Abstract
Rationale
Systemic corticosteroids (SCS) are used for treat preschoolers with acute asthma or wheezing exacerbations, with conflicting results.
Objective
To evaluate the effectiveness of oral corticosteroids (OCS) compared to placebo in preschoolers presenting with acute asthma/wheezing exacerbations.
Methods
Five electronic databases were searched for all placebo-controlled, randomized clinical trials of OCS in children <6 years of age presenting with recurrent wheezing/asthma exacerbations of any severity. Primary outcomes were hospitalizations, unscheduled emergency department (ED) visits in following month, need of additional OCS courses, and length of stay (ED or hospital).
Results
Eleven studies met inclusion criteria (n=1733); four were conducted on an outpatient basis, five in inpatients, and two in the ED. Significant heterogeneity was found when pooling all studies, and thus analysis was stratified by trial setting. Among the outpatient studies, children who received OCS had a higher hospitalization rate (RR: 2.15 [95%CI=1.08-4.29], I2=0%) compared to those to received placebo. Among the ED studies, children who received OCS had a lower risk of hospitalization (RR: 0.58 [0.37-0.92], I2=0%). Among the inpatient studies, children who received OCS needed fewer additional OCS courses than those on placebo (RR: 0.57 [0.40 to 0.81], I2=0%).
Conclusions
Treatment with OCS in the ED or hospital may be beneficial in toddlers and preschoolers with frequent asthma/wheezing exacerbations. However, more studies are needed before OCS can be broadly recommended for this age group. Future trials should be carefully designed to avoid bias and according to our findings regarding administration setting.
Keywords: asthma exacerbation, treatment, oral steroids, young children
INTRODUCTION
The management of asthma/wheezing in infants, toddlers and preschoolers (less than 6 years of age) is crucial due to several factors: an increase of asthma incidence in early ages, principally explained by variation in preschoolers1; the greatest decline in lung function in asthmatics may occur during the preschool period2; preschool age is the age bracket with the poorest asthma control in childhood,3 with 48% of preschoolers with asthma reporting an exacerbation in the preceding year4. The annual rate of emergency department (ED) visits is 23-42 per 1000 for preschoolers vs. less than 15 per 1000 for those aged 6-70 yrs5, with the same pattern hospitalizations5,6. Furthermore, the age at first hospitalization for asthma has decreased over time1.
For decades, it has been standard practice among community pediatricians, allergists and pulmonary specialists to treat infants, toddlers, and preschoolers with acute episodes of wheeze with systemic corticosteroids (SCS), based on strong evidence of their efficacy in school-aged children and adolescents with asthma7. International guidelines recommend the use of SCS in children under 6 years old with severe wheeze/asthma exacerbations that do not respond to short active beta-2 agonists (SABA)8,9, and thus up to 38% of wheezing infants receive OCS during their first year of life10. This practice is based on the general belief that episodes of wheeze in young children are likely early manifestations of asthma caused by the same pathophysiologic process of airway inflammation and narrowing8. However, recent trials evaluating the efficacy of OCS in preschoolers presenting to the ED or outpatient care for acute wheezing/asthma exacerbations have shown conflicting results, and a recent update on the efficacy of OCS in preschoolers does not support their efficacy, suggesting that the heterogeneity of early childhood wheezing might be at least partly responsible11. At present, there is no systematic review with meta-analysis published for this specific age group.
The objective of this systematic review is to evaluate the efficacy of SCS (mainly OCS) use in children up to six years of age (including infants, toddlers and preschoolers) presenting with acute asthma or recurrent wheezing exacerbations. We hypothesized that SCS efficacy may vary according to severity of exacerbation and timing of administration, and thus may be more be effective in some settings than others.
METHODS
Search and Selection Criteria
This study was registered with the International Prospective Register of Systematic Reviews (PROSPERO, http://www.crd.york.ac.uk/PROSPERO) as CRD42015024332. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to perform this review12. The authors identified studies published in MEDLINE, EMBASE, CINAHL, SCOPUS and the Cochrane Controlled Trials Register (CENTRAL) databases and ClinicalTrials.gov until May 2015, using the terms “(oral corticosteroids OR steroids) AND (asthma OR wheeze) AND (infant OR toddler OR preschool).” Additionally, a search of relevant files from the drug manufacturer’s databases (published and unpublished) was performed. Finally, the references of all selected studies were also reviewed, and backward and forward references searches were performed. Language restrictions were not applied. To be included, studies had to meet all the following criteria: 1) children under 6 years of age with recurrent wheezing/asthma exacerbations of any severity presenting to the ED, receiving treatment at home (“outpatient studies”) or hospitalized for an asthma/wheezing exacerbation (“inpatient studies”); 2) randomized clinical trials (RCTs; parallel group or cross-over design) of any duration; 3) comparison of OCS (any type) vs. placebo; and 4) report at least one of the following primary outcomes: need of hospitalization, unscheduled visits to the ED in the four weeks following the trial intervention, length of hospital stay, or need for additional courses of SCS; or the following secondary outcomes: improvement of lung function measured by forced expiratory volume in 1 second (FEV1) or peak expiratory flow (PEF), length of stay during the first ED visit, symptom scores, withdrawals (total and due to adverse effects [AEs]), and safety (AEs and serious AEs [SAEs]). An SAE was defined as any untoward medical occurrence that results in death, is life-threatening, requires inpatient hospitalization, or results in persistent or significant disability or incapacity13. We used study setting (outpatient, ED, or inpatient) as a proxy for exacerbation severity and timing of OCS administration.
Data Extraction and Assessment of Risk of Bias
Titles, abstracts, and citations were independently analyzed by the two authors (JCR and AB). From the full text, all studies were independently assessed for inclusion. Both authors were independently involved in all stages of study selection, data extraction, and risk of bias assessment. The latter was assessed according to recommendations outlined in the Cochrane Handbook14 for the following items: 1) adequacy of sequence generation; 2) allocation concealment; 3) blinding of participants and investigators; 4) blinding of outcome assessment; 5) incomplete outcome data; 6) selective outcome reporting, and other bias. Disagreements were discussed and resolved by the third investigator (EF).
Data Analysis
Analysis was performed by intention to treat and included all participants to minimize bias. Outcomes were pooled using mean differences (MD) (inverse variance method) or Mantel-Haenszel risk ratios (RR). Estimate precision was quantified by 95% confidence intervals (CI). Heterogeneity was measured by the I2 test15 (≤ 25% absence of bias; 26 to 39% unimportant; 40% to 60% moderate; and 60% to 100% substantial bias). A fixed-effects model was used when there was no evidence of significant heterogeneity in the analysis (I2 < 40%); if significant heterogeneity was found a random-effects model was used16,17. A priori subgroup analyses included: type of OCS, age (<2 versus >2 years old), severity of exacerbation (mild versus moderate to severe), and trials sponsored by pharmaceutical industry versus independent trials. The meta-analysis was performed with the Review Manager 5.3.5 software (Cochrane IMS, 2014).
RESULTS
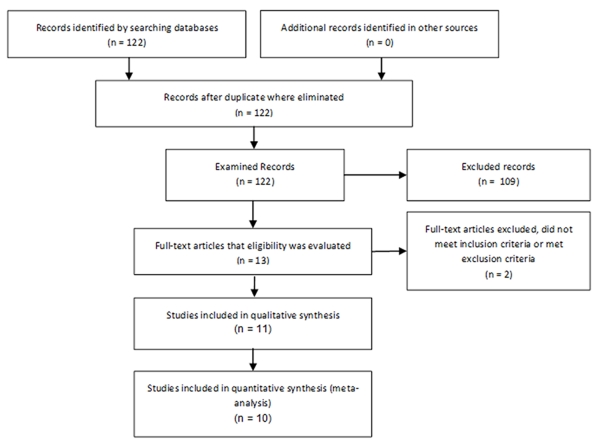
A total of 122 studies were initially identified in the database search and from other sources (Figure 1). Of these, 109 studies were excluded (reasons for exclusion: abstracts, letters, reviews, pooled analysis, no RCTs), leaving thirteen studies for which eligibility was assessed. Two of these studies were excluded because they did not meet inclusion criteria: one included the same population as another included study and added children with no prior wheezing episodes; the other included only participants with none or just one prior wheezing episodes.
FIGURE 1.
Process of study selection.
Included studies
Eleven RCTs published from 1986 to 2013 were included18-28 (Table 1). Six were conducted in the United Kingdom18,20,24-26,28, three in the U.S.19,21,23, one in Israel22 and one in Finland27. A total of 1,733 patients were randomized. Four of the studies were conducted on an outpatient basis18-21, two were realized in the ED 22,23 and five were inpatient studies24-28.
Table 1. Characteristics of Included Studies.
| Study | Design | Patients, n (% Male) |
Mean Age Steroid group (SD or range) |
Mean Age Placebo group (SD or range) |
Selected Comparisons |
|---|---|---|---|---|---|
| Webb 198618 |
R,DB, CO, SC |
38 (74%) | 10.4 mo (4.1) | 9.3 mo (3.7) | Prednisolone 1 mg/kg BID 5d, vs. placebo |
| Grant 199519 |
R,DB, CO, SC |
86 (65%) | NR | NR | Prednisone 2 mg/kg 5d, vs. placebo |
| Oommen 200320 |
R,DB, PG, SC |
225 (65%) | 25 mo (17-37) | 27 mo (19-38) | Prednisolone 20 mg daily 5d, vs. placebo |
| Beigelman 201321 |
R,DB, PG, MC |
215 (NR) 278 (NR) |
29 mo (0.84) 34.5 mo (0.77) |
30 mo (0.84) 34.5 mo (0.77) |
Prednisolone 2 mg/kg 2 d, then 1 mg/kg 2d, prescribed as rescue treatment according to predefined protocol |
| Tal 199022 |
R,DB, PG, SC |
74 (62%) | 23.1 mo (7-54) | Methylprednisolone 4 mg/kg IM once, vs. placebo |
|
| Scarfone 199323 |
R,DB, PG, SC |
75 (72%) | 59 mo (47) | 63 mo (49) | ED: Prednisone 2 mg/kg once, vs. placebo After discharge: all prednisone 1 mg/kg BID 5d |
| Storr 198724 |
R,DB, PG, SC |
140 (69%) | 5.2 y (NR) | 5.4 y (NR) | Prednisolone 30 mg once |
| Gleeson 199025 |
R,DB, PG, SC |
39 (74%) | 4.7 y (0.5) | 5.1 y (0.7) | Hydrocortisone 6 mg/kg IV once then 2 mg/kg IV 4 hourly 1d then prednisolone 1 mg/kg BID 5d, vs. placebo |
| Fox 199626 |
R,DB, PG, SC |
62 (69%) | 7 mo (4-13) | 7 mo (3-14) | Prednisolone 2 mg/kg 5d, vs. placebo |
| Jartti 200727 |
R,DB, PG, SC |
58 (66%) | 2.1 y (1.1) | 2.9 y (1.4) | Prednisolone 2 mg/kg 3d, vs. placebo |
| Panickar 200928 |
R,DB, PG, MC |
443 (65%) | 25.8 mo (13.3) | 26.2 mo (14.7) | Prednisolone 10 mg daily for children 10-24 mo and 20 mg daily for older children 5d, vs. placebo |
AD=after discharge; BID=twice daily; CO=crossover; d=days; DB=double-blind; ED=emergency department; IM=intramuscular; MC=multicenter; mo= months; NR=not reported; PG=parallel group; R=randomized; SC=single center; y=years.
Seven studies used prednisolone18,20,21,24,26-28, two studies used prednisone19,23, one used methylprednisolone22 and one used hydrocortisone followed by prednisolone25. Most studies maintained the treatment for three to five days18-21,23,25-28 and two studies used a single dose of OCS22,24. All studies compared the treatment with OCS to placebo, with the exception of one post hoc analysis study21. One study compared OCS and placebo in the ED23, but both groups received OCS after discharge, so only the first phase of the study was used for analysis. In one ED study22 the OCS was administered within 30 minutes of arrival at the ED, and in the other study this subject was not specified23.
Two studies included only infants and toddlers18,26 and four studies also randomized preschoolers20-22,28. Five studies also included older children by protocol, but finally enrolled only a small proportion of older children or analyzed their results by different age range; therefore they were also included in the present analysis19,23-25,27. Most studies enrolled children with a moderate acute asthma or recurrent wheezing exacerbation18,21-28 and two studies gave no specification on episode severity19,20. Four studies included some children being treated with inhaled corticosteroids (ICS)20,21,27,28, three studies specifically excluded such population22,23,25 and four studies did not mention ICS use18,19,24,26. Among all of the studies, two were sponsored by the pharmaceutical industry24,27, seven received other financial support18-21,25,26,28 and two did not specify funding source22,23. Two studies were post-hoc analyses of two prior protocols21,27: one of them was a subgroup analysis of a larger study comparing OCS with placebo for any wheezing episode, and included only recurrent wheezing episodes27; the other one was a multicenter study, including two different trials, and compared the use of high dose ICS with montelukast and with albuterol alone during acute asthma21. In this latter study21, participants were not randomly assigned to treatment with OCS, and therefore it was included in the review but excluded from the meta-analysis. Methodological quality of included studies is described in Table 2; most studies had low methodological quality.
TABLE 2.
Risk of Bias of the Included Studies
| Study | Random Sequence Generation |
Allocation Concealment |
Blinding of Participants & Personnel |
Blinding of Outcome Assesment |
Incomplete Outcome Data |
Selective Reporting |
Funded by pharmaceutical industry |
Other bias |
|---|---|---|---|---|---|---|---|---|
| Webb 198618 |
U | U | Y | U | N | U | N | - |
| Storr 198724 |
U | U | Y | U | N | U | Y | - |
| Tal 199022 | U | U | Y | Y | N | U | U | - |
| Gleeson 199025 |
U | U | Y | U | N | U | N | - |
| Scarfone 199323 |
U | U | Y | Y | N | U | U | - |
| Grant 199519 |
U | U | Y | U | N | U | N | - |
| Fox 199626 | U | U | Y | U | N | U | N | - |
| Oommen 200320 |
Y | Y | Y | Y | Y | U | N | - |
| Jartti 200727 |
Y | Y | Y | Y | N | U | Y | Subgroup analysis of study designed for comparison of interest |
| Panickar 200928 |
Y | Y | Y | Y | N | U | N | - |
| Beigelman 201321 |
Y | Y | Y | Y | N | Y | N |
Post hoc analysis of study not designed for comparison of interest |
N=No; U=Unknown; Y=Yes.
Primary Outcomes
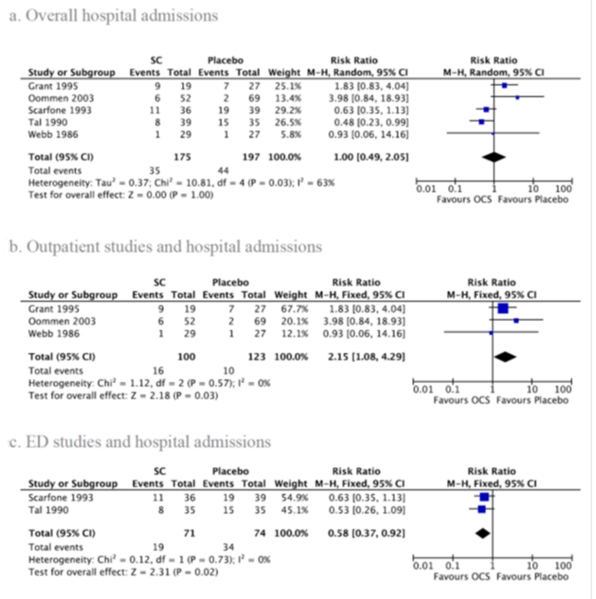
1. Hospital admission
Five studies reported hospital admission rates18-20,22,23 (Figure 2a). There was no significant difference between OCS and placebo (RR: 1.00; 95% CI: 0.49-2.05), and there was significant heterogeneity between studies (I2=63%, p=0.03). Analyzing only outpatient studies18-20, OCS treatment was associated with a higher hospital admission rate (Figure 2b; RR: 2.15; 95% CI: 1.08-4.29) with no heterogeneity (I2=0%, p=0.57). Considering only the two studies conducted in the ED22,23, OCS treatment had a lower risk of hospital admissions (Figure 2c; RR: 0.58; 95% CI: 0.37-0.92) with no heterogeneity (I2=0%, p=0.73). In our subgroup analysis, we found no significant difference between OCS and placebo for children less than 2 years of age vs. older (RR: 0.41; 95% CI: 0.17 to 1.03; I2=0%, p=0.52)18,22. When analyzing studies not sponsored by the pharmaceutical industry, those who received OCS had significantly lower risk of hospital admission than those on placebo (Figure 2b); however, opposite results were found when analyzing studies that did not specify funding (Figure 2c). When excluding the ED study by Scaropone et al.23, in which 37% of the study population was over 5 years of age, the effect of OCS on hospital admission became non-significant. No differences were found by type of OCS. There were insufficient data to perform subgroup analyses by episode severity. No difference was observed after adjusting for methodological quality.
FIGURE 2.
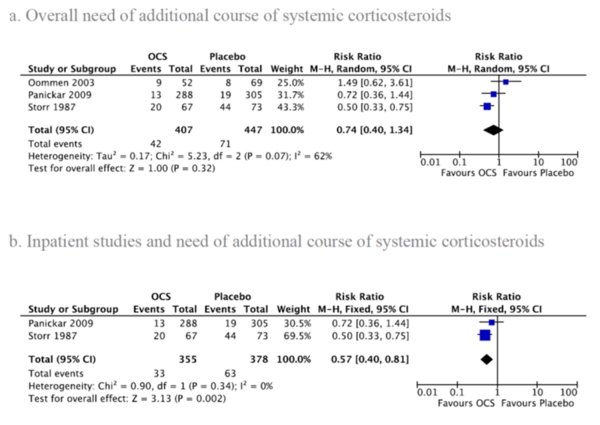
2. Additional course of systemic corticosteroids
One outpatient study20 and two inpatient studies24,28 reported the need for additional courses of SCS (Figure 3a), showing no significant difference between OCS and placebo (RR: 0.74; 95% CI: 0.40 to 1.34). There was significant heterogeneity between studies (I2=62%, p=0.07). Analyzing only inpatient studies24,28, the difference became significant favoring the OCS group (RR: 0.57; 95% CI: 0.40 to 0.81; I2=0%, p=0.34), (Figure 3b). However, when excluding the inpatient study by Storr et al.24, in which 50% of the study population was over 4 years of age, the effect of OCS became non-significant. Subgroup analysis was not performed due to insufficient data.
FIGURE 3.
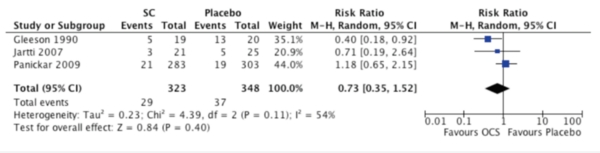
3. Unscheduled visits
Three inpatient studies reported unscheduled visits for asthma symptoms in the month following25,27,28. There was no significant statistical difference between OCS and placebo (RR: 0.73; 95% CI: 0.35 to 1.52; I2=54%, p=0.11) (Figure 4). When excluding the study by Gleeson et al.25, which included some children older than 5 years, the results did not change. No differences were observed when excluding data obtained from Jartti27 (RR: 0.72; 95% CI: 0.25 to 2.07, I2=77%, p=0.04), considering it was performed on a post-hoc basis. On the other hand, one outpatient study reported more ED consults in the OCS group than placebo during the treatment period (0.87 ± 1.5 vs. 0.41 ± 0.81, respectively)19 in children under 6 years of age. Subgroup analysis was not performed due to insufficient data.
FIGURE 4.
Unscheduled visits for asthma symptoms
4. Hospital length of stay
Finally, four studies25-28 reported no differences in hospital length of stay were found between OCS and placebo, while one study reported the OCS group had a shorter stay24; however length of stay was reported differently (means vs medians) and thus we were not able to perform a pooled analysis.
Secondary Outcomes
Outpatient and ED studies did not give any data on pulmonary function. Two inpatient studies found an improvement in PEF measurements in the OCS group24,25, while a third study showed no differences between both groups in impulse oscillometry values27. There were insufficient data to perform a meta-analysis.
Outpatient studies found no differences between OCS and placebo on symptom scores,18,20,21 but ED studies found better symptom improvement in the OCS group22,23. No differences were shown in four inpatient studies24,26-28, while a single one showed a bigger fall in heart rate25. There were insufficient data to perform a meta-analysis of symptom scores due to different scores used.
None of the ED studies reported length of stay during the first consult in the ED. Some studies reported no relevant AEs18,27,28, while one found minor AEs similar in both groups19. There were insufficient data to perform meta-analysis of AEs.
DISCUSSION
The use of OCS in the treatment of recurrent wheezing in infants, toddlers and preschoolers remains controversial. In this specific population, considering the overall group, we did not find evidence that OCS administration is effective compared to placebo in any of our primary outcomes: hospital admissions, additional systemic corticosteroid courses, hospital length of stay, and unscheduled ED visits for asthma symptoms.
Our analysis revealed very significant heterogeneity when pooling all identified studies. Strikingly, this heterogeneity disappeared when the analysis was stratified by the clinical setting in which the trials were performed (i.e. outpatient setting, ED, or inpatient setting). When analyzing studies performed in the ED, OCS treatment was associated with a lower hospitalization rate. Similarly, when analyzing studies performed in the inpatient setting, OCS treatment was associated with a lower need for additional courses of SCS. In the outpatient studies, on the other hand, OCS administration was associated with more hospital admissions, suggesting that OCS may not be beneficial in all clinical settings for this age group. In addition, it must be taken under consideration that the largest study in this group had a high rate of treatment noncompliance20. Also, behavioral changes have been reported in children during OCS therapy, and this factor might affect the clinical decision to admit to the hospital20.
These stratified findings support our a priori hypothesis that OCS may be more effective in certain subgroups depending on exacerbation severity or timing of administration: in this age group, OCS may be more beneficial among children who present with more severe exacerbations that require urgent care or hospitalization. However, asthma in young children is a heterogeneous condition with different underlying pathophysiological pathways, which could explain why some patients may benefit from OCS prescription while others show no response. In a recent study on preschoolers with severe recurrent wheeze, biopsies of children up to 36 months were found to contain more inflammatory cells with fewer eosinophils than those on older preschoolers29. Most asthma/wheezing exacerbations in preschoolers are triggered by episodic viral infections5,30. Moreover, the particular virus causing an asthma/wheezing exacerbation should be considered in this age group, since they produce a different immune response, which could affect the effectiveness of OCS;31 while prednisolone reduces wheezing relapse in children with acute rhinovirus infection, this effect was not observed in children with acute respiratory syncytial virus infection32. Other factors that may affect OCS response include the timing of administration33,34, vitamin D deficiency35, or genetic predisposition36.
In regards to methodology, all included studies were randomized, double-blinded and placebo-controlled studies; however, in most of them allocation concealment was unclear, and therefore their overall reporting quality was suboptimal. While the dose (single or multiple) of OCS used in these eleven RCTs was adequate, as was stated recently37, the different protocols used could also have contributed to the observed heterogeneity. Additionally, one study27 was a post hoc analysis, although results did not change when it was excluded from the meta-analysis. Moreover, although the mean age of participants was within preschool age range, some of the analyzed studies included older children. Three studies23-25 included some proportion of children 6 years of age or older; when excluding those studies the protective effect for hospital admission of OCS among the ED studies and for need of additional course of systemic corticosteroids among the inpatient studies became non-statistically significant. All these differences make it difficult to reach uniform conclusions on the use of OCS for asthma/wheeze in this age group.
There were not enough data to analyze results in terms of lung function or symptom scores. Similarly, available data on adverse effects was not enough to perform a meta-analysis. It is important to consider that OCS may have multiple systemic adverse effects, including alterations in adrenal function and bone metabolism38,39,40. Therefore, it is critically important to accurately identify those preschoolers with asthma/wheezing exacerbations who would benefit from OCS administration.
In summary, current evidence is inadequate to formulate any broad clinical recommendations regarding the use of OCS in infants, toddlers and preschoolers with recurrent episodes of acute wheezing. OCS might potentially be beneficial (lower hospital admission rates and less need for additional courses of systemic steroids) in children with more severe asthma/wheezing exacerbations that present to the ED or require hospitalization; however, in order to answer this question more definitively, it will be important that future studies utilize a standardized case definition, larger sample size, and more homogeneous methodological quality are needed to give a more definitive answer. It will also be imperative to take into account our findings in terms of the setting of OCS administration.
Acknowledgments
Funding Source: Dr. Castro-Rodriguez’s and Dr. Beckhaus’s contribution was supported by grant CI 03-2015 from the Division of Pediatrics. Dr Forno’s contribution was supported by grant HL125666 from the NIH. No sponsorship from the pharmaceutical industry was provided to conduct this study.
LIST OF ABBREVITATIONS
- AEs
adverse effects
- CI
Confidence intervals
- ED
Emergency department
- FEV1
Forced expiratory volume in 1 second
- ICS
Inhaled corticosteroids
- MD
Mean differences
- OCS
Oral corticosteroids
- PEF
Peak expiratory force
- RCTS
Randomized clinical trials
- RR
Risk ratio
- SAEs
Serious adverse effects
- SCS
Systemic corticosteroids
Footnotes
Conflict of Interests: The authors declared have no conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Radhakrishnan DK, Dell SD, Guttmann A, Shariff SZ, Liu K, To T. Trends in the age of diagnosis of childhood asthma. J Allergy Clin Immunol. 2014;134:1057–1062. doi: 10.1016/j.jaci.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Morgan WJ, Stern DA, Sherrill DL, Guerra S, Holberg CJ, Guilbert TW, Taussig LM, Wright AL, Martinez FD. Outcome of asthma and wheezing in the first 6 years of life: follow-up through adolescence. Am J Respir Crit Care Med. 2005;172:1253–1258. doi: 10.1164/rccm.200504-525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuehni CE, Frey U. Age-related differences in perceived asthma control in childhood: guidelines and reality. Eur Respir J. 2002;20:880–889. doi: 10.1183/09031936.02.00258502. [DOI] [PubMed] [Google Scholar]
- 4.Garner R, Kohen D. Changes in the prevalence of asthma among Canadian children. Health Rep. 2008;19:45–50. [PubMed] [Google Scholar]
- 5.Lougheed MD, Garvey N, Chapman KR, Cicutto L, Dales R, Day AG, Hopman WM, Lam M, Sears MR, Szpiro K, To T, Paterson NA. Ontario Respiratory Outcomes Research Network. The Ontario Asthma Regional Variation Study: emergency department visit rates and the relation to hospitalization rates. Chest. 2006;129:909–917. doi: 10.1378/chest.129.4.909. [DOI] [PubMed] [Google Scholar]
- 6.Karaca-Mandic P, Jena AB, Joyce GF, Goldman DP. Out-of-pocket medication costs, medication utilization, and use of healthcare services among children with asthma. JAMA. 2012;307:1284–1291. doi: 10.1001/jama.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teague WG. Prednisone for acute virus-associated wheeze in children: Panacea or one more brick in the wall? J Allergy Clin Immunol. 2015;135:699–700. doi: 10.1016/j.jaci.2014.10.056. [DOI] [PubMed] [Google Scholar]
- 8.National Asthma Education and Prevention Program Guidelines for the diagnosis and management of asthma 2007. J Allergy Clin Immunol. 2007;120(5Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 9.Global Initiative for Asthma [Accessed 2015 June];Global Strategy for Asthma Management and Prevention 2014. Available at: www.ginasthma.org.
- 10.Alvim CG, Nunes S, Fernandes S, Camargos P, Fontes MJ. Oral and inhaled corticoid treatment for wheezing in the first year of life. J Pediatr (Rio J) 2011;87:314–318. doi: 10.2223/JPED.2101. [DOI] [PubMed] [Google Scholar]
- 11.Collins AD, Beigelman A. An update on the efficacy of oral corticosteroids in the treatment of wheezing episodes in preschool children. Ther Adv Respir Dis. 2014;8:182–190. doi: 10.1177/1753465814552283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 13. [Accessed May, 2015];International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Clinical safety data management: Definitions and standards for expedited reporting. Available at: http://www.pmda.go.jp/files/000156623.pdf.
- 14.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thompson SG, Deecks JJ, Altman D. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to metaanalysis. John Wiley & Songs, Ltd; Chichester (West Sussex): 2009. [Google Scholar]
- 17.Deeks JJ, Altman DG, Bradburn MJ. Statistical Methods for Examining Heterogeneity and Combining Results from Several Studies in Meta-Analysis. In: Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health Care: Meta-Analysis in Context, Second Edition. BMJ Publishing Group; London, UK: 2001. [Google Scholar]
- 18.Webb MS, Henry RL, Milner AD. Oral corticosteroids for wheezing attacks under 18 months. Arch Dis Child. 1986;61:15–19. doi: 10.1136/adc.61.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grant CC, Duggan AK, DeAngelis C. Independent parental administration of prednisone in acute asthma: a double-blind, placebo-controlled, crossover study. Pediatrics. 1995;96(2 Pt 1):224–229. [PubMed] [Google Scholar]
- 20.Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1-5 years: randomised controlled trial. Lancet. 2003;362(9394):1433–1433. doi: 10.1016/S0140-6736(03)14685-5. [DOI] [PubMed] [Google Scholar]
- 21.Beigelman A, King TS, Mauger D, Zeiger RS, Strunk RC, Kelly HW, Martinez FD, Lemanske RF, Jr, Rivera-Spoljaric K, Jackson DJ, Guilbert T, Covar R, Bacharier LB. Do oral corticosteroids reduce the severity of acute lower respiratory tract illnesses in preschool children with recurrent wheezing? J Allergy Clin Immunol. 2013;131:1518–1525. doi: 10.1016/j.jaci.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tal A, Levy N, Bearman JE. Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86:350–356. [PubMed] [Google Scholar]
- 23.Scarfone RJ, Fuchs SM, Nager AL, Shane SA. Controlled trial of oral prednisone in the emergency department treatment of children with acute asthma. Pediatrics. 1993;92:513–518. [PubMed] [Google Scholar]
- 24.Storr J, Barrell E, Barry W, Lenney W, Hatcher G. Effect of a single oral dose of prednisolone in acute childhood asthma. Lancet. 1987;1(8538):879–882. doi: 10.1016/s0140-6736(87)92857-1. [DOI] [PubMed] [Google Scholar]
- 25.Gleeson JG, Loftus BG, Price JF. Placebo controlled trial of systemic corticosteroids in acute childhood asthma. Acta Paediatr Scand. 1990;79:1052–1058. doi: 10.1111/j.1651-2227.1990.tb11382.x. [DOI] [PubMed] [Google Scholar]
- 26.Fox GF, Marsh MJ, Milner AD. Treatment of recurrent acute wheezing episodes in infancy with oral salbutamol and prednisolone. Eur J Pediatr. 1996;155:512–516. doi: 10.1007/BF01955192. [DOI] [PubMed] [Google Scholar]
- 27.Jartti T, Lehtinen P, Vanto T, Vuorinen T, Hartiala J, Hiekkanen H, Malmberg P, Mäkelä M, Ruuskanen O. Efficacy of prednisolone in children hospitalized for recurrent wheezing. Pediatr Allergy Immunol. 2007;18:326–334. doi: 10.1111/j.1399-3038.2007.00512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panickar J, Lakhanpaul M, Lambert PC, Kenia P, Stephenson T, Smyth A, Grigg J. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360:329–338. doi: 10.1056/NEJMoa0804897. [DOI] [PubMed] [Google Scholar]
- 29.Lezmi G, Gosset P, Deschildre A, Abou-Taam R, Mahut B, Beydon N, de Blic J. Airway Remodeling in Preschool Children with Severe Recurrent Wheeze. Am J Respir Crit Care Med. 2015;192:164–171. doi: 10.1164/rccm.201411-1958OC. [DOI] [PubMed] [Google Scholar]
- 30.Jartti T, Lehtinen P, Vanto T, Vuorinen T, Hiekkanen H, Hartiala J, Mäkelä MJ, Ruuskanen O. Atopic characteristics of wheezing children and responses to prednisolone. Pediatr Pulmonol. 2007;42:1125–1133. doi: 10.1002/ppul.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jartti T, Paul-Anttila M, Lehtinen P, Parikka V, Vuorinen T, Simell O, Ruuskanen O. Systemic T-helper and T-regulatory cell type cytokine responses in rhinovirus vs. respiratory syncytial virus induced early wheezing: an observational study. Respir Res. 2009;10:85. doi: 10.1186/1465-9921-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jartti T, Lehtinen P, Vanto T, Hartiala J, Vuorinen T, Mäkelä MJ, Ruuskanen O. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25:482–488. doi: 10.1097/01.inf.0000215226.69696.0c. [DOI] [PubMed] [Google Scholar]
- 33.Davis SR, Burke G, Hogan E, Smith SR. Corticosteroid timing and length of stay for children with asthma in the Emergency Department. J Asthma. 2012;49:862–867. doi: 10.3109/02770903.2012.717656. [DOI] [PubMed] [Google Scholar]
- 34.Zemek R, Plint A, Osmond MH, Kovesi T, Correll R, Perri N, Barrowman N. Triage nurse initiation of corticosteroids in pediatric asthma is associated with improved emergency department efficiency. Pediatrics. 2012;129:671–680. doi: 10.1542/peds.2011-2347. [DOI] [PubMed] [Google Scholar]
- 35.Beigelman A, Zeiger RS, Mauger D, Strunk RC, Jackson DJ, Martinez FD, Morgan WJ, Covar R, Szefler SJ, Taussig LM, Bacharier LB. The association between vitamin D status and the rate of exacerbations requiring oral corticosteroids in preschool children with recurrent wheezing. J Allergy Clin Immunol. 2014;133:1489–1492. doi: 10.1016/j.jaci.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ducharme FM, Zemek R, Gravel J, Chalut D, Poonai N, Laberge S, Quach C, Krajinovic M, Guimont C, Lemiére C, Guertin MC. Determinants Of Oral corticosteroid Responsiveness in Wheezing Asthmatic Youth (DOORWAY): protocol for a prospective multicentre cohort study of children with acute moderate-to-severe asthma exacerbations. BMJ Open. 2014;4:e004699. doi: 10.1136/bmjopen-2013-004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cross KP, Paul RI, Goldman RD. Single-dose dexamethasone for mild-to-moderate asthma exacerbations: effective, easy, and acceptable. Can Fam Physician. 2011;57:1134–1136. [PMC free article] [PubMed] [Google Scholar]
- 38.Volovitz B. Inhaled corticosteroids as rescue medication in asthma exacerbations in children. Expert Rev Clin Immunol. 2008;4:695–702. doi: 10.1586/1744666X.4.6.695. [DOI] [PubMed] [Google Scholar]
- 39.Mori H, Tanaka H, Ohno Y, Ito F, Funaguchi N, Endo J, La BL, Minatoguchi S. Effect of intermittent systemic corticosteroid on bone metabolism in bronchial asthma patients. J Asthma. 2009;46:142–146. doi: 10.1080/02770900802492095. [DOI] [PubMed] [Google Scholar]
- 40.Ducharme FM, Chabot G, Polychronakos C, Glorieux F, Mazer B. Safety profile of frequent short courses of oral glucocorticoids in acute pediatric asthma: impact on bone metabolism, bone density, and adrenal function. Pediatrics. 2003;111:376–383. doi: 10.1542/peds.111.2.376. [DOI] [PubMed] [Google Scholar]