Abstract
Aims
The introduction of non-VKA oral anticoagulants (NOACs), which differ from the earlier vitamin K antagonist (VKA) treatments, has changed the approach to stroke prevention in atrial fibrillation (AF). GLORIA-AF is a prospective, global registry programme describing the selection of antithrombotic treatment in newly diagnosed AF patients at risk of stroke. It comprises three phases: Phase I, before the introduction of NOACs; Phase II, during the time of the introduction of dabigatran, the first NOAC; and Phase III, once NOACs have been established in clinical practice.
Methods and results
In Phase I, 1063 patients were eligible from the 1100 enrolled (54.3% male; median age 70 years); patients were from China (67.1%), Europe (EU; 27.4%), and the Middle East (ME; 5.6%). The majority of patients using VKAs had high stroke risk (CHA2DS2-VASc ≥ 2; 86.5%); 13.5% had moderate risk (CHA2DS2-VASc = 1). Vitamin K antagonist use was higher for persistent/permanent AF (47.7%) than that for paroxysmal (23.9%). Most patients in China were treated with antiplatelet agents (53.7%) vs. 27.1% in EU and 28.8% in ME. In China, 25.9% of patients had no antithrombotic therapy, vs. 8.6% in EU and 8.5% in ME.
Conclusion
Phase I of GLORIA-AF shows that VKAs were mostly used in patients with persistent/permanent (vs. paroxysmal) AF and in those with high stroke risk. Furthermore, there were meaningful geographical differences in the use of VKA therapy in the era before the availability of NOACs, including a much lower use of VKAs in China, where most patients either received antiplatelet agents or no antithrombotic treatment.
Keywords: Atrial fibrillation, Stroke, Oral anticoagulation, Registry
What's new?
GLORIA-AF is a prospective, long-term global registry in non-valvular atrial fibrillation (AF) with three separate phases: Phase I, baseline cohort before the introduction of non-VKA oral anticoagulants (NOACs); Phase II, just after the introduction of the first NOAC dabigatran, with 2-year follow-up in dabigatran-treated patients; and Phase III, 3-year follow-up study in patients on any antithrombotic treatment.
In this Phase I part of GLORIA-AF, before availability of NOACs, vitamin K antagonists (VKAs) were mostly used in patients with persistent/permanent (vs. paroxysmal) AF, and in those with high stroke risk.
Compared with the rest of the world, VKA use is much lower in China, where most patients were either treated with antiplatelet agents or received no antithrombotic treatment.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and affects ∼1–2% of the adult population.1 The lifetime risk for development of AF is one in four for those over the age of 40 years.2 The prevalence of AF rises with advancing age, increasing from <1% in those <60 years of age to nearly 20% in those 85 and older.3 Thromboembolic complications—particularly ischaemic stroke and systemic thromboembolism—are a major cause of morbidity and mortality in patients with AF. Patients with AF have a four- to five-fold higher risk for stroke than those without AF.4–6 Up to 15% of all strokes are due to AF, and strokes in patients with AF have worse outcomes with higher mortality rates than strokes in patients without AF.7
Until recently, the treatment choices for stroke prevention in patients with AF were vitamin K antagonists (VKAs, e.g. warfarin) or antiplatelet agents such as aspirin [acetylsalicylic acid (ASA)]. A meta-analysis demonstrated that warfarin decreased the risk of stroke/systemic embolism on average by 64% vs. placebo (all-cause mortality by 26%), while antiplatelet therapy reduced the occurrence of stroke by 22% vs. placebo. When the analysis was confined to the ASA-only studies, ASA reduced stroke by 19% (95% confidence interval, −1 to 35%) vs. placebo,8,9 with no reduction in mortality.
The benefits of VKAs must be weighed against important limitations including a narrow therapeutic window, an unpredictable dose–response, numerous drug–drug and drug–food interactions, and a slow onset and ebbing of action.10 As a result, many patients with AF do not receive VKAs, but receive ASA, antiplatelet agents, or both, or no antithrombotic therapy.4 With the approval of non-VKA oral anticoagulants (NOACs), including thrombin inhibitors such as dabigatran etexilate (dabigatran) and factor Xa inhibitors such as rivaroxaban and apixaban for stroke prevention in patients with non-valvular AF (NVAF), antithrombotic treatment patterns have changed.
In the RE-LY (Randomized Evaluation of Long-Term Anticoagulation Therapy) study, which was the first Phase III study in patients with NVAF, dabigatran 150 mg twice daily was demonstrated to be superior to warfarin, while dabigatran 110 mg twice daily was shown to be non-inferior to warfarin for the prevention of stroke and systemic emboli. The higher dose of dabigatran was associated with comparable risk of major bleeding, whereas the lower dose was associated with fewer major bleeds. Importantly, both doses of dabigatran etexilate considerably reduced the occurrence of intracranial haemorrhage vs. warfarin.11
After drug approval, additional collection of data from clinical practice is essential to characterize the broad spectrum of co-morbidities and other medication use and to understand antithrombotic treatment patterns and responses outside the more controlled setting of clinical trials. Analyses of claims and electronic medical record data, such as the recent analysis of Medicare data for dabigatran etexilate,12 enable rapid and early evaluation of outcomes; however, to collect accurate, pertinent information and to allow for the adequate control of confounding factors, new data should ideally be pursued. A prospective registry enables precise collection of important baseline information in patients managed in the course of routine care (e.g. smoking, alcohol use, and co-medication, including platelet inhibitors).
The Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF) was designed to provide prospective information on a population with recently diagnosed AF. In this article, results from the Phase I cohort of GLORIA-AF are presented, including baseline characteristics and initial antithrombotic management of patients before the approval of NOACs.
Methods
The design of the GLORIA-AF Registry Programme has been reported previously.13 Briefly, GLORIA-AF is an ongoing disease registry of newly diagnosed AF patients run in three separate phases, as follows. In Phase I, conducted before approval of the first NOAC, information has been collected on prescription patterns prior to availability of NOACs on the market. This initial phase used a cross-sectional approach, with no data collected beyond the initial visit. Phase I was conducted only in countries that had not yet received marketing authorization at the time of study initiation; this design therefore limited the participating countries and the enrolment time for entering patients.
Patients
Patients aged ≥18 years with newly diagnosed (≤3 months before the baseline visit) NVAF and at risk for stroke (CHA2DS2-VASc score ≥1) were included. A broad cross-section of consecutively examined patients, treated within the different healthcare settings of each participating country, was included (e.g. general practices, specialist offices, community hospitals, and university hospitals).
Patient symptom burden categories reflect the European Heart Rhythm Association classification.14 Patients were categorized as symptomatic (defined as severe or disabling symptoms that impact daily activities), minimally symptomatic, or asymptomatic.
Patients were excluded if they had (i) mechanical heart valves or valve disease expected to require valve replacement, (ii) received >60 days of VKA treatment in their lifetime for any indication, (iii) AF with a generally reversible cause, (iv) expected life expectancy <1 year at the time of enrolment as assessed by the investigator, or (v) a medical condition other than AF for which chronic use of VKAs was indicated. Patients receiving NOACs were excluded when analysing predictors of VKA use and reasons to forego VKAs.
As Phase I was conducted before approval of the first NOAC, at the time of study initiation, this design restricted country participation, and hence inclusion of patients, to the following: China, The Netherlands, Spain, Germany, Croatia (defined as Europe), Egypt, Lebanon, Turkey, and the United Arab Emirates (defined as the Middle East). At the cross-sectional (or baseline) assessment, clinical and demographic characteristics were recorded, as were type of AF and management approaches.
Data quality control
An academic steering committee designed and provided scientific oversight of all phases of the programme. Regular meetings of the study team and the chair and co-chairs of the steering committee were held throughout the study.
Data were collected in electronic case report forms, and investigators were trained on the electronic data capture system before entering any data. Data quality was monitored electronically as well as through periodic medical and data quality reviews, on-site monitoring, and audits. Investigators were asked to enrol all eligible patients consecutively to limit selection bias at the patient level.
Statistical analysis and study size
For Phase I, data were summarized descriptively, displaying median and interquartile ranges [IQR; given by Q1 (25% quartile) and Q3 (75%)] for continuous variables. Categorical variables were expressed as frequencies and percentages. Statistical analysis was performed using SAS software version 9.2 (SAS Institute Inc.).
For summary of data by antithrombotic therapy, similar treatment regimens were pooled for analysis, resulting in the following four treatment groups, which had been available in the respective time period: (i) VKA, defined as treatment with VKAs alone or in combination with other antiplatelet medications; (ii) ASA, defined as treatment with ASA alone or in combination with other antiplatelet medications; (iii) antiplatelet agents other than ASA, defined as treatment with single or combination antiplatelet medications; and (iv) no treatment. A category ‘missing’ is shown whenever at least one patient with missing data occurred.
Main predictors for VKA use were identified based on multivariable logistic regression analysis using the backward model selection procedure. Sensitivity analyses were conducted using stepwise selection and based on a broader model including relevant covariates based on the results seen in univariable regression. Odds ratios and corresponding 95% confidence intervals are presented.
End of Phase I and thereby overall study size was driven—per the study design—by approval and availability of dabigatran within a country.
Results
From May 2011 (date of first patient in) until January 2013 (date of last patient in), 1100 patients from 64 centres were enrolled in Phase I of the registry; 59 of the centres enrolled at least one eligible patient. Of the enrolled patients, 1063 were eligible for inclusion in the analysis of the Phase I data; for the 37 patients who were not eligible, the most frequent reasons for exclusion were inclusion after the study stop date (n = 15) and informed consent before the site initiation visit (n = 15). Patients were included from China (67.1%), Europe (27.4%), and the Middle East (5.6%). The majority of eligible patients (95.2%; n = 1012/1063) were enrolled by cardiologists, and the type of participating centres can be seen in Table 1.
Table 1.
Enrolling centres
| Number of sites, n (%)a | |
|---|---|
| Total | 59 (100) |
| Type of site | |
| General practice/primary care | 4 (6.8) |
| Specialist office | 14 (23.7) |
| Community hospital | 2 (3.4) |
| University hospital | 36 (61.0) |
| Outpatient healthcare centre | 0 (0.0) |
| Anticoagulation clinic | 0 (0.0) |
| Other | 3 (5.1) |
aOnly sites with at least one eligible patient are displayed.
Of the 1063 enrolled patients (median age 70 years [IQR, 61.0–77.0]; 45.7% female), 74.8% (795) had hypertension, 22.6% (240) diabetes mellitus, and 24.1% (256) congestive heart failure. Further demographics and co-morbidities are summarized in Table 2.
Table 2.
Patient demographic and baseline characteristics
| Characteristic | China (n = 713) | Europe (n = 291) | Middle East (n = 59) | Total (n = 1063) |
|---|---|---|---|---|
| Age, median, years (Q1, Q3) | 69 (59, 77) | 71 (64, 79) | 65 (57, 74) | 70 (61–77) |
| Female, n (%) | 305 (42.8) | 147 (50.5) | 34 (57.6) | 486 (45.7) |
| BMI, median, kg/m2 (Q1, Q3) | 23.9 (21.5, 26.1) | 28.1 (25.4, 31.2) | 27.3 (24.2, 33.3) | 25.0 (22.5–28.0) |
| Medical history, n (%) | ||||
| Previous stroke | 73 (10.2) | 31 (10.7) | 6 (10.2) | 110 (10.3) |
| Myocardial infarction | 59 (8.3) | 32 (11.0) | 8 (13.6) | 99 (9.3) |
| Coronary artery disease | 181 (25.4) | 59 (20.3) | 16 (27.4) | 256 (24.1) |
| Congestive heart failure | 176 (24.7) | 65 (22.3) | 15 (25.4) | 256 (24.1) |
| History of hypertension | 500 (70.1) | 248 (85.2) | 47 (79.7) | 795 (74.8) |
| Diabetes mellitus | 139 (19.5) | 79 (27.1) | 22 (37.5) | 240 (22.6) |
| Chronic GI diseases | 61 (8.6) | 9 (3.1) | 3 (5.1) | 73 (6.9) |
| Type of AF | ||||
| Paroxysmal | 470 (65.9) | 155 (53.3) | 40 (67.8) | 665 (62.6) |
| Persistent | 231 (32.4) | 115 (39.5) | 13 (22.0) | 359 (33.8) |
| Permanent | 12 (1.7) | 21 (7.2) | 6 (10.2) | 39 (3.7) |
| AF ablation | 34 (4.8) | 3 (1.0) | 0 (0.0) | 37 (3.5) |
| Any drug (HAS-BLED) | 406 (56.9) | 147 (50.5) | 32 (54.2) | 585 (55.0) |
AF, atrial fibrillation; BMI, body mass index; GI, gastrointestinal; Q1, 25%-quartile; Q3, 75%-quartile.
The majority of patients overall had symptomatic AF (62.2%; 661/1063), with higher frequency in China (64.8%; 462/713) and in the Middle East (64.4%; 38/59) than in Europe (55.3%; 161/291). Asymptomatic AF was more frequent in Europe (24.1%; 70/291) than in China (13.5%; 96/713) and the Middle East (8.5%; 5/59) (Figure 1).
Figure 1.
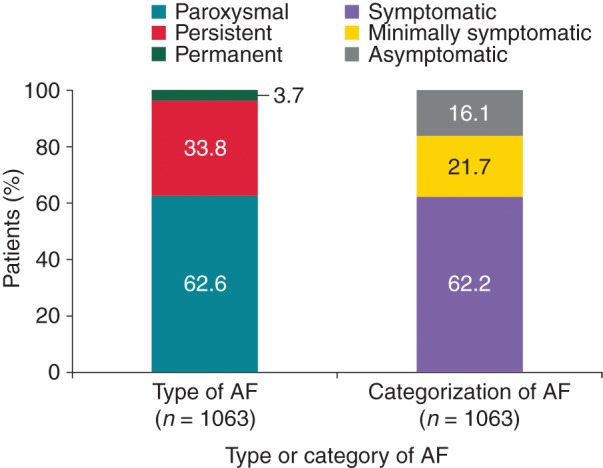
Type and symptom burden of AF.
Stroke and bleeding risk scores
Overall, 54.1% of patients had a high stroke risk (CHADS2 score ≥2). The median (Q1, Q3) CHADS2 score was 2.0 (1.0–3.0) and the median (Q1, Q3) CHA2DS2-VASc score was 3.0 (2.0–4.0). The median (Q1, Q3) HAS-BLED risk score was 1.0 (1.0–2.0). Overall, the majority (80.9%; 860/1063) of patients had low bleeding risk (HAS-BLED score <3). The stroke and bleeding risk score classes per region are summarized in Table 3.
Table 3.
Stroke and bleeding risk scores
| China (n = 713) | Europe (n = 291) | Middle East (n = 59) | Total (n = 1063) | |
|---|---|---|---|---|
| CHADS2 score class, n (%) | ||||
| Low (score = 0) | 84 (11.8) | 16 (5.5) | 2 (3.4) | 102 (9.6) |
| Moderate (score = 1) | 270 (37.9) | 95 (32.6) | 21 (35.6) | 386 (36.3) |
| High (score ≥ 2) | 359 (50.4) | 180 (61.9) | 36 (61.0) | 575 (54.1) |
| CHA2DS2-VASc score class, n (%) | ||||
| Score = 0 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Score = 1 | 184 (25.8) | 36 (12.4) | 6 (10.2) | 226 (21.3) |
| Score ≥ 2 | 529 (74.2) | 255 (87.6) | 53 (89.8) | 837 (78.7) |
| HAS-BLED score class, n (%) | ||||
| Low (score < 3) | 596 (83.6) | 224 (77.0) | 40 (67.8) | 860 (80.9) |
| High (score ≥ 3) | 88 (12.3) | 23 (7.9) | 10 (16.9) | 121 (11.4) |
| Missing | 29 (4.1) | 44 (15.1) | 9 (15.3) | 82 (7.7) |
CHADS2, congestive heart failure, hypertension, age ≥75 years, diabetes, stroke (doubled); CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke (doubled), vascular disease, age 65–74 years, sex category (female); HAS-BLED, hypertension, abnormal renal and liver function (1 point each), stroke, bleeding, labile international normalized ratios, elderly (e.g. age >65 years), drugs or alcohol (1 point each) (where ‘drugs/alcohol’ refers to concomitant use of drugs such as antiplatelet agents, NSAIDs, or alcohol abuse, etc.).
Selection of antithrombotic therapy
Overall, of the 1063 patients, treatment with ASA was the most common at 41.7% of patients (443), followed by 32.8% (349) treated with VKAs and 3.4% (36) treated with antiplatelet agents other than ASA; 20.2% (215) did not receive antithrombotic therapy. The remaining 1.9% of patients (20), comprising 1 patient from Europe and 19 from the Middle East, received a NOAC in various treatment combinations. When assessing antithrombotic treatment choice by region, treatment with VKAs was more common in Europe than in China. Treatment with ASA or no antithrombotic treatment was more common in China than in Europe (Figure 2).
Figure 2.
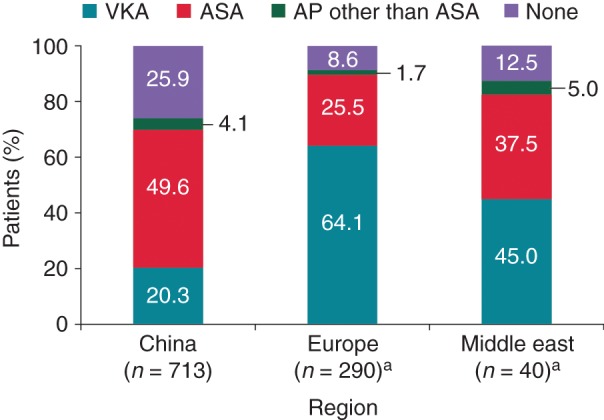
Antithrombotic treatment pattern by region (all regions). aOne patient from Europe and 19 from the Middle East are excluded due to receipt of NOAC. AP, antiplatelet agents.
Overall, among the 1063 eligible patients, VKA monotherapy was more common (24.1%; n = 256) than VKAs in combination with a single antiplatelet agent (7.8%; 83) and with multiple antiplatelet agents (0.9%; 10). Similarly, ASA monotherapy was more common (30.4%; 323) than ASA in combination with other antiplatelet agents (11.3%; 120). Similar patterns in monotherapy were observed within regions. Vitamin K antagonist combination therapy was most frequently ASA. Excluding combinations with VKAs, ASA combination therapy with clopidogrel was the most common. A few patients (3.4%; 36) were treated with antiplatelet agents other than ASA.
Antithrombotic treatment by atrial fibrillation type
The majority of patients had paroxysmal (62.6%) or persistent AF (33.8%), and most were symptomatic (62.2%) or minimally symptomatic (21.7%). When assessing the treatment per type of AF, patients with paroxysmal AF were predominantly treated with ASA, whereas patients with persistent and permanent AF were predominantly treated with VKAs (Figure 3).
Figure 3.
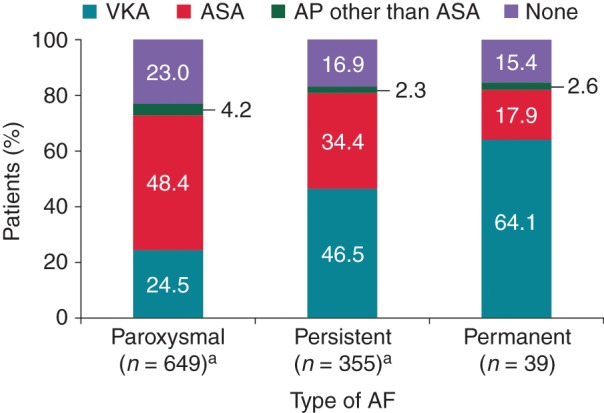
Antithrombotic treatment pattern by AF type (all regions). aSixteen patients with paroxysmal AF and four with permanent AF are excluded from the graph because they received a NOAC.
Stroke risk by treatment
When assessing treatment pattern per stroke risk scores, patients with high risk (CHADS2 score ≥2) comprised the majority of patients in groups that received treatment: 61.3% (214/349) on VKA treatment, 56.7% (51/443) on ASA treatment, and 75.0% (27/36) on antiplatelet agents other than ASA. Nevertheless, 33% of patients receiving no antithrombotic treatment had a CHADS2 score ≥2, and 22.3% of Chinese patients with a CHA2DS2-VASc ≥2 received no antithrombotic treatment. Of the 215 patients with no treatment, according to CHADS2 risk score, 50.7% (109) had a moderate risk and 16.3% (215) had a low risk score of 0.
For high stroke risk patients in China (CHA2DS2-VASc ≥2), there was still a large proportion receiving only ASA (52.0%) or no therapy (22.3%) (Figure 4). In Europe and the Middle East, high stroke risk patients (CHADS2 score ≥2) received ASA in 18.3% (33/180) and 22.2% (8/36) or no therapy in 3.9% (7/180) and 8.3% (3/36), respectively.
Figure 4.
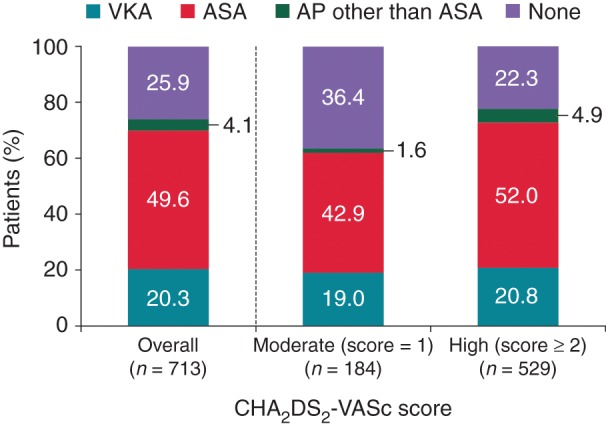
Antithrombotic treatment pattern by stroke risk (CHA2DS2-VASc score) (China). AP, antiplatelet agents; CHA2DS2-VASc, congestive heart failure, hypertension, age ≥75 years (doubled), diabetes, stroke (doubled), vascular disease, age 65–74 years, sex category (female).
Bleeding risk by treatment
In the VKA group, the majority of patients (84.2%) had a low HAS-BLED risk score (HAS-BLED <3), vs. 76.5% in the ASA group and 63.9% in the antiplatelet agents other than ASA-treated group. Of the patients without antithrombotic treatment, 89.8% were classified as low bleeding risk.
Predictors for vitamin K antagonist use
Based on the multivariable logistic regression analysis, the most relevant predictors for VKA use were AF ablation, region, any drugs causing bleeding [i.e. antiplatelet agents and/or non-steroidal anti-inflammatory drugs (NSAIDs)], non-central nervous system (CNS) arterial embolism, and hepatic disease (see Table A1 in the appendix). The univariable logistic regression results are shown in Table A2 in the appendix. Patients with prior ablation or prior non-CNS arterial embolism or who were from Europe were more likely to be prescribed VKAs. Patients taking antiplatelets and/or NSAIDs and patients with hepatic disease were less likely to be prescribed VKAs. Atrial fibrillation ablation, non-CNS arterial embolism, and hepatic disease are rare conditions, with a prevalence of <4% in the study cohort; thus, they have a limited predictive value for VKA use in general.
Reasons to forego vitamin K antagonists
For the 694 eligible patients receiving neither VKAs nor combinations of VKAs, the most frequent reason to forego VKAs was that the patient was currently in stable sinus rhythm. Other frequent, specific reasons to forego VKAs were an expected low stroke risk and patient's unwillingness to take VKAs.
Of the 139 total patients for whom perceived low stroke risk was given as the reason to forego VKA therapy, 35.3% (49) actually had low CHADS2 scores, 58.3% (81) had moderate CHADS2 scores, and 6.5% (9) had high CHADS2 scores. These trends were similar for European and Chinese patients. For the CHA2DS2-VASc, this corresponds overall to 75.5% (105) patients with moderate CHA2DS2-VASc scores, and 24.5% (34 patients) with high CHA2DS2-VASc scores within the group of patients with perceived low stroke risk. In all regions, moderate CHA2DS2-VASc scores were predominant among those perceived as having low stroke risk [China, 82.5% (80/97); Europe, 58.5% (24/41); the Middle East, 100% (1/1)]. Many of these patients had a CHA2DS2-VASc score of 1, defined by female gender alone, which may explain why some were considered low risk.
Reasons to forego vitamin K antagonists in patients with high stroke risk
For the 349 patients with high CHADS2 scores (score ≥2) receiving neither VKAs nor combinations of VKAs, the most frequent reasons to forego VKAs were patient currently in stable sinus rhythm (22.6%; 79) and the non-specific ‘other’ category (39.8% of non-VKA patients; 139). As expected, only for nine patients out of the group of patients with CHADS2 score ≥2, an expected low stroke risk was reported as reason to forgo VKA therapy. Within the subset of patients with CHADS2 score ≥2, an expected high bleeding risk led to the decision to forgo VKA therapy in 44/349 patients. Thereby, in patients at high risk for stroke, investigators considered the bleeding risk to warrant withholding VKA therapy for 12.6% of these patients.
When using CHA2DS2-VASc instead of CHADS2 to describe patients’ stroke risk, similar results were observed; for the 516 patients with high CHA2DS2-VASc scores receiving neither VKAs nor combinations of VKAs, the most frequent reasons to forego VKAs were patient currently in stable sinus rhythm (25.8%; 133) and the non-specific ‘other’ category (38.4%; 198). Within the subset of patients with a high score, an expected high bleeding risk led to the decision to forego VKA therapy in 47/516 patients (9.1%).
Discussion
The main objective of Phase I of the GLORIA-AF Registry Programme was to gather information on demographic and disease characteristics, as well as regional prescribing practices for treatment of NVAF during a time period before the availability of NOACs for the prevention of stroke in patients with AF. Therefore, patient enrolment for this phase of the programme stopped with the approval of the first NOAC (dabigatran) for this indication in a country.
In summary, Phase I enrolled 1063 eligible patients in China, The Netherlands, Spain, Germany, Croatia, Egypt, Lebanon, Turkey, and the United Arab Emirates. The majority of patients (∼70%) were enrolled in China.
The observed patient demographics and medical history are reflective of a newly diagnosed patient population at risk of stroke with a median age of 70 years (IQR, 61.0–77.0) and multiple co-morbidities (Table 2). Overall, treatment with ASA was the most common (41.7%), followed by VKAs (32.8%) and no antithrombotic treatment (20.2%). Despite the fact that the majority of patients overall had moderate or high stroke risk and low bleeding risk, a substantial proportion of patients were not given any therapy. Patients with high CHADS2 risk comprised the majority of patients in all groups that received treatment: 61.3% on VKA treatment, 56.7% on ASA treatment, and 75.0% on antiplatelet agents other than ASA. Similarly, by CHA2DS2-VASc score, patients with high stroke risk comprised the majority of patients in all groups: 86.5% on VKA treatment, 77.9% on ASA treatment, 88.9% on antiplatelet agents other than ASA, and 64.7% on no antithrombotic treatment. Overall, the majority of patients had a low bleeding risk, but patients with a high bleeding risk were more prevalent in the group of ASA-treated patients (19.6%) than in the group of VKA-treated patients (4.6%) and in patients not prescribed any antithrombotic therapy (0%).
There are two important reasons for the low number of patients on VKAs and the higher number on ASA in China. First, anticoagulation therapy is not commonly given to Asian patients with NVAF, probably because of the risk of critical bleeding during treatment, which has been reported to be higher among Asian patients. Warfarin-related intracranial haemorrhage in Asian patients was reported to be 1.75/100 patient-years, which is considerably higher than that in Caucasian patients (0.34/100 patient-years).15 The second reason is that in China, many patients lack access to good laboratory control for VKA therapy and are therefore not prescribed VKAs. Of note, the percentage of patients on VKA therapy in China in our present study—20.3%—is strikingly higher than that in earlier community-based cohort studies in China that found between 0.5 and 2.7%.16,17 This difference may reflect the fact that most patients in the current study were recruited in (university) hospital settings, with better access to healthcare resources, including warfarin control.16,17
Our findings are consistent with a recent report based on claims data showing that ASA use is increasing among newly diagnosed Chinese AF patients, with no relationship to the patient's stroke or bleeding risks, and that warfarin use was very low.18 The suboptimal use of thromboprophylaxis in China has important implications given the increasing prevalence, incidence, and burden of AF in this region.18
Several other aspects of this study deserve comment. First, the most relevant predictors for VKA use based on logistic regression analyses were AF ablation, region, any drugs causing bleeding (i.e. antiplatelet agents and/or NSAIDs), non-CNS arterial embolism, and hepatic disease. Patients with prior ablation or prior non-CNS arterial embolism or who were from Europe were more likely to be prescribed a VKA. Specifically, patients taking antiplatelet agents and/or NSAIDs and patients with hepatic disease were less likely to be prescribed a VKA. Second, VKA initiation differed by type of site and regions since patients enrolled in Europe were more likely to be prescribed a VKA than patients enrolled in China, and patients enrolled at specialists’ offices or university hospitals were more likely to be prescribed a VKA than patients enrolled at general practice/primary care centres. Third, all three risk scores—HAS-BLED, CHADS2, and CHA2DS2-VASc—were associated in univariable logistic regression analyses with VKA use, and the risk profile of a patient is an important factor for prediction of VKA use. Expected high bleeding risk was rarely reported by physicians as the rationale for foregoing treatment with VKAs. Importantly, despite the fact that the majority of patients were classified as having high stroke risk and low bleeding risk, approximately one in five patients remained untreated with thromboprophylaxis, especially in China. A shift to more VKA use in patients with AF was seen when ‘going West’. Finally, there was lower VKA use in those with paroxysmal AF, indicating discrepancies between VKA use in clinical practice and guideline recommendations.
Limitations
There are limitations with the interpretation of these data based on the relatively small study size, especially for the Middle East, and the high representation of patients from China in the overall study population. As the end of Phase I was determined by the approval of dabigatran, the early approval in some countries led to a lower country participation and patient number than originally expected for this phase of the registry programme. Of the participating countries, China was the last country to approve dabigatran, which led to the high representation of Chinese patients in the study. The impact of the introduction of NOACs will be further explored in Phases II and III of GLORIA-AF, to assess treatment patterns after the availability of these agents. Data on variables, including patient educational level, economical class, patient mental condition, and patient living condition, i.e. if they were from urban or rural areas, were not available. The majority of patients (95.2%) were entered by cardiologists, which may have affected the population of the registry.
Conclusion
Phase I of GLORIA-AF demonstrated a wide variety of antithrombotic treatment patterns, depending on region. These geographical differences existed in the use of VKA therapy in the era before the availability of NOACs, with a notably lower use of VKAs in China.
Funding
This trial was funded by Boehringer Ingelheim. Funding to pay the Open Access publication charges for this article was provided by Boehringer Ingelheim Pharma GmbH & Co. KG.
Conflict of interest: M.V.H. has provided consulting for Boehringer Ingelheim. H.-C.D. has received honoraria for participation in clinical trials, contribution to advisory boards, or oral presentations from Abbott, Allergan, AstraZeneca, Bayer Vital, Bristol-Myers Squibb, Boehringer Ingelheim, CoAxia, Corimmun, Covidien, Daiichi-Sankyo, D-Pharm, Fresenius, GlaxoSmithKline (GSK), Janssen-Cilag, Johnson & Johnson, Knoll, Lilly, Merck Sharp & Dohme, Medtronic, MindFrame, Neurobiological Technologies, Novartis, Novo Nordisk, Paion, Parke-Davis, Pfizer, Sanofi Aventis, Schering-Plough, Servier, Solvay, St. Jude, Syngis, Talecris, Thrombogenics, WebMD Global, Wyeth, and Yamanouchi; received financial support for research projects from AstraZeneca, GSK, Boehringer Ingelheim, Lundbeck, Novartis, Janssen-Cilag, Sanofi Aventis, Syngis, and Talecris; and has no ownership interest and does not own stocks of any pharmaceutical company. The Department of Neurology at the University Duisburg-Essen received research grants from the German Research Council, German Ministry of Education and Research, European Union, the National Institutes of Health, Bertelsmann Foundation, and Heinz-Nixdorf Foundation. S.J.D. currently participates in research sponsored by Boehringer Ingelheim. J.L.H. currently conducts research sponsored by Boehringer Ingelheim as a member of the Executive Steering Committee for the GLORIA-AF Registry. C.T. is an employee of Boehringer Ingelheim. N.S. is an employee of Boehringer Ingelheim. E.K. is an employee of Boehringer Ingelheim. D.B.B. is an employee of Boehringer Ingelheim. G.Y.H.L. has served as a consultant for Bayer, Merck, Sanofi, BMS/Pfizer, Daiichi-Sankyo, Biotronik, Medtronic, Portola, and Boehringer Ingelheim; and has been on the speakers’ bureau for Bayer, BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, and Medtronic.
Acknowledgements
Editorial assistance for the formatting of this manuscript was provided by Lawrence Hargett of PAREXEL, with funding from Boehringer Ingelheim.
Appendix
Table A1.
Association between patient characteristics and the decision to forego VKA based on multivariable logistic regression analysis
| Variable | ORa,b | 95% CIa | Odds of being prescribed treatment with VKA |
|---|---|---|---|
| Any drug (HAS-BLED) | 0.066 | (0.0391–0.1058) | Lower for patients on any antiplatelet medication, COX-2 inhibitor, or other NSAID |
| AF ablation | 24.342 | (7.9997–85.7171) | Higher for patients with AF ablation |
| Type of AF | |||
| Paroxysmal | 1.0 | – | Higher for patients with persistent or permanent AF than for those with paroxysmal AF |
| Persistent | 3.141 | (2.0336–4.8950) | |
| Permanent | 1.374 | (0.4011–4.5970) | |
| Region | |||
| China | 1.0 | – | Higher in patients in Europe or the Middle East relative to patients in China |
| Europe | 14.683 | (7.4549–30.0002) | |
| Middle East | 3.275 | (0.8056–12.5629) | |
| Type of site where patients treated | |||
| GP/primary care | 1.0 | – | Lower for patients treated at community hospitals or other sites than at GP/primary care sites; higher for patients treated at specialists’ offices or university hospitals |
| Specialists’ offices | 3.603 | (1.4065–9.4857) | |
| Community hospitals | 0.435 | (0.0317–3.9350) | |
| University hospital | 3.240 | (1.5571–7.1283) | |
| Other sites | 0.537 | (0.0936–2.9496) | |
| Hypertension | |||
| No | 1.0 | – | Higher for patients with either uncontrolled or controlled hypertension |
| Yes, uncontrolled | 1.453 | (0.5901–3.4937) | |
| Yes, controlled | 2.206 | (1.3325–3.7161) | |
| Pacemaker | 0.230 | (0.0484–0.8962) | Lower for patients with pacemaker |
| Congestive heart failure | 1.691 | (1.0379–2.7550) | Higher for patients with congestive heart failure |
| Hepatic disease | 0.086 | (0.0032–0.8791) | Lower for patients with hepatic disease |
| Antihypertensive/heart failure and antiarrhythmic therapy | 1.645 | (1.0298–2.6516) | Higher for patients on antihypertensive/heart failure and antiarrhythmic therapy |
| Diabetes mellitus | 1.654 | (1.0045–2.7231) | Higher for patients with diabetes mellitus |
| Smoking status | |||
| Non-smoker | 1.0 | – | Lower for patients who are current smokers, and higher for patients who are past smokers, than for those who are non-smokers |
| Current smoker | 0.534 | (0.2751–1.0038) | |
| Past smoker | 1.405 | (0.7787–2.5214) | |
| Hyperlipidaemia | 1.667 | (0.9798–2.8354) | Higher for patients with hyperlipidaemia |
| Non-CNS arterial embolism | 12.860 | (0.8195–325.3468) | Higher for patients with non-CNS arterial embolism |
AF, atrial fibrillation; CI, confidence interval; CNS, central nervous system; GP, general practitioner; NSAID, non-steroidal anti-inflammatory drugs; OR, odds ratio; VKA, vitamin K antagonist.
aFrom likelihood ratio test.
bIf not specified differently, OR are for presence vs. absence of patient characteristic.
Table A2.
Association between patient characteristics and the decision to prescribe VKA based on univariable logistic regression analysis
| Variable | ORa,b | 95% CIb | Odds of being prescribed treatment with VKA |
|---|---|---|---|
| HAS-BLED risk score ≥3 | 0.305 | (0.1705–0.5125) | Lower for patients with high bleeding risk (score ≥3) |
| CHADS2 score class | |||
| Low risk | 1.0 | – | Higher for patients with moderate or higher stroke risk than for those with lower stroke risk |
| Moderate risk | 2.959 | (1.6659–5.6219) | |
| High risk | 3.854 | (2.2063–7.2283) | |
| CHA2DS2-VASc score class, score ≥2 | 2.217 | (1.5717–3.1782) | Higher for patients with higher stroke risk than for those with moderate stroke risk |
| AF ablation | 6.671 | (3.2324–15.1401) | Higher for patients with AF ablation |
| Type of AF | |||
| Paroxysmal | 1.0 | – | Higher for patients with persistent or permanent AF than for those with paroxysmal AF |
| Persistent | 2.676 | (2.0347–3.5261) | |
| Permanent | 5.503 | (2.8313–11.1139) | |
| Any drug (HAS-BLED) | 0.167 | (0.1249–0.2211) | Lower for patients on any antiplatelet medication, COX-2 inhibitor, or other NSAID |
| LVH | 2.214 | (1.5265–3.2119) | Higher for patients with LVH |
| Hyperlipidaemia | 2.111 | (1.5675–2.8433) | Higher for patients with hyperlipidaemia |
| Weight class, kg | |||
| <50 | 1.0 | – | Higher for patients in weight class 50 to <100 or ≥100 kg |
| 50 to <100 | 2.688 | (1.3662–5.9207) | |
| ≥100 | 8.205 | (3.1245–23.2589) | |
| BMI class, kg/m2 | |||
| 18.5 to <25 | 1.0 | – | Lower for patients with BMI <18.5 than for those with BMI 18.5 to <25 Higher for patients with BMI ≥25 than for those with BMI 18.5 to <25 |
| <18.5 | 0.450 | (0.1512–1.0812) | |
| 25 to <30 | 1.760 | (1.3138–2.3624) | |
| 30 to <35 | 3.614 | (2.3960–5.4769) | |
| ≥35 | 5.085 | (1.9985–3.9435) | |
| Alcohol use | |||
| No alcohol | 1.0 | – | Higher for patients who consumed <1–1–7, or ≥8 drinks/week than for those who consumed no alcohol |
| <1 drink/week | 2.506 | (1.7352–3.6213) | |
| 1–7 drinks/week | 1.476 | (1.0245–2.1129) | |
| ≥8 drinks/week | 2.577 | (0.7970–8.3315) | |
| Race | |||
| White | 1.0 | – | Lower for patients who were not white |
| Asian | 0.143 | (0.1057–0.1924) | |
| Arab/Middle East | 0.349 | (0.1590–0.7387) | |
| Region | |||
| China | 1.0 | – | Higher for patients in Europe or the Middle East than for patients in China |
| Europe | 7.006 | (5.1970–9.4997) | |
| Middle East | 3.205 | (1.6576–6.1258) | |
| Type of site where patients were treated | |||
| GP/primary care | 1.0 | – | Lower for patients treated at community hospitals than at GP/primary care sites; higher for patients treated at specialists’ offices or other sites |
| Specialists’ offices | 3.655 | (2.1491–6.3601) | |
| Community hospitals | 0.159 | (0.0246–0.5771) | |
| University hospital | 1.004 | (0.6268–1.6494) | |
| Other sites | 4.569 | (1.4410–16.0961) | |
| History of hypertension | 1.841 | (1.3479–2.5412) | Higher for patients with hypertension |
| Hypertension | |||
| No | 1.0 | – | Higher for patients with either uncontrolled or controlled hypertension |
| Yes, uncontrolled | 1.322 | (0.7582–2.2616) | |
| Yes, controlled | 1.896 | (1.3807–2.6287) | |
| CAD | 0.549 | (0.3950–0.7551) | Lower for patients with CAD |
| Any prior VKA therapy during lifetime | 4.121 | (1.7937–10.2542) | Higher for patients with prior VKA therapy during lifetime |
| Chronic gastrointestinal diseases | 0.386 | (0.1951–0.7019) | Lower for patients with chronic gastrointestinal diseases |
| Neurologic disease | 3.490 | (1.3908–9.4516) | Higher for patients with neurologic disease |
| PAD | 4.090 | (1.4414–13.2177) | Higher for patients with PAD |
| Antihypertensive/heart failure and antiarrhythmic therapy | 1.464 | (1.1017–1.9563) | Higher for patients on antihypertensive/heart failure and antiarrhythmic therapy |
| Any chronic concomitant medication | 1.469 | (1.0844–2.0073) | Higher for patients on any chronic concomitant medication |
| Previous stroke timing | |||
| No | 1.0 | – | Higher for patients with recent or past stroke |
| Recent | 2.919 | (1.3881–6.3422) | |
| Past | 1.190 | (0.6072–2.2515) | |
| Smoking status | |||
| Non-smoker | 1.0 | – | Lower for patients who are current smokers and higher for patients who are past smokers than for those who are non-smokers |
| Current smoker | 0.595 | (0.3863–0.8954) | |
| Past smoker | 1.168 | (0.8097–1.6740) | |
| Angina pectoris | 0.639 | (0.4174–0.9559) | Lower for patients with angina pectoris |
| Diabetes mellitus | 1.401 | (1.0348–1.8922) | Higher for patients with diabetes mellitus |
| Non-CNS arterial embolism | 8.034 | (1.1832–157.3896) | Higher for patients with non-CNS arterial embolism |
| Pacemaker | 0.356 | (0.1034–0.9382) | Lower for patients with a pacemaker |
| Congestive heart failure | 1.360 | (1.0119–1.8228) | Higher for patients with congestive heart failure |
| Other concomitant drugs | 1.402 | (1.0117–1.9338) | Higher for patients on other concomitant drugs |
| Creatinine clearance class 1, ml/min | |||
| <30 | 1.0 | – | Higher for patients with creatinine clearance ≥30 |
| 30 to <50 | 1.061 | (0.4368–2.8641) | |
| 50 to <80 | 1.754 | (0.7726–4.5149) | |
| ≥80 | 1.940 | (0.8482–5.0188) | |
| Physician specialty | 1.879 | (0.9853–3.8913) | Higher for patients treated by cardiologists |
| Categorization of AF | |||
| Symptomatic | 1.0 | – | Higher for patients with minimally and asymptomatic AF |
| Minimally symptomatic | 1.164 | (0.8421–1.6003) | |
| Asymptomatic | 1.523 | (1.0723–2.1565) | |
| Psychosocial factors | 3.014 | (0.8564–11.8664) | Higher for patients with psychosocial factors |
| Gender | 1.247 | (0.9633–1.6143) | Higher for female patients |
| DVT | 4.029 | (0.7826–29.1577) | Higher for patients with DVT |
| Previous stroke | 1.418 | (0.9387–2.1248) | Higher for patients with previous stroke |
| MI | 0.706 | (0.4350–1.1142) | Higher for patients without MI |
| Age classc, ≥80 years | 0.763 | (0.5269–1.0897) | Lower for younger patients <80 years |
| Age class 2 | |||
| <65 years | 1.0 | – | Higher for patients between 65 and <75 years |
| 65 to <75 years | 1.095 | (0.7969–1.5028) | |
| ≥75 years | 0.886 | (0.6502–1.2065) | |
| Hepatic disease | 0.359 | (0.0553–1.3461) | Higher for patients without hepatic disease |
| Metabolic and anti-inflammatory therapy | 0.997 | (0.7627–1.3009) | |
| Cancer | 1.191 | (0.6540–2.1133) | Higher for patients with cancer |
| Abnormal kidney function | 0.626 | (0.2263–1.4962) | Higher for patients without abnormal kidney function |
| Hyperthyroidism | 1.347 | (0.5234–3.2881) | Higher for patients with hyperthyroidism |
| Pulmonary embolism | 3.014 | (0.4979–22.9857) | Higher for patients with pulmonary embolism |
| TIA | 1.337 | (0.5760–2.9764) | Higher for patients with TIA |
| Cardioversion | 1.230 | (0.8637–1.7390) | Higher for patients with cardioversion |
| Presence of complex aortic plaque | |||
| No | 1.0 | – | Higher for patients with presence of complex aortic plaque |
| Yes | 1.817 | (0.6505–4.8058) | |
| Not applicable | 1.183 | (0.8406–1.6540) | |
| After AF diagnosis bleeding | 1.498 | (0.2938–6.8292) | Higher for patients with AF diagnosis after bleeding |
| Before AF diagnosis bleeding | 0.724 | (0.2998–1.5809) | Lower for patients with AF diagnosis before bleeding |
AF, atrial fibrillation; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; CNS, central nervous system; DVT, deep vein thrombosis; GP, general practitioner; LVH, left ventricular hypertrophy; MI, myocardial infarction; NSAID, non-steroidal anti-inflammatory drugs; OR, odds ratio; PAD, peripheral arterial disease; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
aFrom likelihood ratio test.
bIf not specified differently, OR are for presence vs. absence of patient characteristics.
cNot assessed for inclusion in multivariable model; for age, it was pre-specified to include the age classification (<65, 65 to <75, ≥75 years).
References
- 1.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–5. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation 2004;110:1042–6. [DOI] [PubMed] [Google Scholar]
- 3.Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J 2006;27:949–53. [DOI] [PubMed] [Google Scholar]
- 4.Peters NS, Schilling RJ, Kanagaratnam P, Markides V. Atrial fibrillation: strategies to control, combat, and cure. Lancet 2002;359:593–603. [DOI] [PubMed] [Google Scholar]
- 5.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- 6.Mandalenakis Z, Von Koch L, Eriksson H, Dellborg M, Caidahl K, Welin L et al. The risk of atrial fibrillation in the general male population: a lifetime follow-up of 50-year-old men. Europace 2015;17:1018–22. [DOI] [PubMed] [Google Scholar]
- 7.Lin HJ, Wolf PA, Kelly-Hayes M, Beiser AS, Kase CS, Benjamin EJ et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke 1996;27:1760–4. [DOI] [PubMed] [Google Scholar]
- 8.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857–67. [DOI] [PubMed] [Google Scholar]
- 9.Laupacis A, Boysen G, Connolly S, Ezekowitz M, Hart R, James K. The efficacy of aspirin in patients with atrial fibrillation. Analysis of pooled data from 3 randomized trials. The Atrial Fibrillation Investigators. Arch Intern Med 1997;157:1237–40. [PubMed] [Google Scholar]
- 10.De Caterina R, Husted S, Wallentin L, Andreotti F, Arnesen H, Bachmann F et al. Parenteral anticoagulants in heart disease: current status and perspectives (Section II). Position paper of the ESC Working Group on Thrombosis-Task Force on Anticoagulants in Heart Disease. Thromb Haemost 2013;109:769–86. [DOI] [PubMed] [Google Scholar]
- 11.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009;361:1139–51. [DOI] [PubMed] [Google Scholar]
- 12.Graham DJ, Reichman ME, Wernecke M, Zhang R, Southworth MR, Levenson M et al. Cardiovascular, bleeding, and mortality risks in elderly Medicare patients treated with dabigatran or warfarin for nonvalvular atrial fibrillation. Circulation 2015;131:157–64. [DOI] [PubMed] [Google Scholar]
- 13.Huisman MV, Lip GY, Diener HC, Dubner SJ, Halperin JL, Ma CS et al. Design and rationale of Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation: a global registry program on long-term oral antithrombotic treatment in patients with atrial fibrillation. Am Heart J 2014;167:329–34. [DOI] [PubMed] [Google Scholar]
- 14.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 2010;12:1360–420. [DOI] [PubMed] [Google Scholar]
- 15.Chiang CE, Wang KL, Lip GY. Stroke prevention in atrial fibrillation: an Asian perspective. Thromb Haemost 2014;111:789–97. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Zhang S, Li Y, Detrano RC, Chen K, Li X et al. Association of obesity and atrial fibrillation among middle-aged and elderly Chinese. Int J Obes (Lond) 2009;33:1318–25. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Z, Hu D. An epidemiological study on the prevalence of atrial fibrillation in the Chinese population of mainland China. J Epidemiol 2008;18:209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo Y, Wang H, Tian Y, Wang Y, Lip GY. Time trends of aspirin and warfarin use on stroke and bleeding events in Chinese patients with new-onset atrial fibrillation. Chest 2015;148:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]


