Abstract
Harms of opioid analgesics, especially high-dose therapy among individuals with comorbidities and older age, are increasingly recognized. However, trends in opioid receipt among HIV-infected patients are not well characterized. We examined trends, from 1999 to 2010, in any and high-dose (≥120 mg/day) opioid receipt among patients with and without HIV, by age strata, controlling for demographic and clinical correlates. Of 127,216 patients, 64 % received at least one opioid prescription. Opioid receipt increased substantially among HIV-infected and uninfected patients over the study; high-dose therapy was more prevalent among HIV-infected patients. Trends in high-dose receipt stratified by three age groups revealed an increasing trend in each age strata, higher among HIV-infected patients. Correlates of any opioid receipt included HIV, PTSD and major depression. Correlates of high-dose receipt included HIV, PTSD, major depression and drug use disorders. These findings suggest a need for appropriate balance of risks and benefits, especially as these populations age.
Keywords: Analgesics, Opioids, Aging, Pain, Chronic, Human immunodeficiency virus
Introduction
Over the last two decades, marked increases in opioid sales have been reported [1–3]. Concomitant with increased prescribing has been an increase in opioid-related adverse events, including overdose deaths, especially with high-dose therapy [4, 5]. Several studies have found a higher incidence of major adverse events in individuals with mental health disorders and substance use disorders [6–8]. Additionally, observational research revealed increased mortality among older adults prescribed opioids compared to non-steroid anti-inflammatory drugs [9], making increasing age another factor to consider in weighing the balance benefit and harm of opioids. These findings have driven safety-minded proposals including dose restrictions, medication labeling changes, and additional physician education [10].
Among HIV-infected populations, there has been a shift from opioid prescribing for end-of-life, palliative care to long-term opioid prescribing for chronic pain, although research has not documented the impact of this shift on prescribing rates. As in general populations, prescribing opioid analgesics to HIV-infected patients requires a careful balance of potential benefit and harm [11–13]. On one hand, aging patients with HIV have a high prevalence of painful conditions for which opioids may be beneficial, including both non HIV-related conditions like osteoarthritis, as well as, for example, HIV-associated painful peripheral neuropathy and painful sequelae of opportunistic infections [14, 15]. On the other hand, patients with HIV have a higher prevalence of risk factors associated with major opioid-related adverse events, including mental health, alcohol and substance use disorders [16]. When present, these factors also place patients at higher risk of developing opioid use disorder [17]. Furthermore, opioids may impair immune function [18] and interact with antiretroviral therapy in sometimes difficult to predict and dangerous ways [19, 20]. Additionally, as patients with HIV live longer, concerns about effects of high-risk medications on geriatric populations who are more prone to falls, fractures, decreased metabolism and altered pharmacodynamics, have increased [21]. Finally, a recent study of long-term opioid receipt demonstrated a higher risk of death among individuals with HIV compared to those without HIV [22].
For these reasons, a better understanding of the pharmacoepidemiology of opioid receipt among HIV-infected patients is critical. To our knowledge, only one prior study has evaluated trends in opioid receipt among HIV-infected patients [23]. This study, however, was in one geographic region and did not incorporate opioid dose trends. Therefore, as a follow-up to a single-year, cross-sectional study [24], the purpose of this prospective cohort study was to examine trends over a 12-year period in any and high-dose opioid receipt for noncancer pain in patients with HIV, by age strata, and to assess the demographic characteristics and clinical conditions associated with these outcomes. Uninfected patients were included as a comparison group, providing information about secular trends during this time period.
Methods
Study Overview
We used data from the Veterans Aging Cohort Study-Virtual Cohort (VACS-VC), described in detail elsewhere [25–27]. Briefly, the VACS-VC is a cohort of HIV-infected patients and uninfected controls matched 1:2 on age, sex, race/ethnicity and site of care identified from Veterans Health Administration (VHA) administrative data. Data for this analysis included fiscal years 1999 through 2010 and incorporated information from the Immunology Case Registry–the VHA HIV registry–and the Decision Support System [28, 29]. The study was approved by the institutional review boards at VA Connecticut Healthcare System and Yale University.
Study Population
VACS-VC has 44,180 HIV-infected and 88,360 matched uninfected patients with clinical data [30]. Patients were excluded if they met any of the following criteria: (1) unclear HIV status; (2) cancer diagnosis, excluding non-epithelial skin cancers; or (3) incomplete opioid pharmacy data.
Prescription Opioid Types
Data on oral and transdermal opioid receipt included the following medications: codeine, hydrocodone, oxycodone, oxycodone sustained action (SA), morphine, morphine SA, fentanyl, hydromorphone, methadone, propoxyphene, and tramadol. We opted to include tramadol, a non-scheduled medication, because it is a weak μ opioid receptor agonist and is frequently prescribed to treat pain. Because of low frequency prescribing, we combined the following opioids into a single “other” category: dihydrocodeine, meperidine, pentazocine, levorphanol, and tapentadol. Opioid types with negligible receipt (e.g. hydrocodone sustained action (SA), hydromorphone SA, oxymorphone, oxymorphone SA, and codeine SA) were excluded. Medications provided for the treatment of opioid dependence (methadone via opioid treatment programs and buprenorphine) were also excluded.
Prescription Opioid Use Profiles
Consistent with published methodology [24], total morphine equivalents were calculated by multiplying the quantity of each prescription by the strength of the prescription (milligram of opioid per unit dispensed). For non-morphine opioids, standard conversion factors were used to estimate milligrams of morphine equivalents dispensed [31]. To determine the milligrams of morphine equivalents of special formulations, including cough elixirs, transdermal fentanyl, and solutions, we consulted published literature [31] and reached consensus when ambiguity remained. Any opioid receipt was defined as receipt of at least one prescription for any outpatient opioid from 1999–2010. High-dose opioid therapy was defined as an average daily dose of at least 120 mg of morphine equivalents, consistent with our previous analysis [24]. Both of these outcomes were assessed across the 12-year analytic frame and then within each year.
Covariates
Demographic variables included gender, age and race/ethnicity (white, black, Hispanic and other) based on self-report. Clinical variables, based on ICD-9 codes, included post-traumatic stress disorder (PTSD); major depression; bipolar disorder; schizophrenia; alcohol use disorders; drug use disorders; smoking, classified as never, current or former; diabetes and pain-related diagnoses. Hepatitis C virus (HCV) was based on ICD-9 codes and laboratory data. We categorized pain-related diagnoses as acute pain-related: abdominal pain, chest pain, fracture, or kidney stones, and chronic pain-related: back pain, extremity pain, headache, menstrual pain, neck pain, neuropathy, osteoarthritis, other pain, rheumatoid arthritis, or temporomandibular joint pain [24]. HIV status was established at entry into the cohort based on serologic testing. Other diagnoses were assigned if they appeared in at least one inpatient or two outpatient encounters in the year prior or 6 months after study enrollment. We included mental health disorders (PTSD, major depression, bipolar disorder and schizophrenia), alcohol use disorders and drug use disorders as covariates as they are known risk factors for adverse effects of opioids [6–8, 17, 32].
Statistical Analyses
First, using all years together as a single cross-section, we performed descriptive statistics to characterize patients overall and by opioid receipt. We used t test for continuous variables, or the Wilcoxon rank sum test for non-normally distributed continuous variables, and Chi square for categorical variables to compare characteristics by opioid receipt, considering p < 0.05 as statistically significant. Then, with each of the 12 years as a serial cross section, we examined trends in proportion of the sample with any and high-dose receipt. Since repeated measures are correlated, GEE is required to avoid underestimating standard errors and overestimating influence [33]. We then performed logistic regression using GEE to assess the association between each covariate and receipt of any and high-dose opioids. To further explore trends by age, we examined proportion of high-dose receipt by HIV status in three age groups: <45, 45–64 and ≥65 years old, meant to represent young, middle aged and older adult populations, respectively. The older adult population was of particular interest given its vulnerability to medication-related toxicity. Statistical analyses were performed using SAS version 9.1.3 (SAS Institute Inc., North Carolina).
Results
The final analytic sample included 127,216 patients. The sample was predominately male (98 %) with a mean age of 46 years; 47 % were black, 39 % white, 8 % Hispanic and 6 % other (Table 1). PTSD was the most common mental health disorder at 7 %. The proportion of patients with alcohol use disorders and drug use disorders was 15 % and 14 %, respectively. The median number of pain-related diagnoses was two. Thirty-three percent of the sample was HIV-infected. Over the 12-year time frame, 64 % of the total sample received opioids (HIV-infected: 65 %, HIV-uninfected: 64 %; p = 0.003); 7 % received high-dose opioids (HIV-infected: 8 %, HIV-uninfected: 6 %; p < 0.001).
Table 1.
Description of the sample
| N = 127,216 | Overall % | (−) Opioid receipt %, N = 45,854
|
(+) Opioid receipt %, N = 81,362
|
||||
|---|---|---|---|---|---|---|---|
| HIV-uninfected N = 30,985 |
HIV-infected N = 14,869 |
p value | HIV-uninfected N = 54,307 |
HIV-infected N = 27,055 |
p value | ||
| Age mean(SD) | 46 (10) | 46.4 (11.6) | 47.1 (10.9) | <0.001 | 46.5 (9.6) | 45.8 (9.8) | <0.001 |
| Sex | 0.67 | 0.30 | |||||
| Female | 2 | 2 | 2 | 3 | 3 | ||
| Male | 98 | 98 | 98 | 97 | 97 | ||
| Race | <0.001 | <0.001 | |||||
| White | 39 | 38 | 35 | 40 | 39 | ||
| Black | 47 | 43 | 48 | 48 | 50 | ||
| Hispanics | 8 | 7 | 7 | 8 | 7 | ||
| Other | 6 | 12 | 10 | 3 | 4 | ||
| PTSD | 7 | 5 | 3 | <0.001 | 10 | 6 | <0.001 |
| Major depression | 6 | 4 | 5 | <0.001 | 7 | 8 | <0.001 |
| Bipolar disorder | 4 | 3 | 3 | 0.38 | 4 | 4 | 0.03 |
| Schizophrenia | 5 | 7 | 3 | <0.001 | 5 | 3 | <0.001 |
| Alcohol use disorders | 15 | 12 | 14 | <0.001 | 15 | 17 | <0.001 |
| Drug use disorders | 14 | 9 | 16 | <0.001 | 13 | 20 | <0.001 |
| Smokinga | <0.001 | <0.001 | |||||
| Never | 28 | 35 | 33 | 27 | 24 | ||
| Current | 55 | 46 | 51 | 56 | 62 | ||
| Former | 17 | 19 | 16 | 17 | 14 | ||
| Hepatitis C infection | 20 | 8 | 24 | <0.001 | 17 | 39 | <0.001 |
| Diabetes | 23 | 16 | 11 | <0.001 | 30 | 20 | <0.001 |
| Pain related diagnosisb | <0.001 | <0.001 | |||||
| No pain diagnosis | 23 | 45 | 56 | 7 | 13 | ||
| Acute pain diagnosis | 6 | 6 | 9 | 3 | 7 | ||
| Chronic pain diagnosis | 71 | 48 | 35 | 90 | 80 | ||
| Number of pain related diagnoses, IQR | 2 (1, 4) | 1 (1, 2) | 1 (0, 2) | <0.001 | 3 (2, 5) | 3 (1, 4) | <0.001 |
| High-dose receipt | 7 | 10 | 13 | <0.001 | |||
17 % missing
Based on ICD-9 codes. Acute pain is comprised of abdominal, chest, kidney and fracture pain. Chronic pain is comprised of back, neck, temporomandibular, extremity, menstrual, rheumatoid arthritis, neuropathy and other pain
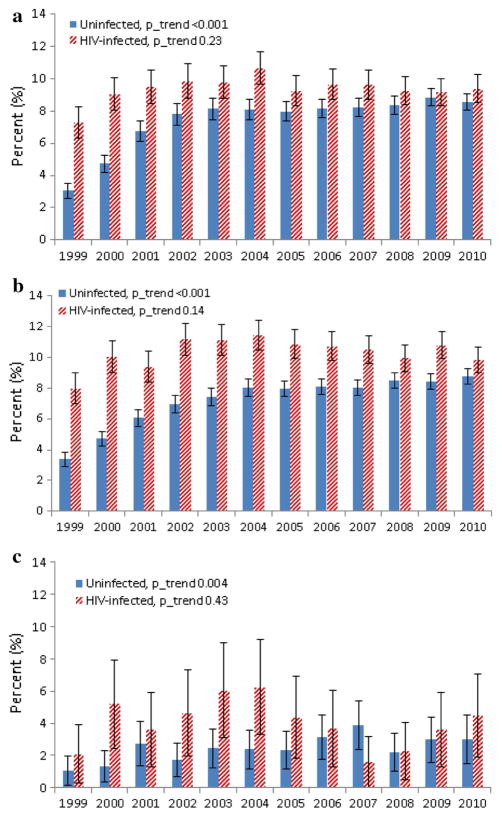
Trends in any opioid receipt were similar when stratified by HIV status (see Fig. 1), with a significant increase over time in both groups. Comparing the beginning to the end of the study, there was a marked increase in the proportion of patients with high-dose receipt as well, but that increase occurred in the first half of the study and then stabilized (Fig. 2). In each of three age strata (<45, 45–64 and ≥65), high-dose receipt generally started higher among HIV-infected patients and was stable but started lower and trended upwards among uninfected patients (Fig. 3a–c). Notably, 4.5 % of HIV-infected individuals 65 and older with any opioid receipt in 2010 had high-dose receipt, compared to 3.0 % of uninfected (p = 0.31).
Fig. 1.

Trends in any opioid receipt, overall and by HIV status
Fig. 2.

Trends in high-dose opioid receipt, overall and by HIV status (asterisk)
Fig. 3.
a High-dose opioid receipt by HIV status, age <45. b High-dose opioid receipt by HIV status, age 45–64. c High-dose opioid receipt by HIV status, age ≥ 65
Among those who received opioids, HIV-infected patients, compared to uninfected patients, were slightly younger, more likely to have major depression, bipolar disorder, alcohol and drug use disorders and HCV, and less likely to have PTSD, schizophrenia or a pain-related diagnosis (Table 1). Also, among those with any opioid receipt, HIV-infected patients were more likely to have high-dose receipt than uninfected-patients (13 % vs. 10 %; p < 0.001). In the full sample, compared to those without opioid receipt, those with opioid receipt were more likely to have HIV infection, were slightly younger and more likely to have mental health, alcohol use and drug use disorders, HCV, diabetes, and chronic pain-related diagnoses. Those with opioid receipt were more likely to be current smokers (p < 0.05 for all comparisons).
In multivariable logistic analyses using GEE, in which HIV was the predictor of interest (Table 2), HIV, PTSD, major depression, smoking (current or former), HCV, diabetes, having an acute pain-related diagnosis, having a chronic pain-related diagnosis, and number of pain-related diagnoses were positively associated with opioid receipt whereas black race, Hispanic ethnicity, other race/ethnicity, alcohol use disorders, drug use disorders and schizophrenia were negatively associated with opioid receipt. Among those receiving opioids, HIV, PTSD, major depression, drug use disorders, smoking (current or former), HCV, and number of pain-related diagnoses were positively associated with high-dose opioid receipt whereas black race, Hispanic ethnicity, other race/ethnicity, schizophrenia and alcohol use disorders were negatively associated with high-dose opioid receipt. We took into account the matched design in a sensitivity analysis and there was no substantial difference in results. Given the pattern observed in the descriptive analysis examining opioid receipt and HIV by age groups, an a posteriori interaction term between HIV and age in relation to high dose opioid receipt was tested. In the unadjusted model with HIV, age and HIV*age, the odds ratio for the interaction term was 1.08 (1.03, 1.13), p = 0.002. However, in the adjusted model the interaction term did not remain statistically significant.
Table 2.
repeated measures analyses of binary outcomes: any and high-dose opioid receipt, 1999–2010, unadjusted and adjusted
| Characteristic | Any opioid receipt N = 127,216
|
High-dose receipt N = 81,362
|
||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted
|
Adjusted
|
Unadjusted
|
Adjusted
|
|||||
| OR (95 % CI) | p value | AOR (95 % CI) | p value | OR (95 % CI) | p value | AOR (95 % CI) | p value | |
| HIV | 1.01 (1.00, 1.02) | 0.18 | 1.14 (1.12, 1.16) | <0.001 | 1.48 (1.41, 1.56) | < 0.001 | 1.29 (1.21, 1.37) | <0.001 |
| Age/10 years | 0.98 (0.98, 0.99) | < 0.001 | 1.01 (1.01, 1.02) | 0.0003 | 0.98 (0.96, 1.00) | 0.09 | 1.00 (0.97, 1.03) | 0.85 |
| Black (ref: white) | 0.90 (0.88, 0.91) | < 0.001 | 0.87 (0.86, 0.89) | <0.001 | 0.51 (0.49, 0.54) | < 0.001 | 0.46 (0.43, 0.49) | <0.001 |
| Hispanic (ref: white) | 0.87 (0.85, 0.89) | < 0.001 | 0.82 (0.80, 0.84) | <0.001 | 0.56 (0.50, 0.62) | < 0.001 | 0.56 (0.50, 0.62) | <0.001 |
| Other (ref: white) | 0.38 (0.37, 0.39) | < 0.001 | 0.71 (0.68, 0.74) | <0.001 | 0.46 (0.39, 0.55) | < 0.001 | 0.60 (0.50, 0.73) | <0.001 |
| PTSD | 1.53 (1.49, 1.56) | < 0.001 | 1.10 (1.08, 1.13) | <0.001 | 1.39 (1.29, 1.50) | < 0.001 | 1.19 (1.09, 1.30) | <0.001 |
| Major Depression | 1.50 (1.46, 1.54) | < 0.001 | 1.09 (1.06, 1.12) | <0.001 | 1.55 (1.43, 1.68) | < 0.001 | 1.26 (1.15, 1.38) | <0.001 |
| Bipolar | 1.23 (1.19, 1.27) | < 0.001 | 1.01 (0.98, 1.04) | 0.50 | 1.16 (1.03, 1.30) | 0.01 | 0.89 (0.79, 1.01) | 0.07 |
| Schizophrenia | 0.84 (0.81, 0.87) | < 0.001 | 0.75 (0.73, 0.77) | <0.001 | 0.65 (0.57, 0.75) | < 0.001 | 0.61 (0.53, 0.71) | <0.001 |
| Alcohol use disorders | 1.17 (1.15, 1.20) | < 0.001 | 0.94 (0.92, 0.96) | <0.001 | 1.15 (1.08, 1.23) | < 0.001 | 0.76 (0.69, 0.83) | <0.001 |
| Drug use disorders | 1.20 (1.18, 1.22) | < 0.001 | 0.93 (0.91, 0.96) | <0.001 | 1.44 (1.36, 1.54) | < 0.001 | 1.36 (1.24, 1.49) | <0.001 |
| Smoking, current (ref: never) | 1.32 (1.30, 1.35) | < 0.001 | 1.24 (1.22, 1.26) | <0.001 | 1.83 (1.70, 1.96) | < 0.001 | 1.56 (1.45, 1.69) | <0.001 |
| Smoking, former (ref: never) | 1.14 (1.12, 1.17) | < 0.001 | 1.03 (1.01, 1.05) | 0.004 | 1.42 (1.30, 1.57) | < 0.001 | 1.23 (1.12, 1.36) | <0.001 |
| Hepatitis C | 1.50 (1.48, 1.53) | < 0.001 | 1.20 (1.18, 1.22) | <0.001 | 1.95 (1.85, 2.05) | < 0.001 | 1.78 (1.68, 1.89) | <0.001 |
| Diabetes | 1.51 (1.49, 1.53) | < 0.001 | 1.13 (1.11, 1.15) | <0.001 | 1.07 (1.01, 1.13) | 0.01 | 1.06 (0.99, 1.12) | 0.08 |
| Acute pain (ref: no pain diagnosis) | 2.55 (2.45, 2.65) | < 0.001 | 1.19 (1.14, 1.25) | <0.001 | 1.05 (0.89, 1.24) | 0.57 | 0.87 (0.69, 1.10) | 0.26 |
| Chronic pain (ref: no pain diagnosis) | 5.72 (5.58, 5.86) | < 0.001 | 1.45 (1.40, 1.50) | <0.001 | 1.29 (1.15, 1.44) | < 0.001 | 0.85 (0.71, 1.02) | 0.08 |
| Number of pain related diagnoses | 1.34 (1.34, 1.35) | < 0.001 | 1.30 (1.29, 1.30) | <0.001 | 1.16 (1.15, 1.17) | < 0.001 | 1.18 (1.17, 1.20) | <0.001 |
Among patients prescribed opioids
Discussion
In a large national cohort over a 12-year time frame, we found a significant increase in opioid receipt among both uninfected and HIV-infected patients, mirroring studies where HIV status was not specifically examined [2, 34, 35]. The proportion and absolute number of HIV-infected patients receiving high-dose opioids also increased substantially over the study period. Expanding on the findings from a single-year study [24], we found that HIV-infected patients were more likely to receive any and high dose opioids compared to HIV-uninfected patients after adjusting for potential confounders over this 12-year period. Of note, opioid receipt remained common in older ages and differences in receipt of high dose opioids by HIV status were particularly pronounced among patients over 45 years of age. Furthermore, we found that individuals with PTSD and major depression–risk factors for serious adverse effects of opioids–were more likely to receive any and high-dose therapy. While there was not a significant difference between HIV-infected and uninfected individuals, any and high-dose opioid receipt were still widely prevalent in older adults with HIV, presenting another potential safety concern given the known increased risk of complications and adverse effects in aging populations [36].
The underlying reasons for our findings are likely myriad. As treatment of HIV infection has improved, leading to increased aging among patients with HIV, it is likely that chronic pain stemming from degenerative joint disease and other common pain generators has increased in prevalence among patients with HIV. As such, increases in the proportion of patients receiving opioids may in part be related in increases in the prevalence of chronic pain. However, a 2.5-fold increase in opioid receipt over 12 years suggests other factors are also contributing. Factors identified in the general pain literature such as widespread public health campaigns targeting the under-treatment of pain, Joint Commission pain management standards tied to hospital reimbursement and aggressive pharmaceutical company marketing may all have contributed to opioids becoming a more prominent pain treatment option [37]. With respect to HIV infection having a higher rate of opioid receipt and high dose therapy, it is possible that providers caring for patients with HIV have a lower threshold for prescribing opioids, perhaps still applying palliative care or harm reduction paradigms [38].
While our data do not allow patient-level inference about the appropriateness of individual opioid regimens, from a public health perspective, the increase in proportion of patients receiving high-dose opioids is noteworthy in light of observational studies identifying an inflection point of 100 mg, above which incidence rates of overdose and overdose-related deaths rise [6, 8]. In addition to the potential for increased rates of acute adverse events these findings signal, there are also emerging data to suggest potential complications of high-dose opioid therapy, such as hypogonadism [39], hyperalgesia [40] and bone demineralization [41], all of which may be particularly problematic in HIV-infected patients given the advancing age of those who are infected, their burden of chronic illness and evidence of accelerated aging [42–44]. Sustained and high-dose opioid therapy is less likely to be discontinued [45], meaning a greater likelihood of continuation into older age where opioids are associated with risk of cardiovascular events, fractures and all-cause mortality, compared to non-opioid analgesics [9]. Our data support this concern as differences in high dose opioid receipt by HIV status were even more pronounced among those over 45 years of age. These findings further support the Institute of Medicine’s call for improved access to evidenced-based, non-pharmacologic pain treatments, especially for populations vulnerable to opioid harms [46].
Mental health disorders, more prevalent among HIV populations in general, have been shown to be associated with serious opioid-related adverse events [6–8, 47] opioid misuse [48–50] and opioid use disorders [17, 32], yet we found an association between PTSD and major depression and any and high-dose opioid receipt even after adjusting for number of pain diagnoses as a marker of pain severity. While it is possible that incompletely captured pain severity explained the increased likelihood of any and high-dose opioid receipt in these groups, the observed pattern exposes an already-vulnerable population to greater risk.
In contrast, we found a negative association between alcohol use disorders and high-dose opioid receipt, suggesting providers may be exercising caution when prescribing opioids to patients with this risk factor for both opioid misuse and overdose [51]. Our findings regarding drug use disorders are more difficult to interpret. This covariate had a positive association with high-dose receipt. The extent to which this finding is related to patient or provider factors deserves further study.
The strongest and most consistent negative associations we observed were between non-white race/ethnicity and opioid receipt. While these findings may suggest the presence of health disparities, we refer to them henceforth as differences since it is not clear whether exposure to opioids is health-promoting or not based on equivocal data on opioid efficacy for chronic non-cancer pain [52]. These differences—given their strength and consistency—may reflect race-influenced variability in pain assessment and treatment, corroborating previous research [53–55] and may also relate to race-related variability in patient preferences and values with respect to pain treatment [56].
Our study has limitations. We relied on administrative and clinical databases which may lack sensitivity and specificity for some diagnoses, including those related to mental health and pain. However, studies have found these sources to be a generally valid [57, 58]. Pain severity, a moderator of the relationship between certain conditions and opioid receipt, is difficult to measure in secondary data [59]. Nonetheless, while increased pain might be the reason a higher proportion of some at-risk subpopulations received opioids, these patients are still at higher risk for adverse effects. Since our sample was entirely veterans who receive medical care in the VA system, it may not generalize to veterans who do not receive care in the VA system and non-veteran populations of patients with HIV. Though, of note, the socio-demographics of patients with HIV in VA, especially in terms of race/ethnicity, are similar to individuals with HIV outside of VA [60]. Additionally, we could not account for opioid receipt from non-VHA sources nor confirm whether medications were taken as prescribed.
Within a large national sample of patients with and without HIV, we found approximately a twofold increase in opioid exposure over a 12-year time frame, persisting into older aged groups, and that HIV infected individuals were more likely to have any and high-dose receipt. Additionally, some sub-groups at elevated risk of serious adverse effects of opioids were more likely to experience any and high-dose exposure. These population-level findings warrant future research into the impact of opioid prescribing on pain among patients with HIV and targeted interventions.
Acknowledgments
This work was supported by a Veterans Health Administration Health Services Research & Development Career Development Award (08-276); other VA support came from VA Health Services Research and Development Center of Innovation (CIN 13-047). This work was also supported by the Society of General Internal Medicine’s Lawrence Linn Award, the Robert Wood Johnson Foundation Clinical Scholars Program, and the Veterans Aging Cohort study, funded by the National Institute on Alcohol Abuse and Alcoholism (U10 AA 13566). This work was presented at the Northeast Regional Society of General Internal Medicine Meeting, New Haven, CT, March 8, 2013 and the Society of General Internal Medicine National Meeting, Denver, CO, April 25, 2013. The authors would like to thank Dr. Eugenia Buta for input on statistical analyses.
Footnotes
Compliance with Ethical Standards
Disclosure The views expressed in this article are those of the authors and do not necessarily reflect positions or policies of the VHA.
References
- 1.Drug Enforcement Administration. [Accessed 12 July 2010];Retail Drug Summary. 2010 http://www.deadiversion.usdoj.gov/arcos/retail_drug_summary/
- 2.Sullivan MD, Edlund MJ, Fan M-Y, DeVries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and medicaid insurance plans: the TROUP study. Pain. 2008;138(2):440–9. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olsen Y, Daumit GL, Ford DE. Opioid prescriptions by U.S. primary care physicians from 1992 to 2001. J Pain. 2006;7(4):225–35. doi: 10.1016/j.jpain.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Paulozzi LJ Centers for Disease Control and Prevention. Drug-induced deaths—United States, 2003–2007. Morb Mortal Wkly Rep Surveill Summ. 2011;60(Suppl):60–1. [PubMed] [Google Scholar]
- 5.Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999–2006. NCHS Data Brief. 2009;22:1–8. [PubMed] [Google Scholar]
- 6.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose. Ann Intern Med. 2010;152:85–92. doi: 10.1059/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seal Karen SY, Cohen G, et al. Association of mental health disorders with prescription opioids and high-risk opioid use in us veterans of Iraq and Afghanistan. JAMA. 2012;307(9):940–7. doi: 10.1001/jama.2012.234. [DOI] [PubMed] [Google Scholar]
- 8.Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–21. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 9.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170:1968–76. doi: 10.1001/archinternmed.2010.391. [DOI] [PubMed] [Google Scholar]
- 10.Becker WC, Fiellin DA. Federal plan for prescriber education on opioids misses opportunities. Ann Intern Med. 2012;157(3):205–6. doi: 10.7326/0003-4819-157-3-201208070-00448. [DOI] [PubMed] [Google Scholar]
- 11.Krashin DL, Merrill JO, Trescot AM. Opioids in the management of HIV-related pain. Pain Physician. 2012;15(3 Suppl):ES157–68. [PubMed] [Google Scholar]
- 12.Lum PJ, Little S, Botsko M, et al. Opioid-prescribing practices and provider confidence recognizing opioid analgesic abuse in HIV primary care settings. JAIDS. 2011;56:S91–7. doi: 10.1097/QAI.0b013e31820a9a82. [DOI] [PubMed] [Google Scholar]
- 13.Basu S, Bruce RD, Barry DT, Altice FL. Pharmacological pain control for human immunodeficiency virus–infected adults with a history of drug dependence. J Subst Abuse Treat. 2007;32(4):399–409. doi: 10.1016/j.jsat.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewitt DJ, McDonald M, Portenoy RK, Rosenfeld B, Passik S, Breitbart W. Pain syndromes and etiologies in ambulatory AIDS patients. Pain. 1997;70(2–3):117–23. doi: 10.1016/s0304-3959(96)03281-2. [DOI] [PubMed] [Google Scholar]
- 15.Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, Kushel MB. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. J Pain. 2011;12(9):1004–16. doi: 10.1016/j.jpain.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bing EGBM, Longshore D, Fleischman JA, Sherbourne CD, London AS, Turner BJ, Eggan F, Beckman R, Vitello B, Morton SC, Orlando M, Bozzette SA, Ortiz-Barron L, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus–infected adults in the united states. Arch Gen Psychiatry. 2001;58(8):721–8. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 17.Edlund MJ, Steffick D, Hudson T, Harris KM, Sullivan M. Risk factors for clinically recognized opioid abuse and dependence among veterans using opioids for chronic non-cancer pain. Pain. 2007;129(3):355–62. doi: 10.1016/j.pain.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Roy S, Ninkovic J, Banerjee S, et al. Opioid drug abuse and modulation of immune function: consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol. 2011;6(4):442–65. doi: 10.1007/s11481-011-9292-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19(1):4–16. doi: 10.1111/j.1521-0391.2009.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kao D, Bartelson BB, Khatri V, et al. Trends in reporting methadone-associated cardiac arrhythmia, 1997–2011: an analysis of registry data. Ann Intern Med. 2013;158(10):735–40. doi: 10.7326/0003-4819-158-10-201305210-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edelman EJ, Gordon KS, Glover J, McNicholl IR, Fiellin DA, Justice AC. The next therapeutic challenge in HIV: polypharmacy. Drugs Aging. 2013;30(8):613–28. doi: 10.1007/s40266-013-0093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisberg DF, Gordon KS, Barry DT, et al. Long-term prescription of opioids and/or benzodiazepines and mortality among HIV-infected and uninfected patients. JAIDS. 2015;69(2):223–33. doi: 10.1097/QAI.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverberg MJ, Ray GT, Saunders K, et al. Prescription long-term opioid use in HIV-infected patients. Clin J Pain. 2012;28(1):39. doi: 10.1097/AJP.0b013e3182201a0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edelman EJ, Gordon K, Becker W, et al. Receipt of opioid analgesics by HIV-infected and uninfected patients. J Gen Intern Med. 2013;28(1):82–90. doi: 10.1007/s11606-012-2189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tetrault JM, Tate JP, McGinnis KA, et al. Hepatic Safety and Antiretroviral Effectiveness in HIV-Infected Patients Receiving Naltrexone. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Butt AA, Chang CC, Kuller L, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171(8):737–43. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freiberg MS, Chang CC, Skanderson M, et al. The risk of incident coronary heart disease among veterans with and without HIV and hepatitis C. Circ Cardiovasc Qual Outcomes. 2011;4(4):425–32. doi: 10.1161/CIRCOUTCOMES.110.957415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith MW, Joseph GJ. Pharmacy data in the VA health care system. Med Care Res Rev. 2003;60(3 Suppl):92S–123S. doi: 10.1177/1077558703256726. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Medicare and Medicaid Services. [Accessed 1 July 2011];ICD-9 Provider and Diagnostic Codes. 2011 https://www.cms.gov/icd9providerdiagnosticcodes/
- 30.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Med Care. 2006;44(8):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 31.von Korff M, Saunders K, Thomas Ray G, et al. De facto long-term opioid therapy for noncancer pain. Clin J Pain. 2008;24(6):521–7. doi: 10.1097/AJP.0b013e318169d03b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boscarino JA, Rukstalis M, Hoffman SN, et al. Risk factors for drug dependence among out-patients on opioid therapy in a large US health-care system. Addiction. 2010;105(10):1776–82. doi: 10.1111/j.1360-0443.2010.03052.x. [DOI] [PubMed] [Google Scholar]
- 33.Diggle P, Heagerty P, Liang K-Y, Zeger S. Analysis of longitudinal data. London: Oxford University Press; 2002. [Google Scholar]
- 34.Caudill-Slosberg MA, Schwartz LM, Woloshin S. Office visits and analgesic prescriptions for musculoskeletal pain in US: 1980 vs. 2000.[see comment] Pain. 2004;109(3):514–9. doi: 10.1016/j.pain.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18(12):1166–75. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gloth FM., III Pharmacological management of persistent pain in older persons: focus on opioids and nonopioids. J Pain. 2011;12(3):S14–20. doi: 10.1016/j.jpain.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Dowell D, Kunins HV, Farley TA. OPioid analgesics—risky drugs, not risky patients. JAMA. 2013;309(21):2219–20. doi: 10.1001/jama.2013.5794. [DOI] [PubMed] [Google Scholar]
- 38.Merlin JS, Turan JM, Herbey I, et al. Aberrant drug-related behaviors: a qualitative analysis of medical record documentation in patients referred to an HIV/chronic pain clinic. Pain Med. 2014;15(10):1724–33. doi: 10.1111/pme.12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katz N, Mazer NA. The impact of opioids on the endocrine system. Clin J Pain. 2009;25(2):170–5. doi: 10.1097/AJP.0b013e3181850df6. [DOI] [PubMed] [Google Scholar]
- 40.Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679–84. [PubMed] [Google Scholar]
- 41.Vuong C, Van Uum SHM, O’Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31(1):98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greene M, Justice AC, Lampiris HW, Valcour V. Management of human immunodeficiency virus infection in advanced age. JAMA. 2013;309(13):1397. doi: 10.1001/jama.2013.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deeks S. Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med. 2009;17(4):118. [PubMed] [Google Scholar]
- 44.Aberg J. Aging, inflammation, and HIV infection. Top Antivi Med. 2012;20(3):101. [PMC free article] [PubMed] [Google Scholar]
- 45.Martin B, Fan M-Y, Edlund M, DeVries A, Braden J, Sullivan M. Long-term chronic opioid therapy discontinuation rates from the TROUP study. J Gen Intern Med. 2011;26(12):1450–7. doi: 10.1007/s11606-011-1771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Committee on Advancing Pain Research Care, Institute of Medicine. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. Washington, DC: National Academies Press; 2011. 030921484X. [PubMed] [Google Scholar]
- 47.Gomes TRDA, Juurlink DN, Dhalla IA, Camacho X, Mamdani MM. Opioid dose and risk of road trauma in canada: a population-based study. JAMA Intern Med. 2013;173(3):196–201. doi: 10.1001/2013.jamainternmed.733. [DOI] [PubMed] [Google Scholar]
- 48.Ives TJ, Chelminski PR, Hammett-Stabler CA, et al. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res. 2006;2006(6):46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care.[see comment] J Gen Intern Med. 2002;17(3):173–9. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008;24(6):497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- 51.Braden JRJFM, et al. EMergency department visits among recipients of chronic opioid therapy. Arch Intern Med. 2010;170(16):1425–32. doi: 10.1001/archinternmed.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: opioid treatment for chronic back pain: prevalence, efficacy, and association with addiction [see comment] Ann Intern Med. 2007;146(2):116–27. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 53.Chen I, Kurz J, Pasanen M, et al. Racial differences in opioid use for chronic nonmalignant pain. J Gen Intern Med. 2005;20(7):593–8. doi: 10.1111/j.1525-1497.2005.0106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Medicine. 2003 Sep;4(3):277–294. doi: 10.1046/j.1526-4637.2003.03034.x. [see comment][erratum appears in Pain Med. 2005;6(1):99 Note: Kaloukalani, Donna A [corrected to Kalauokalani, Donna A]] [DOI] [PubMed] [Google Scholar]
- 55.Burgess DJ, Crowley-Matoka M, Phelan S, et al. Patient race and physicians’ decisions to prescribe opioids for chronic low back pain. Soc Sci Med. 2008;67(11):1852–60. doi: 10.1016/j.socscimed.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 56.Katz JN. PAtient preferences and health disparities. JAMA. 2001;286(12):1506–9. doi: 10.1001/jama.286.12.1506. [DOI] [PubMed] [Google Scholar]
- 57.Frayne SM, Miller DR, Sharkansky EJ, et al. Using administrative data to identify mental illness: what approach is best? Am J Med Qual. 2010;25(1):42–50. doi: 10.1177/1062860609346347. [DOI] [PubMed] [Google Scholar]
- 58.Desai MM, Rosenheck RA, Craig TJ. Case-finding for depression among medical outpatients in the veterans health administration. Med Care. 2006;44(2):175–81. doi: 10.1097/01.mlr.0000196962.97345.21. [DOI] [PubMed] [Google Scholar]
- 59.Goulet J, Brandt CA, Crystal S, et al. Agreement between electronic medical record-based and self-administered pain numeric rating scale: clinical and research implications. Med Care. 2012 doi: 10.1097/MLR.0b013e318277f1ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Centers for Disease Control and Prevention. HIV in the United States: At a Glance. 2015 http://www.cdc.gov/hiv/statistics/basics/ataglance.html.