Abstract
Reproductive tract abnormalities and male infertility have higher incidence in autosomal dominant polycystic kidney disease (ADPKD) patients than in general population. In this work, we revealed that Pkd1, whose mutations account for 85% of ADPKD cases, is essential for male reproductive tract development. Disruption of Pkd1 caused a spectrum of defects in the murine male reproductive tract. The earliest visible defect in Pkd1-/- reproductive tract was cystic dilation of the efferent ducts, which are derivatives of the mesonephric tubules. Epididymis development was delayed or arrested in the Pkd1-/- mice. No sign of epididymal coiling was seen in the Pkd1 null mice. Disruption of Pkd1 in epithelia alone using the Pax2-cre mice was sufficient to cause efferent duct dilation and coiling defect in the epididymis, suggesting that Pkd1 is critical for epithelial development and maintenance in male reproductive tract. In-depth analysis showed that Pkd1 is required to maintain tubulin cytoskeleton and important for Tgf-β/Bmp signal transduction in the epithelia of male reproductive tract. Altogether, our results provide the first direct evidence for developmental roles of Pkd1 in male reproductive tract and provide new insights in reproductive tract abnormalities and infertility in ADPKD patients.
Keywords: Polycystin-1, polycystic kidney disease, epididymis, efferent duct, mice
Introduction
The male reproductive tract is a ductal system consisting of a set of sex accessory tissues, including the efferent ducts, epididymis, and vas deferens. These sex accessory tissues are primarily derived from the Wolffian duct, which is an epithelial tube formed bilaterally from the intermediate mesoderm via mesenchyme to epithelium transformation during early organogenesis (E8.5 to E9.5 in mice) (Hannema and Hughes, 2007; Staack et al., 2003; Wilhelm and Koopman, 2006). The intermediate mesoderm also forms a urogenital ridge, from which three sets of tubular nephric structures develop during embryonic development. The pronephros is the first of the three embryonic kidneys to be established. The pronephros degenerates shortly after being formed, while the mesonephros and metanephros are formed at the intermediate and caudal portions of the intermediate mesoderm (Hannema and Hughes, 2007; Joseph et al., 2009). At E10.5, an epithelial bud outgrows from the caudal end of the Wolffian duct, which is the ureteric bud, and later invades into the metanephric mesenchyme to form the renal collecting duct system through continuous branching morphogenesis (Sakurai, 2003; Staack et al., 2003). The extra-renal segment of the ureteric bud, together with its associated mesenchyme, forms the ureter. The Wolffian duct also connects with the newly formed tubules in the mesonephros (Hannema and Hughes, 2007). Meanwhile, another pair of epithelial structures, the Müllerian ducts, are being formed parallel to the Wolffian ducts by invagination of the coelomic epithelium (Klattig and Englert, 2007; Orvis and Behringer, 2007). During gender specification, the Wolffian ducts and mesonephric tubules degenerate in females, and the Müllerian ducts form the sex accessory tissues including oviducts, uterus, cervix and vagina (Hannema and Hughes, 2007; Klattig and Englert, 2007).
In males, however, the Müllerian ducts degenerate (Klattig and Englert, 2007; Wilhelm and Koopman, 2006). The mesonephric tubules, which attach to the Wolffian duct, form the efferent ducts via a remodeling process connecting the testis with the epididymis. At E15, as the testes descend, the Wolffian duct elongates and twists into two major segments, the segment for epididymis and the segment for vas deferens. The epididymal primordium is further specified into caput, corpus, and cauda segments (Cornwall, 2009; Joseph et al., 2009). The epithelial duct of the caput epididymis starts to coil during E15.5 to E16.5, controlled by testicular testosterone signaling that is relayed by Tgf-β signaling in the epididymis (Tomaszewski et al., 2007). Later, coiling also occurs in other segments of the epididymis, and finally a spatially complex 3-dimensional epithelial duct system is formed (Cornwall, 2009; Joseph et al., 2009). At the caudal end of each Wolffian duct, the seminal vesicle will be formed via epithelial outgrowth during postnatal development.
Sperm are generated in the testes and mature while migrating through the tortuous and lengthy reproductive tract. Necrospermia and reproductive tract cysts have been detected in a significant number of patients with autosomal dominant polycystic kidney disease (ADPKD) and infertility in male ADPKD patients is not uncommon (Belet et al., 2002; Kanagarajah et al., 2011; Manno et al., 2005; Torra et al., 2008; Vora et al., 2008), implying that the ADPKD genes, Pkd1 and Pkd2, might be involved in reproductive system development and homeostasis. ADPKD is one of the most common inherited genetic diseases, affecting up to 1 in 500 people. It is characterized by progressive development of renal cysts, enlargement of kidneys, and gradual loss of renal function. Over half of the cases proceed to end-stage renal disease by the fifth and sixth decades. Mutations in the Pkd1 gene account for over 85% of the ADPKD cases, and the remaining cases are attributable to mutations in Pkd2.
The Pkd1 gene encodes polycystin1 (PC1), a large membrane-spanning glycoprotein with 4303 amino acids and an estimated molecular mass of 460-kDa. The predicted structure of polycystin1 includes a large extracellular N terminus, 11 membrane-spanning domains, and a short cystoplasmic C-terminus. The large extracellular N terminus contains two complete leucine-rich repeat motifs flanked by cysteine-rich sequences, a C-type lectin domain, 16 copies of unique Ig-like PKD domains, an acid cysteine-rich low-density lipoprotein related motif, and a region of homology with a sea urchin receptor for egg jelly (Drummond, 2011; Sandford et al., 1997; Wilson, 2001). These extracellular domains play established roles in protein-protein interactions (Drummond, 2011; Wilson, 2001). The PC1 C-terminus tail contains a G protein binding domain and a coiled-coil domain, which are thought to transduce extracellular signaling into intracellular signaling through activation of G-protein or interactions with cytoplasmic molecules at the coiled-coil domain (Delmas et al., 2002; Qian et al., 1997; Wilson, 2001; Xu et al., 2003). The function of PC1 is critical to maintain structural integrity in a variety of tissues and organs. Although a large number of studies have been conducted to investigate the functions of the ADPKD genes, it is still unclear if ADPKD genes have a developmental role for the reproductive system. In this work, we found that Pkd1 is essential for normal male reproductive tract development. In the absence of Pkd1, the efferent ducts were severely dilated and the epithelial duct of the epididymis failed to elongate and coil. In-depth analysis suggests that Pkd1 is critical for maintaining developmental signaling in male reproductive tract.
Results
Pkd1 expression in the developing male reproductive tract
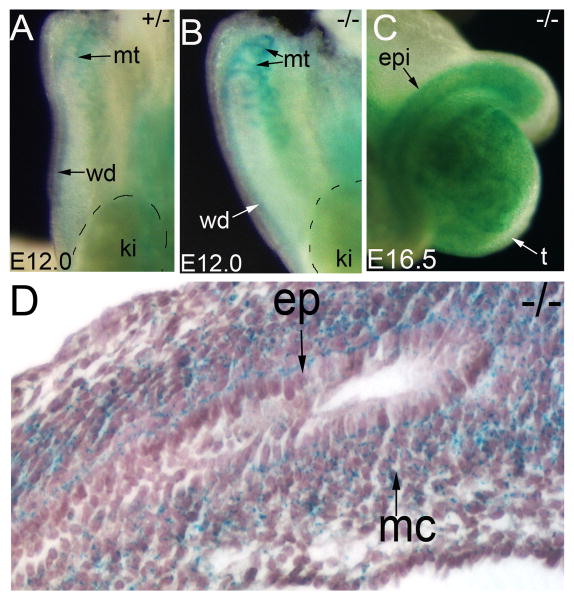
To know which tissue requires Pkd1 during reproductive tract development, we first examined Pkd1 expression by performing LacZ staining on Pkd1LacZ/+ and Pkd1LacZ/LacZ mice. Consistent with a previous study (Chauvet et al., 2002), Pkd1 expression was detected in the Wolffian duct and mesonephric tubules starting as early as E11.5 to E12.5 (Figure 1A, B). As the Pkd1LacZ/LacZ mice have two copies of LacZ genes, staining of Pkd1LacZ/LacZ tissues was much more intense than that of the Pkd1LacZ/+ mice. Later, after sex determination initiated by gonadal differentiation, Pkd1 was seen in the epididymis (Figure 1C, D). Histological examination of the stained tissue revealed that Pkd1 expression shifted from the epithelium to the mesenchyme in the epididymis as development proceeded (Figure 1D). The spatiotemporal dynamics of Pkd1 expression suggests that it regulates both epithelium and mesenchyme development in the developing epididymis.
Figure 1. Constitutive Pkd1 expression in male reproductive tract.
(A, B) LacZ staining of the urogenital primordia at E12.0, showing Pkd1 expression in the mesonephric tubules and the Wolffian duct. (C) Whole mount LacZ staining of Pkd1-/- epididymis at E16.5 (dorsal view). (D) Sectional view of the LacZ stained Pkd1-/- epididymis at E16.5. ep: epithelium; epi: epididymis; ki: kidney; mc:mesenchyme; mt; mesonephric tubules; wd: Wolffian duct;
Cystic dilation of efferent ducts in Pkd1-/- mice
A majority of Pkd1-/- mice died at E15.5 to E16.5 with multiple system defects, including hydrops fetalis and cysts in the kidneys and pancreas, similar to other Pkd1 knockout mouse models (Guay-Woodford, 2003; Wilson, 2008). We found that the reproductive system in heterozygous mice was comparable to that of wild type mice, but the Pkd1-/- mice displayed severe defects in the male reproductive tract.
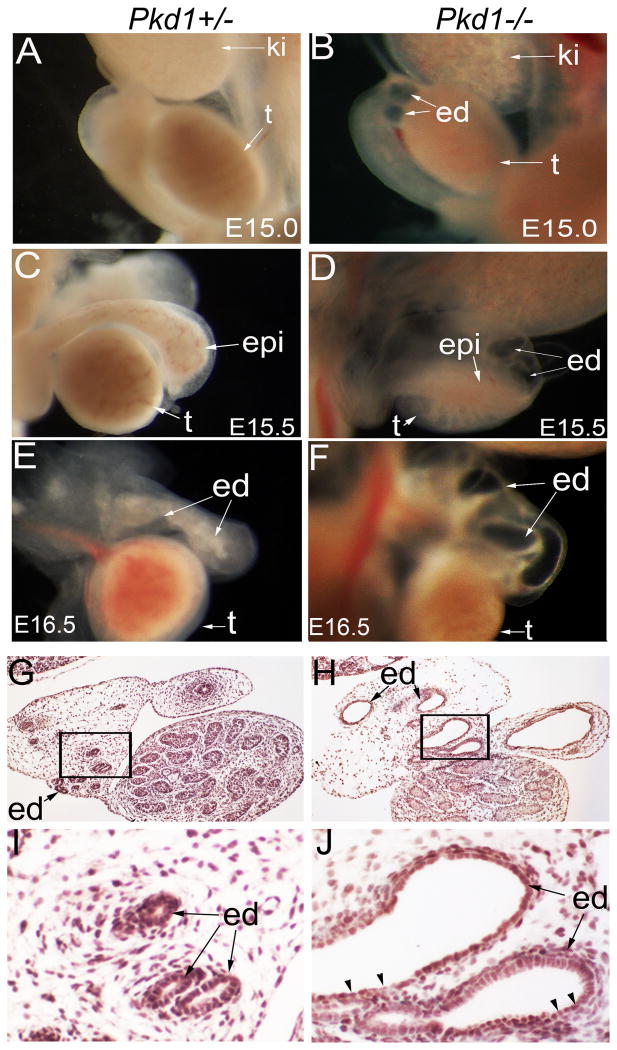
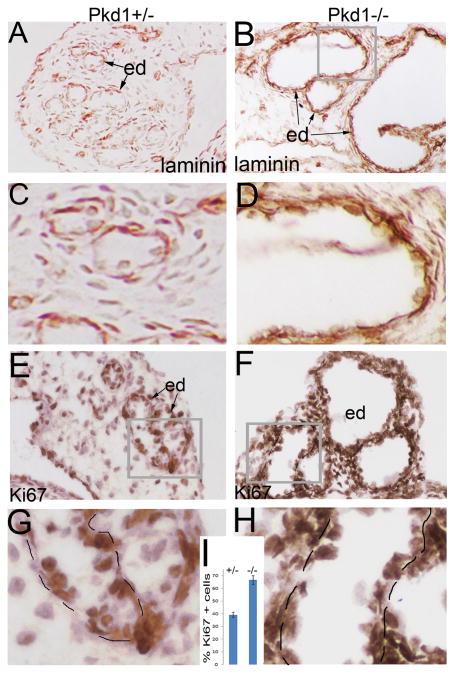
From E15.0, we detected cystic dilation of the efferent ducts in the Pkd1-/- mice (Figure 2A, B), and at E15.5 and E16.5, efferent duct dilation became severe (Figure 2C, D; E, F). At E16.5, transformation from cuboidal cells to flat cells was seen in the cystic epithelium (Figure 2G-J). The basement membrane, formed and maintained by both overlying epithelial cells and underlying mesenchymal cells, is an important structure to maintain epithelial integrity. Alterations in basement membrane have been detected in ADPKD patients and in mouse models of polycystic kidney diseases (Calvet, 1993; Grantham et al., 2011). Further, mice with a mutation in laminin alpha5 develop polycystic kidneys (Shannon et al., 2006). We thus examined basement membrane development by performing laminin staining. Results showed that the basement membrane was thickened and laminated in dilated efferent ducts of the Pkd1-/- mice (Figure 3A, B; C, D). We further performed apoptosis and proliferation assays by TUNEL and Ki67 staining at the stage of E16.5. Apoptosis was undetectable in the efferent ducts in both mutant and control mice (data not shown). However, proliferation rate was significantly increased in the mutant efferent ducts compared to the control (Figure 3E-I). Altogether, these alterations suggest a phenotypic change in efferent duct epithelium of the Pkd1-/- mice.
Figure 2. Efferent duct dilation in the Pkd1-/- mice.
(A, B) Male reproductive system at E15.0. Note efferent duct dilation in the Pkd1-/- mutant before testes fully descended. (C, D) Male reproductive system at E15.5. (E, F) Male reproductive system at E16.5, showing severe dilation of mutant efferent ducts. (G, H) Histology of the male reproductive system at E16.5, showing dilated efferent ducts in the Pkd1-/- mutant. (I, J) High magnification view of the framed areas in (G, H), arrow heads indicate flat epithelial cells. ed: efferent duct; epi: epididymis; ki: kidney; t: testis; wd: Wolffian duct
Figure 3. Laminin and Ki67 staining of the efferent ducts.
(A, B) Laminin staining of the efferent ducts at E16.5. (C, D) High magnification view of the framed areas in (A, B). (E, F) Ki67 staining of the efferent ducts. (G, H) High magnification view of the framed areas in (E, F). (I) Histograms showing proliferation rates in the efferent duct epithelia. ed: efferent duct
Epididymis defects in Pkd1-/- mice
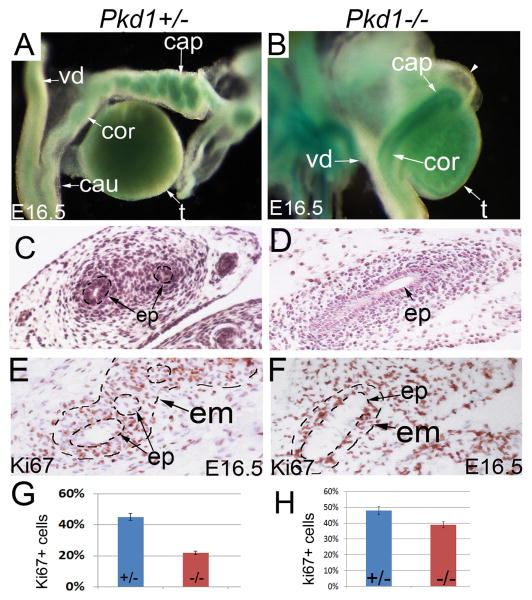
The epididymis is a derivative of the Wolffian duct, and shows high expression of Pkd1 (Chauvet et al., 2002). Normal epididymis development is characterized by continuous elongation and coiling. Coiling was evident at E16.5 in the control mice, initiating from the caput epididymis (Figure 4A, C). By contrast, there was no sign of coiling in the Pkd1-/- epididymis at this stage; a straight epithelial tube was seen in the mutant epididymis (Figure 4B, D). Ki67 staining showed that proliferation rate was significantly decreased in the epithelium of mutant epididymis in comparison to the control at E16.5 (Figure 4E, F, G). Moreover, the adjacent mesenchyme was also poorly formed in the mutants, and showed a significant decrease in cell proliferation (Figure 4D, F, H). We failed to detect abnormal apoptosis in the mutant epididymis (data not shown). Altogether, these results indicate that both epithelium and mesenchyme were defective in the Pkd1-/- epididymis.
Figure 4. Epididymis defects in Pkd1-/- mice.
(A, B) LacZ staining of E16.5 male reproductive system. Dorsal view showing coiling defect of epithelial duct in the mutant epididymis. (C, D) Histology of E16.5 epididymides. (E, F) Ki67 staining of the epididymis at E16.5. (G) Histograms showing proliferation rates in Pkd1+/- and Pkd1-/- epididymis. cap: caput epididymis; cau: cauda epididymis; cor: corpus epididymis; ed: efferent duct; em: epididymal mesenchyme; ep; epithelium; t: testis; vd: vas deferens
Epithelial-specific disruption of Pkd1 causes efferent duct dilation similar to that of the Pkd1-/- mice
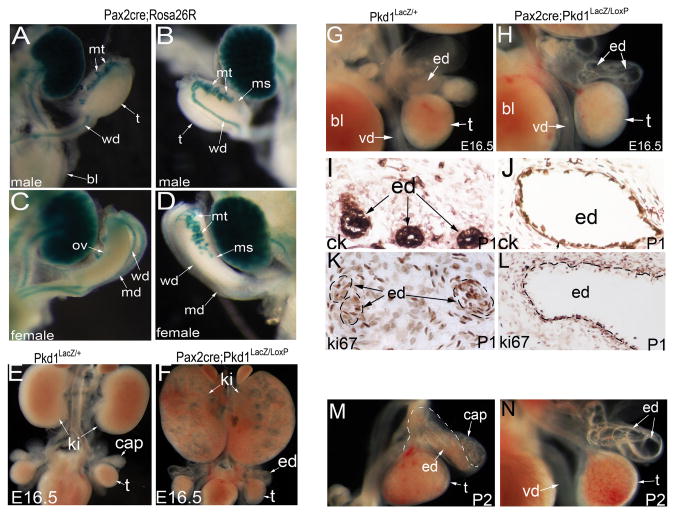
Since Pkd1 expression was seen in both mesenchyme and epithelium, we sought to dissect the roles of Pkd1 in these different tissues by using a conditional knockout approach. We used an epithelial-specific Cre strain for the reproductive tract, the Pax2-cre mice (Ohyama and Groves, 2004). We first confirmed that expression of the Pax2-cre transgene in the reproductive tract is exclusively in the epithelium by performing LacZ staining of the reproductive system of the Pax2-cre; Rosa26R mice. Consistent with previous publications (Airik et al., 2006; Ohyama and Groves, 2004), Pax2-cre specifically targeted the epithelium of the mesonephric tubules, the Wolffian ducts, and the Müllerian ducts, but was absent from the testes and the mesenchyme in the reproductive tract of both male and female mice (Figure 5A-D).
Figure 5. Efferent duct defects in Pax2-cre;Pkd1LacZ/LoxP mice.
(A, B) LacZ staining of male reproductive system in Pax2-cre;Rosa26R mice at E13.5: ventral view(A) and dorsal view (B). (C, D) LacZ staining of female reproductive system in Pax2-cre;Rosa26R mice at E13.5: ventral view (C) and dorsal view (D). (E, F) Urogenital organs in Pkd1LacZ/+ control and Pax2-cre;Pkd1LacZ/LoxP mutant mice at E16.5. (G, H) Ventral view of the genital organs at E16.5, showing dilation of the efferent ducts in the mutant. (I, J) Pan-cytokeratin staining of the efferent ducts at P1. (K, L) Ki67 staining of the efferent ducts at P1. (M, N) Ventral view of the genital organs at P2, showing severe dilation of the efferent ducts in the mutant. bl: bladder; cap: caput epididymis; ck: pan-cytokeratin; ed: efferent duct; ki: kidney; md: Müllerian duct; ms: mesonephros; mt: mesonephric tubules; ov: ovary; t: testis; vd: vas deferens; wd: Wolffian duct
We then crossed Pax2-cre;Pkd1LacZ/+ mice with Pkd1LoxP/LoxP mice to obtain Pax2-cre;Pkd1LacZ/LoxP mutant mice, which contain a Pkd1 germline mutation in one copy and a pair of LoxP sites in the other copy that would enable Pkd1 deletion by Cre expression at Pax2 domains. The Pax2-cre;Pkd1LacZ/LoxP mutant mice died in the first few days after birth. No mutant mice survived beyond 3 days post-partum (dpp). Kidney cystic dilation was detected at E15.5 (data not shown), and was severe at E16.5 (Figure 5E, F). Efferent duct dilation in the Pax2-cre;Pkd1LacZ/LoxP mice was first detected at E15.5 and became obvious from E16.5 (Figure 5E, F; G, H), similar to that seen in the Pkd1-/- mice. This result suggests that epithelial Pkd1 plays a critical role to maintain structural integrity in the efferent ducts. At postnatal stages, efferent duct dilation became very severe and most of the efferent ducts were dilated (Figure 5I-N). The cells of the cystic epithelium were mostly transformed to flat cells as demonstrated by pan-cytokeratin staining (Figure 5I, J). Some epithelial cells were also detached, losing cell-cell adhesion (Figure 5I, J). However, cell proliferation rate in the epithelium of the dilated efferent ducts was comparable to the control at this stage, suggesting cell proliferation slows after significant dilation (Figure 5K, L). Up to the last stage of mutant mouse survival, there was no dilation in the vas deferens (Figure 5M, N).
Epithelial-specific disruption of Pkd1 is sufficient to cause epididymis coiling defect
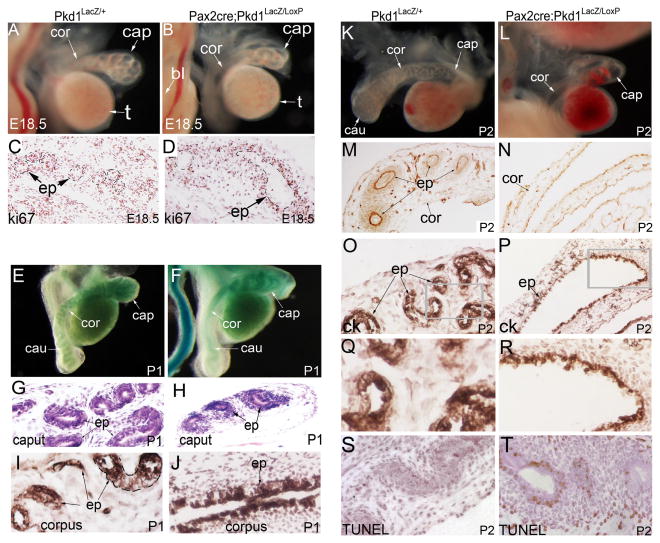
Coiling defect was also present in the epididymis of the Pax2-cre;Pkd1LacZ/LoxP mice. At E16.5, there was no coiling in the Pax2-cre;Pkd1LacZ/LoxP epididymis, similar to that seen in the Pkd1-/- mice (data not shown). Up to E18.5, only a few rounds of coiling events were seen in the mutant caput epididymis; by contrast, in the control, a highly coiled epithelial duct was already present in the caput epididymis (Figure 6A, B). Ki67 staining showed that while proliferation in the mesenchyme was still present, cell proliferation ceased in the mutant epithelium, suggesting epithelial growth arrest at this stage (Figure 6C, D). At postnatal stages, coiling was apparently arrested after initiation in the mutant mice, and was absent in the corpus and cauda regions of the epididymis, whereas a highly coiled duct was seen in all segments of the epididymis in the control (Figure 6E-L). Histologic examination of the epididymis showed that highly condensed mesenchyme was present around the mutant epithelium at P1 (Figure 6G, H), suggesting that the mesenchyme was not significantly affected by epithelial disruption of Pkd1 at this stage. Of note, mesenchyme of the mutant epididymis was more condensed than that of the heterozygous and wildtype controls (Figure 6G, H). This was likely due to lack of epithelial elongation and coiling in the mutant epididymis. The mutant corpus epididymis was often dilated at postnatal stages (Figure 6K, L). Laminin staining suggested that basement membrane was still present in the mutant epididymis (Figure 6M, N). However, pan-cytokeratin staining demonstrated that cell shape changed in the mutant epithelium (Figure 6O, P; Q, R). Epithelial changes were seen before dilation was evident (Figure 6I, J). At P2, we also detected abnormal apoptosis in the mutant epididymis (Figure 6S, T). Altogether, the results showed that coiling was initially delayed in the mutant epididymis and was eventually arrested in the absence of epithelial Pkd1.
Figure 6. Epididymis defects in Pax2-cre;Pkd1LacZ/Loxp mice.
(A, B) Dorsal view of E18.5 male reproductive system showing dilation and coiling defect in the mutant epididymis. (C, D) Ki67 staining of E18.5 epididymis. Note low proliferation in the mutant epithelium. (E, F) LacZ staining of male genital system at P1. Dorsal view revealed coiling defect in the mutant epididymis. (G, H) Histology of the caput epididymis at P1. (I, J) Pan-cytokeratin staining of the corpus epididymis at P1. (K, L) Dorsal view of the epididymis at P2, showing coiling defect and dilation in the mutant epididymis. (M, N) Laminin staining of corpus epididymis at P2. (O, P) Pan-cytokeratin staining of the corpus epididymis at P2. (Q, R) Magnification view of the framed areas in (O and P). Note abnormal epithelium in mutant. (S, T) TUNEL staining of epididymis at P2. bl: bladder; cap: caput epididymis; cau: cauda epididymis; ck: pan-cytokeratin; cor: corpus epididymis; ed: efferent duct; ep: epithelium; LM: laminin
Disruption of Pkd1 induces tubulin-cytoskeleton change and affects Tgf-β/Bmp signaling in male reproductive tract
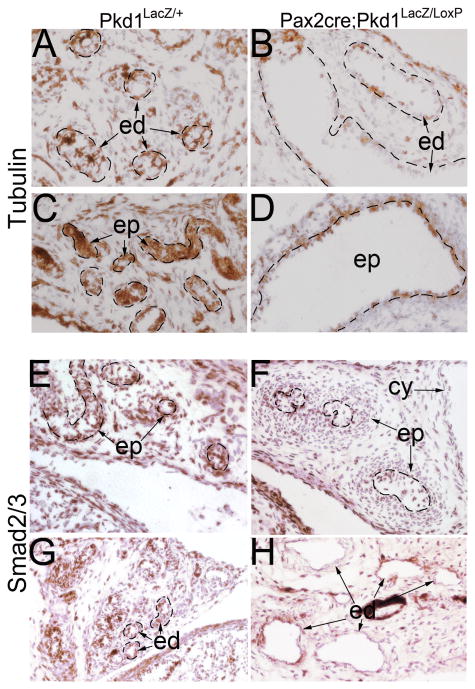
Cell shape change in the Pkd1-/- epithelium suggests Pkd1 might be involved in regulating cytoskeleton dynamics. Indeed, previous studies showed that Pkd1-encoded PC1 regulates cytoskeleton arrangement in renal epithelial cells (Boca et al., 2007; Xu et al., 2001). Here, we examined tubulin cytoskeleton in male reproductive tract of the Pax2-cre;Pkd1LacZ/LoxP mutant mice by immunostaining for α-tubulin, a subunit for microtubule assembling. Results showed that while α-tubulin expression levels were comparable between the mutant and control at most embryonic stages, expression was markedly reduced at postnatal stages in mutant epithelium of the efferent ducts and epididymis (Figure 7A-D), suggesting that tubulin cytoskeleton was affected by loss of Pkd1.
Figure 7. Reduced α-tubulin and Smad2/3 expression in male reproductive system of Pax2-cre;Pkd1LacZ/Loxp mice at P1.
(A, B) α-tubulin staining of the efferent ducts, showing reduced α-tubulin expression in the mutant epithelium. (C, D) α-tubulin staining of the epididymis, showing reduced α-tubulin expression in the mutant epithelium. (E, F) Smad2/3 staining of the epididymis. (G, H) Smad2/3 staining of the efferent ducts. cy: cystic dilation; ed: efferent duct; ep: epithelium
Given that Tgf-β/Bmp signaling pathways are critical for ductal system development and maintenance in male reproductive tract, and disrupting Tgf-β signaling abolishes coiling in the epididymis (Di Giovanni et al., 2011; Tomaszewski et al., 2007), we tested if disruption of Pkd1 affects Tgf-β/Bmp signaling in the male reproductive tract by performing Smad2/3 and phospho-Smad1/5 staining, which are the intracellular mediators of Tgf-β and Bmp signaling respectively. As has been described previously (Tomaszewski et al., 2007), Smad2/3 level was high in the epididymis epithelium, indicative of a high requirement of Tgf-β signaling by the epithelial cells (Figure 7E). However, in the Pkd1-/- epididymis epithelium, Smad2/3 level was markedly reduced beginning in late gestation (Figure 7F, and data not shown), suggesting that Tgf-β signaling was disrupted in the mutant epididymis. Similarly, the level of Smad2/3 was also reduced in the cystic lining epithelium of mutant efferent ducts (Figure 7G, H).
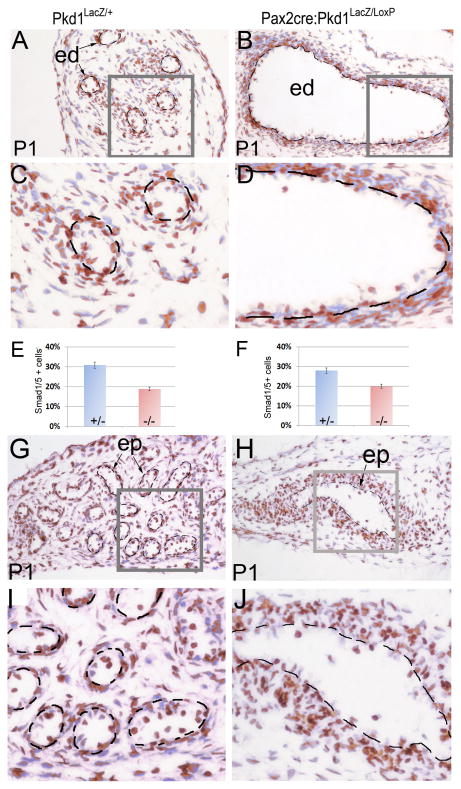
In contrast to Smad2/3, which was predominantly present in the epithelium, phospho-Smad1/5 was detected at a high level in both epithelium and mesenchyme in the efferent duct and epididymis of control mice. However, the ratio of cells positive for phospho-Smad1/5 was significantly decreased in mutant epithelium but not in mesenchyme in Pkd1 epithelial-specific knockout mice in both efferent ducts (Figure 8A-E) and epididymis (Figure 8F-J). This was more obvious in severely dilated ducts, in which positive cells were very few (Figure 8D). Altogether, these results suggest a link between PC1 and Tgf-β/Bmp signaling pathways in the male reproductive tract. Since Tgf-β/Bmp ligands are mainly secreted by local mesenchyme cells that were not affected by epithelial Pkd1 disruption, epithelial responsiveness to Tgf-β/Bmp ligands was likely changed in the mutant (Tomaszewski et al., 2007).
Figure 8. Reduced phospho-Smad1/5 in efferent ducts and epididymis of Pax2-cre;Pkd1LacZ/Loxp mice at P1.
(A, B) Phospho-Smad1/5 staining of the efferent ducts in mutant and control. (C, D) Magnification view of the framed areas in (A, B). (E) Histograms representing the ratios of phospho-Smad1/5/8 positive cells in efferent duct epithelia of mutant and control. (F) Histograms representing the ratios of phospho-Smad1/5 positive cells in the epididymal epithelia of mutant and control. (G, H) Phospho-Smad1/5 staining of the epididymis in mutant and control. (I, J) Magnification view of the framed areas in (G, H). ed: efferent duct; ep: epithelium
Pkd1-/- epididymis fails to respond to Tgf-β1 in culture
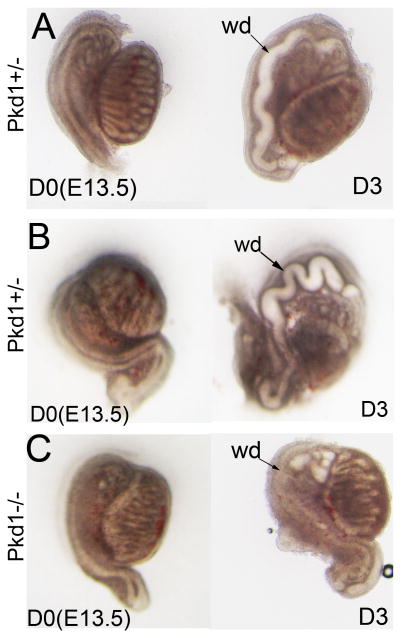
After 3 days of culture, the epididymis began to show signs of coiling in the control but not in the mutant, consistent with the in vivo observation in the Pkd1-/- mice (Figure 9A, and data not shown). Addition of Tgf-β1 enhanced epididymal coiling in cultures of control epididymis (Figure 9B), suggesting that Tgf-β1 can activate the intracellular Tgf-β signalling cascade in culture. However, Tgf-β1 failed to rescue epididymal coiling in the Pkd1-/- mice in the culture system (Figure 9C), supporting the notion that epithelial responsiveness to growth factors was defective in the mutant epididymis.
Figure 9. Male reproductive tract development in culture.
(A) Pkd1+/- reproductive system at E13.5 in a Transwell culture system. (B) Pkd1+/- reproductive system at E13.5 in culture in the presence of Tgf-β1 protein. (C) Pkd1-/- reproductive system at E13.5 in culture in the presence of Tgf-β1 protein. D0: first day in culture; D3: third day in culture; wd: Wolffian duct
Discussion
This work describes for the first time that Pkd1 is critical for normal male reproductive tract development, and provides new insights into reproductive tract abnormalities and infertility in ADPKD patients. A striking finding in male reproductive tract of Pkd1-/- mice was cystic dilation of the efferent ducts. However, initial mesonephric tubulogenesis prior to differentiation into a male reproductive phenotype was normal in the Pkd1-/- mice, suggesting that Pkd1 is not critical for early tubule morphogenesis in the mesonephros. We detected dysregulated proliferation and dedifferentiation in epithelial cells of mutant efferent ducts, which are reminiscent of the kidney cystic changes in ADPKD (Chapin and Caplan, 2010; Chapman, 2007) and indicate that a common mechanism might underlie cystogenesis of mesonephric and metanephric-derived tissues.
Tissue specific deletion of Pkd1 from epithelium alone caused efferent duct defects recapitulating those seen in global knockout mice, implying that epithelial expression of Pkd1 plays a central role in maintaining structural integrity in the efferent ducts. Normally, epithelial cells of the efferent ducts, like that of the nephric tubules, absorb significant amounts of fluid in physiological conditions. Phenotypic changes of the efferent duct epithelia might affect fluid absorption and might, as in polycystic kidney epithelium, even cause fluid secretion (Sullivan and Grantham, 1996; Sullivan et al., 1998). If so, the epithelial changes will facilitate fluid buildup and cystic dilation in the efferent duct system by defective fluid regulation.
Another major discovery of this study is that Pkd1 is required for epididymis development, which is characterized by continuous epithelial elongation and coiling producing an epithelial duct of up to 1 meter in length in mice (Hinton et al., 2011; Joseph et al., 2009). Coiling and spatial arrangement of epididymal duct are governed by a complex signaling network involving testosterone signaling and a number of developmental signaling pathways (Cornwall, 2009; Di Giovanni et al., 2011; Hsia and Cornwall, 2004; Joseph et al., 2009; Kitagaki et al., 2011; Tomaszewski et al., 2007; Xu et al., 2010). Here we showed that Pkd1 is also an essential player in this regulatory network. In the absence of Pkd1, both the epithelium and associated mesenchyme became defective, and epithelial elongation and coiling were disturbed in the epididymis. The data from the epithelial-specific knockout mice further demonstrated that Pkd1 has primary functions for epididymis development, as in this model the testes that produce testosterone and the epididymal mesenchyme that is the primary target of the testicular testosterone in embryonic epididymis were unaffected by epithelial Pkd1 deletion (Cooke et al., 1991). In this epithelial-knockout model, the epithelial duct of the epididymis not only failed to elongate and coil but also displayed cystic dilation starting from late gestation.
Multiple lines of evidence indicate that PC1 regulates cytoskeleton dynamics (Boca et al., 2007; Wilson, 2001; Wilson, 2004; Xu et al., 2001). Cell shape change in the Pkd1-/- epithelium in both efferent ducts and epididymis indicates that PC1 is involved in cytoskeleton control in these tissues. Here, we revealed that PC1 regulates dynamics of α-tubulin cytoskeleton in the epithelium of male reproductive tract. Reduced expression level of α-tubulin in the Pkd1-/- epithelia indicates a reduction of microtubules, which are essential for intracellular transport, mitosis and other cellular processes. Furthermore, microtubules are the key cytoskeleton component for the cilium, an important organelle coordinating signaling pathways during development and in tissue homeostasis. Microtubule reduction also indicates that cilia in the Pkd1-/- epithelia might also change. Our results also suggest that in the male reproductive tract PC1 activity is linked to signal pathways that control epithelial development. Indeed, deficiency of PC1 compromised Tgf-β/Bmp signal pathways, which are essential for ductal system development and epididymal coiling in male reproductive tract (Di Giovanni et al., 2011; Tomaszewski et al., 2007 ). However, molecular pathways linking PC1 activity with developmental pathways and cytoskeleton regulation remain to be determined.
We noted dramatically different effects of loss of Pkd1 function on cell proliferation in different tissues. In the epididymis, deficiency of PC1 caused a significant decrease in cell proliferation, whereas in the efferent ducts and renal epithelium the presence of PC1 appeared to restrict cell proliferation (Bhunia et al., 2002; Nishio et al., 2005). These results indicate that the effect of PC1 on cell proliferation depends on the specific developmental context. Dysregulated proliferation in male reproductive tract of the Pkd1-/- mice might be a prerequisite for cystic dilation upon mechanical stimulation from fluid flow. It is possible that PC1 serves as a negative regulator for stress-induced proliferation in a physiological setting but is essential for cell proliferation in response to developmental signaling.
Fluid flow from the testes not only produces mechanical stimulation but also carries various growth factors to the tissues of male reproductive tract, regulating epithelium development and maintenance (Cornwall, 2009; Joseph et al., 2009; Xu et al., 2011; Xu et al., 2010). The epididymal lumen opens at E13.5 to E14.5, and fluid is present from this stage (Cornwall, 2009; Joseph et al., 2009). Efferent duct dilation and fluid buildup prevent testicular fluid from flowing into the epididymal lumen and also disrupt fluid flow dynamics in the epididymis. Furthermore, fluid regulation defect (absorption/secretion) in the efferent ducts might cause severe dilution of luminal factors important for epididymis development.
In summary, our data for the first time show that Pkd1 is essential for male reproductive tract development. PC1 regulates cellular activity by linking extracellular environmental signaling, including mechanical stress and extracellular matrix signaling, to a set of critical signaling pathways. This mechanism might also exist in adult tissues. Alteration in PC1 signaling by Pkd1 loss of function or gain of function mutations might lead to dysregulation of gene expression in adult tissues, and abnormally activate or inactivate critical signaling pathways causing cystogenesis. Finally, it should be noted that the male reproductive system continues to develop and mature at postnatal stages until puberty. Genital structures, such as the seminal vesicles, ejaculatory ducts and prostate, are still to be developed even at the most advanced stage of our conditional knockout mice survival. Furthermore, maintenance of reproductive organs may require Pkd1 in adulthood. In future studies, inducible Cre systems may be helpful to test these hypotheses and to examine roles of Pkd1 in maintenance of male reproductive system.
Experimental procedures
Mice
Animal use protocol is approved by Johns Hopkins University Animal Care and Use Committee. Pkd1LacZ/+(Pkd1+/-), and Pkd1LoxP/LoxP mice were provided by the Polycystic Kidney Disease Core at Johns Hopkins University (Bhunia et al., 2002; Piontek et al., 2004). Pax2-cre and Rosa26R mice were described previously (Ohyama and Groves, 2004; Soriano, 1999). Mice were genotyped by PCR. All the mice were maintained in a mixed background.
Histology and immunohistochemistry
Tissue preparation for histology and immunohistochemistry was performed using standard procedures. Paraffin sections were used for histologic examination and immunostaining. Anti-laminin (Sigma), pan-cytokeratin (Sigma), α-tubulin (Neomarkers), Smad2/3 and phospho-Smad1/5 antibodies (Cell Signalling Technology) were used for immunodetection on sections. Secondary antibodies were peroxidase-conjugated (Sigma). DAB was used for peroxidase-mediated color reaction. Hematoxylin was used for counterstaining.
Apoptosis and Proliferation assays
Paraffin sections were used for TUNEL staining and proliferation assay. TUNEL staining for apoptosis was performed using DeadEnd Colorimetric Apoptosis Detection System (Promega). The Ki67 antibody (Millipore, AB9260) was used for proliferation assay. Serial sections at intervals of 35μm were used for comparison of proliferation rates on each genotype. Three samples were used for each genotype. The number of Ki67+ cells relative to total number of cells was calculated and used for proliferation rate.
LacZ staining
Urogenital system primordia were dissected out from mouse embryos, fixed with 4% paraformaldehyde in 1× PBS for 1 hour at 4 °C, rinsed three times in 1× PBS, and stained in X-gal reaction solution overnight at room temperature. Stained tissues were visualized in 80% glycerol or sectioned for examination.
Tissue culture
Embryonic genital organ system was dissected out and cultured at the air-medium interface on a Transwell filter system (Corning Incorporated) for 3 days (Nie et al., 2011). DMEM containing 10% FBS, 2 mM glutamine, 100 U penicillin/ml, and 0.1 mg/ml streptomycin was changed every two days. Tgf-β1 protein (Millipore, GF111) was used at a concentration of 200ng/ml.
Statistical analysis
Statistical analysis was performed using two tailed t test.
Acknowledgments
This work was supported by a generous donation from the Lillian Goldman Charitable Trust. This study utilized resources provided by the NIDDK-sponsored Johns Hopkins Polycystic Kidney Disease Research and Clinical Core Center (P30 DK090868). We are grateful to Dr Timothy H. Moran of the Department of Psychiatry and Behavioral Sciences at Johns Hopkins University School of Medicine for use of the microscope and imaging system. We thank Dr Andy Groves at the Baylor College of Medicine and Dr Angelika Doetzlhofer of the Department of Neuroscience at Johns Hopkins University School of Medicine for providing Pax2-cre mice. We thank Dr Noel Rose at the Department of Pathology at Johns Hopkins University School of Medicine for use of the gel imaging system. We also thank Humera Khan for technical assistance in this work.
Abbreviations
- ADPKD
autosomal dominant polycystic kidney disease
- Ig
immunoglobulin
- PC1
polycystin1
- PKD
polycystic kidney disease
Footnotes
Conflict of Interest Statement: Authors declare that there are no conflicts of interest.
References
- Airik R, Bussen M, Singh MK, Petry M, Kispert A. Tbx18 regulates the development of the ureteral mesenchyme. The Journal of clinical investigation. 2006;116:663–74. doi: 10.1172/JCI26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belet U, Danaci M, Sarikaya S, Odabas F, Utas C, Tokgoz B, Sezer T, Turgut T, Erdogan N, Akpolat T. Prevalence of epididymal, seminal vesicle, prostate, and testicular cysts in autosomal dominant polycystic kidney disease. Urology. 2002;60:138–41. doi: 10.1016/s0090-4295(02)01612-6. [DOI] [PubMed] [Google Scholar]
- Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu PN, Germino FJ, Germino GG. PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell. 2002;109:157–68. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- Boca M, D'Amato L, Distefano G, Polishchuk RS, Germino GG, Boletta A. Polycystin-1 induces cell migration by regulating phosphatidylinositol 3-kinase-dependent cytoskeletal rearrangements and GSK3beta-dependent cell cell mechanical adhesion. Molecular biology of the cell. 2007;18:4050–61. doi: 10.1091/mbc.E07-02-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvet JP. Polycystic kidney disease: primary extracellular matrix abnormality or defective cellular differentiation? Kidney international. 1993;43:101–8. doi: 10.1038/ki.1993.17. [DOI] [PubMed] [Google Scholar]
- Chapin HC, Caplan MJ. The cell biology of polycystic kidney disease. The Journal of cell biology. 2010;191:701–10. doi: 10.1083/jcb.201006173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman AB. Autosomal dominant polycystic kidney disease: time for a change? Journal of the American Society of Nephrology : JASN. 2007;18:1399–407. doi: 10.1681/ASN.2007020155. [DOI] [PubMed] [Google Scholar]
- Chauvet V, Qian F, Boute N, Cai Y, Phakdeekitacharoen B, Onuchic LF, Attie-Bitach T, Guicharnaud L, Devuyst O, Germino GG, Gubler MC. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. The American journal of pathology. 2002;160:973–83. doi: 10.1016/S0002-9440(10)64919-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke PS, Young P, Cunha GR. Androgen receptor expression in developing male reproductive organs. Endocrinology. 1991;128:2867–73. doi: 10.1210/endo-128-6-2867. [DOI] [PubMed] [Google Scholar]
- Cornwall GA. New insights into epididymal biology and function. Human reproduction update. 2009;15:213–27. doi: 10.1093/humupd/dmn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Nomura H, Li X, Lakkis M, Luo Y, Segal Y, Fernandez-Fernandez JM, Harris P, Frischauf AM, Brown DA, Zhou J. Constitutive activation of G-proteins by polycystin-1 is antagonized by polycystin-2. The Journal of biological chemistry. 2002;277:11276–83. doi: 10.1074/jbc.M110483200. [DOI] [PubMed] [Google Scholar]
- Di Giovanni V, Alday A, Chi L, Mishina Y, Rosenblum ND. Alk3 controls nephron number and androgen production via lineage-specific effects in intermediate mesoderm. Development. 2011;138:2717–27. doi: 10.1242/dev.059030. [DOI] [PubMed] [Google Scholar]
- Drummond IA. Polycystins, focal adhesions and extracellular matrix interactions. Biochimica et biophysica acta. 2011;1812:1322–6. doi: 10.1016/j.bbadis.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham JJ, Mulamalla S, Swenson-Fields KI. Why kidneys fail in autosomal dominant polycystic kidney disease. Nature reviews. Nephrology. 2011;7:556–66. doi: 10.1038/nrneph.2011.109. [DOI] [PubMed] [Google Scholar]
- Guay-Woodford LM. Murine models of polycystic kidney disease: molecular and therapeutic insights. American journal of physiology. Renal physiology. 2003;285:F1034–49. doi: 10.1152/ajprenal.00195.2003. [DOI] [PubMed] [Google Scholar]
- Hannema SE, Hughes IA. Regulation of Wolffian duct development. Hormone research. 2007;67:142–51. doi: 10.1159/000096644. [DOI] [PubMed] [Google Scholar]
- Hinton BT, Galdamez MM, Sutherland A, Bomgardner D, Xu B, Abdel-Fattah R, Yang L. How do you get six meters of epididymis inside a human scrotum? Journal of andrology. 2011;32:558–64. doi: 10.2164/jandrol.111.013029. [DOI] [PubMed] [Google Scholar]
- Hsia N, Cornwall GA. DNA microarray analysis of region-specific gene expression in the mouse epididymis. Biology of reproduction. 2004;70:448–57. doi: 10.1095/biolreprod.103.021493. [DOI] [PubMed] [Google Scholar]
- Joseph A, Yao H, Hinton BT. Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns. Developmental biology. 2009;325:6–14. doi: 10.1016/j.ydbio.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagarajah P, Ayyathurai R, Lynne CM. Male infertility and adult polycystic kidney disease - revisited: case report and current literature review. Andrologia. 2011 doi: 10.1111/j.1439-0272.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- Kitagaki J, Ueda Y, Chi X, Sharma N, Elder CM, Truffer E, Costantini F, Lewandoski M, Perantoni AO. FGF8 is essential for formation of the ductal system in the male reproductive tract. Development. 2011;138:5369–78. doi: 10.1242/dev.051888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klattig J, Englert C. The Mullerian duct: recent insights into its development and regression. Sexual development : genetics, molecular biology, evolution, endocrinology, embryology, and pathology of sex determination and differentiation. 2007;1:271–8. doi: 10.1159/000108929. [DOI] [PubMed] [Google Scholar]
- Manno M, Marchesan E, Tomei F, Cicutto D, Maruzzi D, Maieron A, Turco A. Polycystic kidney disease and infertility: case report and literature review. Archivio italiano di urologia, andrologia : organo ufficiale [di] Societa italiana di ecografia urologica e nefrologica / Associazione ricerche in urologia. 2005;77:25–8. [PubMed] [Google Scholar]
- Nie X, Xu J, El-Hashash A, Xu PX. Six1 regulates Grem1 expression in the metanephric mesenchyme to initiate branching morphogenesis. Developmental biology. 2011;352:141–51. doi: 10.1016/j.ydbio.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio S, Hatano M, Nagata M, Horie S, Koike T, Tokuhisa T, Mochizuki T. Pkd1 regulates immortalized proliferation of renal tubular epithelial cells through p53 induction and JNK activation. The Journal of clinical investigation. 2005;115:910–8. doi: 10.1172/JCI22850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–9. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- Orvis GD, Behringer RR. Cellular mechanisms of Mullerian duct formation in the mouse. Developmental biology. 2007;306:493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontek KB, Huso DL, Grinberg A, Liu L, Bedja D, Zhao H, Gabrielson K, Qian F, Mei C, Westphal H, Germino GG. A functional floxed allele of Pkd1 that can be conditionally inactivated in vivo. Journal of the American Society of Nephrology : JASN. 2004;15:3035–43. doi: 10.1097/01.ASN.0000144204.01352.86. [DOI] [PubMed] [Google Scholar]
- Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nature genetics. 1997;16:179–83. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- Sakurai H. Molecular mechanism of ureteric bud development. Seminars in cell & developmental biology. 2003;14:217–24. doi: 10.1016/s1084-9521(03)00024-7. [DOI] [PubMed] [Google Scholar]
- Sandford R, Sgotto B, Aparicio S, Brenner S, Vaudin M, Wilson RK, Chissoe S, Pepin K, Bateman A, Chothia C, Hughes J, Harris P. Comparative analysis of the polycystic kidney disease 1 (PKD1) gene reveals an integral membrane glycoprotein with multiple evolutionary conserved domains. Human molecular genetics. 1997;6:1483–9. doi: 10.1093/hmg/6.9.1483. [DOI] [PubMed] [Google Scholar]
- Shannon MB, Patton BL, Harvey SJ, Miner JH. A hypomorphic mutation in the mouse laminin alpha5 gene causes polycystic kidney disease. Journal of the American Society of Nephrology : JASN. 2006;17:1913–22. doi: 10.1681/ASN.2005121298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature genetics. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Staack A, Donjacour AA, Brody J, Cunha GR, Carroll P. Mouse urogenital development: a practical approach. Differentiation; research in biological diversity. 2003;71:402–13. doi: 10.1046/j.1432-0436.2003.7107004.x. [DOI] [PubMed] [Google Scholar]
- Sullivan LP, Grantham JJ. Mechanisms of fluid secretion by polycystic epithelia. Kidney international. 1996;49:1586–91. doi: 10.1038/ki.1996.230. [DOI] [PubMed] [Google Scholar]
- Sullivan LP, Wallace DP, Grantham JJ. Epithelial transport in polycystic kidney disease. Physiological reviews. 1998;78:1165–91. doi: 10.1152/physrev.1998.78.4.1165. [DOI] [PubMed] [Google Scholar]
- Tomaszewski J, Joseph A, Archambeault D, Yao HH. Essential roles of inhibin beta A in mouse epididymal coiling. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11322–7. doi: 10.1073/pnas.0703445104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torra R, Sarquella J, Calabia J, Marti J, Ars E, Fernandez-Llama P, Ballarin J. Prevalence of cysts in seminal tract and abnormal semen parameters in patients with autosomal dominant polycystic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2008;3:790–3. doi: 10.2215/CJN.05311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora N, Perrone R, Bianchi DW. Reproductive issues for adults with autosomal dominant polycystic kidney disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;51:307–18. doi: 10.1053/j.ajkd.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Wilhelm D, Koopman P. The makings of maleness: towards an integrated view of male sexual development. Nature reviews. Genetics. 2006;7:620–31. doi: 10.1038/nrg1903. [DOI] [PubMed] [Google Scholar]
- Wilson PD. Polycystin: new aspects of structure, function, and regulation. Journal of the American Society of Nephrology : JASN. 2001;12:834–45. doi: 10.1681/ASN.V124834. [DOI] [PubMed] [Google Scholar]
- Wilson PD. Polycystic kidney disease. The New England journal of medicine. 2004;350:151–64. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- Wilson PD. Mouse models of polycystic kidney disease. Current topics in developmental biology. 2008;84:311–50. doi: 10.1016/S0070-2153(08)00606-6. [DOI] [PubMed] [Google Scholar]
- Xu B, Abdel-Fattah R, Yang L, Crenshaw SA, Black MB, Hinton BT. Testicular lumicrine factors regulate ERK, STAT, and NFKB pathways in the initial segment of the rat epididymis to prevent apoptosis. Biology of reproduction. 2011;84:1282–91. doi: 10.1095/biolreprod.110.090324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Yang L, Lye RJ, Hinton BT. p-MAPK1/3 and DUSP6 regulate epididymal cell proliferation and survival in a region-specific manner in mice. Biology of reproduction. 2010;83:807–17. doi: 10.1095/biolreprod.110.085613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu GM, Gonzalez-Perrett S, Essafi M, Timpanaro GA, Montalbetti N, Arnaout MA, Cantiello HF. Polycystin-1 activates and stabilizes the polycystin-2 channel. The Journal of biological chemistry. 2003;278:1457–62. doi: 10.1074/jbc.M209996200. [DOI] [PubMed] [Google Scholar]
- Xu GM, Sikaneta T, Sullivan BM, Zhang Q, Andreucci M, Stehle T, Drummond I, Arnaout MA. Polycystin-1 interacts with intermediate filaments. The Journal of biological chemistry. 2001;276:46544–52. doi: 10.1074/jbc.M107828200. [DOI] [PubMed] [Google Scholar]