ABSTRACT
Cytokine-induced killer (CIK) cell immunotherapy represents an effective treatment strategy for treating hepatocellular carcinoma (HCC). However, the therapeutic benefits of CIK cell treatment can be influenced by differences in complex immune microenvironment between patients. Herein, we investigated the relationship between PD-L1 expression and survival benefits of CIK cell immunotherapy in HCC patients. This retrospective study included 448 HCC patients: 217 cases underwent hepatectomy alone; 231 cases received hepatectomy and post-operative CIK cell transfusion. Immunohistochemistry was used to measure PD-L1 expression in tumor tissue sections from all patients. Meanwhile, flow cytometry was performed to explore the relationship between PD-L1 expression and localized inflammatory response in HCC microenvironment. We found a significantly improved prognosis in CIK treatment group compared with surgery alone group. In the CIK treatment group, higher PD-L1 expression was observed in patients who exhibited long-term survival benefit. Survival analysis showed patients with ≥5% PD-L1 expression had better overall survival (OS) and recurrence-free survival (RFS) than patients with 1–5% or <1% PD-L1 expression, particularly in the subgroup with high hepatitis B viral load. By contrast, PD-L1 expression did not show direct impact on the survival of patients in surgery alone group. Additionally, PD-L1 expression was found to be highly associated with hepatitis B viral load and the proportion of tumor-infiltrating lymphocytes in HCC patients. In conclusions, our study indicates that PD-L1 expression may reflect the presence of endogenous host immune response to tumor and serve as a biomarker for predicting survival benefits from adjuvant CIK cell immunotherapy in HCC patients.
Keywords: Adjuvant CIK immunotherapy, hepatocellular carcinoma, immunohistochemistry, PD-L1, survival benefit
Abbreviations
- CIK
cytokine-induced killer cells
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HCC
hepatocellular carcinoma
- MHC
major histocompatibility complex molecules
- NSCLC
non-small cell lung cancer
- PBMCs
peripheral blood mononuclear cells
- PD-1
programmed death-1
- PD-L1
Programmed death-ligand 1
- SIRT
selective intra-arterial radiotherapy
- TACE
transarterial chemoembolization
Introduction
Hepatocellular carcinoma (HCC), a disease with both etiologic and geographic diversity, is currently the sixth most prevalent cancer and the second most common cause of cancer-related deaths, especially in developing countries.1-3 Although many treatment strategies, such as surgery, local ablative therapies, transarterial chemoembolization (TACE), selective intra-arterial radiotherapy (SIRT), systemic chemotherapy, and sorafenib, are used in palliative settings in patients with HCC.4,5 Many patients do not gain long-term clinical benefits from these aggressive interventions, and its overall prognosis remains poor because of the high incidence of intrahepatic recurrence and/or distant metastases.6 Currently, cytokine-induced killer (CIK) cells, which are ex vivo-activated lymphocytes generated by culturing peripheral blood mononuclear cells (PBMCs) with a cytokine cocktail that includes interleukin (IL)-2, interferon (IFN)γ, and anti-CD3 antibodies, has been established to provide potential benefits in immune-based therapies for HCC that show the characteristics of a high proliferation rate, potent antitumor effects, and minimal toxicity.7-9 A recent series of prospective randomized trials and retrospective studies showed that the addition of adjuvant CIK cell immunotherapy can significantly improve the odds of obtaining an effective clinical response, and improve the overall survival (OS) and/or recurrence-free survival (RFS) rates in HCC patients who have undergone hepatectomy.10-13 However, the survival benefits of adjuvant CIK cell immunotherapy for HCC remain variable among patients. Previously, we developed a nomogram that could be used to evaluate survival benefits in individual HCC patients after receiving adjuvant CIK cell immunotherapy.14 However, this study was designed based on several clinicopathological parameters that were related to the prognosis of HCC. As an adoptive immunotherapy, the therapeutic benefits of CIK cell treatment can be influenced by the complex immune microenvironment in patients with cancer, including tumor-associated immune cells and the immune signatures of tumor tissue. 15,16 Thus, immune factors should be accounted for and could represent additional prognostic variables for predicting survival benefits associated with adjuvant CIK cell treatment in patients with HCC.
Programmed death-ligand 1 (PD-L1; also known as B7-H1 or CD274) is expressed on various types of solid tumors, including melanoma, HCC, renal cell carcinoma, non-small cell lung cancer, ovarian cancer, and colorectal cancer. PD-L1 plays an important role in generating the immunosuppressive tumor microenvironment and preventing T cell-mediated cytolysis by binding to programmed death-1 (PD-1) and B7.1 (CD80), which are both negative regulators of T cell activation, migration, proliferation, and the secretion of cytotoxic mediators.17 Emerging clinical and preclinical data suggests that the expression of PD-L1 on tumors is not an oncogenic driver, but is rather a co-opted and maladaptive immune “shield” that protects a tumor from its immune microenvironment.18,19 Recently, a series of clinical studies have confirmed that patients with tumors that overexpress PD-L1 can best benefit from cancer immunotherapy.20-22 In a prospective randomized trial by Garon et al., cell membrane PD-L1 expression in at least 50% of tumor cells was associated with a higher response rate and longer rates of progression-free and OS compared with patients with cell membrane PD-L1 expression less than 50% in patients with advanced non-small cell lung cancer who received pembrolizumab treatment.23 Therefore, PD-L1 expression in tumors might represent a predictive biomarker in cancer immunotherapy. However, the relationship between PD-L1 expression in HCC and the clinical benefits of adjuvant CIK cell immunotherapy has not been validated.
This study aimed to define and validate the expression levels of PD-L1 on HCC cells, and to determine whether PD-L1 expression was associated with the likelihood of a survival benefit in HCC patients who received adjuvant CIK cell treatment.
Results
Patient demographics, clinical characteristics, and prognosis
Demographic data and clinical characteristics from a total of 448 HCC patients are presented in Table 1. All patients received complete hepatectomy and 427 (95.3%) were Child-Pugh classes A; 397 (88.6%) patients were positive for HBsAg and only 6 (1.3%) patients were infected with hepatitis C virus (HCV); 19 (4.8%) patients were alcoholic liver disease and 15 (3.3%) were non-alcoholic steatohepatitis ( NASH ) (Table 1).The clinicopathological parameters between CIK treatment and surgery alone groups were well matched. No statistically significant differences between the two groups were detected for all variables that we tested (p ≥ 0.05, Table 1). Patients in the CIK treatment group had a significantly better OS and RFS compared with those in the surgery alone group (Fig. 1). The median OS and RFS for patients in the CIK treatment group were 44 and 12 mo compared with 31 and 9 mo for patients in the surgery alone group, respectively. The OS rates at 1-, 3-, and 5-y were 80.1%, 54.1%, and 45.4% for the CIK treatment group compared to 71.6%, 46.1%, and 36.6% for the surgery alone group (log-rank test, p = 0.042). The RFS rates at 1-, 3-, and 5-y were 49.4%, 31.6%, and 28.5%, respectively, for the CIK treatment group compared to 40.3%, 21.2%, and 14.8% for the surgery alone group (log-rank test, p = 0.003).
Table 1.
Demographics and clinical characteristics of patients in the CIK treatment and surgery alone groups.
| Clinicopathological variables | Surgery alone group (n = 217) | CIK treatment group (n = 231) | p value |
|---|---|---|---|
| Age (y) | 0.292 | ||
| <50 | 118 | 137 | |
| ≥50 | 99 | 94 | |
| Median (range) | 48 (22–74) | 47 (14–80) | |
| Gender | 0.068 | ||
| Male | 198 | 198 | |
| Female | 19 | 33 | |
| Capsule | 0.880 | ||
| Complete | 183 | 196 | |
| Incomplete | 34 | 35 | |
| Tumor size (cm) | 0.781 | ||
| ≤5 | 93 | 96 | |
| >5 | 124 | 135 | |
| TNM stage | 0.539 | ||
| I | 122 | 136 | |
| II | 31 | 25 | |
| III | 64 | 70 | |
| Tumor number | 0.142 | ||
| Single | 181 | 180 | |
| Multiple | 36 | 51 | |
| Histological differentiation | 0.653 | ||
| Well | 34 | 43 | |
| Moderate | 133 | 133 | |
| Poor | 50 | 55 | |
| Virus infection | 0.090 | ||
| HBV | 195 | 198 | |
| HCV | 1 | 1 | |
| HBV+HCV | 3 | 1 | |
| Non | 18 | 31 | |
| Child-Pugh classes | 0.414 | ||
| A | 205 | 222 | |
| B | 12 | 9 | |
| Alcoholic liver disease | 0.924 | ||
| Yes | 9 | 10 | |
| No | 208 | 221 | |
| NASH | 0.700 | ||
| Yes | 8 | 7 | |
| No | 209 | 224 | |
| AFP (ng/mL) | 0.433 | ||
| Median | 173.3 | 254.5 | |
| Range | 0.64–1,21,000 | 0-1,21,000 | |
| PD-L1 | 0.960 | ||
| <1% | 111 | 116 | |
| 1–5% | 54 | 57 | |
| ≥5% | 52 | 58 |
HBsAg, Hepatitis B surface antigen; AFP, α-fetoprotein; NASH, non-alcoholic steatohepatitis.
Figure 1.
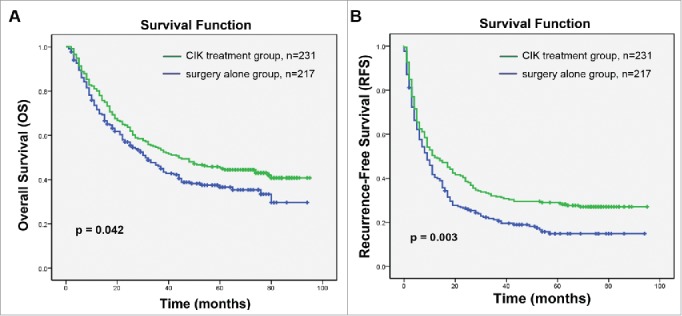
Kaplan–Meier curves of differences in survival among patients with hepatocellular carcinoma (HCC) who received adjuvant CIK cell treatment (CIK treatment group) or hepatectomy alone (surgery alone group). (A) Overall survival (OS) and (B) recurrence-free survival (RFS) curves. Significantly improved OS and PFS were observed in the CIK treatment group versus the surgery alone group.
Adverse events of CIK cell immunotherapy
Adverse events was defined by the protocol with NCI Common Terminology Criteria (version 4.0), which were considered drug-related or that occurred in patients during medical treatment or procedure regardless of relationship to drug. The CIK cell treatment-related adverse drug reactions include chills, pyrexia, myalgia, fatigue, vomiting, and hypersphyxia. Of all the 448 patients, adverse events occurred in 21 cases (9.1%) during they received CIK cell treatment, 18 of which were chills, fatigue and pyrexia, at grade 1 or 2, and regression spontaneously within 12 h. Another three patients appeared hypersphyxia and recovered after receiving symptomatic treatment. No myalgia and vomiting, autoimmune disorder, sign of infection, hepatic functional deterioration, or renal symptoms developed in all patients who received CIK cell infusion.
Patterns and quantification of PD-L1 expression in HCC tissue samples
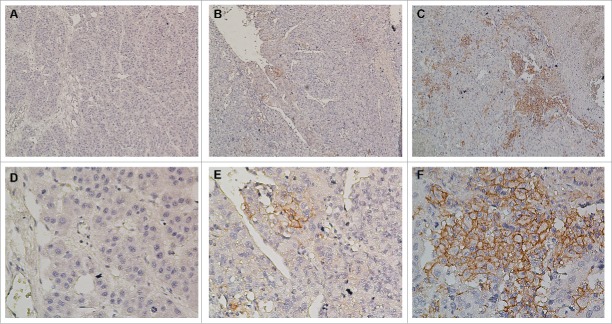
Immunohistochemical studies of HCC tumor tissues showed that PD-L1 expression on hepatoma cells was predominantly shown in the cell membrane with a variably strong component of staining in the cytoplasm (Fig. 2). In addition, PD-L1 was also expressed on some tumor-infiltrating immune cells but was hardly expressed on normal hepatocytes (Fig. S1). Patterns of PD-L1 expression within tumors included absence or regional expression, which was similar to their expression in melanoma,24 NSCLC, renal cell carcinoma, and other tumors.25 Considering the patterns of PD-L1 expression, the percentages of tumor cells exhibiting membranous staining for PD-L1 were quantified, and specimens were scored as ≥5%, 1–5%, and <1 % based on the frequency of total tumor cells that exhibited membranous PD-L1 expression (Fig. 2). Because of the complexity of our scoring algorithm, quantification was performed independently by two experienced researchers who were blinded to the patient outcomes; any discrepancies in scoring were adjudicated. A level of ≥5% PD-L1 expression was selected to define PD-L1 positivity. Among 231 HCC patients in the CIK treatment group, 58 (25.1%) exhibited ≥5% PD-L1 expression, 57 (24.7%) showed 1–5%, and 116 (50.2%) had <1 %. Among 217 HCC patients in the surgery alone group, 52 (24.0%) had ≥5% PD-L1 expression, 54 (24.9%) showed 1–5%, and 111 (51.1%) exhibited <1 %. No significant difference in PD-L1 expression was detected between the two groups (p = 0.96, Table 1).
Figure 2.
Immunohistochemical analysis of PD-L1 expression in primary hepatocellular carcinoma surgical specimens. Positive staining results are reported as the percentage of tumor cells showing membranous PD-L1 expression (frequency). (A and D) PD-L1 expression was scored as <1%, (B and E) PD-L1 expression was scored as 1–5%, (C and F) PD-L1 expression was scored as ≥5%. PD-L1 staining is indicated by the presence of brown chromogen. Blue represents the hematoxylin counterstain (A–C, 100× magnification; D–F, 400× magnification).
Associations between PD-L1 expression on HCC cells and survival benefits from adjuvant CIK cell immunotherapy
Although we demonstrated significantly improved rates of OS and RFS in HCC patients who received CIK cell treatment, not all patients can benefit from adjuvant CIK cell immunotherapy. We hypothesized that PD-L1 expression in HCC tumors may be associated with survival benefits of patients who received adjuvant CIK cell immunotherapy. Thus, using 12 mo of RFS as a cutoff value, we divided all patients in the CIK cell treatment group into long-term survival benefit and minimal survival benefit subgroups, and then investigated the potential correlation of PD-L1 expression with the survival benefit associated with adjuvant CIK treatment.
There was a significant difference in levels of PD-L1 expression between the long-term survival benefit and minimal survival benefit subgroups (p = 0.034; Fig. 3A). Because significant differences between the two subgroups might be affected by certain clinicopathological parameters, such as the tumor capsule (p = 0.039), tumor size (p = 0.008), TNM staging (p < 0.001), tumor number (p = 0.006), and AFP (p = 0.006). To eliminate these inherent biases, a propensity score weighting method was used to normalize all covariates between the two subgroups (Table 2).Then, potential association between PD-L1 expression and the survival benefit of CIK cell treatment was again analyzed. As expected, the raw and normalized results were consistent (p = 0.009; Fig. 3B).
Figure 3.
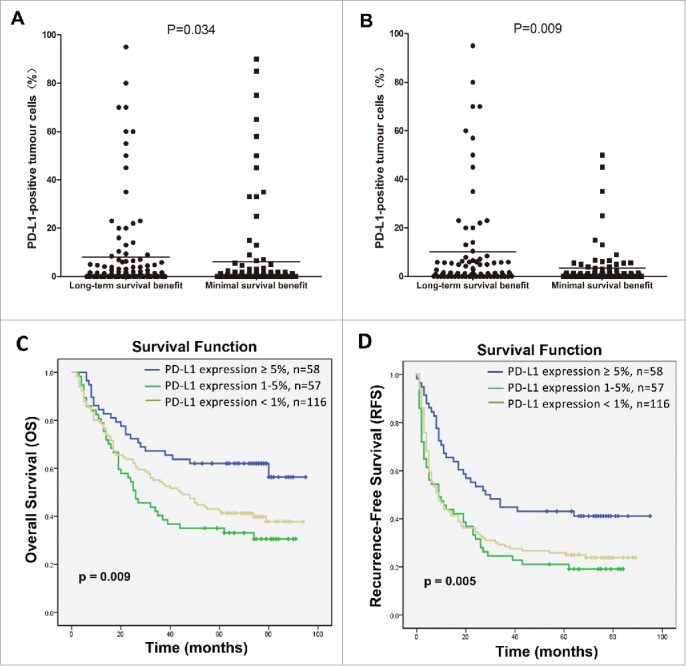
Correlation of PD-L1 expression by HCC cells with the survival benefit from adjuvant CIK cell immunotherapy. Differences in levels of PD-L1 expression between patients showing a long-term versus a minimal survival benefit are shown before (A) and after (B) the propensity score weighting method was used to balance all covariates. C and D depicts the Kaplan–Meier curves for overall survival (OS) and recurrence-free survival (RFS) in the CIK cell treatment group for patients with ≥5%, 1–5% and <1 % PD-L1 expression, respectively.
Table 2.
Demographics and clinical characteristics of patients according to the survival benefit from CIK cell therapy before and after use of PS weighting.
| Clinicopathological variables | Original |
PS weight |
||||
|---|---|---|---|---|---|---|
| Minimal survival benefit (n = 115) | Long-term survival benefit (n = 116) | p value | Minimal survival benefit (n = 83) | Long-term survival benefit (n = 83) | p value | |
| Age (y) | 0.626 | 0.377 | ||||
| Median | 46 | 48 | 46 | 43 | ||
| Range | 25–80 | 14–70 | 28–77 | 14–70 | ||
| Gender | 0.640 | 0.393 | ||||
| Male | 99 | 98 | 68 | 72 | ||
| Female | 18 | 21 | 15 | 11 | ||
| Capsule | 0.039 | 0.822 | ||||
| Complete | 94 | 107 | 71 | 72 | ||
| Incomplete | 23 | 12 | 12 | 11 | ||
| Tumor size (cm) | 0.008 | 0.347 | ||||
| ≤5 | 38 | 59 | 33 | 39 | ||
| >5 | 79 | 60 | 50 | 44 | ||
| TNM stage | <0.001 | 0.791 | ||||
| I | 54 | 84 | 51 | 55 | ||
| II | 12 | 15 | 10 | 8 | ||
| III | 51 | 20 | 22 | 20 | ||
| Tumor number | 0.006 | 0.431 | ||||
| Single | 83 | 102 | 65 | 69 | ||
| Multiple | 34 | 17 | 18 | 14 | ||
| Histological differentiation | 0.104 | 0.684 | ||||
| Well | 17 | 27 | 15 | 19 | ||
| Moderate | 67 | 70 | 50 | 45 | ||
| Poor | 33 | 22 | 18 | 19 | ||
| HBsAg | 0.332 | 0.659 | ||||
| Positive | 97 | 104 | 70 | 72 | ||
| Negative | 20 | 15 | 13 | 11 | ||
| AFP (ng/mL) | 0.006 | 0.475 | ||||
| Median | 460.3 | 100 | 293 | 255 | ||
| Range | 1–1,21,000 | 0–1,21,000 | 1–1,21,000 | 0–1,21,000 | ||
HBsAg, Hepatitis B surface antigen; AFP, α-fetoprotein.
Moreover, survival analysis showed that ≥5% PD-L1 expression was significantly correlated with improved rates of OS and RFS compared with patients showing 1–5% expression or <1 % expression (p = 0.009 and p = 0.005, respectively; Figs. 3C and D). Effects of PD-L1 expression on the survival benefit of CIK cell treatment were further evaluated in univariate and multivariate analyses. PD-L1 expression showed a significant correlation with improved OS and RFS benefits associated with CIK cell treatment in univariate analyses (Table 3 and Table 4), and also represented an independent prognostic factor for the survival of patients who received CIK cell treatment in multivariate analysis (Table 3 and Table 4).
Table 3.
Univariate and multivariate analysis of overall survival (OS) in HCC patients who received adjuvant CIK cell immunotherapy.
| Variables | Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age (≤50 vs. >50) | 1.188 | 0.843–1.676 | 0.326 | |||
| Gender (Male vs. Female) | 1.020 | 0.633–1.641 | 0.937 | |||
| Capsule (Complete vs. Incomplete) | 2.504 | 1.662–3.770 | <0.001 | 1.189 | 0.754–1.876 | 0.456 |
| Tumor size (cm) (≤5 vs. >5) | 2.172 | 1.496–3.152 | <0.001 | 1.151 | 0.755–1.755 | 0.514 |
| TNM stage (I vs. II vs. III) | 2.107 | 1.748–2.540 | <0.001 | 1.952 | 1.492–2.553 | <0.001* |
| Tumor number (Single vs. Multiple) | 2.690 | 1.856–3.898 | <0.001 | 0.899 | 0.563–1.436 | 0.655 |
| Histological differentiation (Well vs. Moderate vs. Poor) | 1.590 | 1.220–2.072 | 0.001 | 1.276 | 0.945–1.722 | 0.111 |
| HBsAg (Positive vs. Negative) | 0.786 | 0.488–1.265 | 0.322 | |||
| AFP (ng/mL) (<400 vs. ≥400) | 1.747 | 1.239–2.462 | 0.001 | 1.313 | 0.969–1.778 | 0.079 |
| PD-L1 (<5% vs. ≥5%) | 0.527 | 0.336–0.827 | 0.005 | 0.526 | 0.329–0.839 | 0.007* |
HR, hazard ratio; CI, confidence interval; HBsAg, Hepatitis B surface antigen; AFP, α-fetoprotein.
Statistically significant, p < 0 .05.
Table 4.
Univariate and multivariate analysis of recurrence-free survival (RFS) in HCC patients who received adjuvant CIK cell immunotherapy.
| Variables | Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | ||
| Age (≤50 vs. >50) | 1.183 | 0.872–1.605 | 0.279 | ||||
| Gender (Male vs. Female) | 0.954 | 0.624–1.460 | 0.829 | ||||
| Capsule (Complete vs. Incomplete) | 1.821 | 1.230–2.696 | 0.003 | 0.911 | 0.591–1.406 | 0.675 | |
| Tumor size (cm) (≤5 vs. >5) | 1.988 | 1.439–2.747 | <0.001 | 1.331 | 0.937–1.892 | 0.110 | |
| TNM stage (I vs. II vs. III) | 1.818 | 1.536–2.152 | <0.001 | 1.723 | 1.371–2.165 | <0.001* | |
| Tumor number (Single vs. Multiple) | 2.100 | 1.490–2.960 | <0.001 | 0.904 | 0.589–1.388 | 0.645 | |
| Histological differentiation (Well vs. Moderate vs. Poor) | 1.386 | 1.099–1.749 | 0.006 | 1.206 | 0.936–1.554 | 0.146 | |
| HBsAg (Positive vs. Negative) | 0.807 | 0.527–1.234 | 0.807 | ||||
| AFP (ng/mL) (<400 vs. ≥400) | 1.518 | 1.120–2.057 | 0.007 | 1.258 | 0.936–1.554 | 0.146 | |
| PD-L1 (<5% vs. ≥5%) | 0.556 | 0.381–0.811 | 0.002 | 0.572 | 0.388–0.845 | 0.005* | |
HR, hazard ratio; CI, confidence interval; HBsAg, Hepatitis B surface antigen; AFP, α-fetoprotein.
Statistically significant, p <0 .05.
To exclude the impact of PD-L1 on the survival of patients with HCC, we also investigated the expression levels of PD-L1 and their potential association with survival in HCC patients who received hepatectomy alone (surgery alone group). Survival analysis showed that PD-L1 expression does not exert a direct effect on the rates of OS and RFS in patients in the surgery alone group (p = 0.298 and p = 0.174, respectively; Fig. 4). In univariate analyses, PD-L1 expression was also not associated with survival benefit in the patients (Tables S1 and 2). These findings indicated that PD-L1 was not related with the survival of patients with HCC, and the difference in levels of PD-L1 expression between the long-term survival benefit and minimal survival subgroups could be attributed to CIK cell treatment.
Figure 4.
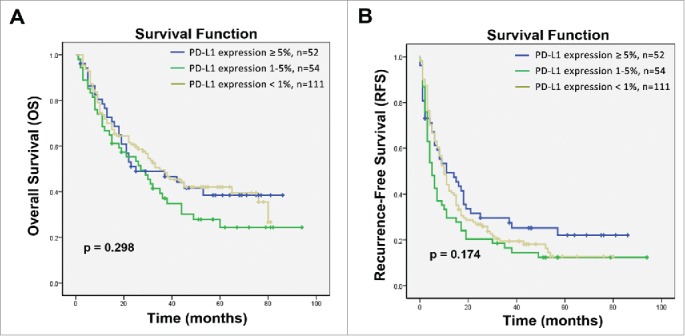
Kaplan–Meier curves for HCC patients who received hepatectomy alone based on expression of PD-L1 on tumor cells. (A) Overall survival (OS) and (B) recurrence-free survival (RFS) curves are shown. No correlation with PD-L1 expression was observed with the OS of RFS rates of patients with HCC; p values were calculated using the log-rank test.
PD-L1 expression can predict the clinical benefit of adjuvant CIK cell immunotherapy for HCC patients
To determine whether PD-L1 expression was associated with clinical benefits of adjuvant CIK cell immunotherapy, all patients, including the CIK treatment and surgery alone groups, were divided into ≥5%, 1–5%, and <1 % subgroups according to the level of PD-L1 expression. The survival benefits between the CIK treatment and surgery alone groups were compared. In the ≥5% subgroup, the OS and RFS for patients in the CIK treatment group were significantly prolonged compared with those of patients in the surgery alone group (Fig. S2A). The median OS and RFS were 65 and 28 mo for patients in CIK treatment group compared with 25 and 11 mo for patients in surgery alone group (p = 0.016 and p = 0.010, respectively). However, in the 1–5% and <1 % subgroups, there were no significant differences in either OS or DFS between the CIK treatment and surgery alone groups (Figs. S2B and C, respectively). These data indicated that HCC patients with ≥5% PD-L1 expression were more likely to benefit from adjuvant CIK cell immunotherapy.
Correlation of PD-L1 expression with immunological status to HBV and its impact on the effects of CIK treatment
Because most patients in this study were chronically infected with HBV, which is well known to be associated with chronic inflammation and immune response in HCC microenvironment, we subsequently investigated whether PD-L1 expression was associated with immunological status (HBsAg, anti-HBsAb, HBeAg, anti-HBeAb, anti-HBcAb, viral load) to HBV. Correlation analysis suggested that PD-L1 expression was positively correlated with hepatitis B viral load (p = 0.017) (Table 5). We further assess the effects of PD-L1 expression on survival benefits from CIK cell immunotherapy stratified by the viral load. In the high viral load subgroup (≥1,000 copy/mL), high PD-L1 expression was significantly correlated with improved OS and RFS benefit from CIK cell treatment compared with low PD-L1 expression (p = 0.004 and p = 0.001, respectively; Fig. 5A), but PD-L1 expression did not significantly affect the OS and RFS of the patients who received CIK cell infusion in the low viral load subgroup (<1,000 copy/mL) (p = 0.385 and p = 0.463, respectively; Fig. 5B).
Table 5.
Correlation of PD-L1 expression and immunological status to HBV.
| Variables | ≥5 % PD-L1 expression (n = 110) | 1–5% PD-L1 expression (n = 111) | <1% PD-L1 expression (n = 227) | p value |
|---|---|---|---|---|
| HBsAg | ||||
| Positive | 99 | 102 | 196 | 0.279 |
| Negative | 11 | 9 | 31 | |
| Anti-HBsAb | 0.332 | |||
| Positive | 5 | 3 | 4 | |
| Negative | 105 | 108 | 223 | |
| HBeAg | 0.576 | |||
| Positive | 16 | 18 | 43 | |
| Negative | 94 | 93 | 184 | |
| Anti-HBcAb | 0.339 | |||
| Positive | 101 | 107 | 211 | |
| Negative | 9 | 4 | 16 | |
| Anti-HBeAb | 0.651 | |||
| Positive | 83 | 82 | 161 | |
| Negative | 27 | 29 | 66 | |
| Viral load (copy/mL) |
0.017 * | |||
| ≥1,000 | 70 | 59 | 107 | |
| <1,000 | 40 | 52 | 120 |
Statistically significant, p <0 .05.
Figure 5.
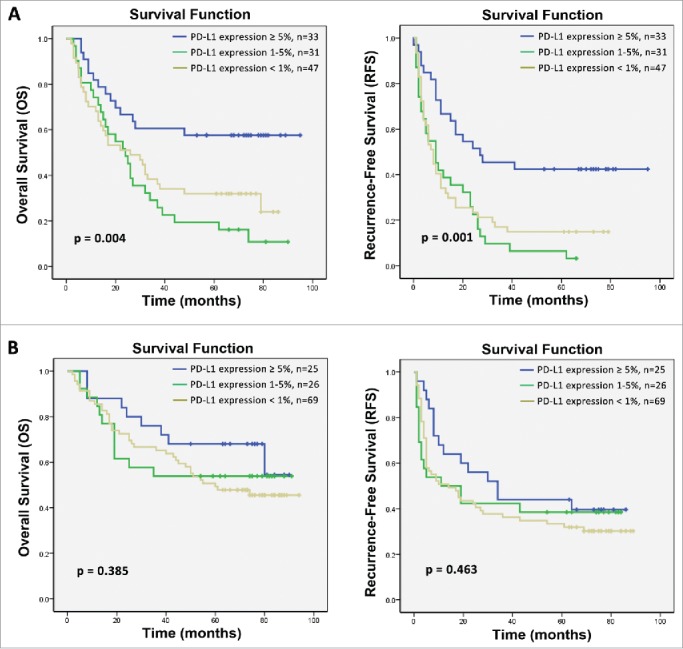
Subgroup analysis to estimate the impact of PD-L1 expression on survival benefits of additional CIK treatment according to hepatitis B viral load. (A) OS and RFS curves for patients with high hepatitis B viral load. (B) OS and RFS curves for patients with low hepatitis B viral load.
Relationship between PD-L1 expression on tumor cells and localized inflammatory response to HCC
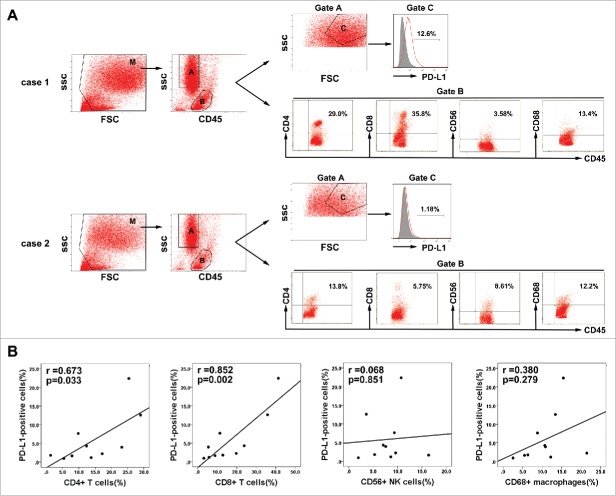
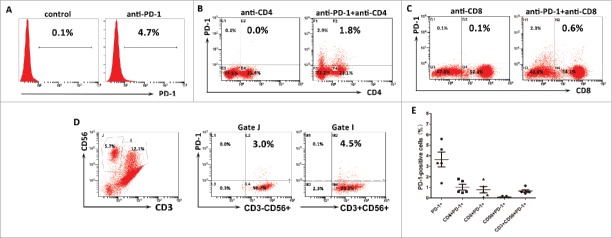
The association of PD-L1 expression with hepatitis B viral load which can induce intrahepatic immune reaction suggested that PD-L1 expression might relate to immunological responses to HBV-associated HCC. To test this hypothesis, we measured the PD-L1 expression on tumor cells and the proportion of tumor-infiltrating inflammatory cells by flow cytometry in 10 fresh HBV-associated HCC tumor tissues. Gating strategies were used for the flow cytometric analysis of the disaggregated tumor samples. Tumor cells were identified based on CD45− and light scatter (FSC/SSC), which were subsequently measured for PD-L1 expression. Tumor-infiltrating leukocytes were gated on the basis of CD45+ and FSC/SSC, which were subsequently assessed for CD4+ T cells, CD8+ T cells, CD56+NK, or CD68+ macrophages(Fig. 6A). We detected a higher percentage of infiltrating CD4+ T cells and CD8+ T cells in the tumor tissues with high PD-L1 expression on tumor cells, but the proportion of infiltrating CD56+NK cells and CD68+ macrophages were not associated with the PD-L1 expression (Fig. 6B).These results indicated that indeed PD-L1 expression marked the existence of adaptive immune response (tumor-infiltrating lymphocytes) in HCC microenvironment. To determine whether PD-L1 expression on tumor would inhibit the antitumor activity of CIK cells by binding to PD-1, we assess the ratio of PD-1 positive cells in the autologous CIK cells of five HCC patients by flow cytometry. Results showed that the ratio of PD-1+ cells among the populations of CIK cells ranged from 1.4 to 5.6% with a median of 3.3%; the ratio of CD4+PD-1+ subset ranged from 0.5 to 1.8% with a median of 0.6%; the ratio of CD8+PD-1+ subset ranged from 0.1 to 1.8% with a median of 0.6%; the ratio of CD56+ PD-1+ subset ranged from 0.01 to 0.12% with a median of 0.04%; and the ratio of CD3+CD56+ PD-1+ subset ranged from 0.42 to 1.16% with a median of 0.58% (Fig. 7).
Figure 6.
Association between PD-L1 expression on tumor cells and infiltration of inflammatory cells in HCC microenvironment. (A) Gating routine for the identification of tumor cells and CD45+ cells is shown. Flow cytometric analysis was performed to measure PD-L1 expression on tumor cells(gate C) and CD4+, CD8+, CD56+, and CD68+ cell subsets gating on the CD45+fraction(gate B). Significantly higher percentages of CD4+ T cells and CD8+ T cells were observed in the HCC tumor tissues with high PD-L1 expression (case 1) compared to that with low PD-L1 expression (case 2). (B) A summary of correlation analysis was shown that PD-L1 positive tumor cells in tumor tissues significantly correlated with tumor-infiltrating CD4+ and CD8+ T cells, but not associated with CD56+ NK cell and CD68+ macrophage (n = 10).
Figure 7.
PD-1 expression on the autologous CIK cells. (A) The percentages of PD-1 positive cells among the populations of CIK cells. (B) The percentages of CD4+PD-1+ CIK cells. (C) The percentages of CD8+PD-1+ CIK cells. (D) The percentages of CD3−CD56+ PD-1+ and CD3+CD56+ PD-1+ CIK cells. (E) PD-1 expression on each subgroup of CIK cells in five HCC patients. These data suggested CIK cells exhibited a low PD-1 expression.
Discussion
Consistent with various previous studies that suggested that the addition of adjuvant CIK cell treatment to hepatectomy could reduce tumor recurrence and improve the survival benefit of HCC patients,26,27 our present study also showed that HCC patients who received CIK cell-based immunotherapy after surgery exhibited a more favorable prognosis. Previously, we used several clinicopathological characteristics of HCC patients to predict the survival benefits of CIK cell treatment,14 but the immunological factors related to the tumor microenvironment that might represent additional prognostic variables were not included. Thus, we decided to estimate potential correlations between PD-L1 expression by HCC tumor cells with the survival benefits of adjuvant CIK cell immunotherapy.
Expression of PD-L1 in surgically resected HCC tissue specimens could be detected on plasma membrane of cancer cells, and it significantly affected the OS and RFS of patients who received adjuvant CIK cell immunotherapy. However, no statistically significant association between PD-L1 expression and the prognosis of patients who underwent surgery alone was detected. Furthermore, we found that patients with ≥5% PD-L1 expression had a more favorable survival benefit from post-operative CIK cell treatment compared with surgery alone. By contrast, another patients with <5% PD-L1 expression may derive some benefit from adjuvant CIK cell treatment, but this benefit would not be statistically significant. These results indicated that our analyses of PD-L1 expression permitted the identification of patients who showed an enhanced likelihood of positively responding to CIK cell-based immunotherapy.
In this present study, we did not detect a direct link between PD-L1 expression and the prognosis of HCC patients in the hepatectomy alone group. With the exception of previous studies of primary invasive melanomas,24 non-small cell lung cancer,28 and cervical cancer,29 this finding differs from many studies of patients with other tumor types, which have suggested that PD-L1 expression was a negative overall prognostic factor.30-32 Additionally, several reports have claimed that the overexpression of PD-L1 was associated with a poor prognosis in patients with HCC.33,34 These conflicting findings may be a consequence of the use of different detection antibodies, staining patterns of PD-L1 expression, or staining protocols that were used. For example, Gao et al.33 used mouse anti-human PD-L1 antibody (eBioscience) for IHC detection; they did not distinguish between membranous and cytoplasmic patterns of PD-L1 expression in HCC tumor cell and they compared “high” to “low” expression of PD-L1 based on the areas and color densities that were calculated using a digital image system. By contrast, our study used a rabbit monoclonal antibody (Cell Signaling Technology), and we developed a scoring system based on a 5% threshold of cell membrane expression for a positive result; 25 we only scored a few (25%) HCC tissues as PD-L1 positive using these criteria.
Nevertheless, we detected a significant correlation between higher PD-L1 expression and improved survival in HCC patients who received post-operative CIK cell transfusion, indicating that PD-L1 expression may represent a predictive biomarker that could imply the benefit of CIK cell immunotherapy. The development and progression of HCC is known to be associated with persistent and chronic viral inflammation.35 Once a neoplasm is established, many mutual interactions between tumor cells and the tumor microenvironment occur during chronic inflammation that may create more favorable conditions for tumor cell survival.36,37 The plasma membrane expression of the immunosuppressive molecule PD-L1 on tumor cells is involved in the PD-L1/PD-1 immune checkpoint. When engaged, this checkpoint inhibits the activation and/or proliferation of tumor-specific T cells, including CD4+ T cells and/or CD8+ T cells. Hence, antitumor T cell responses are diverted into a state of exhaustion or anergy, resulting in the impairment of major histocompatibility complex (MHC)-restricted cytotoxic immunity.38,39 Non-MHC-restricted-directed cytotoxic effector cells, here termed CIK cells, may potentially overcome the aforementioned limitations of cytotoxic immune responses against HCC and they may act via a perforin-dependent cell killing, Fas–Fas ligand interactions, and the production of a large amount of tumoricidal cytokines.40 We hypothesize that higher PD-L1 expression on tumor cells indicates that mechanisms exist to thwart the MHC-restricted antitumor immune response, and that CIK cells might be better poised to induce a productive tumor-specific T cell-mediated response and consequently exert more potent treatment effects for patients. Moreover, our findings showed that only approximately 3% of autologous CIK cells exhibited PD-1 expression, indicating CIK cells would not be induced into exhaustion or anergy by PD-L1/PD-1 pathway.
Additionally, we found that only patients with ≥5% PD-L1 expression could obtain an improved survival benefit from adjuvant CIK cell treatment, suggesting that PD-L1 expression could identify HCC patients who would be more responsive to adjuvant CIK cell treatment. Indeed, accumulating literature suggests the existence of a positive relationship between inflamed tumors that express PD-L1 and respond to immunotherapies, such as high-dose IL-2, tumor vaccination, or PD-1 blockade, and identifies PD-L1 overexpression as a novel prognostic biomarker for cancer immunotherapy.25,41 Although PD-L1 is an immunosuppressive molecule and has been considered to be an independent predictor of poor prognosis in many tumor types, emerging preclinical and clinical findings support an adaptive immune resistance hypothesis for PD-L1 expression, which suggests that PD-L1 is adaptively induced as a consequence of immune responses to tissue inflammation.42 This hypothesis intrinsically implies that PD-L1 expression reflects the process of immunosurveillance or endogenous antitumor immunity within the tumor microenvironment. Consistent with these findings and hypothesis, our study also revealed a strong association between tumor expression of PD-L1and the presence of tumor-infiltrating lymphocytes (CD8+ T-cells and CD4+ T-cells) in HCC microenvironment. Furthermore, we detected a significant correlation between PD-L1 expression and hepatitis B viral load which is viewed as inducing a host immune response. Previous studies have provided evidence to show that the proportion CD8+ T-cells was higher in patients with high hepatitis B viral load.43,44 Thus, PD-L1 expression may represent an endogenous adaptive immunity (infiltrating lymphocytes in response to tumor-selective or HBV-specific antigens).Taube et al.24 argued that patients with higher levels of membranous expression of PD-L1 might be more likely to respond to immunotherapies, which should be interpreted as protection or potentiation of ongoing immunity rather than the generation of de novo antitumor immunity. Indeed, as an adoptive immunotherapy, CIK cells can both recognize and kills tumor targets via a MHC-unrestricted, TCR-independent mechanism, but also can activate antitumor immune responses and enhance immune function by secreting various cytokine, such as interferon (IFN) γ, tumor necrosis factor (TNF)-α, IL-2, and granulocyte-macrophage colony-stimulating factor (GM-CSF).45 Therefore, we believe the HCC patients with high PD-L1 expression had an active antitumor or antiviral immunity and hence they are more susceptible to CIK cell-based immunotherapy which is viewed as protection or potentiation of ongoing immunity. These also explained why the survival benefit of CIK cells treatment is more susceptible to PD-L1 expression in the patients with high hepatitis B viral load.
However, since PD-L1 expression can be induced either constitutively or upon tumor-specific T cell recognition and production of interferons,46 Tumeh et al.47 have found that clinically relevant antitumor activity following therapeutic PD-1 blockade requires pre-existing tumor antigen-restricted T cells and that responses to PD-1 blockade would more tightly upregulate the inducible PD-L1 expression in the presence of tumor-specific T cells. Indeed, as a cancer immunotherapy and a potentiation of ongoing immunity, CIK cells treatment would also change the PD-L1 expression in the tumor microenvironment. Thus, in terms of CIK cell-based immunotherapy outcome, the clinical relevance of PD-L1 expression on tumor cells, activated T cells and CIK cells in tumors remains to be elucidated.
In conclusion, in this single-institution study, we confirmed the expression of PD-L1 in tumor tissue specimens from patients with HCC and demonstrated that PD-L1 expression was not significantly associated with the prognoses of HCC patients. Importantly, our data provide the first clinical evidence linking PD-L1 expression on tumor cells with OS and RFS in HCC patients who received CIK cell treatment, suggesting that PD-L1 expression might represent a predictive biomarker for identifying patients with an enhanced likelihood of exhibiting a survival benefit from adjuvant CIK cell treatment. External validation and prospective randomized studies are warranted to further establish our present findings.
Materials and methods
Case selection
Between December 2001 and May 2009, the medical records of patients with HCC stored in a computerized database at Sun Yat-Sen University Cancer Center were reviewed. This database accurately recorded the clinicopathological data of patients with HCC and included details about gender, age, tumor characteristics, TNM-stage, blood biochemistry, treatment, and outcomes. A diagnosis of HCC was made when at least two radiological images exhibiting the characteristic features of HCC, or one imaging technique showed positive findings together with either α-fetoprotein (AFP) levels ≥400 ng/mL or cytological/histological evidence. All of the HCC patients underwent curative hepatectomy if they had histopathologically confirmed HCC, hepatic functions of Child-Pugh were class A or B, adequate baseline cardio-pulmonary and renal function, the Karnofsky Performance scores were over 90, and no clinical symptoms or signs of sepsis. After surgical treatment, a subpopulation of the patients received at least four cycles of post-operative CIK cell immunotherapy if they had no post-operative dysfunction in any organ, no systemic immunosuppressive therapy, no active autoimmune disease, and no occurrence of serious adverse events during CIK cell immunotherapy. Patients were excluded from this retrospective study based on the following criteria: a history of other malignancies, previous anticancer treatment, with distant metastasis before operation, or receiving CIK treatment after recurrence. After the review process, 1,181 HCC patients met the described criteria and were included continuously in the patient cohort. Among them, 575 patients underwent hepatectomy alone (surgery alone group), whereas the other 606 patients received hepatectomy and post-operative CIK cell immunotherapy (CIK treatment group). For further studies, random number table method was then used to select a half of patients (288 and 303) from surgery alone group and CIK treatment group, respectively. Because a proportion of patients lacked of their paraffin-embedded tumor tissue, 217 patients from surgery alone group and 231 patients from CIK treatment group were finally included for further immunohistochemical study and reviewing retrospectively. To further exclude selection bias, clinicopathological variables and survival were compared between the total patients and the included patients. No statistically significant differences between the two groups were detected for all parameters that we tested (p ≥ 0.05, Table S3).In the CIK cell treatment group, patients with a RFS greater than 12 mo were defined as long-term survival benefit patients, whereas the other cases with a RFS of less than 12 mo were defined as minimal survival benefit patients. All patients in this study provided written informed consent before being subjected to tumor tissue samples. This study was performed in accordance with the Declaration of Helsinki and according to national and international guidelines, and was also approved by the Research Ethics Committee in Sun Yat-sen University Cancer Center (GZR2015-030).
CIK cell preparation and treatment
CIK cell-based immunotherapy is observational clinical treatment in our hospital. It was approved by the Ethics Committee of Sun Yat-Sen University Cancer Center (Guangzhou, China), and the written informed consent was obtained from each patient. The generation and treatment of autologous CIK cells were performed as we described previously.12,48 Briefly, heparinized peripheral blood was obtained from HCC patients after hepatectomy over a two-week period. PBMCs were separated by Ficoll–Hypaque gradient centrifugation, suspended in X-VIVO 15 serum-free medium (Longza, Shanghai, China), and culture with 1,000 U/mL rhIFNγ (Clone-gamma, Shanghai Clone Company, Shanghai, China) for the first 24 h, followed by stimulation with 100 ng/mL mouse anti-human CD3 monoclonal antibody (R&D Systems, Shanghai, China), 1,000 U/mL rhIL-2 (Beijing Sihuan, Beijing, China), and 100 U/mL IL-1α (Life Technologies, Guangzhou, China) to activate the CIK cells. Fresh medium containing 1,000 U/mL rhIL-2 was added every 2 d and the cell density was maintained at 2 × 106 cells/mL. CIK cells were harvested, washed, and resuspended after culture for 14 d. Before cell transfer, a fraction of the CIK cells were collected to assess their number, phenotype, and viability (by the dye exclusion test) of cells, and to test for possible contamination by bacteria, fungi, or endotoxins. Then, autologous CIK cells (range, 1.0–1.5 × 1010 cells) were transferred to patients via intravenous infusion. Generally, patients received at least four cycles of CIK cell treatment at two-week intervals. The CIK cell treatment assignment procedure was showed in Fig. S3. If the patients were disease-stable, they were eligible for additional cycles of CIK maintenance treatment following the procedure described above. Otherwise, the CIK therapy was stopped when the disease is progression or the patients did not want to continue.
Follow-up
All post-operative patients underwent regular follow-up at our outpatient department or follow-up center. During the follow-up period, which included clinical and laboratory examinations along with phone calls, subjects were contacted every 3 mo for the first 2 y, every 6 mo from years 3 to years 5, and annually thereafter. Serum levels of AFP, abdominal ultrasonography, liver function tests, and chest radiography were obtained at each follow-up visit. Additional assessments that consisted of chest computed tomography, positron emission tomography, biopsy, or second hepatectomy were performed when tumor recurrence was suspected or if the recurrent tumor was resectable. OS and RFS were used as the primary end points of interest in this study. OS was defined from the date of surgery to either the date of death or the last follow-up. RFS was defined as the time from surgery to the time of the first detectable recurrence (local or distant) or the date of the last follow-up. Apart from a second hepatectomy, other treatments for recurrent tumors were determined by our multidisciplinary team that included hepatobiliary surgeons, physicians, and radiologists.
Tumor tissue samples and immunohistochemical analysis of PD-L1 expression
All of the 448 patients had tumor tissue samples that could be evaluated by immunohistochemistry. Samples were confirmed by pathological examination, fixed in 10% formalin, and embedded in paraffin. Formalin-fixed, paraffin-embedded (FFPE) tissue sections of 3-µm thickness were cut for immunohistochemical analysis. Sections were deparaffinized in xylene and rehydrated with graded ethanol. Antigen retrieval was performed by immersing sections in EDTA (1 mmol/L, pH 9.0), which were then boiled for 15 min in a microwave oven. After rinsing with PBS, endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide for 10 min, followed by blocking nonspecific staining with goat serum for 30 min. Slides were incubated with a rabbit anti-human PD-L1 mAb at 1:200 dilution (E1L3N, Cell Signaling Technology) overnight in a humidified chamber at 4°C and then were incubated with horseradish peroxidase-conjugated secondary antibody (EnvisionTM Detection Kit, GK500705, Gene Tech) for 30 min after washing three times with PBS. Finally, 3,3′-diaminobenzidine tetrahydrochloride (DAB) was used to develop positive signals, and sections were counter-stained with hematoxylin. Finally, slides were dehydrated, cleared, and evaluated.
Sample disaggregation and flow cytometry
Single-cell suspensions were prepared from 10 fresh HBV-associated HCC tumor tissues obtained from patients who underwent hepatectomy. In brief, tissues were minced into small fragments and digested by incubation for 2 h at 37°C in RPMI 1640 medium containing 10% collagenase I (Sigma-Aldrich Corp, St Louis, MO, USA). After washings in medium plus 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA), the cell suspension was forced through a graded series of cell strainers to separate the cell components from stroma and aggregates. Finally, the isolated single-cells from tissue samples were stained with anti-CD45-APC-CY7,anti-PD-L1-PE, anti-CD4+- FITC, anti-CD8+-PE-CF594, anti-CD56- PE-Cy7 and anti-CD68-PE-Cy5 (all from BD Biosciences, San Diego, CA, USA) and analyzed by flow cytometry.
Phenotype and PD-1 expression analysis of CIK cell
The phenotype and PD-1 expression of the autologous CIK cells from HCC patients was characterized by flow cytometry using anti-CD3-PE-Cy5, anti-CD4+- FITC, anti-CD8+-PE-CF594, anti-CD56- PE-Cy7, and anti-PD-1-PE (all from BD Bioscicence). The ratio of each subgroup (CD3+CD4+PD-1+, CD3+CD8+PD-1+, CD3+CD56+PD-1+, and CD3−CD56+PD-1+) was calculated according to the cell density of each gate.
Statistical analysis
Student's t-test, the Pearson χ2 test, and Fisher's exact test were used to evaluate differences in demographic and clinical variables of the patients. Mann–Whitney test was used to compare PD-L1 expression levels. OS and RFS rates were evaluated using the Kaplan–Meier method and were compared using the log-rank test. Univariate and multivariate regression analyses were performed using a Cox regression model. To reduce some of the inherent bias that could affect the RFS rates of patients, we used a propensity score weighting method to balance observed covariates between the long-term survival benefit and minimal survival benefit patients. All statistical analyses were performed using SPSS version 20.0 (SPSS Inc., Chicago, IL, USA) and the R software package (http://www.r-project.org/). All tests were two-sided with a statistical significance threshold of p < 0.05 used to denote statistically significant differences.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was primarily supported by a grant from the National Natural Science Foundation of China (31270964 and 81572865) and the key project of Natural Science Foundation of Guangdong Province (S2013020012722), and was partially supported by Guangdong Province Science and Technology Plan Project (2012A030400059).
References
- 1.Knox JJ, Cleary SP, Dawson LA. Localized and Systemic Approaches to Treating Hepatocellular Carcinoma. J Clin Oncol 2015; 33:1835-44; PMID:25918289; http://dx.doi.org/ 10.1200/JCO.2014.60.1153 [DOI] [PubMed] [Google Scholar]
- 2.Alejandro F, Josep ML, Jordi B. Hepatocellular Carcinoma. Lancet 2012; 379:1245-55; PMID:22353262; http://dx.doi.org/ 10.1016/S0140-6736(11)61347-0 [DOI] [PubMed] [Google Scholar]
- 3.Jacques F, Hai-Rim S, Freddie B, David F, Colin M, Donald MP. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893-917; PMID:21351269; http://dx.doi.org/ 10.1002/ijc.25516 [DOI] [PubMed] [Google Scholar]
- 4.Ulahannan SV, Duffy AG, McNeel TS, Kish JK, Dickie LA, Rahma OE, McGlynn KA,. Greten TF, Altekruse SF. Earlier presentation and application of curative treatments in hepatocellular carcinoma. Hepatol 2014; 60:1637-44; PMID:24996116; http://dx.doi.org/ 10.1002/hep.27288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A et al.. Sorafenib in Advanced Hepatocellular. N Engl J Med 2008; 359:378-90; PMID:18650514; http://dx.doi.org/ 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 6.Hashem B, El Serag. Hepatocellular carcinoma. N Engl J Med 2011; 365:1118-27; PMID:21992124; http://dx.doi.org/ 10.1056/NEJMra1001683 [DOI] [PubMed] [Google Scholar]
- 7..Ochoa AC, Gromo G, Alter BJ, Sondel PM, Bach FH.. Long-term growth of lymphokine-activated killer (LAK) cells: role of anti-CD3, β-IL 1, interferon-gamma and -β. J Immunol 1987; 138:2728-273; PMID:2435804 [PubMed] [Google Scholar]
- 8.Verneris MR, Ito M., Baker J, Arshi A, Negrin RS, Shizuru JA. Engineering hematopoietic grafts: purified allogeneic hematopoietic stem cells plus expanded CD8+ NK-T cells in the treatment of lymphoma. Biol Blood Marrow Transplant 2001; 7:532-42; PMID:11760085; http://dx.doi.org/ 10.1016/S1083-8791(01)70014-6 [DOI] [PubMed] [Google Scholar]
- 9.Kim JS, Kim YG, Pyo M, Lee HK, Hong JT, Kim Y, Han SB. Adoptive Cell Therapy of Melanoma with Cytokine-induced Killer Cells. Immune Netw 2015; 15:58-65; PMID:25922594; http://dx.doi.org/ 10.4110/in.2015.15.2.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y et al.. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000; 356:802-807; PMID:11022927; http://dx.doi.org/ 10.1016/S0140-6736(00)02654-4 [DOI] [PubMed] [Google Scholar]
- 11.Hui D, Qiang L, Jian W, Ti Z, Da-Lu K. A randomized, controlled trial of postoperative adjuvant cytokine-induced killer cells immunotherapy after radical resection of hepatocellular carcinoma. Dig Liver Dis 2009; 41:36-41; PMID:18818130; http://dx.doi.org/ 10.1016/j.dld.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 12.Pan K, Li YQ, Wang W, Xu L, Zhang YJ, Zheng HX, Zhao JJ, Qiu HJ, Weng DS, Li JJ et al.. The efficacy of cytokine-induced killer cell infusion as an adjuvant therapy for postoperative hepatocellular carcinoma patients. Ann Surg Oncol 2013; 20:4305-11; PMID:23892527; http://dx.doi.org/ 10.1245/s10434-013-3144-x [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015; 148:1383-1391; PMID:25747273; http://dx.doi.org/ 10.1053/j.gastro.2015.02.055 [DOI] [PubMed] [Google Scholar]
- 14.Pan QZ, Wang QJ, Dan JQ, Pan K, Li YQ, Zhang YJ, Zhao JJ, Weng DS, Tang Y, Huang LX et al.. A nomogram for predicting the benefit of adjuvant cytokine-induced killer cell immunotherapy in patients with hepatocellular carcinoma. Sci Rep 2015; 5:9202; PMID:25776856; http://dx.doi.org/ 10.1038/srep09202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao L, Huang G, Shi S, Chen L. Bevacizumab improves the antitumor efficacy of adoptive cytokine-induced killer cells therapy in non-small cell lung cancer models. Med Oncol 2014; 31:777; PMID:24271420; http://dx.doi.org/ 10.1007/s12032-013-0777-3 [DOI] [PubMed] [Google Scholar]
- 16.Shi H, Qi X, Ma B, Cao Y, Wang L, Sun L, Niu H. The status, limitation and improvement of adoptive cellular immunotherapy in advanced urologic malignancies. Chin J Cancer Res 2015; 27:128-37; PMID:25937774; http://dx.doi.org/ 10.3978/j.issn.1000-9604.2014.12.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A 2002; 99:12293-7; PMID:12218188; http://dx.doi.org/ 10.1073/pnas.192461099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, Cachola KE, Murray JC, Tihan T, Jensen MC et al.. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 2007; 13:84-8; PMID:17159987; http://dx.doi.org/ 10.1038/nm1517 [DOI] [PubMed] [Google Scholar]
- 19.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12:252-64; PMID:22437870; http://dx.doi.org/ 10.1038/nrc3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K et al.. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122-33; PMID:23724867; http://dx.doi.org/ 10.1056/NEJMoa1302369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL et al.. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515:558-62; PMID:25428503; http://dx.doi.org/ 10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 22.Postow M, Taube J, Anders R, Taylor C, Wolchok J. Peripheral and tumor immune correlates in patients with advanced melanoma treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) monotherapy or in combination with ipilimumab. J Transl Med 2014; 12:O8; http://dx.doi.org/ 10.1186/1479-5876-12-S1-O8 [DOI] [Google Scholar]
- 23.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L et al.. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372:2018-28; PMID:25891174; http://dx.doi.org/ 10.1056/NEJMoa1501824 [DOI] [PubMed] [Google Scholar]
- 24.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL et al.. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:127-37; PMID:22461641; http://dx.doi.org/ 10.1126/scitranslmed.3003689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN et al.. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515:563-7; PMID:25428504; http://dx.doi.org/ 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y et al.. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet 2000; 356:802-807; PMID:11022927; http://dx.doi.org/ 10.1016/S0140-6736(00)02654-4 [DOI] [PubMed] [Google Scholar]
- 27.Cui J, Wang N, Zhao H, Jin H, Wang G, Niu C, Terunuma H, He H, Li W. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma.Int J Cancer 2014; 134:342-51; PMID:23825037; http://dx.doi.org/ 10.1002/ijc.28372 [DOI] [PubMed] [Google Scholar]
- 28.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res 2004; 10:5094-5100; PMID:15297412; http://dx.doi.org/19825956 10.1158/1078-0432.CCR-04-0428 [DOI] [PubMed] [Google Scholar]
- 29.Karim R, Jordanova ES, Piersma SJ, Kenter GG, Chen L, Boer JM, Melief CJ, van der Burg SH.. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res 2009; 15:6341-7; PMID:19825956; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1652 [DOI] [PubMed] [Google Scholar]
- 30.Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T, Okazaki T, Tokura Y.. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 2010; 116:1757-66; PMID:20143437; http://dx.doi.org/ 10.1002/cncr.24899 [DOI] [PubMed] [Google Scholar]
- 31.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K et al.. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res 2005; 11:2947-53; PMID:15837746; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-1469 [DOI] [PubMed] [Google Scholar]
- 32.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Amer S, Tulbah A et al.. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia 2006; 8:190-8; PMID:16611412; http://dx.doi.org/ 10.1593/neo.05733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS et al.. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009; 15:971-9; PMID:19188168; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-1608 [DOI] [PubMed] [Google Scholar]
- 34.Umemoto Y, Okano S, Matsumoto Y, Nakagawara H, Matono R, Yoshiya S, Yamashita Y, Yoshizumi T, Ikegami T, Soejima Y et al.. Prognostic impact of programmed cell death 1 ligand 1 expression in human leukocyte antigen class I-positive hepatocellular carcinoma after curative hepatectomy. J Gastroenterol 2015; 50:65-75; PMID:24509608; http://dx.doi.org/ 10.1007/s00535-014-0933-3 [DOI] [PubMed] [Google Scholar]
- 35.Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013; 144:512-27; PMID:23313965; http://dx.doi.org/ 10.1053/j.gastro.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungefroren H, Sebens S, Seidl D, Lehnert H, Hass R. Interaction of tumor cells with the microenvironment. Cell Commun Signal 2011; 9:18; PMID:21914164; http://dx.doi.org/ 10.1186/1478-811X-9-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF. The yin and yang of evasion and immune activation in HCC. J Hepatol 2015; 62:1420-1429; PMID:25733155; http://dx.doi.org/ 10.1016/j.jhep.2015.02.038 [DOI] [PubMed] [Google Scholar]
- 38.Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009; 206:1327-37; PMID:19451266; http://dx.doi.org/ 10.1084/jem.20082173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flecken T, Schmidt N, Spangenberg HC, Thimme R. Hepatocellular carcinoma–from immunobiology to immunotherapy. Z Gastroenterol 2012; 50:47-56; PMID:22222798; http://dx.doi.org/ 10.1055/s-0031-1282002 [DOI] [PubMed] [Google Scholar]
- 40.Pievani A, Borleri G, Pende D, Moretta L, Rambaldi A, Golay J, Introna M. Dual-functional capability of CD3+CD56+ CIK cells, a T-cell subset that acquires NK function and retains TCR-mediated specific cytotoxicity. Blood 2011; 118:3301-10; PMID:21821703; http://dx.doi.org/ 10.1182/blood-2011-02-336321 [DOI] [PubMed] [Google Scholar]
- 41.Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO et al.. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother 2012; 61:1019-31; PMID:22146893; http://dx.doi.org/ 10.1007/s00262-011-1172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gajewski TF, Louahed J, Brichard VG. Gene signature in melanoma associated with clinical activity:a potential clue to unlock cancer immunotherapy. Cancer J 2010; 16:399-403; PMID:20693853; http://dx.doi.org/ 10.1097/PPO.0b013e3181eacbd8 [DOI] [PubMed] [Google Scholar]
- 43.Sprengers D, van der Molen RG, Kusters JG, De Man RA, Niesters HG, Schalm SW, Janssen HL. Analysis of intrahepatic HBV-specific cytotoxic T-cells during and after acute HBV infection in humans. J Hepatol 2006; 45:182-9; PMID:16516331; http://dx.doi.org/ 10.1016/j.jhep.2005.12.022 [DOI] [PubMed] [Google Scholar]
- 44.Sprengers D, van der Molen RG, Kusters JG, Hansen B, Niesters HG, Schalm SW, Janssen HL. Different composition of intrahepatic lymphocytes in the immune-tolerance and immune-clearance phase of chronic hepatitis B. J Med Virol 2006; 78:561-8; PMID:16555293; http://dx.doi.org/ 10.1002/jmv.20576 [DOI] [PubMed] [Google Scholar]
- 45.Guo Y, Han W. Cytokine-induced killer (CIK) cells: from basic research to clinical translation. Chin J Cancer 2015; 34:99-107; PMID:25962508; http://dx.doi.org/ 10.1186/s40880-015-0002-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bald T, Landsberg J, Lopez-Ramos D, Renn M, Glodde N, Jansen P, Gaffal E, Steitz J, Tolba R, Kalinke U et al.. Immune cell-poor melanomas benefit from PD-1 blockade after targeted type I IFN activation. Cancer Discov 2014; 4:674-87; PMID:24589924; http://dx.doi.org/ 10.1158/2159-8290.CD-13-0458 [DOI] [PubMed] [Google Scholar]
- 47.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V et al.. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515:568-71; PMID:25428505; http://dx.doi.org/ 10.1038/nature13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan K, Guan XX, Li YQ, Zhao JJ, Li JJ, Qiu HJ, Weng DS, Wang QJ, Liu Q, Huang LX et al.. Clinical activity of adjuvant cytokine-induced killer cell immunotherapy in patients with post-mastectomy triple-negative breast cancer. Clin Cancer Res 2014; 20:3003-11; PMID:24668644; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0082 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.