ABSTRACT
Erdheim–Chester disease (ECD) is a rare non-Langerhans cell histiocytosis (LCH) characterized by tissue infiltration with CD68+ foamy histiocytes. TNF-related chronic inflammation and mutations in the MAP kinase signaling pathway in histiocytes are recognized as the two major pathogenic events. Among pleomorphic clinical manifestations, cardiovascular involvement is frequent and prognostically relevant. Evaluation of ECD clinical course and response to treatment is, however, still challenging. Taking advantage of the two largest cohorts of ECD patients worldwide, we investigated the relevance and the potential of circulating Chromogranin A (CgA), a pro-hormone involved in cardiovascular homeostasis and inflammation, as a biomarker of response to therapy in ECD. Consistent with other TNF-related inflammatory diseases, we found that not only TNF-α and soluble TNF-Receptors (sTNF-Rs), but also CgA plasma levels were significantly increased in ECD patients compared to controls. CgA, but not sTNF-Rs, discriminated cardiovascular involvement in ECD patients and correlated with pro-Brain Natriuretic Peptide (pro-BNP). In a single case, where a cardiac biopsy was available, CgA was found expressed by cardiomyocytes but not by infiltrating histiocytes. In four ECD patients, where serial determination of these parameters was obtained, the kinetics of sTNF-Rs and CgA paralleled response to therapy with anti-cytokine inhibitors; specifically, sTNF-Rs overlapped TNF-associated inflammation, while CgA, together with pro-BNP, closely mirrored response of cardiac disease. Our data indicate that both sTNF-Rs and CgA are linked to ECD pathophysiology. Moreover, CgA, in concert with pro-BNP, can be further exploited to fulfill the unmet clinical need of non-invasive reliable biomarkers of cardiac disease in these patients.
KEYWORDS: Chromogranin A, chronic inflammation, Erdheim–Chester disease, histiocytes, soluble TNF-Receptors, TNF-α
Abbreviations
- CgA
Chromogranin A
- CHF
chronic heart failure
- ECD
Erdheim–Chester disease
- LCH
Langerhans cell histiocytosis
- pro-BNP
pro-Brain Natriuretic Peptide
- sTNF-R
soluble TNF-Receptors
- TNF-α
Tumor Necrosis Factor-α
Introduction
Erdheim–Chester disease (ECD) is a rare form of non-Langerhans cell histiocytosis (LCH), with about 550 cases reported since its first description in 1930.1 Histologically, the disease is characterized by xanthomatous or xantho-granulomatous infiltration of tissues by foamy CD68+ CD1a− non-Langerhans histiocytes or lipid-laden macrophages, surrounded by fibrosis.2-6 Since virtually any organ can be affected, ECD exhibits protean clinical manifestations, including the nearly pathognomonic osteosclerosis especially of the long bones and extra-skeletal manifestations (exophthalmos, xanthelasma, interstitial lung disease, obstructive renal impairment, diabetes insipidus, and cardiovascular and central nervous system (CNS) involvement).2-6 The prognosis is generally severe, particularly when the cardiovascular and central nervous system are involved.5 Given the lack of reliable biomarkers, evaluation of clinical course of ECD and response to treatment is still challenging.5
We and others have identified two major and possibly interconnected pathogenic events, i.e., a full-blown chronic inflammation and a mutation in the Ras–Raf–Mek–Erk signaling pathway (in particular the BRAFV600E mutation) in histiocytes, which is detectable in the majority of ECD patients.7-11 Oncogene-induced senescence has been proposed as a possible link between those pathogenic events,12 which have been therapeutically targeted with cytokine inhibitors and more recently with the BRAFV600E inhibitor vemurafenib.13-16
Chronic local and systemic inflammation is a hallmark of the disease,7,8 with TNF-α playing a key role, as indicated by increased plasma levels of TNF-related cytokines and chemokines, and also of soluble TNF-Receptor I (sTNF-RI) and sTNF-RII, in ECD patients.14 sTNF-Rs derive from proteolytic cleavage of membrane-associated TNF-Rs, which is promoted by TNF-α itself, and can therefore be considered bona fide surrogates of TNF-activity.17 Recently, serum TNF-α and/or sTNF-Rs levels have been found to correlate with increased circulating Chromogranin A (CgA) in inflammatory diseases and cancer.18-21
CgA is 49-kDa acidic polypeptide stored in the secretory granules of chromaffin cells and released in the extracellular environment by exocytosis. This protein can undergo intracellular and extracellular proteolytic processing, originating biologically active fragments which are implicated in several functions, including vascular homeostasis, angiogenesis, and tissue repair.22-25 CgA levels are increased in patients with neuroendocrine tumors, or with hearth or renal failure, rheumatoid arthritis (RA) or other inflammatory diseases.18-22 With regard to the cardiovascular system, serum CgA levels correlate with severity of cardiac dysfunction and are a predictive factor for mortality in patients with chronic heart failure (CHF) and acute coronary syndrome.21,26-28 More recently, immunohistochemical analyses identified cardiomiocytes as an important source of CgA in patients with CHF.29 In these cells, CgA co-localized with the brain natriuretic peptide (BNP) inside cytoplasmic granules in patients but not in normal controls.29
The relationship between CgA and TNF-α/sTNF-Rs in ECD, and also its association with cardiovascular involvement, a prominent clinical manifestation of the disease,30,31 are presently unknown. Taking advantage of the two largest cohorts of ECD patients worldwide, we aimed to assess the expression and kinetics of circulating sTNF-Rs and CgA in ECD patients, in order to explore their potential to fulfill the unmet clinical need of non-invasive reliable biomarkers of disease activity and response to therapy.
Results
Circulating CgA is increased in ECD patients and identifies cardiovascular involvement
We have previously reported the systemic increase in TNF-α, TNF-related cyto-chemokines and sTNF-Rs in 10 ECD patients.14 We here confirm and extend this observation to a larger cohort of patients, including 17 patients from Ospedale San Raffaele (OSR) and 20 from Pitié-Salpêtrière Hospital. Demographic and clinical characteristics of ECD patients recruited to the study are summarized in Table 1. Also in this cohort of patients sTNF-RI and -RII are significantly increased compared to controls (Table 2). Circulating CgA was also significantly increased in ECD patients (Table 2), and correlated with sTNF-RI only (r = 0.433, p ≤0 .05); sTNF-RI and sTNF-RII instead strongly correlated with each other (r = 0.770, p ≤ 0 .001) (Fig. 1A).
Table 1.
Demographic and clinical characteristics of ECD patients.
| Age | Sex | Involvement | Ongoing therapy | |
|---|---|---|---|---|
| 1 | 69 | F | Exophthalmos, diabetes insipidus, bone, pleuro-pericardial effusion | None |
| 2 | 49 | M | Bone, retroperitoneal fibrosis, lung | None |
| 3 | 46 | M | Retroperitoneal fibrosis, diabetes insipidus, exophthalmos, bone | PEG-IFNα |
| 4 | 52 | M | End-stage renal disease, heart (chronic heart failure, pericardial effusion) | None |
| 5 | 79 | F | Heart (CHF), mediastinal localization, bone | Corticosteroids |
| 6 | 65 | F | Bone, lung, heart, xanthelasma, CNS | IFNα |
| 7 | 46 | M | Retroperitoneal fibrosis, renal failure, aortic aneurism, lung, bone | Corticosteroids + IFNα |
| 8 | 61 | M | Heart (CHF), bone, lung, diabetes insipidus, hypogonadotropic hypogonadism, retroperitoneal fibrosis | Methotrexate |
| 9 | 56 | M | Heart (atrial mass and pericardial effusion), retroperitoneal fibrosis, bone | Corticosteroids |
| 10 | 71 | M | Heart (pericardial effusion), bone, retroperitoneal fibrosis | none |
| 11 | 54 | M | CNS, diabetes insipidus | IFNα |
| 12 | 55 | M | Renal failure, retroperitoneal fibrosis, bone, xanthelasma, CNS and heart (atrial mass and pericardial effusion) | IFNα |
| 13 | 27 | M | Diabetes insipidus, CNS, renal failure, bone, pulmonary fibrosis | Corticosteroids |
| 14 | 26 | M | Retroperitoneal fibrosis, CNS, retro-orbital, pericardial effusion, bone | Corticosteroids + IFNα |
| 15 | 45 | M | Diabetes insipidus, pulmonary fibrosis, optic nerves, retroperitoneal fibrosis, bone | Corticosteroids |
| 16 | 58 | F | Retroperitoneal fibrosis, xanthelasma, bone | IFNα |
| 17 | 52 | M | Pan-hypopituitarism, diabetes insipidus, pulmonary infiltration, bone | Corticosteroids |
| 18 | 69 | M | CNS, bone | PEG-IFNα |
| 19 | 25 | F | Skin, exophthalmos, bone | IFNα |
| 20 | 33 | M | CNS, exophthalmos, retroperitoneal fibrosis, bone | Cladribine |
| 21 | 64 | F | Xanthelasma, heart (atrial mass and pericardial effusion), retroperitoneal fibrosis, bone | IFNα |
| 22 | 59 | M | Xanthelasma, bone, heart (pericardial effusion), diabetes insipidus, CNS, retroperitoneal fibrosis | IFNα |
| 23 | 58 | F | Bone, exophthalmos, CNS, retroperitoneal fibrosis | None |
| 24 | 45 | F | Bone, xanthelasma, retroperitoneal fibrosis, diabetes insipidus | PEG-IFNα |
| 25 | 67 | F | Bone, xanthelasma, heart (atrial mass and pericardial effusion), lung, retroperitoneal fibrosis | Corticosteroids |
| 26 | 73 | M | Bone, retroperitoneal fibrosis, xanthelasma, CNS | Corticosteroids + IFNα |
| 27 | 65 | F | Bone, xanthelasma | PEG-IFNα |
| 28 | 60 | M | Bone, diabetes insipidus, heart (pericardial effusion), retroperitoneal fibrosis | PEG-IFNα |
| 29 | 66 | M | Bone, lung, retroperitoneal fibrosis | IFNα |
| 30 | 75 | M | Bone, CNS, diabetes insipidus, retroperitoneal fibrosis | IFNα |
| 31 | 33 | M | Bone, heart (atrial mass), retroperitoneal fibrosis, CNS, xanthelasma | Corticosteroids |
| 32 | 68 | F | Bone, retroperitoneal fibrosis, lung, xanthelasma, exophthalmos | Corticosteroids |
| 33 | 55 | M | Retroperitoneal fibrosis, bone, exophthalmos | None |
| 34 | 56 | M | Heart (atrial mass and pericardial effusion), retroperitoneal fibrosis, aortic aneurism, bone | PEG-IFNα |
| 35 | 52 | M | CNS, diabetes insipidus, bone, exophthalmos | None |
| 36 | 63 | M | Heart (CHF), coated aorta, bone | None |
| 37 | 52 | M | CNS, diabetes insipidus, bone, retroperitoneal fibrosis | PEG-IFNα |
Abbreviations: CNS, central nervous system; CHF, chronic heart failure; IFN, interferon.
Table 2.
Soluble TNF-Rs and Chromogranin A in ECD patients.
| sTNF-RI (ng/mL) | sTNFR-II (ng/mL) | TNF-§ (pg/mL) | CgA (ng/mL) | |
|---|---|---|---|---|
| HD (n = 20) | 1.21 ± 0.09 | 1.83 ± 0.10 | 0.89 ± 0.56 | 35.4 ± 1.7 |
| ECD (n = 37) | 3.88 ± 0.41*** | 4.86 ± 0.35*** | 11.53 ± 3.41* | 153.08 ± 23.7** |
Results are mean ± SEM of soluble TNF Receptor (sTNF-R) I and II, TNF-, and Chromogranin A (CgA) concentrations in plasma from Healthy Donors (HD) and ECD patients, as determined by ELISA
TNF- concentration was determined in 17 ECD patients and 7 HD. Data obtained from ECD patients and controls were compared by the Mann– Whitney U test
statistically significant at p < 0 .05.
statistically significant at p < 0 .01.
statistically significant at p < 0 .001.
Figure 1.
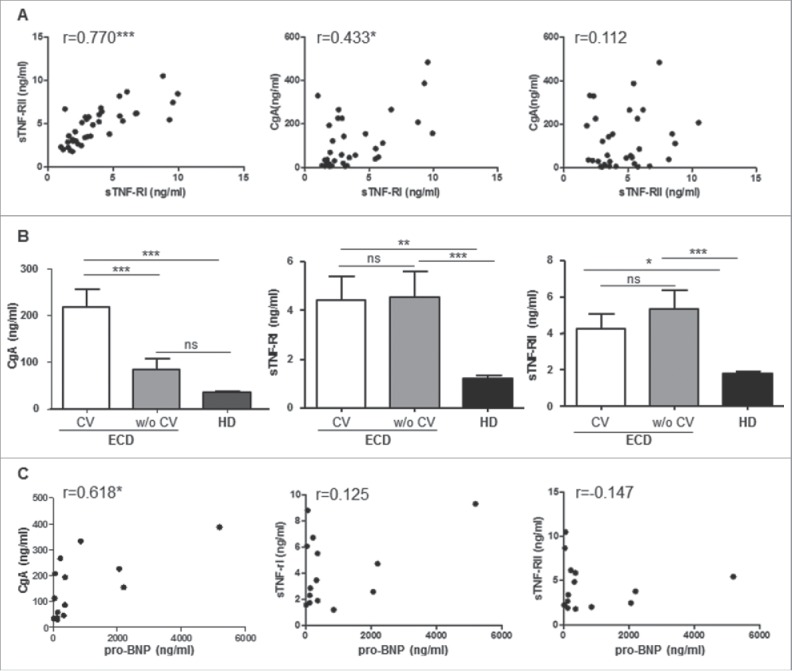
Circulating CgA identifies cardiovascular involvement in ECD patients. (A) Correlation between sTNF-RI and sTNF-RII (left) and between sTNF-RI and CgA (middle) and sTNF-RII and CgA (right) was evaluated by Spearman correlation analysis in 37 ECD patients. (B) circulating CgA, sTNF-RI, and sTNF-RII levels were determined in ECD patients with (n = 8, white columns) or without (w/o) (n = 9, gray columns) cardiovascular involvement (CV) and represented as mean ±SEM . Statistical significance was assessed by ANOVA. Black columns represent the mean of healthy controls (HD) (n = 20). (C) correlation between circulating CgA, sTNF-RI, or sTNF-RII with pro-BNP values was performed by Spearman correlation analysis in 14 patients. *p < 0 .05; **p < 0 .005; ***p < 0 .001.
Given the reported increase in circulating CgA in patients affected by CHF,20 we aimed to determine whether CgA levels in ECD correlate with cardiac disease. Since echocardiography is not sensitive enough to exclude cardiac involvement,5 we analyzed only 17 patients who had cardiac MRI performed at the time of blood sampling. Baseline clinical characteristics of these patients (eight with cardiac involvement and nine without) are summarized in Table 3. CgA concentration in patients with cardiac involvement was significantly higher (217.66 ± 38.29 ng/mL) compared to both HD (35.44 ± 1.72 ng/mL; p ≤ 0 .001) and nine ECD patients without cardiac involvement (84.36 ± 21.48 ng/mL; p ≤ 0 .001) (Fig. 1B, Table 4). Neither sTNF-R could discriminate between the two groups of patients (Fig. 1B, Table 4): sTNF-RI and sTNF-RII concentrations were 4.41 ± 0.99 ng/mL and 4.22 ± 0.84 ng/mL in ECD patients with versus 4.55 ± 1.04 ng/mL and 5.30 ± 1.05 ng/mL in patients without cardiac involvement.
Table 3.
Characteristics of ECD patients with or without cardiovascular involvement.
| ECD withCV involvement(n = 8) | ECD withoutCV involvement(n = 9) | |
|---|---|---|
| Median systolic blood pressure (mmHg) | 135 | 125 |
| Median diastolic blood pressure (mmHg) | 75 | 80 |
| Arterial hypertension (% of patients) | 88% | 56% |
| Median LV ejection fraction (%) | 67 | 65 |
| Median LV end diastolic volume (mL) | 93 | 116 |
| Diastolic dysfunction (% of patients) | 75% | 33% |
| Systolic disfunction (% of patients) | 13% | 11% |
| Median pericardial effusion thickness (mm) | 16.4 | N.A. |
Abbreviations: LV, left ventricular; CV, cardiovascular; N.A., not applicable.
Table 4.
Soluble TNF-Rs and Chromogranin A in ECD patients with/without cardiovascular involvement.
| sTNF-RI (ng/mL) | sTNF-RII (ng/mL) | CgA (ng/mL) | |
|---|---|---|---|
| ECD w CV (n = 8) | 4.41 ± 0 .99** | 4.22 ± 0 .84* | 217.66 ± 38 .29*** |
| ECD w/o CV (n = 9) | 4.55 ± 1 .04*** | 5.30 ± 1 .05*** | 84.36ns ± 21 .48 |
| HD (n = 20) | 1.21 ± 0 .09 | 1.83 ± 0 .10 | 35.44 ± 1 .72 |
Results are mean ± SEM of soluble TNF Receptor (sTNF-R) I and II, and Chromogranin A (CgA) concentrations in plasma from ECD patients with or without cardiovascular involvement (CV) and from Healthy Donors (HD), as determined by ELISA. Statistical significance between ECD patients and controls was assessed by ANOVA.
statistically significant at p < 0 .05.
statistically significant at p < 0 .01.
statistically significant at p < 0 .001.
= not significant.
Since circulating CgA significantly correlates with BNP in patients with cardiomyopathy,29 we determined Pro-BNP levels in 14 ECD patients, including 8 with and 6 without cardiac involvement. Pro-BNP levels were higher in the former group (median value of 613.5 pg/mL, range 42–5196 pg/mL vs. 124 pg/mL, range 17–366 pg/mL). Moreover, pro-BNP levels significantly correlated with circulating CgA (r = 0.618, p ≤ 0 .05) (Fig. 1C). Neither sTNF-RI nor sTNF-RII correlated with pro-BNP (Fig. 1C).
CgA is expressed in cardiomyocytes from an ECD patient
Cardiomyocytes from patients with dilated cardiomyopathy and hypertrophic cardiomyopathy have been identified as a source of CgA.29 We therefore investigated intra-lesional CgA expression on a cardiac biopsy obtained for diagnostic purposes from an ECD patient who presented with a cardiac mass, involving the right atrium and the right intra-ventricular groove.14 Infiltration by foamy histiocytes (Figs. 2A–C), which stained positive for CD68 (Fig. 2B), was evident, leading to ECD diagnosis.5 Notably, intra-lesional cardiomyocytes expressed the reported diffuse granular cytoplasmic immunoreactivity for the anti-CgA antibody 5A8 (Figs. 2C–D),29 at variance with infiltrating histiocytes.
Figure 2.
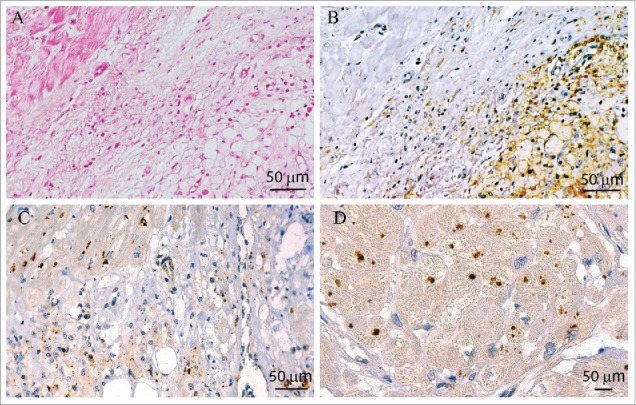
ECD cardiomyocytes express CgA. Immunohistochemistry on ECD myocardial tissue shows a polymorphic xanto-granulomatosous process, composed of vacuolated macrophages and lymphocytes, infiltrating normal myocardial fibers (A–C); (A) Hematoxylin & Eosin staining; (B) CD68 staining is expressed by foamy macrophages; (C) 5A8 staining, directed against the NH2 terminal vasostatin-1 domain of CgA, shows a granular staining of myocyte cytoplasm. In (D) 5A8 staining on cross-section of myocardial fibers at higher magnification.
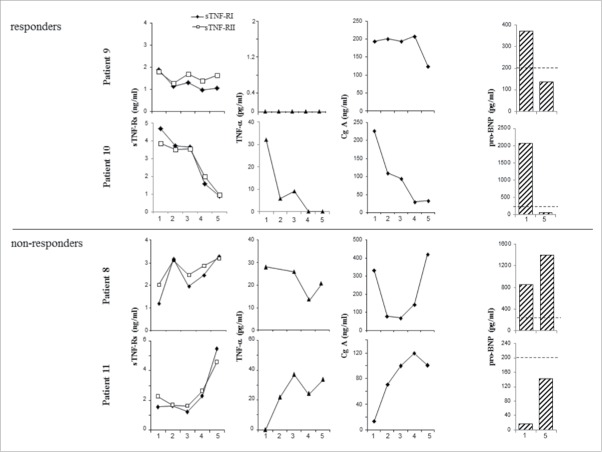
sTNF-Rs and CgA levels parallel response to therapy
To investigate the potential of sTNF-Rs and CgA as biomarkers of response to therapy in ECD patients, we serially determined these parameters in four patients with cardiovascular involvement and divergent response to therapy (infliximab for Patients 8 and 10; tocilizumab for Patients 9 and 11). In particular, Patients 9 and 10 experienced substantial amelioration of their cardiac disease upon treatment; Patient 8 showed an initial response of his cardiac disease, which later progressively worsened, and Patient 11 presented neurological progression under treatment, while showing improvement of cardiac involvement.
As shown in Fig. 3, the time-course of all soluble factors paralleled response to therapy. The kinetics of circulating sTNF-Rs and CgA, however, did not completely overlap. In fact, in patients with prominent cardiovascular involvement (Patient 8 and particularly Patients 9 and 10), CgA and pro-BNP levels marked the clinical course of cardiac disease. Conversely, in Patient 11, where response to treatment discriminated neurological from cardiac involvement, sTNF-Rs levels steadily increased at variance with the decrease of CgA and pro-BNP levels.
Figure 3.
Kinetics of plasma sTNF-Rs and CgA parallel response to therapy in ECD patients. Plasma levels of sTNF-RI, sTNF-RII, TNF-α, and CgA were serially measured in four patients treated with TCZ (Patients 8 and 11) or IFX (Patients 9 and 10) and showing either response to therapy or disease progression. In the x-axis, time points corresponding to 3–6 mo intervals. Pro-BNP levels were also determined at the baseline (point 1) and at the end of the observation (point 5); dotted lines represent the cut-off value (200 ng/mL) for healthy age-matched males.
In all patients, TNF-α plasma levels paralleled circulating sTNF-Rs (Fig. 3).
Discussion
ECD is a rare form of non-LCH, characterized by mutations in the Ras–Raf–Mek–Erk signaling pathway in histiocytes, associated with local and systemic chronic inflammation. We here confirm the key pathogenic role of TNF-α in a large cohort of 37 ECD patients, showing an increase in both plasma TNF-α and sTNF-Rs. TNF-α is produced by intra-lesional ECD histiocytes in the context of a complex cytokine-chemokine storm,7,32 and in turn can modulate the release of down-stream pro-inflammatory molecules and also of sTNF-Rs.14 Excessive TNF-α exerts its detrimental effects particularly on vasculature;17 consistently, we have recently reported that TNF-α released in pericardial fluid from ECD patients promotes vascular leakage in vitro.33 sTNF-Rs counteract TNF-driven inflammation, possibly in concert with CgA, which is released from neuroendocrine cells in response to TNF-α and also inhibits TNF-induced endothelial activation.22-25
Another major source of CgA is represented by stretched cardiomyocytes from patients with dilated and hypertrophic cardiomyopathy, where it co-localizes with BNP.29 The emerging pathophysiological contribution of CgA to cardiovascular homeostasis is further suggested by its inhibitory effect on myocardial contractility and relaxation found in experimental models.34 Of note, we here show for the first time that CgA is expressed also by cardiomyocytes inside ECD intracardiac masses. This finding, together with the correlation of CgA levels with cardiovascular involvement and with pro-BNP, suggests that, also in ECD, CgA is mainly produced and released by diseased myocardium and may be implicated in cardiovascular homeostasis and tissue remodeling.29
Finally, we investigated the potential of sTNF-Rs and CgA as biomarkers for ECD. Assessment of disease activity and response to therapy presently relies on clinical examination and radiologic investigation.5 Recently, a droplet-digital assay for quantitative determination of BRAFV600E mutation in plasma and urine cell-free DNA was proposed as a sensitive biomarker of response to therapy in both ECD and LCH patients,35,36 but this method can be obviously applied only to patients carrying identified mutations, and only to monitor the impact of a drug on mutated histiocytes.
We show here that both sTNF-Rs and CgA levels parallel response to therapy in individual patients, irrespective of the ongoing treatment. These factors, however, are possibly endowed with different clinical implications, the former being markers of ECD-associated inflammation, while the latter, together with pro-BNP, being specifically marker of ECD-associated cardiovascular involvement. Circulating CgA is a well-established marker of neuro-endocrine tumors and is emerging as a diagnostic/prognostic indicator of acute coronary syndromes and CHF.34 Given that cardiovascular involvement in ECD is prognostically significant but often asymptomatic, our data suggest that CgA can be also exploited as an independent biomarker for the disease.
Patients and methods
Patients
We enrolled 37 patients with ECD, diagnosed on the basis of clinical imaging and histopathological criteria, including the accumulation inside lesions of CD68+ CD1a− foamy histiocytes.2-6 Seventeen patients were followed in the Unit of Medicine and Clinical Immunology of the OSR (Milan, Italy) and 20 in the Department of Internal Medicine and French Reference Center for Rare Autoimmune and Systemic Diseases of the Pitié-Salpêtrière Hospital (Paris, France). The study was approved by the locally appointed Ethics Committees in accordance with the Declaration of Helsinki and written informed consent was obtained from all subjects. Twenty age- and sex-matched healthy volunteers were enrolled as controls. In four patients samples were subsequently obtained every 3–6 mo after the baseline; two patients (Patients 9 and 11) were in treatment with the IL-6 receptor inhibitor tocilizumab (TCZ) and two (Patients 8 and 10) with the anti-TNF-α antibody infliximab (IFX) as second-line therapies because of contraindications or unresponsiveness to IFN-α treatment. Clinical evaluation, including collection of laboratory and radiological data (total-body computed tomography-CT-scan, Technetium-99m methylene diphosphonate-99mTc-MDP-bone-scan, fluorine-18-2-fluoro-d-glucose positron emission tomography–FDGPET, brain and cardiac Magnetic Resonance Imaging–MRI) was performed at the baseline and repeated at the time of each blood collection.
Determination of TNF-α, sTNF-Rs, and CgA concentrations
Plasma levels of TNF-α were determined by the Bio-Plex Multiple-Cytokines Assay (Bio-Rad, Hercules, CA).14 Plasma sTNF-Rs and CgA were measured by ELISA as described.14,23
Determination of pro-BNP concentration
Pro-BNP plasma levels were determined through a specific electrochemiluminescence (ECL) method (Elecsys® NT-proBNP, Roche) and analyzed with a Hitachi Cobas E601 analyzer.
Immunohistochemistry
Immunohistochemistry for CgA was performed using the mouse anti-human CgA monoclonal antibody 5A8, directed against the N-terminal domain (vasostatin-1) on paraffin sections of a myocardial sample.27 Infiltrating histiocytes were identified by staining with an anti-human CD68 monoclonal antibody (DAKO, Glostrup, Denmark).10
Statistical analysis
Data are presented as mean ± SEM. We used the Mann–Whitney U test to compare parameters between ECD patients and controls. The correlation between CgA levels, TNF-Rs and pro-BNP in ECD patients was calculated by Spearman correlation analysis and is expressed as r coefficient. A p value below 0.05 was considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Kathy Brewer and the ECD Global Alliance (De Ridder, LA) for their continuous activities for patients with Erdheim–Chester disease and their unceasing support. We also thank Dr Barbara Vergani (Consorzio MIA) for histological analyses; Dr Giliola Calori (San Raffaele Scientific Institute) for her assistance in statistical analyses; and Dr Andrea Motta (San Raffaele Scientific Institute) for pro-BNP determination.
Funding
The work was supported in part by a Grant from the Italian Ministry of Health (GR 2009-1594586 to LD) and by a grant from ECD Global Alliance (to MF & LD).
References
- 1.Chester W. Über lipoidgranulomatose. Virchows Arch Pathol Anat Physiol Klin Med 1930; 279:561-602; http://dx.doi.org/ 10.1007/BF01942684 [DOI] [Google Scholar]
- 2.Veyssier-Belot C, Cacoub P, Caparros-Lefebvre D, Wechsler J, Brun B, Remy M, Wallaert B, Petit H, Grimaldi A, Wechsler B et al.. Erdheim-Chester disease. Clinical and radiologic characteristics of 59 cases. Medicine (Baltimore) 1996; 75:157-69; PMID:8965684; http://dx.doi.org/ 10.1097/00005792-199605000-00005 [DOI] [PubMed] [Google Scholar]
- 3.Haroche J, Arnaud L, Amoura Z. Erdheim-Chester disease. Curr Opin Rheumatol 2012; 24:53-9; PMID:22089098; http://dx.doi.org/ 10.1097/BOR.0b013e32834d861d [DOI] [PubMed] [Google Scholar]
- 4.Cavalli G, Guglielmi B, Berti A, Campochiaro C, Sabbadini MG, Dagna L. The multifaceted clinical presentations and manifestations of Erdheim-Chester disease: comprehensive review of the literature and of 10 new cases. Ann Rheum Dis 2013; 72:1691-5; PMID:23396641; http://dx.doi.org/ 10.1136/annrheumdis-2012-202542 [DOI] [PubMed] [Google Scholar]
- 5.Diamond EL, Dagna L, Hyman DM, Cavalli G, Janku F, Estrada-Veras J, Ferrarini M, Abdel-Wahab O, Heaney ML, Scheel PJ et al.. Consensus guidelines for the diagnosis and clinical management of Erdheim-Chester disease. Blood 2014; 124:483-926; PMID:24850756; http://dx.doi.org/ 10.1182/blood-2014-03-561381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campochiaro C, Tomelleri A, Cavalli G, Berti A, Dagna L. Erdheim-Chester disease. Eur J Intern Med. 2015; 26:223-9; PMID:25865950; http://dx.doi.org/ 10.1016/j.ejim.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 7.Stoppacciaro A, Ferrarini M, Salmaggi C, Colarossi C, Praderio L, Tresoldi M, Beretta AA, Sabbadini MG. Immunohistochemical evidence of a cytokine and chemokine network in three patients with Erdheim-Chester disease: implications for pathogenesis. Arthritis Rheum 2006; 54:4018-22; PMID:17133532; http://dx.doi.org/ 10.1002/art.22280 [DOI] [PubMed] [Google Scholar]
- 8.Arnaud L, Gorochov G, Charlotte F, Lvovschi V, Parizot C, Larsen M, Ghillani-Dalbin P, Hervier B, Kahn JE, Deback C et al.. Systemic perturbation of cytokine and chemokine networks in Erdheim-Chester disease: a single-center series of 37 patients. Blood 2011; 117:2783-90; PMID:21205927; http://dx.doi.org/ 10.1182/blood-2010-10-313510 [DOI] [PubMed] [Google Scholar]
- 9.Haroche J, Charlotte F, Arnaud L, von Deimling A, Hélias-Rodzewicz Z, Hervier B, Cohen-Aubart F, Launay D, Lesot A, Mokhtari K et al.. High prevalence of BRAFV600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood 2012; 120:2700-3; PMID:22879539; http://dx.doi.org/ 10.1182/blood-2012-05-430140 [DOI] [PubMed] [Google Scholar]
- 10.Cangi MG, Biavasco R, Cavalli G, Grassini G, Dal-Cin E, Campochiaro C, Guglielmi B, Berti A, Lampasona V, von Deimling A et al.. BRAFV600E-mutation is invariably present and associated to oncogene-induced senescence in Erdheim-Chester disease. Ann Rheum Dis 2015; 74:1596-602; PMID:24671772; http://dx.doi.org/ 10.1136/annrheumdis-2013-204924 [DOI] [PubMed] [Google Scholar]
- 11.Blombery P, Wong SQ, Lade S, Prince HM. Erdheim-Chester disease harboring the BRAF V600E mutation. J Clin Oncol 2012; 30:e331-2; PMID:23008323; http://dx.doi.org/ 10.1200/JCO.2012.43.2260 [DOI] [PubMed] [Google Scholar]
- 12.Cavalli G, Biavasco R, Borgiani B, Dagna L. Oncogene-induced senescence as a new mechanism of disease: the paradigm of Erdheim-Chester disease. Front Immunol 2014; 5:281; PMID:24982657; http://dx.doi.org/ 10.3389/fimmu.2014.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aouba A, Georgin-Lavialle S, Pagnoux C, Martin Silva N, Renand A, Galateau-Salle F, Le Toquin S, Bensadoun H, Larousserie F, Silvera S et al.. Rationale and efficacy of interleukin-1 targeting in Erdheim-Chester disease. Blood 2010; 116:4070-6; PMID:20724540; http://dx.doi.org/ 10.1182/blood-2010-04-279240 [DOI] [PubMed] [Google Scholar]
- 14.Dagna L, Corti A, Langheim S, Guglielmi B, De Cobelli F, Doglioni C, Fragasso G, Sabbadini MG, Ferrarini M. Tumor necrosis factor α as a master regulator of inflammation in Erdheim-Chester disease: rationale for the treatment of patients with infliximab. J Clin Oncol 2012; 30:e286-90; PMID:22869874; http://dx.doi.org/ 10.1200/JCO.2012.41.9911 [DOI] [PubMed] [Google Scholar]
- 15.Haroche J, Cohen-Aubart F, Emile JF, Arnaud L, Maksud P, Charlotte F, Cluzel P, Drier A, Hervier B, Benameur N et al.. Dramatic efficacy of vemurafenib in both multisystemic and refractory Erdheim-Chester disease and Langerhans cell histiocytosis harboring the BRAF V600E mutation. Blood 2013; 121:1495-500; PMID:23258922; http://dx.doi.org/ 10.1182/blood-2012-07-446286 [DOI] [PubMed] [Google Scholar]
- 16.Haroche J, Cohen-Aubart F, Emile JF, Maksud P, Drier A, Tolédano D, Barete S, Charlotte F, Cluzel P, Donadieu J et al.. Reproducible and sustained efficacy of targeted therapy with vemurafenib in patients with BRAF(V600E)-mutated Erdheim-Chester disease. J Clin Oncol 2015; 33:411-8; PMID:25422482; http://dx.doi.org/ 10.1200/JCO.2014.57.1950 [DOI] [PubMed] [Google Scholar]
- 17.Puimège L, Libert C, Van Hauwermeiren F. Regulation and dysregulation of tumor necrosis factor receptor-1. Cytokine Growth Factor Rev 2014; 25:285-300; PMID:24746195; http://dx.doi.org/19090560 10.1016/j.cytogfr.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 18.Sciola V, Massironi S, Conte D, Caprioli F, Ferrero S, Ciafardini C, Peracchi M, Bardella MT, Piodi L. Plasma chromogranin a in patients with inflammatory bowel disease. Inflamm Bowel Dis 2009; 15:867-71; PMID:19090560; http://dx.doi.org/ 10.1002/ibd.20851 [DOI] [PubMed] [Google Scholar]
- 19.Di Comite G, Rossi CM, Marinosci A, Lolmede K, Baldissera E, Aiello P, Mueller RB, Herrmann M, Voll RE, Rovere-Querini P et al.. Circulating chromogranin A reveals extra-articular involvement in patients with rheumatoid arthritis and curbs TNF-α-elicited endothelial activation. J Leukoc Biol 2009; 85:81-7; PMID:18832606; http://dx.doi.org/ 10.1189/jlb.0608358 [DOI] [PubMed] [Google Scholar]
- 20.Gregorc V, Spreafico A, Floriani I, Colombo B, Ludovini V, Pistola L, Bellezza G, Vigan∫ MG, Villa E, Corti A. Prognostic value of circulating chromogranin A and soluble tumor necrosis factor receptors in advanced nonsmall cell lung cancer. Cancer. 2007; 110:845-53; PMID:17599769; http://dx.doi.org/ 10.1002/cncr.22856 [DOI] [PubMed] [Google Scholar]
- 21.Corti A, Ferrari R, Ceconi C. Chromogranin A and tumor necrosis factor-α (TNF) in chronic heart failure. Adv Exp Med Biol 2000; 482:351-9; PMID:11192595; http://dx.doi.org/ 10.1007/0-306-46837-9_28 [DOI] [PubMed] [Google Scholar]
- 22.Helle KB, Corti A. Chromogranin A: a paradoxical player in angiogenesis and vascular biology. Cell Mol Life Sci 2015; 72:339-48; PMID:25297920; http://dx.doi.org/ 10.1007/s00018-014-1750-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crippa L, Bianco M, Colombo B, Gasparri AM, Ferrero E, Loh YP, Curnis F, Corti A. A new chromogranin A-dependent angiogenic switch activated by thrombin. Blood 2013; 121:392-402; PMID:23190532; http://dx.doi.org/ 10.1182/blood-2012-05-430314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrero E, Scabini S, Magni E, Foglieni C, Belloni D, Colombo B, Curnis F, Villa A, Ferrero ME, Corti A. Chromogranin A protects vessels against tumor necrosis factor α-induced vascular leakage. FASEB J 2004; 18:554-6; PMID:14734634 [DOI] [PubMed] [Google Scholar]
- 25.Helle KB, Corti A, Metz-Boutigue MH, Tota B. The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci 2007; 64:2863-86; PMID:17717629; http://dx.doi.org/ 10.1007/s00018-007-7254-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, Parrinello G, Corti A. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J 2002; 23:967-74; PMID:12069452; http://dx.doi.org/ 10.1053/euhj.2001.2977 [DOI] [PubMed] [Google Scholar]
- 27.Jansson AM, Røsjø H, Omland T, Karlsson T, Hartford M, Flyvbjerg A, Caidahl K. Prognostic value of circulating chromogranin A levels in acute coronary syndromes. Eur Heart J 2009; 30:25-3; PMID:19028779; http://dx.doi.org/ 10.1093/eurheartj/ehn513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dieplinger B, Gegenhuber A, Struck J, Poelz W, Langsteger W, Haltmayer M, Mueller T. Chromogranin A and C-terminal endothelin-1 precursor fragment add independent prognostic information to amino-terminal proBNP in patients with acute destabilized heart failure. Clin Chim Acta 2009; 400:91-6; PMID:19000665; http://dx.doi.org/ 10.1016/j.cca.2008.10.012 [DOI] [PubMed] [Google Scholar]
- 29.Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, Cerra MC, Bellocci F, Crea F, Maseri A. Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J 2007; 28:1117-27; PMID:17389654; http://dx.doi.org/ 10.1093/eurheartj/ehm022 [DOI] [PubMed] [Google Scholar]
- 30.Haroche J, Amoura Z, Dion E, Wechsler B, Costedoat-Chalumeau N, Cacoub P, Isnard R, Généreau T, Wechsler J, Weber N et al.. Cardiovascular involvement, an overlooked feature of Erdheim-Chester disease: report of 6 new cases and a literature review. Medicine (Baltimore). 2004; 83:371-92; PMID:15525849 [DOI] [PubMed] [Google Scholar]
- 31.Berti A, Ferrarini M, Ferrero E, Dagna L. Cardiovascular manifestations of Erdheim-Chester disease. Clin Exp Rheumatol 2015; 33(2 Suppl 89):S-155-63; PMID:25738753 [PubMed] [Google Scholar]
- 32.Dagna L, Girlanda S, Langheim S, Rizzo N, Bozzolo EP, Sabbadini MG, Ferrarini M. Erdheim-Chester disease: report on a case and new insights on its immunopathogenesis. Rheumatology (Oxford) 2010; 49:1203-6; PMID:20097905; http://dx.doi.org/ 10.1093/rheumatology/kep461 [DOI] [PubMed] [Google Scholar]
- 33.Ferrero E, Belloni D, Corti A, Doglioni C, Dagna L, Ferrarini M. TNF-α in Erdheim-Chester disease pericardial effusion promotes endothelial leakage in vitro and is neutralized by infliximab. Rheumatology (Oxford) 2014; 53:198-200; PMID:23893523; http://dx.doi.org/ 10.1093/rheumatology/ket246 [DOI] [PubMed] [Google Scholar]
- 34.Tota B, Angelone T, Cerra MC. The surging role of Chromogranin A in cardiovascular homeostasis. Front Chem 2014; 2:64; PMID:25177680; http://dx.doi.org/ 10.3389/fchem.2014.00064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Janku F, Vibat CR, Kosco K, Holley VR, Cabrilo G, Meric-Bernstam F, Stepanek VM, Lin PP, Leppin L, Hassaine L et al.. BRAF V600E mutations in urine and plasma cell-free DNA from patients with Erdheim-Chester disease. Oncotarget 2014; 5:3607-10; PMID:25003820; http://dx.doi.org/ 10.18632/oncotarget.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyman DM, Diamond EL, Vibat CR, Hassaine L, Poole JC, Patel M, Holley VR, Cabrilo G, Lu TT, Arcila ME et al.. Prospective blinded study of BRAFV600E mutation detection in cell-free DNA of patients with systemic histiocytic disorders. Cancer Discov 2015; 5:64-71; PMID:25324352; http://dx.doi.org/ 10.1158/2159-8290.CD-14-0742 [DOI] [PMC free article] [PubMed] [Google Scholar]