Abstract
Background
Neoadjuvant therapy is administered to breast cancer patients as an induction process before surgery or radiotherapy to reduce tumor size. Human epidermal growth factor receptor-2 (HER-2) negative breast cancer lacks effective standard target therapy. Bevacizumab has a controversial role in the treatment of breast cancer and we conduct a meta-analysis to evaluate the value of adding bevacizumab in neoadjuvant regimen.
Methods
Potentially eligible studies were retrieved using PubMed, EMBASE and Medline. Clinical characteristics of patients and statistical data with pathological complete response (pCR) data were collected. Then a meta-analysis model was established to investigate the correlation between administration of bevacizumab in neoadjuvant therapy and pCR rates in HER-2 negative breast cancer.
Results
Seven eligible studies and 5408 patients were yielded. The pCR rates for “breast” or “breast plus lymph node” were similar. In subgroup analysis, we emphasized on patients with triple-negative breast cancer (TNBC). In the criterion of “lesions in breast” the pooled ORs was 1.55 [1.29, 1.86], P<0.00001 and regarding to the evaluation criterion of “lesions in breast and lymph nodes”, the pooled ORs was 1.48 [1.23, 1.78], P<0.0001, in favor of bevacizumab administration.
Conclusion
According to our pooled results, we finally find that bevacizumab addition as a neoadjuvant chemotherapy component, for induction use with limited cycle to improve the pCR rates and patients may avoid long-term adverse event and long-term invalid survival improvement. Especially in subgroup analysis, pCR rates could be improved significantly and physicians could consider bevacizumab with caution. As patients could avoid the adverse event caused by long-term using of bevacizumab, long-term quality of life improvement may be achieved, especially in TNBC.
Introduction
Breast cancer can be subdivided into human epidermal growth factor receptor 2 (HER-2) positive and HER-2 negative breast cancer due to the important molecular marker HER-2, and around 10%–17% is defined as triple-negative breast cancer (TNBC) that is also negative for estrogen and progesterone receptors It confers a high risk of recurrence and mortality [1]. Neoadjuvant treatment (NT), also called primary systemic treatment, is administered to breast cancer patients as an induction process before surgery or radical radiotherapy to reduce tumor size by allowing for more women to become candidates for breast conserving therapy.
Pathological complete response (pCR) in the breast could be defined as that there is no histologic evidence of invasive tumor foci in the surgical breast specimen (ypT0ypN0/is), while pCR in the breast and axillary nodes was defined as the absence of histologic evidence regarding invasive tumor cells in the surgical breast specimen, axillary nodes identified after neoadjuvant chemotherapy (ypT0ypN0). The pCR rate after NT appears to correlate with improved survival outcome including disease-free (DFS) and overall survival (OS) in local advanced breast cancer patients, and what is more important is that it could act effectively as an indicator for operation [2]. Several recent studies and trials suggested pCR rate used as a surrogate marker for trials comparing different schedules of primary systemic therapy.
Nowadays, choice of treatment for cancer is to combine traditional chemo-radiotherapy with addition of target therapy. For breast cancer, trastuzumab [3, 4] and everolimus [5] were demonstrated to improve the clinical remission rate and survival outcomes in the long-term scale. In a phase III trial, 54.8% HER-2 positive patients receiving trastuzumab plus chemotherapy achieved pCR, but only 19.3% HER-2 positive patients who received chemotherapy alone achieved pCR [6]. The addition of trastuzumab has almost doubled the pCR rates in patients with HER-2 positive breast cancer [6–8].
Meanwhile, bevacizumab, a monoclonal antibody aimed to target vascular endothelial growth factor receptor (VEGFR), still has a controversial role in the treatment of breast cancer. In 2008, Bevacizumab was approved by US Food and Drug Administration (FDA) to treat patients with metastatic breast cancer, which at that time was under an accelerated plan that allows for approval based on data that are not complete enough for the full approval. Later on, the approval of bevacizumab was revoked in 2011 because further studies demonstrated that there was no significant difference regarding overall survival or quality-of-life [9, 10]. Some recent studies demonstrated increased pathological complete response (pCR) rates when adding bevacizumab to the NT in patients with Her-2 negative expression, especially in triple-negative breast cancer (TNBC) type. Bevacizumab was chosen as a candidate choice to further increase the rate of pCR rates in patients with the Her2-negative subtypes.
Previous studies demonstrate that pCR rates ranged from 18% to 61% in various chemotherapy regimens or with different ER/PR expression status. Some conflicting reports still remain in these studies, although patients who received both carboplatin and bevacizumab had the highest pCR rates, the combination did not demonstrate any synergy. The objective of this study was to perform a meta-analysis of recent clinical trials to evaluate the potential value of bevacizumab in NT for patients with HER-2 negative status.
Materials and Methods
Search strategy
Pubmed, Medline and EMBASE were searched for the last time on Jun 25, 2016. The search strategy included the following keywords, which are variably combined by “breast cancer”, “neoadjuvant”, “bevacizumab”, and “pathological complete response”.
Selection criteria
Studies were considered eligible if they met all of the following inclusion criteria, (i) all the patients were local advanced breast cancer patients receiving NT, (ii) receptor expression pattern included were HER-2 negative, (iii) the research investigated the data regarding pCR rate, which is the measurement of the effect of bevacizumab schedule, and (iv) study designs were prospective randomized controlled trials or case control studies. Studies were excluded based on any of the following criteria, (i) were review articles, case report or letters, (ii) lacked key information for calculation pooled ORs from pCR, (iii) single arm studies without control, and (iv) with duplicated data regarding one population.
Data extraction
All included studies were independently reviewed by two investigators (Huang JW and Ma XL) for data extraction. If there was any discrepancy, we discussed it and further to reach a consensus. The data were independently extracted from eligible studies by two investigators (Huang JW and Ma XL). The primary data were odds ratio (OR) with 95% confidence interval (CI) of pCR after neoadjuvant chemotherapy regimen, with bevacizumab or not.
The additional data obtained from these articles included, first author, publication year, patient source (region), percentage of treatment regimen with bevacizumab, study type, TNM stage, details of neoadjuvant chemotherapy regimens, methods to determine pCR, patients number, who achieved pCR /total patients number in bevacizumab and control group respectively, pCR rates. The statistical data for OR regarding the relationship between bevacizumab administration and pCR rate were also obtained, such as patients number, who achieved pCR /total patients number in bevacizumab and control group respectively.
Statistical Methods
The pCR number/total numbers were required in our analysis. The included studies provided the remission and total number of patients in both bevacizumab and control group, and we utilized these primary data to calculate pooled ORs using methods developed by Williamson et al. (2002) [11], and Tierney et al. (2007) [12].
In analysis of pCR rates in patients, the significant outcome was defined as a P value<0.05. A pooled ORs>1 frequently indicated the administration of bevacizumab was related to a relatively better pCR rates. Therefore, we use the term “positive” to describe that bevacizumab administration in neoadjuvant regimen could predicting a better pathological complete response outcome, and “negative” for no correlation between the two neoadjuvant chemotherapy regimens. P<0.10 or I2>50% indicates that heterogeneity existing in pooled ORs result (Higgins et al., 2003) [13]. When homogeneity was fine (p≥0.10, I2≤50%), a fixed-effects model was applied to secondary analysis; otherwise, a random-effects model was chosen. In terms of publication bias, if the p value greater than 0.10, then the publication bias was accepted in the analysis.
All the earlier calculated ORs were used as measure index to describe the correlation between pCR rates with Bevacizumab adding. The calculation process for the current meta-analysis was performed using REVIEW MANAGER (version 5.0 for Windows; the Cochrane collaboration, Oxford, UK). With regard to publication bias, it was measured using the Begg’s funnel plot, which was performed by STATA 11.0 (STATA Corporation, College Station, TX).
Result
Eligible Studies
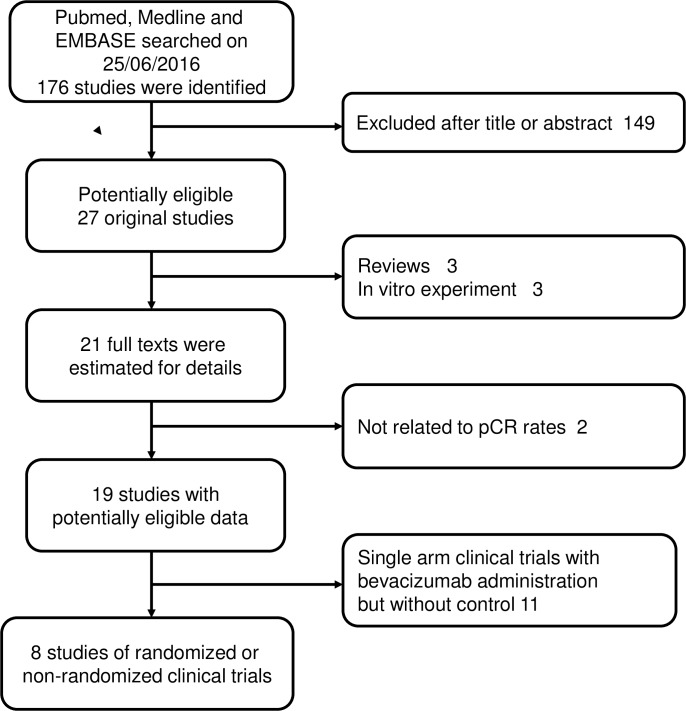
The initial search yielded 176 studies in PubMed, Medline and EMBASE. After a reviewing the abstracts, 27 potentially relevant studies were identified as eligible candidates and underwent a full-text review. Eighteen studies were excluded for the following reasons: three were reviews, eleven were single arm clinical trials without comparison statistics, two were not related to pathologic response rate and three studies were in vitro experiment (Fig 1).
Fig 1. Process of studies selection.
Finally, eight eligible published articles were included [14–20]. In addition, 11 single arm clinical trials containing bevacizumab administration without control has been included as well [16, 21–30]. These eligible control studies were published from 2009 to 2015 and included a total of 5408 patients, ranging from 36 to 1916 per study (median, 703). Five included studies involving eight sets of data related to pooled ORs for pCR rates within breast (ypT0ypN0/is) and five studies with six sets of data dealing with OR data related to pCR rates in the breast and axillary lymph node (ypT0ypN0). The basic clinical characteristics of patients and other useful information were shown in Table 1.
Table 1. Basic clinical characteristics of patients.
| Author | Diagnostic method | Date | Attitude | Study design | +/- Beva | Age | Type of breast cancer | Receptor statue | Treatment regimen | Stage | pCR define criteria | Beva group | Without beva group | pCR rate | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR | total | PCR | total | |||||||||||||
| Bahri S [15] | MRI | 2009 | Negative | Non-RCT | 16/20 | 43 | IDC and ILC | HER-2 negative | +/- Beva | Stage II-IV | Breast | 5 | 17 | 8 | 20 | 29.4 |
| Harry D. Bear [16] | Core biopsy | 2012 | Positive | RCT | 604/602 | HER-2 negative | all regimens -AC +/- Beva | cT1—T3,M0 | Breast | 204 | 591 | 168 | 595 | 34.5 | ||
| Breast & node | 167 | 584 | 134 | 591 | 26.7 | |||||||||||
| Dox | Breast | 63 | 199 | 68 | 201 | 31.6 | ||||||||||
| Dox + capecitabine | Breast | 74 | 201 | 48 | 204 | 36.8 | ||||||||||
| Dox + gemcitabine | Breast | 73 | 204 | 54 | 197 | 35.8 | ||||||||||
| TNBC | Breast | 126 | 240 | 116 | 247 | 52.5 | ||||||||||
| Breast & node | 105 | 240 | 97 | 247 | 43.8 | |||||||||||
| non-TNBC | Breast | 84 | 364 | 54 | 355 | 23.0 | ||||||||||
| Jeon Hor Chen [17] | MRI and biopsy | 2007 | Negative | Non-RCT | 26/25 | 48 | IBC | HER-2 negative | AC+Taxol or Nab-paclitaxel+Ca+/-beva | Stage II-IV | Breast | 1 | 4 | 7 | 12 | 25 |
| B. Gerber [18] | Core biopsy | 2013 | Positive | RCT | 323/340 | 48 | Primary IBC | TNBC | EC—Dox +/- Beva | Breast | 135 | 323 | 104 | 340 | 41.8 | |
| Breast & node | 127 | 323 | 94 | 340 | 39.3 | |||||||||||
| Gunter von Minckwitz [19] | Core biopsy | 2014 | Positive | RCT | 954/962 | 49 | IBC | HER-2 negative | Breast & node | 357 | 956 | 284 | 969 | 18.4 | ||
| William M. Sikov [20] | IHC | 2014 | Positive | Phase II RCT | 215/218 | 40–59 | IBC | TNBC | Taxol +/-Beva | Stage II–III | Breast | 50 | 10 5 | 42 | 107 | 47.6 |
| Breast & node | 43 | 105 | 39 | 107 | 41 | |||||||||||
| Taxol + Ca +/-Beva | Breast | 67 | 110 | 53 | 111 | 61 | ||||||||||
| Breast & node | 60 | 110 | 49 | 111 | 54.1 | |||||||||||
| Bernd Gerber [21] | Core biopsy | 2014 | Positive | RCT | 394/349 | 48 | IDC or IDLC and ILC | HER-2 negative | EC+/-Beva—Dox | cT1-T4 | Breast & node | 94 | 394 | 70 | 349 | 23.9 |
| Baljit Singh [22] | NR | 2014 | Positive | RCT | 173/187 | NR | NR | TNBC | +/- Beva | NR | Breast | 97 | 152 | 73 | 162 | 64 |
| Breast & node | 87 | 152 | 70 | 162 | 57 | |||||||||||
IHC, immunohistochemistry; RCT, randomized controlled clinical trial; IDC, invasive ductal cancer; ILC, infiltrating lobular cancer; IDLC, invasive ductal-lobular cancer; IBC, invasive breast carcinoma; TNBC, triple negative breast cancer; beva, bevacizumab; +/- beva, with or without bevacizumab; pCR, pathologic complete response; Dox, docetaxel; AC, doxorubicin + cyclophosphamide; EC, etoposide + carboplatin; Nab-paclitaxel, Abraxane-Ab + a new formulation of albumin-bound nanoparticle of paclitaxel; Ca, carboplatin; NR, not reference.
Correlation between Bevacizumab administration status and pathological complete response
Seven sets of accommodated data showed pathological complete response in patients who were scheduled to receive neoadjuvant chemotherapy plus bevacizumab or neoadjuvant chemotherapy alone. As two different definitions of pCR rates are common: one assessment rule is that no noninvasive residuals could be found in breast (ypT0ypN0/is) and another suggests that no residuals in breast and axillary lymph nodes (ypT0ypN0), we assessed both conditions respectively.
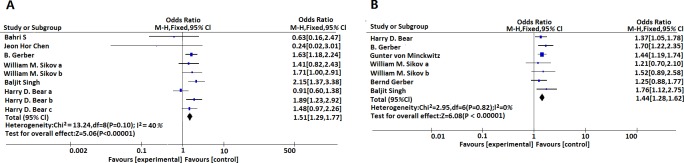
After integrating data, we found that pooled ORs to predict the pCR rates for both “breast” and “breast plus lymph node” were similar and the mathematic value for two settings were 1.51 [1.29, 1.77], (I2 = 40%, P<0.00001) and 1.44 [1.28, 1.62], (I2 = 0%, P<0.00001), respectively. (Fig 2)
Fig 2.
(A) Meta-analysis estimates the relationship between bevacizumab administration and pCR rates (defined as ypT0ypN0/is) in HER-2 negative breast cancer. (B) Meta-analysis estimates the relationship between bevacizumab administration and pCR rates (defined as ypT0ypN0) in HER-2 negative breast cancer. ypT0ypN0/is, absence of histologic evidence of invasive tumor foci in the surgical breast specimen; ypT0ypN0, absence of histologic evidence of invasive tumor cells in the surgical breast specimen and axillary nodes.
Subgroup analysis
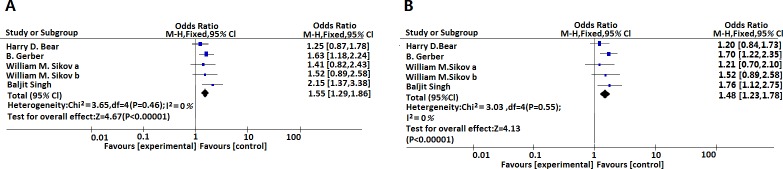
In subgroup analysis, we emphasized the patients with triple-negative breast cancer. In the group of “pCR in the breast (ypT0ypN0/is)”, the pooled ORs is 1.55 [1.29, 1.86], (I2 = 0%, P<0.00001) and in terms of the evaluation group of “pCR in the breast and axillary nodes (ypT0ypN0/is)”, the pooled ORs was 1.48 [1.23, 1.78], (I2 = 0%, P<0.00001). Both standards were effective for prediction patient pCR rates after administration of bevacizumab as a component for neoadjuvant chemotherapy (p<0.05). (Fig 3, Table 2)
Fig 3.
(A) Meta-analysis estimates the relationship between bevacizumab administration and pCR rates (defined as ypT0ypN0/is) in triple negative breast cancer. (B) Meta-analysis estimates the relationship between bevacizumab administration and pCR rates (defined as ypT0ypN0) in triple negative breast cancer. ypT0ypN0/is, absence of histologic evidence of invasive tumor foci in the surgical breast specimen; ypT0ypN0, absence of histologic evidence of invasive tumor cells in the surgical breast specimen and axillary nodes.
Table 2. pCR (95% Cl) for evaluation the use of bevacizumab in neoadjuvant treatment.
| Receptor status | pCR definition | Study N. | Model | HR (95% Cl) | P value | Heterogeneity (I2, p) | Conclusion |
|---|---|---|---|---|---|---|---|
| HER-2 negative | pCR in the breast | 9 | Fixed | 1.51 [1.29, 1.77] | <0.00001 | 40%, 0.10 | Positive |
| HER-2 negative | pCR in the breast and axillary nodes | 7 | Fixed | 1.44 [1.28, 1.62] | <0.00001 | 0%, 0.82 | Positive |
| Triple-negative | pCR in the breast | 5 | Fixed | 1.55 [1.29, 1.86] | <0.00001 | 0%, 0.46 | Positive |
| Triple-negative | pCR in the breast and axillary nodes | 5 | Fixed | 1.48 [1.23, 1.78] | <0.0001 | 0%, 0.55 | Positive |
N, number; HER-2, human epidermal growth factor receptor-2; pCR, pathological complete response; CI, confidence interval.
Results of single arm studies
Eleven studies aimed to evaluate the remission rate of one group of people with HER-2 negative breast cancers and/or triple-negative subgroup [19, 21–28]. The basic characteristics, including first author, publication year, patient source (region), percentage of treatment regimen with bevacizumab, study type, TNM stage, details of neoadjuvant chemotherapy regimens, methods to determine pCR, patients number who achieved pCR and pCR rate were shown in Table 3. The pCR rates ranged from 9%-37% in the breast (ypT0ypN0/is) and 18%-42% for breast and/or axillary lymph nodes, while the rates were 55% for breast and various from 47%-50% in the breast and/or axillary lymph nodes (ypT0ypN0). (Table 4)
Table 3. Basic patients’ characteristics regarding clinical data.
| Author | Diagnostic method | Receptor statue | Date | Age | Study design | Stage | Treatment | pCR define cretiria | Beva group | pCR rate (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| pCR | Total | ||||||||||
| Mrozek E bu [23] | Core biopsy | HER-2 negative | 2014 | 48 | Single-arm phase II trial | Stage II-III | Nab-P + Ca + beva | Breast and/or axillary lymph nodes | 6 | 33 | 18% |
| TNBC | Breast and/or axillary lymph nodes | 6 | 12 | 50% | |||||||
| Priya Rastogi [24] | Core biopsy | HER-2 negative | 2011 | 50 | Single-arm phase II trial | Stage IIIA-IIIC | ATC + Cap—beva | Breast | 4 | 45 | 9% |
| Sanchez-Rovira [25] | Core biopsy | HER-2 negative | 2013 | 46 | Single-arm phase II trial | Stage IIA-IIIC | AC-Dox + beva | Breast and/or axillary lymph nodes | 16 | 66 | 24% |
| Issam Makhoul [26] | SLNB | HER-2 negative | 2013 | 45 | Single-arm phase II trial | Stage IIA-IIIC | ATC + beva | Breast and/or axillary lymph nodes | 13 | 31 | 42% |
| TNBC | Breast and/or axillary lymph nodes | 8 | 14 | 47% | |||||||
| Clavare-zza M [27] | Core biopsy | HER-2 negative | 2013 | 48.5 | Single-arm phase II trial | Stage IIIA-IIIC | FEC + T + beva | Breast and/or axillary lymph nodes | 10 | 49 | 21% |
| TNBC | Breast and/or axillary lymph nodes | 7 | 15 | 47% | |||||||
| Jeon Hor Chen [17] | MRI-guided biopsy | HER-2 negative | 2007 | 51 | Stage II-IV | AC + beva | Breast | 1 | 4 | 25% | |
| Makhoul I [29] | HER-2 negative | 2014 | 46.5 | Single-arm phase II trial | Stage II-III | ATC + beva | Breast | 10 | 27 | 37% | |
| TNBC | Breast | 6 | 11 | 55% | |||||||
| Kim HR [30] | TNBC | 2013 | 45 | Single-arm phase II trial | Stage II-III | Dox + Ca + beva | Breast and/or axillary lymph nodes | 8 | 19 | 42% | |
| Greil R [31] | Sentinel node biopsy | HER-2 negative | 2009 | 48 | Single-center, phase II | Stage II-III | Dox + Ca + beva | Breast | 4 | 18 | 22% |
| Guarneri V [59] | Core-needle biopsy | TNBC | 2015 | 49.4 | Single-arm phase II trial | Stage II-IIIC | CaT+beva | Breast and/or axillary lymph nodes | 22 | 44 | 50% |
| Bertucci F [28] | Core biopsy | HER-2 negative | 2016 | 49 | Single-arm phase II trial | Stage II-IIIC | FEC + beva | Breast and axillary lymph nodes | 19 | 100 | 19% |
pCR, pathologic complete response; SLNB, sentinel lymph node biopsy; nab-P, nanoparticle albumin-bound paclitaxel; beva, bevacizumab; Ca, carboplatin; FEC, 5-fluorouracil, epirubicin and cyclophosphamide; AC, doxorubicin+ cyclophosphamide; ATC, doxorubicin + docetaxel + cyclophosphamide; ATC+Cap, doxorubicin + docetaxel + cyclophosphamide capecitabine; FEC+T, 5-fluorouracil, epirubicin + cyclophosphamide + Taxol; Dox, dcetaxel; CaT, carboplatin + Paclitaxel.
Table 4. pCR (95% Cl) for evaluation the response rate of adding bevacizumab in single-arm study.
| Receptor status | pCR definition | Study N. | Response rate range (%) | Mean±SD response rate (%) | |
|---|---|---|---|---|---|
| HER-2 negative | pCR in the breast | 5 | 9~37 | 23.3±11.5 | |
| HER-2 negative | pCR in the breast and axillary nodes | 4 | 18~42 | 26.3±10.8 | |
| Triple-negative | pCR in the breast | 1 | 55 | 55±0 | |
| Triple-negative | pCR in the breast and axillary nodes | 5 | 42~50 | 46.5±3.3 | |
N, number; HER-2, human epidermal growth factor receptor-2; pCR, pathological complete response; CI, confidence interval; SD, standard deviations.
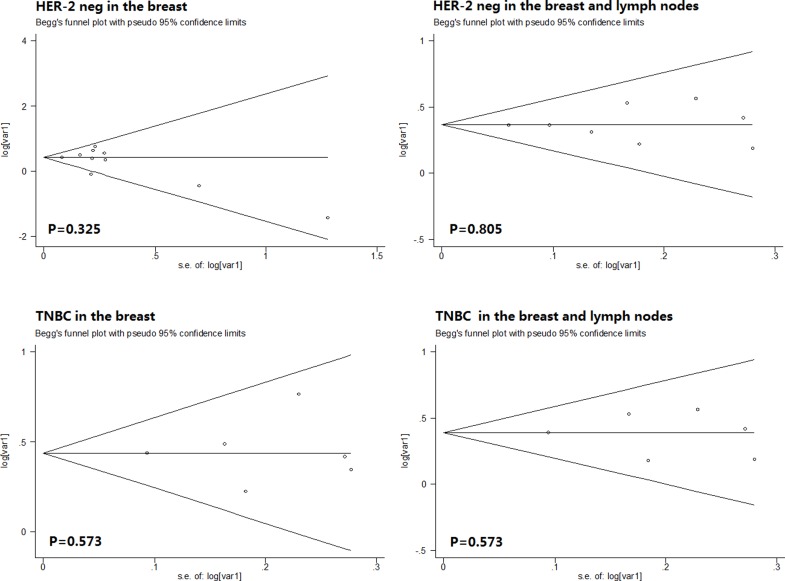
Assessment of publication bias
On the basis of Begg’s funnel plot, p value greater than 0.10 indicates that the publication bias was accepted in the analysis. According to the Begg’s funnel plot analysis, publication bias did not emerge in the pCR in the breast of HER-2 negative breast cancer cohort (0.325), pCR in the breast and axillary lymph nodes of HER-2 negative breast cancer cohort (0.805), pCR in the breast of TNBC cohort (0.573) or pCR in the breast and axillary lymph nodes of TNBC cohort (0.573). (Fig 4)
Fig 4. Estimated Begg’s funnel plots of publication bias regarding pCR in the breast of HER-2 negative breast cancer cohort,breast and axillary lymph nodes of HER-2 negative breast cancer cohort, breast of TNBC cohort, breast and axillary lymph nodes of TNBC cohort respectively.
Discussion
Whether adding bevacizumab into standard neoadjuvant chemotherapy regimen could benefit patients with HER-2 negative breast cancer is controversial, discrepant results regarding bevacizumab addition or not was reported previously. Thus, we conducted a meta-analysis to find out the effect of adding this monoclonal antibody as a target component together with basic chemotherapy. According to our study result, we approved the value of adding bevacizumab into neoadjuvant chemotherapy regimen for patients with HER-2 negative breast cancer, especially people with triple-negative breast cancer (TNBC), which is consistent with previous studies [28, 31, 32].
Marker expression could be used to direct drug administration especially for target therapy, and the utility of target therapy could significantly increase the survival outcomes [33–35] with largely improved quality of life [36, 37]. In breast cancer, HER-2 is a widely used marker for categorizing, as HER-2 positive expression statue is in favor of the usage of trastuzumab [38, 39]. Bevacizumab, a monoclonal antibody proposed to target against vascular endothelial growth factor (VEGF) A and could impair the effect of VEGFR and go against the activated genes of angiogenesis [40, 41]. HER-2 negative breast cancer, especially TNBC are highly invasive type for their high ability of proliferation and enhanced level of VEGFR to prompt angiogenesis [42, 43]. However, as bevacizumab has poor selective characteristics, the toxicity and adverse event are long been discussed. The long-time administration of bevacizumab is difficult for patients to continue, because of the common, high-grade (grade 3 or higher) toxicity and adverse events such as diarrhea, hypertension, and peripheral sensory neuropathy [44].
The dose, time and duration for bevacizumab delivery are three quite important factors influencing the treatment regimen decision. In details, the number of cycle patients received, the utility of bevacizumab in neoadjuvant or adjuvant chemotherapy, and the finish time of it would have considerable effect on the combination with basic chemotherapy and patient remission, survival outcomes. As what was shown from the pooled results, the addition of bevacizumab would improve the pCR rates. Though the CT-NeoBC pooled analysis demonstrated that patients, whoever achieved pathological complete response either in the breast (ypT0ypN0/is) or in the breast and axillary lymph nodes (ypT0ypN0) group had improved survival [45]. It has been demonstrated before that patients, regardless of benefitting from short-term pCR or not, seem to fail to show an inspiring outcomes as no significance has been found regarding the invasive disease-free survival and overall survival [46]. In one recent meta-analysis, the neoadjuvant bevacizumab delivery could improve PFS but not OS regarding to years of survival [47]. However, one previous study reported an opposite result that whether patients achieve pCR after neoadjuvant chemotherapy had a strong positive correlation with surgical rates and survival outcomes (p < 0.05) [48], which give us further clue that patients with pCR after neoadjuvant chemotherapy could increase surgical rate and may further improve the survival outcomes.
While the role of pCR rates to become an independent surrogate marker for predicting survival outcomes is controversial, previous evidence showed established advantage of neoadjuvant chemotherapy (only chemotherapy was administrated) of converting patients who were initially ineligible for breast conserving operations into candidate of this operation on both sides of shrinkage of solid tumor and decrease the incidence of positive nodes [49–51], which is thought to be the first treatment for cancer patients without distant metastasis. Thus, pCR rates after effective neoadjuvant chemotherapy, as in our analysis, the combination of chemotherapy plus bevacizumab, could provide an indication for breast-conserving surgery. In a recent report from National Cancer Database showed that patients who reached pCR, the lumpectomy rate was higher compared to patients who did not achieve pCR (41.0% vs. 26.8%, p < 0.001). In addition, Rouzier et al reported that whether patients had complete pCR after neoadjuvant chemotherapy could be an independent predict factor of loco-regional recurrence in patients who underwent breast conserving surgery or mastectomy [52].
In concordance with our result, pCR could be improved in neoadjuvant treatment regimen, which contains bevacizumab and integrating studies described above, we think that pCR is a valuable surrogate marker predicting breast conserving surgery and would further increase quality of life. Thus, based on these clinical trials, short-term results support the adjuvant bevacizumab administration while long-term results go against with the benefit for patients. Thus, for bevacizumab delivery sequence: patients could benefit a lot from neoadjuvant chemotherapy, which plays a role in only 1–2 cycles induction, and after this induction therapy, patients who had better pCR outcomes. Take sever toxicity and patients tolerance into consideration, this short cycle of induction regimen, compared with relative long cycle adjuvant chemotherapy, would be optimal schedule for patients in case of efficiency and toxicity.
In subgroup analysis, patients with TNBC showed better pCR outcomes compared with the whole population. In previous studies, in the result of [NSABP] B-40 trial, patients with TNBC receiving standard chemotherapy plus bevacizumab had significant higher pCR rates compared with no bevacizumab group (P = 0.003). Whereas, GBG44, another randomized controlled study showed no significance between patients with or without bevacizumab (P = 0.43). In accordance with previous studies, our pooled result, with a larger population, indicated that adding of bevacizumab as a component in neoadjuvant chemotherapy would benefit patient with pCR and confer them more opportunities for breast surgery. The pCR is even more important in this subgroup, as data from National Cancer Database showed a strong correlation between improved outcomes in patients with aggressive breast cancer subtypes (TNBC and Her-2 positive breast cancer) and the pCR achievement [15]. One recent meta-analysis of randomized trials regarding bevacizumab plus chemotherapy versus chemotherapy alone to treat non-metastatic breast cancer showed similar trends with ours [53].
In spite that those VEGFR blockers may enhance the severe bleeding after surgery, bevacizumab administration as a neoadjuvant component may be a high risk factor, Cortés J et al demonstrated that the surgery could be performed on patients with metastatic breast cancer who underwent bevacizumab therapy before, for the low risk of severe bleeding or wound-healing complications after the surgery [54].
In addition, as this is a meta-analysis, some limitations still exist. Primarily, only published data within those prospective or retrospective studies were included in our meta-analysis, without individual data. In addition, we combined both retrospective and the randomized trials in our meta-analysis and this could also contribute to the bias of this meta-analysis as these two type of studies may not be the same and result in data mixed bias. Also, in pooled data calculation process, we prefer multivariate data if they were available. Otherwise, our calculate data is from the univariate data without adjusting with some other influencing factors, such as age, sex, and Histologic grade. This would bring in a source of bias, for multivariate studies tests the prognostic value independently while univariate studies consider single factor.
Currently, the cost of bevacizumab is a big obstacle preventing its using. Small molecule target therapies designed for breast cancer (including TNBC), such as trastuzumab, are now approved or under clinical trials. Thus, we need further clinical trials to confirm the effectiveness and bright future of bevacizumab. If neoadjuvant regimens contain bevacizumab are really effective and improve pCR or even survival outcomes, the effect overweighs the cost and we could consider bevacizumab.
Conclusion
In spite of all the limitations and biases of our meta-analysis, we finally find that bevacizumab addition as a neoadjuvant chemotherapy component, for induction use with limited cycle to improve the pCR rates and patients may avoid long-term adverse event and long-term invalid survival improvement. Especially in subgroup analysis, pCR rates could be improved significantly and physicians could consider bevacizumab with caution. As patients could avoid the adverse event caused by long-term using of bevacizumab, long-term quality of life improvement may be achieved, especially in TNBC. Combined with current results, more clinical trials could also focus on the choice of chemotherapy, that is, the effect of bevacizumab plus which chemotherapy could improve pCR rates obviously in a relative cost-effective way.
Supporting Information
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files
Funding Statement
This study was supported by the National Natural Science Foundation of China, Beijing, China (Grant No. 81101991) and Research Award Fund for New Young Teachers in Higher Education Institutions, China (Grant No. 20120181120024). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the article.
References
- 1.Brodowicz T, Lang I, Kahan Z, Greil R, Beslija S, Stemmer SM, et al. Selecting first-line bevacizumab-containing therapy for advanced breast cancer: TURANDOT risk factor analyses. Br J Cancer. 2014;111(11):2051–7. Epub 2014/10/01. 10.1038/bjc.2014.504 bjc2014504 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boughey JC, McCall LM, Ballman KV, Mittendorf EA, Ahrendt GM, Wilke LG, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;260(4):608–14; discussion 14–6. Epub 2014/09/10. doi: 10.1097/SLA.0000000000000924 00000658-201410000-00006 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Minckwitz G, Rezai M, Fasching PA, Huober J, Tesch H, Bauerfeind I, et al. Survival after adding capecitabine and trastuzumab to neoadjuvant anthracycline-taxane-based chemotherapy for primary breast cancer (GBG 40—GeparQuattro). Ann Oncol. 2014;25(1):81–9. Epub 2013/11/26. 10.1093/annonc/mdt410 mdt410 [pii]. . [DOI] [PubMed] [Google Scholar]
- 4.Perez EA, Romond EH, Suman VJ, Jeong JH, Davidson NE, Geyer CE Jr, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29(25):3366–73. Epub 2011/07/20. 10.1200/JCO.2011.35.0868 JCO.2011.35.0868 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maass N, Harbeck N, Mundhenke C, Lerchenmuller C, Barinoff J, Luck HJ, et al. Everolimus as treatment for breast cancer patients with bone metastases only: results of the phase II RADAR study. J Cancer Res Clin Oncol. 2013;139(12):2047–56. Epub 2013/09/28. 10.1007/s00432-013-1518-x . [DOI] [PubMed] [Google Scholar]
- 6.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–84. Epub 2010/02/02. 10.1016/S0140-6736(09)61964-4 S0140-6736(09)61964-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 7.Buzdar AU, Valero V, Ibrahim NK, Francis D, Broglio KR, Theriault RL, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13(1):228–33. Epub 2007/01/04. 13/1/228 [pii] 10.1158/1078-0432.CCR-06-1345 . [DOI] [PubMed] [Google Scholar]
- 8.von Minckwitz G, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro study. J Clin Oncol. 2010;28(12):2015–23. Epub 2010/03/24. 10.1200/JCO.2009.23.8303 JCO.2009.23.8303 [pii]. . [DOI] [PubMed] [Google Scholar]
- 9.Rose S. FDA pulls approval for avastin in breast cancer. Cancer Discov. 2011;1(7):OF1-2. Epub 2012/05/16. 10.1158/2159-8290.CD-ND112311OL-08 2159-8290.CD-ND112311OL-08 [pii]. . [DOI] [PubMed] [Google Scholar]
- 10.Sharma SP. Avastin saga reveals debate over clinical trial endpoints. J Natl Cancer Inst. 2012;104(11):800–1. Epub 2012/06/08. 10.1093/jnci/djs265 djs265 [pii]. . [DOI] [PubMed] [Google Scholar]
- 11.Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta-analysis with time-to-event outcomes. Stat Med. 2002;21(22):3337–51. Epub 2002/10/31. 10.1002/sim.1303 . [DOI] [PubMed] [Google Scholar]
- 12.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16 Epub 2007/06/09. 1745-6215-8-16 [pii] 10.1186/1745-6215-8-16 ; PubMed Central PMCID: PMC1920534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. Epub 2003/09/06. 10.1136/bmj.327.7414.557 327/7414/557 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahri S, Chen JH, Mehta RS, Carpenter PM, Nie K, Kwon SY, et al. Residual breast cancer diagnosed by MRI in patients receiving neoadjuvant chemotherapy with and without bevacizumab. Ann Surg Oncol. 2009;16(6):1619–28. Epub 2009/04/01. 10.1245/s10434-009-0441-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Killelea BK, Yang VQ, Mougalian S, Horowitz NR, Pusztai L, Chagpar AB, et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the National Cancer Database. J Am Coll Surg. 2015;220(6):1063–9. Epub 2015/04/15. doi: 10.1016/j.jamcollsurg.2015.02.011 S1072-7515(15)00145-3 [pii]. . [DOI] [PubMed] [Google Scholar]
- 16.Chen JH, Feig B, Agrawal G, Yu H, Carpenter PM, Mehta RS, et al. MRI evaluation of pathologically complete response and residual tumors in breast cancer after neoadjuvant chemotherapy. Cancer. 2008;112(1):17–26. Epub 2007/11/15. 10.1002/cncr.23130 . [DOI] [PubMed] [Google Scholar]
- 17.Gerber B, Loibl S, Eidtmann H, Rezai M, Fasching PA, Tesch H, et al. Neoadjuvant bevacizumab and anthracycline-taxane-based chemotherapy in 678 triple-negative primary breast cancers; results from the geparquinto study (GBG 44). Ann Oncol. 2013;24(12):2978–84. Epub 2013/10/19. 10.1093/annonc/mdt361 mdt361 [pii]. . [DOI] [PubMed] [Google Scholar]
- 18.von Minckwitz G, Eidtmann H, Rezai M, Fasching PA, Tesch H, Eggemann H, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366(4):299–309. Epub 2012/01/27. 10.1056/NEJMoa1111065 . [DOI] [PubMed] [Google Scholar]
- 19.Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33(1):13–21. Epub 2014/08/06. 10.1200/JCO.2014.57.0572 JCO.2014.57.0572 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerber B, von Minckwitz G, Eidtmann H, Rezai M, Fasching P, Tesch H, et al. Surgical outcome after neoadjuvant chemotherapy and bevacizumab: results from the GeparQuinto study (GBG 44). Ann Surg Oncol. 2014;21(8):2517–24. Epub 2014/04/18. 10.1245/s10434-014-3606-9 . [DOI] [PubMed] [Google Scholar]
- 21.Mrozek E, Layman R, Ramaswamy B, Lustberg M, Vecchione A, Knopp MV, et al. Phase II trial of neoadjuvant weekly nanoparticle albumin-bound paclitaxel, carboplatin, and biweekly bevacizumab therapy in women with clinical stage II or III HER2-negative breast cancer. Clin Breast Cancer. 2014;14(4):228–34. Epub 2014/04/08. doi: 10.1016/j.clbc.2014.02.005 S1526-8209(14)00030-5 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rastogi P, Buyse ME, Swain SM, Jacobs SA, Robidoux A, Liepman MK, et al. Concurrent bevacizumab with a sequential regimen of doxorubicin and cyclophosphamide followed by docetaxel and capecitabine as neoadjuvant therapy for HER2- locally advanced breast cancer: a phase II trial of the NSABP Foundation Research Group. Clin Breast Cancer. 2011;11(4):228–34. Epub 2011/06/21. doi: 10.1016/j.clbc.2011.04.001 S1526-8209(11)00027-9 [pii]. . [DOI] [PubMed] [Google Scholar]
- 23.Sanchez-Rovira P, Segui MA, Llombart A, Aranda E, Anton A, Sanchez A, et al. Bevacizumab plus preoperative chemotherapy in operable HER2 negative breast cancer: biomarkers and pathologic response. Clin Transl Oncol. 2013;15(10):810–7. Epub 2013/02/12. 10.1007/s12094-013-1006-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makhoul I, Klimberg VS, Korourian S, Henry-Tillman RS, Siegel ER, Westbrook KC, et al. Combined neoadjuvant chemotherapy with bevacizumab improves pathologic complete response in patients with hormone receptor negative operable or locally advanced breast cancer. Am J Clin Oncol. 2015;38(1):74–9. Epub 2013/04/09. 10.1097/COC.0b013e31828940c3 . [DOI] [PubMed] [Google Scholar]
- 25.Clavarezza M, Turazza M, Aitini E, Saracchini S, Garrone O, Durando A, et al. Phase II open-label study of bevacizumab combined with neoadjuvant anthracycline and taxane therapy for locally advanced breast cancer. Breast. 2013;22(4):470–5. Epub 2013/05/07. doi: 10.1016/j.breast.2013.03.012 S0960-9776(13)00080-5 [pii]. . [DOI] [PubMed] [Google Scholar]
- 26.Bertucci F, Fekih M, Autret A, Petit T, Dalenc F, Levy C, et al. Bevacizumab plus neoadjuvant chemotherapy in patients with HER2-negative inflammatory breast cancer (BEVERLY-1): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2016;17(5):600–11. Epub 2016/04/02. 10.1016/S1470-2045(16)00011-5 S1470-2045(16)00011-5 [pii]. . [DOI] [PubMed] [Google Scholar]
- 27.Makhoul I, Griffin RJ, Siegel E, Lee J, Dhakal I, Raj V, et al. High-circulating Tie2 Is Associated With Pathologic Complete Response to Chemotherapy and Antiangiogenic Therapy in Breast Cancer. Am J Clin Oncol. 2016;39(3):248–54. Epub 2014/03/01. 10.1097/COC.0000000000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim HR, Jung KH, Im SA, Im YH, Kang SY, Park KH, et al. Multicentre phase II trial of bevacizumab combined with docetaxel-carboplatin for the neoadjuvant treatment of triple-negative breast cancer (KCSG BR-0905). Ann Oncol. 2013;24(6):1485–90. Epub 2013/02/06. 10.1093/annonc/mds658 mds658 [pii]. . [DOI] [PubMed] [Google Scholar]
- 29.Greil R, Moik M, Reitsamer R, Ressler S, Stoll M, Namberger K, et al. Neoadjuvant bevacizumab, docetaxel and capecitabine combination therapy for HER2/neu-negative invasive breast cancer: Efficacy and safety in a phase II pilot study. Eur J Surg Oncol. 2009;35(10):1048–54. Epub 2009/03/03. doi: 10.1016/j.ejso.2009.01.014 S0748-7983(09)00033-X [pii]. . [DOI] [PubMed] [Google Scholar]
- 30.Guarneri V, Dieci MV, Bisagni G, Boni C, Cagossi K, Puglisi F, et al. Preoperative carboplatin-paclitaxel-bevacizumab in triple-negative breast cancer: final results of the phase II Ca.Pa.Be study. Ann Surg Oncol. 2015;22(9):2881–7. Epub 2015/01/13. 10.1245/s10434-015-4371-0 . [DOI] [PubMed] [Google Scholar]
- 31.Schneider BP, Gray RJ, Radovich M, Shen F, Vance G, Li L, et al. Prognostic and predictive value of tumor vascular endothelial growth factor gene amplification in metastatic breast cancer treated with paclitaxel with and without bevacizumab; results from ECOG 2100 trial. Clin Cancer Res. 2013;19(5):1281–9. Epub 2013/01/24. 10.1158/1078-0432.CCR-12-3029 1078-0432.CCR-12-3029 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montagna E, Cancello G, Bagnardi V, Pastrello D, Dellapasqua S, Perri G, et al. Metronomic chemotherapy combined with bevacizumab and erlotinib in patients with metastatic HER2-negative breast cancer: clinical and biological activity. Clin Breast Cancer. 2012;12(3):207–14. Epub 2012/04/24. doi: 10.1016/j.clbc.2012.03.008 S1526-8209(12)00084-5 [pii]. . [DOI] [PubMed] [Google Scholar]
- 33.Gianni L, Eiermann W, Semiglazov V, Lluch A, Tjulandin S, Zambetti M, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15(6):640–7. Epub 2014/03/25. doi: 10.1016/S1470-2045(14)70080-4 S1470-2045(14)70080-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 34.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–8. Epub 2010/06/25. doi: 10.1056/NEJMoa0909530 362/25/2380 [pii]. . [DOI] [PubMed] [Google Scholar]
- 35.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–46. Epub 2012/01/31. doi: 10.1016/S1470-2045(11)70393-X S1470-2045(11)70393-X [pii]. . [DOI] [PubMed] [Google Scholar]
- 36.Oizumi S, Kobayashi K, Inoue A, Maemondo M, Sugawara S, Yoshizawa H, et al. Quality of life with gefitinib in patients with EGFR-mutated non-small cell lung cancer: quality of life analysis of North East Japan Study Group 002 Trial. Oncologist. 2012;17(6):863–70. Epub 2012/05/15. 10.1634/theoncologist.2011-0426 theoncologist.2011-0426 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thongprasert S, Duffield E, Saijo N, Wu YL, Yang JC, Chu DT, et al. Health-related quality-of-life in a randomized phase III first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients from Asia with advanced NSCLC (IPASS). J Thorac Oncol. 2011;6(11):1872–80. Epub 2011/10/21. doi: 10.1097/JTO.0b013e31822adaf7 S1556-0864(15)32250-4 [pii]. . [DOI] [PubMed] [Google Scholar]
- 38.Hurvitz SA, Dirix L, Kocsis J, Bianchi GV, Lu J, Vinholes J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol. 2013;31(9):1157–63. Epub 2013/02/06. 10.1200/JCO.2012.44.9694 JCO.2012.44.9694 [pii]. . [DOI] [PubMed] [Google Scholar]
- 39.Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–52. Epub 2014/10/22. doi: 10.1200/JCO.2014.55.5730 JCO.2014.55.5730 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cobleigh MA, Langmuir VK, Sledge GW, Miller KD, Haney L, Novotny WF, et al. A phase I/II dose-escalation trial of bevacizumab in previously treated metastatic breast cancer. Semin Oncol. 2003;30(5 Suppl 16):117–24. Epub 2003/11/13. S0093775403004469 [pii]. . [DOI] [PubMed] [Google Scholar]
- 41.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3(5):391–400. Epub 2004/05/12. 10.1038/nrd1381 nrd1381 [pii]. . [DOI] [PubMed] [Google Scholar]
- 42.Greenberg S, Rugo HS. Triple-negative breast cancer: role of antiangiogenic agents. Cancer J. 2010;16(1):33–8. Epub 2010/02/19. 10.1097/PPO.0b013e3181d38514 . [DOI] [PubMed] [Google Scholar]
- 43.Nalwoga H, Arnes JB, Stefansson IM, Wabinga H, Foulkes WD, Akslen LA. Vascular proliferation is increased in basal-like breast cancer. Breast Cancer Res Treat. 2011;130(3):1063–71. Epub 2011/08/30. 10.1007/s10549-011-1740-7 . [DOI] [PubMed] [Google Scholar]
- 44.Martin M, Roche H, Pinter T, Crown J, Kennedy MJ, Provencher L, et al. Motesanib, or open-label bevacizumab, in combination with paclitaxel, as first-line treatment for HER2-negative locally recurrent or metastatic breast cancer: a phase 2, randomised, double-blind, placebo-controlled study. Lancet Oncol. 2011;12(4):369–76. Epub 2011/03/25. 10.1016/S1470-2045(11)70037-7 S1470-2045(11)70037-7 [pii]. . [DOI] [PubMed] [Google Scholar]
- 45.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. Epub 2014/02/18. 10.1016/S0140-6736(13)62422-8 S0140-6736(13)62422-8 [pii]. . [DOI] [PubMed] [Google Scholar]
- 46.von Minckwitz G, Loibl S, Untch M, Eidtmann H, Rezai M, Fasching PA, et al. Survival after neoadjuvant chemotherapy with or without bevacizumab or everolimus for HER2-negative primary breast cancer (GBG 44-GeparQuinto)dagger. Ann Oncol. 2014;25(12):2363–72. Epub 2014/09/17. 10.1093/annonc/mdu455 mdu455 [pii]. . [DOI] [PubMed] [Google Scholar]
- 47.Chen XS, Yuan Y, Garfield DH, Wu JY, Huang O, Shen KW. Both carboplatin and bevacizumab improve pathological complete remission rate in neoadjuvant treatment of triple negative breast cancer: a meta-analysis. PLoS One. 2014;9(9):e108405 Epub 2014/09/24. 10.1371/journal.pone.0108405 PONE-D-14-15993 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luangdilok S, Samarnthai N, Korphaisarn K. Association between Pathological Complete Response and Outcome Following Neoadjuvant Chemotherapy in Locally Advanced Breast Cancer Patients. J Breast Cancer. 2014;17(4):376–85. Epub 2014/12/31. 10.4048/jbc.2014.17.4.376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15(7):2483–93. Epub 1997/07/01. . [DOI] [PubMed] [Google Scholar]
- 50.Truong PT, Woodward WA, Thames HD, Ragaz J, Olivotto IA, Buchholz TA. The ratio of positive to excised nodes identifies high-risk subsets and reduces inter-institutional differences in locoregional recurrence risk estimates in breast cancer patients with 1–3 positive nodes: an analysis of prospective data from British Columbia and the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 2007;68(1):59–65. Epub 2007/02/27. S0360-3016(06)03631-5 [pii] 10.1016/j.ijrobp.2006.12.017 . [DOI] [PubMed] [Google Scholar]
- 51.Vergine M, Scipioni P, Garritano S, Colangelo M, Di Paolo A, Livadoti G, et al. Breast-conserving surgery after neoadjuvant chemotherapy in patients with locally advanced cancer. Preliminary results. G Chir. 2013;34(9–10):254–6. Epub 2014/03/19. 6060 [pii]. [PMC free article] [PubMed] [Google Scholar]
- 52.Rouzier R, Mathieu MC, Sideris L, Youmsi E, Rajan R, Garbay JR, et al. Breast-conserving surgery after neoadjuvant anthracycline-based chemotherapy for large breast tumors. Cancer. 2004;101(5):918–25. Epub 2004/08/27. 10.1002/cncr.20491 . [DOI] [PubMed] [Google Scholar]
- 53.Cao L, Yao GY, Liu MF, Chen LJ, Hu XL, Ye CS. Neoadjuvant Bevacizumab plus Chemotherapy versus Chemotherapy Alone to Treat Non-Metastatic Breast Cancer: A Meta-Analysis of Randomised Controlled Trials. PLoS One. 2015;10(12):e0145442 Epub 2015/12/31. 10.1371/journal.pone.0145442 PONE-D-15-39485 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cortes J, Caralt M, Delaloge S, Cortes-Funes H, Pierga JY, Pritchard KI, et al. Safety of bevacizumab in metastatic breast cancer patients undergoing surgery. Eur J Cancer. 2012;48(4):475–81. Epub 2011/12/27. doi: 10.1016/j.ejca.2011.11.021 S0959-8049(11)00940-3 [pii]. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files