into naïve animals increased time to rejection and simultaneously reduced TVS severity and salivary gland viral titers at the time of rejection, we propose that the hydrogen peroxide RCMV vaccine potently stimulates both arms of the immune system. We did not examine antiviral T cell responses; however, increased protection from accelerated rejection in T cell transfer experiments clearly demonstrates their functionality. It is established that immunosuppressive drugs given to prevent acute cellular graft rejection result in reduced active T cells, and negatively impacts recipient cell‐mediated immunity 37. Importantly, anti‐CMV T cell immunity levels substantially drop in kidney transplant patients shortly after transplantation 38. It takes >1 year for antiviral‐T cell levels to recover and in most individuals pretransplant levels are never reached. Similar findings have been observed in heart transplant recipients with initial low levels of anti‐CMV T cell immunity that failed to develop protective immune responses following transplantation, resulting in increased frequency of CMV reactivation 39. Results from these human studies indicate the potential benefit of a CMV vaccine in reducing viral reactivation in patients with functional pretransplant antiviral immune responses, which wane after transplant 22. We chose to focus on the vaccine‐associated impact on CMV‐specific antibody responses since passive transfer of antiserum was highly efficacious.
In the current study, vaccine‐induced CMV‐specific antibody responses were not neutralizing or sterilizing; however, these antibodies provided robust protection from CMV‐induced vasculopathy and CR. We propose that the mechanism involves a modulation in down‐stream antibody effector mechanisms that include antibody‐dependent cellular cytotoxicity (ADCC), which was also suggested to explain data obtained during the MF59‐gB human clinical trial 40. We demonstrate that anti‐RCMV antibody responses in the 70‐day group were more robust with broader specificity compared to the 35‐day group. Importantly, the 70‐day vaccination strategy had robust recall antibody responses displaying quicker antiviral antibody kinetics and rapid formation of neutralizing activity by POD 21. Interestingly, the increase in antiviral antibody titers and induction of neutralizing antibodies following transplantation of the RCMV‐infected heart occurred in the presence of the immunosuppressant CsA, indicating that T cell help was not required for memory B cell activation in the 70‐day vaccine group. This finding suggests that vaccine‐derived anti‐CMV memory B cells are capable of robust proliferation/differentiation into antibody‐secreting cells, further strengthening the vaccine efficacy. Studies in latently infected B‐cell deficient mice on immunosuppression receiving immune serum resulted in a significant reduction in salivary gland, lung and spleen viral titers compared to wild‐type controls 41, suggesting that this effect was antibody‐based. A MCMV latency model by Reddehase et al compared the risk of virus reactivation in BALB/c mice that had experienced a primary infection as either neonates or adults 42. They observed a higher incidence of virus recurrence in neonatally‐infected mice due to a higher latent viral load in spite of a fourfold higher titer of neutralizing antibody, suggesting that neutralizing antibodies do not prevent virus reactivation. However, recurrent infection remained confined to the organs hosting viral reactivation and serum‐transfer from wild‐type vs. B‐cell deficient donor mice identifying antiviral antibody as the mediator preventing virus spread. These findings may explain the effect of active induction of antiviral antibodies by our vaccine in preventing graft disease. In human SOT patients, passive transfer of IVIG is used to treat drug‐resistant HCMV disease, which supports this concept 43.
Antibody and T cell responses are induced when H2O2‐inactivated virus vaccine is combined with FCA, which is composed of inactivated mycobacteria. Freund's adjuvant is effective in stimulating cell‐mediated immunity and potentiates production of certain Ig‐subtypes, but it is not approved for human use. Therefore, we tested the ability of clinically relevant adjuvants to boost immunogenicity of H2O2‐inactived CMV vaccine. The combination of MPL plus alum resulted in significant protection against CMV‐mediated CR and TVS. MPL displays greatly reduced toxicity while maintaining most immunostimulatory activities of lipopolysaccharide by signaling through Toll‐like receptor‐4. Alum promotes humoral responses to alum‐adsorbed deposits of antigens due to induction of inflammatory signals. Our findings of the synergistic effect of MPL/alum are likely due to their complementary immunostimulatory effects. Future studies will define the nature of antiviral B cell activation and determine whether anti‐CMV antibody responses impede alloreactivity by blocking inflammation. There is increasing precedent that antibodies have anti‐inflammatory properties, and these anti‐inflammatory properties may be involved in the vaccine's protective effects 44. Our vaccination strategy induced immune responses in both arms of the immunologic repertoire. Our findings demonstrate that a vaccine that induces durable antibody‐mediated viral immunity would have long‐term benefits to human transplant recipients by reducing episodes of CMV reactivation and the consequent chronic allograft rejection.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. O.H.S.U. and M.K.S. have a financial interest in Najít Technologies, Inc., a company developing H2O2‐inactivated vaccines. This potential individual and institutional conflict of interest has been reviewed and managed by O.H.S.U.
Supporting information
Figure S1: Neutralization potential of Anti‐RCMV gB‐specific rat monoclonal antibody is higher in the presence of complement.
Figure S2: Analysis of Anti‐RCMV serum antibody isotype by ELISA for vaccinated rats.
Acknowledgments
This work was supported by research grants from the National Institutes of Health to D.N.S. (HL083194) and S.L.O. (HL 66238‐01).
References
- 1. Bruning JH, Persoons MCJ, Lemstrom KB, Stals FS, De Clereq E, Bruggeman CA. Enhancement of transplantation associated atherosclerosis by CMV, which can be prevented by antiviral therapy in the form of HPMPC. Transplant Int 1994; 7: 365–370. [DOI] [PubMed] [Google Scholar]
- 2. Lemstrom K, Koskinen P, Krogerus L, Daemen M, Bruggeman CA, Hayry PJ. Cytomegalovirus antigen expression, endothelial cell proliferation, and intimal thickening in rat cardiac allografts after cytomegalovirus infection. Circ 1995; 92: 2594–2604. [DOI] [PubMed] [Google Scholar]
- 3. Lemstrom KB, Bruning JH, Bruggeman CA, Lautenschlager IT, Hayry PJ. Cytomegalovirus Infection Enhances Smooth Muscle Cell Proliferation and Intimal Thickening of Rat Aortic Allografts. J Clin Invest 1993; 92: 549–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Orloff SL. Elimination of donor‐specific alloreactivity by bone marrow chimerism prevents cytomegalovirus accelerated transplant vascular sclerosis in rat small bowel transplants. J Clin Virol 1999; 12: 142 (G3–25). [Google Scholar]
- 5. Orloff SL, Streblow DN, Soderberg‐Naucler C, et al. Elimination of donor‐specific alloreactivity prevents cytomegalovirus‐accelerated chronic rejection in rat small bowel and heart transplants. Transplantation 2002; 73: 679–688. [DOI] [PubMed] [Google Scholar]
- 6. Orloff SL, Yin Q, Coreless CL, Loomis CB, Rabkin JM, Wagner CR. A rat small bowel transplant model of chronic rejection: Histopathologic characteristics. Transplantation 1999; 68: 766–779. [DOI] [PubMed] [Google Scholar]
- 7. Orloff SL, Yin Q, Corless CL, Orloff MS, Rabkin JM, Wagner CR. Tolerance induced by bone marrow chimerism prevents transplant vascular sclerosis in a rat model of small bowel transplant chronic rejection. Transplantation 2000; 69: 1295–1303. [DOI] [PubMed] [Google Scholar]
- 8. Orloff SL, Hwee YK, Kreklywich C, et al. Cytomegalovirus latency promotes cardiac lymphoid neogenesis and accelerated allograft rejection in CMV naive recipients. Am J Transplant 2011; 11: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zamora MR. Cytomegalovirus and lung transplantation. Am J Transplant 2004; 4: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 10. Limaye AP. Antiviral resistance in cytomegalovirus: An emerging problem in organ transplant recipients. Semin Respir Infect 2002; 17: 265–273. [DOI] [PubMed] [Google Scholar]
- 11. Limaye AP. Ganciclovir‐resistant cytomegalovirus in organ transplant recipients. Clin Infect Dis 2002; 35: 866–872. [DOI] [PubMed] [Google Scholar]
- 12. Limaye AP, Corey L, Koelle DM, Davis CL, Boeckh M. Emergence of ganciclovir‐resistant cytomegalovirus disease among recipients of solid‐organ transplants. Lancet 2000; 356: 645–649. [DOI] [PubMed] [Google Scholar]
- 13. Limaye AP, Raghu G, Koelle DM, Ferrenberg J, Huang ML, Boeckh M. High incidence of ganciclovir‐resistant cytomegalovirus infection among lung transplant recipients receiving preemptive therapy. J Infect Dis 2002; 185: 20–27. [DOI] [PubMed] [Google Scholar]
- 14. Plotkin SA, Plotkin SL. The development of vaccines: How the past led to the future. Nat Rev Microbiol 2011; 9: 889–893. [DOI] [PubMed] [Google Scholar]
- 15. Griffiths P, Plotkin S, Mocarski E, et al. Desirability and feasibility of a vaccine against cytomegalovirus. Vaccine 2013; 31: B197–B203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Plotkin SA, Higgins R, Kurtz JB, et al. Multicenter trial of Towne strain attenuated virus vaccine in seronegative renal transplant recipients. Transplantation 1994; 58: 1176–1178. [PubMed] [Google Scholar]
- 17. Plotkin SA, Smiley ML, Friedman HM, et al. Prevention of cytomegalovirus disease by Towne strain live attenuated vaccine. Birth defects original article series 1984; 20: 271–287. [PubMed] [Google Scholar]
- 18. Jacobson MA, Sinclair E, Bredt B, et al. Antigen‐specific T cell responses induced by Towne cytomegalovirus (CMV) vaccine in CMV‐seronegative vaccine recipients. J Clin Virol 2006; 35: 332–337. [DOI] [PubMed] [Google Scholar]
- 19. Jacobson MA, Sinclair E, Bredt B, et al. Safety,immunogenicity of Towne cytomegalovirus vaccine with or without adjuvant recombinant interleukin‐12. Vaccine 2006; 24: 5311–5319. [DOI] [PubMed] [Google Scholar]
- 20. Pass RF. Development and evidence for efficacy of CMV glycoprotein B vaccine with MF59 adjuvant. J Clin Virol 2009; 46: S73–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sabbaj S, Pass RF, Goepfert PA, Pichon S. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T‐cell responses to cytomegalovirus in chronically infected women. J Infect Dis 2011; 203: 1534–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kharfan‐Dabaja MA, Boeckh M, Wilck MB, et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem‐cell transplantation: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet Infect Dis 2012; 12: 290–299. [DOI] [PubMed] [Google Scholar]
- 23. Reap EA, Dryga SA, Morris J, et al. Cellular and humoral immune responses to alphavirus replicon vaccines expressing cytomegalovirus pp65, IE1, and gB proteins. Clin Vaccine Immunol 2007; 14: 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus‐specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med 2005; 202: 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adler SP, Starr SE, Plotkin SA, et al. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J Infect Dis 1995; 171: 26–32. [DOI] [PubMed] [Google Scholar]
- 26. Plotkin SA, Starr SE, Friedman HM, Gonczol E. Weibel RE. Protective effects of Towne cytomegalovirus vaccine against low‐passage cytomegalovirus administered as a challenge. J Infect Dis 1989; 159: 860–865. [DOI] [PubMed] [Google Scholar]
- 27. Hansen SG, Powers CJ, Richards R, et al. Evasion of CD8+ T cells is critical for superinfection by cytomegalovirus. Science 2010; 328: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Amanna IJ, Raue HP, Slifka MK. Development of a new hydrogen peroxide‐based vaccine platform. Nat Med 2012; 18: 974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bruggeman CA. Cytomegalovirus and latancy: An overview. Virch Archiv B Cell Pathol 1993; 64: 325–333. [DOI] [PubMed] [Google Scholar]
- 30. Bruggeman CA, Meijer H, Bosman F, van Boven CP. Biology of rat cytomegalovirus infection. Intervirology 1985; 24: 1–9. [DOI] [PubMed] [Google Scholar]
- 31. Meyer C, Grey F, Kreklywich CN, et al. Cytomegalovirus microRNA expression is tissue specific and is associated with persistence. J Virol 2011; 85: 378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bruggeman CA, Schellekens H, Grauls G, Debie WM, van Boven CP. Rat cytomegalovirus: induction of and sensitivity to interferon. Antiviral Res 1983; 3: 315–324. [DOI] [PubMed] [Google Scholar]
- 33. Streblow DN, Kreklywich C, Yin Q, et al. Cytomegalovirus‐mediated upregulation of chemokine expression correlates with the acceleration of chronic rejection in rat heart transplants. J Virol 2003; 77: 2182–2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Armstrong AT, Strauch AR, Starling RC, Sedmak DD, Orosz CG. Morphometric analysis of neointimal formation in murine cardiac allografts. Transplant 1997; 63: 941–947. [DOI] [PubMed] [Google Scholar]
- 35. Streblow DN, Kreklywich CN, Smith P, et al. Rat cytomegalovirus‐accelerated transplant vascular sclerosis is reduced with mutation of the chemokine‐receptor R33. Am J Transplant 2005; 5: 436–442. [DOI] [PubMed] [Google Scholar]
- 36. Duchini A, Goss JA, Karpen S, Pockros PJ. Vaccinations for adult solid‐organ transplant recipients: current recommendations protocols. Clin Microbiol Rev 2003; 16: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Boppana SB, Britt WJ. Recent approaches and strategies in the generation of antihuman cytomegalovirus vaccines. Methods Mol Biol. 2014; 1119: 311–348. [DOI] [PubMed] [Google Scholar]
- 38. Abate D, Saldan A, Fiscon M, et al. Evaluation of cytomegalovirus (CMV)‐specific T cell immune reconstitution revealed that baseline antiviral immunity, prophylaxis, or preemptive therapy but not antithymocyte globulin treatment contribute to CMV‐specific T cell reconstitution in kidney transplant recipients. J Infect Dis 2010; 202: 585–594. [DOI] [PubMed] [Google Scholar]
- 39. Abate D, Fiscon M, Saldan A, et al. Human cytomegalovirus‐specific T‐cell immune reconstitution in preemptively treated heart transplant recipients identifies subjects at critical risk for infection. J Clin Microbiol 2012; 50: 1974–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Griffiths PD, Stanton A, McCarrell E, et al. Cytomegalovirus glycoprotein‐B vaccine with MF59 adjuvant in transplant recipients: A phase 2 randomised placebo‐controlled trial. Lancet 2011; 377: 1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jonjic S, Pavic I, Polic B, Crnkovic I, Lucin P, Koszinowski UH. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J Exp Med 1994; 179: 1713–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Reddehase MJ, Balthesen M, Rapp M, Jonjic S, Pavic I, Koszinowski UH. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J Exp Med 1994; 179: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Radha R, Jordan S, Puliyanda D, et al. Cellular immune responses to cytomegalovirus in renal transplant recipients. Am J Transplant 2005; 5: 110–117. [DOI] [PubMed] [Google Scholar]
- 44. Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: The intravenous IgG paradox. J Exp Med 2007; 204: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Neutralization potential of Anti‐RCMV gB‐specific rat monoclonal antibody is higher in the presence of complement.
Figure S2: Analysis of Anti‐RCMV serum antibody isotype by ELISA for vaccinated rats.


