ABSTRACT
Lymphatic vasculature plays a crucial role in the immune response, enabling transport of dendritic cells (DCs) and antigens (Ags) into the lymph nodes. Unfortunately, the lymphatic system has also a negative role in the progression of cancer diseases, by facilitating the metastatic spread of many carcinomas to the draining lymph nodes. The lymphatics can promote antitumor immune response as well as tumor tolerance. Here, we review the role of lymphatic endothelial cells (LECs) in tumor progression and immunity and mechanism of action in the newest anti-lymphatic therapies, including photodynamic therapy (PDT).
KEYWORDS: Anti-lymphatic therapies, CCR7, lymphatic endothelial cells, photodynamic therapy, vascular endothelial growth factor
Abbreviations
- Ags
antigens
- AKT
protein kinase B
- AMD
age macular degeneration
- Ang1/2
angiopoietin-1/2
- APCs
antigen-presenting cells
- CCL5/19/21
chemokine (C–C motif) ligand 5/19/21
- CCR4/5/l7
C–C chemokine receptor type 4/5/7
- COX-2
cyclooxygenase-2
- CD
cluster of differentiation
- DCs
dendritic cells
- ERK1/2
extracellular-signal-regulated kinases
- HIF-1
hypoxia-inducible transcription factor
- ICAM-1
intercellular adhesion molecule 1
- IRES
internal ribosome entry site
- LECs
lymphatic endothelial cells
- LEN
lenalidomide
- MDSCs
myeloid-derived suppressor cells
- MHC
major histocompatibility complex
- mTOR
mammalian target of rapamycin kinase
- PDT
photodynamic therapy
- PGE2
prostaglandin E2
- PROX-1
prospero homeobox 1
- SMCs
smooth muscle cells
- SOX18
SRY-related HMG-box
- STAT3
signal transducer and activator of transcription 3
- TAMs
tumor-associated macrophages
- TCM
tumor-conditioned media
- Tie2
angiopoietin receptors
- TGFBIp
transforming growth factor-β-induced protein
- TGF-β
transforming growth factor β
- TNF
tumor necrosis factor
- TNFR1
tumor necrosis factor receptor 1
- VEGF-A/VEGF-C/VEGF-D
vascular endothelial growth factor C/D
- VEGFR
vascular endothelial growth factor receptor
Introduction
One of the main causes of cancer evasion is the ability of tumor cells to spread to local and distant tissues and organs. Therefore, for several decades, a major focus of cancer research was to understand the mechanisms which unlock the ability of tumor cells to form metastases. Many studies showed that the lymphatic development can be re-activated during tumor lymphangiogenesis and, as it was widely reviewed before, the tumor lymphangiogenesis strongly depends on vascular endothelial growth factor C/D (VEGF-C/D) and vascular endothelial growth factor receptor 3 (VEGFR-3) pathway.1 In lymphangiogenesis, the pivotal role is also played by the SRY-related HMG-box (SOX18), which through activation of prospero homeobox 1 (Prox-1) transcription, induces expression of lymphatic-specific genes, e.g. podoplanin. 2
Recent research has been focused on uncovering the active role of lymphatic endothelium in tumor cells transport and modulation of antitumor immune response.3 The communication between immune system and tumor is initiated with blind-ended capillaries of afferent lymphatics that merge into larger collecting vessels and connect tissue with lymph nodes. This lymphatic link between tumor and the distant microenvironment is associated with flow of tumor antigens (Ags), cytokines and enzymes but is also crucial for the progression and dissemination of tumor cells. In face of the discovery of several key lymphatic-specific molecular markers, the number of studies on lymphatic biology has been recently augmented. Therefore, we review the role of lymphatic endothelial cells (LECs) in tumor progression and immunity as well as the requirements for tumor cells to enter initial afferent lymphatic vessels. We discuss the newest achievements in anti-lymphatic therapies underlying some innovative treatments such as photodynamic therapy (PDT).
Structure and function of lymphatic vessels
Normal tissue fluid homeostasis in the human body is dependent on mutual reinforcing functions of blood and lymph vessels system. In contrast to blood vessels responsible for delivery of oxygen, nutrients, hormones and cells to the body tissues, lymphatic vessels are specialized in uptake of tissue fluid with macromolecules, microbes and other substances from interstitial space.4
The lymphatic vessels are present in tissues that frequently come in contact with foreign Ags, such as the skin and mucous membranes. Moreover, lymphatic vessels are found in all vascularized tissues, including recently discovered central nervous system lymphatic vasculature, with notable exception of bone marrow.5 Tissue fluid first enters lymphatic capillaries, comprised of LECs organized in highly permeable single-layered, blind-ended and thin-walled sacs. LECs are attached to the extracellular matrix through elastic anchoring filaments. These fibrillin-stranded structures protect vessels from collapse and stretch under high-interstitial pressure. This leads to the opening of endothelial flaps, allowing fluid and macromolecules to enter the vessel's lumen.6 The lymph is further transferred to larger pre-collector vessels that contain occasional valves, a basement membrane and sparse coverage of smooth muscle cells (SMCs). The pre-collectors converge into the lymphatic collecting vessels, with LECs forming continuous “zipper-like” junctions, surrounded by a basement membrane and supported by incessant layer of SMCs. The lymph flow is achieved by SMCs contractility, vasomotion and the activity of surrounding skeletal muscles. The bileaflet valves in collecting vessels prevent the backflow of lymph.7 The collectors pass the lymph to the lymph nodes and further to lymphatic trunks and right lymphatic and thoracic ducts, where lymph is eventually drained back into the venous circulation at the venous angles.1 Therefore, the central function of lymphatic vascular system is to sustain tissue-fluid homeostasis. Additionally, lacteal lymphatic vessels absorb and transport fat soluble vitamins and dietary fat, such as chylomicrons, from the small intestine, bypassing the liver that normally clear hydrophilic substances collected by blood from the intestine. In addition, the lymphatic system is essential for proper functioning of the immune system. Its role is especially invaluable in the immune response as lymphatic vessels enable leukocytes trafficking and transport of Ags and antigen-presenting cells (APCs) from the interstitium to the lymph nodes, where they communicate with naive lymphocytes. Until recently, the role of lymphatic endothelium in immune response modulation was underestimated. It was thought that LECs' function was restricted only to the passive transport of immune cells and Ags.8 Conversely, it was shown that interstitial pressure and fluid flow can activate LECs, leading to increase of fluid and solute permeability, uptake and expression of adhesion molecules required for immune cell migration.9 Furthermore, current data suggest that LECs suppress dendritic cells (DCs) maturation and further priming of CD8+ T cells, express components of antigen-presenting machinery, major histocompatibility complex (MHC) class I and II molecules, and may be significant contributors to peripheral tolerance.10 Lately, Swartz group provided data suggesting that LECs can constantly uptake and cross-present exogenous Ags to CD8+ T cells, under normal conditions, implying the contribution of LECs in sustaining of CD8+ T-cell tolerance to exogenous Ags present in the lymph.8
The lymphatic system plays an important role not only in physiological but also in pathological conditions. The fluid production reaches up to two-thirds of the total volume of interstitial fluid every day. 11 Hence, any dysregulation of extracellular fluid balance, caused by insufficient lymphatic vessel function, leads to interstitial accumulation of fluid and to lymphedema. Additionally, a high-protein edema fluid triggers an inflammatory reaction, a subsequent fibrosis, an adipose tissue augmentation, an impaired immune response and wound healing.12 Moreover, during the inflammation, the gene expression profile of LECs is changed and the enhancement of C-C chemokine receptor type 7 (CCR7)-positive DCs migration to lymphatic vessels is mainly induced by increased secretion of a CCL21 chemokine.13
The tumor lymphatic vessels development
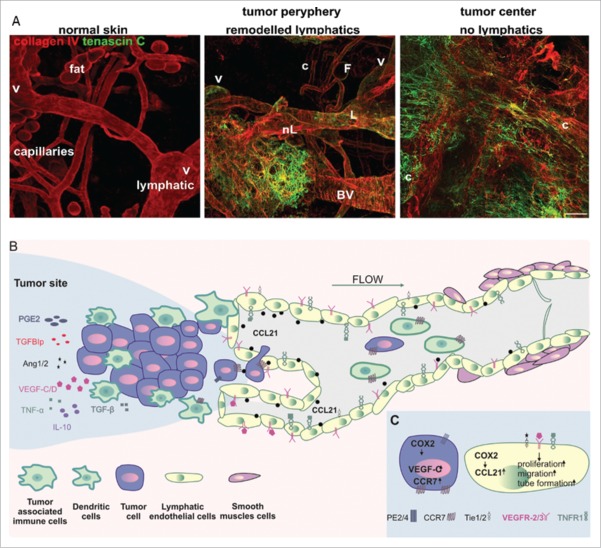
Recent research has defined a tumor lymphangiogenesis as a multifactorial process occurring due to the interactions between tumor, endothelial and immune cells. These cells are the source of protein factors that lead to LECs proliferation, migration and vessels development. Due to a high-interstitial fluid pressure of most solid tumors, the lymphatic vessels in the tumor mass are collapsed and have a limited functionality.9 Thus, they are unable to transport cancer cells to distant organs and their role has remained unclear, whereas lymphatics at the tumor margin (peritumoral) facilitate the spread of cancer cells (Fig. 1A).14
Figure 1.
(A) The lymphatic vessel in normal and mouse melanoma tumor tissue. Left. In normal skin, basement membrane-supported lymphatic vessels (L) can be morphologically distinguished after identification of intravascular valves (V) from blood capillaries (c) and adipocytes (F). Normal tissue is also free from tenascin C, extracellular matrix characteristic for tumor remodeled tissue. Middle. At the tumor margin, new extracellular matrix is deposited and pre-existing lymphatic (L) and blood vessels (BV) are remodeled. Also, new, poorly organized vessels are formed (nL). Right. In the center of the tumor, deposition of new matrix paralleled the loss of organized tissue architecture with large tortuous granulation tissue-like blood (c) vessels and a collapse of lymphatics. (B) The overview of LECs, tumor and tumor-associated immune cells' interactions during tumor lymphangiogenesis and lymphatic vessels enlargement. Secretion of a variety of cytokines and growth factors mobilize tumor cells as well as dendritic cells to get inside the initial lymphatic vessels. (C) COX-2 increases level of prostaglandin receptor (EP2) and enhances expression of VEGF-C, CCR7 as well as CCL21. Binding of VEGFR-3, Tie1/2 and TNFR1 ligands induces LECs proliferation and capability of tube formation.
Tumor cells and tumor-infiltrating myeloid cells stimulate direct formation of peritumoral lymphatic vessels via secretion of IL-10, VEGF, TGF-β and PGE2 (Fig. 1B).15 However, the central role in the embryonic and postnatal lymphatic formation is played by VEGFR-3, that is phosphorylated through the interaction with secreted proteins: VEGF-C and VEGF-D. Nevertheless, the lymphangiogenesis can be additionally initiated by the activation of multiple types of receptor tyrosine kinases such as VEGFR-2, insulin-like growth factor receptor, fibroblast growth factor receptor 3 and angiopoietin receptors (Tie1 and Tie2). However, in view of the fact that tumor cells as well as tumor-associated cells (fibroblasts, immune cells) can overexpress VEGF-C, the VEGF-C-VEGFR-3 pathway has been so far best described.16 The overexpression of VEGF genes seems to be related directly to conditions of tumor environment. The physiological environment of even microscopic tumors can be characterized by high-interstitial pressure and hypoxia that promote tumor growth and metastatic dissemination. The correlation between hypoxia-inducible transcription factor (HIF-1) and lymphangiogenesis was extensively studied.17 HIF-1 was shown to stimulate transcription of important lymphangiogenic factors: platelet derived growth factor B, Prox-1 and SOX18. 18,19
However, it seems that hypoxia can augment the VEGF-C protein levels also via a HIF-1 independent effect on VEGF-C IRES-dependent initiation of translation. Moreover, this hypoxia-induced switching from cap-dependent to IRES-dependent VEGF-C translation was even higher in tumor cells that had metastasized to the lymph nodes compared to tumor cells that were present in the primary tumor.20 These results are in line with the pre-clinical studies on melanoma xenografts showing that lymph nodes metastases correlate with the size of hypoxic tissue fraction in the center of tumor as well as microvascular density in the tumor periphery.21 Interestingly, VEGFR-3 and VEGF-C expressed by metastatic tumor cells, acts as autocrine stimulation mechanisms that may induce tumor cells proliferation and invasiveness.22 Therefore, much focus has been placed lately on VEGF-C implication in the lymphangiogenesis and tumorigenesis. In addition, there are data indicating the role of pro-inflammatory TNF-α-TNFR1 signaling pathway in the VEGF-C-induced lymphangiogenesis. TNF-α stimulates LECs migration and morphological changes in a VEGFR-3-independent manner. The downregulation of TNF-α expression in ovarian tumors restrains lymphangiogenesis and lymphatic metastasis. However, the blocking of VEGFR-3 still suppresses TNF-α-induced lymphatic metastasis.23 Another, newly described secreted protein, involved in the process of tumor lymphangiogenesis is transforming growth factor-β-induced protein (TGFBIp). Tumors expressing TGFBIp develop more metastases via induction of LECs migration and tube formation as well as increase of lymphatic vessels permeability.24 Interestingly, the TGFBIp-induced effect on tumor dissemination can be abolished by lithium treatment, that suppress the metastatic potential of colon cancer without affecting the growth rate of tumor cells.25
The tumor-generated unique conditions of draining lymph nodes, promoting metastases
It has been evident that cancer cells coordinate the pre-metastatic niche formation through the secretion of a variety of cytokines, enzymes and growth factors 26 Pre-treatment of animals with tumor-conditioned media (TCM) not only increases lymphangiogenesis in draining lymph nodes and peripheral areas of tumors but also accelerates metastasis of MDA-MB-231 and SUM-149 in mammary fat pad xenografts models.27 Moreover, it is suggested that in lymph nodes lymphangiogenesis occurs even before the arrival of tumor cells and leads to the formation of the pre-metastatic niche. Interestingly, tumor cells may influence the gene expression profile of LECs. In rat model of gastric cancer, the significant differences between expression profile of LECs, from control and metastatic tumor, were detected in over 800 of genes.28 In LECs from pre-metastatic organs, treated with TCM, expression of CCL5 and VEGF-induced tumor cell recruitment and colonization, is increased. Additionally, these changes in LECs expression profile were observed as a result of tumor-cell-derived-IL-6 action through the phosphorylation of STAT3.29 Although, the role of IL-6 in cancer development has been still controversial, multiple studies have shown that elevated level of IL-6 correlates with a poor clinical prognosis.30 Furthermore, high-tumor expression of IL-6 in human mammary tumors results in mobilization of tumor-associated suppressive myeloid cells and accelerates formation of metastases.31,32 Importantly, induction of IL-6 expression in non-metastatic mouse mammary tumor EMT6 cell line causes recruitment of MDSCs and tumor cell spreads, comparable to that triggered by metastasizing 4T1 cancer cells.33
The VEGF-C-stimulated tumor lymphangiogenesis establishes and increases the efficacy of communication between tumor and lymph nodes. Moreover, initial studies suggest that VEGF-C together with enhanced lymph flow of tumor Ags may promote a suppressive function of LECs, leading to direct inhibition of activated CD8+ T cells and induction of immune tolerance to tumor Ags.3 Although further studies are needed to confirm these data, all these observations can indicate that tumor cells as well as tumor-associated cells influence LECs, which next may actively participate in formation of metastases.
Lymphatic vessels' role in antitumor immune response and metastases formation
Recent research has highlighted that lymphatic vessels can actively contribute to the disease progression through management of leukocyte accumulation and retention within inflamed tissue. Moreover, it seems that lymphatic vessels actively participate in tumor spread as well as induction of immune tolerance. Although, secretion of VEGF-C and VEGF-D by tumor has been correlated with lymphatic metastases both in humans and in animal models, the mechanism of tumor cell escape into lymphatic vessels has still remained unclear.34 Thus, a great deal of interest has been focused on the elucidation of mechanism of tumor cells interaction with LECs (Table 1).
Table 1.
Molecules potentially involved in tumor cells trafficking through the lymphatic vessels.
| Molecule involved in cell migration | Role in leucocytes trafficking by lymphatic vessels | Expression on tumor cells | Influence on tumor progression |
|---|---|---|---|
| CCR7 | Upregulated CCR7 during activation and maturation of DCs to respond to lymphatic-secreted CCL21 and elicits directional migration62 | Melanoma, colorectal, mammary, gastric, non-small cell lung, head and neck cancers, thyroid and squama cell carcinomas42,63 | Expression is correlated with lymph node metastasis. |
| CCR4 | Selectively expressed on Th2 cells and regulatory T cells | Breast, lung, gastric cancer 64 | Expression is associated with lung and lymph nodes metastasis. In breast cancer, correlated with HER2 positive tumors and poor prognosis |
| ICAM-1 | ICAM-1 knockout mice have defects in lymph node recruitment of DCs43 | Melanoma, breast cancer, gastric cancer, esophageal cancer, colorectal carcinoma65 | The results are controversial. In some cases ICAM-1 plays a major role in invasion of cancerous cells while in others decreased expression inhibits formation of metastases66 |
| COX-2 | COX-2 plays an important role in leucocytes migration and adhesion, likely by modulating p110g PI3K–mediated cell signaling67 | Breast cancer, esophageal cancer pancreatic cancer, various colorectal tumors, adenocarcinoma, prostate and bladder cancers68 | Upregulates CCR7 via EP2/EP4 receptor signaling pathways and enhances lymphatic metastasis |
| CD99 | Involved in transmigration of monocytes, neutrophils, lymphocytes and DCs | Ewing sarcoma, synovial sarcoma and low-grade fibromyxoid sarcoma69 | Knocking down CD99 in Ewing sarcoma reduces tumor ability to form metastases |
CCR7 was one of the first described molecules, overexpressed by tumor cells that increases their lymphatic-dependent metastatic potential. It is suggested that CCR7 is upregulated in many cancer cells and guide them up lymphatic-secreted CCL21 gradients.35 In breast cancer, the expression of CCR7 was reported to be increased by cyclooxygenase-2 (COX-2) activity via prostaglandin E2 and E4 receptors (Fig. 1C).36 High expression of this enzyme was correlated with lymph node metastasis, poor prognosis and short survival.37 The anti-CCR7 antibody as well as knockdown of CCR7 in COX-2-overexpressing MDAMB-231 cells significantly attenuates the effect of migration and invasion of breast cancer cells.36 Additionally, COX-2 enhances secretion of VEGF-C, that in turn increases expression of CCR7 ligand, and CCL21 by LECs as well as development of new lymphatic vessels.38 Tumor cells also secrete CCR7 ligands to generate autologous gradients of CCL19/21 chemokines that promote their migration toward functional lymphatics.39 The selective presentation of CCR7 ligands on LECs can be explained by the expression of non-typical chemokine receptor D6. Due to the expression of D6, only cells that are CCR7 positive (e.g., mature DCs) are able to migrate to lymph nodes.40 However, D6 expressed by tumor cells, was shown to inhibit the proliferation of tumor cells and was negatively correlated with prognosis in breast cancer.41
Another chemokine receptor involved in tumor cells spread through lymphatic vessels is CCR4. CXCL12 through CCR4 leads to actin polymerization and pseudopodia formation of breast tumor cells and have a significant influence on metastasis to regional lymph nodes and lungs.42
Although the chemokine-dependent migration mechanism of tumor cells appears to be similar to those used by DCs, this similarity to DCs cannot be established as a rule for other molecules. For example ICAM-1 promotes the exit of leukocytes from tissue to lymphatics.43 However, in case of tumor, the role of adhesion molecules in metastatic dissemination has not been yet fully understood. In melanoma, the high expression of ICAM-1 correlates with the risk of metastasis, while breast cancer patients with CAMs-positive tumors have a better prognosis.44 Nevertheless, it is probable that some molecules like CD99 may be involved in migration of leucocytes as well as tumor cells. CD99 has been described as a transmigration mediator of monocytes, neutrophils, lymphocytes and DCs.45 The results with anti-CD99 antibodies suggest a homophilic interaction between CD99 on the leukocytes and CD99 on the endothelial cells.46 Interestingly, the truncated isoform of CD99 enhances migration and metastasis ability of cancer cells, while the full length of CD99 acts as an onco-suppressor in a wider group of tumors.47 However, the direct mechanism of tumor cells and LECs interaction mediated by CD99 has not been yet investigated.
The anti-lymphangiogenic therapies
Monoclonal antibodies and recombinant proteins
Angiogenesis and lymphangiogenesis play a pivotal role in tumor cells growth and dissemination, therefore, factors involved in these processes are potential targets in antitumor therapies. Since VEGF-C/VEGF-D-VEGFR-3 signaling axis is the most prominent in lymphangiogenesis, neutralization of this pathway in metastatic disease is provided with either anti-VEGF or anti-VEGFR antibodies or soluble form of VEGF receptor—sVEGFR-3. Interestingly, VEGFR-3 and VEGFR-2 together promote proliferation and migration of LECs, therefore simultaneously blocking both of the receptors seems to be more effective in inhibition of peritumoral lymphangiogenesis than single anti-VEGFR treatment (Table 2). Bevacizumab, an anti-VEGF antibody, is applied in the treatment of colon, non-small cell lung, kidney and ovarian cancer. Moreover, there are some ongoing clinical trials using bevacizumab in combination with different drugs, such as dexamethasone or VGX-100. VGX-100 is an anti-VEGF-C antibody, which is currently in phase I of clinical trials.48 However, in the mentioned combined therapies, VEGF-D is still active and may induce lymphatic vessels formation, therefore these therapies only partially block peritumoral lymphangiogenesis. Another agent with promising effect in pre-clinical studies is AMG-386 (Trebananib) an Angiopoietin-1/2-neutralizing peptibody, consisting of Ang-binding sequence fused with Fc region of antibody. It prevents Ang1/2 binding to Tie-2 receptor, therefore inhibits the growth of tumor in mouse xenograft models and currently is in phase III of clinical trials in ovarian cancer treatment.49 Moreover, AMG-386 is under current investigation in glioblastoma therapy combined with bevacizumab, however this study has not been completed yet.50 There are some interesting results indicating that sVEGFR-3 poses soluble extracellular ligand-binding domain, able to trap VEGF-C and leads to its inactivation, what results in inhibition of lymphangiogenesis. Also, ramucirumab, the fully human monoclonal antibody that binds to VEGFR-2 inhibits not only angiogenesis but may affect lymphangiogenesis. 51
Table 2.
Overview of lymphangiogenesis inhibitors investigated in pre-clinical studies.
| Drug name | Molecular target | Treatment | Mechanism of action | Outcome |
|---|---|---|---|---|
| Soluble VEGFR-370 | VEGF-C | Pre-clinical studies in endometrial cancer model | Inhibits lymphatic endothelial cell growth in vitro | Suppresses in vivo lymph node and lung metastasis |
| Canstatin71 | Ang1/Ang2 | Pre-clinical studies in colon carcinoma model | Reduces tumor blood and lymphatic vessel densities | Reduces final volume and weight of tumors |
| Endostatin72 | VEGFR-3 | Pre-clinical | Causes inhibition of bFGF-induced corneal angiogenesis and lymphangiogenesis | — |
| 16K hPRL73 | VEGFR-3 | Pre-clinical studies in melanoma model | Induces apoptosis and inhibits proliferation, migration and tube formation of human dermal lymphatic microvascular endothelial cells | Prevents lymphatic metastasis |
| SAR13167574 | VEGFR-3 | Pre-clinical studies in breast cancer model | Reduces TAM infiltration | Reduces lymph node and lung metastasis |
| cVE-19975 | VEGF-D | Pre-clinical studies in neuroblastoma model | Inhibits lymphangiogenesis | Prevents lymphatic metastasis of neuroblastoma |
| Nrp276 | Semaphorin | Pre-clinical studies in breast cancer model | Inhibits VEGF-C-induced phosphorylation of VEGFR-3, ERK1/2, and AKT | Tumor cells expressing sema3C contained a lower concentration of lymph vessels and form lymph nodes metastasis much less effectively |
| Biomimetic peptide SP201253 | c-MET | Pre-clinical studies in breast cancer model | Inhibits blood and lymphatic endothelial cell viability, migration, adhesion and tube formation | Inhibits lymphangiogenesis in primary tumors |
| Rapamycin77 | mTOR | Pre-clinical studies in head and neck cancer model | Inhibits lymphangiogenesis | Prevents dissemination to the cervical lymph nodes |
Chemotherapy
Another group of antitumor lymphangiogenesis drugs are small molecule receptor tyrosine kinase inhibitors targeting VEGFR-3 including regorafenib used in the treatment of metastatic colorectal cancer and gastrointestinal stromal tumors and axitinib applied in renal cell carcinoma therapy. A promising choice for anti-lymphangiogenic therapy is lenalidomide (LEN). This immunomodulatory agent currently is used in the treatment of multiple myeloma, transfusion-dependent myelodysplastic syndrome and mantle cell lymphoma. In LECs, LEN is shown to reduce levels of PROX-1 factor, podoplanin and VEGFR-3. Several studies indicate that LEN affects not only LECs but also tumor-associated macrophages (TAMs), which are primarily responsible for the secretion of VEGF-C. Additionally, LEN triggers various effects on the immune system, which may contribute to its therapeutic outcome. It stimulates CD4+ and CD8+ T lymphocytes and also increases the expression of IL-2 and IFNγ.52
Novel anti-lymphatic agent, collagen IV biomimetic peptide (SP2012), inhibits metastases to lungs in breast cancer tumor xenograft model and leads to LECs apoptosis.53 Moreover, other well-known kinase inhibitors including sorafenib, sunitinib and pazopanib are already approved for the treatment of various cancer by Food and Drug Administration (Table 3). These drugs, well known for their anti-angiogegenic action, also prevent phosphorylation of VEGFR-3, leading to lymphangiogenesis inhibition.54,55
Table 3.
Overview of lymphangiogenesis inhibitors investigated in clinical studies.
| Drug name | Molecular target | As a monotherapy | As a combined therapy |
|---|---|---|---|
| VGX-100 | VEGF-C | — | With bevacizumab – Phase I ongoing (NCT01514123) in treatment of advanced solid tumors |
| Lenalidomide | VEGF-C | Lenalidomide is used in pre-clinical studies to inhibit growth of peritumoral lymphatic vessels | |
| Bevacizumab | VEGF | Bevacizumab is an inhibitor of angiogenesis studied in various pre-clinical trials as anti-lymphangiogenic drug. Approved for various treatment: breast, lung, colorectal, renal and brain cancer | |
| AMG-386 (Trebananib) | Ang1/Ang2 | Treatment of endometrial adenocarcinoma – Phase II ongoing (NCT01210222). | Used in the treatment of renal cell carcinoma with sorafenib – Phase II completed (NCT00467025). No data have been published so far |
| Treatment of advanced solid tumors – Phase I completed (NCT00102830). No data have been published so far | Used in the treatment of renal cell carcinoma with sunitinib – Phase II ongoing (NCT00853372) | ||
| MEDI3617 | Ang1/Ang2 | — | Used in treatment of melanoma with tremelimumab – Phase I ongoing (NCT02141542). |
| Used in the treatment of advanced solid tumors with bevacizumab/paclitaxel/carboplatin/gemcitabine – Phase I completed (NCT01248949). No data have been published so far | |||
| CVX-060 | Ang1/Ang2 | Treatment of advanced solid tumors – Phase I completed (NCT00879684). 0.3, 1, 3, 6, 12, 15 mg/kg of b.w. intravenous infusion in Stage 1 and 15 mg/kg of b.w. intravenous infusion in Stage 2, administered once-weekly in a 4-week cycle | — |
| Sorafenib | VEGFR-3 | Sorafenib inhibits VEGFR-2 and VEGFR-3, blocks proliferation of different tumor cells and inhibits tumor lymphangiogenesis.78 Was approved for renal cell carcinoma and hepatocellular carcinoma treatment. | |
| Sunitinib | VEGFR-3 | In pre-clinical studies, sunitinib blocked VEGFR-2 and VEGFR-3 phosphorylation induced by VEGF-C or VEGF-D and inhibited LECs proliferation and migration.79 Was clinically approved for renal cell carcinoma and gastrointestinal stromal tumor treatment | |
| Axitinib | VEGFR-3 | In pre-clinical studies used as VEGFR-3 inhibitor.55 Approved for renal cell carcinoma treatment | |
| Regorafenib | VEGFR-3 | Regorafenib is used in pre-clinical studies to inhibit VEGFR-2 and VEGFR-3 autophosphorylation, VEGFR-3 intracellular signaling and to block LECs migration.80 | |
| Pazopanib | VEGFR-2/ VEGFR-3 | Pazopanib exert anti-angiogenic and anti-lymphangiogenic potential in pre-clinical studies as VEGFR-2 and VEGFR-3 inhibitor.55 In clinical studies is use in combination with bevacizumab. Approved for renal cell carcinoma treatment | |
| IMC-3C5 | VEGFR-3 | Treatment of neoplasma – Phase I completed (NCT01288989). No data have been published so far | — |
All these experimental and clinical studies highlighted the critical role of lymphatic vasculature in tumor metastatic spreading and point them as antitumor therapies target. Although lymphatic vessels are an important element of human immune system function, little is known so far about the impact of lymphatic destruction on immune response to cancer cells.
Photodynamic therapy as a new anti-lymphatic approach
Recently described approach used to damage lymphatic vessels is PDT. PDT is a light-based therapeutic modality approved for the treatment of various solid tumors as well as non-oncological conditions such as age macular degeneration (AMD). In the clinical settings, PDT procedure requires administration of a photosensitizing drug, that selectively accumulates in the tumor tissue, and irradiation of the lesion with a visible light of an appropriate wavelength.56 Light-excited photosensitizer transfers its energy to the molecular oxygen, leading to formation of reactive oxygen species. Antitumor effects of PDT result from direct tumor damage, collapse of tumor vasculature and induction of antitumor immune response.57 Antivascular effect of PDT has been extensively studied during the past decades, whereas anti-lymphatic action of PDT is a recently described phenomenon. While pre-existing lymphatic vessels cannot be eradicated with anti-lymphangiogenic agents, PDT can be applied for the destruction of even pre-existing tumor lymphatic vessels. Tammela et al. have been the first to publish that verteporfin-PDT can damage tumor-associated lymphatic vessels in mice and pigs. Moreover, PDT of tumor lymphatic vasculature led to the eradication of intra-lymphatic tumor cells and prevented metastasis of mouse melanoma cells and subsequent recurrence. Interestingly, Tammela et al. also have shown that combination treatment of PDT and AdVEGFR-3-Ig reduces the surface area of peritumoral lymphatic capillaries when compared with single treatment.58 Furthermore, as PDT is an established procedure in various ophthalmological diseases such as AMD, Bucher et al. have used PDT to induce regression of corneal lymphatic vessels without affecting blood vessels in mouse model.
In our previous study, we have identified the optimal conditions to selectively close lymphatic collecting vessels without injuring the blood vasculature in a mouse ear dermis.59 We have shown that PDT selectively ablates lymphatic vessels and subsequently leads to closure of lymphatic drainage in a particular region. According to our recent results, both apoptosis and autophagy are involved in cell death induced by verteporfin-PDT in LECs.60 In addition to Tammela studies presenting that PDT-damaged lymphatic vessels regrowth after stimulation with VEGF-C, we have shown that lymphatic vessels eventually regenerate, without any additional treatment, by recanalization of blocked collectors leading to restoration of lymphatic drainage.58,59 Therefore, in order to avoid tumor metastasis and further relapse, treatment combining PDT with anti-lymphangiogenic agents seems to be rational. Such combination therapy would be a promising approach as, according to current literature, there are several known pharmacological inhibitors of lymphangiogenesis as well as it is possible to obtain recombinant proteins able to suppress formation of lymphatic vasculature.
However, we would like to emphasize an undiscussed problem concerning anti-lymphangiogenic therapies' influence on development of specific antitumor immune response. Since Burnet and Thomas extended “immune surveillance hypothesis,” suggesting that cancer cells are recognized as “foreign” by the immune system, various immune therapies have been extensively studied. Therefore, new effective therapies, stimulating immune system and overcoming tumor suppression, become available including, exciting recent developments, immune checkpoint blockade.61 PDT is also thought to be an immune therapy as it can induce strong local inflammatory reaction that under some unique circumstances and in combination with immune-modulating drugs can lead to the development of systemic antitumor immune response and the formation of memory responses.57 A prolonged damage of tumor lymphatic vasculature may totally abrogate immune cell trafficking from the tumor to the draining lymph nodes and can subsequently lead to impaired development of antitumor immune response (unpublished results). Thus, we speculate that, even though anti-lymphangiogenic therapies can overcome tumor tolerance and prevent metastasis, it may affect antitumor immune response leading to poor PDT outcome.
Conclusions
In the light of the latest findings, the lymphatic vessels are not only passive channels but also can participate in the development of antitumor immune response. It is becoming clear that the process of cells' entry into the lymphatic vessels requires active and highly complex interactions with LECs. However, in contrast to blood vessels the mechanisms of tumor cell intravasation has not been well described. Hence, it is not known what type of junctions are preferred between tumor cell interactions and whether the initial or/and collecting vessels are the gates for metastatic cells entry. Therefore, to better understand the molecular control of immune and tumor cells interaction with LECs, further research is warranted.
The latest discoveries have demonstrated the potential of novel therapeutic strategies to prevent formation of tumor metastases. As we discussed here, the VEGF-C-stimulated tumor lymphangiogenesis may have important bearing on formation of pre-metastatic niche in draining lymph nodes, and promotes progression and dissemination of tumor cells. Nevertheless, the lymphatic vessels' role in the process of antigen presentation and antitumor adaptive immune response development should not be diminished, especially in the light of recently described effective immunotherapies. Thus, the currently generated inhibitors of VEGF-C-VEGFR-3 pathway are probably just the beginning of therapies modulating lymphatic function and hopefully the nearest future will bring great advances in this field.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The experiments (Fig. 1A) were carried out in mice according to a protocol approved by the Committee for Animal Experiments for the Canton Vaud, Switzerland (authorization 2687).
Funding
This work was supported by grant from the Polish -Swiss Research Program (PSPB-057/2010) and MUW grant 1M19/PM2/16/16.
References
- 1.Stacker SA, Williams SP, Karnezis T, Shayan R, Fox SB, Achen MG. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat Rev Cancer 2014; 14:159-72; PMID:24561443; http://dx.doi.org/ 10.1038/nrc3677 [DOI] [PubMed] [Google Scholar]
- 2.Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M et al.. Sox18 induces development of the lymphatic vasculature in mice. Nature 2008; 456:643-7; PMID:18931657; http://dx.doi.org/ 10.1038/nature07391 [DOI] [PubMed] [Google Scholar]
- 3.Lund AW, Duraes FV, Hirosue S, Raghavan VR, Nembrini C, Thomas SN, Issa A, Hugues S, Swartz MA. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Rep 2012; 1:191-9; PMID:22832193; http://dx.doi.org/ 10.1016/j.celrep.2012.01.005 [DOI] [PubMed] [Google Scholar]
- 4.Maby-El Hajjami H, Petrova TV. Developmental and pathological lymphangiogenesis: from models to human disease. Histochem Cell Biol 2008; 130:1063-78; PMID:18946678; http://dx.doi.org/ 10.1007/s00418-008-0525-5 [DOI] [PubMed] [Google Scholar]
- 5.Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS et al.. Structural and functional features of central nervous system lymphatic vessels. Nature 2015; 523:337-41; PMID:26030524; http://dx.doi.org/ 10.1038/nature14432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E et al.. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 2007; 204:2349-62; PMID:17846148; http://dx.doi.org/ 10.1084/jem.20062596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dieterich LC, Detmar M. Tumor lymphangiogenesis and new drug development. Adv Drug Deliv Rev 2016 Apr 1; 99(Pt B):148-60; PMID:26705849; http://dx.doi.org/ 10.1016/j.addr.2015.12.011 [DOI] [PubMed] [Google Scholar]
- 8.Hirosue S, Vokali E, Raghavan VR, Rincon-Restrepo M, Lund AW, Corthesy-Henrioud P, Capotosti F, Halin Winter C, Hugues S, Swartz MA. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. J Immunol 2014; 192:5002-11; PMID:24795456; http://dx.doi.org/ 10.4049/jimmunol.1302492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund AW, Swartz MA. Role of lymphatic vessels in tumor immunity: passive conduits or active participants? J Mammary Gland Biol Neoplasia 2010; 15:341-52; PMID:20835756; http://dx.doi.org/ 10.1007/s10911-010-9193-x [DOI] [PubMed] [Google Scholar]
- 10.Fletcher AL, Malhotra D, Turley SJ. Lymph node stroma broaden the peripheral tolerance paradigm. Trends Immunol 2011; 32:12-8; PMID:21147035; http://dx.doi.org/ 10.1016/j.it.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rovenska E, Rovensky J. Lymphatic vessels: structure and function. Isr Med Assoc J 2011; 13:762-8; PMID:22332449 [PubMed] [Google Scholar]
- 12.Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: a comprehensive review. Ann Plast Surg 2007; 59:464-72; PMID:17901744; http://dx.doi.org/ 10.1097/01.sap.0000257149.42922.7e [DOI] [PubMed] [Google Scholar]
- 13.Vigl B, Aebischer D, Nitschke M, Iolyeva M, Rothlin T, Antsiferova O, Halin C. Tissue inflammation modulates gene expression of lymphatic endothelial cells and dendritic cell migration in a stimulus-dependent manner. Blood 2011; 118:205-15; PMID:21596851; http://dx.doi.org/ 10.1182/blood-2010-12-326447 [DOI] [PubMed] [Google Scholar]
- 14.Duong T, Koopman P, Francois M. Tumor lymphangiogenesis as a potential therapeutic target. J Oncol 2012; 2012:204946; PMID:22481918; http://dx.doi.org/ 10.1155/2012/204946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoppmann SF, Birner P, Stockl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 2002; 161:947-56; PMID:12213723; http://dx.doi.org/ 10.1016/S0002-9440(10)64255-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell 2005; 7:121-7; PMID:15710325; http://dx.doi.org/ 10.1016/j.ccr.2005.01.017 [DOI] [PubMed] [Google Scholar]
- 17.Min Y, Ghose S, Boelte K, Li J, Yang L, Lin PC. C/EBP-delta regulates VEGF-C autocrine signaling in lymphangiogenesis and metastasis of lung cancer through HIF-1alpha. Oncogene 2011; 30:4901-9; PMID:21666710; http://dx.doi.org/ 10.1038/onc.2011.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schito L, Rey S, Tafani M, Zhang H, Wong CC, Russo A, Russo MA, Semenza GL. Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells. Proc Natl Acad Sci USA 2012; 109:E2707-16; PMID:23012449; http://dx.doi.org/ 10.1073/pnas.1214019109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bridges JP, Lin S, Ikegami M, Shannon JM. Conditional hypoxia inducible factor-1alpha induction in embryonic pulmonary epithelium impairs maturation and augments lymphangiogenesis. Dev Biol 2012; 362:24-41; PMID:22094019; http://dx.doi.org/ 10.1016/j.ydbio.2011.10.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morfoisse F, Kuchnio A, Frainay C, Gomez-Brouchet A, Delisle MB, Marzi S, Helfer AC, Hantelys F, Pujol F, Guillermet-Guibert J et al.. Hypoxia induces VEGF-C expression in metastatic tumor cells via a HIF-1alpha-independent translation-mediated mechanism. Cell reports 2014; 6:155-67; PMID:24388748; http://dx.doi.org/ 10.1016/j.celrep.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 21.Rofstad EK, Galappathi K, Mathiesen BS. Tumor interstitial fluid pressure – a link between tumor hypoxia, microvascular density, and lymph node metastasis. Neoplasia 2014; 16:586-94; PMID:25117980; http://dx.doi.org/ 10.1016/j.neo.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Trappen PO, Steele D, Lowe DG, Baithun S, Beasley N, Thiele W, Weich H, Krishnan J, Shepherd JH, Pepper MS et al.. Expression of vascular endothelial growth factor (VEGF)-C and VEGF-D, and their receptor VEGFR-3, during different stages of cervical carcinogenesis. J Pathol 2003; 201:544-54; PMID:14648657; http://dx.doi.org/ 10.1002/path.1467 [DOI] [PubMed] [Google Scholar]
- 23.Ji H, Cao R, Yang Y, Zhang Y, Iwamoto H, Lim S, Nakamura M, Andersson P, Wang J, Sun Y et al.. TNFR1 mediates TNF-alpha-induced tumour lymphangiogenesis and metastasis by modulating VEGF-C-VEGFR3 signalling. Nat Commun 2014; 5:4944; PMID:25229256; http://dx.doi.org/ 10.1038/ncomms5944 [DOI] [PubMed] [Google Scholar]
- 24.Maeng YS, Aguilar B, Choi SI, Kim EK. Inhibition of TGFBIp expression reduces lymphangiogenesis and tumor metastasis. Oncogene 2016; 35:196-205; PMID:25772247; http://dx.doi.org/ 10.1038/onc.2015.73 [DOI] [PubMed] [Google Scholar]
- 25.Maeng YS, Lee R, Lee B, Choi SI, Kim EK. Lithium inhibits tumor lymphangiogenesis and metastasis through the inhibition of TGFBIp expression in cancer cells. Scientific Rep 2016; 6:20739; PMID:26857144; http://dx.doi.org/ 10.1038/srep20739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steeg PS. Cancer biology: emissaries set up new sites. Nature 2005; 438:750-1; PMID:16341000; http://dx.doi.org/ 10.1038/438750b [DOI] [PubMed] [Google Scholar]
- 27.Lee E, Pandey NB, Popel AS. Pre-treatment of mice with tumor-conditioned media accelerates metastasis to lymph nodes and lungs: a new spontaneous breast cancer metastasis model. Clin Exp Metastasis 2014; 31:67-79; PMID:23963763; http://dx.doi.org/ 10.1007/s10585-013-9610-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Zhang C, He Y, Wu H, Wang Z, Song W, Li W, He W, Cai S, Zhan W. Lymphatic endothelial cell-secreted CXCL1 stimulates lymphangiogenesis and metastasis of gastric cancer. Int J Cancer J Int du Cancer 2012; 130:787-97; PMID:21387301; http://dx.doi.org/ 10.1002/ijc.26035 [DOI] [PubMed] [Google Scholar]
- 29.Lee E, Fertig EJ, Jin K, Sukumar S, Pandey NB, Popel AS. Breast cancer cells condition lymphatic endothelial cells within pre-metastatic niches to promote metastasis. Nat Commun 2014; 5:4715; PMID:25178650; http://dx.doi.org/ 10.1038/ncomms5715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schafer ZT, Brugge JS. IL-6 involvement in epithelial cancers. J Clin Investig 2007; 117:3660-3; PMID:18060028; http://dx.doi.org/ 10.1172/JCI34237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X et al.. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia 2013; 15:848-62; PMID:23814496; http://dx.doi.org/ 10.1593/neo.13706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fertig EJ, Lee E, Pandey NB, Popel AS. Analysis of gene expression of secreted factors associated with breast cancer metastases in breast cancer subtypes. Scientific reports 2015; 5:12133; PMID:26173622; http://dx.doi.org/ 10.1038/srep12133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh K, Lee OY, Shon SY, Nam O, Ryu PM, Seo MW, Lee DS. A mutual activation loop between breast cancer cells and myeloid-derived suppressor cells facilitates spontaneous metastasis through IL-6 trans-signaling in a murine model. Breast Cancer Res 2013; 15:R79; PMID:24021059; http://dx.doi.org/ 10.1186/bcr3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amioka T, Kitadai Y, Tanaka S, Haruma K, Yoshihara M, Yasui W, Chayama K. Vascular endothelial growth factor-C expression predicts lymph node metastasis of human gastric carcinomas invading the submucosa. Eur J Cancer 2002; 38:1413-9; PMID:12091074; http://dx.doi.org/ 10.1016/S0959-8049(02)00106-5 [DOI] [PubMed] [Google Scholar]
- 35.Shields JD, Emmett MS, Dunn DB, Joory KD, Sage LM, Rigby H, Mortimer PS, Orlando A, Levick JR, Bates DO. Chemokine-mediated migration of melanoma cells towards lymphatics–a mechanism contributing to metastasis. Oncogene 2007; 26:2997-3005; PMID:17130836; http://dx.doi.org/ 10.1038/sj.onc.1210114 [DOI] [PubMed] [Google Scholar]
- 36.Pan MR, Hou MF, Chang HC, Hung WC. Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. J Biol Chem 2008; 283:11155-63; PMID:18319253; http://dx.doi.org/ 10.1074/jbc.M710038200 [DOI] [PubMed] [Google Scholar]
- 37.Denkert C, Winzer KJ, Muller BM, Weichert W, Pest S, Kobel M, Kristiansen G, Reles A, Siegert A, Guski H et al.. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease free survival and overall survival in patients with breast carcinoma. Cancer 2003; 97:2978-87; PMID:12784332; http://dx.doi.org/ 10.1002/cncr.11437 [DOI] [PubMed] [Google Scholar]
- 38.Tutunea-Fatan E, Majumder M, Xin X, Lala PK. The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis. Mol Cancer 2015; 14:35; PMID:25744065; http://dx.doi.org/ 10.1186/s12943-015-0306-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shields JD, Fleury ME, Yong C, Tomei AA, Randolph GJ, Swartz MA. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 2007; 11:526-38; PMID:17560334; http://dx.doi.org/ 10.1016/j.ccr.2007.04.020 [DOI] [PubMed] [Google Scholar]
- 40.McKimmie CS, Singh MD, Hewit K, Lopez-Franco O, Le Brocq M, Rose-John S, Lee KM, Baker AH, Wheat R, Blackbourn DJ et al.. An analysis of the function and expression of D6 on lymphatic endothelial cells. Blood 2013; 121:3768-77; PMID:23479571; http://dx.doi.org/ 10.1182/blood-2012-04-425314 [DOI] [PubMed] [Google Scholar]
- 41.Wu FY, Ou ZL, Feng LY, Luo JM, Wang LP, Shen ZZ, Shao ZM. Chemokine decoy receptor d6 plays a negative role in human breast cancer. Mol Cancer Res 2008; 6:1276-88; PMID:18708360; http://dx.doi.org/ 10.1158/1541-7786.MCR-07-2108 [DOI] [PubMed] [Google Scholar]
- 42.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN et al.. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001; 410:50-6; PMID:11242036; http://dx.doi.org/ 10.1038/35065016 [DOI] [PubMed] [Google Scholar]
- 43.Johnson LA, Clasper S, Holt AP, Lalor PF, Baban D, Jackson DG. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med 2006; 203:2763-77; PMID:17116732; http://dx.doi.org/ 10.1084/jem.20051759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan J, Jiang Y, Ye M, Liu W, Feng L. The clinical value of lymphatic vessel density, intercellular adhesion molecule 1 and vascular cell adhesion molecule 1 expression in patients with oral tongue squamous cell carcinoma. J Cancer Res Therapeutics 2014; 10 Suppl:C125-30; PMID:25450269; http://dx.doi.org/ 10.4103/0973-1482.145827 [DOI] [PubMed] [Google Scholar]
- 45.Winger RC, Harp CT, Chiang MY, Sullivan DP, Watson RL, Weber EW, Podojil JR, Miller SD, Muller WA. Cutting edge: CD99 is a novel therapeutic target for control of T cell-mediated central nervous system autoimmune disease. J Immunol 2016; 196:1443-8; PMID:26773145; http://dx.doi.org/ 10.4049/jimmunol.1501634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lou O, Alcaide P, Luscinskas FW, Muller WA. CD99 is a key mediator of the transendothelial migration of neutrophils. J Immunol 2007; 178:1136-43; PMID:17202377; http://dx.doi.org/ 10.4049/jimmunol.178.2.1136 [DOI] [PubMed] [Google Scholar]
- 47.Scotlandi K, Zuntini M, Manara MC, Sciandra M, Rocchi A, Benini S, Nicoletti G, Bernard G, Nanni P, Lollini PL et al.. CD99 isoforms dictate opposite functions in tumour malignancy and metastases by activating or repressing c-Src kinase activity. Oncogene 2007; 26:6604-18; PMID:17471235; http://dx.doi.org/ 10.1038/sj.onc.1210481 [DOI] [PubMed] [Google Scholar]
- 48.Hajrasouliha AR, Funaki T, Sadrai Z, Hattori T, Chauhan SK, Dana R. Vascular endothelial growth factor-C promotes alloimmunity by amplifying antigen-presenting cell maturation and lymphangiogenesis. Investig Ophthalmol Visual Sci 2012; 53:1244-50; PMID:22281820; http://dx.doi.org/ 10.1167/iovs.11-8668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mita AC, Takimoto CH, Mita M, Tolcher A, Sankhala K, Sarantopoulos J, Valdivieso M, Wood L, Rasmussen E, Sun YN et al.. Phase 1 study of AMG 386, a selective angiopoietin 1/2-neutralizing peptibody, in combination with chemotherapy in adults with advanced solid tumors. Clin Cancer Res 2010; 16:3044-56; PMID:20501621; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-3368 [DOI] [PubMed] [Google Scholar]
- 50.Dieras V, Wildiers H, Jassem J, Dirix LY, Guastalla JP, Bono P, Hurvitz SA, Gonçalves A, Romieu G, Limentani SA et al.. Trebananib (AMG 386) plus weekly paclitaxel with or without bevacizumab as first-line therapy for HER2-negative locally recurrent or metastatic breast cancer: a phase 2 randomized study. Breast 2015; 24:182-90; PMID:25747197; http://dx.doi.org/ 10.1016/j.breast.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 51.Spratlin JL, Cohen RB, Eadens M, Gore L, Camidge DR, Diab S, Leong S, O'Bryant C, Chow LQ, Serkova NJ et al.. Phase I pharmacologic and biologic study of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor-2. J Clin Oncol 2010; 28:780-7; PMID:20048182; http://dx.doi.org/ 10.1200/JCO.2009.23.7537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song K, Herzog BH, Sheng M, Fu J, McDaniel JM, Chen H, Ruan J, Xia L. Lenalidomide inhibits lymphangiogenesis in preclinical models of mantle cell lymphoma. Cancer Res 2013; 73:7254-64; PMID:24158094; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-0750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee E, Lee SJ, Koskimaki JE, Han Z, Pandey NB, Popel AS. Inhibition of breast cancer growth and metastasis by a biomimetic peptide. Scientific Rep 2014; 4:7139; PMID:25409905; http://dx.doi.org/ 10.1038/srep07139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilhelm SM, Adnane L, Newell P, Villanueva A, Llovet JM, Lynch M. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol Cancer Ther 2008; 7:3129-40; PMID:18852116; http://dx.doi.org/ 10.1158/1535-7163.MCT-08-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mihaly Z, Sztupinszki Z, Surowiak P, Gyorffy B. A comprehensive overview of targeted therapy in metastatic renal cell carcinoma. Current cancer drug targets 2012; 12:857-72; PMID:22515521; http://dx.doi.org/ 10.2174/156800912802429265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wachowska M, Muchowicz A, Golab J. Targeting epigenetic processes in photodynamic therapy-induced anticancer immunity. Front Oncol 2015; 5:176; PMID:26284197; http://dx.doi.org/ 10.3389/fonc.2015.00176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D et al.. Photodynamic therapy of cancer: an update. CA Cancer J Clin 2011; 61:250-81; PMID:21617154; http://dx.doi.org/ 10.1017/S0009840X10002799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norrmen C, Tammela T, Petrova TV, Alitalo K. Biological basis of therapeutic lymphangiogenesis. Circulation 2011; 123:1335-51; PMID:21444892; http://dx.doi.org/ 10.1161/CIRCULATIONAHA.107.704098 [DOI] [PubMed] [Google Scholar]
- 59.Kilarski WW, Muchowicz A, Wachowska M, Mezyk-Kopec R, Golab J, Swartz MA, Nowak-Sliwinska P. Optimization and regeneration kinetics of lymphatic-specific photodynamic therapy in the mouse dermis. Angiogenesis 2014; 17:347-57; PMID:23892627; http://dx.doi.org/ 10.1007/s10456-013-9365-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wachowska M, Osiak A, Muchowicz A, Gabrysiak M, Domagala A, Kilarski WW, Golab J. Investigation of cell death mechanisms in human lymphatic endothelial cells undergoing photodynamic therapy. Photodiagnosis and photodynamic therapy 2016; Jun;14:57-65; PMID:26868051; http://dx.doi.org/ 10.1016/j.pdpdt.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Van der Jeught K, Bialkowski L, Daszkiewicz L, Broos K, Goyvaerts C, Renmans D, Van Lint S, Heirman C, Thielemans K, Breckpot K. Targeting the tumor microenvironment to enhance antitumor immune responses. Oncotarget 2015; 6:1359-81; PMID:25682197; http://dx.doi.org/ 10.18632/oncotarget.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999; 99:23-33; PMID:10520991; http://dx.doi.org/ 10.1016/S0092-8674(00)80059-8 [DOI] [PubMed] [Google Scholar]
- 63.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett 2007; 256:137-65; PMID:17629396; http://dx.doi.org/ 10.1016/j.canlet.2007.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li JY, Ou ZL, Yu SJ, Gu XL, Yang C, Chen AX, Di GH, Shen ZZ, Shao ZM. The chemokine receptor CCR4 promotes tumor growth and lung metastasis in breast cancer. Breast Cancer Res Treat 2012; 131:837-48; PMID:21479551; http://dx.doi.org/ 10.1007/s10549-011-1502-6 [DOI] [PubMed] [Google Scholar]
- 65.Johnson JP. Cell adhesion molecules of the immunoglobulin supergene family and their role in malignant transformation and progression to metastatic disease. Cancer Metastasis Rev 1991; 10:11-22; PMID:1680575; http://dx.doi.org/ 10.1007/BF00046840 [DOI] [PubMed] [Google Scholar]
- 66.Rosette C, Roth RB, Oeth P, Braun A, Kammerer S, Ekblom J, Denissenko MF. Role of ICAM1 in invasion of human breast cancer cells. Carcinogenesis 2005; 26:943-50; PMID:15774488; http://dx.doi.org/ 10.1093/carcin/bgi070 [DOI] [PubMed] [Google Scholar]
- 67.Diaz-Munoz MD, Osma-Garcia IC, Iniguez MA, Fresno M. Cyclooxygenase-2 deficiency in macrophages leads to defective p110gamma PI3K signaling and impairs cell adhesion and migration. J Immunol 2013; 191:395-406; PMID:23733875; http://dx.doi.org/ 10.4049/jimmunol.1202002 [DOI] [PubMed] [Google Scholar]
- 68.Godbey WT, Atala A. Directed apoptosis in Cox-2-overexpressing cancer cells through expression-targeted gene delivery. Gene Ther 2003; 10:1519-27; PMID:12900768; http://dx.doi.org/ 10.1038/sj.gt.3302012 [DOI] [PubMed] [Google Scholar]
- 69.Kanamori M, Suzuki K, Yasuda T, Hori T. CD99-positive soft tissue sarcoma with chromosomal translocation between 1 and 16 and inversion of chromosome 5. Oncology Lett 2012; 3:1213-5; PMID:22783420; http://dx.doi.org/ 10.3892/ol.2012.641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi K, Mizukami H, Saga Y, Takei Y, Urabe M, Kume A, Machida S, Fujiwara H, Suzuki M, Ozawa K. Suppression of lymph node and lung metastases of endometrial cancer by muscle-mediated expression of soluble vascular endothelial growth factor receptor-3. Cancer Sci 2013; 104:1107-11; PMID:23614535; http://dx.doi.org/ 10.1111/cas.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hwang-Bo J, Yoo KH, Park JH, Jeong HS, Chung IS. Recombinant canstatin inhibits angiopoietin-1-induced angiogenesis and lymphangiogenesis. Int J Cancer J Int du Cancer 2012; 131:298-309; PMID:21823121; http://dx.doi.org/ 10.1002/ijc.26353 [DOI] [PubMed] [Google Scholar]
- 72.Han KY, Azar DT, Sabri A, Lee H, Jain S, Lee BS, Chang JH. Characterization of the interaction between endostatin short peptide and VEGF receptor 3. Protein and peptide letters 2012; 19:969-74; PMID:22512651; http://dx.doi.org/ 10.2174/092986612802084465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kinet V, Castermans K, Herkenne S, Maillard C, Blacher S, Lion M, Noël A, Martial JA, Struman I. The angiostatic protein 16K human prolactin significantly prevents tumor-induced lymphangiogenesis by affecting lymphatic endothelial cells. Endocrinology 2011; 152:4062-71; PMID:21862622; http://dx.doi.org/ 10.1210/en.2011-1081 [DOI] [PubMed] [Google Scholar]
- 74.Espagnolle N, Barron P, Mandron M, Blanc I, Bonnin J, Agnel M, Kerbelec E, Herault JP, Savi P, Bono F et al.. Specific inhibition of the VEGFR-3 tyrosine kinase by SAR131675 reduces peripheral and tumor associated immunosuppressive myeloid cells. Cancers 2014; 6:472-90; PMID:24589997; http://dx.doi.org/ 10.3390/cancers6010472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kashima K, Watanabe M, Satoh Y, Hata J, Ishii N, Aoki Y. Inhibition of lymphatic metastasis in neuroblastoma by a novel neutralizing antibody to vascular endothelial growth factor-D. Cancer Sci 2012; 103:2144-52; PMID:22937829; http://dx.doi.org/ 10.1111/cas.12010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mumblat Y, Kessler O, Ilan N, Neufeld G. Full-length Semaphorin-3C is an inhibitor of tumor lymphangiogenesis and metastasis. Cancer Res 2015; 75:2177-86; PMID:25808871; http://dx.doi.org/ 10.1158/0008-5472.CAN-14-2464 [DOI] [PubMed] [Google Scholar]
- 77.Patel V, Marsh CA, Dorsam RT, Mikelis CM, Masedunskas A, Amornphimoltham P, Nathan CA, Singh B, Weigert R, Molinolo AA et al.. Decreased lymphangiogenesis and lymph node metastasis by mTOR inhibition in head and neck cancer. Cancer Res 2011; 71:7103-12; PMID:21975930; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-3192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li XP, Jing W, Sun JJ, Liu ZY, Zhang JT, Sun W, Zhu W, Fan YZ. A potential small-molecule synthetic antilymphangiogenic agent norcantharidin inhibits tumor growth and lymphangiogenesis of human colonic adenocarcinomas through blocking VEGF-A,-C,-D/VEGFR-2,-3 “multi-points priming” mechanisms in vitro and in vivo. BMC Cancer 2015; 15:527; PMID:26187792; http://dx.doi.org/ 10.1186/s12885-015-1521-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kodera Y, Katanasaka Y, Kitamura Y, Tsuda H, Nishio K, Tamura T, Koizumi F. Sunitinib inhibits lymphatic endothelial cell functions and lymph node metastasis in a breast cancer model through inhibition of vascular endothelial growth factor receptor 3. Breast Cancer Res 2011; 13:R66; PMID:21693010; http://dx.doi.org/ 10.1186/bcr2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmieder R, Hoffmann J, Becker M, Bhargava A, Muller T, Kahmann N, Ellinghaus P, Adams R, Rosenthal A, Thierauch KH et al.. Regorafenib (BAY 73-4506): antitumor and antimetastatic activities in preclinical models of colorectal cancer. Int J Cancer J Int du Cancer 2014; 135:1487-96; PMID:24347491; http://dx.doi.org/ 10.1002/ijc.28669 [DOI] [PMC free article] [PubMed] [Google Scholar]