ABSTRACT
Tumor-associated macrophages (TAM) are immunosuppressive cells that can massively accumulate in the tumor microenvironment. In patients with ovarian cancer, their density is correlated with poor prognosis. Targeting mediators that control the generation or the differentiation of immunoregulatory macrophages represents a therapeutic challenge to overcome tumor-associated immunosuppression. The ectonucleotidase CD39 hydrolyzes ATP into extracellular adenosine that exhibits potent immunosuppressive properties when signaling through the A2A adenosine receptor. We report here that CD14+ CD163+ TAM isolated from ovarian cancer patients and macrophages generated in vitro with M-CSF, express high levels of the membrane ectonucleotidase CD39 compared to classically activated macrophages. The CD39 inhibitor POM-1 and adenosine deaminase (ADA) diminished some of the immunosuppressive functions of CD14high CD163high CD39high macrophages, such as IL-10 secretion. We identified the cytokine IL-27, secreted by tumor-infiltrating neutrophils, located close to infiltrating CD163+ macrophages, as a major rheostat of CD39 expression and consequently, on the acquisition of immunoregulatory properties by macrophages. Accordingly, the depletion of IL-27 downregulated CD39 and PD-L1 expression as well as IL-10 secretion by M-CSF-macrophages. Collectively, these data suggest that CD39, drived by IL-27 and CD115 ligands in ovarian cancer, maintains the immunosuppressive phenotype of TAM. This work brings new information on the acquisition of immunosuppressive properties by tumor-infiltrating macrophages.
KEYWORDS: ATP, adenosine, CD39, IL-27, IL-10, M-CSF, ovarian cancer, tumor-associated macrophages
Introduction
Macrophages are involved in numerous functions, ranging from tissue homeostasis to microbe eradication. Accordingly, they can exhibit different states of polarization,1 with M1 and M2 representing two extreme states of a continuum spectrum of activation. M1-polarized macrophages, also named “classically activated macrophages,” are characterized by an IL-12high IL-10low phenotype. They play a protective role against microbes and tumors via the production of inflammatory cytokines. M2-polarized macrophages, referred to as “alternatively activated macrophages,” exhibit immunoregulatory properties, and are characterized by an IL-10high IL-12low phenotype and the ability to suppress Th1 immunity. M2-type macrophages are involved in the resolution of inflammation and the protection against some parasites. They also promote angiogenesis, tissue remodeling and wound healing.2,3
Macrophages are plastic cells. Their phenotype and functions can be modulated, depending on their microenvironment. As an example, type I and type II IFNs can revert M2-type macrophages into immunostimulatory cells.4 To the same extent, GM-CSF prevents the differentiation of human monocytes into immunosuppressive macrophages.5
At the tumor site, the accumulation of immunosuppressive cells abrogates effector cell functions and contributes to maintain tumor-immune tolerance.6 In various solid tumors, such as breast and ovarian cancers, tumor-associated macrophages (TAM) accumulate more than any other immune cell type, and their density is correlated with poor prognosis.7 TAM derive from circulating monocytes recruited locally by CCL2, M-CSF and/or VEGF.8 TAM exhibit an immunoregulatory phenotype and suppress antitumor immune responses by altering the cytotoxic properties of CTLs and by promoting apoptosis or inhibiting the proliferation of effector T cells. Via its ability to switch the differentiation of monocytes from DCs into macrophages, to favor the expression of inhibitory molecules on myeloid cells and to prevent T cell priming, IL-10 appears as the prominent immunoregulatory mediator expressed by TAM. Moreover, TAM favor the local recruitment of Treg cells by secreting CCL22.9 We have previously reported that the CD115 ligands M-CSF and IL-34, drive monocytes into IL-10high IL-12low immunoregulatory macrophages which are similar to ovarian cancer TAM.5,10,11 They represent a robust model to decipher in vitro the biology of immunoregulatory macrophages.
Accumulating data have underlined the impact of the local tumor-associated metabolism in regulating the recruitment and/or functional polarization of immune cells, among which ATP and its metabolites have emerged as potent regulators of immune cells.12,13 However, and to the best of our knowledge, the role of tumor-associated ATP metabolites on functional macrophage polarization has not been addressed in detail.
In an inflammatory context, extracellular ATP is considered as a potent danger signal that activates immune cells via P2 receptors.14-16 It was previously described that, under activation or stress, numerous cell types and particularly monocytes, release ATP.16,17 ATP is important for immune cell response, it attracts antigen-presenting cells and potentiates the initiation of protective antitumor immune responses.18,19 Even though inflammation is important for pathogens and tumor cells eradication, a chronic inflammation could be detrimental to the host. Consequently, ATP has to be removed from the extracellular milieu to avoid unabated inflammation. Landmark studies of Ohta and Sitkovsky highlighted the importance of adenosine in immunoregulation.20 ATP could be rapidly shifted into an immunosuppressive mediator upon catabolism into adenosine, a well-known potent inhibitor of immune cells.20-29 This observation led to analyze the cellular-mediated ATP catabolism. ATP is essentially hydrolyzed by the membrane-bound nucleotidase CD39 (NTPDase1, nucleoside triphosphate diphosphohydrolase 1) into ADP and AMP, the latter being dephosphorylated by the ecto-5′nucleotidase CD73 into adenosine.29-31 Ohta, Sitkovsky and others showed that, among the different adenosine receptors (A1, A2A, A2B and A3),26 the adenosine receptor 2A has a major role in the attenuation of inflammation and tissue damage.20,21,32-35 These studies evidenced the CD39-CD73-adenosine receptor axis blockade as a promising therapeutic target.31,36-42
In line with these studies and with the objective to better understand the mechanism involved in the acquisition by TAM of an immunoregulatory phenotype, this study aimed at evaluating the impact of ATP and ATP metabolites on TAM functions.
In tumors, all these partners are present: (i) ATP is mainly released by dying cells and infiltrated immune cells,12,13 (ii) CD39 and CD73 are expressed by numerous primary tumor cells 43,44 and by some infiltrating immunosuppressive cells 45-48 and (iii) adenosine receptor 2A is expressed by immune-infiltrating cells.41 Furthermore, an accumulation of extracellular adenosine in tumor microenvironment and the subsequent purinergic signaling are involved in the regulation of immune cell functions.20,31,36,40
In this context, we hypothesized that the CD39-CD73-adenosine receptor axis plays a strategic role in the acquisition of immunoregulatory properties by tumor-infiltrating macrophages. The objective of this study was to investigate the role of CD39 in the polarization of human macrophages generated using CD115 ligands and of TAM isolated from ovarian cancer patients.
Results
M-CSF-macrophages and ovarian cancer TAM express higher levels of CD39 than GM-CSF-macrophages
We analyzed, by flow cytometry, the expression of CD39 by M-CSF-macrophages and GM-CSF-macrophages, used as prototypes of M2-polarized and M1-polarized macrophages, respectively. Results showed that, at the end of the differentiation process (day 7), the expression of CD39 was higher on M-CSF-macrophages, compared to GM-CSF-macrophages (RFI = 5.8 ± 0.82 and RFI = 2.96 ± 0.37, respectively; mean ± SEM, n = 11) (Fig. 1A). Interestingly, the expression of CD39 was increased during the differentiation process on M-CSF-macrophages (Fig. 1B). On the contrary, CD39 expression remained stable on GM-CSF-macrophages during the 7-d differentiation process and was only slightly increased compared to freshly isolated monocytes (Fig. 1B). We previously reported that M-CSF-macrophages are similar to TAM isolated from ovarian cancer patients.11 M-CSF-macrophages and TAM are CD14high CD163high that produce high amounts of IL-10, but not IL-12, upon stimulation with LPS (Fig. 2C and data not shown).11 We observed that the levels of CD39 on ovarian cancer TAM isolated from patients are similar to the ones of M-CSF-macrophages (RFI = 6.28 compared to 5.8, respectively; mean, n = 5 and n = 11, respectively) (Fig. 1A). IHC confirmed, in ovarian cancer tissues, that CD14+ macrophages express CD39 (Fig. S1).
Figure 1.
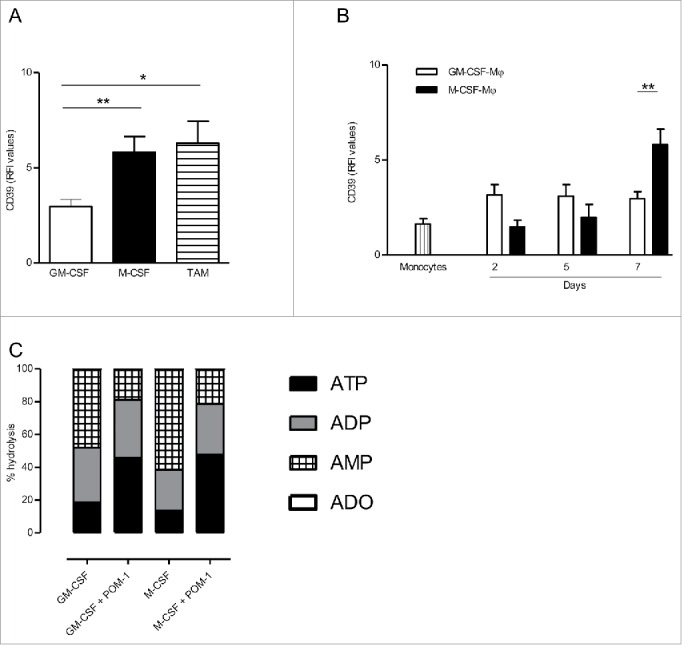
CD39 is more expressed in M-CSF-Mφ and TAM from ovarian cancer patients compared to GM-CSF-Mφ (A) The expression of CD39 was analyzed by flow cytometry on GM-CSF-Mφ, M-CSF-Mφ and TAM isolated from patients. Results are expressed in relative fluorescent intensity (RFI values are the ratio of antibody/isotype control antibody) (mean ± SEM, n = 11 for M-CSF-Mφ, GM-CSF-Mφ and n = 5 for TAM), *p < 0 .05 compared to GM-CSF-Mφ (B) M-CSF-Mφ polarization results in the acquisition of CD39 expression whereas GM-CSF-Mφ polarization results in its maintenance. CD39 expression was analyzed by flow cytometry. Results are expressed in RFI values, as mean ± SEM (n = 4 for monocytes, n = 11 for GM-CSF-Mφ and n = 11 for M-CSF-Mφ). (C) HPLC analysis of Etheno-ATP (ATP), -ADP (ADP), -AMP (AMP) and -adenosine (Ado) are expressed in percentage compared to the initial dose of ATP (200 µM = 100%) during 20 min. in GM-CSF-Mφ and M-CSF-Mφ (mean of two representative donors). In certain conditions, CD39 inhibitor (POM-1) was used at 10 µM.
Figure 2.
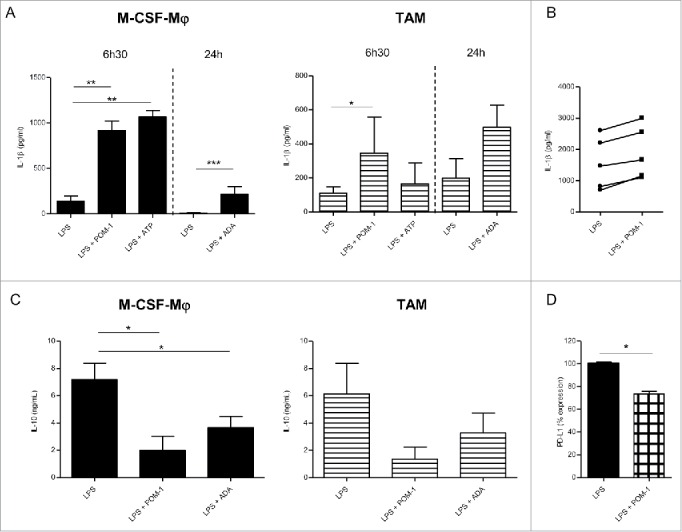
Secretion profile after CD39 blockade with POM-1 in GM-CSF-Mφ, M-CSF-Mφ and TAM isolated from patients (A) M-CSF-Mφ and TAM were stimulated with LPS (200 ng/mL) with or without POM-1 (10 µM), ATP (1 mM) for 6 h. M-CSF-Mφ and TAM isolated from patients were stimulated with LPS (200 ng/mL) with or without ADA (2.5 UI/mL) for 24 h. (B) GM-CSF-Mφ were stimulated with LPS (200 ng/mL) with or without POM-1 (10 µM) for 6 h. (A and B) IL-1β secretion in supernatant was analyzed by ELISA (pg/mL). Results are expressed in pg/mL (mean ± SEM, M-CSF-Mφ: n = 4 for POM-1 and ATP experiments, n = 9 for ADA experiment; TAM: n = 3 for POM-1 and ATP; GM-CSF-Mφ: n = 5, *p <0.05 compared to LPS alone). (C) M-CSF-Mφ and TAM isolated from patients were stimulated with LPS (200 ng/mL) with or without POM-1 (10 µM) or ADA (2.5 UI/mL) for 24 h. The secretion of IL-10 was analyzed by ELISA. Results are expressed in ng/mL as mean ± SEM (M-CSF-Mφ: n = 7 for POM-1 experiment and n = 8 for ADA experiment; TAM: n = 4 for POM-1 experiment and n = 5 for ADA experiment). (D) M-CSF-Mφ were stimulated with LPS (200 ng/mL) with or without POM-1 (10 µM) during 24 h. Expression of PD-L1 by M-CCSF-Mφ was analyzed by flow cytometry. Results are expressed in mean ± SEM of percentage of RFI compared to LPS-stimulated M-CSF-Mφ as 100% (n = 4).
GM-CSF-macrophages and M-CSF-macrophages express functional CD39
In order to evaluate the functionality of CD39, we quantified the degradation of exogenous etheno-ATP into ADP, AMP and adenosine (ADO) by GM-CSF-macrophages and M-CSF-macrophages. After 20 min., more than 80% of ATP is hydrolyzed by both macrophages subsets (Fig. 1C). Furthermore, M-CSF-macrophages hydrolyzed extracellular etheno-ATP more efficiently than GM-CSF-macrophages (3.9 µM/min vs. 2.9 µM/min, respectively; mean of two representative experiments) while producing more AMP compared to GM-CSF-macrophages from 0 min to 20 min (61.2% vs. 47.7%, respectively). These results confirmed that a high level of CD39 expression by M-CSF-macrophages was associated to a high ATPase activity compared to GM-CSF macrophages. Finally, the addition of the CD39 inhibitor POM-1 prevented extracellular ATP and ADP from hydrolysis in both M-CSF- and GM-CSF-macrophages (Fig. 1C and Fig. S2). This result confirms the role of CD39 in the metabolism of extracellular ATP in by M-CSF-macrophages.
Previous study has reported that the secretion of IL-1β is correlated to the concentration of extracellular ATP,49 therefore quantification of mature IL-1β in culture supernatants allowed us to monitor extracellular ATP release by macrophages. Therefore, we evaluated the impact of CD39 inhibition on ATP dependent IL-1β secretion by M-CSF-macrophages (Fig. 2A, left panel). As previously reported,50 LPS triggered IL-1β secretion by GM-CSF-macrophages (Fig. 2B and Fig. S3A) but induced low levels of IL-1β by M-CSF-macrophages (Fig. 2A, left panel). However, no IL-1β was produced by non-stimulated macrophages (data not shown). Interestingly, IL-1β secretion was increased in M-CSF-macrophages upon stimulation with LPS in the presence of POM-1 (6.59-fold increase; mean, n = 5) (Fig. 2A, left panel). A moderated but reproducible increase of IL-1β secretion was observed using TAM isolated from ovarian cancer patients stimulated in the presence of POM-1 (3.65-fold increase; mean, n = 3) (Fig. 2A, right panel). By contrast, POM-1 only marginally modulated IL-1β secretion by GM-CSF-macrophages (1.3-fold increase; mean, n = 5) (Fig. 2B and Fig. S3A). These results suggest an autocrine role of ATP and of its metabolites in regulation of M-CSF-macrophages properties.
CD39 is involved in the acquisition of the immunoregulatory phenotype by M-CSF-macrophages and ovarian cancer TAM
We therefore evaluated whether CD39 is involved in the acquisition of an immunoregulatory phenotype by M-CSF-macrophages and TAM isolated from ovarian cancer patients. Interestingly, inhibiting CD39 with POM-1 significantly decreased the production of IL-10 by M-CSF-macrophages in response to LPS (7.18 ± 1.22 ng/mL and 1.99 ± 1.04 ng/mL without and with POM-1, respectively; mean ± SEM, n = 7; p value = 0.011) (Fig. 2C left panel). A similar but not statistically significant effect was observed on ovarian cancer TAM (6.14 ± 2.22 ng/mL compared to 1.36 ± 0.88 ng/mL with and without POM-1, respectively; mean ± SEM, n = 4) (Fig. 2C, right panel) and GM-CSF-macrophages (4.66 ± 0.93 ng/mL compared to 0.68 ± 0.38 ng/mL; mean ± SEM, n = 5; p value = 0.03) (Fig. S3B). This decrease of IL-10 production was associated with a decrease of IL-10 mRNA expression in all macrophage subsets (Fig. S3C). In contrast, the expression of the costimulatory molecules CD80 and CD86 was not modulated by POM-1 (data not shown). In parallel, we observed a decrease of PD-L1 expression on LPS-stimulated M-CSF-macrophages or LPS-stimulated CD39low M-CSF-macrophages upon treatment with POM-1 (Fig. 2D). By contrast, the expression of IL-12, both at the mRNA and protein levels, by all macrophage subsets, was not modulated by POM-1 (Fig. S3D and E and data not shown). These results suggest that CD39 plays a central role in regulating the expression of immunoregulatory molecules by M2-type macrophages, with a marginal effect if any on the expression of costimulatory molecules.
The addition of exogenous ATP slightly decreased IL-10 production but did not induce IL-12 production by M-CSF-macrophages and ovarian cancer-isolated TAM (data not shown) and did not affect IL-12 secretion by GM-CSF-macrophages (Fig. S3D). As a control, ATP significantly increased IL-1β secretion by LPS-activated M-CSF-macrophages (139.4 ± 58.3 pg/mL to 1070 ± 68.14 pg/mL in the absence or presence of ATP, respectively, mean ± SEM ; n = 4; p value = 0.005), TAM (from 111.4 ± 36.2 pg/mL to 239.6 ± 90.9 pg/mL; n = 3) and GM-CSF-macrophages (from 1557 ± 309.2 pg/mL to 8236 ± 3906 pg/mL; n = 4) (Fig. 2A and Fig. S3A). These data suggested that the hydrolysis of ATP may be pivotal in regulating the immunoregulatory phenotype of M-CSF-macrophages. As macrophages express adenosine receptors and that adenosine receptor stimulation regulates IL-10 secretion,51 we addressed the role of adenosine generated following ATP hydrolysis on the cellular fate of macrophages by using adenosine deaminase (ADA) that degrades adenosine, and caffeine that inhibits adenosine receptor. Results showed that inhibition of adenosine receptor during M-CSF-macrophages activation inhibits IL-10 secretion (Fig. S4). Likewise, the presence of ADA during the activation led to a similar decrease of IL-10 secretion by LPS-stimulated M-CSF-macrophages, ovarian cancer-isolated TAM (Fig. 2A) and GM-CSF-macrophages (Fig. S3A). Adenosine depletion showed no effect on IL-12 secretion by GM-CSF-macrophages (Fig. S3D) nor triggered IL-12 secretion by M-CSF-macrophages and ovarian cancer-isolated TAM (data not shown). Surprisingly, adenosine depletion triggered IL-1β secretion by M-CSF-macrophages and slightly increased IL-1β secretion by GM-CSF-macrophages upon stimulation with LPS (Fig. 2A, and Fig. S3A). The increase of IL-1β production was associated with an increase of IL-1β mRNA expression by both macrophage subsets (Fig. S3F).
Collectively, these results suggest that ATP metabolites, generated in a CD39-dependent manner, are involved in the acquisition of immunoregulatory properties by M-CSF-macrophages and ovarian cancer TAM.
The expression of CD39 is regulated by interleukin-27
Results underlined the pivotal role played by CD39 in controling the immunoregulatory properties of M-CSF-macrophages and ovarian cancer TAM. Previous studies have reported that IL-27 receptor knock-out down-modulates the expression of CD39 on dendritic cells and regulatory T cells.52,53 LPS-stimulated human monocytes produce IL-27 (Fig. S5A) and LPS-stimulated macrophages expressed the transcript encoding IL-27, IL-27RA and gp130 (data not shown). We thus investigated the role of IL-27 in the CD39-mediated acquisition of an immunoregulatory phenotype by M-CSF-macrophages.
Neutralizing IL-27 during the 7-d culture of monocytes reduced membrane and mRNA CD39 expression by M-CSF-macrophages (Figs. 3A–B). Moreover, the neutralization of IL-27 decreased the ability of M-CSF-macrophages to produce IL-10 (Fig. 3C Left panel), to express IL-10 mRNA (Fig. 3C, right panel) and to express PD-L1 (Fig. 3D) in response to LPS. The modulation of PD-L1 expression by M-CSF-macrophages was comparable to one observed in the presence of POM-1 (18.1% decrease with anti-IL-27 Ab compared to 26.4% decrease with POM-1) (Figs. 2D and 3D) without affecting CD14 and CD163 expression (data not shown). These data suggest that autocrine IL-27 controls, at least in part, the acquisition of some immunoregulatory features by macrophage via the upregulation of CD39 expression.
Figure 3.
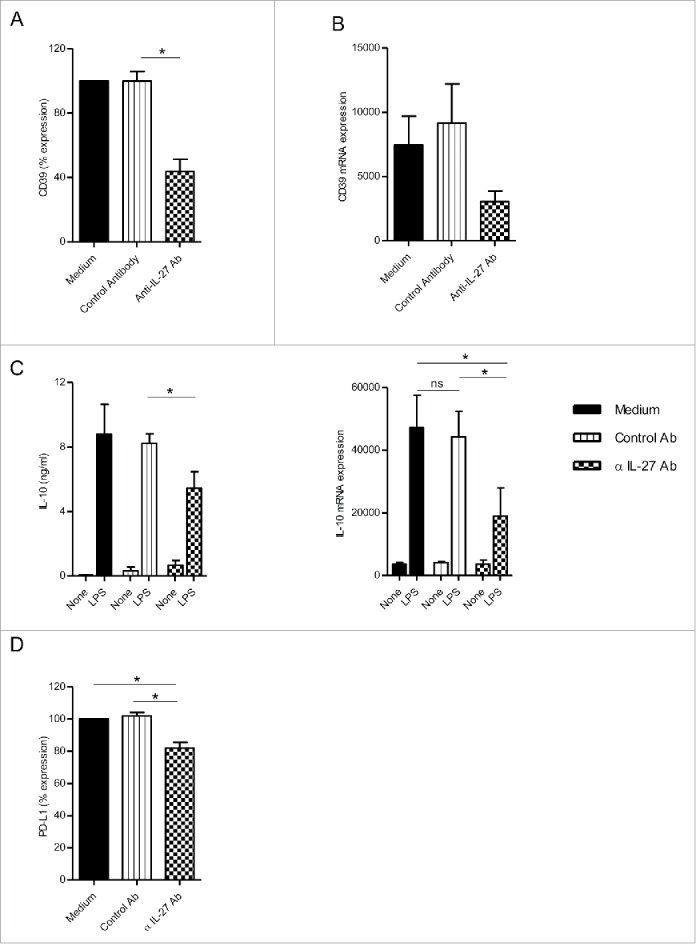
IL-27 blockade during macrophage differentiation in the presence of M-CSF-Mφ decreased CD39 expression. (A) M-CSF-Mφ was cultivated during 7 d with M-CSF in the presence of control medium, an anti-IL-27 antibody (5 µg/mL) or its control antibody. CD39 expression was analyzed by flow cytometry. Results are expressed in percent of CD39 expression in monocytes differentiated during 7 d with M-CSF as mean ± SEM (n = 4). (B) CD39 mRNA expression was analyzed by RT-qPCR. Results are expressed in mRNA expression normalized to GAPDH, as mean ± SEM (n = 4). (C, left panel) After stimulation of all type of macrophages generated with LPS (200 ng/mL) during 24 h, the secretion of IL-10 was analyzed by ELISA. Results are expressed in ng/mL as mean ± SEM (n = 5). (C, right panel) IL-10 mRNA expression was analyzed by RT-qPCR. Results are expressed in mRNA expression normalized to GAPDH, as mean ± SEM (n = 4). (D) All type of macrophages generated were stimulated with LPS (200 ng/mL) during 24 h. Expression of PD-L1 by M-CSF-Mφ was analyzed by flow cytometry. Results are expressed in percentage of RFI compared to LPS-stimulated M-CSF-Mφ as 100% (n = 5). *p < 0 .05 compared to differentiation process with control antibody or medium alone.
We thus evaluated IL-27 expression in ovarian cancer tissues. IHC revealed that infiltrating neutrophils are the main IL-27-producing cells in the tumors (Fig. 4A, middle and right panels). Interestingly, infiltrating CD163+ macrophages are in close vicinity of infiltrating neutrophils (Fig. 4A, middle and right panels). Only few IL-27-producing cells were detected in ovarian tissues from healthy subjects (Fig. 4A, left panel). In agreement with previous studies, we observed that peripheral blood neutrophils from healthy subjects constitutively express IL-27 (Fig. S5B). These data indicate that tumor-infiltrating neutrophils represent the major source of IL-27 in ovarian cancer tissue.
Figure 4.
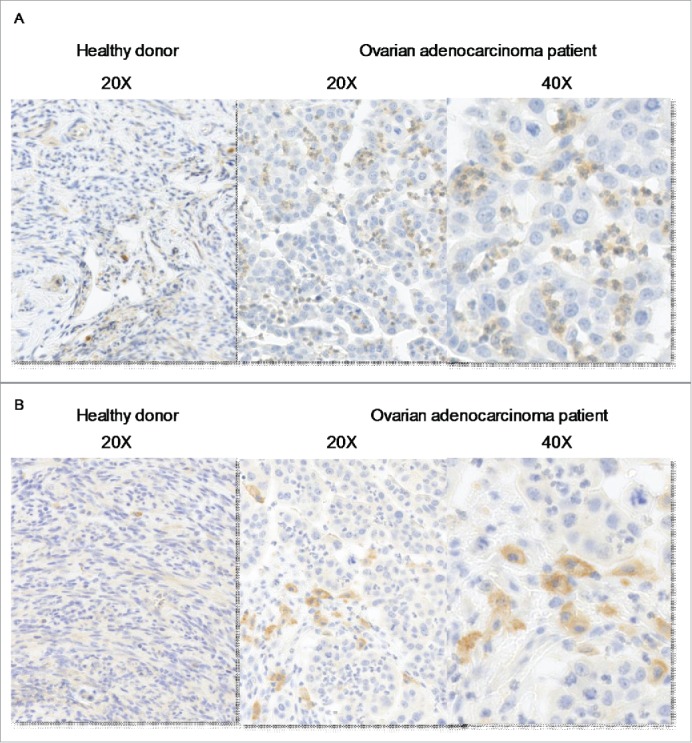
IL-27 is expressed by tumor-infiltrating neutrophils in vicinity of CD163+ macrophages. Immunochemical analysis of IL-27 (A) and CD163 (B) in normal tissue (left panel) and in ovarian adenocarcinomas (right panels).
Discussion
In the present work, we report that the ectonucleotidase CD39, overexpressed by ovarian cancer TAM and by M-CSF-macrophages, is involved in the acquisition of some immunoregulatory features by these immunoregulatory cells. Moreover, we observed that, during the in vitro differentiation of monocytes into macrophages, the level of CD39 expression was controlled by autocrine IL-27. In spite of uprising their level of activation, CD39 blockade shifts human macrophages from an immunosuppressive toward a more inflammatory phenotype; however contrary to murine macrophages,54 they remain unable to produce IL-12.
Even though human macrophages express low levels of membrane CD73,47,55 we confirmed that macrophages express CD73 mRNA and that its expression is increased upon LPS stimulation. In line with this result we showed that (i) CD73 metabolites were diluted in the cell culture supernatant of macrophages and (ii) that CD73 inhibition prevents the production of Il-10 by activated macrophages (personal unpublished data). We thus focused this study on CD39 rather than on CD73 31 or adenosine receptor blockade.20 Indeed, blocking CD39 maintains a pool of extracellular ATP suitable for inflammasome activation and prevents the production of immunomodulatory adenosine. The capacity of TLR agonists to induce ATP release and to activate caspase-1, an activated cascade required to induce the release of active IL-1β by myeloid cells, has for long been subjected to controversy.15,56 After stimulation with LPS alone, GM-CSF-macrophages but not M-CSF-macrophage produced IL-1β. This result suggested that GM-CSF-macrophages release their endogenous ATP after LPS activation, a result in agreement with Riteau et al. and Lévesque et al.57,58 In the present study, the blockade of CD39 and the depletion of adenosine rendered human TAM and M-CSF-macrophages able to secrete IL-1β, a process that reflects their ability to release ATP. These results confirmed the importance of endogenous ATP for the tuning of immunostimulatory properties by human macrophages.
Extracellular nucleotides also regulate the fate of immune cells,14,35 and immunosuppressive properties of adenosine in solid carcinomas have been extensively described.20 Moreover, hypoxia modulates immune cell sensitivity to adenosine via increase of HIF1a expression which leads to modulation of CD73 and ADORA2A. Hypoxia has emerged as a major inducer of tumor immune suppression. Interestingly, studies have reported that Ado is involved in maintaining T cell immune suppression in hypoxic conditions. Based on these data, we could hypothesize that hypoxia may also participate/amplify the macrophage-dependent tumor-induced immune suppression induced by ATP metabolites.31,36
CD39 belongs to the arsenal of regulatory T cells and ovarian cancer cells to generate an immunosuppressive environment.43,46,59 Here, we describe that ovarian tumor-infiltrating macrophages are also active players of this process. Newly recruited monocytes in the tumor are in contact with ATP-derived nucleotides which may participate to the acquisition of an IL-10high IL-12low immunosuppressive phenotype.10,60 As CD39 is located upstream the ATP metabolism, we hypothesized that manipulating the ATP/AMP ratio may modulate the immunosuppressive properties of M-CSF-macrophages. Accordingly, we observed that CD39 inhibition or adenosine depletion enhanced the capacity of activated TAM and M-CSF-macrophages to produce IL-1β and reduced their capacity to produce IL-10. The field of cancer immunology has recently been fed with studies on the roles of the ectonucleotidases CD39 and CD73 on the biology of tumor-associated immune cells, showing that CD39 and/or adenosine receptors are major negative regulators of T cells, myeloid cells and cancer cells that could be targeted within the tumor microenvironment.20,21,43 To support our choice to target CD39, it has been shown that CD73 (an ectonucleotidase hydrolyzing AMP into Adenosine) may be dispensable for M2-type macrophage polarization.61
Numerous studies have underlined the role of IL-27 in the generation of an immunoregulatory microenvironment in physiological and pathological settings. IL-27 limits tissue inflammation and autoimmunity in various settings 62 and IL-27 inhibits Th17 cells and promotes Treg cells differentiation in tumors and inflamed skin.63,64 IL-27 also confers immunosuppressive properties to several immune cells. Although the anti-inflammatory role of IL-27 has been reported during the course of inflammation,52,65 knowledge is limited about its role during the regulation of myeloid cell polarization and in the regulation of innate immunity. IHC analysis of ovarian cancer tissues revealed that tumor-infiltrating neutrophils represent the main source of IL-27.
IL-27 potentiates immunosuppression at least by inducing the acquisition of CD39 at the surface on cells present in the tumor microenvironment.43,59 However, this effect might depend on the target cells and on the microenvironment.66 IL-27 polymorphisms affecting IL-27 have been associated to an increased susceptibility to ovarian cancer.67 As proposed by Kalliolas et al.,66 IL-27 may exhibit opposite effects on innate and adaptive immune cells. It is more likely that monocytes/macrophages express IL-27 upon stimulation. These results support the hypothesis that IL-27 might exert, at least, its immunosuppressor properties by increasing CD39 expression on TAM. As a matter of fact, IL-27 acts as a rheostat for CD39.
PD-L1 appears as an important immune checkpoint. Modulation of PD-L1 expression may impact the fate of tumor cells and infiltrating immune cells from the microenvironment in adenocarcinomas.68-71 After CD39 blockade or IL-27 depletion, the reduced PD-L1 expression by macrophages might affect the ratio of costimulatory vs. coinhibitory molecules in the context of inflammation.
Ovarian cancer is the gynecological malignancy with the worse prognosis. TAM density was originally correlated with bad prognosis. Currently, different approaches have been proposed to avoid TAM polarization or to neutralize some immunosuppressive or pro-tumor factors produced by TAM (e.g. IL-10, VEGF, MMPs, IDO).60 For this concern, to identify strategies that may inhibit their recruitment, block their generation, reverse their trophic and immunosuppressive roles or interfere with their survival may represent new therapeutic opportunities.10 As a matter of fact, our study identifying the role of membrane CD39 in the acquisition and maintenance of an immunosuppressive phenotype by M-CSF-macrophages and TAM, could define ATP metabolism and IL-27 as privileged targets.54,55
Experimental procedures / materials and methods
Cell isolation and macrophage generation
Peripheral blood mononuclear cells (PBMC) were obtained from healthy human volunteers (Blood collection center, Angers, France; agreement ANG-2003-2). Ascites were collected from ovarian cancer patients with written informed consent in accordance with the Angers University Hospital ethics committee requirements and the declaration of Helsinki (Agreement 2013/38) and through the Center de Ressources Biologiques (CRB) Santé of Rennes (Agreement BB-0033-00056).
Mononuclear cells were isolated using standard Lymphocyte Separation Medium (PAA Laboratories, Pashing, Austria). CD14+ monocytes and CD14+ TAM were isolated by magnetic sorting (Miltenyi Biotec, Bergisch Gladbach, Germany). M-CSF-macrophages and GM-CSF-macrophages were generated by culturing monocytes (1 × 106 cells/mL) for 7 d in RPMI 1640 medium supplemented with 10% fetal calf serum and antibiotics (all from Lonza, Verviers, Belgium) in the presence of 50 ng/mL M-CSF (ORF Genetics Kopavogur, Iceland) or 20 ng/mL GM-CSF (Cellgenix, Freiburg, Germany), respectively. In some experiments, in order to evaluate the role of IL-27 during the 7-d differentiation process, cells were cultured in the presence of 2.5 or 5 µg/mL anti-IL-27 (R&D Systems, Abingdon, UK) or isotype control Abs (Sigma-Aldrich, Saint-Louis, MO).
Neutrophils were isolated from blood by 3% dextran (Amersham Biosciences) density-gradient sedimentation. Contaminating red blood cells were lysed by hypo-osmotic shock. Purity was determined by FACS analysis on forward scatter/side scatter parameters; purity was routinely >96 %.
Cell activation
Macrophages were activated or not for 6 h or 24 h with 200 ng/mL LPS (from E. coli serotype O111:B4; Sigma-Aldrich). In some experiments, macrophages were cultured for 6 h or 24 h in the presence of 10 µM POM-1 (Tocris, Bristol, UK), a potent CD39 antagonist,52 or for 24 h in the presence of 0.25 UI/mL ADA (Sigma-Aldrich) containing less than 11 ng/mL endotoxin or Caffeine (Sigma-Aldrich), a pan adenosine receptor antagonist during activation with LPS.
Cytokine quantification
IL-1β, IL-10, IL-12 (Diaclone, Dijon, France) and IL-27 (R&D Systems) were quantified with ELISA.
Flow cytometry analysis
Cells were incubated with FITC-labeled anti-CD80, PE-labeled anti-CD86, PE-labeled anti-CD39 (all from BD PharMingen, San Diego, CA), BV421-labeled anti-PD-L1 (Biolegend, San Diego, CA), FITC-labeled anti-CD14 (Milteny biotec) or APC-labeled anti-CD163 (R&D Systems) mAbs. Data were acquired using a FACS Canto flow cytometer (BD Biosciences, San Jose, CA) and analyzed with the FlowJo software (Tree Star, Ashland, OR). Results are expressed as relative fluorescence intensities (RFI) determined as follows: (A/B), where A and B were the MFI values obtained with specific and isotype control mAbs, respectively. MFI values were determined on 7-AAD− viable cells.
Analysis of mRNA expression
After cell lysis using the Trizol reagent (Life technologies, Saint-Aubin, France), total RNA were extracted using RNeasy Micro kit according to the manufacturer recommendations (Qiagen, Hilden, Germany) and reverse transcribed using Superscript II reverse transcriptase (Life technologies). The expression of IL-1β, IL-10, IL-12p35, IL-12p40, IL-27p28, CD39 and CD163 mRNA was analyzed by qPCR using the iQ™ SYBR® Green Supermix (Biorad, Marnes-la-Coquette, France); primer sequences are available upon request. Specific gene expression was calculated using the 2−ΔΔCT method using GAPDH as calibrator.
HPLC measurements
Nucleotidase activity of in vitro generated M-CSF-Mϕ and GM-CSF-Mφ was assessed by measuring fluorescent etheno-ATP hydrolysis. Etheno-ATP was obtained by incubating ATP (100 mM) for 30 min at 72°C in the presence of 1 M chloroacetaldehyde and 25 mM Na2HPO4 (pH 4.0), as previously described.72 M-CSF-Mϕ and GM-CSF-Mϕ (500 × 103 cells) were incubated at 37°C with 200 µM etheno-ATP, in the presence of 10 µM POM-1. At different time points (0, 10, 20, 50 min), a fraction of the supernatant was collected and precipitated on ice with 10% trichloroacetic acid (TCA; Sigma-Aldrich). Etheno-ATP, -ADP, -AMP and -Adenosine were separated by HPLC Waters purification systems (Alliance analytical, Fremont, CA) and C18 column (Sigma-Aldrich). Areas under the curve corresponding to each etheno nucleotide and etheno adenosine were integrated. Results were expressed as a percentage of each nucleotide present in the supernatants at the different time points, taking 100% as the dose of Etheno-ATP added at the start of the experiment and remaining without cells.
Immunohistochemistry (IHC)
Paraffin-embedded ovary biopsies from patients with ovarian adenocarcinomas or from healthy subjects were from the Department of Tissue Pathology of the University Hospital of Angers. After deparaffinization following Bond Max protocol (Leica) slides were treated with citrate for 30 min for IL-27-CD39-CD14 staining and with EDTA for 20 min for CD163 staining. IHC was performed using rabbit anti-human IL-27 (Abcam, Paris France), rabbit anti-human CD163 (Interchim, Montluçon, France), rabbit anti-human CD39 (Atlas antibodies) or mouse anti CD14 (Abcam) antibodies. Bound antibodies were detected with the HRP substrate 3, 3′-diaminobenzidine (brown) or Red Permanent (Red) After counter-staining with hematoxylin coloration, sections were mounted on coverslips and analyzed under a light microscope or NanoZoomer (Hamamatsu Photonics, Hamamatsu, Japan).
Immunofluorescence analysis
PNN were seeded on poly-L-lysine (Sigma-Aldrich) coated sterile at 1 × 106 cells per mL in complete medium SVF 2%. Membrane Fc receptors were blocked with Human Immunoglobulin (IgH, Sigma-Aldrich). Cells were incubated with mouse anti-human CD66b PE or its control isotype for 15 min. and fixed with PFA 4% for 15 min. at room temperature. Cell permeabilization was obtained after 30 min. with PBS 0,1% saponin plus 2% BSA. Cells were incubated with goat anti-human anti-IL-27 (R&D) or its control isotype for 30 min. Cells were then incubated with anti-goat FITC for 30 min. Samples were mounted with proLong gold (Thermo Fischer Scientific) and analyzed with Leica TCS SP8 confocal microscope.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA). Data are shown as mean ± SEM and were analyzed by Mann-Whitney test to analyze non-parametric data or one-way analysis of variance (ANOVA) with a post-hoc Bonferroni’s multiple comparison tests. Statistical significance was considered when p values were less than 0.05.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Dimitri Breard and David Macherel for HPLC experiments. The authors also thank members of the cellular and molecular facility (PACeM) of the University of Angers and members of the Cellular and Tissular Imaging core facility (MicroPICell) of the University of Nantes. The authors are grateful to Simon Blanchard, Isabelle Fremaux and Laurence Preisser for expert technical assistance.
Author contributions
Conceived and designed the experiments: SMDA, PJ, MG, YD, JT. Performed the experiments: SMDA, GK, CR, LB, LP. Analyzed the data: SMDA, GK, CR, LB, DH, AC, PJ, MG, YD, JT. Contributed reagents/materials/analysis tools: VC, NI, PD, AC. Wrote the paper: SMDA, PJ, MG, YD, JT.
Funding
This work was supported by institutional grants from INSERM and the University of Angers, and by a grant from the regional council Pays de la Loire (IMBIO-DC program) a grant from the Ligue contre le Cancer (Comité Départemental du Maine-et-Loire), and a grant from the Institut Recherche en Santé Respiratoire des Pays de la Loire. SMDA received a financial support from the regional council Pays de la Loire (IMBIO-DC program).
References
- 1.Romagnani P, Annunziato F, Piccinni MP, Maggi E, Romagnani S. Th1/Th2 cells, their associated molecules and role in pathophysiology. Eur Cytokine Netw 2000; 11:510-1; PMID:11203198 [PubMed] [Google Scholar]
- 2.Lacey DC, Achuthan A, Fleetwood AJ, Dinh H, Roiniotis J, Scholz GM, Chang MW, Beckman SK, Cook AD, Hamilton JA. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J Immunol 2012; 188:5752-65; PMID:22547697; http://dx.doi.org/ 10.4049/jimmunol.1103426 [DOI] [PubMed] [Google Scholar]
- 3.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012; 122:787-95; PMID:22378047; http://dx.doi.org/ 10.1172/JCI59643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E, Ponsoda S, Delneste Y, Hebbar M, Jeannin P. Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J Cancer 2009; 125:367-73; PMID:19378341; http://dx.doi.org/ 10.1002/ijc.24401 [DOI] [PubMed] [Google Scholar]
- 5.Foucher ED, Blanchard S, Preisser L, Garo E, Ifrah N, Guardiola P, Delneste Y, Jeannin P. IL-34 induces the differentiation of human monocytes into immunosuppressive macrophages. antagonistic effects of GM-CSF and IFNgamma. PLoS One 2013; 8:e56045; PMID:23409120; http://dx.doi.org/ 10.1371/journal.pone.0056045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005; 5:263-74; PMID:15776005; http://dx.doi.org/ 10.1038/nrc1586 [DOI] [PubMed] [Google Scholar]
- 7.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol 2008; 18:349-55; PMID:18467122; http://dx.doi.org/ 10.1016/j.semcancer.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer 2004; 40:1660-7; PMID:15251154; http://dx.doi.org/ 10.1016/j.ejca.2004.03.016 [DOI] [PubMed] [Google Scholar]
- 9.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity 2013; 39:61-73; PMID:23890064; http://dx.doi.org/ 10.1016/j.immuni.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N et al.. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007; 110:4319-30; PMID:17848619; http://dx.doi.org/ 10.1182/blood-2007-02-072587 [DOI] [PubMed] [Google Scholar]
- 11.Metcalf D. Control of granulocytes and macrophages: molecular, cellular, and clinical aspects. Science 1991; 25:529-33; PMID:1948028; http://dx.doi.org/23562161 10.1126/science.1948028 [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K et al.. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013; 38:729-41; PMID:23562161; http://dx.doi.org/ 10.1016/j.immuni.2013.03.003 [DOI] [PubMed] [Google Scholar]
- 13.Ma YT, Adjemian S, Yang H, Catani JPP, Hannani D, Martins I, Michaud M, Kepp O, Sukkurwala AQ, Vacchelli E et al.. ATP-dependent recruitment, survival and differentiation of dendritic cell precursors in the tumor bed after anticancer chemotherapy. Onco Immunol 2013; 2:e24568; PMID:23894718; http://dx.doi.org/ 10.4161/onci.24568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Idzko M, Ferrari D, Eltzschig HK. Nucleotide signalling during inflammation. Nature 2014; 509:310-7; PMID:24828189; http://dx.doi.org/ 10.1038/nature13085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Netea MG, Nold-Petry CA, Nold MF, Joosten LAB, Opitz B, van der Meer JHM, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I et al.. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 2009; 113:2324-35; PMID:19104081; http://dx.doi.org/ 10.1182/blood-2008-03-146720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccini A, Carta S, Tassi S, Lasiglie D, Fossati G, Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc Natl Acad Sci U S A 2008; 105:8067-72; PMID:18523012; http://dx.doi.org/ 10.1073/pnas.0709684105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazarowski ER, Sesma JI, Seminario-Vidal L, Kreda SM. Molecular mechanisms of purine and pyrimidine nucleotide release. Adv Pharmacol 2011; 61:221-61; PMID:21586361; http://dx.doi.org/ 10.1016/B978-0-12-385526-8.00008-4 [DOI] [PubMed] [Google Scholar]
- 18.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P et al.. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009; 461:282-U165; PMID:19741708; http://dx.doi.org/ 10.1038/nature08296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aymeric L, Apetoh L, Ghiringhelli F, Tesniere A, Martins I, Kroemer G, Smyth MJ, Zitvogel L. Tumor cell death and ATP release prime dendritic cells and efficient anticancer immunity. Cancer Res 2010; 70:855-8; PMID:20086177; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-3566 [DOI] [PubMed] [Google Scholar]
- 20.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MMK, Huang XJ, Cladwell S, Liu KB, Smith P. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A 2006; Aug 29; 103:13132-7; PMID:16916931; http://dx.doi.org/ 10.1073/pnas.0605251103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature 2001; 414:916-20; PMID:11780065; http://dx.doi.org/ 10.1038/414916a [DOI] [PubMed] [Google Scholar]
- 22.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-α production via adenosine A(2a) receptor-dependent and independent mechanisms. Faseb J 2000; 14:2065-74; PMID:11023991; http://dx.doi.org/ 10.1096/fj.99-0508com [DOI] [PubMed] [Google Scholar]
- 23.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-α and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol 1996; 157:4634-40; PMID:890684312939345 [PubMed] [Google Scholar]
- 24.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med 2003; 198:783-96; PMID:12939345; http://dx.doi.org/ 10.1084/jem.20030891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasko G, Pacher P. Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol 2012; 32:865-9; PMID:22423038; http://dx.doi.org/ 10.1161/ATVBAHA.111.226852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol 2004; 25:33-9; PMID:14698282; http://dx.doi.org/ 10.1016/j.it.2003.11.003 [DOI] [PubMed] [Google Scholar]
- 27.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov 2008; 7:759-70; PMID:18758473; http://dx.doi.org/ 10.1038/nrd2638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol 2004; 22:657-82; PMID:15032592; http://dx.doi.org/ 10.1146/annurev.immunol.22.012703.104731 [DOI] [PubMed] [Google Scholar]
- 29.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med 2013; 19:355-67; PMID:23601906; http://dx.doi.org/ 10.1016/j.molmed.2013.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Resta R, Yamashita Y, Thompson LF. Ecto-enzyme and signaling functions of lymphocyte CD73. Immunol Rev 1998; 161:95-109; PMID:9553767; http://dx.doi.org/ 10.1111/j.1600-065X.1998.tb01574.x [DOI] [PubMed] [Google Scholar]
- 31.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 2010; 29:5346-58; PMID:20661219; http://dx.doi.org/ 10.1038/onc.2010.292 [DOI] [PubMed] [Google Scholar]
- 32.Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M et al.. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 2005; 129:26-33; PMID:16012931; http://dx.doi.org/ 10.1053/j.gastro.2005.05.032 [DOI] [PubMed] [Google Scholar]
- 33.Odashima M, Otaka M, Jin M, Komatsu K, Wada I, Matsuhashi T, Horikawa Y, Hatakeyama N, Oyake J, Ohba R et al.. Selective A2A adenosine agonist ATL-146e attenuates acute lethal liver injury in mice. J Gastroenterol 2005; 40:526-9; PMID:15942719; http://dx.doi.org/ 10.1007/s00535-005-1609-9 [DOI] [PubMed] [Google Scholar]
- 34.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood 1997; 90:1600-10; PMID:9269779 [PubMed] [Google Scholar]
- 35.Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res 2014; 74:7250-9; PMID:25377469; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res 2008; Oct 1:5947; PMID:18829471; http://dx.doi.org/20080644 10.1158/1078-0432.CCR-08-0229 [DOI] [PubMed] [Google Scholar]
- 37.Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A 2010; 107:1547-52; PMID:20080644; http://dx.doi.org/ 10.1073/pnas.0908801107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene 2013; 32:1743-51; PMID:22751118; http://dx.doi.org/ 10.1038/onc.2012.269 [DOI] [PubMed] [Google Scholar]
- 39.Hausler SF, Del Barrio IM, Diessner J, Stein RG, Strohschein J, Honig A, Dietl J, Wischhusen J. Anti-CD39 and anti-CD73 antibodies A1 and 7G2 improve targeted therapy in ovarian cancer by blocking adenosine-dependent immune evasion. Am J Transl Res 2014; 6:129-39; PMID:24489992 [PMC free article] [PubMed] [Google Scholar]
- 40.Young A, Mittal D, Stagg J, Smyth MJ. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov 2014; 4:879; PMID:25035124; http://dx.doi.org/ 10.1158/2159-8290.CD-14-0341 [DOI] [PubMed] [Google Scholar]
- 41.Beavis PA, Milenkovsky N, Henderson Ma, John LB, Allard B, Loi S, Kershaw MH, Stagg J, Darcy PK. Adenosine receptor 2A blockade increase the eficacy of anti-PD-1 through enhanced antitumor T-cell responses. Cancer Immunol Res 2015; 3:506; PMID:25672397; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0211 [DOI] [PubMed] [Google Scholar]
- 42.Allard B, Pommey S, Smyth MJ, Stagg J. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res 2013; 19:5626; PMID:23983257; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-0545 [DOI] [PubMed] [Google Scholar]
- 43.Bastid J, Regairaz A, Bonnefoy N, Dejou C, Giustiniani J, Laheurte C, Cochaud S, Laprevotte E, Funck-Brentano E, Hemon P et al.. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res 2015; 3:254-65; PMID:25403716; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0018 [DOI] [PubMed] [Google Scholar]
- 44.Zhang B, Cheng B, Li F, Ding J, Feng Y, Zhuo G, Wei H, Zhao K. High expression of CD39/ENTPD1 in malignant epithelial cells of human rectal adenocarcinoma. Tumor Biol 2015; 36:9411-9; PMID:26113408; http://dx.doi.org/20546740 10.1007/s13277-015-3683-9 [DOI] [PubMed] [Google Scholar]
- 45.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Muller CE, Murakami T, Robson SC. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterol 2010; 139:1030-40; PMID:20546740; http://dx.doi.org/ 10.1053/j.gastro.2010.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M et al.. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007; 204:1257-65; PMID:17502665; http://dx.doi.org/ 10.1084/jem.20062512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zanin RF, Braganhol E, Bergamin LS, Campesato LF, Filho AZ, Moreira JC, Morrone FB, Sevigny J, Schetinger MR, de Souza Wyse AT et al.. Differential macrophage activation alters the expression profile of NTPDase and ecto-5′-nucleotidase. PLoS One 2012; 7:e31205; PMID:22348056; http://dx.doi.org/ 10.1371/journal.pone.0031205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thibaudin M, Chaix M, Boidot R, Vegran F, Derangere V, Limagne E, Berger H, Ladoire S, Apetoh L, Ghiringhelli F. Human ectonucleotidase-expressing CD25high Th17 cells accumulate in breast cancer tumors and exert immunosuppressive functions. Onco Immunol 2016; 5:e1055444; PMID:26942062; http://dx.doi.org/ 10.1080/2162402X.2015.1055444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006; 440:237-41; PMID:16407889; http://dx.doi.org/ 10.1038/nature04516 [DOI] [PubMed] [Google Scholar]
- 50.Foucher ED, Blanchard S, Preisser L, Descamps P, Ifrah N, Delneste Y, Jeannin P. IL-34- and M-CSF-induced macrophages switch memory T cells into Th17 cells via membrane IL-1alpha. Eur J Immunol 2014; 45(4):1092-102; PMID:25545357; http://dx.doi.org/17056121 10.1002/eji.201444606 [DOI] [PubMed] [Google Scholar]
- 51.Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther 2007; 113:264-75; PMID:17056121; http://dx.doi.org/ 10.1016/j.pharmthera.2006.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mascanfroni ID, Yeste A, Vieira SM, Burns EJ, Patel B, Sloma I, Wu Y, Mayo L, Ben-Hamo R, Efroni S et al.. IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat Immunol 2013; 14:1054-63; PMID:23995234; http://dx.doi.org/ 10.1038/ni.2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sekar D, Hahn C, Brune B, Roberts E, Weigert A. Apoptotic tumor cells induce IL-27 release from human DCs to activate Treg cells that express CD69 and attenuate cytotoxicity. Eur J Immunol 2012; 42:1585-98; PMID:22678911; http://dx.doi.org/ 10.1002/eji.201142093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen HB, Briggs KT, Marino JP, Ravid K, Robson SC, Mosser DM. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood 2013; 122:1935-45; PMID:23908469; http://dx.doi.org/ 10.1182/blood-2013-04-496216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dubois-Colas N, Petit-Jentreau L, Barreiro LB, Durand S, Soubigou G, Lecointe C, Klibi J, Rezai K, Lokiec F, Coppee JY et al.. Extracellular Adenosine Triphosphate Affects the Response of Human Macrophages Infected With Mycobacterium tuberculosis. J Infect Dis 2014; 210:824-33; PMID:24604822; http://dx.doi.org/ 10.1093/infdis/jiu135 [DOI] [PubMed] [Google Scholar]
- 56.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009; 27:519-50; PMID:19302047; http://dx.doi.org/ 10.1146/annurev.immunol.021908.132612 [DOI] [PubMed] [Google Scholar]
- 57.Riteau N, Baron L, Villeret B, Guillou N, Savigny F, Ryffel B, Rassendren F, Le Bert M, Gombault A, Couillin I. ATP release and purinergic signaling: a common pathway for particle-mediated inflammasome activation. Cell Death Dis 2012; 3:e403; PMID:23059822; http://dx.doi.org/ 10.1038/cddis.2012.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levesque SA, Kukulski F, Enjyoji K, Robson SC, Sevigny J. NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur J Immunol 2010; 40:1473-85; PMID:20201036; http://dx.doi.org/ 10.1002/eji.200939741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hausler SF, Montalban del Barrio I, Strohschein J, Anoop Chandran P, Engel JB, Honig A, Ossadnik M, Horn E, Fischer B, Krockenberger M et al.. Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother 2011; 60:1405-18; PMID:21638125; http://dx.doi.org/ 10.1007/s00262-011-1040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity 2014; 41:49-61; PMID:25035953; http://dx.doi.org/ 10.1016/j.immuni.2014.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eichin D, Laurila JP, Jalkanen S, Salmi M. CD73 activity is dispensable for the polarization of M2 macrophages. PLoS One 2015; 10:e0134721; PMID:26258883; http://dx.doi.org/ 10.1371/journal.pone.0134721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hunter CA, Kastelein R. Interleukin-27: balancing protective and pathological immunity. Immunity 2012; 37:960-9; PMID:23244718; http://dx.doi.org/ 10.1016/j.immuni.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol 2010; 11:854-61; PMID:20676095; http://dx.doi.org/ 10.1038/ni.1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kido M, Takeuchi S, Sugiyama N, Esaki H, Nakashima H, Yoshida H, Furue M. T cell-specific overexpression of interleukin-27 receptor α subunit (WSX-1) prevents spontaneous skin inflammation in MRL/lpr mice. Br J Dermatol 2011; 164:1214-20; PMID:21332454; http://dx.doi.org/ 10.1111/j.1365-2133.2011.10244.x [DOI] [PubMed] [Google Scholar]
- 65.Jung JY, Robinson CM. IL-12 and IL-27 regulate the phagolysosomal pathway in mycobacteria-infected human macrophages. Cell Commun Signal 2014; 12:16; PMID:24618498; http://dx.doi.org/ 10.1186/1478-811X-12-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalliolias GD, Ivashkiv LB. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J Immunol 2008; 180:6325-33; http://dx.doi.org/ 10.4049/jimmunol.180.9.6325 [DOI] [PubMed] [Google Scholar]
- 67.Zhang Z, Zhou B, Wu Y, Gao Q, Zhang K, Song Y, Zhang L, Xi M. Prognostic value of IL-27 polymorphisms and the susceptibility to epithelial ovarian cancer in a Chinese population. Immuno Genetics 2014; 66:85-92; PMID:24352695; http://dx.doi.org/ 10.1007/s00251-013-0753-2 [DOI] [PubMed] [Google Scholar]
- 68.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K et al.. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455-65; PMID:22658128; http://dx.doi.org/ 10.1056/NEJMoa1200694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res 2013; 73:6900-12; PMID:23975756; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, Yamaguchi K, Murakami R, Yamamoto A, Kharma B et al.. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res 2013; 19:1363-74; PMID:23340297; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2199 [DOI] [PubMed] [Google Scholar]
- 71.Maine CJ, Aziz NH, Chatterjee J, Hayford C, Brewig N, Whilding L, George AJ, Ghaem-Maghami S. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunotherapy 2014; 63:215-24; PMID:24297569; http://dx.doi.org/6731811 10.1007/s00262-013-1503-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Levitt B, Head RJ, Westfall DP. High-pressure liquid chromatographic-fluorometric detection of adenosine and adenine nucleotides: application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal Biochem 1984; 137:93-100; PMID:6731811; http://dx.doi.org/ 10.1016/0003-2697(84)90352-X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


