Abstract
Engineered nanomaterials significantly entered commerce at the beginning of the 21st century. Concerns about serious potential health effects of nanomaterials were widespread. Now, approximately 15 years later, it is worthwhile to take stock of research and efforts to protect nanomaterial workers from potential risks of adverse health effects. This article provides and examines timelines for major functional areas (toxicology, metrology, exposure assessment, engineering controls and personal protective equipment, risk assessment, risk management, medical surveillance, and epidemiology) to identify significant contributions to worker safety and health. The occupational safety and health field has responded effectively to identify gaps in knowledge and practice, but further research is warranted and is described. There is now a greater, if imperfect, understanding of the mechanisms underlying nanoparticle toxicology, hazards to workers, and appropriate controls for nanomaterials, but unified analytical standards and exposure characterization methods are still lacking. The development of control-banding and similar strategies has compensated for incomplete data on exposure and risk, but it is unknown how widely such approaches are being adopted. Although the importance of epidemiologic studies and medical surveillance is recognized, implementation has been slowed by logistical issues. Responsible development of nanotechnology requires protection of workers at all stages of the technological life cycle. In each of the functional areas assessed, progress has been made, but more is required.
Keywords: Engineered nanomaterials, History, Toxicology, Epidemiology, Societal implications of nanotechnology
Introduction
The commercial exploitation of nanotechnology has attracted marked public interest since around the year 2000. Workers, including researchers and their students, were the first people exposed to the products of this new technology. If nanotechnology was to be responsibly developed, workers had to be protected (Schulte and Salamanca-Buentello 2006). The concern that engineered nanomaterials (ENMs) could be hazardous stemmed from awareness of the respiratory and cardiovascular effects of ultrafine air pollutants; industrial experience with health effects from welding fumes, and diesel particles, including metal fume fever; acute pulmonary inflammation from fumed silica; and various animal studies showing translocation of gold nanoparticles from nasal mucosa to the brain (De Lorenzo 1970) and respiratory effects due to ultrafine zinc (Amdur et al. 1988). Ultrafine particles were found to have greater pulmonary toxicity than larger respirable (fine) particles when measured by the mass dose; particle volume and particle surface area were found to be more predictive dose metrics (Morrow 1988; Oberdörster and Yu 1990; Duffin et al. 2002). Scientific literature on occupational exposures to and health effects from existing aerosol particulates and fibers provided a basis for evaluating the potential hazards of new nanoscale aerosols and highlighted gaps in the current body of knowledge (Maynard and Kuempel 2005).
Caution was initially sounded in 2000, when the United States National Science Foundation stated, “As currently envisioned, nanomaterials are likely to possess at least three properties that will generate novel safety and governance challenges: invisibility, micro-locomotion, and self-replication” (Roco and Bainbridge 2001). In 2004, the insurance company Swiss Re was more explicit, stating: “Presumably, nanoparticles must be handled with the same care given to bio-organisms or radioactive substances” (Hett 2004). In that same year, The Royal Society and The Royal Academy of Engineering warned, “There are uncertainties about the risk of nanoparticulates currently in production that need to be addressed immediately to safeguard workers and consumers and support regulatory decisions. … The evidence that has been reviewed suggests that manufactured nanoparticles and nanotubes are likely to be more toxic per unit mass than particles of the same chemical at larger size and will, therefore, present a greater hazard.… Free particles in the nanometer size range do raise health, environmental, and safety concerns, and their toxicology cannot be inferred from that of particles of the same chemical at larger size” (The Royal Society and The Royal Academy of Engineering 2004).
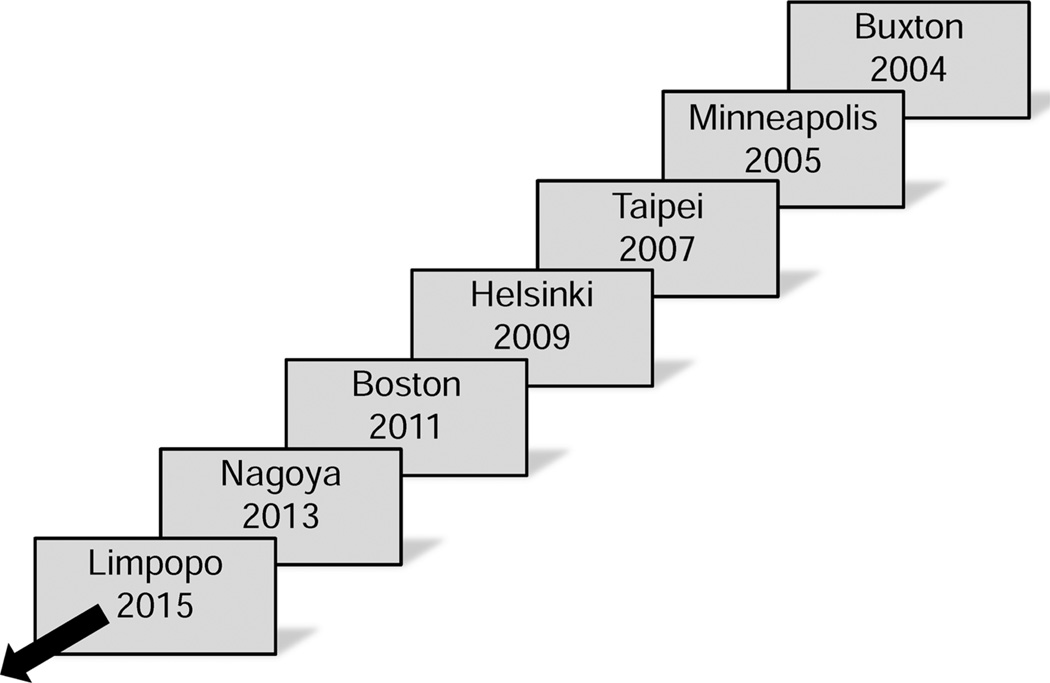
In 2004, when scientists from various countries met in Buxton, UK, for the first in a special series of nanotechnology occupational and environmental health (NanOEH) conferences of the occupational and environmental communities (Fig. 1), there were already over 200 claimed products identified as “nano-enabled” in commerce, indicating that workers were handling nanomaterials (Woodrow Wilson Center 2013). Moreover, the global market for nanomaterials was predicted to grow to an estimated $4.4 trillion by 2018 (Lux Research Inc. 2014). This meant that scientists and government officials had to answer not only whether the material was harmful, but also whether there were exposure and risk, and simultaneously, they had to devise strategies for handling nanomaterials safely. These NanOEH conferences represented periodic ad-hoc gatherings of the occupational safety and health community to address potential hazards of a new technology. They served to stimulate networking and discussion of emerging findings and needs in nanosafety research.
Fig. 1.
Recurring International NanOEH conferences
After almost two decades of commercial activity, it is worth assessing whether progress has been made on the critical issues of occupational safety and health in nanotechnology. In this article, we examine the history of the occupational safety and health efforts regarding ENMs from 2000 to 2015. To better explore progress in this field, eight important topical areas are distinguished: toxicology, metrology, exposure assessment, engineering controls and protective equipment, risk assessment, risk management, medical surveillance, and epidemiology. For each topical area, major milestones have been identified, including significant technical or research achievements as well as policy recommendation or adoption by national agencies, international government organizations, or professional non-governmental organizations. The selection of these milestones is based on the experience and judgment of the authors and includes consideration of the import assigned to actions and works by professional communities, as well as the volume of citations (as indicated by Google Scholar, Assessed August–September 2015) for academic literature. The selected milestones are then synthesized into timelines to allow a historical portrayal of critical findings and actions that have advanced knowledge in that area. Although this is a qualitative approach, this method provides a better context than would be available by a strictly quantitative review of the academic literature. Moreover, it allows one to more readily see not only how the field came to reach its current state, but also the current tasks and challenges in each area that remain to be addressed.
Toxicology
The initial question from workers, employers, and other decision-makers was whether ENMs are harmful and, if so, what mechanisms and properties explained their toxicity. Many of the toxicological methods and models used to investigate nanomaterial health risks have their origins in studies of the toxicology of particles and fibers (Kreyling et al. 2002a; Oberdörster et al. 2005b). Studies investigating the unique or enhanced properties of nanomaterials published prior to 2000, have led to the current field of nanotoxicology (Donaldson et al. 1998; Oberdörster et al. 1992, 1994). A major focus of these studies has been effects on the respiratory tract, because of the history of occupational respiratory diseases associated with aerosol exposures. Nanoparticles are respirable, which enables them to reach the gas-exchange (alveolar) region of the respiratory tract (Oberdörster et al. 2005a). Nanoparticles have also been observed to reach the interstitium and the blood, which may represent a path for the translocation of inhaled nanoparticles from the lung to secondary organs (Donaldson et al. 2004; Kreyling et al. 2010; Mercer et al. 2010, 2013). Carbon nanotubes (CNTs) also appear to rapidly promote interstitial fibrosis, and specific multi-walled CNTs (MWCNTs) have been shown to promote lung cancer (Sargent et al. 2014). Beyond this, inhalation of nanoparticles has been linked to cardiovascular effects (Li et al. 2007; Nurkiewicz et al. 2008). The toxicological behavior of ENMs and natural or incidental ultrafine particulate matter has been observed to be quite similar (Gwinn and Vallyathan 2006). It is thought that inhalation is the main route of exposure, as insoluble nanoparticles do not generally traverse the intact skin (Rouse et al. 2007). However, there does not appear to have been a comprehensive study of cutaneous exposure, which leaves unanswered certain questions about reliability of cross-species models for nanoparticles, effects of nanoparticle functionalization on penetration, and the degree to which different forms of skin damage might affect penetration.
Nanoparticle toxicity is increasingly thought of as a confluence of multiple contributory mechanisms stemming from the physicochemical properties of a given nanomaterial. Much-like inhalation of fine and ultrafine particles, nanoparticle size does affect (but does not fully govern) the toxicity (Maynard and Kuempel 2005; Oberdörster et al. 2005a). For instance, insoluble nanoparticles have been shown to have inhalation toxicity proportional to the total surface area (and thus inversely proportional to individual particle size) (Oberdörster et al. 1992; Driscoll 1996; Tran et al. 1999). Additional important factors that influence particle toxicity include surface reactivity, solubility, and shape (Duffin et al. 2002; Maynard and Kuempel 2005). Some indicated mechanisms are: (1) release of ions into solution; (2) generation of oxidative stress by facilitating the generation of electron–hole pairs to create reactive oxygen species in the microenvironment; (3) semiconductive properties causing ejection of excited electrons to create superoxides, directly catalyzing electron transport in redox regulators; and (4) fiber-induced biological responses. Each of these mechanisms may, in turn, promote an immune response. These mechanisms appear to be associated with the particle’s physicochemical properties (the size of the particle, shape, porosity, chemical composition, and any functionalization), but the cellular response to these toxic insults may be the primary dictator of outcomes Donaldson et al. (1998, 2004, Nel et al. 2006, 2009; Palomaki et al. 2011; Schins and Knaapen 2007). By 2010, the knowledge of toxicology had grown considerably, and reviews began to focus on the integration of that knowledge with other occupational health-related fields and the gaps that existed (Seaton 2006; Bergamaschi 2009; Savolainen et al. 2010a).
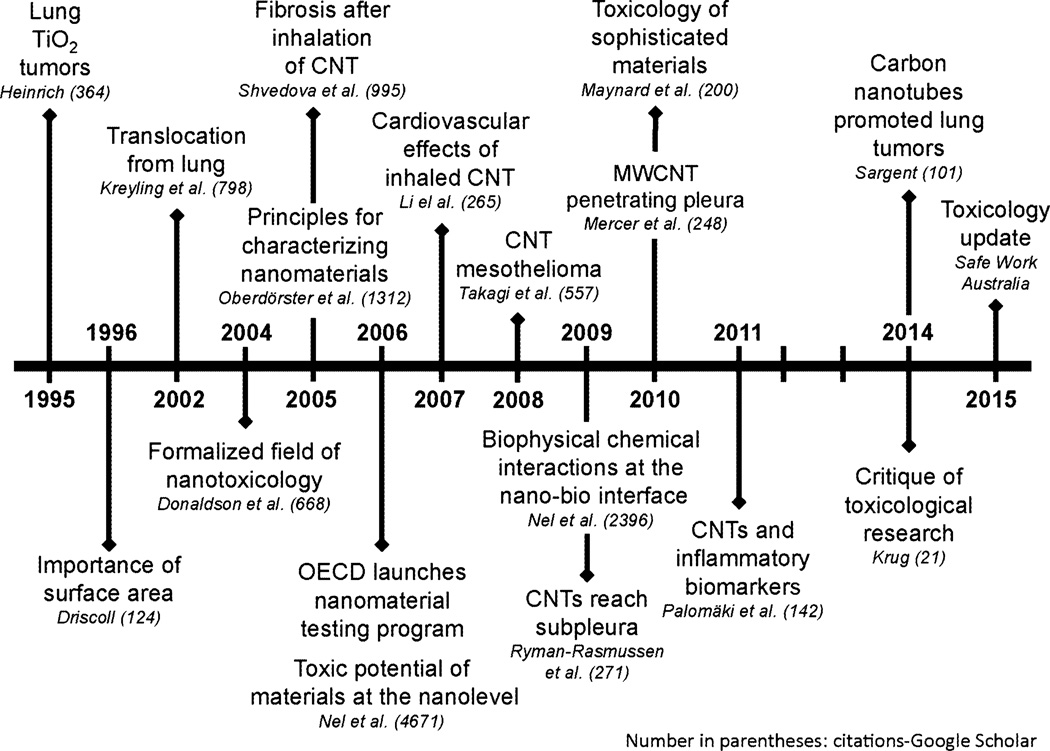
Figure 2 shows the timeline for toxicology progress. Critical points in the timeline were initiation of nanomaterial testing by the Organization for Economic Co-operation and Development (OECD) in 2006, findings of possible carcinogenicity from animal studies conducted in 2008–2013, and critique of the toxicological research in 2014, which called for a better particle characterization (Krug 2014). A commentary by Yu et al. in the following years recognized a disconnection: despite tremendous advancement in the field, there were only questionable data relating the experimental findings to human hazards (Yu et al. 2015). Bridging this gap is going to become increasingly important, not only for occupational health, but also for medical applications of ENMs (Cattaneo et al. 2010). At the current stage, there is a large body of literature on the toxicology of ENMs (both quantitative and mechanistic). However, gaps remain both in the ability to extrapolate toxicity of selected ENMs to related classes of ENMs and in translating our knowledge from the petri dish, microarrays, or laboratory animal to the human worker.
Fig. 2.
Progress in ENM-related toxicology
Metrology
By 2000, it was clear that metrology was going to be critical for understanding NanOEH. ENMs were quite varied, at the limits of many instruments’ ability to measure, and it was still unclear which properties were essential for understanding toxicity and exposure and for devising control schemes. The question was: What nanomaterial physiochemical properties should be measured, and how? This question directly impacted both laboratory toxicology studies, requiring a thorough characterization of study materials to understand exposure–response relationships, and workplace exposure assessments, which require differentiation of the ENMs of interest from other nanoscale particles in workplace atmospheres. From toxicology studies, it was becoming clear that multiple ENM physicochemical properties could affect the toxicological response. As such, initial approaches to characterization of ENMs sought to measure as many properties as possible to enable understanding of potential exposure– response relationships (Bouwmeester et al. 2011). Such strategies are not practical, economically viable, or technically feasible and, therefore, necessitate more targeted characterization strategies. With regard to assessments of ENMs in workplace atmospheres, investigators were initially faced with the challenge that many sampling and analytical methods lacked specificity for the ENMs of interest, which made it difficult to differentiate them from airborne incidental “background” nanoparticles (Methner et al. 2007).
Though the best metric(s) for understanding ENM health effects are still unknown and likely vary with material and health endpoint (Jiang et al. 2008), increasingly, there have been efforts to better define ENM characterization strategies. For example, many investigators have attempted to define a minimum set of nanomaterial properties (surface area, elemental composition, particle size, particle-size distribution, zeta potential, and crystallinity) to thoroughly characterize an ENM for toxicology studies (Stefaniak et al. 2013). It is now recognized that to overcome weaknesses in sampling and analytical methods, because of non-specificity, field exposure studies need to be carefully designed to compensate for background nanoparticle exposures; in addition, findings potentially must be reevaluated with more specific, but costlier and time-consuming analytical methods, such as electron microscopy (Methner et al. 2007). This is also necessary, because many real-time instruments [such as scanning mobility particle (SMP) analyzers and condensation particle counters (CPCs)] used in field studies are limited to assaying a single parameter (such as particle-size distribution) and cannot develop the detailed characterization data set required for more rigorous exposure assessment and risk assessment.
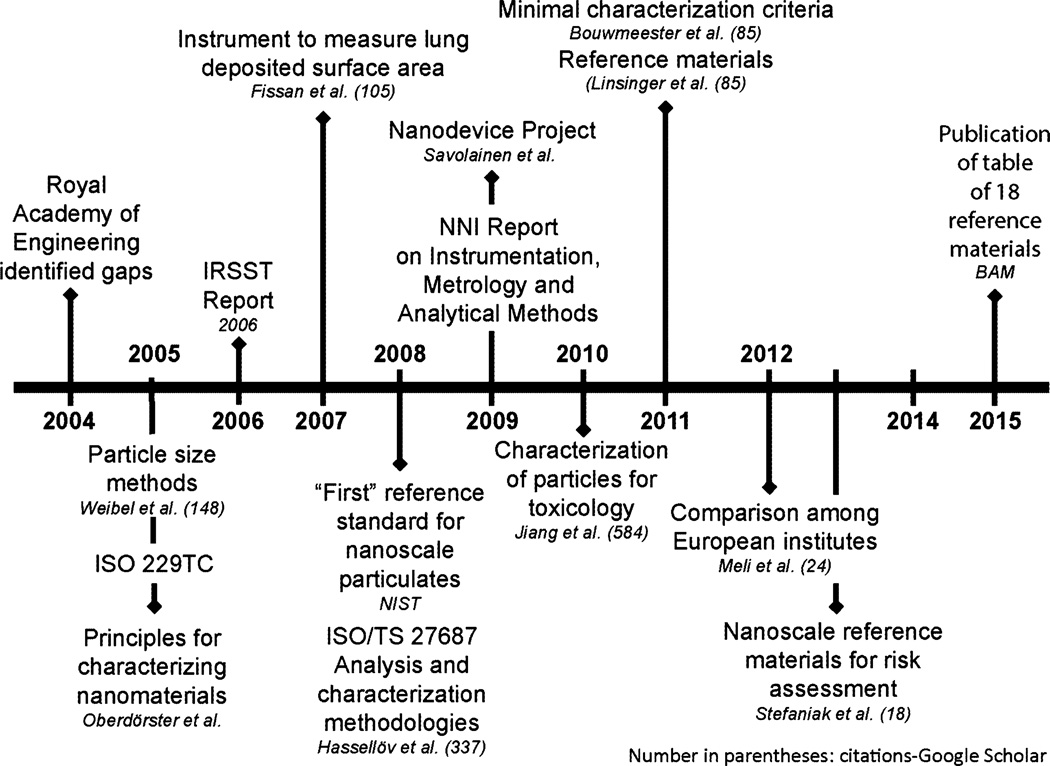
Figure 3 is the timeline for progress in nanomaterial metrology. A significant progress has been made in developing reference materials to support particle identification, in refining toxicology characterization strategies, and in clarifying field exposure measurements. Going forward, the ability to develop a complete picture of toxicity and exposure results from numerous tests by different laboratories—and to reach a broader understanding of the exposures associated with specific materials and processes—will require a set of common standards (Linsinger et al. 2011; Stefaniak et al. 2013). These standards may be in the form of existing and new reference materials, study protocols, and agreed-upon guidance. The National Institute of Standards and Technology (NIST) began introducing nanoparticle reference materials in 2008, with the release of three sizes of gold nanoparticles (NIST and National Cancer Institute [NCI] 2008). Since then, at least 18 such well-characterized materials have entered common usage throughout the US and Europe, including carbon nanotubes (CNTs), metals and metal oxides, and cellulose nanocrystals (Germany Federal Institute for Materials Research and Testing [BAM] 2015). In addition, the European Union’s Joint Research Centre’s Nanohub has begun to amass a repository of well-characterized nanomaterials specifically to “promote better reproducibility and reliability of nanomaterials safety testing” (Joint Research Centre 2014). Reference materials may be essential for overcoming the lack of standardized measuring practices, allowing interlaboratory comparison and instrument calibration. The NanOEH community needs to decide whether reference materials are really necessary or if “study,” “benchmark,” or “representative test” materials are sufficient for an intended purpose (Roebben et al. 2013). Test methods are becoming available from ISO and ASTM International, and guidance documents are freely available from the U.S. National Cancer Institute (http://ncl.cancer.gov/assay_cascade.asp) and consortiums, such as the Center for Environmental Implications of NanoTechnology (http://www.ceint.duke.edu/allprotocols). Finally, continued development of standards for terminology will be critical for ensuring sound metrology that best supports toxicological characterization and workplace exposure measurements.
Fig. 3.
Progress in ENM-related metrology
Exposure assessment
Initially, there was little understanding of ENM exposures, although the ability to protect workers and make decisions requires well-developed methods of exposure assessment. Once metrology enabled the study of ENM exposures, characterization commenced immediately. Initial forays into assessing exposures to ENMs borrowed heavily from the techniques of sampling for particulate matter and asbestos (Kuhlbusch et al. 2004; NIOSH 2009a). Maynard et al., who examined a single-walled carbon nanotube (SWCNT)–generating operation using an SMP analyzer for particle counting and sizing and filter capture, demonstrated workplace air concentrations up to 53 µg/m3 (Maynard et al. 2004). In 2007, Methner et al. studied a carbon nanofiber (CNF) facility using a real-time CPC as well as filter capture, followed by offline analysis with transmission electron microscopy, and discovered that the largest amount of particle aerosolization occurred during saw use (Methner et al. 2007). These studies demonstrated that ENM aerosols were measurable and often linked to specific tasks. However, the studies demonstrated a weakness in the ability to measure exposures in a worker’s personal breathing zones, as well as the need for a more consistent sampling protocol that could differentiate background concentrations of incidental, non-engineered nanoparticles (such as diesel exhaust matter). Several studies by Brenner et al. demonstrated an approach to systematic exposure assessment for ENMs in semiconductor-related tasks (Brenner et al. 2015; Shepard and Brenner 2014a, b). Similar methods were demonstrated by Lee et al. in an investigation of a plant creating silver ENMs (Lee et al. 2012).
In addition, the advances in toxicology brought into question whether ‘mass per unit volume of air’ was a sufficient exposure metric. Maynard and Aitken outlined the numerous physicochemical parameters that seemed to be related to toxicology and developed a categorization scheme that would indicate the most critical parameter to analyze for a given category of material (Maynard and Aitken 2007). It was also recognized that no single measuring tool available could attain all the desired parameters and that the field would benefit from an economical, portable tool for simultaneous examination of particle mass, and number and development of area-to-volume ratios (Maynard and Aitken 2007). To date, such a device remains elusive. Brouwer et al. also examined critical deficiencies in the field, noting that in spite of the data governing nanoparticle behavior, the lack of sufficient field data or a unified sampling approach made it difficult to draw meaningful conclusions from the current exposure data sets (Brouwer et al. 2009).
The need for a more comprehensive and unified approach was addressed by several groups. In the US, the National Institute for Occupational Safety and Health (NIOSH) developed and demonstrated the Nanoparticle Emission Assessment Technique (NEAT), which specifically highlighted the need for surveying discrete tasks in a workplace as well as the need for analysis with multiple tools (including particle counters and filter samples analyzed by electron microscopy) of reference samples from the material of interest (Methner et al. 2009). The EU-sponsored NANOSH (Finnish Institute of Occupational Health 2010) emphasized the need for harmonized decision logic, measurement strategy, and data reporting, as well as the use of multi-instrumental analysis (Brouwer et al. 2009). In 2012, Brouwer et al. reported that the First International Scientific Workshop on Harmonization of Strategies to Measure and Analyze Exposure to (Manufactured) Nano-objects in Workplace Air had recommended a multi-metric approach combined with specifics in sampling approaches and contextual data gathering (Brouwer et al. 2012). OECD followed the guidance of Brouwer et al. by recommending a tiered approach consisting of an initial survey of work tasks and identification of potential sources of exposure, followed by rapid assessment of a few key parameters (such as concentration measurements) and then, detailed assessment of all potentially related parameters (surface chemistry testing, imaging, etc.) (Organisation for Economic Co-operation and Development 2015).
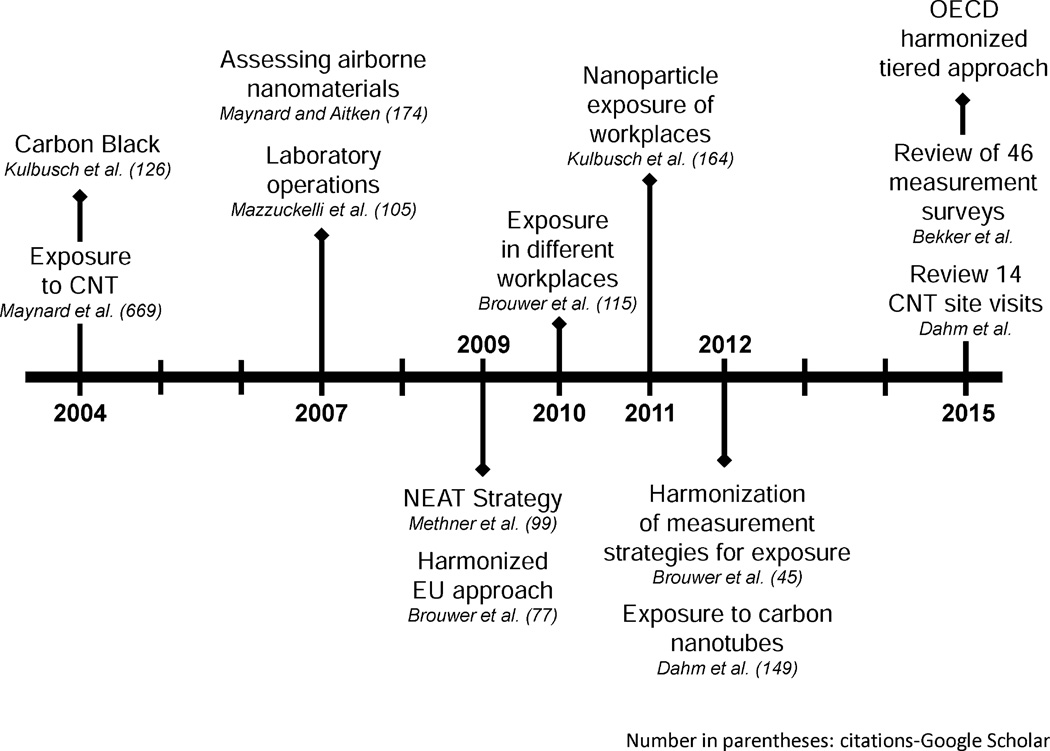
An example of these practices can be observed in the 2012 report by Dahm et al., in which careful decision-making and multiple techniques enabled the research group to demonstrate statistically significant correlations in exposure between elemental carbon levels and CNT/CNF exposures (Dahm et al. 2012). As a direct consequence of such quality studies, the amount of available exposure data is increasing rapidly, and therefore, the field is becoming more sophisticated. In 2015, two studies were published that compared numerous sites and sampling events to derive high-confidence conclusions (Bekker et al. 2015; Dahm et al. 2015); Bekker et al. were able to define the highest-exposure task in their study. Such a comprehensive sampling strategy, consistently applied and using a multifaceted methodology, represents the future of occupational nanoparticle exposure assessment. Advances in the field of exposure assessment for ENMs are presented as a timeline in Fig. 4. Despite these advances, the body of published exposure data focuses extensively on CNTs and TiO2 and, to a lesser extent, on a few other commercial nanomaterials, such as noble metals (silver), metals and metallic oxides (iron, zinc, aluminum), carbon black, and other carbonaceous materials, such as fullerenes (Bekker et al. 2015; Brouwer 2010; Kuhlbusch et al. 2011). There remains a need to expand investigations of exposures to other types of ENMs, such as multilayered or functionalized nanoparticles.
Fig. 4.
Progress in ENM-related exposure assessment
Engineering controls and personal protective equipment
Once exposures could be assessed, it was critical to evaluate which control measures could mitigate exposure. Occupational safety and health practitioners drew upon the long and successful history of controlling fine dusts and dry powder formulations in pharmaceutical, nuclear, coating, pigmentation, and cosmetic industries to identify effective exposure containment and control strategies for industries involved in generating or using ENMs. This was supported by the accumulating body of evidence, indicating that nanoparticles followed laws of aerosol physics in their airborne behavior and, therefore, were susceptible to established exposure control technologies. Further investigations concluded that worker exposures were directly linked to specific material handling practices and processing tasks (NIOSH 2009a). Many of these processes were directly analogous to classic manufacturing processes, and as with many historical workplace contaminants, the potential for inhalation exposure became a focus. However, because of the greater respiratory deposition of nanoparticles versus microparticles, more emphasis was placed on exposure control strategies that could capture the smaller aerosols. A secondary concern was dermal exposure, because of the ability of some types of nanoparticles to enter through damaged skin. Initial exposure control recommendations included the use of local exhaust ventilation (LEV), equipped with high-efficiency particulate air (HEPA) filters, if needed; use of damp-cleaning methods or HEPA-equipped vacuums for cleaning; and use of impermeable materials for gloves (NIOSH 2009a).
These initial recommendations were validated by recent studies. A study by Methner in 2008 found that in the process of generating 15- to 50-nm-diameter metal-alloy spheres, a single process (cleaning a reactor) generated the most airborne nanoparticles. The study also showed that installation of LEV with a HEPA filter (since discharge was impractical in the self-contained system) could result in a mean airborne reduction of 88 % mass-concentration (µg/m3) (Methner 2008). Another investigation, by Rengasamy et al. (2008), found that N95 and P100 filtering face-piece respirators typically removed in excess of 95 % of nanoparticles, with the effectiveness increasing as particle diameter decreased below 30 nm (Rengasamy et al. 2008). Safe Work Australia discussed the methods by which nanoparticle trapping occurs and further recommended exploration of the use of electrostatic precipitators and, for some processes, full enclosure as well as eye and facial protection (Safe Work Australia 2009).
The uncertainty of exposure risk for a given ENM can complicate determination of the appropriate controls. A control-banding approach introduced by Paik et al. (2008) ranks materials on a 1–4 scale, based on both the particle toxicity and the probability of exposure, with conservatively high assumptions for unknown parameters. Each control band is then matched with a specific set of recommended controls (Paik et al. 2008). This technique is part of a growing toolbox of accepted methods for controlling ENMs, despite uncertainties about specific nanomaterials (NIOSH 2013a).
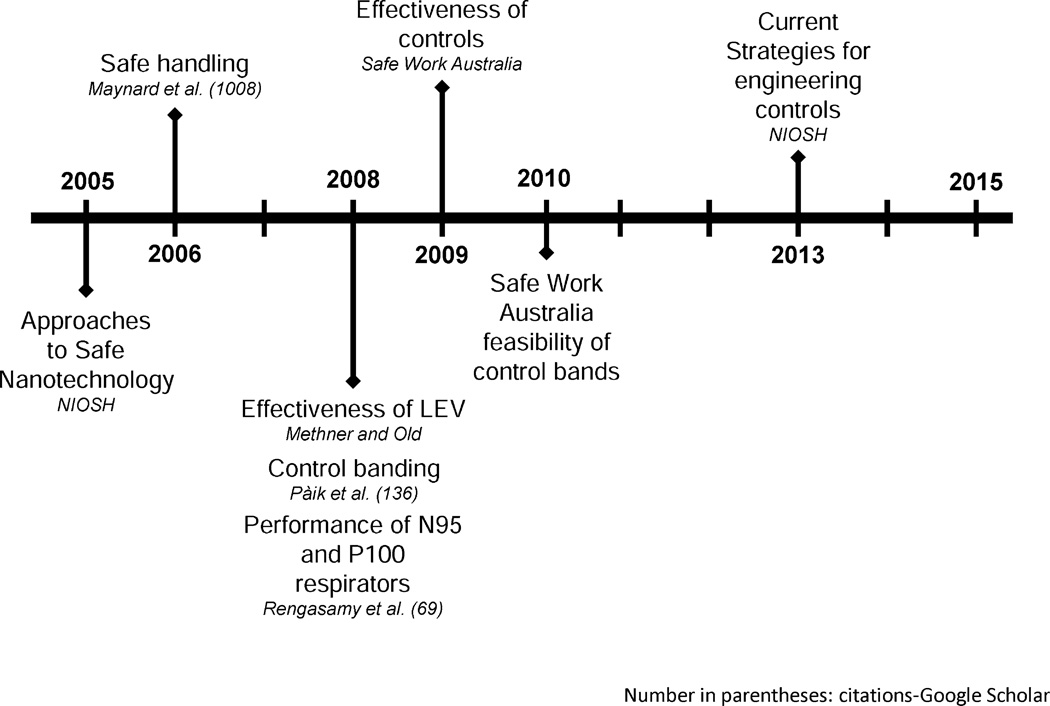
A growing body of studies is available from NIOSH and the United Kingdom Health and Safety Executive (HSE) to guide decisions on controls and selection of personal protective equipment (PPE) both for general nanomaterial use and specific processes of interest. These recommendations are strongly rooted in the identification of exposure risks that are both task-derived and toxicology-dependent, and they help bring to bear scientifically demonstrated methods of mitigation through engineering controls, followed by PPE. In addition, efforts are ongoing to develop a method for sharing best practices in occupational health and to develop the technologies necessary to enable better assessment of the efficacy of engineering controls (Sirviö and Savolainen 2011). This cooperative development will complement other global efforts to develop standards to assess and mitigate ENM hazards. Advances in the field of engineering controls and PPE for ENMs are presented as a timeline in Fig. 5.
Fig. 5.
Progress in ENM-related engineering control and PPE
Risk assessment
One of the driving questions in the early 2000s was whether workers exposed to ENMs were at risk of adverse health effects and to what extent those effects depend on the nanoscale properties. The limited data and uncertainty on the toxicity and environmental behavior of ENMs made risk assessment difficult initially. However, standard risk assessment methods and dosimetry models that account for differences in the lung doses in animals and humans have been used in quantitative risk assessments of ultrafine or fine particles (Kuempel et al. 2006). These methods were used to estimate the particle-size-specific pulmonary disease risk of titanium dioxide (TiO2) on the basis of retained particle surface area lung burden (Dankovic et al. 2007). Particle volume lung retention was the dose metric used by Pauluhn to derive an occupational exposure limit (OEL) for MWCNT, using the lung particle overload hypothesis (Morrow 1988; Pauluhn 2010). Default uncertainty factor approaches have also been applied to derive OELs for several types of nanoparticles (Aschberger and Christensen 2011). NIOSH has published recommended exposure limits for ultrafine and fine TiO2 and for carbon nanotubes and nanofibers based on quantitative risk assessments (NIOSH 2011, 2013b). As the number of nanotoxicology studies continues to increase, the challenge is how to effectively translate these research findings to occupational safety and health practice (Schulte et al. 2010).
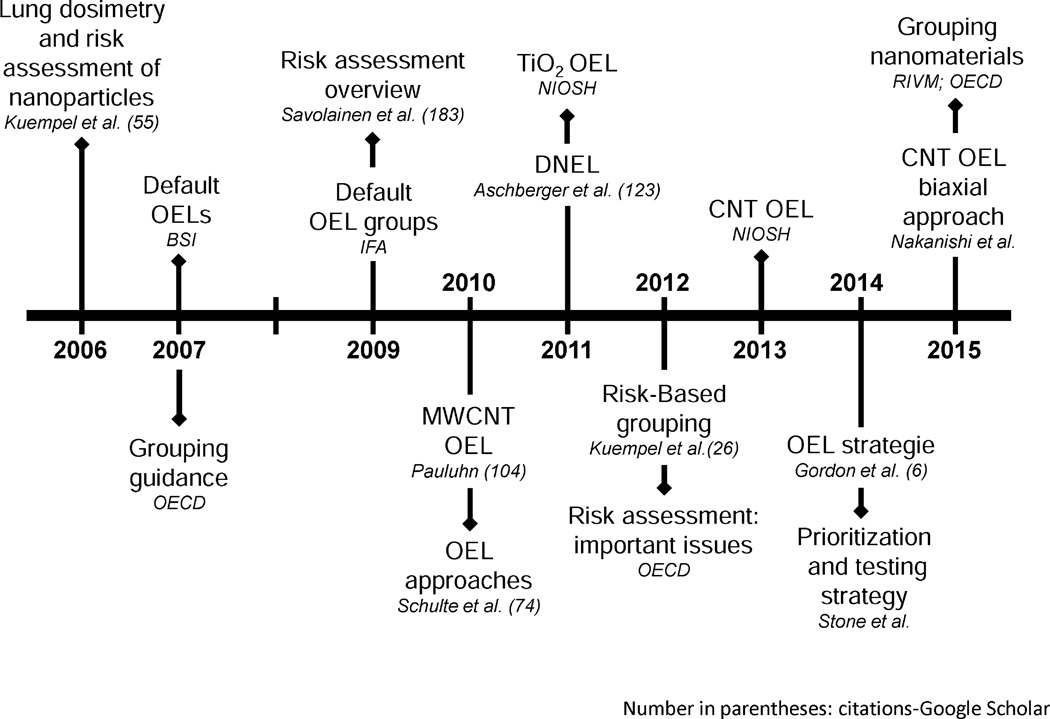
To address the paucity of data for most nanomaterials, the OECD has recommended categorizing materials into similar categories based on physicochemical properties to extrapolate reasonable estimates of the bioactivity of a nanomaterial (Organisation for Economic Co-operation and Development 2007). The British Standards Institute (BSI) and, soon after, the Institute for Occupational Safety and Health of the German Social Accident Insurance (IFA) proposed broad categories of nanomaterials with provisional exposure limits. They also examined the parameters that indicate the risk a material may pose, with a particular emphasis on respiratory risks and mention of others, including those that might be sensitizers or explosion hazards (BSI 2007; Netherlands National Institute for Public Health and the Environment 2014). Hazard-based grouping and risk-based grouping of nanomaterials by comparative potency analyses of data-rich benchmark materials with data-poor nanomaterials have been proposed to supplement evidence from nanotoxicology research (Kuempel et al. 2012a). A similar concept is the “biaxial approach,” which was developed to incorporate detailed examinations of representative materials with simpler assays of new nanomaterials (Nakanishi et al. 2015). “Read across” approaches are also being used to interpolate unknown material bioactivity from other materials with similar physicochemical, exposure, and hazard properties; and such approaches, while not universally accepted, offer a useful way to interpret the often incomplete data on ENMs (Organisation for Economic Co-operation and Development 2012; Sellers et al. 2015; Stone et al. 2014). Advances in risk assessment for ENMs are presented as a timeline in Fig. 6.
Fig. 6.
Progress in ENM-related risk assessment
Risk management
With the increasing awareness of potential adverse health effects from ENMs, employers wanted to know how to manage any related risks. Protecting against occupational illness by identifying, managing, and preventing risks from ENM is the ultimate goal in occupational safety and health. Initially, although there was some general risk management experience with fine and ultrafine particles, HSE and NIOSH examined nanomaterial toxicities and exposures and concluded that the available data were insufficient to be certain of proper exposure mitigation methods and engineering controls, highlighting risk management as an important area of future study (NIOSH 2011; UK Health and Safety Executive (HSE) 2004). Independent health researchers concurred, and Maynard observed that because of the rapid rate of ENM development, it was critical for an analytical framework to be developed quickly and to incorporate exposure mitigation models that had a long lifespan (Maynard et al. 2006).
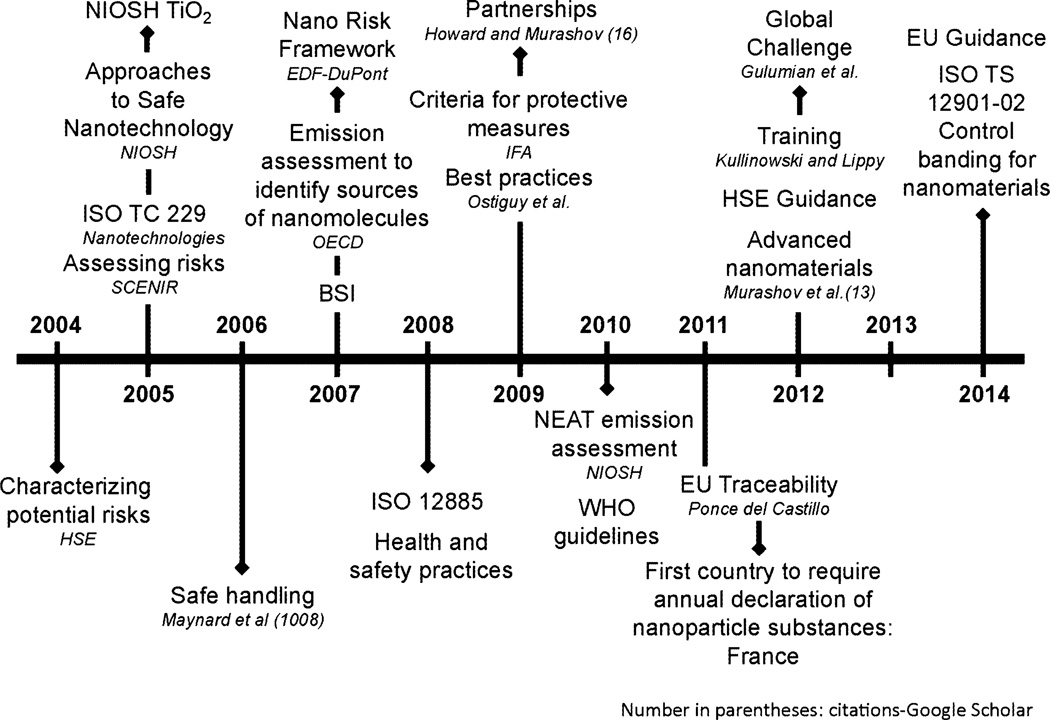
With cognizance of the knowledge base needed for risk management, organizations, such as the ISO Technical Committee 229 Working Group moved to address issues of nanomaterial nomenclature, metrology, and environmental health and safety by proposing a set of guidelines and the use of control banding (International Organization for Standardization (ISO) 2012). Initial risk management recommendations were maximally protective, often incorporating full containment of potential ENM exposures and emphasizing the importance of medical surveillance (NIOSH 2009a). The introduction of models, such as the Nano Risk Framework, promoted the use of a systematic approach used by the chemical industry to manage the introduction of new materials (Environmental Defense—DuPont Nano Partnership 2007). Such frameworks compensate for a lack of knowledge in specific toxicities and exposure, which, despite the advent of well-validated methods of exposure assessment and control using NEAT, remains a persistent problem (Gulumian et al. 2012; Methner et al. 2010a, b). Application of these frameworks has accelerated the development and proposal of a standardized control-banding approach to manage ENMs (International Organization for Standardization (ISO) 2013).
At this time, guidelines for managing occupational exposure to ENM have been issued, with special guidance for specific nanomaterials (CNTs and high-aspect-ratio nanomaterials) (Institute for Occupational Safety and Health of the German Social Accident Insurance (IFA) 2009; UK Health and Safety Executive [HSE] 2013). In addition, information on risk management is being published that targets the employer, employee, and health and safety professionals, enabling better training and helping facilitate better risk management programs (European Commission on Employment 2014a, b; Kulinowski and Lippy 2011). Advances in risk management for ENMs are presented as a timeline in Fig. 7. These efforts are bearing fruit in the development by universities and research institutions of sophisticated risk management plans with nuanced decision-making logics rooted in established concepts, such as control banding (Grieger et al. 2015; Groso and Meyer 2013; Groso et al. 2010).
Fig. 7.
Progress in ENM-related risk management
Medical surveillance
A question that arose early in the development of ENMs was whether workers’ health should be monitored. Medical surveillance (tracking the health of workers over time) is a critical tool for evaluating the health effects on workers potentially exposed to occupational hazards. In the case of ENMs, the limited data set available on hazards, exposure concentrations, and risk management effectiveness increased the perceived need for a general medical monitoring program for nanomaterial workers. However, that same lack of information in other areas also extends to understanding pathologies associated with ENM exposure. To date, no specific human illnesses have been definitely associated with ENM exposure, although some biomarkers of effect that may be associated with adverse effects have been identified (see section on epidemiology). Consequently, specific medical screenings (clinical tests) have not yet been recommended for most ENMs, but general medical surveillance is a prudent precautionary measure for workers exposed to ENMs (Nasterlack et al. 2008; NIOSH 2009b). To generate a more comprehensive data set for future surveillance efforts, the creation of a registry with information on workers potentially exposed to ENMs is an option being explored in various countries (Boutou-Kempf et al. 2011; Health Council of the Netherlands 2012).
Specific medical screening of workers exposed to selected ENMs may be warranted if an ENM is composed of a compound that has known toxicity and is already subject to medical screening (Trout 2011). Similarly, specific materials whose potential pathologies are well understood might be subject to more specific medical screening. Examples of such nanomaterials are CNTs and CNFs, because of the human pulmonary effects anticipated on the basis of extensive toxicological research (NIOSH 2013b).
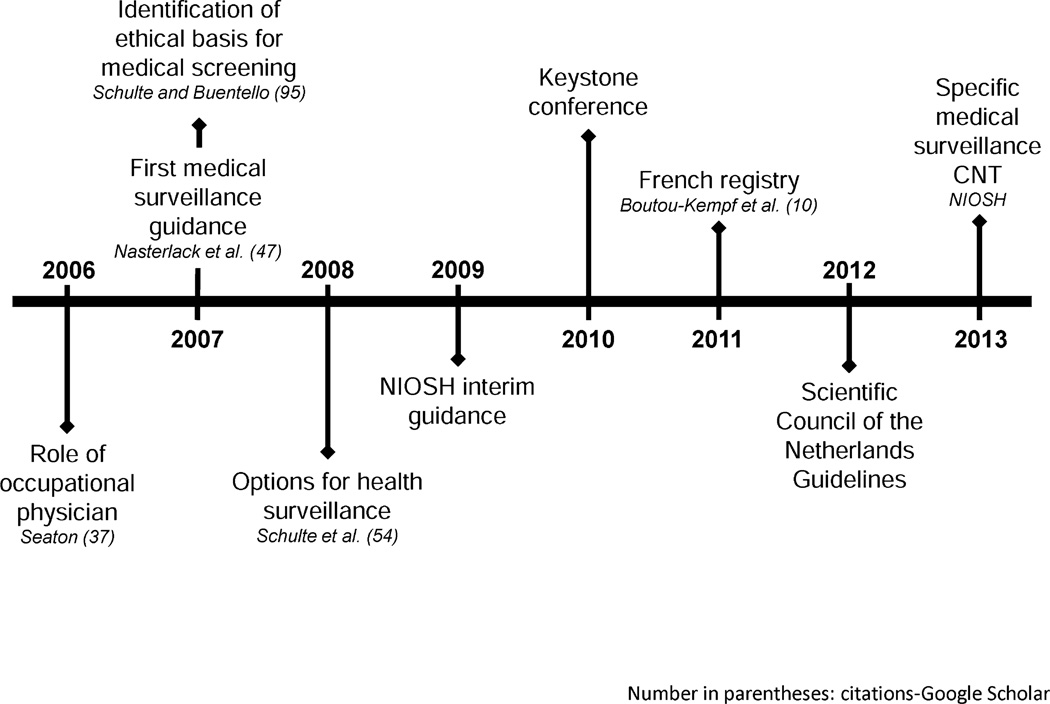
It is also essential to carefully consider the structure of any medical surveillance, to ensure that the information gathered does not become the primary object rather than the maintenance of worker health (Nasterlack 2011; Schulte and Salamanca-Buentello 2006). Although there is a strong ethical impetus to use all available methods to characterize ENM health outcomes, caution must also be used to avoid errors in data analysis when well-defined health endpoints from an exposure are unknown (Fischman et al. 2011). However, when used properly, medical surveillance is a powerful tool for risk management and can also serve as a mechanism to assess the health status of a group of workers exposed to a specific ENM. Establishment of such a program can provide critical knowledge to workers and involve them in the process of establishing a safe work environment (Schulte and Salamanca-Buentello 2006). Advances in medical surveillance for ENM workers are presented as a timeline in Fig. 8.
Fig. 8.
Progress in ENM-related medical surveillance
Epidemiology
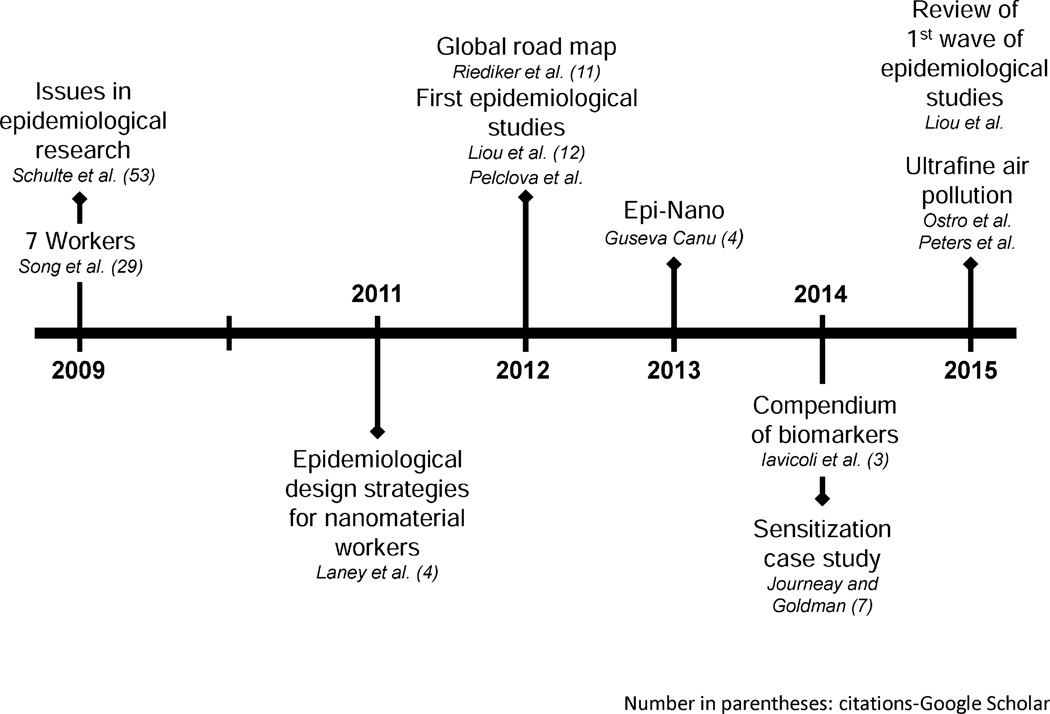
Whether worker populations exposed to ENMs were at risk of adverse health effects was another early question that has awaited an answer. Because of the relatively short period of occupational exposure to ENMs for most workers and the time required for development of long-latency outcomes, such as pulmonary fibrosis and cancer, insufficient time has passed to measure many occupational diseases, and few epidemiologic studies have been conducted. Challenges in developing epidemiological studies are significant. Most prominent among the logistical challenges are the difficulty of identifying a subject population (which is linked to the small number of workers exposed to a specific ENM employed in many diverse industries) and the requirement of consistent tasks with relevant materials and exposures for a long period of time to elucidate chronic outcomes (Schulte et al. 2009). These challenges are due to the fact that there is not a single “nanotechnology industry,” but rather a collection of different industries that are enabled by nanotechnology, resulting in difficulties finding large groups of workers exposed to the same ENM.
Factors that could improve the development of epidemiological studies include: consistency in documenting ENM characteristics, a lack of confounding information on other toxicants in the workplace, and a proper range of exposure concentrations (that is, not entirely attenuated or rendered inconsistent by controls or PPE) (Laney et al. 2011; Schulte et al. 2009). Prior to the study published by Liou et al. (2012), few studies had focused on nanomaterial workers, although studies had been conducted on naturally occurring or incidental exposures to nanoparticles, largely in the form of air pollution (Liou et al. 2012).
A more generalized plan was introduced by Riediker et al. (2012), which broke the general need for epidemiologic studies into subquestions divided into categories more closely linked to materials and exposure assessment, determination of endpoints and markers, and elements of risk management, study design, and data analysis. This context recognized the need for advances in each field to determine the ideal analytical techniques for exposure assessment, determination of exposure biomarkers, and data handling methods as well as cross-talk between each to yield a more useful final result (Riediker et al. 2012).
In the last several years, a significant progress has been made in many of the critical areas outlined by Riediker et al. (2012). France has begun to implement a broad, compulsory surveillance plan for workers exposed to CNTs, which could potentially be broadened to other ENMs, forming a large database for epidemiologic data mining (Canu et al. 2013). Iavicoli released a comprehensive survey of biomarkers useful for potential ENM-related epidemiology categorized into markers of exposure for specific materials, common outcomes, and genotoxicity (Iavicoli et al. 2014). A case study reported by Journeay and Goldman demonstrated that certain ENMs might also have the ability to act as sensitizers (Journeay and Goldman 2014), suggesting this as a possible health outcome to be monitored by future epidemiology studies. In addition, in 2015, Ostro et al. and Peters et al. each published an epidemiologic study linking ultrafine exposure to negative cardiovascular outcomes in female teachers and type II diabetics (Ostro et al. 2015; Peters et al. 2015).
In a review of early ENM-related epidemiological studies, Liou et al. compared 11 cross-sectional studies and 8 longitudinal studies, finding that the use of biomarkers was prevalent and a small number of nanomaterials had been tested (including but not limited to silver, CNTs, and TiO2), and all suffered from limited exposure assessment and sample size (Liou et al. 2015).
Nanomaterial usage is increasingly diversified, with many nanomaterials potentially having several different uses for multiple key industries. Thus, identification of an appropriate sample group for epidemiological studies will remain a challenge for the near term (Schulte et al. 2009), and significantly more data and studies are needed in this area. Advances in the field of ENM worker epidemiology are presented as a timeline in Fig. 9.
Fig. 9.
Progress in ENM-related epidemiology
Conclusions
Although a great deal of progress has been made in NanOEH during the past two decades, further research and development are still required in each of the eight identified areas. There are now many published toxicology reports, which have identified possible mechanisms of action. Large numbers of diverse materials and models have been tested, but the potential hazards of the vast majority of nanomaterials are unknown. To build on the work thus far, it is necessary to synthesize a standard approach that combines knowledge of hazards, exposures, and risk assessment, which can then be used to formalize a clear decision-making logic model that will allow prioritization of resources to manage ENM-associated risks (Kuempel et al. 2012b). There is a substantial lack of long-term animal studies, which weakens the strength of the toxicological findings. Although sampling and analytical instrumentation and methodology have become increasingly sophisticated, allowing for better occupational exposure assessments and more laboratory precision, metrology, similarly, needs to be evaluated and standardized. Common ENM reference materials and study protocols need to be adopted to enable data to be more easily compared, interpreted, and applied.
Although exposure assessment methods with sophisticated approaches and logics have been developed, the total volume of data is still small and remains isolated, preventing meta-analysis. As much of the analysis has been focused on a few classes of nanomaterials, such as CNTs, attention should be paid to a broader range of ENMs. Adoption of forward-looking risk management approaches, such as control banding, and more standardized analytical frameworks to characterize ENMs has been a significant step in coping with uncertainty about ENMs. The best occupational risk management frameworks are still broad and would benefit from further refinement.
Although the usefulness of medical surveillance for workers exposed to ENMs is accepted among health practitioners, a basis for specific medical screening guidance is still lacking. Epidemiologic investigation of the nanomaterials workforce is a relatively young field and suffers from a deficit of data (such as information on exposures with types of industries/processes/job tasks). However, a framework for designing, executing, and analyzing studies has taken form in the literature. Finally, a critical measure beyond these eight areas is global compliance with available risk management guidance. This guidance is important, because there are still many uncertainties about potential health effects of ENMs. Few studies have examined the level of adherence to such guidelines by academia and industry, and such assessments are crucial to understand whether the knowledge gained from toxicological research and workplace exposure assessments is being disseminated in a way that is both comprehensible and practical for use by employers and in helping to protect the workers.
Responsible development of nanotechnology requires protection of workers at all stages of technological development. In each of the functional areas assessed, progress has been made but more is required. While our knowledge has grown, there is still a need for a better and faster hazard assessment, capabilities to handle of diverse ENMs and matrices, and understanding of actual worker exposures, in addition to a more standardized system of methods to allow these data to be more effectively gathered and compared by different researchers in distant locations. A maturing NanOEH field has two critical and interdependent needs: (1) continuing targeted and high-quality research into the areas of toxicology, metrology, medical surveillance, and epidemiology, and (2) harmonization of methods for conducting assays, assessing exposure, and assessing risk. A good start has been made, but more effort is needed.
Acknowledgments
The authors thank Nicole Romero for assistance with the figures.
Footnotes
Disclaimer The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Contributor Information
P. A. Schulte, Email: pas4@cdc.gov, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV, USA.
G. Roth, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV, USA
L. L. Hodson, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV, USA
V. Murashov, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV, USA
M. D. Hoover, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV, USA
R. Zumwalde, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV, USA
E. D. Kuempel, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV, USA
C. L. Geraci, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV, USA
A. B. Stefaniak, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV, USA
V. Castranova, School of Pharmacy, West Virginia University, Morgantown, WV, USA
J. Howard, National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention, Morgantown, WV, USA
References
- Amdur MO, Chen LC, Guty J, Lam HF, Miller PD. Speciation and pulmonary effects of acidic SOx formed on the surface of ultrafine zinc oxide aerosols. Atmos Environ (1967) 1988;22:557–560. [Google Scholar]
- Aschberger K, Christensen FM. Approaches for establishing human health no effect levels for engineered nanomaterials. J Phys. 2011;304:012078. [Google Scholar]
- BAM Bundestalt für material forschung and prüfung. http://www.nano-refmat.bam.de/en/category_10_nanobjects_nanoparticles_nanomaterials.htm. [Google Scholar]
- Bekker C, Kuijpers E, Brouwer DH, Vermeulen R, Fransman W. Occupational exposure to nano-objects and their agglomerates and aggregates across various life cycle stages. A Broad-Scale Exposure Study. Ann Occup Hyg. 2015;59:681–704. doi: 10.1093/annhyg/mev023. [DOI] [PubMed] [Google Scholar]
- Bergamaschi E. Occupational exposure to nanomaterials: present knowledge and future development. Nanotoxicology. 2009;3:194–201. [Google Scholar]
- Boutou-Kempf O, Marchand JL, Radauceanu A, Witschger O, Imbernon E. Group Health Risks of N Development of a French epidemiological surveillance system of workers producing or handling engineered nanomaterials in the workplace. J Occup Environ Med/Am Coll Occup Environ Med. 2011;53:S103–S107. doi: 10.1097/JOM.0b013e31821b1d68. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, et al. Minimal analytical characterization of engineered nanomaterials needed for hazard assessment in biological matrices. Nanotoxicology. 2011;5:1–11. doi: 10.3109/17435391003775266. [DOI] [PubMed] [Google Scholar]
- Brenner SA, Neu-Baker NM, Caglayan C, Zurbenko IG. Occupational exposure to Airborne nanomaterials: an assessment of worker exposure to aerosolized metal oxide nanoparticles in semiconductor Wastewater treatment. J Occup Environ Hyg. 2015;12:469–481. doi: 10.1080/15459624.2015.1018515. [DOI] [PubMed] [Google Scholar]
- British Standards Institute (BSI) Nanotechnologies—Part 2: guide to safe handling and disposal of manufactured nanomaterials. London: British Standards Institution; 2007. [Google Scholar]
- Brouwer D. Exposure to manufactured nanoparticles in different workplaces. Toxicology. 2010;269:120–127. doi: 10.1016/j.tox.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Brouwer D, van Duuren-Stuurman B, Berges M, Jankowska E, Bard D, Mark D. From workplace air measurement results toward estimates of exposure? Development of a strategy to assess exposure to manufactured nano-objects. J Nanopart Res. 2009;11:1867–1881. [Google Scholar]
- Brouwer D, et al. Harmonization of measurement strategies for exposure to manufactured nano-objects; report of a workshop. Ann Occup Hyg. 2012;56:1–9. doi: 10.1093/annhyg/mer099. [DOI] [PubMed] [Google Scholar]
- Canu IG, Boutou-Kempf O, Delabre L, Ducamp S, Iwatsubo Y, Marchand JL, Imbernon E. French registry of workers handling engineered nanomaterials as an instrument of integrated system for surveillance and research. J Phys. 2013;429:012066. [Google Scholar]
- Castillo AMPD. Nanomaterials and workplace health & safety: What are the issues for workers? European Trade Union Institute; 2013. [Google Scholar]
- Cattaneo AG, Gornati R, Sabbioni E, Chiriva-Internati M, Cobos E, Jenkins MR, Bernardini G. Nanotechnology and human health: risks and benefits. J Appl Toxicol. 2010;30:730–744. doi: 10.1002/jat.1609. [DOI] [PubMed] [Google Scholar]
- Dahm MM, Evans DE, Schubauer-Berigan MK, Birch ME, Fernback JE. Occupational exposure assessment in carbon nanotube and nanofiber primary and secondary manufacturers. Ann Occup Hyg. 2012;56:542–556. doi: 10.1093/annhyg/mer110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm MM, Schubauer-Berigan MK, Evans DE, Birch ME, Fernback JE, Deddens JA. Carbon nanotube and nanofiber exposure assessments: an Analysis of 14 site visits. Ann Occup Hyg. 2015;59:705–723. doi: 10.1093/annhyg/mev020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankovic D, Kuempel E, Wheeler M. An approach to risk assessment for TiO2 . Inhal Toxicol. 2007;1(19 Suppl):205–212. doi: 10.1080/08958370701497754. [DOI] [PubMed] [Google Scholar]
- De Lorenzo AJD. The olfactory nerve and the blood-brain barrier. In: Wolstenholme GEW, Knight J, editors. Taste and smell in vertebrates. London: Churchill; 1970. pp. 151–176. [Google Scholar]
- Donaldson K, Li XY, MacNee W. Ultrafine (nanometre) particle mediated lung injury. J Aerosol Sci. 1998;29:553–560. [Google Scholar]
- Donaldson K, Stone V, Tran CL, Kreyling W, Borm PJ. Nanotoxicology. Occup Environ Med. 2004;61:727–728. doi: 10.1136/oem.2004.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll KE. Role of inflammation in the development of rat lung tumors in response to chronic particle exposure. Inhal Tox. 1996;8:139. [Google Scholar]
- Duffin R, Tran C, Clouter A, Brown D, MacNee W, Stone V, Donaldson K. The importance of surface area and specific reactivity in the acute pulmonary inflammatory response to particles. Ann Occup Hyg. 2002;46:242–245. [Google Scholar]
- Environmental Defense—DuPont Nano Partnership. NANO risk framework. Washington, DC: 2007. [Google Scholar]
- European Commission on Employment SAI. Guidance on the protection of the health and safety of workers from the potential risks related to nanomaterials at work—Guidance for employers and health and safety practitioners. Brussels: European Commission; 2014a. [Google Scholar]
- European Commission on Employment SAI. Working safely with manufactured nanomaterials—guidance for workers. Brussels: European Commission; 2014b. [Google Scholar]
- Federal Ministry for Economic Affairs and Energy. [Accessed 16 Nov 2015]; http://www.nanorefmat.bam.de/en/category_10_nanoobjects_nanoparticles_nanomaterials.htm. [Google Scholar]
- Finnish Institute of Occupational Health. NANOSH. Finnish Institute of Occupational Health; 2010. [Accessed 2 Dec 2015]. http://www.ttl.fi/partner/nanosh/sivut/default.aspx. [Google Scholar]
- Fischman M, Storey E, McCunney RJ, Kosnett M. National Institute for Occupational Safety and Health Nanomaterials and Worker Health Conference–medical surveillance session summary report. J Occup Environ Med/Am Coll Occup Environ Med. 2011;53:S35–S37. doi: 10.1097/JOM.0b013e31821b1b0a. [DOI] [PubMed] [Google Scholar]
- Fissan H, Neumann S, Trampe A, Pui DYH, Schin WG. Rationale and principle of an instrument measuring lung deposited nanoparticle surface area. J Nanopart Res. 2007;9:55–59. [Google Scholar]
- Germany Federal Institute for Materials Research and Testing [BAM] Nanoscaled Reference Materials. [Accessed 16 Nov 2015];Federal Institute for Materials Research and Testing, Federal Ministry for Economic Affairs and Energy. 2015 http://www.nano-refmat.bam.de/en/category_10_nanoobjects_nanoparticles_nanomaterials.htm. [Google Scholar]
- Gordon SC, Butala JH, Carter JM, Elder A, Gordon T, Sayre PG, et al. Workshop report: strategies for setting occupational exposure limits for engineered nanomaterials. Reg Toxicol Pharmcol. 2014;68:305–311. doi: 10.1016/j.yrtph.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Grieger KD, Sayes CM, Chen E, Ensor DS, Jayanty R. Safe Handling of Engineered Nanomaterials: Turning Knowledge into Practice. 2015 [Google Scholar]
- Groso A, Meyer T. Concerns related to safety management of engineered nanomaterials in research environment. J Phys. 2013;429:012065. [Google Scholar]
- Groso A, Petri-Fink A, Magrez A, Riediker M, Meyer T. Management of nanomaterials safety in research environment. Part Fibre Toxicol. 2010;7:40. doi: 10.1186/1743-8977-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulumian M, Kuempel ED, Savolainen K. Global challenges in the risk assessment of nanomaterials: relevance to South Africa. S Afr J Sci. 2012;108:1–9. [Google Scholar]
- Gwinn MR, Vallyathan V. Nanoparticles: health effects: pros and cons. Environ Health Perspect. 2006 doi: 10.1289/ehp.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassellöv M, Readman JW, Ranville JK, Tiede K. Nanoparticle analysis and characterization methodologies in environmental risk assessment of engineered nanoparticles. Exotoxicology. 2008;17:344–361. doi: 10.1007/s10646-008-0225-x. [DOI] [PubMed] [Google Scholar]
- Health Council of the Netherlands. Working with nanoparticles: exposure registry and health monitoring. The Hague: Health Council of the Netherlands; 2012. [Google Scholar]
- Heinrich U, Fuhst R, Rittinghausen S, Creutzenberg O, Bellmann B, Koch W, Levsen K. Chronic inhalation exposure of wistar rates and 2 different strains of mice to diesel-engine exhaust, carbon-black, and titaniumdioxide. Inhal Toxicol. 1995;7:533–556. [Google Scholar]
- Hett A. Nanotechnology: small matter, many unknowns. Zurich: Swiss Reinsurance Company; 2004. [Google Scholar]
- Howard J, Murashov V. National nanotechnology partnership to protect workers. J Nanopart Res. 2009;11:1673–1683. [Google Scholar]
- HSE. Nanoparticles: an occupational hygiene review. Norwich: 2004. [Google Scholar]
- HSE. Using nanomaterials at work vol HSG272. Norwich: UK health and safety executive (HSE); 2013. [Google Scholar]
- Iavicoli I, Leso V, Manno M, Schulte PA. Biomarkers of nanomaterial exposure and effect: current status. J Nanopart Res. 2014;16:1–33. [Google Scholar]
- Institute for Occupational Safety and Health of the German Social Accident Insurance (IFA) Criteria for assessment of the effectiveness of protective measures. [Accessed 20 Oct 2015];2009 http://www.dguv.de/ifa/Fachinfos/Nanopartikel-am-Arbeitsplatz/Beurteilung-von-Schutzmaßnahmen/index-2.jsp. [Google Scholar]
- International Organization for Standardization (ISO) ISO/TR 12885:2008—Nanotechnologies—Health and safety practices in occupational settings relevant to nanotechnologies. 2008a [Google Scholar]
- International Organization for Standardization (ISO) ISO/TS 27687:2008—Nanotechnologies—Terminology and definitions for nano-objects—Nanoparticle, nanofibre and nanoplate. 2008b [Google Scholar]
- International Organization for Standardization (ISO) ISO/TC 229 Nanotechnologies. 2012 [Google Scholar]
- International Organization for Standardization (ISO) ISO/TS 12901-2—Nanotechnologies—Occupational risk management applied to engineered nanomaterials—Part 2: use of the control banding approach. Switzerland: 2013. [Google Scholar]
- Jiang J, Oberdörster G, Biswas P. Characterization of size, surface charge, and agglomeration state of nanoparticle dispersions for toxicological studies. J Nanopart Res. 2008;11:77–89. [Google Scholar]
- Joint Research Centre. JRC Nanomaterials Repository. [Accessed 2 Dec 2015];Joint Research Centre, European Commission. 2014 https://ec.europa.eu/jrc/en/scientific-tool/jrc-nanomaterials-repository. [Google Scholar]
- Journeay WS, Goldman RH. Occupational handling of nickel nanoparticles: a case report. Am J Ind Med. 2014;57:1073–1076. doi: 10.1002/ajim.22344. [DOI] [PubMed] [Google Scholar]
- Kaiser J-P, Roesslein M, Diener L, Wick P. Human health risk of ingested nanoparticles that are added as multifunctional agents to paints: an in vitro study. PloS One. 2013;8:e83215. doi: 10.1371/journal.pone.0083215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreyling W, et al. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health Part A. 2002a;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- Kreyling W, Semmler M, Erbe F, Mayer P, Takenaka S, Oberdörster G, Ziesenis A. Minute translocation of inhaled ultrafine insoluble iridium particles from lung epithelium to extrapulmonary tissues. Ann Occup Hyg. 2002b;46:223–226. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- Kreyling WG, Hirn S, Schleh C. Nanoparticles in the lung. Nat Biotechnol. 2010;28:1275–1276. doi: 10.1038/nbt.1735. [DOI] [PubMed] [Google Scholar]
- Krug HF. Nanosafety research—are we on the right track? Angew Chem. 2014;53:12304–12319. doi: 10.1002/anie.201403367. [DOI] [PubMed] [Google Scholar]
- Krug HF, Wick P. Nanotoxicology: an interdisciplinary challenge. Angewandte Chemie. 2011;50:1260–1278. doi: 10.1002/anie.201001037. [DOI] [PubMed] [Google Scholar]
- Kuempel ED, Tran CL, Castranova V, Bailer AJ. Lung dosimetry and risk assessment of nanoparticles: evaluating and extending current models in rats and humans. Inhal Toxicol. 2006;18:717–724. doi: 10.1080/08958370600747887. [DOI] [PubMed] [Google Scholar]
- Kuempel E, Geraci C, Schulte P. Nanotechnology-toxicological issues and environmental safety and environmental safety. Dordrecht: Springer; 2007. Risk assessment approaches and research needs for nanomaterials: an examination of data and information from current studies; pp. 119–145. [Google Scholar]
- Kuempel ED, Castranova V, Geraci CL, Schulte PA. Development of risk-based nanomaterial groups for occupational exposure control. J Nanopart Res. 2012a;14:1–15. doi: 10.1007/s11051-012-1029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuempel ED, Geraci CL, Schulte PA. Risk assessment and risk management of nanomaterials in the workplace: translating research to practice. Ann Occup Hyg. 2012b;56:491–505. doi: 10.1093/annhyg/mes040. [DOI] [PubMed] [Google Scholar]
- Kuhlbusch TA, Neumann S, Fissan H. Number size distribution, mass concentration, and particle composition of PM1, PM2.5, and PM10 in bag filling areas of carbon black production. J Occup Environ Hyg. 2004;1:660–671. doi: 10.1080/15459620490502242. [DOI] [PubMed] [Google Scholar]
- Kuhlbusch TA, Asbach C, Fissan H, Gohler D, Stintz M. Nanoparticle exposure at nanotechnology workplaces: a review. Part Fibre Toxicol. 2011;8:22. doi: 10.1186/1743-8977-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulinowski K, Lippy B. Training workers on risks of nanotechnology. Bethesda: DHHS; 2011. [Google Scholar]
- Laney AS, McCauley LA, Schubauer-Berigan MK. Workshop summary: epidemiologic design strategies for studies of nanomaterial workers Journal of occupational and environmental medicine/American College of. Occup Environ Med. 2011;53:S87–S90. doi: 10.1097/JOM.0b013e31821b1af5. [DOI] [PubMed] [Google Scholar]
- Lee JH, Ahn K, Kim SM, Jeon KS, Lee JS, Yu IJ. Continuous 3-day exposure assessment of workplace manufacturing silver nanoparticles. J Nanopart Res. 2012;14:1–10. [Google Scholar]
- Li Z, et al. Cardiovascular effects of pulmonary exposure to single-wall carbon nanotubes. Environ Health Perspect. 2007;115:377–382. doi: 10.1289/ehp.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao HY, et al. Six-month follow-up study of health markers of nanomaterials among workers handling engineered nanomaterials. Nanotoxicology. 2014;8(Suppl 1):100–110. doi: 10.3109/17435390.2013.858793. [DOI] [PubMed] [Google Scholar]
- Linsinger TPJ, Roebben G, Solans C, Ramsch R. Reference materials for measuring the size of nanoparticles. TrAC Trends Anal Chem. 2011;30:18–27. [Google Scholar]
- Liou S-H, et al. Epidemiological study of health hazards among workers handling engineered nanomaterials. J Nanopart Res. 2012;14:1–15. [Google Scholar]
- Liou S-H, Tsai CSJ, Pelclova D, Schubauer-Berigan MK, Schulte PA. Assessing the first wave of epidemiological studies of nanomaterial workers. J Nanopart Res. 2015;17:1–19. doi: 10.1007/s11051-015-3219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux Research Inc. Nanotechnology Update: Corporations up their spending as revenues for nano-enabled products increase. New York: Lux Research Inc; 2014. [Google Scholar]
- Martin J, Bello D, Bunker K, Shafer M, Christiani D, Woskie S, Demokritou P. Occupational exposure to nanoparticles at commercial photocopy centers. J Haz Mat. 2015;298:351–360. doi: 10.1016/j.jhazmat.2015.06.021. [DOI] [PubMed] [Google Scholar]
- Maynard AD, Aitken RJ. Assessing exposure to airborne nanomaterials: current abilities and future requirements. Nanotoxicology. 2007;1:26–41. [Google Scholar]
- Maynard AD, Kuempel ED. Airborne nanostructured particles and occupational health. J Nanopart Res. 2005;7:587–614. [Google Scholar]
- Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single- walled carbon nanotube material. J Toxicol Environ Health Part A. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- Maynard AD, et al. Safe handling of nanotechnology. Nature. 2006;444:267–269. doi: 10.1038/444267a. [DOI] [PubMed] [Google Scholar]
- Methner MM, Birch ME, Evan DE, Ku BK, Crouch KG, Hoover MD. Case study: identification and Characterization of potential sources of worker exposure to carbon nanofibers during polymer composite laboratory operations. In: Mazzuckeli LF, editor. J Occup Environ Hyg. 12. Vol. 4. 2007. pp. 0125–0130. [DOI] [PubMed] [Google Scholar]
- Meli F, Klein T, Buhr E, Frase CG, Gleber G, Krumrey M, et al. Traceable size determination of nanoparticles, a comparison among European metrology institutes. Meas Sci Technol. 2012;23(12) http://iopscience.iop.org/article/10.1088/0957-0233/23/12/12005/meta. [Google Scholar]
- Mercer RR, et al. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part Fibre Toxicol. 2010;7:28. doi: 10.1186/1743-8977-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer RR, et al. Extrapulmonary transport of MWCNT following inhalation exposure. Part Fibre Toxicol. 2013;10:38. doi: 10.1186/1743-8977-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methner MM. Engineering case reports. Effectiveness of local exhaust ventilation (LEV) in controlling engineered nanomaterial emissions during reactor cleanout operations. J Occup Environ Hyg. 2008;5:D63–D69. doi: 10.1080/15459620802059393. [DOI] [PubMed] [Google Scholar]
- Methner MM, Birch ME, Evans DE, Ku BK, Crouch K, Hoover MD. Identification and characterization of potential sources of worker exposure to carbon nanofibers during polymer composite laboratory operations. J Occup Environ Hyg. 2007;4:D125–D130. doi: 10.1080/15459620701683871. [DOI] [PubMed] [Google Scholar]
- Methner M, Hodson L, Dames A, Geraci C. Nanoparticle emission assessment technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials—Part B: results from 12 field studies. J Occup Environ Hyg. 2009;7:163–176. doi: 10.1080/15459620903508066. [DOI] [PubMed] [Google Scholar]
- Methner M, Hodson L, Dames A, Geraci C. Nanoparticle Emission Assessment Technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials–Part B: results from 12 field studies. J Occup Environ Hyg. 2010a;7:163–176. doi: 10.1080/15459620903508066. [DOI] [PubMed] [Google Scholar]
- Methner M, Hodson L, Geraci C. Nanoparticle emission assessment technique (NEAT) for the identification and measurement of potential inhalation exposure to engineered nanomaterials—part A. J Occup Environ Hyg. 2010b;7:127–132. doi: 10.1080/15459620903476355. [DOI] [PubMed] [Google Scholar]
- Moller KL, Thygesen LC, Schipperijn J, Loft S, Bonde JP, Mikkelsen S, Brauer C. Occupational exposure to ultrafine particles among airport employees–combining personal monitoring and global positioning system. PLoS One. 2014;9:e106671. doi: 10.1371/journal.pone.0106671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow PE. Possible mechanisms to explain dust overloading of the lungs. Toxicol Sci. 1988;10:369–384. doi: 10.1016/0272-0590(88)90284-9. [DOI] [PubMed] [Google Scholar]
- Murashov V, Schulte P, Howard J. Progression of occupational risk management with advances in nanomaterials. J Occup Environ Hyg. 2012;9:D12–D22. doi: 10.1080/15459624.2012.638217. [DOI] [PubMed] [Google Scholar]
- Nakanishi J, et al. Risk Assessment of the Carbon Nanotube Group Risk analysis: an official publication of the Society for Risk Analayis. Risk Anal. 2015 doi: 10.1111/risa.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasterlack M. Role of medical surveillance in risk management. J Occup Environ Med/Am Coll Occup Environ Med. 2011;53:S18–S21. doi: 10.1097/JOM.0b013e31821b1d54. [DOI] [PubMed] [Google Scholar]
- Nasterlack M, Zober A, Oberlinner C. Considerations on occupational medical surveillance in employees handling nanoparticles. Int Arch Occup Environ Health. 2008;81:721–726. doi: 10.1007/s00420-007-0245-5. [DOI] [PubMed] [Google Scholar]
- Nel A, Xia T, Madler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- Nel AE, et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- Netherlands National Institute for Public Health and the Environment. Nanotechnology. Netherlands National Institute for Public Health and the Environment, Ministry of Health, Welfare, and Sport; 2014. [Accessed 2015 Oct 30]. http://www.rivm.nl/en/Topics/N/Nanotechnology/Workplace. [Google Scholar]
- NIOSH. Approaches to safe nanotechnology: managing the health and safety concerns associated with engineered nanomaterials. Cincinnati, OH: DHHS (NIOSH); 2009a. [Google Scholar]
- NIOSH. Current intelligence bulletin 60: interim guidance for medical screening and hazard surveillance for workers potentially exposed to engineered nanoparticles. 60. Cincinnati, OH: DHHS (NIOSH); 2009b. [Google Scholar]
- NIOSH. Current intelligence bulletin 63: occupational exposure to Titanium Dioxide. 63. Cincinnati, OH: DHHS (NIOSH); 2011. [Google Scholar]
- NIOSH. Current strategies for enginnering controls in nanomaterial production and downstream handling processes, 2014-02 edn. Cincinnati, OH: DHHS (NIOSH); 2013a. [Google Scholar]
- NIOSH. Occupational exposure to carbon nanotubes and nanofibers. 65. Cincinnati, OH: DHHS (NIOSH); 2013b. [Google Scholar]
- NIST (National Institute of Standards and Technology) NIST reference materials are ‘Gold Stand’ for bio-nanotech. [Accessed 5 June 2016];2008 http://www.nist.gov/pml/div683/gold/010808.cfm.
- NNI. Report of the National Nanotechnology Initiative Workshop November 17–18, 2009. Washington, DC: 2009. Nanomaterials and human health instrumentation, metrology, and analytical methods. [Google Scholar]
- Nurkiewicz TR, Porter DW, Hubbs AF, Cumpston JL, Chen BT, Frazer DG, Castranova V. Nanoparticle inhalation augments particle-dependent systemic microvascular dysfunction. Part Fibre Toxicol. 2008;5:1. doi: 10.1186/1743-8977-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Yu CP. The carcinogenic potential of inhaled diesel exhaust: a particle effect? J Aerosol Sci. 1990;21:S397–S401. [Google Scholar]
- Oberdörster G, Ferin J, Gelein R, Soderholm SC, Finkelstein J. Role of the alveolar macrophage in lung injury: studies with ultrafine particles. Environ Health Perspect. 1992;97:193. doi: 10.1289/ehp.97-1519541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Ferin J, Soderholm S, Gelein R, Cox C, Baggs R, Morrow PE. Increased pulmonary toxicity of inhaled ultrafine particles: due to lung overload alone? Ann Occup Hyg. 1994;38:295–302. [Google Scholar]
- Oberdörster G, et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: elements of a screening strategy. Part Fibre Toxicol. 2005a;2:8. doi: 10.1186/1743-8977-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ Health Perspect. 2005b;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development. Guidance and grouping of chemicals. 80. Paris: 2007. [Google Scholar]
- Organisation for Economic Co-operation and Development. Important issues on risk assessment of manufactured nanomaterials. Vol. 33. Paris: 2012. [Google Scholar]
- Organisation for Economic Co-operation and Development. Harmonized tiered approach to measure and assess the potential exposure to airborne emissions of engineered nano-objects and their agglomerates and aggregates at workplaces. Paris, France: Organisation for Economic Co-operation and Development; 2015. [Google Scholar]
- Ostiguy C, Roberge B, Ménard L, Endo C. Best Practices Guide to Synthetic Nanoparticle Risk Management, L’Institut de recherche Robert-Sauvé en santé et en sécurité du travail (IRSST) Report R-599. 2009 [Google Scholar]
- Ostro B, Hu J, Goldberg D, Reynolds P, Hertz A, Bernstein L, Kleeman MJ. Associations of mortality with long-term exposures to fine and ultrafine particles, species and sources: results from the California Teachers Study Cohort. Environ Health Perspect. 2015;123:549–556. doi: 10.1289/ehp.1408565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik SY, Zalk DM, Swuste P. Application of a pilot control banding tool for risk level assessment and control of nanoparticle exposures. Ann Occup Hyg. 2008;52:419–428. doi: 10.1093/annhyg/men041. [DOI] [PubMed] [Google Scholar]
- Palomaki J, et al. Long, needle-like carbon nanotubes and asbestos activate the NLRP3 inflammasome through a similar mechanism. ACS Nano. 2011;5:6861–6870. doi: 10.1021/nn200595c. [DOI] [PubMed] [Google Scholar]
- Pauluhn J. Multi-walled carbon nanotubes (Baytubes): approach for derivation of occupational exposure limit. Regul Toxicol Pharm. 2010;57:78–89. doi: 10.1016/j.yrtph.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Pelclova D, Fenclova Z, Vickova Stepanka, Zdimal V, Schwarz J, Pusman J, Zikova N, Syslova K, Kuzma M, Navatil T, Zakharov S, Kacer P. Markers of oxidative stress are elevated in workers exposed to nanoparticles; NANOCON 2012, Conference Proceedings; 2012. pp. 654–658. http://www.nanocon.eu/files/proceedings/04/reports/628.pdf. [Google Scholar]
- Ponce Del Castillo AM. ETUI Policy Brief Issue 2/2011. Brussels: 2011. Nano governance: how should the EU implement nanomaterial traceability. [Google Scholar]
- Peters A, et al. Elevated particle number concentrations induce immediate changes in heart rate variability: a panel study in individuals with impaired glucose metabolism or diabetes. Part Fibre Toxicol. 2015;12:7. doi: 10.1186/s12989-015-0083-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy S, King WP, Eimer BC, Shaffer RE. Filtration performance of NIOSH-approved N95 and P100 filtering facepiece respirators against 4 to 30 nanometer-size nanoparticles. J Occup Environ Hyg. 2008;5:556–564. doi: 10.1080/15459620802275387. [DOI] [PubMed] [Google Scholar]
- Riediker M, et al. A road map toward a globally harmonized approach for occupational health surveillance and epidemiology in nanomaterial workers. J Occup Environ Med/Am Coll Occup Environ Med. 2012;54:1214–1223. doi: 10.1097/JOM.0b013e31826e27f1. [DOI] [PubMed] [Google Scholar]
- Roco MC, Bainbridge WS. Societal Implications of Nanoscience and Nanotechnology. In: Foundation NS, editor. Nanoscale Science, Engineering, and Technology Workshop Report. Virginia: Arlington; 2001. [Google Scholar]
- Roebben G, Rasmussen K, Kestens V, Linsinger TPJ, Rauscher H, Emons H, Stamm H. Reference materials and representative test materials: the nanotechnology case. J Nanopart Res. 2013;15:1–13. [Google Scholar]
- Rouse JG, Yang J, Ryman-Rasmussen JP, Barron AR, Monteiro-Riviere NA. Effects of mechanical flexion on the penetration of fullerene amino acid-derivatized peptide nanoparticles through skin. Nano Lett. 2007;7:155–160. doi: 10.1021/nl062464m. [DOI] [PubMed] [Google Scholar]
- Ryman-Rasmussen JP, et al. Inhaled carbon nanotubes reach the subpleural tissue in mice. Nat Nanotechnol. 2009;4:747–751. doi: 10.1038/nnano.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe Work Australia. Engineered nanomaterials: evidence on the effectiveness of workplace controls toprevent exposure. Australia: Commonwealth of Australia; 2009. [Google Scholar]
- Safe Work Australia. Engineered nanomaterials: feasibility of establishing exposure standards and using control banding in Australia. Australia: Commonwealth of Australia; 2010. [Google Scholar]
- Sargent LM, et al. Promotion of lung adenocarcinoma following inhalation exposure to multi-walled carbon nanotubes. Part Fibre Toxicol. 2014;11:b114. doi: 10.1186/1743-8977-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen K, et al. Nanotechnologies, engineered nanomaterials and occupational health and safety—a review. Saf Sci. 2010a;48:957–963. [Google Scholar]
- Savolainen K, Alenius H, Norppa H, Pylkkanen L, Tuomi T, Kasper G. Risk assessment of engineered nanomaterials and nanotechnologies–a review. Toxicology. 2010b;269:92–104. doi: 10.1016/j.tox.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Savolainen K, et al. Nanosafety in Europe 2015–2025: towards safe and sustainable nanomaterials and nanotechnology innovations. Helsinki: Finnish Institute of Occupational Health; 2013. [Google Scholar]
- Schins RP, Knaapen AM. Genotoxicity of poorly soluble particles. Inhal Toxicol. 2007;19(Suppl 1):189–198. doi: 10.1080/08958370701496202. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Salamanca-Buentello F. Ethical and scientific issues of nanotechnology in the workplace. Environ Health Perspect. 2006;115:5–12. doi: 10.1289/ehp.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte PA, Trout D, Zumwalde R, Kuempel E, Geraci CL, Castranova V, et al. Options for occupational health surveillance of workers potentially exposed to engineered nanoparticles: state of the science. J Occup Environ Med. 2008;50:517–526. doi: 10.1097/JOM.0b013e31816515f7. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Schubauer-Berigan MK, Mayweather C, Geraci CL, Zumwalde R, McKernan JL. Issues in the development of epidemiologic studies of workers exposed to engineered nanoparticles. J Occup Environ Med. 2009;51:323–335. doi: 10.1097/JOM.0b013e3181990c2c. [DOI] [PubMed] [Google Scholar]
- Schulte PA, Murashov V, Zumwalde R, Kuempel ED, Geraci CL. Occupational exposure limits for nanomaterials: state of the art. J Nanopart Res. 2010;12:1971–1987. [Google Scholar]
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) The appropriateness of existing methodologies to assess the potential risks associated with engineered and adventitious products of nanotechnologies. [Accessed 5 June 2016];2005 http://ec.europa.edu/health/ph_risk/committees/04_scenhir/docs/scenhir_0_033.pdg. [Google Scholar]
- Seaton A. Nanotechnology and the occupational physician. Occup Med. 2006;56:312–316. doi: 10.1093/occmed/kql053. [DOI] [PubMed] [Google Scholar]
- Sellers K, Deleebeeck NME, Messiaen M, Jackson M, Bleeker EAJ, Sijm DTHM, Broekhuizen FAV. National Institute for Public Health and the Environment. The Netherlands: Bilthoven; 2015. Grouping nanomaterials: A strategy towards grouping and read-across. [Google Scholar]
- Shepard M, Brenner S. Cutaneous exposure scenarios for engineered nanoparticles used in semiconductor fabrication: a preliminary investigation of workplace surface contamination. Int J Occup Environ Health. 2014a;20:247–257. doi: 10.1179/2049396714Y.0000000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard MN, Brenner S. An occupational exposure assessment for engineered nanoparticles used in semiconductor fabrication. Ann Occup Hyg. 2014b;58:251–265. doi: 10.1093/annhyg/met064. [DOI] [PubMed] [Google Scholar]
- Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289(5):L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- Sirviö S, Savolainen K. NANODEVICE: novel concepts, methods, and technologies for the production of portable, easy-to-use devices for the measurement and analysis of airborne engineered nanoparticles in workplace air. J Phys. 2011;304:012081. [Google Scholar]
- Song Y, Li X, Du X. Exposure to nanoparticles is related to pleural effusion, pulmonary fibrosis and granuloma. Eur Respir J. 2009;34:559–567. doi: 10.1183/09031936.00178308. [DOI] [PubMed] [Google Scholar]
- Stefaniak AB, et al. Nanoscale reference materials for environmental, health and safety measurements: needs, gaps and opportunities. Nanotoxicology. 2013;7:1325–1337. doi: 10.3109/17435390.2012.739664. [DOI] [PubMed] [Google Scholar]
- Stone V, et al. ITS-NANO-Prioritising nanosafety research to develop a stakeholder driven intelligent testing strategy. Part Fibre Toxicol. 2014;11:9. doi: 10.1186/1743-8977-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi A, Hirose A, Nishimura T, Fukumori N, Ogata A, Ohashi N, Kitajima S, Kanno J. Induction of mesothelioma in p53± mouse by intraperitoneal application of multiwall carbon nanotubes. J Toxicol Sci. 2008;33:105–116. doi: 10.2131/jts.33.105. [DOI] [PubMed] [Google Scholar]
- The Royal Society and The Royal Academy of Engineering. Nanoscience and nanotechnologies: opportunities and uncertainties. London: The Royal Society and The Royal Academy of Engineering; 2004. [Google Scholar]
- Tran CL, Cullen RT, Buchanan D, Jones AD, Miller BG, Searl A, Davis JMG, Donaldson K. Contract Research Report 216/1999. Suffolk: Health and Safety Executive; 1999. Litigation and prediction of pulmonary responses to dust. Part II. In: investigations into the pulmonary effects of low toxicity dusts. Parts I and II. [Google Scholar]
- Trout DB. General principles of medical surveillance: implications for workers potentially exposed to nanomaterials Journal of occupational and environmental medicine/ American College of. Occup Environ Med. 2011;53:S22–S24. doi: 10.1097/JOM.0b013e31821b1e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UK Health and Safety Executive (HSE) Nanoparticles: an occupational hygiene review. Norwich: 2004. [Google Scholar]
- UK Health and Safety Executive [HSE] Using nanomaterials at work vol HSG272. Norwich: HSE; 2013. [Google Scholar]
- US National Institute of Standards and Technology [NIST] 308.1—Nanomaterials (less than or equal to 100 nm) US National Institute of Standards and Technology (NIST); 2015. [Accessed 13 Nov 2015]. https://www-s.nist.gov/srmors/viewTable.cfm?tableid=231. [Google Scholar]
- US National Institute of Standards and Technology [NIST], National Cancer Institute [NCI] NIST issues first nanoparticle reference materials for biomedical research. National Nanotechnology Institute (NNI); 2008. http://www.nano.gov/sites/default/files/nistissuesfirstnanoparticlereference materials.pdf. [Google Scholar]
- Weibel A, Bouchet R, Boulc’h F, Knauth P. The big problem of small particles: a comparison of methods for determination of particle size in nanocrystalline anatase powders. Chem Mater. 2005;17(9):2378–2385. [Google Scholar]
- Woodrow Wilson Center. The Project on Emerging Nanotechnologies: Consumer Product Inventory. [Accessed 30 Oct 2015];2013 http://www.nanotechproject.org/cpi/ [Google Scholar]
- World Health Organization (WHO) WHO Guidelines on Nanomaterials and Worker’s Health. 2015
- Yu IJ, Gulumian M, Shin S, Yoon TH, Murashov V. Occupational and environmental health effects of nanomaterials. Biomed Res Int. 2015;2015:789312. doi: 10.1155/2015/789312. [DOI] [PMC free article] [PubMed] [Google Scholar]