Abstract
After the April 2010 explosion on the Deepwater Horizon oil rig, and subsequent release of millions of barrels of oil, two Corexit oil dispersant formulations were used in unprecedented quantities both on the surface and sub-surface of the Gulf of Mexico. Although the dispersant formulations contain four classes of surfactants, current studies to date focus on the anionic surfactant, bis-(2-ethylhexyl) sulfosuccinate (DOSS). Factors affecting the integrity of environmental and laboratory samples for Corexit analysis have not been systematically investigated. For this reason, a quantitative analytical method was developed for the detection of all four classes of surfactants, as well as the hydrolysis products of DOSS, the enantiomeric mixture of α- and β-ethylhexyl sulfosuccinate (α-/β-EHSS). The analytical method was then used to evaluate which practices for sample collection, storage, and analysis resulted in high quality data. Large volume, direct injection of seawater followed by liquid chromatography tandem mass spectrometry (LC-MS/MS) minimized analytical artifacts, analysis time, and both chemical and solid waste. Concentrations of DOSS in the seawater samples ranged from 71 – 13,000 ng/L, while the nonionic surfactants including Span 80, Tween 80, Tween 85 were detected infrequently (26% of samples) at concentrations from 840 – 9100 ng/L. The enantiomers α-/β-EHSS were detected in seawater, at concentrations from 200 – 1,900 ng/L, and in both Corexit dispersant formulations, indicating α-/β-EHSS were applied to the oil spill and may be not unambiguous indicator of DOSS degradation. Best practices are provided to ensure sample integrity and data quality for environmental monitoring studies and laboratory that require the detection and quantification of Corexit-based surfactants in seawater.
1 Introduction
In response to the Deepwater Horizon oil rig explosion, and subsequent release of oil into the Gulf of Mexico, an unprecedented quantity of oil dispersant was applied to both the surface oil slick and at the wellhead in order to mitigate the environmental impact of the oil spill (Operational Science Advisory Team, 2010). During the spill 7 million liters of Corexit 9500 and 9527 oil dispersant was applied, 4.1 million liters was applied to the surface while 2.9 million was applied sub-surface (National Commission on the BP Deepwater Horizon Oil Spill and Offshore Drilling, 2011). Multiple studies show low to moderate toxicity of Corexit oil dispersants, both as the dispersant alone and when mixed with crude oil (US EPA, 2011; Anderson et al., 2009; George-Ares and Clark, 2000; Goodrich et al., 1991; Wooten et al., 2012). The environmental impact of the application of oil dispersant at these unprecedented volumes is unknown.
Analytical tools are necessary to study the environmental distribution and fate of the oil dispersant constituents in the Gulf of Mexico. In addition, these tools are necessary to support laboratory studies on dispersant components such as toxicity testing and biodegradation experiments. Prior to the spill, there were few analytical methods available for these purposes (Place et al., 2010), primarily due to the fact that the constituents of both Corexit dispersants were proprietary. After the spill, the United State Environmental Protection Agency (US EPA) reported the components of the oil dispersants, which included four surfactants: bis-(2-ethylhexyl) sulfosuccinate (DOSS), sorbitan monooleate (Span 80), sorbitan monooleate polyethoxylate (Tween 80), and sorbitan trioleate polyethoxylate (Tween 85) (US Environmental Protection Agency, 2011). In addition, the US EPA set the aquatic life benchmark for chronic exposure of DOSS to 40,000 ng/L and a reporting limit of 20,000 ng/L (benchmarks were not set for the other surfactant components of Corexit dispersants) (Operational Science Advisory Team, 2010). Since 2010, multiple analytical methods that have been developed in order to detect levels of Corexit oil dispersants in Gulf of Mexico seawater, although these studies mainly focused on DOSS as the indicator for the presence of Corexit 9500 and 9527.(Hayworth and Clement, 2012; Kujawinski et al., 2011; Mathew et al., 2012; Ramirez et al., 2013) To the best of our knowledge, analytical methods for nonionic surfactants, including Tween 80 and Tween 85,(Crescenzi et al., 1995; Petrovic and Barceló, 2001; Petrovic et al., 2002) have not been developed for seawater analysis. To fully characterize the complex mixture of the dispersant formulations, analytical methods are needed for detecting all the dispersant constituents which exhibit varying chemical properties.
There is little information about the fate of these dispersants in aquatic environments. The chemical and biological transformation pathways, and the resultant toxicity of these transformation products, have not been characterized. However, Hales (1993) proposed the biodegradation pathway of linear dialkyl sulfosuccinates and others have reported the presence of the hydrolysis products of DOSS, α-/β-ethylhexyl sulfosuccinate (α-/β-EHSS) (Campo et al., 2013). In addition to being degradation products, α-/β-EHSS can occur potentially as intermediates in the synthesis of DOSS. Analytical methods need to be developed to detect and track these degradation products, as well as the parent compounds in the dispersant, in order to better understand the environmental fate of the dispersants. However, at present, no commercially-available standards exist for α-/β-EHSS nor are there isotopically-labeled internal standards for α-/β-EHSS.
The objective of this study was to develop an analytical method for the quantitative detection of the surfactants components in seawater, as well as investigate the complexities of sample collection, handling, and storage. Current methods to date use sample preparation steps such as solid-phase extraction (Kujawinski et al., 2011; Ramirez et al., 2013) or direct injection after sample dilution (Mathew et al., 2012; Ramirez et al., 2013). Large-volume injection liquid chromatography (LVI-LC) is an alternative to solid-phase extraction that has been demonstrated for environmental contaminants in surface water and wastewater systems (Backe et al., 2011; Busetti et al., 2012; Chiaia et al., 2008), but not yet for seawater. The instrumental method utilizes large-volume injection liquid chromatography (LVI-LC) with mass spectrometry for a sensitive analytical method capable of analyzing seawater for all surfactant components in Corexit dispersants with minimal sample preparation. In addition to the chemical components in the oil dispersant mixtures, an analytical standard for α-EHSS and its 13C-labeled analog were synthesized for use in quantifying α-/β-EHSS. Best practices to ensure sample integrity and data quality during sample collection, handling, and storage were developed and validated. The capabilities of this analytical method were then demonstrated by the analysis of select seawater samples and Corexit commercial formulations.
2 Methods and materials
2.1 Chemicals and Standards
2.1.1 Analytical Standards
A pure (98.1%) solid standard of bis-(2-ethylhexyl) sodium sulfosuccinate (DOSS) was obtained from Sigma Aldrich (Saint Louis, MO). Liquid standards of sorbitan monooleate (Span 80; purity: 70.5%), sorbitan monooleate polyethoxylate (Tween 80; purity: 74%), and sorbitan monooleate polyethoxylate (Tween 85; purity: 67%) were obtained from Sigma Aldrich (St. Louis, MO). A standard containing 13C4-labeled DOSS was provided by Ed Furlong and James Gray at the United States Geological Survey National Water Quality Laboratory (Denver, CO) that was synthesized by Cambridge Isotope Laboratories, Inc (Andover, MA). Quantitative standards for the DOSS hydrolysis products, α- and β-ethylhexyl sulfosuccinate (α-/β-EHSS) were synthesized in laboratory as described below.
HPLC-grade isopropanol, acetonitrile, acetone, and methanol were purchased from Sigma Aldrich. Laboratory 18-MΩ, deionized (DI) water was obtained by an in-house Millipore Synergy unit with an LC-Pak polisher (EMD Millipore Corp, Billerica, MA). High purity ammonium acetate was also purchased from Sigma Aldrich. Instant Ocean® salt mix (Spectrum Brands Company, Madison, WI) was provided by Robert Tanguay at Oregon State University.
Parent stock standards were prepared from solid or concentrate in solvent; DOSS standards were prepared in methanol while Span 80, Tween 80, and Tween 85 were prepared in isopropanol and α-EHSS was prepared in deionized water. Although others report DOSS standards are unstable in solution for longer than 24 h (Kujawinski et al., 2011), preliminary work indicated that all solvent-based standards were stable for over 1.5 months at 4 °C (Figure S5 in Supporting Information (SI)). Analytical standards were prepared in 25% isopropanol and 75% ocean salt solution (created by mixing 15.2 g of Instant Ocean® in DI water). These analytical standards were analyzed within 8 hours.
2.1.2 α-/β-EHSS synthesis and purification
a-EHSS [i.e., sodium 1-carboxy-2-(2-ethylhexyloxycarbonyl)ethanesulfonate] was prepared from maleic anhydride by the method of Baczko et al. (2001) and this same approach was applied to [13C]4-maleic anhydride to create [13C]4-a-EHSS. Both compounds were isolated as colorless powders by precipitation of their disodium salts and the unlabeled material was quantified by 1H NMR spectral analysis (700 MHz, D2O-CD3OD) using 4-(dimethylamino)benzaldehyde as an internal standard (powder was 2.152 μmol/mg in a-EHSS with remainder inorganic sodium salts). β-EHSS was prepared via a three-step sequence from maleic anhydride that comprised of alcoholysis with 4-methoxybenzyl alcohol, N,N′-dicyclohexyldiimide (DCC) coupling of the resulting monoester with (±)-2-ethylhexan-1-ol, and selective removal of the 4-methoxybenzyl group from the mixed diester by treatment with trifluoroacetic acid. Details for this synthetic chemistry will be reported elsewhere.
2.2 Best Practices: Sample Handling and Storage
2.2.1 Analytical Standard Stability
Standards made from pure solid (for DOSS and α-/β-EHSS) or liquid (for Span 80, Tween 80, and Tween 85) were made in 25-mL volumetric flasks with methanol (for DOSS), DI water (for α-/β-EHSS), or isopropanol (for Span 80, Tween 80, and Tween 85). Standards were made at three different dates to compare the long term stability of the stock and stored at 4 °C until analysis. Standards from multiple long-term time points were compared to standards made on the day of analysis. Working standards, consisting of 25% isopropanol and 75% Instant Ocean or 100% Instant Ocean, were made in multiple 6-mL glass autosampler vials and analyzed over time while left at 4 °C (room temperature α-/β-EHSS) for on the autosampler tray. Each solvent system was analyzed at least 4 times over 12 hours.
2.2.2 Environmental Sample (Seawater) Storage Stability
To determine short-term storage stability (13 h), samples containing all analytes were made in 100% Oregon Coast seawater at the second lowest concentration level in order to simulate samples taken for environmental monitoring. The samples were stored in 50-mL HDPE centrifuge tubes and three samples of each standard were stored at room temperature (20 °C), 4 °C, and at −20 °C. For each treatment, one sample at each of three time points over 13 h was prepared as described below in Section 2.3 and analyzed in triplicate.
To determine the long-term stability of seawater samples, open ocean water collected from the Oregon coast was spiked with all analytes and the mixture was separated into multiple 50-mL centrifuge tubes. All long-term stability samples were then stored at −20 °C until analysis. During each analysis, for a total of 7 months, individual samples were thawed in the presence of isopropanol, as described below in Section 2.4.
2.3 Corexit Formulation Analysis
In order to determine the concentration of each analyte in the whole Corexit 9500 and 9527 commercial formulations (donated by Ronald Tjeerdema of the University of California at Davis) were diluted in methanol. Then, analytical samples were made in 25% isopropanol:75% Instant Ocean at nominally 1 mg/L and 100 μg/L total Corexit concentrations. The samples were prepared in the Instant Ocean solution in order to include the formulation analysis within the seawater analysis (as standards were made in Instant Ocean to mimic ion suppression). The higher concentration was used to determine α-/β-EHSS concentrations and the lower concentration was used to determine surfactant concentrations. All samples were analyzed using the same method as for field samples as described below.
2.4 Field Sample Collection and Preparation
Gulf of Mexico seawater samples were collected on the R/V Walton Smith between May 25, 2010 and June 6, 2010. The samples were collected by a CTD-Niskin rosette system at multiple sites and varying depths.(Joye et al., 2011) The collected water was then split into BD Falcon 50-mL polypropylene centrifuge tubes (BD Biosciences, San Jose, CA) and frozen immediately. The samples were kept frozen until they were shipped with blue ice to Oregon State University. Field blanks consisting of laboratory water (using a MilliQ Advantage A10 water purification system) were made on the ship and frozen until shipment. Samples were shipped frozen and stored at −20 C upon receipt.
To reduce or eliminate analyte loss, the frozen seawater samples (in the 50 mL centrifuge vials) were first weighed to determine volume, and then transferred (while frozen) into a 250 mL HDPE bottle. The centrifuge vials were then rinsed with 3 aliquots of isopropanol (final isopropanol volume equivalent to 25% of the final sample volume) and the rinsate was added to the frozen seawater sample in the 250 mL bottle. Field sample preparation steps significantly impacted the recovery of analytes from the seawater samples. Prior to analysis, 5 mL aliquots of seawater sample/isopropanol were transferred to a 6 mL glass autosampler vial and spiked with labeled internal standard solutions. For this study, no autosampler vial caps were used because they were identified as a potential source of DOSS contamination.
2.5 Instrumental Analysis
2.5.1 Large-Volume Injection Liquid Chromatography with Tandem Mass Spectrometry (LVI-LC-MS/MS)
Chromatographic separations were performed using an Agilent 1100 HPLC system (Agilent Technologies, Inc., Santa Clara, CA). The HPLC was upgraded with large volume injection and multidraw kits for injecting volumes up to 1,800 μL. An Agilent Zorbax C18 guard column (4.6 mm ID x 12.5 mm length x 5-μm particle size) was placed in front of a Targa C18 analytical column (2.1 mm ID x 150 mm x 5-μm particle size; Higgins Analytical, Inc., Mountain View, CA). The guard column was replaced approximately every 100 injections. Because the HPLC gave significant background levels of DOSS, an additional Agilent Zorbax C18 guard column, with the same dimensions as described above, was placed in the flow path after the solvent mixer and purge valve but prior to the autosampler as described by Powley et al.(Powley et al., 2005) With this setup, DOSS contamination originating from within the HPLC eluted after the DOSS analyte peak (Figure S7 in SI).
The HPLC mobile phase consisted 0.5 mM ammonium acetate in DI water (A) and acetonitrile (B). The gradient program followed a starting composition of 5% B that was held for the first 7 min, increased to 50% B in 0.5 min, increased to 60% B in 9.5 min, followed by an increase to 97.5% B that was then held for 10 min before the composition returned to 5% B in 1 min for a total run time of 36 min. In addition to the solvent gradient, the flow rate was 0.5 mL/min for the first 17 min before it was increased to 0.75 mL/min for the rest of the analytical run. In order to reduce solvent dwell time (the time it takes for changes in the gradient to reach the analytical column) the autosampler switch valve was set to bypass the autosampler injector system at 7 min. To reduce analyte carryover, the autosampler switch valve switched back to send the mobile phase through the injector system at 17.5 min (Figure S8 and S9 in SI). Without this “main-pass” switch, nonionic analyte carryover ranged from 4 – 40% of the original concentration. With the switch, the nonionic analytes retained in the injection system were pushed onto the column with the 97.5% acetonitrile mobile phase so that they eluted with the analytes retained on the column.
To prevent fouling of the sample cones by the nonvolatile salts in seawater, the initial flow from the column was diverted to waste, after 9.5 min the flow was switched to the mass spectrometer. In addition, from 16 to 23.5 min the flow was diverted to waste during the injector system cleaning step (the first 7.5 min of the main-pass switch). The entire LC-MS/MS timeline is visually shown in SI.
Mass spectrometric detection for DOSS, Span 80, Tween 80, and Tween 85 was performed with a Waters Acquity Triple Quadrupole Mass Spectrometer (Waters Corporation, Milford, MA), while α-/β-EHSS was determined on a Waters Micromass Quattro Mass Spectrometer. Two separate MS/MS systems were used rather than one because the α-EHSS and its 13C4-a-EHSS internal standard were synthesized after the surfactant analyses were complete. All experiments were repeated to determine the analytical figures of merit for a-EHSS but the analyses were performed on an identical LVI-LC system that was interface with the Quattro Micro MS/MS.
DOSS, 13C4-DOSS, α-/β-EHSS, and 13C4-α-EHSS were detected in negative ionization mode with multiple reaction monitoring (MRM) mode. Span 80 was detected in positive ionization mode with MRM mode with two MRM transitions. Tween 80 and Tween 85 represent a homologous series of compounds with varying polyethoxylate chain lengths and therefore could not be identified by a single MRM transition. Alternatively, a common fragment ion (m/z 309) was identified for both Tween 80 and Tween 85, as reported by Borisov et al.(Borisov et al., 2011) Therefore, precursor ion scanning (positive ionization) was used to scan for all precursor masses (m/z 400–1300) that fragmented into m/z 309 in order to quantify the homologous series of Tween 80 and Tween 85. MS parameters and timeline for all analytes are reported in the SI.
2.4.2 Quantification and Quality Control
Preliminary observations indicated that, even with a 95% aqueous wash step, residual salts suppressed ionization of the nonionic analytes (Figure S5 in SI). Because internal standards exist for DOSS and α-/β-EHSS, the ion suppression could be compensated for, but the nonionic surfactants (Span 80, Tween 80, and Tween 85) do not have commercially available isotopically-labeled internal standards and therefore the ion suppression can greatly impact quantification. For purposes of compensating for the strong ion suppression of seawater, due to the high ionic strength, all analytical standards were made in 25% isopropanol and 75% Instant Ocean for matrix-matched calibration.
Calibration curves consisted of at least 5 calibration standards and required a correlation coefficient of 0.99 or greater in order to be used for quantification. All calibration curves were 1/x weighted, and standards whose calculated concentrations were beyond 30% of the intended concentration were removed from the calibration curve calculation. Calibration curves spanned from the lower limit of quantification (LLOQ) to the upper limit of quantification (ULOQ) for DOSS (67–34,000 ng/L), α-/β-EHSS (150–25,000 ng/L), Span 80 (3,000–60,000 ng/L), Tween 80 (2,700–400,000 ng/L), and Tween 85 (700 –150,000 ng/L) (Table 1). Each calibration standard was spiked to give a final concentration of 100 ng/L 13C4-DOSS and 500 ng/L 13C4-a-EHSS.
Table 1.
Whole method performance indicated by limit of detection (LOD), lower limit of quantification (LLOQ), upper limit of quantification (ULOQ), recovery, precision, and the method for individual analyte quantification.
| Compound | LOD (ng/L) | LLOQ (ng/L) | ULOQ (ng/L) | Recovery (% ± 95% CI) | RSD (%) | Quantification Method |
|---|---|---|---|---|---|---|
| DOSS | 67* | 67 | 34,000 | 88 ± 10 | 10 | ISC: 13C4-DOSS |
| α-/β-EHSS | 16† | 150† | 25000 | 98 ± 6 | 1.4 | ISC: 13C4-α-EHSS |
| Span 80 | 1,250 | 3,000 | 60,000 | 91 ± 21 | 23 | Ext. Cal |
| Tween 80 | 987 | 2,700 | 400,000 | 119 ± 13 | 10 | Ext. Cal |
| Tween 85 | 99 | 700 | 150,000 | 106 ± 20 | 17 | Ext. Cal |
DOSS LOD is equal to DOSS LOQ due to background variability.
ISC: 13C4-DOSS - internal standard calibration using 13C4-DOSS as internal standard; ISC: 13C4-α-EHSS – internal standard calibration using 13C4-α-EHSS as an internal standard; and Ext. Cal. - external standard calibration
Blank and check standards were used for quality control purposes and consisted of at least 20% of the total samples run in any given sequence. Check standards consisted of 25% isopropanol:75% Instant Ocean solution that was spiked with all analytes. For DOSS and α-/β-EHSS quantification, the calculated concentration for the check standards were required to be within 30% of the spiked concentration. For Span 80, Tween 80, and Tween 85 there were no internal standards available; therefore, the check standard criteria required concentrations to be within 35%. Due to concerns about DOSS contamination, blanks, consisting of isopropanol:Instant Ocean solution and spiked with 13C4-DOSS, were used regularly to verify that background DOSS concentration levels were below the LLOQ and that there was no carryover of any of the analytes. Failure to meet QC criteria required corrective action until QC checks were brought back into control before proceeding with sample analysis
2.5.3 Method Performance Evaluation
To determine accuracy of the whole method, four samples of blank Oregon Coast seawater were spiked with all analytes at low concentration levels (equivalent to the second lowest standard). For α-/β-EHSS measurements, Oregon Coast seawater was spiked in the absence of all other analytes at a concentration equivalent to the third lowest standard. Recovery was determined as the ratio of calculated analyte concentration to spiked analyte concentration. Precision was reported as the relative standard deviations (RSD) of the four replicate analyses (Table 1).
In order to calculate limits of detection (LOD) and quantification, ten blank samples, consisting of 25% isopropanol and 75% Oregon Coast seawater, were analyzed to determine a baseline background signal (i.e. noise) for all of the analytes. The area of the background signal for each analyte was integrated and a standard deviation of the area was calculated. A low-range calibration curve spanning ≤ 2 orders of magnitude for all analytes was then developed with analytical standards prepared in 25% isopropanol and 75% Instant Ocean solution. The LOD and lower limit of quantification (LLOQ) were estimated by multiplying the background peak area standard deviation by 3.3 and 10, respectively, and dividing this value by the slope of the low-range calibration curve.(Health Canada, 1999)
3 Results and Discussion
3.1 Analytical Method Performance
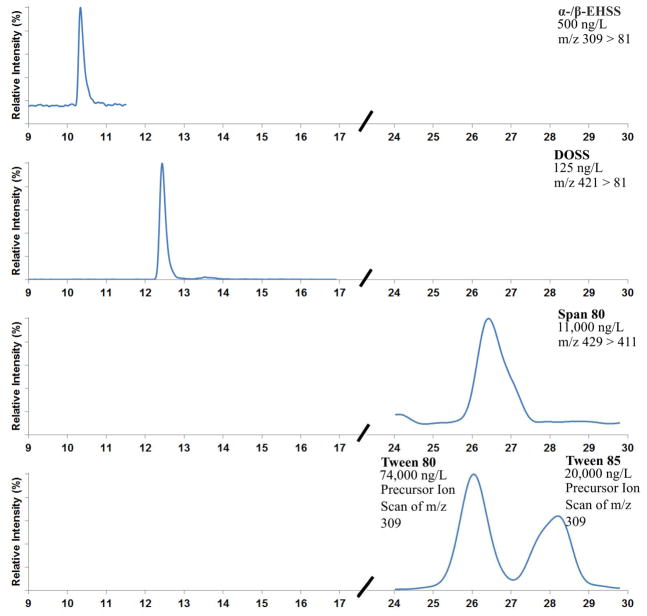
To the best of our knowledge, this is the first study to quantitatively detect all surfactant analytes of Corexit dispersant formulations in seawater. All analytes were chromatographically separated (Figure 1) without adverse effects related to the direct injection of seawater. DOSS, α-/β-EHSS, and Span 80 are single compounds that could be identified using the common multiple reaction monitoring (MRM) mode. The detection of the homologous series of Tween 80 and Tween 85 was more challenging because the complex mixture of polyethoxylates made MRM detection for each individual compound impractical. Furthermore, analytical standards for the Tweens and Span are not commercially available. The precursor ion scanning technique, which detected all mixture components that produce the m/z 309 fragment ion, provided an alternative to MRM for the detection of Tween 80 and Tween 85 (Figure S10 in SI).
Figure 1.
An LVI-HPLC/MSMS chromatogram of all analytes in an analytical standard consisting of 25% isopropanol and 75% Instant Ocean.
LVI-LC is a tool for the sensitive detection of analytes in environmental aqueous samples that avoids extensive sample preparation. The injection of non-volatile salts is of a concern for any analytical method utilizing mass spectrometric detection as salt sprayed into the ionization chamber can lead to sample cone fouling and corrosion. Utilizing the post-column divert valve built into the mass spectrometer, the initial flow, containing most of the salt, was diverted to waste away from the mass spectrometer. This was a vital step in the protection of the MS system during sample analysis. After months of analyses there was no significant deposition of salt on the sample cones.
While column fouling is also a concern with large volume injection, a single analytical column was used for approximately 1 year (~ 2500 large volume injections) without observing diminishing chromatographic peak quality. Guard columns could be used for approximately 100 injections before peak shape deterioration (primarily peak tailing and splitting). Even with the above described instrumental protection procedures, ionization suppression was observed for the nonionic analytes (Figure S5 in SI). We propose that the decrease in sensitivity is due to the formation of sodium-adducted compounds, which result from low levels of residual salts that retained with the analytes and co-eluted into the mass spectrometer. Sodium-adducted compounds have been previously reported to decrease fragmentation efficiency.(Grimalt et al., 2005; Pozo et al., 2008)
3.1.2 Method Accuracy and Precision
Whole method accuracy, as indicated by percent recovery, ranged from 88 – 119% (Table 1). Whole method precision, as indicated by RSD, ranged from 1.4 – 23% (Table 1). Higher RSD values were observed with Tween 85 (17%) and Span 80 (23%), which is due to the poorer sensitivity to these compounds as well as the lack of an internal standard to accommodate for between-injection differences in ionization efficiency. The developed method provides similar recovery of DOSS (88 ±10%, mean ± 95% CI) as those for previously reported methods (80 – 100% recovery).(Gray et al., 2010; Kujawinski et al., 2011; Mathew et al., 2012) In contrast, this LVI method required no sample preparation other than the addition of isopropanol, resulting in higher throughput of the present method. The addition of isopropanol, which ensured analyte stability in seawater, was half the dilution than that employed by Mathew et al. (2012).
The use of 13C4-DOSS as an internal standard for the nonionic compounds was evaluated and the labeled compound did not adequately describe the variation of any of the nonionic compounds, therefore it could not function as an internal standard for any Span 80 or the Tweens. Future research examining the presence and fate of the nonionic analytes will require analytical standards for the individual Tween 80 and Tween 85 polyethoxylate homologues and isotopically-labeled internal standards for these analytes.
Recovery values for the nonionic analytes were better in the isopropanol:Instant Ocean solution than in an ammonium acetate buffer solution, suggesting that the high salt content of the seawater is the primary source of ion suppression and requires matrix-matched calibration (SI).
3.1.3 Limits of Detection/Quantification
Limits of detection (LOD) and lower limits of quantification (LLOQ) ranged from 16 to 1,300 ng/L and 67 to 3,000 ng/L, respectively (Table 1). The background contamination level of DOSS had a mean estimated concentration of 10 ng/L. Due to the high variability (130% RSD) of the DOSS background contamination the LOD was conservatively raised to be equal to the LLOQ at 67 ng/L (Table 1). The use of laboratory blanks, travel blanks, and sample blanks were extremely important eliminating sources of DOSS contamination, which were found to occur on container surfaces and in organic solvents. The LOD for DOSS is higher than that reported by Kujawinski et al. (2011) at 3 ng/L and Ramirez et al. (2013) at 7 ng/L (by SPE), although is below other methods with detection limits of 440 ng/L (Ramirez et al., 2013), 250 ng/L (Gray et al., 2010) and 20,000 ng/L (Mathew et al., 2012). Because comparable methods do not exist for α-/β-EHSS or the nonionic surfactants in seawater, comparisons of the LOD and LLOQs obtained was not possible.
The sensitivity of DOSS and EHSS were multiple orders of magnitude better than those of the nonionic analytes (Span 80, Tween 80, Tween 85). This is most likely due to the poorer ionization efficiency and broader peak shape of the nonionic analytes. In addition, the peaks designated as Tween 80 and Tween 85 represent a broad series of polyethoxylate compounds, which results in a broader overall peak.
3.1.4 Best Practices
3.1.4.1 Sources of DOSS Contamination
Gray et al. (2010) reported the presence of DOSS as a potential contaminant during sample processing. During this study, multiple potential sources of DOSS were identified. These sources included: incomplete cleaning of glassware, PTFE-coated autosampler septa, laboratory deionized water (from three different DI water systems), and general handling of glassware. Various procedures were established in order to eliminate and/or compensate for the DOSS contamination sources. All glassware was cleaned with the following procedures: detergent soak in laboratory tap water, rinsed with warm laboratory tap water, rinsed with laboratory DI water, baked for 12 hours at 400 C, rinsed with methanol, and rinsed with 25% isopropanol/75% cleaned (see below) Instant Ocean solution. All Instant Ocean solutions were made by mixing the commercial Instant Ocean salt with laboratory DI water and then mixed with ENVI-Carb SPE bulk packing (Sigma Aldrich, Saint Louis, MO), using approximately 0.1 g ENVI-Carb per 100 mL of Instant Ocean solution. The solution was stirred for at least 1 hr before it was vacuum filtered and collected in a cleaned Erlenmeyer flask and stored at room temperature. All samples were put in cleaned 6 mL glass autosampler vials without septa. Due to the use of the pump contamination column (see Section 2.5.1), DI water used for the mobile phase did not need to be cleaned.
Care should be used for handling all samples to minimize sources of cross contamination, including using clean glassware, minimizing the number of sample transfers, and changing gloves regularly throughout the sample preparation process. The above procedures were all found to minimize the presence of DOSS contamination in this laboratory, although DOSS signals in blank controls were identified regularly. The use of blank controls for DOSS analytical methods is extremely important in order to provide high-quality, quantitative data and low limits of detection.
3.1.4.2 Analytical Standard Stability
Parent stock standards were stable within an acceptable range over 44 days of analysis when stored at 4 °C (Figure S3 in SI). It was therefore assumed that all standards would be stable for long term storage in 100% organic solvent (water for α-/β-EHSS) when stored at the designated temperature. The addition of 25% isopropanol to Instant Ocean was necessary for the stability of all analytes in the working standards (Figure S4 in SI).
3.1.4.3 Seawater Sample Stability
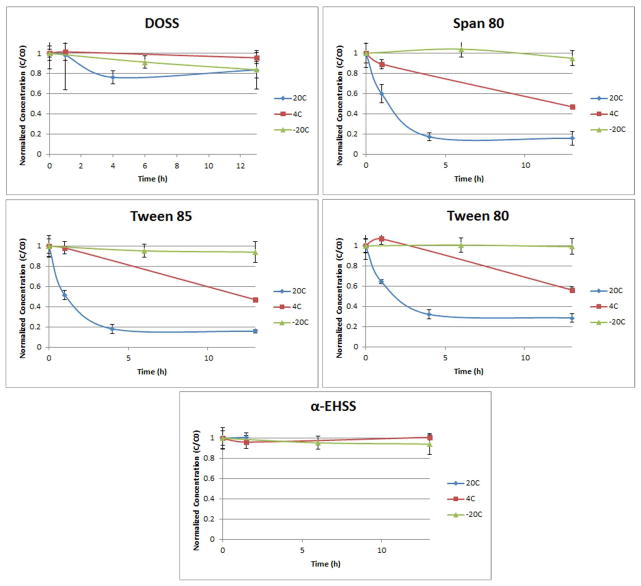
Initial experiments indicated rapid loss of all nonionic surfactants (not DOSS or EHSS) from spiked ocean water when sitting at room temperature (Figure 2). Rinsing the HDPE vials with isopropanol recovered DOSS but not the nonionic Span and Tweens. The recovery of DOSS is attributed to desorption of DOSS from the HDPE vial but the lack of nonionic surfactant recovery may be due to biodegradation because the seawater had not been sterilized. The addition of isopropanol may not only solubilize DOSS but it may also inhibit microbial activity, thus ensuring the integrity of seawater samples containing Corexit components. Therefore, the addition of isoproposal to recover DOSS and quench microbial activity was used to evaluate three seawater sample storage temperatures including room temperature (20 °C, 4 °C, and −20 °C).
Figure 2.
Short-term stability (≤ 13 h) of analytes in seawater in HDPE centrifuge tubes at various temperatures. After storage but prior to analysis, all samples were transferred to a 250 mL HDPE bottle and the centrifuge tube was rinsed with isopropanol and more isopropanol was added to give 25% v/v. Analyte concentrations (C) were normalized to initial concentrations (Co).
The method of sample thawing into 100% isopropanol for the final sample composition produced the most consistent results with full recovery of all analytes. If the loss was due to biodegradation, the isopropanol sterilizes the solution and therefore ceases any further biodegradation activity upon thawing.
For Tween 80 and Tween 85, there were no significant changes in concentration after 7 months at −20 °C in seawater (as determined by the slope, p > 0.05). For Span 80, there was a significant negative slope (p < 0.05) that would result in a 64% decrease in concentration over the 7 months of analysis. For DOSS, there was a significant negative slope (p < 0.05) that would result in a 21% decrease in concentration over the 7 months of analysis. These findings suggest that while samples are stable for the short term when frozen at −20 C, long-term storage of these samples can be detrimental to the quality of the data.
3.2 Method Demonstration
3.2.1 Corexit 9500 and 9527 Formulations
Whole Corexit 9500 and Corexit 9527 formulations were determined to contain 18% and 17% (w/w) DOSS, respectively. Both Corexit 9500 and Corexit 9527 contained detectable quantities of α-/β-EHSS at 0.28% (w/w) and 0.17% (w/w), respectively. It was beyond the scope of the current study to determine whether the presence of α-/β-EHSS was due to synthetic impurities or the degradation of DOSS during storage of the Corexit formulations. The nonionic surfactants were detected in the Corexit 9500 at 4.4% (w/w, Span 80), 18% (w/w, Tween 80), and 4.6% (w/w, Tween 85) and in the Corexit 9527 formulation at 2.7% (w/w, Span 80), 11% (w/w, Tween 80), and 4.3% (w/w, Tween 85). It should be noted that these concentrations may vary between batches and the reported values may not be representative of all Corexit formulations used in the Gulf.
3.2.2 DOSS in Gulf of Mexico Seawater
Quantifiable concentrations of DOSS were detected in over half of the seawater samples analyzed, with concentrations ranging from 71 to 13,000 ng/L (Table 2). The majority of the samples containing detectable DOSS concentrations were at depths deeper than or equal to 1,000 m, with a mean concentration of 4,100 ng/L (n=8). The mean concentration at the more shallow depths was 110 ng/L (n=4). The measured DOSS concentrations of depth seawater samples are consistent with those previously reported by Kujawinski et al. (2011) and are at concentrations below the detection limits reported by Mathew et al. (2012).
Table 2.
Concentrations of DOSS, α-/β-EHSS, Span 80, Tween 80, and Tween 85 for each sampling location, with sample conditions of depth and distance from the Deepwater Horizon well head (designated MC252).
| Sample Station | Cast | Distance to MC252 well head (m) | Depth (m) | [DOSS] ng/L | [α-/β-EHSS] ng/L | [SPAN80] ng/L | [TWEEN80] ng/L | [TWEEN85] ng/L |
|---|---|---|---|---|---|---|---|---|
| WS58 | 75 | 410 | 600 | nd | nd | nd | nd | nd |
| 900 | nd | nd | nd | nd | nd | |||
| 1210 | 7,700 | < LLOQ | nd | nd | 860 | |||
| 1400 | nd | nd | nd | nd | nd | |||
| WS6 | 73 | 610 | 1180 | 13,000 | < LLOQ | nd | 4,800 | 840 |
| WS76 | 86 | 1290 | 50 | nd | < LLOQ | nd | nd | nd |
| 1000 | nd | nd | nd | 9,100 | nd | |||
| 1100 | 100 | nd | nd | 5,900 | nd | |||
| 1200 | 11,000 | < LLOQ | nd | nd | 2,900 | |||
| WS78 | 90 | 13320 | 90 | nd | 1900 | nd | nd | nd |
| 600 | 95 | 530 | nd | nd | nd | |||
| 1130 | nd | < LLOQ | nd | nd | nd | |||
| WS79 | 91 | 15790 | 90 | 71 | < LLOQ | nd | nd | nd |
| 90 | 110 | nd | nd | nd | nd | |||
| 600 | nd | < LLOQ | nd | 3,500 | nd | |||
| 900 | 76 | < LLOQ | nd | nd | nd | |||
| 1050 | 170 | NA | nd | nd | nd | |||
| WS16 | 89 | 17700 | 100 | nd | < LLOQ | nd | nd | nd |
| 600 | 170 | < LLOQ | nd | nd | nd | |||
| 1025 | 76 | < LLOQ | nd | nd | nd | |||
| 1100 | nd | 200 | nd | nd | nd | |||
| 1200 | 220 | < LLOQ | nd | nd | nd | |||
| 1300 | 200 | nd | nd | nd | nd |
< LLOQ designates the analyte was below the lower limit of quantification but above the limit of detection, while “nd” indicates the analyte was below the limit of detection. NA indicates the analyte was not measured in this sample.
3.2.3 α-/β-EHSS in Gulf of Mexico Seawater
There were multiple detections of α-/β-EHSS that were above the LOD of 16 ng/L (n=15; Table 2) in the analyzed seawater. Quantifiable concentrations of α-/β-EHSS were detected in 3 samples with a concentration range from 200 – 1900 ng/L. Although most α-/β-EHSS detections correspond with DOSS detections, there were samples that contained detectable quantities of α-/β-EHSS without DOSS and vice versa.
While the other analytes portrayed sample stability issues in laboratory seawater standards, α-/β-EHSS compounds did not display any loss of concentration in seawater. This observation suggests that α-/β-EHSS are more water soluble and will be in the aqueous phase longer than any of the parent analytes. Because detectable quantities of α-/β-EHSS were observed in the Corexit formulations, the detection of α-/β-EHSS in seawater cannot be used as an unambiguous indicator of DOSS degradation in the environment. α-EHSS, but not β-EHSS, was also at detectable levels in DOSS analytical standards, most likely as an synthetic impurity (approximately 400 ppm concentration in the solid DOSS standard). Therefore, care needs to be taken when analyzing laboratory samples from toxicity or biodegradation studies for α-/β-EHSS because it occurs in Corexit and in DOSS analytical standards.
3.2.4 Nonionic Compounds in Gulf of Mexico Seawater
There were no detectable quantities of Span 80 in any of the analyzed samples (Table 2). Samples that were positive for the nonionic analytes contained concentrations for Tween 80 that ranged from 3,500 to 9,100 ng/L (n=4) and Tween 85 that ranged from 840 to 2,900 ng/L (n=3, Table 2). There was no significant correlation between concentrations of Tween 80 and Tween 85 (correlation coefficient r2=0.48, n=6). While there was a greater number of analyte detections observed at the lower depths, the purpose of the sampling program was not to obtain sufficient monitoring data to develop a correlation between depth and analyte concentration.
The difficulty of stabilization of the nonionic compounds in seawater, combined with their relatively high LLOQs, is consistent with the relatively few observations of the nonionic analytes in seawater. The degradation of the nonionic surfactants in various conditions has been previously reported by many researchers and this is consistent with the rapid loss of the nonionic analytes in non-sterilized laboratory seawater (Kerwin, 2008). Others have found that the rapid biological loss of sorbitan polyethoxylates, such as Tween 80 and Tween 85, due to the degradation by esterase enzymes.(Tellingen et al., 1999) This is consistent with the rapid loss of the nonionic analytes in non-sterilized laboratory seawater.
4 Conclusions
The analytical protocol used in this study provides a sensitive and rugged method for the detection and quantification of the multiple surfactant components in Corexit oil dispersant in seawater samples. The analyte stability findings suggest that protocols for sample handling and instrumental analysis can greatly impact the quality of the data produced. A more thorough, and more current, set of Gulf of Mexico seawater samples (both surface and at depth) would provide a better understanding of the spatial distribution of the surfactants. In addition, future studies to determine the chemical and bio-degradation of DOSS for the formation of β-EHSS and α-EHSS, as well as degradation of the nonionic surfactants, are necessary to determine the environmental implications of these measurements.
Supplementary Material
Acknowledgments
The authors would like to thank James Gray and Ed Furlong (USGS) for collaborative discussions and provision of the 13C4-DOSS standard; Samantha Joye and Kim Hunter (UGA) for the collection and provision of Gulf of Mexico seawater samples; and Ronald Tjeerdema (UCD) for early collaborations related to Corexit oil dispersant and donation of the Corexit 9500 and 9527 formulation. Gulf of Mexico sample collection, aboard the R/V Walton Smith, was supported by a grant from the National Science Foundation OCE-1043224 given to Samantha B. Joye. In addition, this study was supported, in part, by a grant from the BP/The Gulf of Mexico Research Initiative (as part of the University of Mississippi-led consortium for research entitled ‘Ecosystem Impacts of Oil and Gas Inputs to the Gulf (ECOGIG)’) contribution number 217, the OSU Superfund Research Program Award Number P42ES016465 from the National Institute of Environmental Health Sciences, and the N.L. Tartar Research Fellowship from Oregon State University. The data shown in Table2 can be found at this location https://data.gulfresearchinitiative.org/data/R1.X132.34:0036/ and the data for Table 1, Figure 1 and 2, and in the Supporting Information can be found at this location https://data.gulfresearchinitiative.org/data/R1.X132.138:0001/. Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number T32ES007060. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- US Environmental Protection Agency. [May 8 2012];Questions and Answers on Dispersants. 2011 http://www.epa.gov/bpspill/dispersants-qanda.html.
- Anderson BS, Arenella-Parkerson D, Phillips BM, Tjeerdema RS, Crane D. Preliminary investigation of the effects of dispersed Prudhoe Bay Crude Oil on developing topsmelt embryos, Atherinops affinis. Environ Pollut. 2009;157:1058–1061. doi: 10.1016/j.envpol.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Backe WJ, Ort C, Brewer AJ, Field JA. Analysis of Androgenic Steroids in Environmental Waters by Large-Volume Injection Liquid Chromatography Tandem Mass Spectrometry. Analytical Chemistry. 2011;83:2622–2630. doi: 10.1021/ac103013h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baczko K, Chasseray X, Larpent C. Synthesis and surfactant properties of symmetric and unsymmetric sulfosuccinic diesters, Aerosol-OT homologues. Journal of the Chemical Society, Perkin Transactions. 2001;2:2179–2188. [Google Scholar]
- Borisov OV, Ji JA, Wang YJ, Vega F, Ling VT. Toward Understanding Molecular Heterogeneity of Polysorbates by Application of Liquid Chromatography–Mass Spectrometry with Computer-Aided Data Analysis. Analytical Chemistry. 2011;83:3934–3942. doi: 10.1021/ac2005789. [DOI] [PubMed] [Google Scholar]
- Busetti F, Backe W, Bendixen N, Maier U, Place B, Giger W, Field J. Trace analysis of environmental matrices by large-volume injection and liquid chromatography–mass spectrometry. Analytical and Bioanalytical Chemistry. 2012;402:175–186. doi: 10.1007/s00216-011-5290-y. [DOI] [PubMed] [Google Scholar]
- Campo P, Venosa AD, Suidan MT. Biodegradability of Corexit 9500 and Dispersed South Louisiana Crude Oil at 5 and 25 °C. Environmental Science & Technology. 2013;47:1960–1967. doi: 10.1021/es303881h. [DOI] [PubMed] [Google Scholar]
- Health Canada. Therapeutic Products Programme Guideline: Validation of Analytical Procedures: Methodology. Ottawa, Ontario: 1999. [Google Scholar]
- Chiaia AC, Banta-Green C, Field J. Eliminating Solid Phase Extraction with Large-Volume Injection LC/MS/MS: Analysis of Illicit and Legal Drugs and Human Urine Indicators in US Wastewaters. Environmental Science & Technology. 2008;42:8841–8848. doi: 10.1021/es802309v. [DOI] [PubMed] [Google Scholar]
- Crescenzi C, Di Corcia A, Samperi R, Marcomini A. Determination of Nonionic Polyethoxylate Surfactants in Environmental Waters by Liquid Chromatography/Electrospray Mass Spectrometry. Analytical Chemistry. 1995;67:1797–1804. [Google Scholar]
- National Commission on the BP Deepwater Horizon Oil Spill and Offshore Drilling. The Use of Surface and Subsea Dispersants During the BP Deepwater Horizon Oil Spill. 2011. [Google Scholar]
- George-Ares A, Clark JR. Aquatic toxicity of two Corexit® dispersants. Chemosphere. 2000;40:897–906. doi: 10.1016/s0045-6535(99)00498-1. [DOI] [PubMed] [Google Scholar]
- Goodrich MS, Melancon MJ, Davis RA, Lech JJ. The Toxicity, Bioaccumulation, Metabolism and Elimination of Dioctyl Sodium Sulfosuccinate (DSS) in Rainbow Trout (Oncorhynchus Mykiss) Water Research. 1991;25:119–124. [Google Scholar]
- United States Geological Survey. Determination of the Anionic Surfactant Di(Ethylhexyl) Sodium Sulfosuccinate in Water Samples Collected from Gulf of Mexico Coastal Waters Before and After Landfall of Oil from the Deepwater Horizon Oil Spill, May to October, 2010. 2010. [Google Scholar]
- Grimalt S, Pozo ÓJ, Marín JM, Sancho JV, Hernández F. Evaluation of Different Quantitative Approaches for the Determination of Noneasily Ionizable Molecules by Different Atmospheric Pressure Interfaces Used in Liquid Chromatography Tandem Mass Spectrometry: Abamectin as Case of Study. Journal of the American Society for Mass Spectrometry. 2005;16:1619–1630. doi: 10.1016/j.jasms.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Hales SG. Biodegradation of the anionic surfactant dialkyl sulphosuccinate. Environmental Toxicology and Chemistry. 1993;12:1821–1828. [Google Scholar]
- Hayworth JS, Clement TP. Provenance of Corexit-related chemical constituents found in nearshore and inland Gulf Coast waters. Marine Pollution Bulletin. 2012;64:2005–2014. doi: 10.1016/j.marpolbul.2012.06.031. [DOI] [PubMed] [Google Scholar]
- Joye SB, MacDonald IR, Leifer I, Asper V. Magnitude and oxidation potential of hydrocarbon gases released from the BP oil well blowout. Nature Geosci. 2011;4:160–164. [Google Scholar]
- Kerwin BA. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: Structure and degradation pathways. Journal of Pharmaceutical Sciences. 2008;97:2924–2935. doi: 10.1002/jps.21190. [DOI] [PubMed] [Google Scholar]
- Kujawinski EB, Soule MCK, Valentine DL, Boysen AK, Longnecker K, Redmond MC. Fate of Dispersants Associated with the Deepwater Horizon Oil Spill. Environmental Science & Technology. 2011;45:1298–1306. doi: 10.1021/es103838p. [DOI] [PubMed] [Google Scholar]
- Mathew J, Schroeder DL, Zintek LB, Schupp CR, Kosempa MG, Zachary AM, Schupp GC, Wesolowski DJ. Dioctyl sulfosuccinate analysis in near-shore Gulf of Mexico water by direct-injection liquid chromatography–tandem mass spectrometry. Journal of Chromatography A. 2012;1231:46–51. doi: 10.1016/j.chroma.2012.01.088. [DOI] [PubMed] [Google Scholar]
- Operational Science Advisory Team, Unified Area Command. Summary Report for Sub-Sea and Sub-Surface Oil and Dispersant Detection: Sampling and Monitoring. 2010. [Google Scholar]
- Petrovic M, Barceló D. Analysis of ethoxylated nonionic surfactants and their metabolites by liquid chromatography/atmospheric pressure ionization mass spectrometry. Journal of Mass Spectrometry. 2001;36:1173–1185. doi: 10.1002/jms.234. [DOI] [PubMed] [Google Scholar]
- Petrovic M, Fernández-Alba AR, Borrull F, Marce RM, Mazo EG, Barceló D. Occurrence and distribution of nonionic surfactants, their degradation products, and linear alkylbenzene sulfonates in coastal waters and sediments in Spain. Environmental Toxicology and Chemistry. 2002;21:37–46. [PubMed] [Google Scholar]
- Place B, Anderson B, Mekebri A, Furlong ET, Gray JL, Tjeerdema R, Field J. A Role for Analytical Chemistry in Advancing our Understanding of the Occurrence, Fate, and Effects of Corexit Oil Dispersants. Environmental Science & Technology. 2010;44:6016–6018. doi: 10.1021/es102319w. [DOI] [PubMed] [Google Scholar]
- Powley CR, George SW, Ryan TW, Buck RC. Matrix Effect-Free Analytical Methods for Determination of Perfluorinated Carboxylic Acids in Environmental Matrixes. Analytical Chemistry. 2005;77:6353–6358. doi: 10.1021/ac0508090. [DOI] [PubMed] [Google Scholar]
- Pozo OJ, Deventer K, Van Eenoo P, Delbeke FT. Efficient Approach for the Comprehensive Detection of Unknown Anabolic Steroids and Metabolites in Human Urine by Liquid Chromatography–Electrospray-Tandem Mass Spectrometry. Analytical Chemistry. 2008;80:1709–1720. doi: 10.1021/ac7020757. [DOI] [PubMed] [Google Scholar]
- Ramirez C, Batchu S, Gardinali P. High sensitivity liquid chromatography tandem mass spectrometric methods for the analysis of dioctyl sulfosuccinate in different stages of an oil spill response monitoring effort. Analytical and Bioanalytical Chemistry. 2013;405:4167–4175. doi: 10.1007/s00216-013-6841-1. [DOI] [PubMed] [Google Scholar]
- Tellingen Ov, Beijnen JH, Verweij J, Scherrenburg EJ, Nooijen WJ, Sparreboom A. Rapid Esterase-sensitive Breakdown of Polysorbate 80 and Its Impact on the Plasma Pharmacokinetics of Docetaxel and Metabolites in Mice. Clinical Cancer Research. 1999;5:2918–2924. [PubMed] [Google Scholar]
- Wooten K, Finch B, Smith P. Embryotoxicity of Corexit 9500 in mallard ducks (Anas platyrhynchos) Ecotoxicology. 2012;21:662–666. doi: 10.1007/s10646-011-0822-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.