ABSTRACT
Antibody-drug conjugates (ADC), combining the specificity of tumor recognition by monoclonal antibodies (mAb) and the powerful cytotoxicity of anticancer drugs, are currently under growing interest and development. Here, we studied the potential of Chi-Tn, a mAb directed to a glyco-peptidic tumor-associated antigen, to be used as an ADC for cancer treatment. First, we demonstrated that Chi-Tn specifically targeted tumor cells in vivo. Also, using flow cytometry and deconvolution microscopy, we showed that the Chi-Tn mAb is rapidly internalized – condition necessary to ensure the delivery of conjugated cytotoxic drugs in an active form, and targeted to early and recycling endosomes. When conjugated to saporin (SAP) or to auristatin F, the Chi-Tn ADC exhibited effective cytotoxicity to Tn-positive tumor cells in vitro, which correlated with the level of tumoral Tn expression. Furthermore, the Chi-Tn mAb conjugated to auristatin F also exhibited efficient antitumor activity in vivo, validating for the first time the use of an anti-Tn antibody as an effective ADC.
KEYWORDS: Biodistribution, internalization, MMAF, monoclonal antibody-drug conjugate, Tn antigen
Introduction
Over the last two decades, monoclonal antibody (mAb) therapy was established as an effective treatment against cancer.1,2 Therapeutic mAbs can be used as naked antibodies, inhibiting tumor growth directly by inducing apoptosis upon binding to their specific antigen, and/or indirectly by stimulating the immune system. In addition, antibodies recognizing targets/receptors expressed at the surface of malignant cells can be used as antibody-drug conjugates (ADC) to target potent cytotoxic agents selectively to the tumors without affecting healthy cells. The challenge for ADC strategies is to increase antitumor efficacy with diminished toxicity.3
Antibodies can be conjugated to plant or bacterial toxins (ricin and saporin or diphtheria toxin and pseudomonas exotoxin A, respectively), to radioisotopes (β emitters 90Y and 131I, or α-particles 213Bi and 211At), to anticancer chemotherapies (doxorubicin, methotrexate or vinblastine), and to toxic drugs or their derivatives, such as calicheamicin (causing double-strand DNA breaks), auristatins and maytansines (inhibitors of microtubule assembly) or α-amanitin (inhibitor of RNA polymerase II).4
Several requirements need to be fulfilled for a mAb to be used as an ADC, including high tumor selectivity, high expression of its target cell surface antigen and internalization of antigen-ADC complexes into the cancer cell where the drug will be active.4,5 Upon ligand binding, membrane receptors are internalized within early endosomes. Some receptors are then recycled back to the cell surface, while others are sorted into late endosomes and lysosomes where degradation occurs.6
Conjugation of drugs to targeting antibodies is designed, taking into account the subcellular compartment reached by the ADC after internalization into the cell, so that the drug can be released from the carrier under an active form.7 Thus, to facilitate the drug release within early endosomes, pH-sensitive linkers (for example acid-labile bonds such as hydrazide) have been incorporated between the drug and the mAb, leading to conjugates stable at pH = 7.4 in the blood, and cleaved at pH = 5 in endosomes.5,8 Alternatively, other linkers containing cleavable functions as disulfides, thioethers or dipeptides, have been used that are cleaved inside the lysosomes, while releasing the drug moiety.4,5 Drugs can be also attached to the mAb through the non-cleavable maleimidocaproyl linker (mc), which is related to the maleimidocaproyl-Valine-Citrulline (mc-Val-Cit) linker.9 The maleimide is designed to react with a thiol function on the antibody and the carboxylic acid end of the linker with the secondary amine of auristatin. Identifying tumor-specific surface proteins that are efficiently internalized and drive their ligands to the appropriate endosomes for drug delivery is a major challenge for the development of effective cancer ADCs.
Tn (CD175) is a tumor-associated carbohydrate antigen composed of an N-acetyl-galactosamine residue (GalNAc) linked by O-glycosylation to serine or threonine amino acids, mainly found in mucin-type glycoproteins.10,11 The Tn antigen is normally cryptic in mucin-type O-glycans and becomes expressed at the surface of tumor cells because of alterations along the O-glycosylation pathways, resulting in defective elongation of carbohydrate chains. Importantly, Tn is highly expressed in various epithelial cancers (primarily ovarian, breast, prostate, colorectal, and lung cancers), and virtually absent in normal tissues.12-17 Moreover, the Tn antigen is involved in the adhesion of tumor cells to the lymphatic endothelium, and Tn-antigen expression seems to correlate with metastatic potential and poor prognosis.10,15,18 Thus, these data make the Tn-antigen an attractive target for a therapeutic antibody.
Several anti-Tn mAbs with different fine specificities for the Tn antigen have been generated,16,18-22 and two of them were reported to increase survival of mice inoculated with a Jurkat tumor cell line 19 or a lung carcinoma cell line.23 However, none of these antibodies have been validated for their use as an ADC. In a previous work, we demonstrated that naked Chi-Tn mAb, a mouse/human IgG1 chimeric anti-Tn mAb 22 was able to induce the rejection of TA3Ha mammary tumor in mice and this effect was dependent on activating FcγRs.14 In the present study, we further explored the therapeutic potential of the Chi-Tn mAb, but using the antibody as an ADC to selectively target cytotoxic molecules into tumor cells. We showed that the naked Chi-Tn mAb was effectively and rapidly internalized, reaching the early, but not the late, endocytic compartments in vitro. Furthermore, in vivo, the Chi-Tn mAb selectively accumulated in the solid tumor, but not in healthy tissue. When conjugated to saporin (SAP) or to auristatin F, the Chi-Tn ADC exhibited effective cytotoxicity to Tn-positive tumor cells in vitro. Finally, the Chi-Tn mAb conjugated to MMAF also induced a delay of tumor growth in vivo, validating for the first time the use of an anti-Tn antibody as an effective ADC.
Results
The chimeric mAb Chi-Tn specifically binds Tn expressed at different levels on various tumor cell lines
The Tn antigen is present in numerous solid and hematologic tumors,10,12-17,24-26 but its level of expression varies among the different tumors and Tn can be found on various different peptide sequences. Consequently, to study if the chimeric mAb Chi-Tn can be used as a potential ADC to selectively target cytotoxic molecules into tumor cells, we first confirmed and extended our previous results 14 showing that this mAb recognized Tn at the surface of different tumor cell lines (Fig. 1A). Jurkat cells (human T-ALL) displayed the strongest Tn-specific binding of the Chi-Tn mAb to the cell surface, and the human ovarian cancer cell lines, Shin-3 and OvCar-3, also bound the Chi-Tn mAb, although with a lower intensity. Tn surface expression was also detected by Chi-Tn mAb in breast cancer cell lines, either from human origin, such as MCF7 cells 14 or from murine origin, such as TA3Ha cells (Fig. 1A).
Figure 1.

Chi-Tn mAb specifically binds Tn expressed at different levels on various tumor cell lines. (A) Jurkat, Shin-3, OvCar-3 or TA3Ha cells were labeled with the Chi-Tn mAb or a control antibody (IvIg for human cells or trastuzumab for murine cells) at 20 µg/mL, then with a GaH-Fc-PE secondary antibody. DAPI-negative living cells were acquired by flow cytometry and the PE mean fluorescence intensity (M.F.I.) was determined and is indicated for each sample. Numbers in the quadrants: % of cells. (B) The number of Tn motifs expressed at the cell surface of different tumor cell lines was estimated by quantitative flow cytometry using a murine anti-Tn mAb.
It was previously shown that the ADC activity of therapeutic Abs correlates with the expression level of its cognate tumoral antigen.27-29 Thus, we estimated the amount of surface-expressed Tn antigen by quantitative flow cytometry (Fig. 1B).27,29 The Jurkat cells expressed the highest number of Tn molecules at the cell surface, approximately 5 × 105 per cell, while Shin-3 or OvCar-3 cells express 6 × 104 molecules per cell. The murine TA3Ha tumor cells express an intermediate level with 1.5 × 105 molecules/cell. This quantification by flow cytometry was strongly correlated with the results obtained by Scatchard analysis using radiolabeled Chi-Tn (unpublished results from F. Davodeau, Nantes).
In vivo, the Chi-Tn mAb specifically targets tumor cells
To study the in vivo targeting capacity of the Chi-Tn mAb, we used an experimental tumor model consisting in the subcutaneous (s.c.) injection of the Shin-3 human solid tumor cells to nude mice. By day 12 after the injection, when solid tumors were already palpable, mice were injected intraperitoneal (i.p.) with Chi-Tn mAb or control mAb (Herceptin®, anti-Her2 mAb) and 2 d later, tumor and other organs were analyzed by immunohistochemistry (IHC, Fig. 2A). As shown in Fig. 2B, established solid tumors still highly expressed the Tn antigen, while Tn expression was not detected in other tissues/organs distal to the site of injection of the Shin-3 cells, such as liver, spleen or lungs (data not shown). To evaluate the in vivo biodistribution of the Chi-Tn mAb, we directly labeled the tissue sections with a PE-coupled secondary GaH-Fc Ab F(ab')2 specific for the human Fc part of the Chi-Tn mAb. As shown in Fig. 2C, the i.p.-injected Chi-Tn mAb was recovered in tumor sections while it was not detected in other organs (liver, spleen, and lungs, Fig. 2D). Moreover, no mAb was detected in tumors from mice injected with the hIgG1 control mAb (data not shown). These data indicate that in vivo, the Chi-Tn mAb specifically targets s.c. tumors, whereas it has no detectable binding on normal tissues and suggest that Chi-Tn could be an attractive candidate mAb to deliver radiation, drugs or toxins to tumor sites, without toxicity to normal tissue.
Figure 2.

In vivo biodistribution of the Chi-Tn mAb. (A) Nude mice were grafted s.c. with 4 × 106 Shin-3 tumor cells, and were injected i.p. on day 12 with the Chi-Tn mAb or the control mAb at 20 mg/kg. On day 14, solid tumor and organs were removed and sectioned for immunofluorescence (IF) studies. (B) To detect Tn-positive cells, tissue sections were labeled with the Chi-Tn mAb, and with GaH-Fc-Biot and Sa-Cy3. (C) and (D) To detect the presence of the Chi-Tn mAb (or control mAb, red), tumor (C) or tissues (D) sections were labeled directly with GaH-Fc-Biot and Sa-Cy3. Nuclei were labeled using DAPI (blue), and images were acquired by microscopy.
The Chi-Tn mAb is rapidly internalized in cancer cells
To use the Chi-Tn mAb as an ADC, it has to be internalized effectively in its target cells to deliver the cytotoxic compound. We then analyzed the outcome of the Chi-Tn mAb after its binding to cell surface of tumor cells. For that, Jurkat, Shin-3, and TA3Ha cells were first incubated at 4°C with Chi-Tn mAb, then transfered to 37°C, and the membrane-bound Chi-Tn mAb was quantified at different time points by flow cytometry. As shown in Fig. 3A, only 20% of the Chi-Tn mAb initially bound was detected after 5 min at 37°C, at the cell surface of the three different tumor cell lines tested. The percentage of the Chi-Tn mAb remaining at the plasma membrane after 1 h at 37°C reached 15, 4.4, and 10% on Jurkat, Shin-3, and TA3Ha cells, respectively (Fig. 3A). These results showing that the Chi-Tn mAb rapidly disappears from the plasma membrane at 37°C, suggest that the mAb is either internalized into the cells or released into the extracellular medium.
Figure 3.
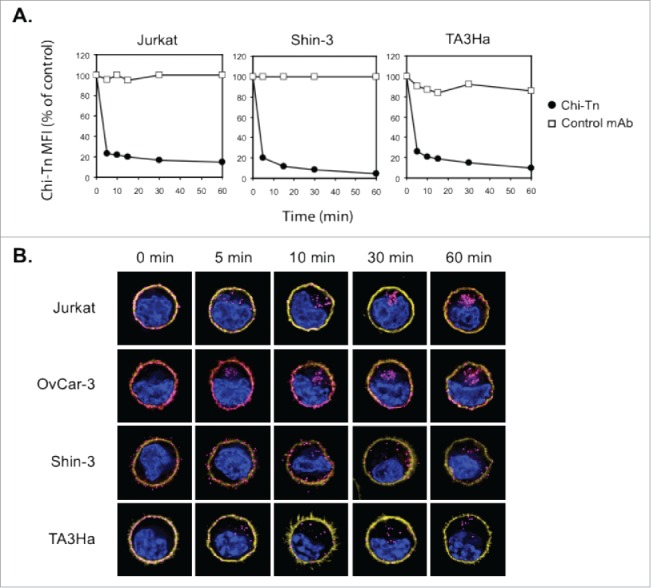
The Chi-Tn mAb is internalized into cancer cells. (A) Jurkat, Shin-3 or TA3Ha cells were incubated for 15 min on ice with the Chi-Tn mAb or with a control antibody (IvIg for human cells or trastuzumab for murine cells) at 20 µg/mL, washed, then transferred to 37°C for the indicated times. Cells were then labeled with GaH-Fc-PE, and the Chi-Tn mAb M.F.I. was determined by flow cytometry in the DAPI-negative living cells gate. For each sample, the Chi-Tn M.F.I. is expressed as a percentage of the Chi-Tn M.F.I. obtained for cells not transferred to 37°C (control cells). (B) Jurkat, OvCar-3, Shin-3 or TA3Ha cells were incubated on ice with the Chi-Tn mAb for 15 min, washed and transferred to 37°C for the indicated period of time. Cells were then fixed to glass coverslips and labeled. Yellow: actin network; pink: membrane-bound or internalized Chi-Tn mAb; blue: DAPI. Arrows indicate examples of internalized Chi-Tn mAb.
To determine if the Chi-Tn mAb was internalized into tumor cells, the antibody was bound to Jurkat, OvCar-3, Shin-3, or TA3Ha cell surface at 4°C, prior transfer of the cells to 37°C during various times. Analysis by deconvolution microscopy (Fig. 3B) showed that initially, at 4°C, the Chi-Tn mAb was localized at the plasma membrane of the cells. After 5 min incubation at 37°C, the Chi-Tn mAb was observed in intracellular structures distributed throughout the cytoplasm. Consistent with flow cytometry results, Chi-Tn mAb internalization increased with time and was more noticeable in cells originally displaying higher amounts of the Tn antigen at the plasma membrane (see Fig. 1). After 15 min at 37°C, the Chi-Tn mAb was internalized in about 77, 86, 44, and 79% of Jurkat, OvCar-3, Shin-3, and TA3Ha cells, respectively (data not shown). After around 30 min at 37°C, the Chi-Tn mAb-containing vesicles were readily observed forming clusters close to the juxta-nuclear region in all the studied cell lines. We conclude that the Chi-Tn mAb binds to the plasma membrane of tumor cells, and is then rapidly internalized.
The Chi-Tn mAb localizes to early and recycling endosomes
After endocytosis, ligand-receptor complexes are internalized and sorted to early endosomes. Receptors are then either recycled back to the plasma membrane through recycling endosomes, or delivered to late endosomes and lysosomes for degradation.6
We investigated the nature of the compartment(s) targeted by the Chi-Tn mAb after internalization using markers of early endosomes, recycling endosomes or late endosome/lysosomes. After internalization in Jurkat cells, Chi-Tn mAb accumulated in transferrin-positive compartments, indicating its presence in early endosomes and/or recycling endosomes.30 (Fig. 4A). Chi-Tn mAb was also present in Rab-11-positive recycling endosomes 30 (Fig. 4B), but with a lower proportion of co-localization than in the early endosomes. These co-localizations started as soon as 5 min and lasted for up to 4 h after transfer at 37°C. On the contrary, Chi-Tn mAb could not be detected in late endosomal/lysosomal LAMP-1+ compartments, even after 4 h of incubation at 37°C (Fig. 4C). We observed the same pattern of intracellular localization of Chi-Tn in TA3Ha cells, with a co-localization in early endosomes and no detection in LAMP-1-positive compartments (data not shown). Therefore, internalized Chi-Tn, accumulates in early and recycling endosomes and is delivered inefficiently to late endosomes and lysosomes.
Figure 4.
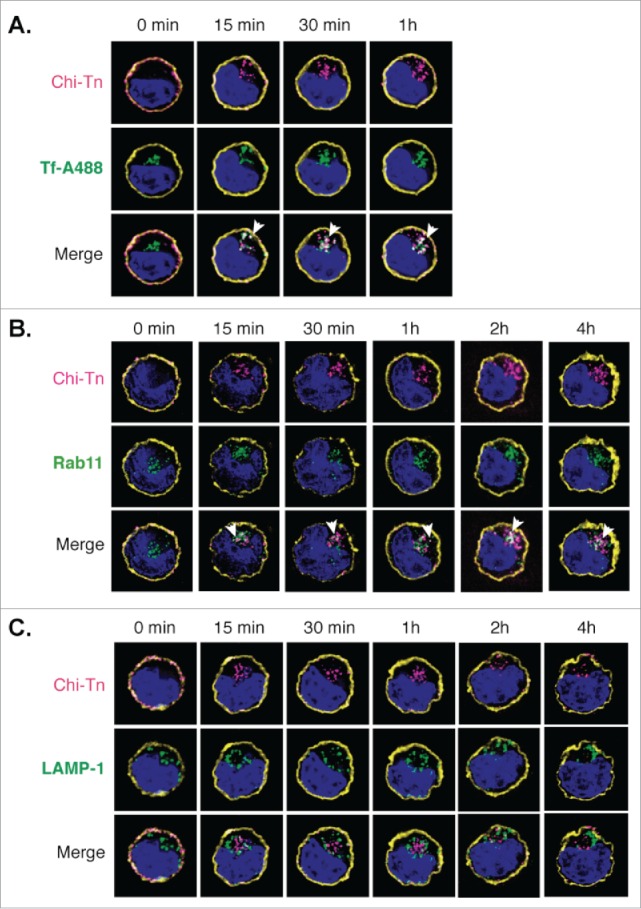
Analysis of the Chi-Tn mAb targeting to endosomal compartments Jurkat cells were labeled with the Chi-Tn mAb, transferred to 37°C for the indicated times, then actin (yellow) and Chi-Tn (pink) were detected in fixed cells. Early endosomes were detected using Tf-A488 (green, A), recycling endosomes with Rab11 (green, B), and late endosomes with anti-LAMP-1 mAb (green, C). Blue: DAPI. Images were acquired by deconvolution 3D-microscopy. Examples of co-localization (in white) are indicated by arrows.
The Chi-Tn mAb coupled to saporin inhibits tumor cell growth in vitro
Since Chi-Tn mAb is effectively and rapidly internalized, we evaluated if it could be used as an ADC. To do so, Chi-Tn was coupled to SAP, a ribosome-inactivating protein (RIP) that inhibits protein synthesis and prevents cell proliferation.31
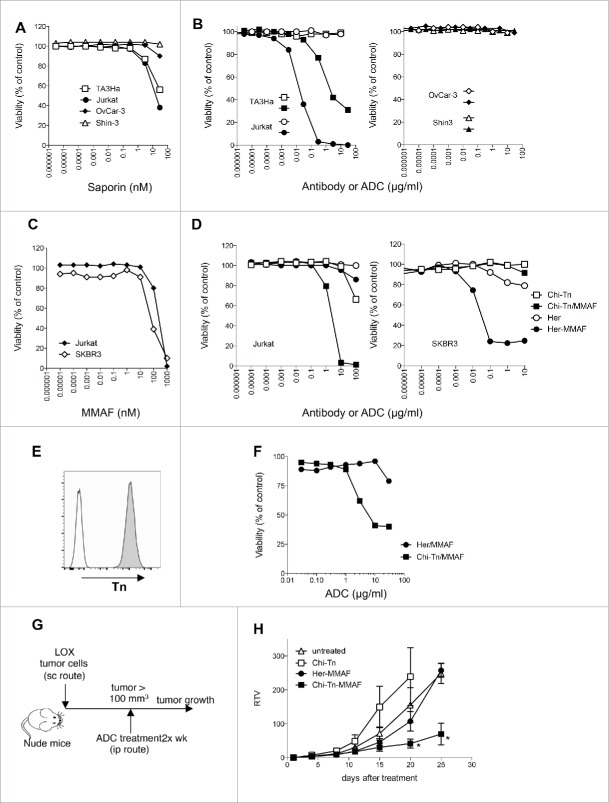
We first assessed whether SAP was directly cytotoxic to different cancer cell lines. For that, viability of TA3Ha, Jurkat, OvCar-3, and Shin-3 cells was measured after 3 d of culture in the presence of different concentrations of SAP. Fig. 5A shows that unconjugated SAP, which has limited access to the cell cytoplasm, does not markedly affect the viability of the different cell lines at concentrations lower than 1 nM. However, at higher concentrations, Jurkat and TA3Ha cell growth is inhibited by the drug, indicating that these cells are more sensitive to SAP at higher doses as compared to OvCar-3 and Shin-3 cells.
Figure 5.
In vitro and in vivo cytotoxicity of Chi-Tn-ADC on cancer cells (A–D) In vitro cytotoxicity of Chi-Tn ADC. (A) TA3Ha, Jurkat, OvCar-3 or Shin-3 cells were cultured with the indicated concentrations of free saporin (SAP). (B) Jurkat, TA3Ha (left panel), and OvCar-3 and Shin-3 cells (right panel) were cultured with the indicated concentrations of Chi-Tn mAb (open symbol) or the Chi-Tn/SAP conjugate (filled symbol). (C) Jurkat and SKBR3 cells were cultured with free MMAF. (D) Jurkat (left panel) and SKBR3 (right panel) cells were cultured with Chi-Tn/MMAF or Her/MMAF conjugate, naked Chi-Tn or Her mAb. Cell viability was assessed after 3 d of culture. Results are expressed as percentage of viable cells compared to untreated cells. (E–H) In vivo cytotoxicity of Chi-Tn/MMAF against Tn+ LOX tumor cells. (E) LOX cells express a high and stable level of Tn. LOX cells were labeled with the Chi-Tn mAb or a control antibody at 20 µg/mL, then with a GaH-Fc-PE secondary antibody and Tn expression was measured by flow cytometry. (F) LOX cells were cultured with the Chi-Tn/MMAF or Her/MMAF conjugate at the indicated concentrations. Cell viability was then assessed after 3 d at 37°C. Results are expressed as percentage of viable cells compared to untreated cells. (G) Experimental schedule of the in vivo antitumor assay. Nude mice were grafted with the LOX Tn+ human cell line and then treated twice a week with the Chi-Tn/MMAF (n = 8) or control Her/MMAF (n = 6) conjugates, with the naked Chi-Tn mAb (n = 6), or were left untreated (n = 7). (H) Tumor growth is depicted as Relative Tumor Volume (RTV), as explained in the Materials and Methods section. Statistical significance was calculated with Mann-Whitney test; significant statistical difference was observed on days 20 and 25 between Her-MMAF and Chi-Tn-MMAF treated groups (*p < 0,05).
Conjugation of SAP to the Chi-Tn mAb, importantly increased the drug cytotoxicity, which was not due to the mAb itself because, as previously described,14 the Ab alone was ineffective at inhibiting in vitro cell proliferation of Jurkat, TA3Ha, OvCar-3, and Shin-3 cells (Fig. 5B). SAP-conjugated Chi-Tn mAb (Chi-Tn/SAP, molar ratio: SAP/Chi-Tn = 2.7) induced a dose-dependent inhibition of the growth of the Tn-expressing tumor cell lines Jurkat and TA3Ha after a 3 d-treatment in vitro, but had no apparent effect on OvCar-3 and Shin-3 cells (Fig. 5B). The Chi-Tn/SAP IC50 values were 7 × 10−11 M for Jurkat cells and 8 × 10−9 M for TA3Ha cells. Notably, SAP alone only inhibited Jurkat and TA3Ha cell growth at much higher molar concentrations (IC50: 3–4 × 10−8 M) (Fig. 5A). Furthermore, Chi-Tn/SAP conjugates showed no cytotoxic effect on the Tn-negative EL-4 cell line (data not shown), while SAP alone inhibited the growth of these cells at concentrations similar to those inhibiting Jurkat and TA3Ha cell proliferation. Of note, the Chi-Tn/SAP conjugate showed high toxicity when administered to mice (data not shown), even at the low doses previously reported.32
Collectively, these results demonstrate that Chi-Tn/SAP immunotoxin displays Tn-specific cell cytotoxicity in vitro, for concentrations of SAP lower than those required for cell growth inhibition using SAP alone. However, as in our hands the Chi-Tn/SAP conjugate is highly toxic for mice, we abandoned this strategy and conjugated the Chi-Tn mAb to a different cytotoxic drug.
The Chi-Tn mAb coupled to auristatin F inhibits tumor cell growth in vitro
Another Chi-Tn ADC was prepared by coupling the mAb to the monomethyl auristatin F drug (MMAF), an antimitotic agent that inhibits cell division by blocking the polymerization of tubulin.9 MMAF is a monomethyl auristatin E (MMAE) analog with a charged C-terminal phenylalanine residue that attenuates the cytotoxic activity compared to the uncharged counterpart MMAE, because it reduces the passive plasma membrane crossing.9,33 Since we showed that internalized Chi-Tn mAb accumulates in early and recycling endosomes, while being delivered inefficiently to late endosomes and lysosomes, we chose a protease-independent linker to conjugate the Chi-Tn mAb and the Herceptin® control to MMAF. Indeed, the mc linker is stable in the extracellular fluid, but is cleaved once the conjugate has entered a tumor cell, thus releasing the anti-mitotic compound.9,34 We prepared ADC-MMAF that contained an average of five MMAF molecules per antibody (Chi-Tn or Her).
As shown in Fig. 5C, free MMAF is cytotoxic to Tn-positive Jurkat and Her2 positive SKBR3 cells, with IC50 values of 4.5 × 102 and 8.3 × 101 nM, respectively indicating that these cells are sensitive to the MMAF drug. Contrary to the Chi-Tn mAb alone, which was ineffective at inhibiting the proliferation of the Tn-expressing tumor cells Jurkat, except at the highest tested dose, the Chi-Tn mAb conjugated to MMAF induced a dose-dependent cytotoxicity on these cells after a 3 d-treatment in vitro (Fig. 5D). Although the Chi-Tn/MMAF recognized Tn at the surface of the Shin-3 or OvCar-3 cells Tn-expressing cell lines as effectively as the naked Chi-Tn mAb (data not shown), the conjugate Chi-Tn/MMAF did not exhibit cytotoxicity toward these cell lines (data not shown). Importantly, the Chi-Tn/MMAF conjugate was specific for Tn expressing tumor cells (Fig. 5D). Indeed, in the same experiment, MMAF conjugated to a non-Tn binding mAb (Her/MMAF) did not have cytotoxic activity to Tn-positive Jurkat cells, indicating that cell killing was subsequent to binding to Tn. In parallel, Her/MMAF conjugate was cytotoxic to Her2-expressing SKBR3 tumor cells (Fig. 5D). These results show that the Chi-Tn/MMAF ADC displays Tn-specific cell cytotoxicity in vitro. Therefore, in vivo tumor targeting using Chi-Tn/MMAF conjugate should allow the use of lower doses of the cytotoxic MMAF, specifically delivering it into the Tn positive tumor cells and thus limiting adverse toxic effects.
In vivo antitumor efficacy of Chi-Tn/MMAF ADC
We showed above that Chi-Tn/MMAF ADC exhibited in vitro cytotoxicity on Jurkat, but not on OvCar-3 or Shin-3 cells. To evaluate the in vivo antitumor activity of the ADC, we grafted Jurkat cells s.c. in Nude mice, but the cells did not grow. Thus, we moved to the LOX melanoma cell line, which expresses a high and stable level of Tn at its surface (Fig. 5E) and forms tumors in Nude mice. We first demonstrated that the Chi-Tn/MMAF conjugate showed cytotoxic activity on the LOX cells in vitro, with cytotoxicity increasing with the dose of ADC (Fig. 5F). This effect was specific to Tn binding, since the ADC containing the irrelevant Ab, Her/MMAF, did not exhibit cytotoxicity except at the highest tested dose. Then, the Chi-Tn/MMAF was investigated in the LOX xenograft model in Nude mice (Fig. 5G). After detection of a palpable tumor, mice were treated with the Chi-Tn/MMAF twice a week along the experiment. Mice did not present any overt clinical signs of adverse events associated to the ADC treatment. We observed that the Her/MMAF conjugate and the non-conjugated Chi-Tn treated mice behaved similarly to the untreated mice (no statistical significance). However, tumor-bearing mice treated with Chi-Tn/MMAF showed an important delay of the tumor growth, highlighting the antitumor activity of the drug-conjugated Chi-Tn antibody (Fig. 5H).
Tn is highly expressed in human cancer tissues, yet at different levels
To further investigate which could be the more adapted clinical setting for the use of anti-Tn drug-conjugate antibodies, we analyzed by IHC the expression level of Tn in paraffin-embedded tumor samples from patients with breast, ovary or endometrium cancers. As shown in Fig. 6, Tn was detected in a great majority of these tumor types, ranging from 80 to 90% of positivity in the tested tumor sections (85% for breast, 90% for ovary, and 83% for endometrium cancers). Although the level of Tn expression was variable among the different samples, we observed that Tn+ ovary tumors consistently exhibited the highest intensity of staining. In the tumors, Tn immunostaining was constantly localized in the cytoplasm, with frequent enhancement of the labeling at the plasma membrane. Exclusive staining at the plasma membrane was not observed. Variability in labeling score was related to the number of cells exhibiting significant staining more than variations in staining intensity. Of note, Tn expression could also be detected in non-tumor cells of the epithelium of normal fallopian tubes. The labeling of other non-tumor tissue was always at a distinctly weaker signal compared to tumor cells. These results emphasize that, as observed with the different tumor cell lines, the different tumor types show varying degree of Tn expression that could condition response to anti-Tn drug-conjugated Abs.
Figure 6.

Analysis of Tn expression in cancer samples from patients. Breast, ovary, and endometrium tumor samples were analyzed by IHC. (A) Representative images of Tn expression in tumor and in healthy breast, ovary and endometrium tissues. (B) Quantification of Tn expression in the different tumor samples.
Discussion
Ideal antibodies for delivering cytotoxic drugs specifically to tumors should target abundantly expressed tumor-specific surface receptors that are internalized efficiently resulting in effective drug delivery. The Tn antigen (GalNac-O-Ser/Thr) is expressed in a great variety of adenocarcinomas 10,12,13,15,16 and in some hematologic malignancies,24-26 but not in healthy tissue. Each anti-Tn antibody is unique in its recognition pattern and function.35 Here, we show that the Chi-Tn mAb that we have generated recognizes Tn on a variety of solid tumors with high specificity,14,36 it is rapidly internalized into Tn-positive tumor cells, and is localized mostly in early endosomes. Given these results indicating that the Chi-Tn mAb is a good candidate to selectively deliver powerful cytotoxic agent to tumor cells, we used an armed antibody approach. Indeed, we show in this study that Chi-Tn mAb inhibits Tn-positive tumor cell growth in vitro when coupled to SAP or MMAF cytotoxic drugs, whereas Tn-negative cells remain unaffected. We also describe a unique ADC that inhibits tumor growth in vivo by specifically delivering the microtubule disrupting agent auristatin F to Tn-expressing tumors. This constitutes the first evidence that an anti-Tn antibody can be successfully conjugated to a cytotoxic drug to be used as a novel antitumor therapeutic agent.
The chimerization of the original murine anti-Tn 83D4 IgM Ab with human Fc generated the Chi-Tn mAb, which recognizes the Tn Ag on tumor cells with a 10-fold reduced affinity (Kd 2.2 × 10−8 M) as compared to the original IgM (Kd 3.1 × 10−9 M37). The moderate affinity might be correlated to the glycopeptidic nature of the Ag as compared to rituximab or trastuzumab, which are directed against protein (affinity of 10−9–10−10 M), since another IgG1 chimeric anti-Tn mAb was reported to have a low affinity (10−7 M).19 Even though intermediate affinities may be detrimental for binding and internalization, it was shown that low affinity mAbs may penetrate solid tumors more efficiently.38
Usually Abs bound to their ligand enter cells along the endocytic pathway, until they reach the acidic lysosomes where final degradation occurs.39 Of note, in the case of the Chi-Tn mAb, we observed that after binding to the surface-expressed Tn, the antibody was rapidly internalized and detected mainly in early endosomes and, to a lesser extent, in recycling endosomes. However, although we did not observe the Chi-Tn mAb in lysosomes, even after overnight incubation at 37°C, we cannot exclude that the mAb reaches lysosomes and is too rapidly degraded to be detectable. Nevertheless, the clinically used mAb trastuzumab directed against HER2 has been described as being similarly internalized in early/recycling endosomes and then recycled to the plasma membrane in breast carcinoma cell lines, without reaching lysosomes.40 Other Abs were also described to localize in early and/or recycling vesicles41,42 and still have antitumor effects, indicating that drug targeting into early endosomes may represent an efficient alternative pathway to obtain antitumor responses with ADC therapy.
Lysosomal compartments are also often the site of cleavage of ADC, especially when the linker contains a dipeptide such as the mc/Val-Cit/PAB used to bind MMAE or MMAF to Abs.27,43 Therefore, since the Chi-Tn mAb mainly localized to early endosomes, we chose to couple the Chi-Tn mAb using the protease-independent mc linker. Indeed, this linker has been previously described to appropriately conjugate Abs to auristatin and to be as effective as the dipeptide-based linkers, with the advantage of decreased toxic adverse effects.9,44 We first used Chi-Tn mAb conjugated to the plant toxin SAP, a ribosome-inactivating enzyme largely studied recently.45 Indeed, SAP is very stable, maintains its enzymatic activity after conjugation, and resists to proteolytic degradation, thus producing very efficient conjugates for the killing of target cells.45 We showed that the Chi-Tn mAb can be used as an efficient ADC to induce tumor cytotoxicity, as Chi-Tn/SAP immunotoxin strongly inhibited the proliferation of Tn-positive Jurkat and TA3Ha cells in vitro. Unfortunately, we could not evaluate the Chi-Tn/SAP activity in vivo, as this conjugate was toxic for mice. Indeed, we tested different Chi-Tn/SAP doses that, in accordance to the existing literature, had been previously used successfully, without toxicity (for review see reference45). We have no clear explanations for this unattended experimental observation, which could be due to specificities of this drug-conjugated construct (Ab, linker), mouse genetic background and/or tumor cell type.
We then evaluated auristatin derivatives, and selected MMAF, less toxic as a free drug than the analog MMAE molecule, resulting in reduced adverse effects on healthy tissues in case of release in the extracellular environment before entering the target tumor cell.9,43 The optimal drug loading per antibody had already been described for MMAF, with the best therapeutic index (ratio between an effective antitumor activity and the maximal tolerated dose) obtained with four molecules per antibody46 as compared to its counterpart with eight drug molecules per antibody. In our hands, our Chi-Tn/MMAF conjugate contained an average of five molecules per antibody – a molar ratio that was previously shown to have antitumor activity with less adverse toxicity46 and potently inhibited the proliferation of Tn-positive tumor cell lines in vitro. Interestingly, the cytotoxic effect of the Chi-Tn mAb conjugated to MMAF was dependent on the level of expression of Tn. Indeed, we observed that the ADC inhibited proliferation of Jurkat and LOX cells, which express high levels of the target antigen; but not of Shin-3 and OvCar-3, which express low Tn levels, although these cells were sensitive to the free drug. This suggests that the level of antigen targeted by the ADC is crucial to achieve an effective antitumor effect by the Chi-Tn-drug conjugate, in agreement with previous studies.27-29
Our results are the first to show that Chi-Tn-based ADC exhibit antitumor activity in a mice model using the Tn-expressing LOX human tumor xenograft. Along these lines, similar results obtained in in vivo models with antibodies targeting other tumor-associated antigens, such as CD22, PMSA, MUC16, and 5T4, conjugated to MMAF or to MMAE,47 have supported the clinical development of these antibodies, as illustrated by numerous ongoing clinical trials in various tumor types (http://www.seattlegenetics.com/pipeline). Furthermore, an anti-CD30 mAb bound to MMAE (Brentuximab vedotin, SGN-35, Seattle Genetics/Takeda) with high therapeutic efficiency in Hodgkin's lymphoma and anaplastic large cells lymphoma (ALCL), has been recently approved for clinical use in more than 55 countries, including the US and European countries.
Overall, our results documenting the high tumor selectivity of the Chi-Tn mAb, its rapid internalization, and targeting to early and recycling endosomes, as well as its effective in vitro and in vivo antitumor effect when linked to a cytotoxic drug, establish the proof of concept that an anti-Tn mAb can be used as an ADC. Interestingly, we observed that Tn was highly expressed in different tumor types, such as breast, ovary, and endometrium carcinomas but with variable patterns of distribution and intensity; and also that the antitumor activity of Chi-Tn/MMAF positively correlated with the level of Tn expression by the tumor. Consequently, for clinical application, Tn expression in individual tumors could represent a good predictive biomarker that should be taken into account in order to propose Chi-Tn-ADC to the appropriate patients who might benefit from this treatment, providing better antitumor efficacy with reduced adverse toxicity.
Materials and methods
Cells and mice
TA3Ha (murine breast cancer, kindly given by C. Leclerc, Institut Pasteur, Paris, France), Jurkat (human T-lymphoid leukemia), OvCar-3 and Shin-3 (human ovarian carcinomas), SKBR3 (human breast adenocarcinoma) and LOX (melanoma, kindly given by O. Fodstad, The Norwegian Radium Hospital, Oslo, Norway) 48 cell lines were cultured in RPMI 1640 (Gibco) containing Glutamax and 10% fetal calf serum (FCS). EL-4 (murine T-lymphoid leukemia) cells were cultured in the same medium supplemented with β-2-mercaptoethanol (Gibco).
Swiss nu/nu female mice (Charles River laboratories, France) were used in agreement with European and National regulations for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (facility license No. C75-05-18). Care and use of animals also complied with internationally established principles of replacement, reduction, and refinement in accordance with the Guide for the Care and Use of Laboratory Animals (NRC 2011).
Antibodies
Trastuzumab (Herceptin®, anti-Her2) was purchased from Hoffman-La Roche. IvIg (Tegeline®, human immunoglobulins) was obtained from LFB (Courtaboeuf, France). The Chi-Tn mAb is a mouse/human chimeric IgG1 specific for the Tn-antigen (Oppezzo, 2000), which was produced and purified by RD Biotech (Besançon, France) following our technical indications.
Flow cytometry
To analyze the Chi-Tn mAb binding to tumor cell plasma membrane, Jurkat (106), Shin-3, OvCar-3 or TA3Ha (4 × 105) cells were incubated for 15 min on ice with the Chi-Tn mAb or a control antibody (IvIg for human cells or trastuzumab for murine cells) at 20 µg/mL in PBS containing 0.5% BSA and 0.01% azide (PBS-BSA-azide). After washing, cells were labeled using a secondary F(ab')2 goat anti-serum specific for the human Fc receptor for IgG (GaH-Fc) coupled to phycoerythrin (PE) (GaH-Fc-PE, Jackson ImmunoResearch Laboratories). DAPI (4′,6′-diamidino-2-phenylindole)-negative living cells were acquired using a FACS Canto™ cytometer (BD Bioscience), and analyzed using the FlowJo software (Tree Star Inc.).
Copy number of Tn antigen was determined by quantitative flow cytometry using a murine anti-Tn mAb kindly provided by R. Lo-Man and C. Leclerc (Institut Pasteur, Paris, France) with the Qifikit (DAKO) following the manufacturer's instructions.
To analyze Chi-Tn mAb internalization, Jurkat (106), Shin-3 or TA3Ha (4 × 105) cells were incubated for 15 min on ice with the Chi-Tn mAb or with control antibodies at 20 µg/mL, washed, then transferred at 37°C in a 5% CO2 atmosphere for the indicated periods of time. The incubation was stopped using cold PBS-BSA-azide; then the cells were labeled with GaH-Fc-PE. DAPI-negative living cells (104) were acquired by flow cytometry and analyzed as above.
Immunohistochemistry
Cancer samples were obtained from surgical specimens removed in patients treated at the Institut Curie (Paris, France). According to French regulation, patients were informed of the research performed and did not express opposition. Tissue sections from formalin fixed paraffin-embedded tumor samples were labeled with the 83D4 murine anti-Tn mAb37 and revealed with a kit containing the biotinylated secondary Ab and the avidin coupled to peroxidase (Vectastain Elite ABC kit, VECTOR Laboratories) following the manufacturer's instructions. Peroxidase was revealed with DAB chromogen (DAKO). Samples were considered as Tn positive when ≥ 50% of tumor cells are labeled.
Biodistribution of the Chi-Tn mAb
Swiss nu/nu female mice were injected s.c. into the flank on day 0 with 4 × 106 Shin-3 ovarian tumor cells. 12 d after tumor xenograft, when solid tumors were grown, mice were injected i.p. with the Chi-Tn mAb or the isotype control mAb (trastuzumab, Herceptin, Her) at 20 mg/kg in sterile PBS. 2 d after (day 14), mice were euthanized. Solid tumor and organs (liver, spleen, and lung) were removed, paraffin-embedded and sectioned. To detect Tn-positive cells, tissue sections were labeled with the Chi-Tn mAb followed by biotinylated GaH-Fc (GaH-Fc-Biot) and Streptavidin-Cy3 (Sa-Cy3). To detect in vivo the presence of the Chi-Tn mAb (or Herceptin), tissue sections were labeled directly with GaH-Fc-Biot and Sa-Cy3. Nuclei were then stained with DAPI, and tissues were mounted to slides.
Images were acquired using a histology microscope Eclipse 90i (Nikon Instruments Europe) equipped with a CDD camera (CoolSNAP HQ2, Roper Scientific), and a dry objective (x10 CFI Plan Apo, NA 0.45).
Three-dimensional deconvolution microscopy
To visualize the Chi-Tn mAb internalization into tumor cells by fluorescence microscopy, Jurkat (106), OvCar-3, Shin-3 or TA3Ha (4 × 105) cells were incubated on ice with Chi-Tn at 40 µg/mL for 15 min, washed with cold medium, and then were incubated at 37°C for the indicated period of time. Internalization was stopped by adding cold PBS-BSA-azide. After washing with PBS containing 0.01% azide (PBS-azide), cells were allowed to adhere for 30 min at room temperature to poly-L-lysine coated glass coverslips, fixed and permeabilized using PBS containing 0.2% BSA and 0.05% saponin (PBS-BSA-saponin). Actin network was labeled using Phalloïdin coupled to Alexa Fluor 647 (Phal-A647, Molecular Probes, Invitrogen). The membrane-bound or internalized Chi-Tn mAb was revealed using GaH-Fc-Biot and Sa-Cy3. Cells were then stained with DAPI, and mounted to glass slides with Mowiol.
Early endosomes were detected using Transferrin-Alexa fluor 488 (Tf-A488, Molecular Probes), added to the cells at 10 µg/mL during the internalization period at 37°C. Recycling endosomes or late endosomes were revealed by adding Rab11 or LAMP-1 antibodies, respectively, (BD Biosciences) at 2.5 µg/mL on fixed cells, then with the corresponding Alexa Fluor 488-conjugated secondary antibody.
Images were acquired using a 3D microscope Eclipse 90i (Nikon) equipped with a CDD camera (CoolSNAP HQ2, Roper Scientific), a piezzo flexure objective scanner (Physik Instrumente), and an oil-immersion objective (x100 CFI Plan Apo VC, NA 1.4). Deconvolutions were performed on stacks of images taken with 0.2 µm plane spacing, using the three-dimensional deconvolution module of MetaMorph® software (Universal Imaging Corp.) and the fast iterative constrained PSF-based algorithm.
Antibody-drug conjugates
The Chi-Tn mAb was coupled to SAP toxin through a disulfide linker, by Advanced Targeting Systems (ATS), with a molar ratio SAP/Chi-Tn = 2.7.
The Chi-Tn mAb coupled to MMAF was produced in our lab and also by AGV Discovery. To prepare the conjugates with MMAF, 10 mg of mAb (Chi-Tn or Her) were thiolated for 2 h at room temperature with 40 M excess of Traut's Reagent. The excess of Traut's Reagent was removed by gel filtration (GE Healthcare) equilibrated in the conjugation buffer. The thiolated antibody was then incubated with a 30 M excess of mc-MMAF diluted in DMSO (Concortis Biosystems) at room temperature overnight. Then, modified antibodies were purified from the excess of the drug by gel filtration after elution with 0.1 M sodium phosphate/0.15 M NaCl/10 mM EDTA (pH 7.6) buffer. The protein concentration was estimated using the BCA protein assay. The molarity of the drug functionality groups after the coupling reaction was determined by Maldi-Tof. The typical overall yield of this antibody modification procedure was five drugs by antibody (Chi-Tn or Herceptin). Modified antibodies were stable for several weeks at 4°C.
Cell viability assay
Jurkat, TA3Ha, OvCar-3 or Shin-3 cells were plated in a 96-W flat bottom tissue culture plates at 104 cells/well in triplicate. The mAb conjugated to drug or free drug were added at the indicated concentration, and plates were incubated for 3 d at 37°C. Cell viability was then assessed by adding the CellTiter Blue® Cell Viability reagent according to the manufacturer's instructions (Promega) during the last 2 h 30 min of culture for Jurkat cells or the last 4 h for the other tumor cells. The dye reduction was measured on a fluorescent plate reader. Results are expressed as a percentage of viability compared to untreated cells incubated in the same conditions without any treatment during the 3 d.
In vivo tumor therapeutic experiment
Swiss nu/nu female mice (n = 6–8) received a s.c. injection of 0.2 × 106 LOX cells. When the tumor reached a volume approximately of 100 mm3 as calculated according to the following formulae: V = (L × l2)/2, where L and l are the largest and the smallest perpendicular tumor diameters respectively, mice were injected i.p. with the Abs coupled to the MMAF (4 mg/Kg), twice a week. Growth curves plotted the mean volume of Relative tumor volume (RTV) calculated according to RTV = (Vx/V1), where Vx is the tumor volume on day X and V1 is the tumor volume at initiation of the therapy (day 1).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Cytometry Platform, the Nikon Imaging Center, and the Animal facilities of the Institut Curie. We acknowledge the CIC IGR-Curie 1428 (Center Of Clinical Investigation), Institut Curie, Paris; the ECOS-Sud program, Université de Paris, ANR-2010-EMMA-015-01, Medicen Paris Region, SiRIC (Grant INCa-DGOS-4654), ANR-10-IDEX-0001-02 PSL*, and ANR-11-LABX-0043. We also specifically thank André Nicolas from the Platform of Experimental Pathology of the Institut Curie and Virginie Premel for their help in IHC.
Funding
This work was supported by fundings from the Institut National de la Santé et de la Recherche Médicale (INSERM); the Association pour la Recherche sur le Cancer (ARC); the Institut Curie; the Agence Nationale pour la Recherche (ANR Emergence program), and the Institut National du Cancer (INCa).
References
- 1.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12:278-87; PMID:22437872; http://dx.doi.org/ 10.1038/nrc3236 [DOI] [PubMed] [Google Scholar]
- 2.Vacchelli E, Eggermont A, Galon J, Sautes-Fridman C, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: monoclonal antibodies in cancer therapy. Oncoimmunology 2013; 2:e22789; PMID:23482847; http://dx.doi.org/ 10.4161/onci.22789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Govindan SV, Goldenberg DM. Designing immunoconjugates for cancer therapy. Expert Opin Biol Ther 2012; 12:873-90; PMID:22679911; http://dx.doi.org/ 10.1517/14712598.2012.685153 [DOI] [PubMed] [Google Scholar]
- 4.Perez HL, Cardarelli PM, Deshpande S, Gangwar S, Schroeder GM, Vite GD, Borzilleri RM. Antibody-drug conjugates: current status and future directions. Drug Discov Today 2014; 19:869-81; PMID:24239727; http://dx.doi.org/ 10.1016/j.drudis.2013.11.004 [DOI] [PubMed] [Google Scholar]
- 5.Chari RV. Targeted cancer therapy: conferring specificity to cytotoxic drugs. Acc Chem Res 2008; 41:98-107; PMID:17705444; http://dx.doi.org/ 10.1021/ar700108g [DOI] [PubMed] [Google Scholar]
- 6.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol 2004; 5:121-32; PMID:15040445; http://dx.doi.org/ 10.1038/nrm1315 [DOI] [PubMed] [Google Scholar]
- 7.Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev 2007; 59:748-58; PMID:17659804; http://dx.doi.org/ 10.1016/j.addr.2007.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ulbrich K, Etrych T, Chytil P, Pechar M, Jelinkova M, Rihova B. Polymeric anticancer drugs with pH-controlled activation. Int J Pharm 2004; 277:63-72; PMID:15158969; http://dx.doi.org/ 10.1016/j.ijpharm.2003.02.001 [DOI] [PubMed] [Google Scholar]
- 9.Doronina SO, Mendelsohn BA, Bovee TD, Cerveny CG, Alley SC, Meyer DL, Oflazoglu E, Toki BE, Sanderson RJ, Zabinski RF et al.. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem 2006; 17:114-24; PMID:16417259; http://dx.doi.org/ 10.1021/bc0502917 [DOI] [PubMed] [Google Scholar]
- 10.Ju T, Otto VI, Cummings RD. The Tn antigen-structural simplicity and biological complexity. Angew Chem Int Ed Engl 2011; 50:1770-91; PMID:21259410; http://dx.doi.org/ 10.1002/anie.201002313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Springer GF. T and Tn, general carcinoma autoantigens. Science 1984; 224:1198-206; PMID:6729450; http://dx.doi.org/ 10.1126/science.6729450 [DOI] [PubMed] [Google Scholar]
- 12.Beuzelin-Yvraut M, Bourguignat A, Phillips E, Roseto A, Osinaga E. Immunocytological analysis of the Tn associated antigen 83D4 in serous effusions from patients with cancer: comparison with Tn soluble glycoprotein. J Clin Pathol 1995; 48:433-7; PMID:7629290; http://dx.doi.org/ 10.1136/jcp.48.5.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croce MV, Rabassa ME, Price MR, Segal-Eiras A. MUC1 mucin and carbohydrate associated antigens as tumor markers in head and neck squamous cell carcinoma. Pathol Oncol Res 2001; 7:284-91; PMID:11882908; http://dx.doi.org/ 10.1007/BF03032385 [DOI] [PubMed] [Google Scholar]
- 14.Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, Pritsch O, Osinaga E, Amigorena S. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res 2011; 71:5134-43; PMID:21697279; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-4222 [DOI] [PubMed] [Google Scholar]
- 15.Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, Takahashi HK, Bigbee WL, Kim YS. Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res 1989; 49:197-204; PMID:2908846 [PubMed] [Google Scholar]
- 16.Li Q, Anver MR, Butcher DO, Gildersleeve JC. Resolving conflicting data on expression of the Tn antigen and implications for clinical trials with cancer vaccines. Mol Cancer Ther 2009; 8:971-9; PMID:19372570; http://dx.doi.org/ 10.1158/1535-7163.MCT-08-0934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pancino GF, Osinaga E, Vorauher W, Kakouche A, Mistro D, Charpin C, Roseto A. Production of a monoclonal antibody as immunohistochemical marker on paraffin embedded tissues using a new immunization method. Hybridoma 1990; 9:389-95; PMID:2210779; http://dx.doi.org/ 10.1089/hyb.1990.9.389 [DOI] [PubMed] [Google Scholar]
- 18.Danussi C, Coslovi A, Campa C, Mucignat MT, Spessotto P, Uggeri F, Paoletti S, Colombatti A. A newly generated functional antibody identifies Tn antigen as a novel determinant in the cancer cell-lymphatic endothelium interaction. Glycobiology 2009; 19:1056-67; PMID:19528665; http://dx.doi.org/ 10.1093/glycob/cwp085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ando H, Matsushita T, Wakitani M, Sato T, Kodama-Nishida S, Shibata K, Shitara K, Ohta S. Mouse-human chimeric anti-Tn IgG1 induced anti-tumor activity against Jurkat cells in vitro and in vivo. Biol Pharm Bull 2008; 31:1739-44; PMID:18758069; http://dx.doi.org/ 10.1248/bpb.31.1739 [DOI] [PubMed] [Google Scholar]
- 20.Avichezer D, Springer GF, Schechter B, Arnon R. Immunoreactivities of polyclonal and monoclonal anti-T and anti-Tn antibodies with human carcinoma cells, grown in vitro and in a xenograft model. Int J Cancer J Int Du Cancer 1997; 72:119-27; PMID:9212232; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 21.Nakada H, Inoue M, Numata Y, Tanaka N, Funakoshi I, Fukui S, Mellors A, Yamashina I. Epitopic structure of Tn glycophorin A for an anti-Tn antibody (MLS 128). Proc Natl Acad Sci USA 1993; 90:2495-9; PMID:7681597; http://dx.doi.org/ 10.1073/pnas.90.6.2495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oppezzo P, Osinaga E, Tello D, Bay S, Cantacuzene D, Irigoin F, Ferreira A, Roseto A, Cayota A, Alzari P et al.. Production and functional characterization of two mouse/human chimeric antibodies with specificity for the tumor-associated Tn-antigen. Hybridoma 2000; 19:229-39; PMID:10952411; http://dx.doi.org/ 10.1089/02724570050109620 [DOI] [PubMed] [Google Scholar]
- 23.Welinder C, Baldetorp B, Borrebaeck C, Fredlund BM, Jansson B. A new murine IgG1 anti-Tn monoclonal antibody with in vivo anti-tumor activity. Glycobiol 2011; 21:1097-107; PMID:21470982; http://dx.doi.org/ 10.1093/glycob/cwr048 [DOI] [PubMed] [Google Scholar]
- 24.Aller CT, Kucuk O, Springer GF, Gilman-Sachs A. Flow cytometric analysis of T and Tn epitopes on chronic lymphocytic leukemia cells. Am J Hematol 1996; 52:29-38; PMID:8638608; http://dx.doi.org/ [DOI] [PubMed] [Google Scholar]
- 25.Inoue M, Nakada H, Tanaka N, Yamashina I. Tn antigen is expressed on leukosialin from T-lymphoid cells. Cancer Res 1994; 54:85-8; PMID:8261467 [PubMed] [Google Scholar]
- 26.Lawrie CH, Marafioti T, Hatton CS, Dirnhofer S, Roncador G, Went P, Tzankov A, Pileri SA, Pulford K, Banham AH. Cancer-associated carbohydrate identification in Hodgkin's lymphoma by carbohydrate array profiling. Int J Cancer J Int Du Cancer 2006; 118:3161-6; PMID:16395706; http://dx.doi.org/ 10.1002/ijc.21762 [DOI] [PubMed] [Google Scholar]
- 27.Asundi J, Reed C, Arca J, McCutcheon K, Ferrando R, Clark S, Luis E, Tien J, Firestein R, Polakis P. An antibody-drug conjugate targeting the endothelin B receptor for the treatment of melanoma. Clin Cancer Res 2011; 17:965-75; PMID:21245091; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-2340 [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Clark S, Wong T, Chen Y, Chen Y, Dennis MS, Luis E, Zhong F, Bheddah S, Koeppen H et al.. Armed antibodies targeting the mucin repeats of the ovarian cancer antigen, MUC16, are highly efficacious in animal tumor models. Cancer Res 2007; 67:4924-32; PMID:17510422; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-4512 [DOI] [PubMed] [Google Scholar]
- 29.Ryan MC, Kostner H, Gordon KA, Duniho S, Sutherland MK, Yu C, Kim KM, Nesterova A, Anderson M, McEarchern JA et al.. Targeting pancreatic and ovarian carcinomas using the auristatin-based anti-CD70 antibody-drug conjugate SGN-75. Br J Cancer 2010; 103:676-84; PMID:20664585; http://dx.doi.org/ 10.1038/sj.bjc.6605816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol 2009; 10:597-608; PMID:19696797; http://dx.doi.org/ 10.1038/nrm2755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Endo Y, Mitsui K, Motizuki M, Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J Biol Chem 1987; 262:5908-12; PMID:3571242 [PubMed] [Google Scholar]
- 32.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer 2006; 6:559-65; PMID:16794638; http://dx.doi.org/ 10.1038/nrc1891 [DOI] [PubMed] [Google Scholar]
- 33.Oflazoglu E, Stone IJ, Gordon K, Wood CG, Repasky EA, Grewal IS, Law CL, Gerber HP. Potent anticarcinoma activity of the humanized anti-CD70 antibody h1F6 conjugated to the tubulin inhibitor auristatin via an uncleavable linker. Clin Cancer Res 2008; 14:6171-80; PMID:18809969; http://dx.doi.org/ 10.1158/1078-0432.CCR-08-0916 [DOI] [PubMed] [Google Scholar]
- 34.Doronina SO, Bovee TD, Meyer DW, Miyamoto JB, Anderson ME, Morris-Tilden CA, Senter PD. Novel peptide linkers for highly potent antibody-auristatin conjugate. Bioconjug Chem 2008; 19:1960-3; PMID:18803412; http://dx.doi.org/ 10.1021/bc800289a [DOI] [PubMed] [Google Scholar]
- 35.Mazal D, Lo-Man R, Bay S, Pritsch O, Deriaud E, Ganneau C, Medeiros A, Ubillos L, Obal G, Berois N et al.. Monoclonal antibodies toward different Tn-amino acid backbones display distinct recognition patterns on human cancer cells. Implications for effective immuno-targeting of cancer. Cancer Immunol Immunother 2013; 62:1107-22; PMID:23604173; http://dx.doi.org/ 10.1007/s00262-013-1425-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charpin C, Pancino G, Osinaga E, Bonnier P, Lavaut MN, Allasia C, Roseto A. Monoclonal antibody 83D4 immunoreactivity in human tissues: cellular distribution and microcytophotometric analysis of immunoprecipitates on tissue sections. Anticancer Res 1992; 12:209-23; PMID:1567169 [PubMed] [Google Scholar]
- 37.Osinaga E, Bay S, Tello D, Babino A, Pritsch O, Assemat K, Cantacuzene D, Nakada H, Alzari P. Analysis of the fine specificity of Tn-binding proteins using synthetic glycopeptide epitopes and a biosensor based on surface plasmon resonance spectroscopy. FEBS Lett 2000; 469:24-8; PMID:10708749; http://dx.doi.org/ 10.1016/S0014-5793(00)01248-5 [DOI] [PubMed] [Google Scholar]
- 38.Rudnick SI, Lou J, Shaller CC, Tang Y, Klein-Szanto AJ, Weiner LM, Marks JD, Adams GP. Influence of affinity and antigen internalization on the uptake and penetration of Anti-HER2 antibodies in solid tumors. Cancer Res 2011; 71:2250-9; PMID:21406401; http://dx.doi.org/ 10.1158/0008-5472.CAN-10-2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim SH, Vaughan AT, Ashton-Key M, Williams EL, Dixon SV, Chan HT, Beers SA, French RR, Cox KL, Davies AJ et al.. Fc gamma receptor IIb on target B cells promotes rituximab internalization and reduces clinical efficacy. Blood 2011; 118:2530-40; PMID:21768293; http://dx.doi.org/ 10.1182/blood-2011-01-330357 [DOI] [PubMed] [Google Scholar]
- 40.Austin CD, De Maziere AM, Pisacane PI, van Dijk SM, Eigenbrot C, Sliwkowski MX, Klumperman J, Scheller RH. Endocytosis and sorting of ErbB2 and the site of action of cancer therapeutics trastuzumab and geldanamycin. Mol Biol Cell 2004; 15:5268-82; PMID:15385631; http://dx.doi.org/ 10.1091/mbc.E04-07-0591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iglesias-Bartolome R, Crespo PM, Gomez GA, Daniotti JL. The antibody to GD3 ganglioside, R24, is rapidly endocytosed and recycled to the plasma membrane via the endocytic recycling compartment. Inhibitory effect of brefeldin A and monensin. FEBS J 2006; 273:1744-58; PMID:16623710; http://dx.doi.org/ 10.1111/j.1742-4658.2006.05194.x [DOI] [PubMed] [Google Scholar]
- 42.Perera RM, Zoncu R, Johns TG, Pypaert M, Lee FT, Mellman I, Old LJ, Toomre DK, Scott AM. Internalization, intracellular trafficking, and biodistribution of monoclonal antibody 806: a novel anti-epidermal growth factor receptor antibody. Neoplasia 2007; 9:1099-110; PMID:18084617; http://dx.doi.org/ 10.1593/neo.07721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee TD, Siegall CB et al.. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 2003; 21:778-84; PMID:12778055; http://dx.doi.org/ 10.1038/nbt832 [DOI] [PubMed] [Google Scholar]
- 44.Jackson D, Gooya J, Mao S, Kinneer K, Xu L, Camara M, Fazenbaker C, Fleming R, Swamynathan S, Meyer D et al.. A human antibody-drug conjugate targeting EphA2 inhibits tumor growth in vivo. Cancer Res 2008; 68:9367-74; PMID:19010911; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-1933 [DOI] [PubMed] [Google Scholar]
- 45.Polito L, Bortolotti M, Pedrazzi M, Bolognesi A. Immunotoxins and other conjugates containing saporin-s6 for cancer therapy. Toxins (Basel) 2011; 3:697-720; PMID:22069735; http://dx.doi.org/ 10.3390/toxins3060697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF et al.. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res 2004; 10:7063-70; PMID:15501986; http://dx.doi.org/ 10.1158/1078-0432.CCR-04-0789 [DOI] [PubMed] [Google Scholar]
- 47.Bouchard H, Viskov C, Garcia-Echeverria C. Antibody-drug conjugates–a new wave of cancer drugs. Bioorg Med Chem Lett 2014; 24:5357-63; PMID:25455482; http://dx.doi.org/ 10.1016/j.bmcl.2014.10.021 [DOI] [PubMed] [Google Scholar]
- 48.Fodstad O, Aamdal S, McMenamin M, Nesland JM, Pihl A. A new experimental metastasis model in athymic nude mice, the human malignant melanoma LOX. Int J Cancer J Int Du Cancer 1988; 41:442-9; PMID:3346110; http://dx.doi.org/ 10.1002/ijc.2910410322 [DOI] [PubMed] [Google Scholar]