ABSTRACT
Most skin malignancies are caused by external and often preventable environmental agents. Multiple reports demonstrated that cutaneous T-cell lymphomas (CTCL) can occur in married couples and cluster in families. Furthermore, recent studies document geographic clustering of this malignancy in Texas as well as in other areas of the United States. Multiple infectious, occupational, and medication causes have been proposed as triggers or promoters of this malignancy including hydrochlorothiazide diuretics, Staphylococcus aureus, dermatophytes, Mycobacterium leprae, Chlamydia pneumoniae, human T-Cell lymphotropic virus type 1 (HTLV1), Epstein-Barr virus (EBV), and herpes simplex virus (HSV). In this report, we review recent evidence evaluating the involvement of these agents in cancer initiation/progression. Most importantly, recent molecular experimental evidence documented for the first time that S. aureus can activate oncogenic STAT3 signaling in malignant T cells. Specifically, S. aureus Enterotoxin type A (SEA) was recently shown to trigger non-malignant infiltrating T cells to release IL-2 and other cytokines. These signals upon binging to their cognate receptors on malignant T cells are then able to activate STAT3 and STAT5 oncogenic signaling and promote cancer progression and IL-17 secretion. In light of these findings, it might be important for patients with exacerbation of their CTCL symptoms to maintain high index of suspicion and treat these individuals for S. aureus colonization and/or sepsis with topical and systemic antibiotics.
Keywords: Cutaneous T-cell lymphoma (CTCL), HTLV-1 and geographic clustering, IL-17, Mycosis fungoides (MF), S. aureus, Sézary syndrome (SS), STAT3, viruses
Skin, respiratory, and gastrointestinal tracts are the three main organ systems that are constantly interfacing with the environment. Therefore, it is not surprising that many malignancies in these organs are the result of external and often preventable agents such as tobacco smoke and asbestos that cause lung cancers and mesotheliomas, exposure to polycyclic aromatic hydrocarbons responsible for colon cancers or Helicobacter pylori producing gastric cancers. For skin too, in the last several decades scientists established a definitive link between a number of etiologic agents and malignancies. Ultraviolet radiation was proven to cause melanomas, basal cell, and squamous cell carcinomas. Viruses such as human papilloma virus (HPV) and Merkel cell polyomavirus were shown to cause squamous cell and Merkel cell cancers, respectively.
Skin is also a very dynamic immune organ, where many lymphocytes reside and are constantly poised to fend off attacks from bacterial, mycobacterial, fungal, or viral pathogens. It was previously documented that the skin surface of a normal adult individual contains approximately 20 billion T cells, which is nearly twice the number that is present in the entire circulation.1 Deregulation of signaling pathways in B and T lymphocytes in the skin can lead to cutaneous lymphomas. For instance, recent evidence indicates that primary cutaneous marginal zone B-cell lymphoma could be triggered by Borrelia burgdorferi.2 Also, for a number of systemic T cell lymphomas that often manifest with skin lesions such as adult T-cell leukemia/lymphoma or natural killer T-cell lymphoma of nasal type viral etiology and, specifically, involvement of HTLV1 or EBV, respectively, have been extensively documented.3,4
CTCL are a heterogeneous group of non-Hodgkin lymphoproliferative disorders characterized by localization of neoplastic T lymphocytes to the skin. Mycosis fungoides (MF), primary cutaneous anaplastic large cell lymphoma (cALCL) and a leukemic form, Sézary Syndrome (SS), are the most common variants and account for ∼80% of all cutaneous lymphomas.5 Previous epidemiologic studies based on the Surveillance, Epidemiology and End Results (SEER) databases documented that until recently, CTCL was on the rise in the United States and around the world.6 Multiple studies have shown a ∼3-fold increase in CTCL incidence during the last 25–30 y.6,7 Different regional variations in CTCL incidence have been reported. Whereas approximately 14–16 cases per million individuals per year were diagnosed in San Francisco, California, only 6–7 cases per million individuals per year were diagnosed in Iowa during 2000 and 2009.6 Recent studies also revealed that CTCL may occur conjointly in married couples and can also cluster in families.8 This malignancy was also previously described in immunosuppressed patients (e.g., organ transplant recipients and HIV+ individuals).9
Interestingly, recent research demonstrated geographic clustering of CTCL cases in Texas.8,10 Specifically, based on the analysis of 1990 patients using two distinct cancer registries, we documented geographic clustering of patients in several communities across the state. This included areas of Katy, Spring, and Houston Memorial area, where CTCL incidence rates were 5–20 times higher than expected for the population rate. Furthermore, this analysis demonstrated that two densely populated adjacent zip codes near El Paso, Texas were completely spared by CTCL during the period of 1995 to 2010.8,10 El Paso is located in a hot desert climate adjacent to the borders of New Mexico and Mexico. Hispanic individuals represent >80% of population in this area. Notably, El Paso, TX was documented to be one of the sunniest cities in the country.10 El Paso is the fourth sunniest city in the United States and is the sunniest one in Texas with 84% of annual sunshine, which could be a protective or a therapeutic factor for MF.10 Other recent studies also suggested geographic clustering for this rare cancer in Pittsburgh, Pennsylvania11 and in the Västernorrland county of Sweden.12 These combined results suggest that there might be an important environmental trigger/tumor initiating factor for this cancer.
The pathogenesis of CTCL is only partially understood. It was noted that certain human leukocyte antigen (HLA) class II alleles were associated with CTCL;13 therefore, suggesting that one of the molecular pathogenesis mechanisms may involve inappropriate T-cell activation via antigen presentation followed by an accumulation of neoplastic memory T cells. However, the antigen(s) remains unknown and would likely differ among patients. Infectious agents previously suggested as potential triggers/promoters of CTCL include Staphylococcus aureus, dermatophytes, M. leprae, C. pneumoniae, HTLV-1, EBV, and herpes simplex virus (HSV).9,14
Indeed, many studies attempted to systematically evaluate HTLV-1, HTLV-2, human immunodeficiency virus (HIV), HSV-1 and 2, varicella zoster virus (VZV), EBV, cytomegalovirus (CMV), human herpesvirus (HHV) 6, 7, and 8, Hepatitis C virus (HCV), polyomaviruses, endogenous retroviruses as well as C. pneumoniae and Borrelia burgdorferi involvement in triggering CTCL (reviewed in ref.9). Unfortunately, many of these studies yielded variable and often inconsistent results, while a number of studies failed to show any association with pathogenic organisms. Also, a number of positive studies had significant limitations, which could affect the interpretation of results. Nonetheless, some of these findings are intriguing and may hold the key to understanding CTCL pathogenesis.
HTLV-1 is endemic in Japan, sub-Saharan Africa, South America, and the Caribbean areas.15 Some patients with smoldering HTLV-1-associated adult T-cell lymphoma present with MF-like skin lesions,16,17 but based on other studies, this and other viruses have not been identified in the vast majority of MF cases,18 as discussed in a recent review.9 Also, interestingly, the co-occurrence of leprosy and MF was reported in U.S. patients,19-21 and treatment of leprosy also led to resolution of MF in some patients.19,20 Others have proposed that C. pneumoniae, which often resides in the lungs, can provide an antigen that stimulates proliferation of Sézary T cells.14
Erythrodermic MF and SS patients are colonized by S. aureus 13,22 at rates similar to atopic dermatitis, another Th2 lymphocyte-driven skin disease, where aberrant production of antimicrobial peptides and lack of Th1 immune response enables the growth of this bacterium.23,24 In MF/SS, as the disease progresses to advanced stages (i.e., tumor stage and/or erythroderma) a switch from a Th1 to a Th2 immune response has been consistently observed.25,26 It was proposed that miR-155-mediated downregulation of STAT4 and upregulation of STAT5 and STAT3 oncogenes drives this process.25,26 This important switch makes CTCL patients highly susceptible to S. aureus colonization and sepsis and promotes eosinophilia and pruritus in these individuals. Similarly to atopic dermatitis, treatment of MF/SS patients with first generation cephalosporins often improves pruritus as well as the extent and morphology of lymphoma lesions.22-24,27
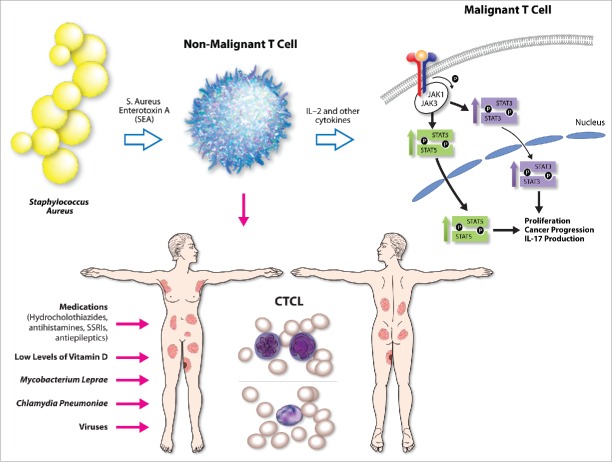
Until recently, the literature only described an association between MF/SS and S. aureus, but no mechanistic studies establishing a causal link between the two were available. A recent article in Blood by Ødum and colleagues demonstrated for the first time that S. aureus can directly activate oncogenic STAT3 signaling in malignant cells and can upregulate IL-17 expression.28 The authors demonstrated that Staphylococcal enterotoxin A type (SEA), but not other staphylococcal toxins (e.g., SEB, SEC, SED, or TSST-1), was present in the CTCL skin isolates.28 In addition, the SEA was able to activate STAT3 and upregulate IL-17 production in both patient-derived malignant T cells and immortalized cell lines.28 Using various co-culture models, the authors were able to demonstrate that SEA impacts malignant T cells indirectly, by activating infiltrating bystander non-malignant T cells, which in response to this stimulus produce IL-2 and other regulatory cytokines.28 These cytokines signal in a paracrine fashion and stimulate nearby malignant T cells to upregulate JAK3/STAT3 signaling, which leads to IL-17 upregulation.28 This process could be blocked at various points using specific siRNAs or tofacenib, a clinical grade JAK3 inhibitor.28 The cancer promoting role of JAK3/STAT3 signaling and IL-17 production in MF/SS has recently been described in numerous reports.25,26,29-33 The above study for the first time describes a causal link between the SEA and STAT3 oncogene activation and explains the mechanism behind IL-17 upregulation in a subset of advanced CTCL patients (Fig. 1).
Figure 1.
Summary of proposed potential etiologic triggers/promoters for CTCL include medications, viruses, low levels of vitamin D, Mycobacterium leprae, Chlamydia pneumonia, and Staphylococcus aureus. Recent evidence documents that S. Aureus enterotoxin type A (SEA) triggers non-malignant infiltrating T cells to release IL-2 and other cytokines that upon binging to their cognate receptors on malignant T cells are able to activate STAT3 and STAT5 oncogene signaling and promote cancer progression and IL-17 secretion.
S. aureus is a common pathogen that can also be found as part of normal skin flora.34 It is believed that high prevalence of this bacterial infection in advanced CTCL is explained by the immunocompromised state and impaired skin barrier. Based on the available molecular and clinical evidence, it is hypothesized that this pathogen promotes malignant inflammation, exacerbates itch, and the extent of body surface area involvement by the lymphoma. However, there is little evidence to suggest that S. aureus is critical for the initial transformation event.
Further evidence for S. aureus involvement in cancer progression originates from various studies that examined the ability of Staphylococcal toxins to act as super antigens, and by binding to a TCR-Vβ chain, activate and lead to a proliferation of malignant T cells in SS patients.13,27,35 The study by Ødum and colleagues further suggests that toxin-mediated activation of malignant cells does not rely on the expression of a single, toxin specific TCR-Vβ chain in malignant cells, but on the expression of multiple toxin binding TCR-Vβ chains expressed in bystander non-malignant tumor infiltrating T cells.28
As mentioned above, sun exposure, might be a protective factor for CTCL. In a recent study, Duvic et al. investigated an association between vitamin D deficiency and MF/SS.36 This study documented that vitamin D deficiency was observed in ~77% of CTCL patients, and is comparable to vitamin D deficiency in other cancer patients (~75% of patients).36 In contrast, extensive literature in the field documents that vitamin D deficiency is usually present in only ~30–40% of individuals in the general United States population.37 In recent years, vitamin D has been recognized as an emerging anticancer and immunomodulatory agent. Notably, increased sun exposure was noted to be a protective factor for other non-Hodgkin lymphomas.38 As immunomodulator, topical calcipotriene ointment is routinely used for the treatment of psoriasis.39 Specifically, vitamin D was shown to induce expression of cathelicidin, an important antimicrobial peptide that protects the skin against various pathogens, including S. aureus.40 Vitamin D deficiency could contribute to the increased S. aureus colonization and sepsis, which are both common in advanced CTCL patients.22,28,41
In addition to infections, various medications were proposed to trigger MF including antihistamines, antiepileptics, antihypertensives (e.g. calcium channel blockers and angiotensin-converting enzyme inhibitors), and serotonin reuptake inhibitors.42-44 The majority of evidence is at the level of case reports and needs further validation. However, in a recent case-control study, Duvic et al. demonstrated an intriguing link between hydrochlorothiazide and MF, suggesting it could be triggering early stage MF lesions.42 Their study indicates that after one or more years of exposure rare patients develop classic MF lesions.42 The results show that hydrochlorothiazide use is mostly associated with stage I disease. After cessation of this medication the MF skin eruption cleared in a number of patients or went into remission, while re-challenge with hydrochlorothiazide led to a recurrence or exacerbation of the MF/SS.42,45
In conclusion, based on existing evidence of geographic clustering and occurrence of CTCL in unrelated family members it is important to continue to search for potential external triggers for this skin cancer. We believe that MF/SS is the end result of a reaction to inappropriate T-cell activation followed by accumulation of memory T helper cells. While several antigens have been proposed to trigger this disease no single antigen has been proven to definitively cause this cancer.
Furthermore, it is important to consider the above discussed evidence in managing CTCL patients. Specifically, in evaluating all newly diagnosed individuals with CTCL, a thorough medication history should be performed and all thiazide diuretics should be stopped and replaced by another class of blood pressure lowering medications. Also, for immunosuppressed patients, it may be important to reduce the dose of immunosuppressive medications in the case of organ transplant recipients and/or to optimize immune function in HIV patients using anti-retroviral medications. If patients are found to be vitamin D deficient, it is appropriate to prescribe vitamin D supplementation therapy. For patients originating from Japan, sub-Saharan Africa, South America, and the Caribbean regions it may be important to rule out HTLV-1 infection. Also, based on the recent mechanistic evidence for S. aureus involvement in driving the cancer progression it may be beneficial to decolonize patients using bleach baths, mupirocin 2% ointment application to nares, and other methods that are currently used to treat atopic dermatitis patients. Finally, for patients presenting with an exacerbation of their malignancy (i.e., increased itch and/or increased body surface involvement by the lymphoma) it is imperative to maintain high index of suspicion and treat these individuals for S. aureus colonization and/or sepsis with topical and systemic antibiotics.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the new investigator funding program from the Ottawa Hospital Research Institute to Dr Litvinov and the Canadian Dermatology Foundation research grants to Dr Sasseville and Dr Litvinov, Joan Sealy Trust Cancer Research Fund grant to Dr Litvinov, Nordic Foundation “Tandem-program” and Danish Cancer Society “Knaeck Cancer Program” grants to Dr Ødum.
References
- 1.Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol 2010; 130:362-70; PMID:19675575; http://dx.doi.org/ 10.1038/jid.2009.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogle MA, Riddle CC, Triana EM, Jones D, Duvic M. Primary cutaneous B-cell lymphoma. J Am Acad Dermatol 2005; 53:479-84; PMID:16112357; http://dx.doi.org/ 10.1016/j.jaad.2005.04.043 [DOI] [PubMed] [Google Scholar]
- 3.Suzuki R. Pathogenesis and treatment of extranodal natural killer/T-cell lymphoma. Seminars Hematol 2014; 51:42-51; PMID:24468315; http://dx.doi.org/ 10.1053/j.seminhematol.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 4.Tsukasaki K, Tobinai K. Human T-cell lymphotropic virus type I-associated adult T-cell leukemia-lymphoma: new directions in clinical research. Clin Cancer Res 2014; 20:5217-25; PMID:25320371; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-0572 [DOI] [PubMed] [Google Scholar]
- 5.Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, Zackheim H, Duvic M, Estrach T, Lamberg S et al.. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007; 110:1713-22; PMID:17540844; http://dx.doi.org/ 10.1182/blood-2007-03-055749 [DOI] [PubMed] [Google Scholar]
- 6.Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol 2013; 149:1295-9; PMID:24005876; http://dx.doi.org/ 10.1001/jamadermatol.2013.5526 [DOI] [PubMed] [Google Scholar]
- 7.Scarisbrick JJ, Prince HM, Vermeer MH, Quaglino P, Horwitz S, Porcu P, Stadler R, Wood GS, Beylot-Barry M, Pham-Ledard A et al.. Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sezary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. J Clin Oncol 2015; 33:3766-73; PMID:26438120; http://dx.doi.org/ 10.1200/JCO.2015.61.7142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litvinov IV, Tetzlaff MT, Rahme E, Habel Y, Risser DR, Gangar P, Jennings MA, Pehr K, Prieto VG, Sasseville D et al.. Identification of geographic clustering and regions spared by cutaneous T-cell lymphoma in Texas using 2 distinct cancer registries. Cancer 2015; 121:1993-2003; PMID:25728286; http://dx.doi.org/ 10.1002/cncr.29301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirvish JJ, Pomerantz RG, Falo LD Jr, Geskin LJ. Role of infectious agents in cutaneous T-cell lymphoma: facts and controversies. Clinics Dermatol 2013; 31:423-31; PMID:23806159; http://dx.doi.org/ 10.1016/j.clindermatol.2013.01.009 [DOI] [PubMed] [Google Scholar]
- 10.Litvinov IV, Tetzlaff MT, Rahme E, Jennings MA, Risser DR, Gangar P, Netchiporouk E, Moreau L, Prieto VG, Sasseville D et al.. Demographic patterns of cutaneous T-cell lymphoma incidence in Texas based on two different cancer registries. Cancer Med 2015; 4:1440-7; PMID:26136403; http://dx.doi.org/ 10.1002/cam4.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreau JF, Buchanich JM, Geskin JZ, Akilov OE, Geskin LJ. Non-random geographic distribution of patients with cutaneous T-cell lymphoma in the Greater Pittsburgh Area. Dermatol Online J 2014; 20:13030/qt4nw7592w; PMID:25046454 [PubMed] [Google Scholar]
- 12.Gip L, Nilsson E. [Clustering of mycosis fungoides in the County of Vasternorrland]. Lakartidningen 1977; 74:1174-6; PMID:850446 [PubMed] [Google Scholar]
- 13.Jackow CM, Cather JC, Hearne V, Asano AT, Musser JM, Duvic M. Association of erythrodermic cutaneous T-cell lymphoma, superantigen-positive Staphylococcus aureus, and oligoclonal T-cell receptor V β gene expansion. Blood 1997; 89:32-40; PMID:8978274 [PubMed] [Google Scholar]
- 14.Abrams JT, Balin BJ, Vonderheid EC. Association between Sezary T cell-activating factor, Chlamydia pneumoniae, and cutaneous T cell lymphoma. Ann N Y Acad Sci 2001; 941:69-85; PMID:11594584; http://dx.doi.org/ 10.1111/j.1749-6632.2001.tb03712.x [DOI] [PubMed] [Google Scholar]
- 15.Gessain A, Cassar O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Frontiers Microbiol 2012; 3:388; PMID:23162541; http://dx.doi.org/ 10.3389/fmicb.2012.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zucker-Franklin D, Pancake BA. The role of human T-cell lymphotropic viruses (HTLV-I and II) in cutaneous T-cell lymphomas. Seminars Dermatol 1994; 13:160-5; PMID:7986683 [PubMed] [Google Scholar]
- 17.Detmar M, Pauli G, Anagnostopoulos I, Wunderlich U, Herbst H, Garbe C, Stein H, Orfanos CE. A case of classical mycosis fungoides associated with human T-cell lymphotropic virus type I. Br J Dermatol 1991; 124:198-202; PMID:2004007; http://dx.doi.org/ 10.1111/j.1365-2133.1991.tb00434.x [DOI] [PubMed] [Google Scholar]
- 18.Pawlaczyk M, Filas V, Sobieska M, Gozdzicka-Jozefiak A, Wiktorowicz K, Breborowicz J. No evidence of HTLV-I infection in patients with mycosis fungoides and Sezary syndrome. Neoplasma 2005; 52:52-5; PMID:15739027 [PubMed] [Google Scholar]
- 19.Grossman D, Rapini RP, Osborne B, Duvic M. Emergence of leprosy in a patient with mycosis fungoides. J Am Acad Dermatol 1994; 30:313-5; PMID:8294589; http://dx.doi.org/ 10.1016/S0190-9622(94)70030-3 [DOI] [PubMed] [Google Scholar]
- 20.Levy ML, Rosen T, Tschen JA, McGavran MH, Kalter DC. Hansen's disease following lymphoma. J Am Acad Dermatol 1986; 15:204-8; PMID:3745525; http://dx.doi.org/ 10.1016/S0190-9622(86)70157-6 [DOI] [PubMed] [Google Scholar]
- 21.Siriphukpong S, Pattanaprichakul P, Sitthinamsuwan P, Karoopongse E. Granulomatous mycosis fungoides with large cell transformation misdiagnosed as leprosy. J Medical Association Thailand = Chotmaihet Thangphaet 2010; 93:1321-6; PMID:21114213 [PubMed] [Google Scholar]
- 22.Talpur R, Bassett R, Duvic M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sezary syndrome. Br J Dermatol 2008; 159:105-12; PMID:18489588; http://dx.doi.org/ 10.1111/j.1365-2133.2008.08612.x [DOI] [PubMed] [Google Scholar]
- 23.Lee M, Van Bever H. The role of antiseptic agents in atopic dermatitis. Asia Pacific Allergy 2014; 4:230-40; PMID:25379483; http://dx.doi.org/ 10.5415/apallergy.2014.4.4.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powers CE, McShane DB, Gilligan PH, Burkhart CN, Morrell DS. Microbiome and pediatric atopic dermatitis. J Dermatol 2015; 42(12):1137-42; PMID:26388516; http://dx.doi.org/ 10.1111/1346-8138.13072 [DOI] [PubMed] [Google Scholar]
- 25.Litvinov IV, Cordeiro B, Fredholm S, Odum N, Zargham H, Huang Y, Zhou Y, Pehr K, Kupper TS, Woetmann A et al.. Analysis of STAT4 expression in cutaneous T-cell lymphoma (CTCL) patients and patient-derived cell lines. Cell Cycle 2014; 13:2975-82; PMID:25486484; http://dx.doi.org/ 10.4161/15384101.2014.947759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Netchiporouk E, Litvinov IV, Moreau L, Gilbert M, Sasseville D, Duvic M. Deregulation in STAT signaling is important for cutaneous T-cell lymphoma (CTCL) pathogenesis and cancer progression. Cell Cycle 2014; 13:3331-5; PMID:25485578; http://dx.doi.org/ 10.4161/15384101.2014.965061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tokura Y, Yagi H, Ohshima A, Kurokawa S, Wakita H, Yokote R, Shirahama S, Furukawa F, Takigawa M. Cutaneous colonization with staphylococci influences the disease activity of Sezary syndrome: a potential role for bacterial superantigens. Br J Dermatol 1995; 133:6-12; PMID:7669641; http://dx.doi.org/ 10.1111/j.1365-2133.1995.tb02485.x [DOI] [PubMed] [Google Scholar]
- 28.Andreas WO, Thorbjørn K, Lise ML, Ivan VL, Simon F, David LP, Claudia N, Robert G, Nigel PM, Denis S et al.. Staphylococcus aureus enterotoxin A (SEA) stimulates STAT3 activation and IL-17 expression in cutaneous T-cell lymphoma. Blood. 2016. Mar 10; 127:1287-96; PMID:26738536; http://dx.doi.org/23801634 10.1182/blood-2015-08-662353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krejsgaard T, Litvinov IV, Wang Y, Xia L, Willerslev-Olsen A, Koralov SB, Kopp KL, Bonefeld CM, Wasik MA, Geisler C et al.. Elucidating the role of interleukin-17F in cutaneous T-cell lymphoma. Blood 2013; 122:943-50; PMID:23801634; http://dx.doi.org/ 10.1182/blood-2013-01-480889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Litvinov IV, Pehr K, Sasseville D. Connecting the dots in cutaneous T cell lymphoma (CTCL): STAT5 regulates malignant T cell proliferation via miR-155. Cell cycle 2013; 12:2172-3; PMID:23803726; http://dx.doi.org/ 10.4161/cc.25550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sibbesen NA, Kopp KL, Litvinov IV, Jonson L, Willerslev-Olsen A, Fredholm S, Petersen DL, Nastasi C, Krejsgaard T, Lindahl LM et al.. Jak3, STAT3, and STAT5 inhibit expression of miR-22, a novel tumor suppressor microRNA, in cutaneous T-Cell lymphoma. Oncotarget 2015; 6:20555-69; PMID:26244872; http://dx.doi.org/ 10.18632/oncotarget.4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willerslev-Olsen A, Litvinov IV, Fredholm SM, Petersen DL, Sibbesen NA, Gniadecki R, Zhang Q, Bonefeld CM, Wasik MA, Geisler C et al.. IL-15 and IL-17F are differentially regulated and expressed in mycosis fungoides (MF). Cell Cycle 2014; 13:1306-12; PMID:24621498; http://dx.doi.org/ 10.4161/cc.28256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litvinov IV, Netchiporouk E, Cordeiro B, Dore MA, Moreau L, Pehr K, Gilbert M, Zhou Y, Sasseville D, Kupper TS. The Use of Transcriptional Profiling to Improve Personalized Diagnosis and Management of Cutaneous T-cell Lymphoma (CTCL). Clin Cancer Res 2015; 21:2820-9; PMID:25779945; http://dx.doi.org/ 10.1158/1078-0432.CCR-14-3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphreys H. Staphylococcus aureus: the enduring pathogen in surgery. Surg 2012; 10:357-60; PMID:23079115; http://dx.doi.org/ 10.1016/j.surge.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 35.Tokura Y, Heald PW, Yan SL, Edelson RL. Stimulation of cutaneous T-cell lymphoma cells with superantigenic staphylococcal toxins. J Invest Dermatol 1992; 98:33-7; PMID:1728639; http://dx.doi.org/ 10.1111/1523-1747.ep12494184 [DOI] [PubMed] [Google Scholar]
- 36.Talpur R, Cox KM, Hu M, Geddes ER, Parker MK, Yang BY, Armstrong PA, Liu P, Duvic M. Vitamin D deficiency in mycosis fungoides and Sezary syndrome patients is similar to other cancer patients. Clin Lymphoma Myeloma Leukemia 2014; 14:518-24; PMID:25442486; http://dx.doi.org/22982792 10.1016/j.clml.2014.06.023 [DOI] [PubMed] [Google Scholar]
- 37.Mitchell DM, Henao MP, Finkelstein JS, Burnett-Bowie SA. Prevalence and predictors of vitamin D deficiency in healthy adults. Endocrine Practice 2012; 18:914-23; PMID:22982792; http://dx.doi.org/ 10.4158/EP12072.OR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kricker A, Armstrong BK, Hughes AM, Goumas C, Smedby KE, Zheng T, Spinelli JJ, De Sanjosé S, Hartge P, Melbye M et al.. Personal sun exposure and risk of non Hodgkin lymphoma: a pooled analysis from the Interlymph Consortium. Int J Cancer J Int Du Cancer 2008; 122:144-54; PMID:17708556; http://dx.doi.org/ 10.1002/ijc.23003 [DOI] [PubMed] [Google Scholar]
- 39.Murphy G, Reich K. In touch with psoriasis: topical treatments and current guidelines. J Eur Acad Dermatol Venereol 2011; 25 Suppl 4:3-8; PMID:21507077; http://dx.doi.org/ 10.1111/j.1468-3083.2011.04059.x [DOI] [PubMed] [Google Scholar]
- 40.Guo C, Gombart AF. The antibiotic effects of vitamin D. Endocrine Metab Immune Disorders Drug Targets 2014; 14:255-66; PMID:25008764; http://dx.doi.org/16497887 10.2174/1871530314666140709085159 [DOI] [PubMed] [Google Scholar]
- 41.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C et al.. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006; 311:1770-3; PMID:16497887; http://dx.doi.org/ 10.1126/science.1123933 [DOI] [PubMed] [Google Scholar]
- 42.Jahan-Tigh RR, Huen AO, Lee GL, Pozadzides JV, Liu P, Duvic M. Hydrochlorothiazide and cutaneous T cell lymphoma: prospective analysis and case series. Cancer 2013; 119:825-31; PMID:22952039; http://dx.doi.org/ 10.1002/cncr.27740 [DOI] [PubMed] [Google Scholar]
- 43.Magro CM, Crowson AN. Drugs with antihistaminic properties as a cause of atypical cutaneous lymphoid hyperplasia. J Am Acad Dermatol 1995; 32:419-28; PMID:7868710; http://dx.doi.org/ 10.1016/0190-9622(95)90063-2 [DOI] [PubMed] [Google Scholar]
- 44.Rijlaarsdam U, Scheffer E, Meijer CJ, Kruyswijk MR, Willemze R. Mycosis fungoides-like lesions associated with phenytoin and carbamazepine therapy. J Am Acad Dermatol 1991; 24:216-20; PMID:1826111; http://dx.doi.org/ 10.1016/0190-9622(91)70029-2 [DOI] [PubMed] [Google Scholar]
- 45.Peck JR, Frank MP, Peck LR. Was treatment the trigger? Mycosis fungoides. Am J Med 2013; 126:1048-9; PMID:24083643; http://dx.doi.org/ 10.1016/j.amjmed.2013.08.007 [DOI] [PubMed] [Google Scholar]