Abstract
Aims
The longevity of generators is a crucial determinant of the cost-effectiveness of therapy with implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-D). We evaluated the trend of device-measured residual battery capacity and longevity projections over 5-year follow-up. We also investigated possible factors associated with battery drain.
Methods and results
Data from 4851 patients in the European LATITUDE® database who were followed up for a minimum of 3 years were analysed. The factors associated with battery drain (i.e. year-to-year decrease in residual battery capacity), and thus potentially impacting on device longevity, were mainly the pacing parameters in CRT-D devices and the number of shocks delivered and diverted in both ICD and CRT-D (all P < 0.01 on linear regression analysis). Over the first 5 years, the longevity estimates provided by devices showed low intra-patient variability and increased with time. The estimates exceeded 10 years for CRT-D and 13 and 12 years for single- and dual-chamber ICDs, respectively. In CRT-D patients, the expected patient age on replacement was 80 ± 12 years, and the expected probability of undergoing device replacement was 63 ± 13% for New York Heart Association (NYHA) II patients and 37 ± 16% for NYHA III patients. For comparison, the probabilities of replacing a CRT-D lasting 5 years were 78 ± 8 and 59 ± 13%, respectively (both P < 0.001).
Conclusion
Battery drain was mainly associated with pacing output in CRT-D devices and with the number of capacitor charges in both ICD and CRT-D devices. The longevity estimates provided by the devices were consistent and conservative. According to these estimates, among CRT-D recipients a low proportion of patients should require device replacement.
Keywords: Implantable cardioverter-defibrillator, Cardiac resynchronization therapy, Longevity, Battery, Remote monitoring
Introduction
Implantable cardioverter-defibrillators (ICDs) and cardiac resynchronization therapy defibrillators (CRT-D) are currently part of the standard approach to the prevention of sudden cardiac death and the treatment of heart failure, as their efficacy has been verified in large trials.1 Nonetheless, since device replacement is associated with a considerable risk of complications2 and increases costs for healthcare systems, device lifespan is a crucial determinant of the cost-effectiveness of therapy.
Analysing data collected in clinical practice may help in evaluating the current approach adopted by implanting centres for the management of cardiac devices, the performance of currently available technology, and the implications for device recipients. Previous studies have shown that ICD and CRT-D longevity may vary significantly between manufacturers,3–5 thus impacting on healthcare costs.6 However, currently available data refer to heterogeneous populations, cardiac devices, and time periods.
The present analysis was aimed at evaluating the trend of residual ICD battery capacity during follow-up in a population with devices from Boston Scientific Corporation (Natick, MA, USA), and at identifying factors associated with battery drain. We also analysed the consistency over time of the longevity estimations provided by devices and calculated the expected rate of CRT-D device replacements.
Methods
Study design
The present study analysed data from implanted ICD and CRT-D devices manufactured by Boston Scientific that regularly communicates information from patients' homes through the remote monitoring system. The system consists of a home monitor, which allows full ICD interrogation and the transmission of data through a standard telephone connection or the mobile network at scheduled intervals or, in the case of programmable alert conditions, without patient intervention. Device information is stored in a central server and can be reviewed on a secure website via the internet. The decision to allocate a patient to remote follow-up is made by the implanting physician at the time of device implantation. In December 2014, the European LATITUDE® database, which stores all data sent in by LATITUDE®-compatible devices implanted in European hospitals, was interrogated in order to retrieve data on all ICD and CRT-D devices followed up for 3 years or more.
The following de-identified patient data were obtained: status of pacing parameters and values of pacing and episode counters at the pre-discharge visit and at yearly interrogations—specifically, stimulation mode, pulse amplitude and duration, pacing impedance for the right ventricular channel, right atrial (in dual-chamber ICD and CRT-D), and left ventricular channel (in CRT-D). In addition, the cumulative percentage of atrial and ventricular pacing calculated since the previous interrogation was obtained, together with the mean heart rate and the number of delivered and diverted shocks. Moreover, the value of residual battery capacity at each interrogation (automatically calculated from the total charge drained from the battery and the battery voltage) was retrieved, together with the estimated device longevity. This latter is a function of the characteristic battery discharge curve7 and is automatically evaluated by the system at the time of interrogation on the basis of the capacity consumed and the current device programming.
Ethics
Patients enrolled in the LATITUDE® system in Europe provide their consent to analyse the data on an anonymized/aggregate basis for statistical, research, and clinical reporting purposes. Participating centres endorsed a data use agreement that allows de-identified data to be used for research purposes.
Objectives
In this study, we evaluated the year-to-year changes in parameter programming and the rates of recorded events. We also investigated the trend of residual battery capacity, in order to identify factors associated with battery drain. In addition, we analysed the changes in longevity estimates provided by the device in order to investigate their consistency over time, and we estimated the expected rate of device replacements on the basis of the longevity estimates for CRT-D devices. In each CRT-D patient, the expected probability of undergoing device replacement was measured as the probability of patient survival at the time of the projected battery depletion. The survival probability was estimated by means of the VALID-CRT risk score,8 i.e. a multifactorial risk stratification tool for CRT recipients. The score allows the probability of survival to be accurately estimated on the basis of the following variables: age, gender, left ventricular ejection fraction, New York Heart Association (NYHA) class, the presence of atrial fibrillation, the presence of atrioventricular junction ablation, coronary artery disease, diabetes, and implantation of a CRT-D. The score was calculated for a typical profile of a patient with heart failure: a male CRT-D recipient with coronary artery disease, no diabetes, and ejection fraction of 26% (the mean value of the VALID-CRT cohort). The age was the value recorded in the LATITUDE® system at the time of implantation; other clinical data were derived from programming. The score was calculated for two different scenarios: patients with NYHA class II and III heart failure.
Statistical analysis
Descriptive statistics are reported as means ± standard deviations for normally distributed continuous variables, or medians with 25th–75th percentiles in the case of skewed distribution. The quartile coefficient of dispersion (QCD), defined as (75th − 25th percentiles)/(75th + 25th percentile), was used as a dimensionless measurement of distribution dispersion. Categorical variables are reported as percentages. The rate of events is reported as the number of events observed per patient-year. One-way ANOVA and the nonparametric Kruskal–Wallis equality-of-populations rank test were used to test for differences in the case of normal and non-normal distributions of continuous variables, respectively. Differences in proportions were compared by means of χ2 analysis. The rates of events were analysed by using the Comparison of Incidence Rates (Large Sample) Test. Multiple linear regression was used to identify variables associated with battery drain, i.e. year-to-year decrease in residual battery capacity. A one-way sensitivity analysis was carried out to take into account the uncertainty of the variables used to calculate the VALID-CRT score. The variables considered in the base-case estimate were changed one at a time. Specifically, the impact of the following variables was tested: ejection fraction in the range from 13 to 35% (the 95% confidence interval of the VALID-CRT cohort), female gender, the absence of coronary artery disease, and the presence of diabetes. The results of the one-way sensitivity analysis were plotted on the Tornado charts. A P-value of <0.05 was considered significant for all tests. All statistical analyses were performed by means of STATISTICA software, version 7.1 (StatSoft, Inc., Tulsa, OK).
Results
Study population
At the time of the analysis, data of 20 860 patients with defibrillators from Boston Scientific from participating centres were stored in the European LATITUDE® database. Of these, data from 4851 consenting patients who started to send data within 1 month of implantation and who were followed up for a minimum of 3 years were included in the analysis (4 years for 1124 patients and 5 years for 231 patients). The database included 1537 single-chamber ICDs, 1289 dual-chamber ICDs, and 2025 CRT-D systems. The device models are reported in Table 1. On implantation, the mean age of the patients was 61 ± 17 years in the single-chamber group, 65 ± 15 years in the dual-chamber group, and 70 ± 12 years in the CRT-D group (P < 0.001).
Table 1.
Numbers of devices in analysis
| Device model | Battery capacity (Ah) | Devices in analysis (4851) |
|---|---|---|
| CRT-D | 2025 | |
| Cognis | 2.0 | 1260 |
| Energen | 1.9 | 279 |
| Punctua | 1.9 | 50 |
| Incepta | 1.9 | 436 |
| Dual-chamber ICD | 1289 | |
| Teligen | 1.7 | 774 |
| Energen | 1.8 | 231 |
| Punctua | 1.8 | 13 |
| Incepta | 1.8 | 271 |
| Single-chamber ICD | 1537 | |
| Teligen | 1.7 | 955 |
| Energen | 1.8 | 259 |
| Punctua | 1.8 | 25 |
| Incepta | 1.8 | 298 |
Programming of pacing parameters
At the pre-discharge examination, 1664 (82%) devices in the CRT-D group were programmed in an atrio-biventricular pacing mode and 361 (18%) in a ventricular-only pacing mode. In dual-chamber ICDs, dual-chamber pacing was programmed in 1201 (93%) patients, ventricular-only pacing in 77 (6%), and atrial-only pacing in 11 (1%). A rate–response mode was programmed in 556 (27%) CRT-D devices, 212 (16%) dual-chamber ICDs, and 39 (3%) single-chamber ICDs at the pre-discharge examination. In general, the pacing mode was changed at least once during follow-up (including the activation or deactivation of the rate–response mode) in 482 (24%) CRT-D devices, 266 (21%) dual-chamber ICDs, and 36 (2%) single-chamber ICDs. In CRT-D devices, the majority of cases of pacing mode reprogramming took place during the first year of follow-up (0.20 programming changes/patient-year vs. <0.06 changes/patient-year in any of the following years, P < 0.001). A similar pattern was observed in dual-chamber ICDs (0.16 changes/patient-year vs. <0.07 changes/patient-year in any of the following years, P < 0.001). In single-chamber ICDs, the rate of reprogramming was 0.01 changes/patient-year during the first year and remained stable during the following years (P = 0.250).
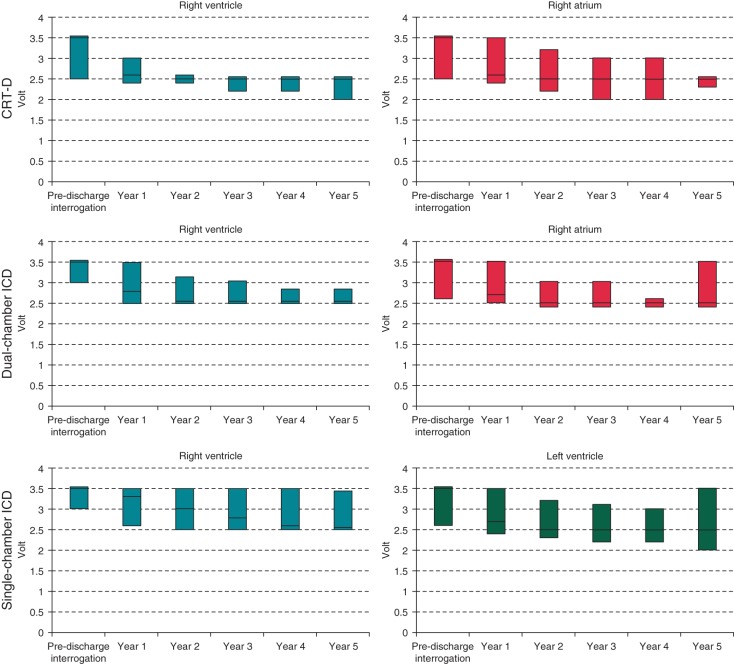
At the pre-discharge examination, the median pulse amplitude for all pacing channels was 3.5 V, i.e. the nominal value for the devices in analysis. From the first year onwards, the amplitude values significantly declined in all pacing channels and in all device groups (Figure 1) (all P < 0.001). In contrast, the median pulse width of 0.4 ms, which is the nominal value, remained unmodified during follow-up. The values of pacing impedance for right atrial and ventricular leads significantly declined during follow-up (P < 0.001 for all device groups), but remained stable for left ventricular leads in the CRT-D group (P = 0.920).
Figure 1.
Trends of pacing amplitudes during follow-up (median, 25th–75th percentiles).
The median heart rate remained stable during follow-up in the three groups. The percentage of atrial pacing was 7 [0–43%] in the dual-chamber ICD group and 8 [0–38%] in the CRT-D group, and remained stable during follow-up. The percentage of ventricular pacing was 0 [0–4%] in the single-chamber ICD group and 0 [0–15%] in the dual-chamber ICD group 1 year after implantation, with no significant changes during follow-up. In the CRT-D group, the ventricular pacing percentage was 97 [92–99%] and increased from the second year (98 [94–99%], P < 0.001) onwards.
Shock therapies
Before the pre-discharge interrogation, shocks were delivered—most probably during defibrillation testing—by 732 (48%) single-chamber ICDs, 729 (57%) dual-chamber ICDs, and 808 (40%) CRT-D (all P < 0.001). The rates of shocks delivered after discharge were 1.1 (95% CI, 1.0–1.1) shocks/patient-year in CRT-D devices, 1.9 (95% CI, 1.8–2.0) shocks/patient-year in dual-chamber ICDs, and 1.4 (95% CI, 1.4–1.5) shocks/patient-year in single-chamber ICDs (all P < 0.001 for paired comparisons).
Residual battery capacity
The trends of residual battery capacity in the three device groups are reported in Figure 2. The decline in median battery capacity was more marked in the CRT-D group (from 2.1Ah pre-discharge to 1.3Ah at 5 years) than in the dual-chamber ICD group (from 1.8Ah pre-discharge to 1.2Ah at 5 years) or in the single-chamber ICD group (from 1.8Ah pre-discharge to 1.3Ah at 5 years). Moreover, the variability of residual capacity values at 5 years was higher in CRT-D devices (QCD: 0.057) and in dual-chamber ICDs (QCD: 0.046) than in single-chamber ICDs (QCD: 0.016).
Figure 2.
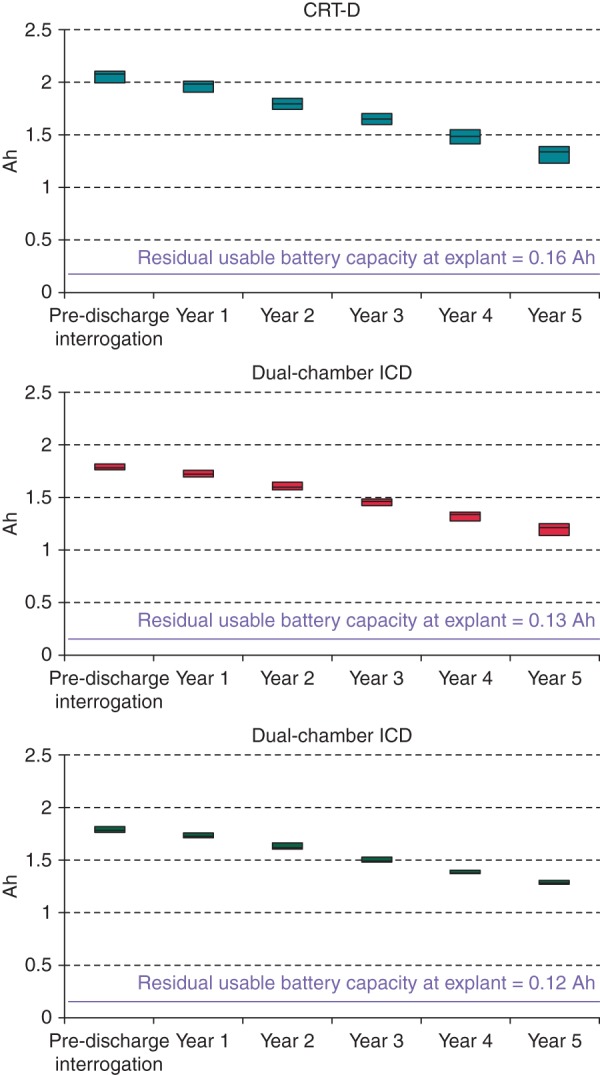
Trends of residual battery capacity (median, 25th–75th percentiles).
The factors associated with battery drain, measured as the year-to-year decrease in residual battery capacity, are reported in Table 2. Among the pacing parameters, left ventricular pulse amplitude and width and right ventricular pulse width were associated with battery drain in CRT-D devices. In dual-chamber ICDs, only right atrial pulse width was significantly associated with battery drain. The percentage of ventricular pacing and the heart rate were associated with battery drain in CRT-D and dual-chamber ICDs. In this latter group, the percentage of atrial pacing and the activation of a rate–response mode were also associated. In all groups, the number of shocks delivered and that of shocks diverted were both associated with battery drain.
Table 2.
Factors associated with battery drain, measured as the year-to-year decrease in residual battery capacity (mAh)
| CRT-D |
ICD-DR |
ICD-VR |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | P | Coefficient | SE | P | Coefficient | SE | P | |
| Year | 48.06 | 1.75 | <0.001 | 8.37 | 1.09 | <0.001 | 6.56 | 0.73 | <0.001 |
| Right ventricular amplitude, V | −2.36 | 2.75 | 0.392 | 1.36 | 1.83 | 0.456 | 0.35 | 0.84 | 0.678 |
| Right ventricular width, ms | 19.64 | 7.80 | 0.012 | 1.54 | 4.87 | 0.752 | 3.10 | 3.61 | 0.391 |
| Right ventricular impedance, Ohm | – | – | – | – | – | – | – | – | – |
| Left ventricular amplitude, V | 11.80 | 1.41 | <0.001 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Left ventricular width, ms | 20.57 | 3.10 | <0.001 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Left ventricular impedance, Ohm | – | – | – | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Right atrial amplitude, V | −3.22 | 2.70 | 0.234 | −1.10 | 1.85 | 0.553 | n.a. | n.a. | n.a. |
| Right atrial width, ms | 8.42 | 7.18 | 0.241 | 22.62 | 5.99 | <0.001 | n.a. | n.a. | n.a. |
| Right atrial impedance, Ohm | – | – | – | – | – | – | n.a. | n.a. | n.a. |
| Ventricular pacing, % | 0.20 | 0.07 | 0.004 | 0.17 | 0.04 | <0.001 | 0.00 | 0.07 | 0.981 |
| Atrial pacing, % | 0.09 | 0.07 | 0.222 | 0.11 | 0.03 | <0.001 | n.a. | n.a. | n.a. |
| Rate response mode, ON | 1.96 | 3.90 | 0.616 | 4.05 | 1.99 | 0.042 | −0.02 | 5.92 | 0.997 |
| Heart rate, bpm | 0.35 | 0.14 | 0.017 | 0.27 | 0.09 | 0.002 | 0.04 | 0.05 | 0.469 |
| Shock delivered, n | 2.65 | 0.34 | <0.001 | 2.34 | 0.30 | <0.001 | 2.54 | 0.16 | <0.001 |
| Shock diverted, n | 1.74 | 0.84 | 0.039 | 1.02 | 0.35 | 0.004 | 0.86 | 0.31 | 0.006 |
SE, standard error; n.a., not applicable.
Estimated device longevity
The trends of estimated longevity for the three device groups are reported in Figure 3. Single- and dual-chamber ICDs showed longer projected service lives (13 and 12 years, respectively, on implantation) than CRT-D (median 9 years). In the three device groups, the longevity estimates showed a slight decrease at 1-year follow-up and an increase thereafter. This was particularly noticeable in CRT-D devices, with a median estimate that exceeded 10 years at 5 years. In general, the difference in longevity estimates between the last interrogation and the baseline was 0.8 years [0.1–1.4] for CRT-D, 0.2 years [−0.3–0.9] for dual-chamber ICDs, and 0.7 years [0.2–1.3] for single-chamber ICDs. As shown in Figure 3, the variability of estimated longevity increased during follow-up for CRT-D and dual-chamber ICDs (QCD at 5 years: 0.098 and 0.073, respectively), but not for single-chamber ICDs (QCD: 0.015).
Figure 3.
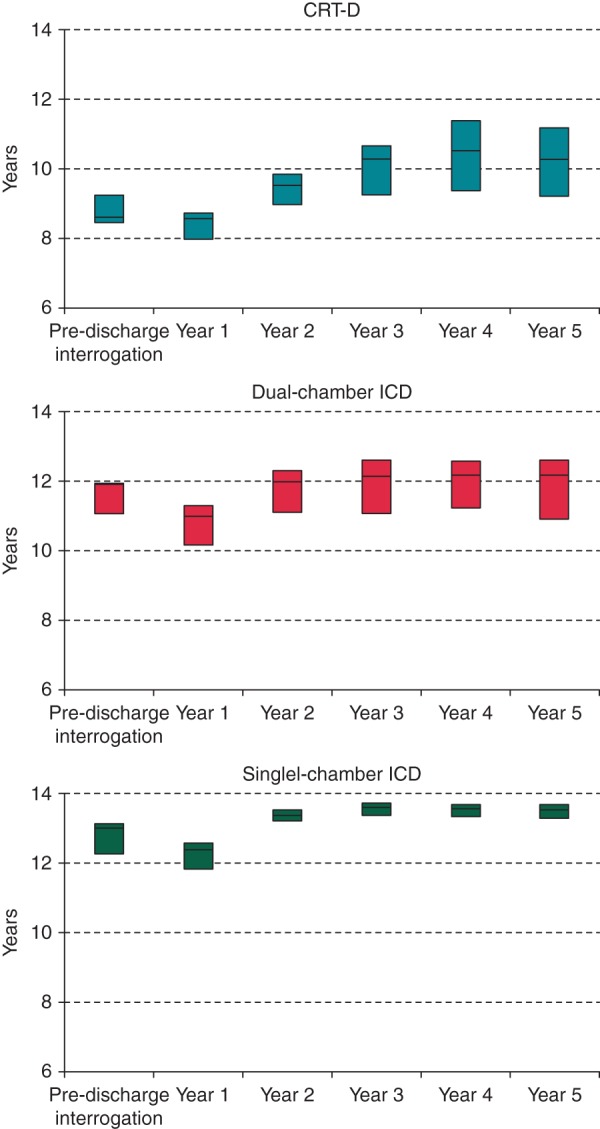
Trends of estimated longevity (median, 25th–75th percentiles).
Expected rate of device replacements for cardiac resynchronization therapy defibrillators devices
On the basis of the longevity projections of CRT-D devices, the mean age on replacement was calculated to be 80 ± 12 years. The expected probability of undergoing device replacement (i.e. patient survival probability at the time of projected battery depletion, according to the VALID-CRT score) was 63 ± 13% in the base case for an NYHA class II patient and 37 ± 16% for an NYHA class III patient. For comparison, in the study population the probability of replacement of a device lasting 5 years would be 78 ± 8% in NYHA class II patients and 59 ± 13% in NYHA class III patients (both P < 0.001). In the perspective of a device lasting 7 years, the probability of replacement would be 71 ± 10% in NYHA class II patients and 48 ± 14% in NYHA class III patients (both P < 0.001).
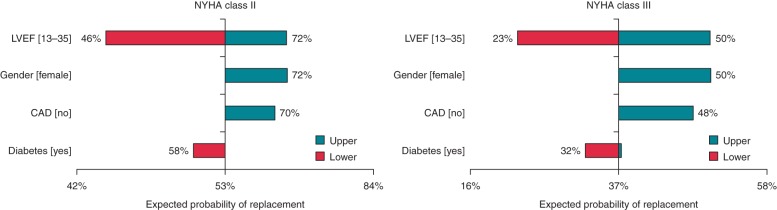
The results of one-way sensitivity analysis are reported in Figure 4. As expected, base-case results were particularly sensitive to variations in the ejection fraction (ranging from 13 to 35%), with the expected probability of replacement varying from 46 to 72% for NYHA class II, and from 23 to 50% for NYHA class III.
Figure 4.
One-way sensitivity analysis of expected probability of replacement for the NYHA class II (left panel) and III (right panel) scenario.
Discussion
The present analysis reports original data retrieved through the LATITUDE® system on the medium-term performance of ICD and CRT-D from Boston Scientific belonging to more recent device families. We showed that, in a ‘real-world’ setting, the factors associated with battery drain, and thus with potential impact on device longevity, were mainly the pacing parameters in the case of CRT-D devices and the number of shocks delivered and diverted in the case of both ICD and CRT-D. This resulted in a higher inter-individual variability in the estimated longevity of CRT-D and dual-chamber ICD than of single-chamber ICD. Moreover, over the first 5 years after implantation, the longevity estimates provided by the devices were consistent (i.e. showed low intra-patient variability) and conservative (i.e. increased with time). Specifically, the estimates exceeded 10 years for CRT-D and 13 and 12 years for single- and dual-chamber ICDs, respectively. Taking into account the patient survival probability, this should result in a low proportion of patients requiring device replacement.
Confirming previous data obtained in the ‘pre-remote monitoring era’,9 our findings showed that the majority of device programming changes took place early after implantation and became less frequent afterwards in CRT-D. Moreover, as expected, reprogramming was extremely rare in single-chamber ICD. We found that, in the majority of patients, the pacing parameters were initially set to the nominal values and then adjusted within the first year. Most frequently, the pacing output was reduced, showing that the operators were reassured by the early performances of the system and decided to reprogramme the device to save energy.
The analysis of shocks recorded by the devices revealed that in about half of patients no shocks were delivered before the pre-discharge interrogation, and that the proportion of patients with events was lower in the CRT-D group. This finding is in agreement with the reported decline of defibrillation testing in current clinical practice,10 especially for CRT-D patients.11 During follow-up, the rate of shocks delivered ranged from ∼1 to 2 shocks/patient-year.
The devices in analysis were from Boston Scientific, and all belonged to more recent device families equipped with high-capacity (>1.7Ah) lithium manganese dioxide batteries. The greater decline in residual battery capacity observed in the CRT-D group was expected, because of the need for continuous biventricular pacing and because pacing thresholds tend to be higher in the left ventricle. Indeed, the factors significantly associated with battery drain in the CRT-D group included variables associated with ventricular pacing: pulse amplitude and width, percentage of pacing and heart rate. This finding confirms recent data that showed the association between left ventricular pacing output and early battery depletion over the long term.3 The impact of pacing on battery drain, as well as the inter- and intra-patient variabilities of pacing parameters, explains the higher variability of residual battery capacity and projected longevity in CRT-D at 5 years. In the dual-chamber ICD group, the atrial pacing output, but not the ventricular pacing output had a great impact on battery drain, as the percentage of stimulation was higher in the atrium and the proportion of patients in whom a rate–response mode was activated was not negligible.
Although the burden of defibrillator therapy was not responsible for observed longevity differences in previous ICD studies,4,12 in our patients the number of shocks delivered was associated with the year-to-year decrease in residual battery capacity. Moreover, in our analysis, battery drain was also associated with the number of shocks diverted, which, like shocks delivered, trigger capacitor charges. This is in agreement both with previous studies that showed an association between the frequency of capacitor reformations and the time to battery depletion,12 and trials that demonstrated that reducing capacitor charges prolonged battery longevity.13 In addition, this result is interesting, as diverted shocks have also been shown to be associated with inappropriately delivered shocks.14 Recently, the MADIT-RIT trial showed that two programming strategies, namely increasing the detection rate or lengthening the monitoring delay before shock delivery, were similarly superior to conventional ICD programming in reducing inappropriate therapy.15 Future studies should clarify what is the best ICD programming in order to reduce not only delivered shocks but also diverted ones and possibly save energy.
The recent adoption of lithium manganese dioxide cells for ICD is driven by a number of factors.7 Their electrochemical behaviour is well characterized and has proved to be stable under diverse conditions. Moreover, their discharge characteristics are predictable, which is crucial to reliably projecting service life. Our analysis confirmed the consistency of longevity estimates of Boston Scientific ICDs over the first 5 years after implantation. However, these estimates are based on current device programming, and therefore, before pacing parameter optimization (mainly in CRT-D), they may slightly underestimate the real service life of the device.
Having noticed that a substantial proportion of ICD recipients outlive their first device, in 2005 Hauser proposed a longevity goal of 10 years for batteries, in order to provide a defibrillator that lasts a lifetime in the majority of patients.16 In our series, we reported extremely long service life projections in real-life conditions and programming: >10 years for CRT-D, 12 years for dual-, and 13 years for single-chamber ICDs. These performances can be mainly ascribed to increased battery capacity, which has already been shown to improve longevity,16 and to the chemistry of lithium manganese dioxide adopted by Boston Scientific, which has recently been demonstrated to ensure longer service life than alternative solutions from other manufacturers.3,5
Single- and dual-chamber ICD recipients are extremely diverse, as they vary from primary to secondary prevention patients and from young subjects with channelopathies to severe heart failure patients with systolic dysfunction. In contrast, the clinical profile of CRT-D patients is far more homogenous. We therefore applied the VALID-CRT risk score8 in order to estimate the survival probability of a typical CRT-D patient in two different scenarios: NYHA class II and III heart failure. Thus, we populated the model with known patient characteristics or with mean values of clinical variables. Our results show that, on the basis of device longevity projections, only 37% of patients with severe heart failure (NYHA class III) will probably undergo replacement of their CRT-D, while among patients with mild heart failure (NYHA class II) this proportion rises to 63%. In absolute terms, this means that, even with modern-generation ICD technology, the mismatch between device service life and the survival of ICD recipients16 still exists, as the life expectancy of ICD recipients has improved in recent years.17 Nonetheless, we showed that, in the same hypotheses, a CRT-D device lasting 5 years would necessitate replacement in 59% of NYHA class III patients and 79% of NYHA class II patients. Recent studies have shown that modern-generation CRT-D equipped with batteries that do not use lithium manganese dioxide still offers longevities of only 5 years,3,5,18 thus requiring at least 20% more replacements than the devices included in the present analysis. Recently, Boriani et al.6 determined the cost impact of extending defibrillator longevity in various clinical scenarios. Specifically, over a 15-year time horizon, they found that a CRT-D device lasting 10 years would yield a relative saving of ∼35% in NYHA II patients and 30% in NYHA III patients in comparison with a 5-year CRT-D, and a saving of ∼22% in NYHA II and 18% in NYHA III patients in comparison with a 7-year device.
Limitations
Some limitations of this study should be noted. First, the baseline clinical characteristics of the patients were not analysed. However, the results should have not been affected by any bias, as previous studies had failed to show any association between clinical characteristics and ICD lifespans.19 Moreover, a sensitivity analysis was carried out in order to account for the uncertainty of the variables used for the calculation of the device replacement rate. Secondly, it should be mentioned that not all device parameters were included in the analysis. However, we assumed that the variables included could adequately describe the ICD battery depletion process. Thirdly, it was only possible to describe the trend in programmed parameters, as we did not collect information on physician rationales or specific reasons for ICD reprogramming. In particular, possible changes in pacing mode or output could have been performed after routine technical or clinical assessment or after detection of lead or system failures, but we could not distinguish between these events. Fourthly, although the majority of findings are not device specific, this analysis was performed on some ICD models from a single manufacturer, i.e. Boston Scientific, and thus some results could not be generally applicable. In particular, the ICDs in analysis were not endowed with automatic algorithms for output adjustment that may have an impact on the frequency of parameter reprogramming and the longevity of devices. Finally, although the present analysis showed that the longevity projections were consistent over the first 5 years, their long-term validity requires confirmation.
Conclusions
The present analysis showed that the factors associated with battery drain were mainly the pacing parameters in the case of CRT-D devices and the number of capacitor charges in the case of both ICD and CRT-D. Moreover, the longevity estimates provided by the devices from Boston Scientific were consistent and conservative, exceeding 10 years for CRT-D and 13 and 12 years for single- and dual-chamber ICDs, respectively. On taking into account patient survival probability, among CRT-D recipients this should result in a low proportion of patients requiring device replacement and in considerable long-term savings for payers.
Funding
The work was supported by Boston Scientific.
Conflict of interest: G.B. receives consultancy fees from Boston Scientific and Medtronic. P.R. is a consultant to Medtronic and Sorin. M.B. declares involvement in educational activity and speaker bureau of Boston Scientific and Medtronic. M.Z. reports educational activity from Medtronic. L.P. receives research grant support and honoraria from Medtronic, Inc., Sorin Group, Boston Scientific Corp, and St Jude Medical, Inc., S.V. is an employee of Boston Scientific.
Acknowledgements
The authors would like to thank Francesco Accardi (Service and Digital Health Engagement, Boston Scientific) and the Boston Scientific LATITUDE® operations team for the management of device data and their technical support, which made this study possible.
References
- 1.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Europace 2013;15:1070–118. [DOI] [PubMed] [Google Scholar]
- 2.Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R et al. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation 2010;122:1553–61. [DOI] [PubMed] [Google Scholar]
- 3.Landolina M, Curnis A, Morani G, Vado A, Ammendola E, D'Onofrio A et al. Longevity of implantable cardioverter-defibrillators for cardiac resynchronization therapy in current clinical practice: an analysis according to influencing factors, device generation, and manufacturer. Europace 2015;17:1251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biffi M, Ziacchi M, Bertini M, Sangiorgi D, Corsini D, Martignani C et al. Longevity of implantable cardioverter-defibrillators: implications for clinical practice and health care systems. Europace 2008;10:1288–95. [DOI] [PubMed] [Google Scholar]
- 5.von Gunten S, Schaer BA, Yap SC, Szili-Torok T, Kühne M, Sticherling C et al. Longevity of implantable cardioverter defibrillators: a comparison among manufacturers and over time. Europace 2016;18:710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boriani G, Braunschweig F, Deharo JC, Leyva F, Lubinski A, Lazzaro C. Impact of extending device longevity on the long-term costs of implantable cardioverter-defibrillator therapy: a modelling study with a 15-year time horizon. Europace 2013;15:1453–62. [DOI] [PubMed] [Google Scholar]
- 7.Root MJ. Lithium-manganese dioxide cells for implantable defibrillator devices—discharge voltage models. J Power Sources 2010;195:5089–93. [Google Scholar]
- 8.Gasparini M, Klersy C, Leclercq C, Lunati M, Landolina M, Auricchio A et al. Validation of a simple risk stratification tool for patients implanted with cardiac resynchronization therapy: the VALID-CRT risk score. Eur J Heart Fail 2015;17:717–24. [DOI] [PubMed] [Google Scholar]
- 9.Lunati M, Gasparini M, Santini M, Landolina M, Perego GB, Pappone C et al. Follow-up of CRT-ICD: implications for the use of remote follow-up systems. Data from the InSync ICD Italian Registry. Pacing Clin Electrophysiol 2008;31:38–46. [DOI] [PubMed] [Google Scholar]
- 10.Brignole M, Occhetta E, Bongiorni MG, Proclemer A, Favale S, Gasparini M et al. Decline of defibrillation testing in the clinical practice: an 8-year nation-wide assessment. Europace 2014;16:1103–4. [DOI] [PubMed] [Google Scholar]
- 11.Brignole M, Raciti G, Bongiorni MG, De Martino G, Favale S, Gasparini M et al. Defibrillation testing at the time of implantation of cardioverter defibrillator in the clinical practice: a nation-wide survey. Europace 2007;9:540–3. [DOI] [PubMed] [Google Scholar]
- 12.Schaer BA, Koller MT, Sticherling C, Altmann D, Joerg L, Osswald S. Longevity of implantable cardioverter-defibrillators, influencing factors, and comparison to industry-projected longevity. Heart Rhythm 2009;6:1737–43. [DOI] [PubMed] [Google Scholar]
- 13.Guédon-Moreau L, Lacroix D, Sadoul N, Clémenty J, Kouakam C, Hermida JS et al. A randomized study of remote follow-up of implantable cardioverter defibrillators: safety and efficacy report of the ECOST trial. Eur Heart J 2013;34:605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan RM, Seth M, Berg K, Stolen KQ, Jones PW, Russo AM et al. Does change in device detected frequency of non-sustained or diverted episodes serve as a marker for inappropriate shock therapy? Analyses from the INTRINSIC RV and ALTITUDE-REDUCES Trials. Europace 2014;16:668–73. [DOI] [PubMed] [Google Scholar]
- 15.Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP et al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275–83. [DOI] [PubMed] [Google Scholar]
- 16.Hauser RG. The growing mismatch between patient longevity and the service life of implantable cardioverter-defibrillators. J Am Coll Cardiol 2005;45:2022–5. [DOI] [PubMed] [Google Scholar]
- 17.Neuzner J. The mismatch between patient life expectancy and the service life of implantable devices in current cardioverter-defibrillator therapy: a call for larger device batteries. Clin Res Cardiol 2015;104:456–60. [DOI] [PubMed] [Google Scholar]
- 18.Alam MB, Munir MB, Rattan R, Flanigan S, Adelstein E, Jain S et al. Battery longevity in cardiac resynchronization therapy implantable cardioverter defibrillators. Europace 2014;16:246–51. [DOI] [PubMed] [Google Scholar]
- 19.Horlbeck FW, Mellert F, Kreuz J, Nickenig G, Schwab JO. Real-world data on the lifespan of implantable cardioverter-defibrillators depending on manufacturers and the amount of ventricular pacing. J Cardiovasc Electrophysiol 2012;23:1336–42. [DOI] [PubMed] [Google Scholar]