Abstract
Background
The optimization of medication use during care transitions represents an opportunity to improve overall health-related outcomes. The utilization of clinical pharmacists during care transitions has demonstrated benefit, although the optimal method of integration during the care transition process remains unclear.
Objective
To evaluate the impact of pharmacist-provided telephonic medication therapy management (MTM) on care quality in a care transitions program (CTP) for high-risk older adults.
Methods
This prospective, randomized, controlled study was conducted from December 8, 2011, through October 25, 2012, in a primary care work group at a tertiary care academic medical center in the midwestern United States. High-risk elderly (aged ≥60 years) patients were randomized to a pharmacist-provided MTM program via telephone or to usual care within an existing outpatient CTP. The primary outcome was the quality of medication prescribing and utilization based on the Screening Tool to Alert Doctors to the Right Treatment (START) and the Screening Tool of Older Persons’ Prescriptions (STOPP) scores. The secondary outcomes were medication utilization using a modified version of the Medication Appropriateness Index, hospital resource utilization within 30 days of discharge, and drug therapy problems.
Results
Of 222 eligible high-risk patients, 25 were included in the study and were randomized to the pharmacist MTM intervention (N = 13) or to usual care (N = 12). No significant differences were found between the 2 groups in medications meeting the STOPP or START criteria. At 30-day follow-up, no significant differences were found between the 2 cohorts in medication utilization quality indicators or in hospital utilization. At 30-day follow-up, 3 (13.6%) patients had an emergency department visit or a hospital readmission since discharge. In all, 22 patients completed the study. Medication underuse was common, with 20 START criteria absent medications evident for all 25 patients at baseline, representing 15 (60%) patients with ≥1 missing medications. Overall, 55 drug therapy problems were identified at baseline, 24 (43.6%) of which remained unresolved at 30-day follow-up.
Conclusion
The use of a pharmacist-provided MTM program did not achieve a significant difference compared with usual care in an existing CTP; however, the findings demonstrated frequent utilization of inappropriate medications as well as medication underuse, and many drug therapy problems remained unresolved. The small size of the study may have limited the ability to detect a difference between the intervention and usual care groups.
Keywords: care transition program, high-risk elderly patients, medication therapy management, medication utilization, pharmacist-based, START criteria, STOPP criteria, usual care
KEY POINTS
-
▸
Optimizing medication use and guidance by pharmacists during care transitions offer opportunities to improve patient outcomes.
-
▸
This study evaluated the impact of pharmacist-based MTM on the care of high-risk elderly patients during care transitions.
-
▸
Of 222 eligible patients, 25 were randomized to the pharmacist intervention or to usual care.
-
▸
At the 30-day follow-up, no significant differences were found between the 2 cohorts in medication utilization quality indicators or hospitalization.
-
▸
Overall, 3 patients had an emergency department visit or a hospital readmission since discharge.
-
▸
However, of the 55 drug therapy problems identified at baseline, 24 (43.6%) were still unresolved at 30-day follow-up.
-
▸
Based on the STOPP criteria, 68% of the patients were prescribed at least 1 inappropriate medication, a rate higher than in previous research.
-
▸
Furthermore, based on START criteria, 60% of patients had underused prescribed medications.
-
▸
These findings suggest a need to identify specific patient populations that may derive the most benefit from a pharmacist-based MTM program during care transitions.
A key measure of the 2010 Affordable Care Act is the improvement of care transitions within the healthcare continuum.1 Optimal medication utilization is important during these care transitions. Elderly patients have shown a higher risk for drug-related adverse events with certain medications.2–7 Thus, Medicare Part D prescription drug plans are required to offer medication therapy management (MTM) to patients who meet certain criteria.8,9 The Medicare criteria for MTM eligibility vary by plan type and by disease, and include a diagnosis of a minimum number of chronic diseases, the use of a specific minimum number of prescription medications, and the likelihood of exceeding a predetermined annual medication cost threshold.10 Patients in care transitions often meet the MTM eligibility criteria; however, the optimal model of pharmacist integration during the care transition process remains unclear.
Previous studies incorporated components of MTM into hospital discharge planning. Two studies have analyzed the impact of a pharmacist intervention via telephone shortly after hospital discharge.11,12 These studies suggest that a decrease in hospital utilization occurred within 30 days of discharge, with evidence of cost-savings related to follow-up by a pharmacist.11,12 In one study, home-based intervention with a nurse and a pharmacist in patients with congestive heart failure was associated with reductions in unplanned readmissions and out-of-hospital deaths within 6 months of hospital discharge.13 A targeted care bundle for high-risk elderly patients that included medication counseling and reconciliation with a clinical pharmacist was associated with a decrease in unplanned acute healthcare utilization up to 30 days after discharge.14 Zillich and colleagues used a telephonic MTM intervention for patients receiving home healthcare, which demonstrated a 3-fold reduction in hospital readmissions among the lowest-risk cohort.15
A variety of screening tools have been developed to improve medication quality and reduce the prevalence of drug-related adverse events. The Screening Tool of Older Persons’ Prescriptions (STOPP) was developed to identify potentially inappropriate medications, and the Screening Tool to Alert Doctors to the Right Treatment (START) was created to identify potential prescribing omissions.
The STOPP criteria include 65 standards that are used to identify potentially inappropriate medications in an elderly patient, including drug-to-disease interactions, drug-to-drug interactions, excessive doses of a medication, and excessive duration of medication use.16 For identifying potentially inappropriate medications, we selected the STOPP criteria instead of the Beers criteria, because recent studies revealed a greater correlation between drug-related adverse events and potentially inappropriate medications defined with the STOPP criteria than with the Beers criteria, suggesting that the STOPP criteria may be more helpful clinically.17 The START criteria assess potential prescribing omissions and identify medications that are clinically indicated for specific patient populations to encourage their proper prescribing.18
The STOPP and START tools are scored by totaling the number of medications that meet certain criteria, with each potentially inappropriate medication and potential prescribing omission generating 1 point. Previous research indicates that a 0.5 decrease in STOPP score yielded a 17% risk reduction in medication-related hospital admissions.19 Although the START criteria were developed using evidence-based disease management, the clinical impact of this intervention remains to be explored.
Our objective was to assess the impact of comprehensive pharmacist-provided telephonic MTM on care quality in an outpatient care transitions program (CTP) in high-risk adults aged ≥60 years.
Methods
In this prospective, randomized, controlled trial, patients were randomized in a 1 to 1 ratio to the MTM intervention or the usual care cohort. The study was conducted from December 8, 2011, through October 25, 2012, in a primary care work group at a tertiary care academic medical center in the midwestern United States. The primary care work group included the family medicine and primary care internal medicine departments. The study was reviewed and approved by the Institutional Review Board.
Patient Population
The target enrollment for the study was set at 50 independent-living elderly adults (aged ≥60 years) who were enrolled in the local CTP. The determination of eligibility for the CTP required stratification of patients during hospitalization using the Elders Risk Assessment (ERA) index, which was developed to identify elderly patients who are at high risk for an emergency department visit or a hospital readmission.20 Patients who were newly enrolled in the CTP were targeted for participation in this study.
Each patient was reimbursed $25 as compensation for the time commitment required for the completion of the study. Patients were offered enrollment in the CTP during their hospitalization if they were empaneled in the primary care work group, resided within a 20-minute drive, and were predicted to be at risk for high healthcare utilization (ie, had an ERA index score of ≥16). The study coordinator recruited participants for the study and obtained informed consent during hospitalization or via a telephone call shortly after discharge from the hospital.
Patients were randomly assigned to either the intervention group or to the usual care group by a study coordinator. Randomization was completed during the phone call by the study coordinator, who opened a sealed envelope that contained an indication of which group the patient was assigned to.
The study statistician used a random number generator to determine the allocation sequence. The trial was unblinded (ie, the participants and the investigators were aware of the intervention), and the patients received a telephone call from the pharmacist if they were randomized to the intervention group. However, all outcomes were assessed while blinded to the intervention or the usual care group allocations.
Data Collection
The intervention group received an MTM consultation with a pharmacist by telephone, preferably within 3 (and up to 7) business days after hospital discharge. This intervention was developed using successful methods of pharmacist integration during care transitions,11,12 while complementing the services of an existing CTP, to assess the impact on the quality of medication use. The pharmacist obtained the necessary information and clinical assessments from each patient's electronic medical record to complete a comprehensive review of all prescription, nonprescription, and herbal medications taken. This systematic review of medications included the identification, resolution, and prevention of drug-related problems, including adverse events or the use of potentially inappropriate medications.
In addition, the electronic medical record was investigated for potential prescribing omissions. This review was the foundation for the phone consultation with the patient to ensure medication optimization. Decisions were based on the pharmacist's clinical judgment after considering practice guidelines, 2 clinical support databases (Truven Health Analytics’ Micromedex21 and Wolters Kluwer Lexi-Drugs22), or the highest-quality evidence available, as well as patient preferences. Recommendations were communicated by the pharmacist via a secure messaging function within the electronic medical record to the CTP provider for review on completion of the phone consultation.
The study included 5 pharmacists—2 had delivered the interventions and 3 performed the analysis; all the pharmacists had pharmacy doctoral degrees and were required to complete an MTM certification program.
The usual care group was defined as the preexisting CTP without pharmacist intervention. Patients enrolled in the CTP during their hospitalization received a home visit by a nurse practitioner within 3 business days after their discharge. As part of the visit, the nurse practitioner reviewed the patient's medications and made changes as deemed appropriate. The changes were implemented directly or were discussed with the patient's primary care provider, depending on clinical judgment. Follow-up telephone calls at scheduled intervals were implemented, depending on the needs of the individual patients.
The study coordinators functioned only in a research capacity, independent of the pharmacist intervention or usual care groups. They performed recruitment and obtained consent from participants. They collected patient characteristics at baseline within 7 days of hospital discharge and at 5 weeks after the intervention with a follow-up phone call. The information collected by the study coordinator included demographic factors, such as age, race, marital status, medication adherence, and patient-reported hospital or emergency department utilization.
Outcome Measures
Two independent pharmacists assessed the medication quality indicators for each patient at baseline after hospital discharge (before pharmacist intervention and/or the nurse practitioner home visit) and at 30 days after discharge.
The primary outcome was to identify potentially inappropriate medications with the STOPP criteria and potential prescribing omissions using the START criteria.
The secondary outcomes included an assessment of medication utilization quality with the Medication Appropriateness Index (MAI), a 10-item instrument used to further assess each prescribed medication.23 Modifications to the MAI were similar to those used previously in another study.24 The assessment, which was reduced to 3 items to minimize interview burden, was based on the following questions:
Is there an indication for the drug?
Is the medication effective for the condition?
Is there unnecessary duplication with other drugs?
For the STOPP and START assessments, the medications involved included routinely administered systemic drugs other than topical treatments. The MAI assessment was applied to systemic drugs (other than over-the-counter or topical treatments) that were used on a regular basis.
Additional secondary end points included an assessment of healthcare resource utilization within 30 days of discharge, defined by (1) emergency department visits, (2) hospital readmissions, and (3) the composite of emergency department visits and hospital readmissions. These outcomes were self-reported during the 5-week follow-up telephone call from the study coordinator.
Medication adherence was determined by utilizing an adapted Morisky Medication Adherence Scale (MMAS) at baseline and at the 5-week follow-up phone call.25 The traditional MMAS was adapted to be a global assessment on medication adherence, in lieu of specific disease state medication adherence. The drug therapy problems that were identified during the pharmacist intervention were categorized using standard MTM definitions.26
The study data were managed using Research Electronic Data Capture (REDCap) tools that are hosted at our institution.27 REDCap is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources.
Statistical Analysis
The START and STOPP scores were calculated for each patient. The possible scores ranged from 0 to 22 for the START criteria and from 0 to 65 for the STOPP criteria. Using the standard deviation estimates of 2.1 and 1.6 for the START and STOPP scores,28 respectively, a total sample size of 25 patients would allow the detection of mean between-group differences of 1.7 and 1.3, respectively, with 80% power.
The statistical methods used to compare the groups were the Wilcoxon rank-sum test for continuous or discrete ordinal variables and the Pearson's chi-squared test for discrete nominal variables. In all cases, 2-tailed P values of <.05 were considered statistically significant. The continuous data are reported as the median (interquartile range), and the categorical data are reported as the frequency and percentage of the group represented. The data analysis was generated using SAS version 9.2 for UNIX (SAS Institute, Inc; Cary, NC).
Results
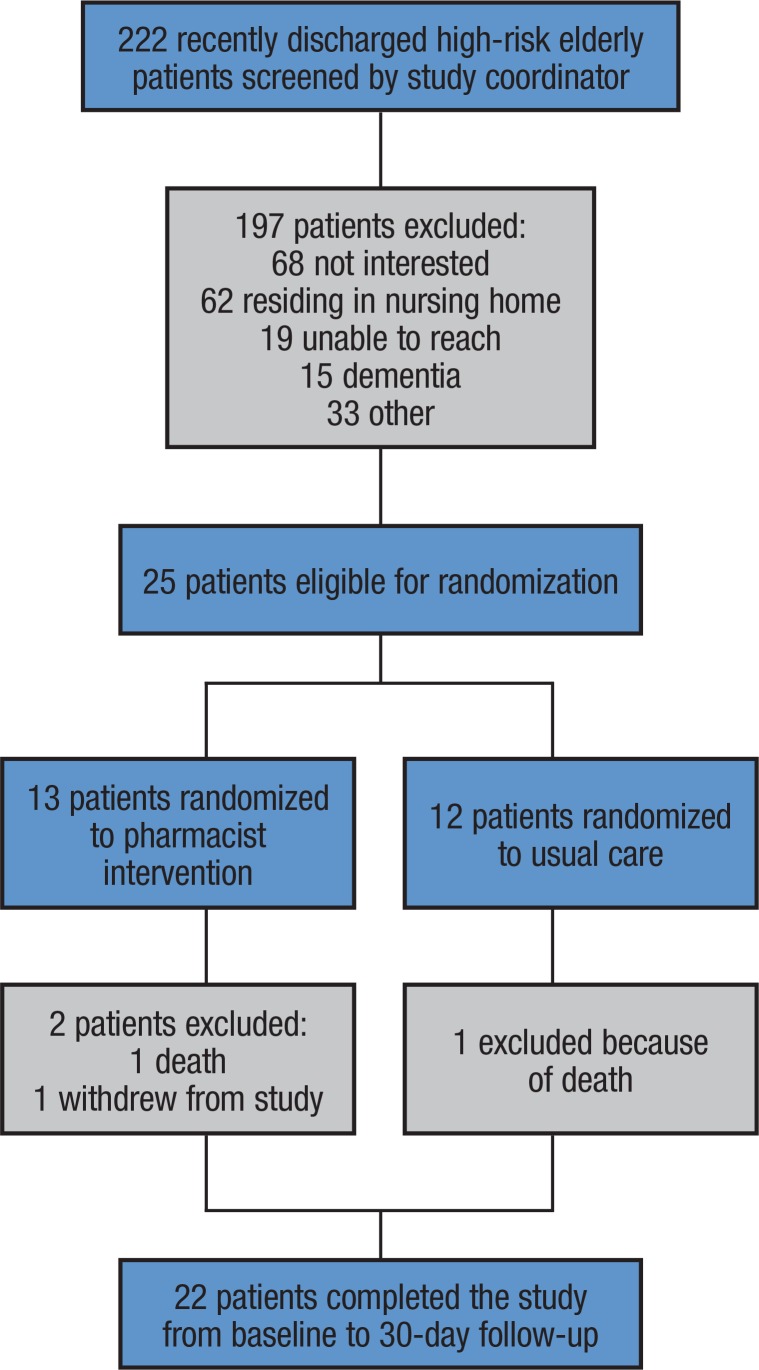
Of the 222 patients eligible for the study, 197 patients were excluded from participation because they were either uninterested, were residing in a skilled nursing facility, unable to reach, had a clinical diagnosis of dementia or a terminal illness, or were unable or unwilling to provide informed consent (Figure). Of the 25 patients who were randomized to the MTM intervention (N = 13) or to usual care (N = 12), 2 died (1 from each cohort) and 1 patient in the intervention group voluntarily withdrew from the study.
Figure. Study Population Diagram.
Baseline patient characteristics are listed in the Table in the Appendix (see www.AHDBonline.com). Table 1 compares baseline and 30-day follow-up outcomes for the 2 study cohorts. The study participants were predominantly male, white, and married, with a median age of approximately 84 years. At baseline, the patients in the pharmacist-intervention group used a median of 17 (interquartile range, 12–20) medications compared with 15.5 (interquartile range, 13–18.5) medications in the control group.
Table 1.
Assessment of 25 Recently Discharged, High-Risk Elderly Patients at Baseline and at 30-day Follow-up
| Baseline | 30-day follow-up visit | |||||
|---|---|---|---|---|---|---|
| Characteristic | Pharmacist intervention (N = 13) | Usual care (N = 12) | P value | Pharmacist intervention (N = 11) | Usual care (N = 11) | P value |
| Total medications listed on home medication list, median (IQR) | 17 (12–20) | 15.5 (13–18.5) | .96 | 18 (12–20) | 17 (13–18) | .95 |
| Total required daily doses, median (IQR) | 17 (14.5–20.5) | 13.8 (12–18) | .59 | 15.3 (11–20.5) | 14 (12–21) | .97 |
| Prescription medications assessed, median (IQR) | 9 (6–11) | 8.5 (7.5–10.5) | .68 | 9 (5–12) | 8 (8–11) | .87 |
| OTC/herbal medications assessed, median (IQR) | 3 (3–4) | 3 (2–4) | .59 | 3 (2–4) | 3 (2–4) | >.99 |
| Topical/as-needed medications excluded, median (IQR) | 4 (2–6) | 3 (2–5.5) | .98 | 5 (2–5) | 4 (3–6) | .59 |
| Do you sometimes forget to take any of your medications? | ||||||
| Yes, N (%) | 4 (31) | 1 (8) | .16 | 1 (9) | 2 (18) | .53 |
| No, N (%) | 9 (69) | 11 (92) | 10 (91) | 9 (82) | ||
| Over the past 2 weeks, were there any days you did not take your medications? | ||||||
| Yes, N (%) | 1 (8) | 0 (0) | .33 | 1 (9) | 0 (0) | .31 |
| No, N (%) | 12 (92) | 12 (100) | 10 (91) | 11 (100) | ||
| Have you ever cut back or stopped taking your medication without telling your doctor because you felt worse when you took it? | ||||||
| Yes, N (%) | 2 (15) | 0 (0) | .16 | 2 (18) | 0 (0) | .14 |
| No, N (%) | 11 (85) | 12 (100) | 9 (82) | 11 (100) | ||
| When you travel or leave home, do you sometimes forget to bring your medications? | ||||||
| Yes, N (%) | 1 (8) | 0 (0) | .33 | 0 (0) | 0 (0) | >.99 |
| No, N (%) | 12 (92) | 12 (100) | 11 (100) | 11 (100) | ||
| Did you take all of your medications yesterday? | ||||||
| Yes, N (%) | 12 (92) | 11 (92) | .95 | 10 (91) | 11 (100) | .31 |
| No, N (%) | 1 (8) | 1 (8) | 1 (9) | 0 (0) | ||
| Do you sometimes stop taking your medications because you feel they are no longer needed? | ||||||
| Yes, N (%) | 0 (0) | 1 (8) | .29 | 1 (9) | 0 (0) | .31 |
| No, N (%) | 13 (100) | 11 (92) | 10 (91) | 11 (100) | ||
| Taking medication every day is a real inconvenience for some people. Do you ever feel hassled about sticking to your medication plans? | ||||||
| Yes, N (%) | 1 (8) | 0 (0) | .33 | 2 (18) | 0 (0) | .14 |
| No, N (%) | 12 (92) | 12 (100) | 9 (82) | 11 (100) | ||
| How often do you have difficulty remembering all of your prescribed medications? | ||||||
| Never or rarely, N (%) | 12 (92) | 10 (83) | .49 | 10 (91) | 10 (91) | >.99 |
| Once in a while, N (%) | 1 (8) | 2 (17) | 1 (9) | 1 (9) | ||
| MMAS score | ||||||
| 0, N (%) | 0 (0) | 1 (8) | .14 | 0 (0) | 0 (0) | .65 |
| 1, N (%) | 7 (54) | 9 (75) | 8 (73) | 9 (82) | ||
| 2, N (%) | 3 (23) | 0 (0) | 2 (18) | 1 (9) | ||
| 3, N (%) | 3 (23) | 2 (17) | 0 (0) | 1 (9) | ||
| 4, N (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 5, N (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 6, N (%) | 0 (0) | 0 (0) | 1 (9) | 0 (0) | ||
IQR indicates interquartile range; MMAS, adapted Morisky Medication Adherence Scale; OTC, over-the-counter.
As shown in Table 1, the total number of medications and the number of required daily doses were not significantly different between the 2 groups at baseline and at 30-day follow-up. The between-group differences in medication adherence were also not significant. At the 30-day follow-up, no significant differences were found between the pharmacist-intervention and usual care groups in the number of STOPP medications or missing START medications (Table 2).
Table 2.
Primary and Secondary Outcomes for 25 Recently Discharged, High-Risk Elderly Patients at 30-Day Follow-Up
| Baseline | 30-day follow-up visit | |||||
|---|---|---|---|---|---|---|
| Outcome | Pharmacist intervention (N = 13) N (%) | Usual care (N = 12) N (%) | P value | Pharmacist intervention (N = 11) N (%) | Usual care (N = 11) N (%) | P value |
| STOPP medications on the patient's list | ||||||
| 0 | 6 (46) | 2 (17) | .09 | 5 (45) | 2 (18) | .26 |
| 1 | 5 (38) | 5 (42) | 4 (36) | 6 (55) | ||
| 2 | 1 (8) | 3 (25) | 1 (9) | 2 (18) | ||
| 3 | 1 (8) | 1 (8) | 1 (9) | 0 (0) | ||
| 4 | 0 (0) | 1 (8) | 0 (0) | 1 (9) | ||
| START medications missing from the list | ||||||
| 0 | 5 (38) | 5 (42) | .91 | 4 (36) | 6 (55) | .44 |
| 1 | 6 (46) | 5 (42) | 4 (36) | 3 (27) | ||
| 2 | 1 (8) | 2 (17) | 0 (0) | 0 (0) | ||
| 3 | 1 (8) | 0 (0) | 3 (27) | 2 (18) | ||
| 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| MAI criterion 1 (no indication) | ||||||
| 0 | 11 (85) | 8 (67) | .29 | 10 (91) | 10 (91) | >.99 |
| 1 | 2 (15) | 4 (33) | 1 (9) | 1 (9) | ||
| MAI criterion 2 (medication not effective for condition) | ||||||
| 0 | 9 (69) | 10 (83) | .42 | 8 (73) | 10 (91) | .31 |
| 1 | 2 (15) | 1 (8) | 1 (9) | 0 (0) | ||
| 2 | 1 (8) | 1 (8) | 1 (9) | 1 (9) | ||
| 3 | 0 (0) | 0 (0) | 1 (9) | 0 (0) | ||
| 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| 5 | 1 (8) | 0 (0) | 0 (0) | 0 (0) | ||
| MAI criterion 3 (unnecessary duplication of other drugs) | ||||||
| 0 | 11 (85) | 8 (67) | .40 | 9 (82) | 10 (91) | .55 |
| 1 | 1 (8) | 4 (33) | 1 (9) | 1 (9) | ||
| 2 | 0 (0) | 0 (0) | 1 (9) | 0 (0) | ||
| 3 | 1 (8) | 0 (0) | 0 (0) | 0 (0) | ||
| 30-day hospital readmission | ||||||
| Yes | NA | NA | NA | 2 (18) | 1 (9) | .53 |
| No | 9 (82) | 10 (91) | ||||
| 30-day emergency department visit | ||||||
| Yes | NA | NA | NA | 1 (9) | 1 (9) | >.99 |
| No | 10 (91) | 10 (91) | ||||
| Composite 30-day emergency department visit or hospital readmission | ||||||
| Yes | NA | NA | NA | 2 (18) | 1 (9) | .53 |
| No | 9 (82) | 10 (91) | ||||
MAI indicates Medication Appropriateness Index; NA, not applicable; START, Screening Tool to Alert Doctors to the Right Treatment; STOPP, Screening Tool of Older Persons’ Prescriptions.
The secondary outcomes of the modified MAI assessment and the 30-day rates of emergency department visits, hospital readmissions, or the composite of both showed no significant differences between the 2 groups. Table 3 summarizes the drug therapy problems that were identified by the MTM consultation, which represented the pharmacist intervention. Our results show that 24 (43.6%) of the 55 identified drug therapy problems persisted at the 30-day follow-up (Table 3).
Table 3.
Drug Therapy Problems Identified During Pharmacist Intervention
| Descriptiona | Drug therapy problems, N |
|---|---|
| There is no valid medical indication for the drug therapy at this time | 4 |
| Multiple drugs are being used for a condition that requires single-drug therapy | 2 |
| The medical condition is more appropriately treated with nondrug therapy | 0 |
| Drug therapy is being taken to treat an avoidable adverse reaction associated with another medication | 1 |
| Drug abuse, alcohol use, or smoking is causing the problem | 0 |
| A medical condition requires the initiation of drug therapy | 7 |
| Preventive drug therapy is required to reduce the risk for a new condition | 3 |
| A medical condition requires additional pharmacotherapy to attain synergistic or additive effects | 0 |
| The drug is not the most effective for the medical problem | 1 |
| The medical condition is refractory to the drug | 0 |
| The dosage form of the drug is inappropriate | 2 |
| The drug is not effective for the indication being treated | 0 |
| The dose is too low to produce the desired response | 7 |
| The dosage interval is too infrequent to produce the desired response | 1 |
| The drug interaction reduces the amount of active drug available | 0 |
| The duration of drug therapy is too short to produce the desired response | 0 |
| The drug causes an undesirable reaction that is not dose related | 3 |
| A safer drug is required because of risk factors | 7 |
| A drug interaction causes an undesirable reaction that is not dose related | 0 |
| The dosage regimen was administered or changed too rapidly | 0 |
| The drug causes an allergic reaction | 0 |
| The drug is contraindicated because of risk factors | 1 |
| Additional laboratory monitoring is recommended to prevent adverse drug reactionb | 12 |
| The drug's dose is too high | 0 |
| The dosing frequency is too short | 0 |
| The duration of drug therapy is too long | 1 |
| A drug interaction occurs, resulting in a toxic reaction to the drug | 0 |
| The dose of the drug was administered too rapidly | 0 |
| The patient does not understand the instructions | 1 |
| The patient prefers not to take the medication | 0 |
| The patient forgets to take the medication | 1 |
| The drug is too expensive for the patient | 1 |
| The patient cannot swallow or self-administer the drug appropriately | 0 |
| The drug is not available for the patient | 0 |
| Total drug therapy problems/total medications assessed among 13 patients | 55/191 (28.8%) |
| Drug therapy problems unresolved at 30 days after discharge | 24 (43.6%) |
Adapted from Brown TR, ed. Handbook of Institutional Pharmacy Practice. 4th ed. Bethesda, MD: American Society of Health-System Pharmacists; 2006.
Denotes addition to standard definitions of drug therapy problems.26
Discussion
We found no difference between the 2 groups in medications meeting the STOPP or START criteria. A similar study used a retrospective chart review to evaluate similar medication quality parameters, including the START and STOPP criteria, in elderly veterans.28 That study found a significant decline in the STOPP score from 1.2 at their initial home-based primary care visit and the 0.895 score at their follow-up visit (P = .001).28
A major factor that might have affected the results of this study was the implementation rate of the pharmacist's recommendations. The patients were reassessed at 30 days after hospital discharge by review of the electronic medical record to determine the status of their medication therapy problems compared with baseline. There were 55 drug therapy problems identified by the pharmacist at baseline, and 43.6% remained unresolved 30 days after discharge (Table 3). This suboptimal rate might have reduced the impact of the pharmacist's interventions and the subsequent statistical differences between the 2 groups.
A 2013 systematic review concluded that the level of collaboration between the general practitioner and the pharmacist regarding medication review yields higher implementation rates of medication recommendations.29 A few of the key elements of collaboration to improve the recommendation implementation rate included sharing of the medical records, recruitment of patients by the provider, and a case conference between the provider and the pharmacist.29
Although we were unable to discern a significant difference in our study, the number of STOPP criteria medications identified is concerning. The pharmacist identified 28 medications that met the STOPP criteria among all 25 patients at baseline, with 17 (68%) patients being prescribed at least 1 inappropriate medicine. This prevalence is higher than the 35% to 57% found previously by researchers who evaluated the inappropriate use of medications in elderly patients.16,24
Medication underuse was also common, with 20 START criteria medications missing among all 25 patients at baseline, which represents 15 (60%) patients with at least 1 missing medication. This rate of medication underuse is similar to the 63% that was found in previous research,30 suggesting that the underuse of appropriate medications is common. The secondary outcomes were not significantly different between the 2 groups, with 3 (13.6%) of the 22 patients available at the 30-day follow-up reporting an emergency department visit or a hospital readmission since the initial discharge. These results are similar to our previously published readmission rates after care transitions.31
The results from our elderly cohort showed that each patient was taking approximately 9 prescription medications, suggesting an association with an increased number of inappropriate medications. Steinman and colleagues demonstrated the frequency of inappropriate medication use increases with the total number of medications taken. However, the frequency of potential prescribing omissions does not vary with the total number of medications taken.24 Our study demonstrated a fairly balanced percentage of potentially inappropriate medications versus potential prescribing omissions (approximately 68% vs 60%, respectively).
Our findings suggest that the implementation of a telephonic MTM consultation into an already resource-intensive, comprehensive CTP may not represent the optimal integration strategy. One area of potential further research is to identify specific patient populations that derive the most benefit from a pharmacist-provided MTM program during care transitions.
Limitations
The primary limitation to the study is the small sample size, which yielded inadequate statistical power to allow us to draw definitive conclusions. Our initial intervention included an in-home assessment by the study coordinator to perform the baseline functional measurements. This requirement limited patient recruitment, and the intervention was modified to perform all baseline and follow-up assessments via telephone. However, this modification did not significantly improve patient recruitment. Thus, this study can be considered preliminary, and its data can be regarded as hypothesis-generating.
In addition, the pharmacist's intervention was part of a multifaceted pilot CTP, and it is difficult to discern the absolute impact of this integration.
Several other methodologic limitations deserve notation. The 3 MAI-derived subscales that identify medications that are not indicated, are ineffective, or are duplicative have not been independently validated as standalone measures. However, this combination has been used in previous research,24 and each of the 3 items has excellent intrarater and interrater reliability.23,32
Notably, 2 of the 12 patients who were randomized to the usual care group had participated in MTM in the past 12 months. These 2 patients might have confounded the results, and might have introduced bias between the 2 treatment groups.
Finally, the study was conducted at a single center, which might have also limited the results.
Conclusion
Although many studies have examined methods to improve care transitions from a hospital to home, an optimal standard of practice has not yet been determined. Utilizing screening tools such as START, STOPP, or MAI may be helpful in transitioning patients, but it is also important to recognize the individual needs of each patient. Inappropriate medication use and underuse were common within the elderly cohort in our study. Although pharmacist-provided MTM consultation did not demonstrate a statistically significant benefit, the negative findings may be attributable to a lack of statistical power because of the small sample size or to the suboptimal integration into the care model. Future research should include a method to identify the specific patients who would benefit most from intervention by a pharmacist.
Acknowledgments
The authors would like to thank Stephanie M. Quigg, Betty A. Wirt, and Ivana T. Croghan, PhD.
Author Disclosure Statement
Dr Haag, Dr Davis, Dr Hoel, Dr Armon, Dr Odell, and Mr Dierkhising reported no conflicts of interest. Dr Takahashi is on the Medical Board of Axial, LLC.
Contributor Information
Jordan D. Haag, Clinical Pharmacist, Department of Pharmacy, Mayo Clinic in Rochester, MN.
Amanda Z. Davis, Clinical Pharmacist, Department of Pharmacy, Mayo Clinic in Rochester, MN.
Robert W. Hoel, Clinical Pharmacist, Department of Pharmacy, Mayo Clinic in Rochester, MN.
Jeffrey J. Armon, Clinical Pharmacist, Department of Pharmacy, Mayo Clinic in Rochester, MN.
Laura J. Odell, Clinical Pharmacist, Department of Pharmacy, Mayo Clinic in Rochester, MN.
Ross A. Dierkhising, Statistician, Division of Biomedical Statistics and Informatics, Mayo Clinic in Rochester, MN.
Paul Y. Takahashi, Consultant, Division of Primary Care Internal Medicine, Mayo Clinic in Rochester, MN.
References
- 1. Patient Protection and Affordable Care Act, Pub L No. 111–148, 124 Stat 119. https://govtrack.us/congress/bills/111/hr3590/text. Accessed July 20, 2011.
- 2. Chutka DS, Evans JM, Fleming KC, Mikkelson KG. Symposium on geriatrics—Part I: Drug prescribing for elderly patients. Mayo Clin Proc. 1995; 70: 685–693. [DOI] [PubMed] [Google Scholar]
- 3. Chutka DS, Takahashi PY, Hoel RW. Inappropriate medications for elderly patients. Mayo Clin Proc. 2004; 79: 122–139. [DOI] [PubMed] [Google Scholar]
- 4. Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003; 163: 2716–2724. Erratum in: Arch Intern Med. 2004; 164: 298. [DOI] [PubMed] [Google Scholar]
- 5. Page RL, 2nd, Ruscin JM. The risk of adverse drug events and hospital-related morbidity and mortality among older adults with potentially inappropriate medication use. Am J Geriatr Pharmacother. 2006; 4: 297–305. [DOI] [PubMed] [Google Scholar]
- 6. Hilmer SN, Mager DE, Simonsick EM, et al; for the Health ABC Study. Drug burden index score and functional decline in older people. Am J Med. 2009; 122: 1142–1149.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Steinman MA, Hanlon JT. Managing medications in clinically complex elders: “there's got to be a happy medium.” JAMA. 2010; 304: 1592–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Medicare Prescription Drug, Improvement, and Modernization Act of 2003, Pub L No. 108–173, 117 Stat 2066.
- 9. Smith SR, Clancy CM. Medication therapy management programs: forming a new cornerstone for quality and safety in Medicare. Am J Med Qual. 2006; 21: 276–279. [DOI] [PubMed] [Google Scholar]
- 10. Centers for Medicare & Medicaid Services. Medication therapy management. http://cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovContra/MTM.html. Accessed January 23, 2014.
- 11. Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Am J Med. 2001; 111(9 suppl 2): 26S–30S. [DOI] [PubMed] [Google Scholar]
- 12. Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009; 150: 178–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med. 1998; 158: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 14. Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med. 2009; 4: 211–218. [DOI] [PubMed] [Google Scholar]
- 15. Zillich AJ, Snyder ME, Frail CK, et al. A randomized, controlled pragmatic trial of telephonic medication therapy management to reduce hospitalization in home health patients. Health Serv Res. 2014; 49: 1537–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gallagher P, O'Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers’ criteria. Age Ageing. 2008; 37: 673–679. [DOI] [PubMed] [Google Scholar]
- 17. Petrarca AM, Lengel AJ, Mangan MN. Inappropriate medication use in the elderly. Consult Pharm. 2012; 27: 583–586. [DOI] [PubMed] [Google Scholar]
- 18. Barry PJ, Gallagher P, Ryan C, O'Mahony D. START (screening tool to alert doctors to the right treatment)—an evidence-based screening tool to detect prescribing omissions in elderly patients. Age Ageing. 2007; 36: 632–638. [DOI] [PubMed] [Google Scholar]
- 19. Gillespie U, Alassaad A, Hammarlund-Udenaes M, et al. Effects of pharmacists’ interventions on appropriateness of prescribing and evaluation of the instruments’ (MAI, STOPP and STARTs’) ability to predict hospitalization—analyses from a randomized controlled trial. PLoS One. 2013; 8: e62401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crane SJ, Tung EE, Hanson GJ, et al. Use of an electronic administrative database to identify older community dwelling adults at high-risk for hospitalization or emergency department visits: the elders risk assessment index. BMC Health Serv Res. 2010; 10: 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Truven Health Analytics. Micromedex 2.0. www.micromedexsolutions.com. Accessed December 8, 2011.
- 22. Wolters Kluwer. Lexi-Drugs. http://online.lexi.com. Accessed December 8, 2011.
- 23. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992; 45: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 24. Steinman MA, Landefeld CS, Rosenthal GE, et al. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc. 2006; 54: 1516–1523. [DOI] [PubMed] [Google Scholar]
- 25. Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008; 10: 348–354. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26. Cipolle RJ, Strand LM, Morley PC. Pharmaceutical Care Practice: The Clinician's Guide. 2nd ed. New York, NY: McGraw-Hill; 2004. [Google Scholar]
- 27. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009; 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brahmbhatt M, Palla K, Kossifologos A, et al. Appropriateness of medication prescribing using the STOPP/START criteria in veterans receiving home-based primary care. Consult Pharm. 2013; 28: 361–369. [DOI] [PubMed] [Google Scholar]
- 29. Kwint H-F, Bermingham L, Faber A, et al. The relationship between the extent of collaboration of general practitioners and pharmacists and the implementation of recommendations arising from medication review: a systematic review. Drugs Aging. 2013; 30: 91–102. [DOI] [PubMed] [Google Scholar]
- 30. Shrank WH, Asch SM, Adams J, et al. The quality of pharmacologic care for adults in the United States. Med Care. 2006; 44: 936–945. [DOI] [PubMed] [Google Scholar]
- 31. Takahashi PY, Haas LR, Quigg SM, et al. 30-day hospital readmission of older adults using care transitions after hospitalization: a pilot prospective cohort study. Clin Interv Aging. 2013; 8: 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fitzgerald LS, Hanlon JT, Shelton PS, et al. Reliability of a modified medication appropriateness index in ambulatory older persons. Ann Pharmacother. 1997; 31: 543–548. [DOI] [PubMed] [Google Scholar]