Abstract
Background
Severe asthma is a complex heterogeneous disease associated with older age and obesity. The presence of eosinophilic (type 2) inflammation in some but not all patients with severe asthma predicts responsiveness to current treatments, but new treatment approaches will require better understanding of non-type 2 mechanisms of severe asthma. We considered the possibility that systemic inflammation - which occurs in subgroups of obese and older patients - modifies asthma to make it worse. Interleukin 6 (IL6) is a biomarker of systemic inflammation and metabolic dysfunction, and we aimed to explore the relationship between IL6, metabolic dysfunction, and asthma severity.
Methods
We generated a reference range in health for plasma IL6 in a cohort of healthy controls (n=93). We compared the clinical characteristics of asthmatics with plasma IL6 levels below and above the upper limit of normal (“IL6 low” and “IL-high” asthma) in two asthma cohorts - predominantly non-severe asthmatics recruited at the University of California San Francisco (UCSF)(n=249) and predominantly severe asthmatics recruited by the Severe Asthma Research Program (SARP)(n=387).
Findings
The upper 95th centile value for plasma IL6 in the healthy cohort was 3·1pg/mL, and 14% of UCSF cohort and 26% of the SARP cohort had plasma IL6 levels above this upper limit. The “IL6-high” patients in both asthma cohorts had a significantly higher body mass index and a higher prevalence of metabolic disease than the IL6-low patients (all p values < 0.01). IL6-high patients also had significantly lower lung function and more frequent asthma exacerbations than IL6-low patients (all p values < 0·01). Although 75% of IL6-high asthmatics were obese, 63% of obese patients were IL6-low. Among obese patients, the forced expired volume in one second (FEV1) was significantly lower in IL6-high than in IL6-low patients (mean FEV1 70·8 [S.D. 19·5] vs. 78·1 [19·7] % predicted, p = 0·002), and the percentage of patients reporting an asthma exacerbation in the past 1-2 years was higher in IL6-high than in IL6-low patients (66 vs. 48%, p = 0·003). Among non-obese asthmatics, FEV1% and asthma exacerbation outcomes were also significantly worse in IL6-high than in IL6-low patients (mean FEV1 66·4 [SD 23·1] vs. 83·2 [20·4] % predicted, p< 0·01; 59 vs. 34 %, p=0·008).
Interpretation
Systemic IL6 inflammation and clinical features of metabolic dysfunction - occurring most commonly among a subset of obese asthmatics but also in a small subset of non-obese patients - is associated with more severe asthma. IL6 inhibitors or treatments that improve metabolic dysfunction represent rational clinical trials to pursue for a subset of patients with severe asthma, and plasma IL6 is a biomarker that could guide patient stratification.
Introduction
Asthma is a heterogeneous disease with variability in clinical features and in underlying cellular and molecular mechanisms. Type 2 inflammation is clearly important in asthma, but a significant subgroup of asthmatics do not have type 2 inflammation in their airways and do not respond to treatments targeting this pathway (1). Many of these “Th2-low” asthma patients have severe disease and have significant unmet treatment needs. Obesity is a prominent clinical trait in severe asthma (2), but the mechanism of the association between obesity and severe forms of asthma is uncertain. One possibility is that obesity-related systemic inflammation contributes to development of severe asthma. Low-grade systemic inflammation occurs in a subset of obese patients because adipocytes and inflammatory macrophages in adipose tissue secrete a variety of pro-inflammatory cytokines (including interleukin 6 [IL6]) (3). Although this low-grade systemic inflammation is known to be associated with development of insulin resistance, dyslipidemia, atherosclerosis, type 2 diabetes, and hypertension (3), surprisingly little attention has been paid to the role of systemic inflammation and metabolic dysfunction as a risk for development of severe asthma. Previous studies in relatively small sample sizes have described increases in plasma IL6 in patients with asthma (4), and genetic studies have revealed that IL6 pathway genes are associated with asthma (5), but these studies have not examined the relationship between airway and systemic levels of IL6 in asthma, the role of systemic IL6 inflammation in explaining the variable effects of obesity on asthma severity, and the relationship between IL6 inflammation and type 2 inflammation in asthma.
Materials and Methods
Study Design
Subjects studied included a reference (healthy) cohort and two asthma cohorts - predominantly non-severe asthmatics recruited at University of California, San Francisco (UCSF) (n=249) and predominantly severe asthmatics recruited by the Severe Asthma Research Program (SARP) (n=387). These cohorts were highly characterized with data available for outcomes related to lung function, asthma control, and asthma exacerbations, as well as data related to metabolic health. Data on history of diabetes was only available in the SARP cohort. Both cohorts also had blood and airway bio-specimens available for analysis. Details about recruitment methods, subject enrollment, study measurements, and study procedures are provided in the supplementary appendix.
UCSF Subjects
93 healthy control subjects and 249 asthma subjects had been recruited to research studies in the UCSF Airway Clinical Research Center between 2005-2014. All studies included 1-2 baseline visits, which used standardized protocols for clinical characterization and collection, processing, and storage of blood and induced sputum. Healthy subjects had no history of pulmonary disease, no history of atopic disease or allergic rhinitis, and had normal airway responses to inhaled methacholine. Asthma subjects had a prior physician diagnosis of asthma and either bronchial hyperresponsiveness (BHR) to methacholine or reversible airflow obstruction. These subjects had no lifetime history of any other pulmonary disease.
SARP Subjects
387 adult asthma subjects were recruited to the Severe Asthma Research Program (SARP) between November 1, 2012 and October 1, 2014 by seven clinical research centers (including UCSF) in the United States. The SARP protocol is an ongoing, six visit, 3-year, longitudinal cohort study in which 60% of subjects have severe asthma as defined by the American Thoracic Society/European Respiratory Society (ATS/ERS) consensus (6). The SARP protocol included two baseline visits in which patients underwent detailed characterization studies and provide samples of blood and induced sputum. The data reported here is from these two baseline visits.
Plasma IL6 and C-reactive protein (CRP) Measurements
Assays were performed at UCSF using aliquots of Ethylenediaminetetraacetic acid (EDTA) treated plasma and the high sensitivity human IL6 Quantikine ELISA Kit (R&D Systems, Minneapolis, MN) (lower/upper limit of detection, 0·16/10·5 pg/mL). CRP was measured in plasma from 38 UCSF healthy subjects and 123 UCSF asthma patients using the Quantikine ELISA kit (R&D Systems, Minneapolis, MN) (lower/upper limit of detection, 0·78/20 ng/L).
Induced Sputum Measures
Total and differential cell counts were quantified in both UCSF and SARP patients using methods previously described (7,8). IL6 protein was measured in induced sputum supernatants from 109 UCSF asthma patients using the human IL6 DuoSet Kit (R&D Systems Minneapolis, MN) (lower limit of detection, 9·38 pg/mL). The reliability of sputum IL6 protein measures was confirmed by spike and recovery experiments (supplemental appendix, Table S1). IL6 gene expression was measured from RNA isolated from induced sputum cell pellets from 210 SARP asthma patients and 55 UCSF asthma patients using previously described methods (9).
Statistical Methods
Analyses were performed using JMP 10 software package (SAS Institute, Cary, NC) and Stata 12.0 (StataCorp College Station TX), and P values less than 0·05 were taken as statistically significant. Three group comparisons between subjects in the UCSF Health, UCSF Asthma, and SARP Asthma cohorts were made using an ANOVA followed by a Bonferroni correction for continuous variables and a Fisher's exact test for categorical variables. Two group comparisons between IL6 high and IL6 low asthma were made with; a student t-test for numeric variables with approximately normal distributions, a Wilcoxon rank-sum for numeric variables with non-normal distributions, and a Fisher's exact test for dichotomous variables. Correlation was performed using the Pearson's correlation to assess the relationship between numeric variables with approximately normal distributions. Numeric variables with non-normal distributions were log transformed to achieve normality prior to Pearson's correlation analysis. Best-fit lines were constructed using least squares regression. Linear and logistic regression modeling was used to assess the relationship between natural log transformed plasma IL6 levels and measures of asthma control. Models were constructed with and without adjustment for the covariates of age, BMI, blood eosinophil cell counts, and corticosteroid use based upon biologic plausibility and using directed acyclic graphs (Fig S2). The dependent variables (outcome) for the linear regression models were FEV1, and forced vital capacity (FVC) % predicted. The dependent variables for the logistic regression modeling were asthma exacerbation (defined as a course of systemic corticosteroids) in the past one year (SARP cohort) or two years (UCSF asthma cohort), asthma hospitalization in the past year, asthma-related emergency department visit in the past year, and ATS/ERS criteria for severe asthma. Interaction terms were constructed for the interaction between the predictor variables of plasma IL-6 and body mass index on the dependent variables of asthma severity. Including these interaction terms did not significantly alter the models and therefore were not included. Although all participants had plasma IL-6 measured, not all participants had data for every study outcome, and analyses used available data (missing data is described in the table footnotes and summarized in Table S2).
Role of the funding source
The funding source was research grants awarded by the US. National Institutes of Health - Heart lung and Blood Institute (NHLBI). The NHLBI was involved in providing funding for the Severe Asthma Research Program (SARP) and program officers from NHLBI designed the structure of the SARP. An NHLBI program officer also participates as a member of the SARP steering committee. The NHLBI was not involved in patient recruitment, data collection, data analysis, data interpretation, or manuscript preparation.
Results
Subject Demographics
The UCSF healthy cohort and the UCSF asthma cohort had a similar mean age, but the SARP cohort was older and heavier than both the UCSF healthy cohort and the UCSF asthma cohort (Table 1). The older age and higher body weight of the SARP cohort reflects the recruitment methods for SARP, which emphasized inclusion of patients with severe asthma who are known to be older and heavier.
Table 1.
Characteristics of Healthy and Asthma Subjects
| Characteristic | Healthy | Asthma | |
|---|---|---|---|
| UCSF (N = 93) | UCSF (N = 249) | SARP (N= 387) | |
| Age (years) | 35·9 (12·1) | 37·3 (14·2) | 47·2 (13·6)* |
| Female sex - no. (%) | 52 (56) | 155 (62) | 255 (66) |
| Race - no. (%)‡ | |||
| American Indian and Alaska Native | 1 (1) | 4 (2) | 2 (1) |
| Asian | 16 (17) | 35 (14) | 16 (4) |
| African American | 3 (3) | 27 (11) | 89 (23) |
| Caucasian | 64 (69) | 155 (62) | 255 (66) |
| Native Hawaiian or other Pacific Islander | 0 (0) | 3 (2) | 0 (0) |
| Mixed race | 5 (5) | 17 (7) | 25 (6) |
| Unknown/refused to answer | 4 (4) | 8 (3) | 0 (0) |
| BMI (kg/m2) | 25·4 (5·5) | 28·9 (6·9)* | 32·3 (8·4)*† |
| Use of ICS no. (%) | 141 (57) | 345 (89)† | |
| % with BMI > 30 | 15 (16) | 82 (33) | 207 (53) |
| Spirometry data | |||
| FEV1 (% predicted) | 99·4 (13·8) | 83·8 (18·3)* | 75·9 (21·7)*† |
| FVC (% predicted) | 102·6 (12·7) | 97·7 (15·4)* | 90·2 (19·7)*† |
| FEV1/FVC | 0·97 (0·10) | 0·85 (0·12)* | 0·83 (0·13)* |
| Score on ACT§ | 18·9 (3·9) | 16·8 (4·9)C | |
| Blood markers of atopy¶ | |||
| Eosinophils (× 106/L) | 133 (106) | 292 (220)* | 292 (275)* |
| Serum IgE (IU/mL) | 51 (74) | 360 (474)* | 342 (581)* |
| FeNO (ppm)∥ | 17 (13) | 46 (41)* | 30 (30)*† |
| Asthma exacerbations – no. (%)** | |||
| ≥1 exacerbation in past two years | 88 (35) | ||
| ≥1 exacerbation in past year | 194 (50) | ||
| Necessitating hospitalization past year | 46 (12) | ||
| Necessitating ER visit past year | 95 (25) | ||
Data reported as mean (SD) unless otherwise indicated. FEV1 = forced expiratory volume in 1 s. BMI = body mass index. FVC = forced vital capacity. ICS = inhaled corticosteroids. ACT = asthma control test. FeNO = fraction of nitric oxide in exhaled air. ER = emergency room.
Indicates statistically different from UCSF Healthy (p<0 05).
Indicates statistically different between UCSF Asthma and SARP Asthma (p<0 05).
Race was statistically different between SARP Asthma and either UCSF Healthy or UCSF Asthma.
Scores on ACT range from 25 to 5 with lower scores indicating worse asthma control – 98 of the UCSF patients did not have this score recorded.
2 of the UCSF healthy subjects and 18 of the UCSF asthma patients did not have blood eosinophils measured; 4 of the UCSF healthy subjects, 18 of the UCSF asthma patients, and 4 of the SARP asthma patients did not have serum IgE levels measured.
23 of the UCSF healthy subjects, 60 of the UCSF asthma patients, and 3 of the SARP asthmatics did not have FeNO measured.
The UCSF asthma patients completed a questionnaire which asked about asthma exacerbations requiring treatment with prednisone in the past 2 years; the SARP asthma patients completed a questionnaire which asked about asthma exacerbations requiring treatment with prednisone in the past year and also captured information about hospitalizations and ER visits for treatment of asthma in the past year.
Plasma IL6 (but not sputum IL6) is increased in a subset of asthmatics
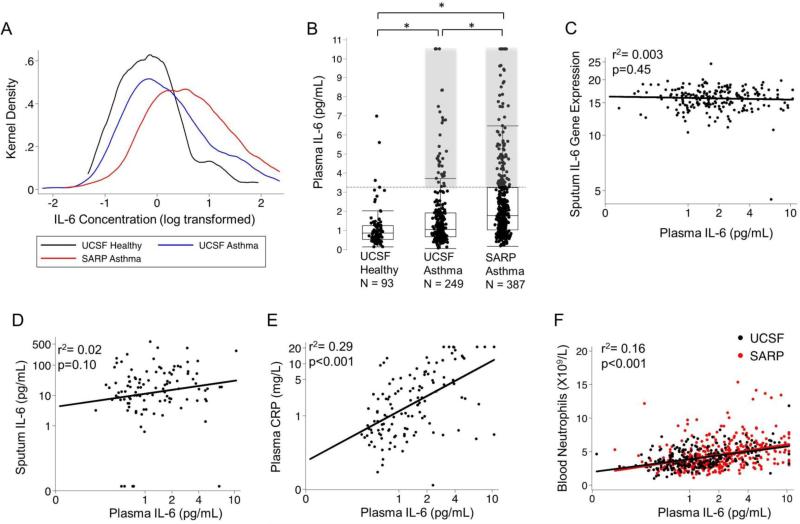
To calculate a reference interval for plasma IL6 in health, we followed the guidelines of the national committee for clinical laboratory standards(10). First, we log transformed plasma IL6 values in healthy subjects to normalize the distribution and to determine the upper 95th centile value as the upper limit of normal (Fig 1A). This approach yielded 3·1pg/mL as the cutoff value. We found that 14% (36/249) of the UCSF asthma patients and 26% (102/387) of the SARP asthma patients were IL-6 high, i.e. plasma IL6 above 3·1 pg/mL (Fig 1B, Table 2). The remaining asthma subjects with plasma IL6 levels below 3·1 pg/mL were classified as “IL6 low”. Among patients with asthma we found no significant correlation between plasma IL6 measurements and sputum IL6 gene expression measurements (r2=0·003, p=0·45)(Fig 1C) and sputum IL6 protein measurements (r2=0·03, p=0·10)(Fig 1D), making airway cells an unlikely source of the high systemic IL6. To explore if the plasma IL6 levels detected were associated with downstream markers of IL6 activity, we measured plasma CRP levels (11) in a subset (n=123) of the UCSF asthma cohort and a subset (n=38) of the healthy subjects. When compared to healthy subjects, we found that median (IQR) CRP levels were significantly higher in asthma than in health (1·6 [0·5-5·0] vs. 0·5, [0·3-1·9] mg/L, p = 0·004). CRP levels were was also higher in IL6-high asthma than in IL6-low asthma (12·9,[2·2-20·9] vs.1·1 [0·5-3·4] mg/L, p<0·001) and significantly correlated with plasma IL6 levels ( r2 = 0·29 p<0·001)(Fig 1E). Plasma IL6 measures were also significantly correlated with blood neutrophils (r2 = 0·16, p<0·001)(Fig 1F).
Fig. 1. Increases in plasma IL6 in a subset of asthmatics that is not related to airway measures of IL6.
(A) Log transformed plasma IL6 measurements shown as kernel density plots to illustrate the distribution of the IL6 concentrations in plasma in the three cohorts. (B) Plasma IL6 values in UCSF healthy subjects, UCSF asthma patients, and SARP asthma patients. The horizontal dashed line indicates the upper 95% centile value for plasma IL6 in the healthy subjects. The shaded areas highlight the 36 (14%) UCSF asthma patients and the 102 (26%) SARP asthma patients who had plasma IL6 levels above the upper reference limit. *p<0·05 from ANOVA comparison. (C) Lack of correlation between plasma IL6 protein levels and log2 normalized gene expression levels for IL6 in sputum cells (n=210, in the SARP asthma cohort , black best-fit line). (D) Lack of correlation between plasma IL6 protein levels and sputum IL6 protein in sputum (n=109, UCSF asthma cohort , black best-fit line). (E) Plasma IL6 levels are positively and significantly correlated with plasma CRP (n= 123, UCSF asthma cohort, black best-fit line). (F) Plasma IL6 protein levels are positively and significantly correlated with blood neutrophils in the UCSF asthma cohort (black symbols and black best-fit line) and the SARP cohort (red symbols and red best-fit line). Best-fit lines were created in each cohort separately and the pearson's coefficient was calculated with the cohorts combined.
Table 2.
Characteristics of the IL6 High and IL6 Low asthma
| Characteristic | UCSF | SARP | ||||
|---|---|---|---|---|---|---|
| IL6 Low (n=213) | IL6 High (n=36) | p-value | IL6 Low (n=285) | IL6 High (n=102) | p-value | |
| Age (years) | 36·6 (14·2) | 41·6 (13·4) | 0·05 | 46·5 (13·9) | 49·1 (12·7) | 0·09 |
| Female sex - no. (%) | 133 (62) | 22 (61) | 0·99 | 176 (62) | 79 (77) | 0·005 |
| Obesity Measures* | ||||||
| BMI (kg/m2) | 27·6 (5·7) | 36·3 (8·6) | <0·001 | 30·3 (6·8) | 37·9 (9·8) | <0·001 |
| BMI ≥ 30 - no. (%) | 54 (25) | 28 (78) | <0·001 | 124 (44) | 83 (81) | <0·001 |
| Waist Circumference (cm) | 96 (16) | 111 (18) | <0·001 | |||
| Use of ICS - no. (%) | 113 (53) | 28 (78) | 0·006 | 251 (88) | 94 (92) | 0·35 |
| Score on ACT† | 19·2 (3·7) | 17·6 (4·5) | 0·07 | 17·4 (4·8) | 15·4 (4·8) | <0·001 |
| Spirometry data‡ | ||||||
| FEV1 (% predicted) | 85·9 (17·3) | 71·3 (19·4) | <0·001 | 78·1 (21·7) | 69·5 (20·6) | <0·001 |
| FEV1 BD-reversibility (%) | 17·4 (16·7) | 22·2 (20·2) | 0·02 | |||
| FVC (% predicted) | 99·6 (14·2) | 86·8 (17·9) | <0·001 | 93·2 (19·5) | 82·0 (17·9) | <0·001 |
| FVC BD-reversibility (%) | 7·7 (10·2) | 10·8 (12·5) | 0·02 | |||
| FEV1/FVC | 0·86 (0·12) | 0·81 (0·13) | 0·05 | 0·83 (0·12) | 0·83 (0·14) | 0·98 |
| Blood cell counts (× 106/L)§ | ||||||
| Total white blood cells | 6676 (1641) | 8891 (1981) | <0·001 | 6868 (2006) | 9139 (3161) | <0·001 |
| Neutrophils | 3806 (1324) | 5442 (1784) | <0·001 | 3992 (1740) | 5671 (2600) | <0·001 |
| Eosinophils | 289 (225) | 309 (190) | 0·62 | 286 (281) | 308 (257) | 0·48 |
| Serum IgE Measures¶ | ||||||
| Total IgE (IU/mL) | 385 (586) | 437 (587) | 0·63 | 308 (567) | 439 (611) | 0·05 |
| ≥1 positive ImmunoCap no. (%) | 228 (81) | 82 (82) | 0·88 | |||
| FeNO (ppm)∥ | 45 (38) | 52 (57) | 0·44 | 31 (31) | 29 (28) | 0·67 |
| Sputum cell counts (%)** | ||||||
| Eosinophils | 4·1 (6·4) | 3·5 (3·8) | 0·65 | 4·6 (10·2) | 3·1 (5·9) | 0·22 |
| Neutrophils | 40 (21) | 34 (20) | 0·19 | 54 (25) | 57 (26) | 0·35 |
| Outcomes of metabolic dysfunction‡‡ | ||||||
| History of hypertension - no. (%) | 19 (9) | 13 (36) | <0·001 | 77 (27) | 53 (52) | <0·001 |
| Systolic BP (mm Hg) | 115 (11) | 122 (13) | 0·007 | 123 (15) | 128 (16) | 0·005 |
| Diastolic BP (mm Hg) | 77 (9) | 80 (8) | 0·02 | 77 (10) | 78 (11) | 0·17 |
| History of diabetes mellitus - no. (%) | 19 (7) | 14 (14) | 0·04 | |||
| Asthma exacerbations – no. (%)§§ | ||||||
| ≥1 exacerbation past two years | 68 (32) | 20 (56) | 0·008 | |||
| ≥1 exacerbation past year | 125 (44) | 69 (68) | <0·001 | |||
| Necessitating hospitalization past year | 25 (9) | 21 (21) | 0·004 | |||
| Necessitating ER visit past year | 59 (21) | 36 (35) | 0·005 | |||
| Asthma Severity - no. (%)¶¶ | ||||||
| Severe | 150 (53) | 71 (70) | ||||
Data are reported as mean (SD) unless otherwise indicated. BMI = body mass index. ICS = inhaled corticosteroids. ACT = asthma control test. FEV1 = forced expiratory volume in 1 s. FVC = forced vital capacity. BD = bronchodilator. IgE = immunoglobulin E. FeNO = fraction of nitric oxide in exhaled air. BP = blood pressure. ER = emergency room.
Waist circumference was not available for the UCSF asthma patients.
ACT was not measured in 98 of the UCSF asthma patients.
In the SARP asthma patients the FEV1 and FVC were measured before and after a maximum reversibility test using up to 8 puffs of albuterol, as detailed in the text; one of the SARP patients did not have this data recorded.
Blood cell counts were not measured in 18 of the UCSF asthma patients.
Total Serum IgE was not measured in 18 of the UCSF asthma patients and 4 of the SARP asthma patients. ImmunoCAP Specific IgE is reported as the number and percentage of patients with at least one positive result when tested for 15 specific allergens. ImmunoCap measures were not performed in 4 SARP asthma patients.
Measures of FeNO were not made in 60 of the UCSF asthma patients, and 3 of the SARP asthma patients.
Cell counts in induced sputum cells were not available in 102 UCSF asthma patients and 94 SARP asthma patients.
Hypertension history data was not recorded in 7 of the UCSF asthma patients. BP measures were not recorded in 2 of the UCSF asthma patients, and the UCSF patients did not have data for history of diabetes available.
One UCSF asthma subject did not have exacerbation data recorded.
The classification of asthma severity was determined using criteria from the American Thoracic Society/European Thoracic Society guidelines.
“IL6-high” asthma is characterized by obesity, metabolic dysfunction, and severe asthma
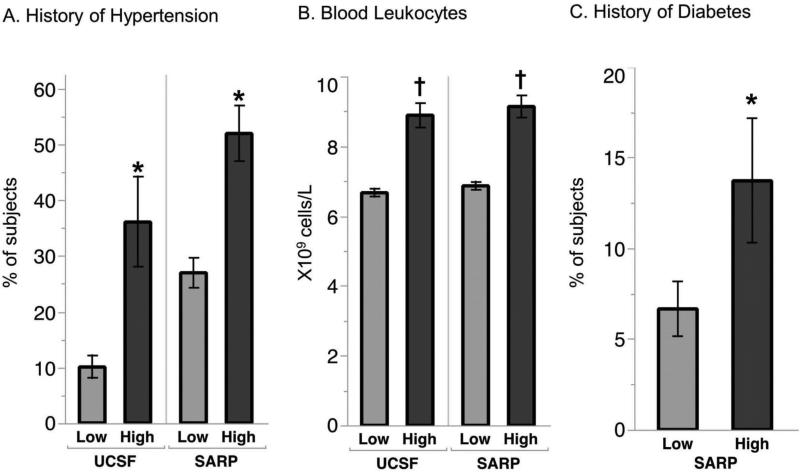
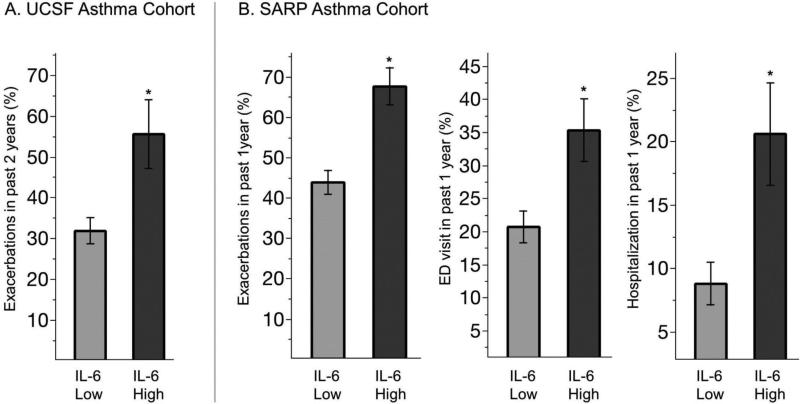
Compared to IL6-low asthma, we found that IL6-high asthma in the SARP cohort was characterized by a higher prevalence of women (Table 2); but race did not differ between IL6-high and IL6-low subgroups in either asthma cohort. Compared to IL6-low asthma, we found that IL6-high asthma in both the UCSF and SARP cohorts was characterized by significant increases in BMI, a higher prevalence of hypertension, and by higher values for systolic blood pressure and blood leukocytes (Table 2, Fig 2A-C). Diabetes mellitus in the SARP cohort occurred more frequently in IL6-high subgroup than in the IL6-low subgroup (history of diabetes was not measured in UCSF cohort). FEV1 and FVC values in both asthma cohorts were lower in the IL6-high subgroups than the IL6 low subgroups (Table 2), and the maximum bronchodilator reversibility test in the SARP cohort showed large increases in FEV1 and FVC with bronchodilators (Table 2). In addition, the percentage of patients who qualified as having severe asthma by ATS/ERS criteria was higher in the IL6-high subgroup in the SARP cohort than the IL6-low subgroup. Furthermore, asthma exacerbations were more frequent and ACT scores were lower in the IL6-high subgroups in both cohorts than in the IL6 low subgroups (Table 2, Fig 3). Sputum eosinophil %, blood eosinophil numbers, sputum neutrophil %, FeNO, or serum levels of IgE in both cohorts did not differ significantly in the IL6-high and -low subgroups (Table 2).
Fig 2. Increased metabolic dysfunction in IL6-high asthma.
(A) A history of hypertension was significantly more common in IL6 high than in IL6 low asthma in both the UCSF and SARP cohorts, Data represented as % of subjects ± SE. (B) Total blood leukocytes number was higher in IL6 high asthma than in IL6 low asthma in the UCSF and SARP cohorts, mean, ± SE (C) A history of diabetes was significantly more common in IL6 high than in IL6 low asthma in the SARP cohort. *p<0·05 from chi-square test †p<0·05 from t-test. Data represented as % of subjects ± SE.
Fig. 3. More frequent asthma exacerbations in IL6-high asthma.
(A) The percentage of asthma patients that required systemic corticosteroid treatment in the past 2 years was higher in IL6 high asthma compared to IL6 low asthma in the UCSF cohort (B) The percentage of asthma patients that required systemic corticosteroid treatment, emergency department treatment, or hospitalization for an asthma exacerbation in the past year in IL6 high asthma was higher than in IL6 low asthma in the SARP cohort. *p<0·05 from chi-square test. Data represented as % of subjects ± SE.
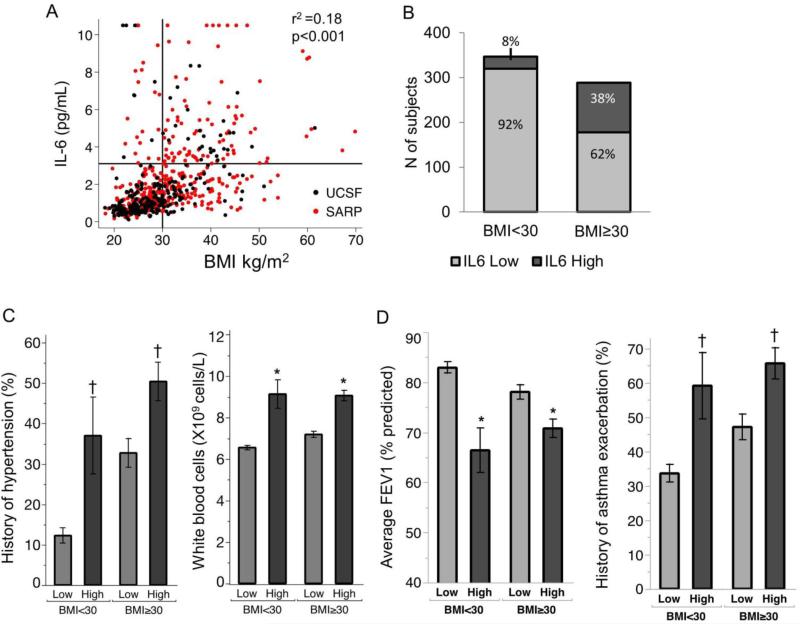
Systemic IL6 levels are associated with metabolic dysfunction and severe asthma in both obese and non-obese patients
Plasma IL6 was significantly and positively correlated with BMI in both cohorts (Fig 4A), and 78% (28/36) and 81% (83/102) of the IL6-high subgroups in the UCSF and SARP asthma patients were obese, respectively (Table 2). But 62% (178/289) of obese patients had normal plasma IL6 (Fig 4B), and this prompted us to explore more deeply the relationship between IL6 inflammation, metabolic dysfunction, and asthma severity in obese and non-obese patients. To do this, we compared outcomes of metabolic dysfunction and asthma severity in IL6-high and IL6-low asthma, and we stratified patients into obese and non-obese subgroups. In addition, we examined relationships between IL6 and asthma severity outcomes while controlling for BMI in regression models. Compared to obese IL6-low asthmatics, we found that obese IL6-high asthmatics had strong signals for metabolic dysfunction, including a frequent history of hypertension, and increases in total blood leukocytes and blood neutrophils (Fig 4C, Table 3). In addition, we found that asthma outcomes were consistently worse in obese IL6-high asthma than in obese IL6-low asthma (Fig 4D, Table 3). Furthermore, we found that indicators of metabolic dysfunction and more severe asthma were also characteristics of non-obese IL6-high asthmatics (Fig 4C-D, Table 3), indicating that IL6 is associated with metabolic dysfunction and severe asthma, even in the absence of obesity.
Fig. 4. Increased plasma IL6 is associated with metabolic dysfunction and more severe asthma in both obese and non-obese patients.
(A) Plasma IL6 levels are positively and significantly correlated with body mass index (BMI) in UCSF asthma patients (black symbols) and SARP asthma patients (red symbols) pearson's coefficient was calculated with the cohorts combined. (B) The proportion of asthma patients with high plasma IL6 levels was much higher in obese asthmatics (BMI >30) than in non-obese asthmatics (BMI <30). (C) Compared to IL6-low asthma, IL6-high asthma is characterized by increased metabolic dysfunction in both obese and non-obese subgroups, as demonstrated by a higher frequency of hypertension and leukocytosis. (D) Asthma severity in IL6-high asthma is worse than in IL6-low asthma in both obese and non-obese subgroups, as demonstrated by lower FEV1 values and a higher percentage of subjects with a history of an asthma exacerbation. The exacerbation history is defined as a prednisone requiring asthma exacerbation in either the past 2 years (UCSF cohort) or the past in past year (SARP cohort).*p<0·05 from Wilcoxon rank sum test †p<0·05 from chi square test. Data represented as % of subjects ± SE, for binary variables and mean ± SEM for continuous variables.
Table 3.
Clinical variables in IL6 High and IL6 Low asthma sub-grouped by obesity
| Characteristic | BMI<30 | BMI≥30 | ||||
|---|---|---|---|---|---|---|
| IL6 Low (n=320) | IL6 High (n=27) | p-value | IL6 Low (n=178) | IL6 High (n=111) | p-value | |
| Age (years) | 41·1 (15·4) | 49·0 (17·6) | 0·01 | 44·3 (13·2) | 46·7 (12·1) | 0·12 |
| Female sex - no. (%) | 199 (62) | 11 (41) | 0·04 | 110 (62) | 90 (81) | <0·001 |
| BMI (kg/m2) | 25·4 (2·9) | 25·5 (2·3) | 0·80 | 36·0 (5·5) | 40·4 (8·2) | <0·001 |
| Spirometry data | ||||||
| FEV1 (% predicted) | 83·2 (20·4) | 66·4 (23·1) | <0·001 | 78·3 (19·7) | 70·8 (19·5) | 0·002 |
| FVC (% predicted) | 98·4 (17·2) | 85·4 (21·7) | <0·001 | 91·4 (17·7) | 82·7 (17·0) | <0·001 |
| FEV/FVC | 0·84 (0·12) | 0·76 (0·14) | 0·002 | 0·85 (0·12) | 0·84 (0·13) | 0·85 |
| Using ICS - no. (%) | 223 (70) | 23 (85) | 0·12 | 141 (79) | 99 (89) | 0·04 |
| Score on ACT* | 18·5 (4·1) | 16·6 (4·7) | 0·03 | 16·9 (5·1) | 15·6 (4·8) | 0·04 |
| ≥1 Exacerbation in the past 1-2 years - no. (%)† | 108 (34) | 16 (59) | 0·01 | 85 (48) | 73 (66) | 0·003 |
| Blood cell counts (× 106/L)‡ | ||||||
| Total white blood cells | 6557 (1750) | 9137 (3532) | <0·001 | 7191 (1992) | 9064 (2755) | <0·001 |
| Neutrophils | 3734 (1479) | 5804 (3117) | <0·001 | 4232 (1710) | 5568 (2230) | <0·001 |
| Eosinophils | 305 (276) | 367 (270) | 0·19 | 268 (245) | 293 (233) | 0·39 |
| Outcomes of metabolic dysfunctionE | ||||||
| History of Hypertension no. (%) | 39 (12) | 10 (37) | 0 002 | 57 (33) | 56 (51) | 0·004 |
| Systolic BP (mm Hg) | 117 (13) | 122 (16) | 0·05 | 125 (14) | 127 (15) | 0·22 |
| Diastolic BP (mm Hg) | 75 (9) | 75 (9) | 0·96 | 80 (10) | 80 (11) | 0·79 |
Data are reported as mean (SD) unless otherwise indicated. BMI = body mass index. FEV1 = forced expiratory volume in 1 s. FVC = forced vital capacity. ICS = inhaled corticosteroids. ACT = asthma control test. BP = blood pressure.
ACT scores were not available for 98 patients.
See footnote to table 1 for details about questionnaire data for asthma exacerbations.
Blood cell counts were not available for 18 patients.
§ Hypertension history data was not available for 7 patients.
Confounding does not explain the association between IL6 and increased asthma severity
Older age, increased body mass, and increased type-2 inflammation have all been associated with increased plasma IL-6 levels and increased asthma severity (Fig S2). To determine if these factors confounded the association between plasma IL-6 measures and asthma severity, we performed linear and logistic regression models using continuous measures of plasma IL-6 as the predictor of interest. We found that the relationship between increased IL-6 and worse asthma remained robust even after controlling for these potential confounders (Table 4).
Table 4.
Relationship between continuous plasma IL6 measures and outcomes of asthma severity, controlled for confounding variables
| Asthma Outcome* | UCSF Asthma (n=249) | SARP (n=387) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unadjusted | Model 1 | Model 2 | Model 3† | Unadjusted | Model 1 | Model 2 | Model 3 | Model 4 | |
| FEV1 % predicted | −6·7 (−9·5 to −4·0), p<0·001 | −6·0 (−9·2 to −2·8), p<0·001 | −4·5 (−7·6 to −1·4), p=0·005 | −4·4 (−7·4 to −1·3), p=0·005 | −8·6 (−11·2 to −6·1), p<0·001 | −8·7 (−11·6 to −5·7), p<0·001 | −6·8 (−9·8 to −3·8), p<0·001 | −6·5 (−9·6 to −3·5), p<0·001 | −5·2 (−8·2 to −2·2), p=0·001 |
| FVC% predicted | −6·2 (−8·5 to −4·0), p<0·001 | −4·7 (−7·3 to −2·0), p=0·001 | −3·5 (−6·1 to −0·9), p=0·03 | −3·3 (−5·9 to −0·7), p=0·01 | −9·0 (−11·2 to −6·7), p<0·001 | −7·7 (−10·3 to −5·1), p<0·001 | −5·6 (−8·2 to −3·0), p<0·001 | −5·8 (−8·5 to −3·2), p<0·001 | −4·9 (−7·6 to −2·3), p<0·001 |
| ≥1 Exacerbation in past 2 years O.R.‡ | 1·4 (1·0 to 1·9), p=0·04 | 1·4 (1·0 to 2·1), p=0·07 | 1·5 (1·0 to 2·1), p=0·06 | 1·5 (1·0 to 2·4), p=0·04 | |||||
| ≥1 Exacerbation in past year O.R. | 2·2 (1·7 to 2·9), p<0·001 | 2·0 (1·4 to 2·7), p<0·001 | 1·9 (1·4 to 2·6), p<0·001 | 2·0 (1·4 to 2·7), p<0·001 | 1·7 (1·2 to 2·4), p=0·002 | ||||
| ≥1 Asthma hospitalization in past year O.R. | 2·0 (1·4 to 3·0), p<0·001 | 2·0 (1·3 to 3·0), p=0·003 | 2·0 (1·3 to 3·2), p=0·002 | 2·2 (1·4 to 3·4), p=0·001 | 1·8 (1·1 to 3·0), p=0·03 | ||||
| ≥1 Asthma ER visit in past year O.R. | 1·9 (1·4 to 2·5), p<0·001 | 1·5 (1·1 to 2·1), p=0·02 | 1·6 (1·1 to 2·3), p=0·008 | 1·7 (1·2 to 2·5), p=0·003 | 1·5 (1·0 to 2·2), p=0·03 | ||||
| Severe Asthma O.R.§ | 2·3 (1·7 to 3·1), p<0·001 | 2·2 (1·6 to 3·0), p<0·001 | 1·9 (1·4 to 2·7), p<0·001 | 2·2 (1·5 to 3·3), p<0·001 | 1·8 (1·3 to 2·6), p=0·001 | ||||
Effect size per one natural log increase in plasma IL6 levels (pg). (95% Confidence Interval)
BMI = body mass index. ICS = inhaled corticosteroid. ER = emergency room. OR = odds ratio. FEV1 = forced expiratory volume in 1 s. FVC = forced vital capacity.
Linear and Logistic Regression models for UCSF and SARP datasets..
18 UCSF Asthma participates had blood cell counts missing.
One UCSF Asthma participate did not have exacerbation data recorded.
Severe Asthma determined by criteria from the American Thoracic Society/European Thoracic Society guidelines.
Model One adjusts for the covariate of BMI.
Model Two adjusts for the covariates BMI and age.
Model Three adjusts for the covariates BMI, age, blood eosinophil cell counts, and ICS use.
Model Four adjusts for the covariates BMI, age, blood eosinophil cell counts, and chronic systemic corticosteroid use.
Adjusting for gender or race did not significantly change any of the associations between IL6 and asthma outcomes.
Plasma IL6 measures are unrelated to measures of type 2 inflammation in asthma
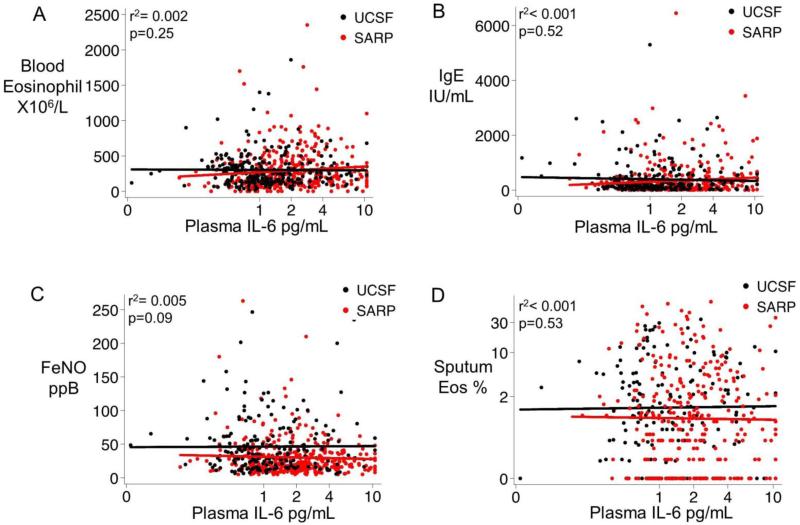
Using a Pearson correlation coefficient, we found no significant relationship between plasma IL6 and outcomes of type 2 inflammation in blood or airway bio-specimens, including blood eosinophils (r2=0·002, p=0.25), blood IgE levels (r2<0·001, p=0·52), FeNO (r2=0·005 p=0·09), and sputum eosinophils (r2<0·001, p=0·53)(Fig 5 A-D).
Fig. 5. Plasma IL6 measures are unrelated to measures of type 2 inflammation in asthma.
(A) Plasma IL6 levels are not related to blood eosinophil cell counts. (B) Plasma IL6 levels are not related to serum IgE levels, (C) Plasma IL6 levels are not related to exhaled nitric oxide levels. (D) Plasma IL6 levels are not related to sputum eosinophil cell percentages. UCSF asthma cohort is represented by black symbols and black best-fit lines and the SARP cohort is represented by red symbols and red best-fit lines. Best-fit lines were created in each cohort separately and the pearson's coefficient was calculated with the cohorts combined.
Directed acyclic graph (DAG) analysis
The possible relationships between systemic IL6 measures, age, body mass index, and type 2 inflammation that informed our analysis are illustrated by the DAG in Fig S2A. The relationships that are supported by our data are illustrated in the modified DAG shown in Figure S2B, which shows most importantly that IL6 is not related to type 2 inflammation.
Discussion
A dominant concept in asthma is that mechanisms of disease that originate in the lung drive airway pathology and airway dysfunction and explain the marked heterogeneity in clinical traits. In particular, there has been a heavy emphasis on research into mechanisms of type 2 inflammation in asthma, an emphasis that has now led to new treatments targeting various members of the type 2 pathway. It is well known, however, that many patients with asthma do not have type 2 inflammation, and that these patients will not benefit from type 2 directed treatments (12,13). New insights about mechanisms of non-type 2 inflammation are now needed as a first step toward developing treatment options for patients with Th2-low asthma. Unfortunately, efforts to date to uncover new disease mechanisms using experimental approaches that focus on analyses of lung bio-specimens have not been very productive. Here, we report that systemic IL6 inflammation occurs in a significantly sized subgroup of patients with asthma, most of whom are obese. We also report that systemic IL6 inflammation marks patients who have both metabolic dysfunction and severe asthma. Taken together, our findings help explain the heterogeneous effects of obesity on disease severity in asthma and point to treatment of systemic IL6 inflammation and metabolic dysfunction as novel therapeutic strategies for asthma.
More than 75% of the IL6-high asthma subgroups in our two asthma cohorts were obese, but only a minority of obese asthma patients (38%) had high plasma IL6 levels. This subset of obese asthmatics with high IL6 levels was more likely to have hypertension and systemic leukocytosis, which are indicators of metabolic dysfunction (14,15). Importantly, we found that the highest signals for asthma severity were in this IL6-high subset of obese asthmatics with metabolic dysfunction. Taking IL6 as a biomarker of metabolic dysfunction (16), our data clearly show that asthma in obese patients with metabolic dysfunction is more severe than in obese patients without metabolic dysfunction. This finding helps explain why obesity is associated with severe asthma in some patients but not in others, and it emphasizes the importance of metabolic and inflammatory complications of obesity over complications arising from chest wall loading from adipose tissue. Notably, our data also show that asthma in non-obese patients with metabolic dysfunction is more severe than in non-obese patients without metabolic dysfunction. This interpretation is consistent with our data showing that the relationships between plasma IL6 and measures of asthma severity remain robustly significant when BMI is controlled for in regression models.
We found no relationship between plasma measures of IL6 and measures of type 2 inflammation (airway and systemic) in the two asthma cohorts and no relationship between measures of plasma IL6 protein and measures of IL6 gene transcripts or IL6 protein in the airway. These data indicate that the increase in systemic IL6 levels in IL6-high asthma is not occurring as a result of upstream type 2 inflammation or as a result of spillover of IL6 inflammation from the lungs. Our data overall suggest an “outside in” mechanism of lung dysfunction in IL6-high asthma in which the proinflammatory mediators driving disease severity originate in extrapulmonary organs. For example, the origin of the increased IL6 in IL6-high asthma is most likely inflammatory macrophages in white adipose tissue (17). Circulating IL6 could mediate airway pathology through effects on endothelial cells, epithelial cells, and other structural airway cells (18,19). It could also influence T cell function, including regulatory T cells and Th17 cells (20,21). Another “outside in” mechanism of lung dysfunction in IL6-high asthma could involve insulin resistance, an important consequence of metabolic dysfunction. Insulin resistance has been linked to poor lung function in asthma (22), and the mechanism of this effect is a subject of speculation that upregulation of insulin-related metabolic signaling cascades has adverse consequences for smooth muscle cells and other airway structural cells (23). Finally, we acknowledge that IL6 is not the only cytokine that is upregulated in patients with metabolic dysfunction. Cytokines such as TNFα or leptin may also be increased in patients with IL6-high asthma, and the activities of these cytokines could be a mechanism of disease in IL6-high asthma.
Because IL6 is a pro-inflammatory factor, differentiation factor, and growth factor (24), it is a therapeutic target in rheumatoid arthritis (25), systemic juvenile arthritis (26), and Crohn's disease (27). Our data provide a rationale for also testing the efficacy of IL6 inhibitors in severe asthma (28). In addition, because IL6 is a marker of metabolic dysfunction, we provide a rationale for exploring the efficacy of treatments for metabolic dysfunction as strategies to lessen disease severity in asthma.
In conclusion, our study suggests a role for systemic IL6 inflammation as a mediator of disease severity in a subgroup of asthma patients. This role may be causal according to Hill's criteria for causality (29), based on the large effect size, the consistent finding for the association between IL6 and asthma severity in two asthma cohorts, the specific clinical features of the IL6-high subgroup, the clear dose response for IL6 measures and outcomes of asthma severity, and the biologic plausibility for IL6 as a mediator of asthma pathology. However, definitive proof that IL6 has a causal role in mediating asthma severity will need to await the results of randomized controlled trials testing the efficacy of IL6 inhibition in asthma. We provide strong rationale for such trials here and our data indicate how plasma IL6 could be used as a biomarker to enrich for patients with IL6-high asthma.
Supplementary Material
Research in context.
Evidence before this study
Asthma is a common disease that is often well controlled with currently available treatments, but a subgroup of patients has severe disease with unmet treatment needs. New treatments for severe asthma require improved understanding of disease mechanisms and easily measured biomarkers to guide personalized treatment plans. Obesity is a well-recognized risk factor for severe asthma, but many obese asthmatics do not have severe disease, and the molecular drivers of the heterogeneity of clinical severity in obese asthma are poorly understood. Low-grade systemic inflammation is a complication of obesity that results in metabolic diseases such as hypertension, atherosclerosis, and type 2 diabetes mellitus. The possibility that systemic inflammation could lead to more severe forms of asthma has not been studied in detail.
Interleukin 6 (IL6) is a biomarker of obesity-related systemic inflammation and metabolic dysfunction. It is also a pleotropic cytokine and a plausible mediator of airway dysfunction in asthma. Previous studies in small numbers of patients have shown an increase in plasma or serum levels of IL6 in asthma, and genetic studies have found associations between IL6 pathway genes and asthma.
Added value of this study
We show in two asthma cohorts that systemic IL6 inflammation occurs in a subset of asthmatics that are characterized by obesity and metabolic dysfunction. These “IL6-high” asthmatics have much lower lung function and more frequent exacerbations than “IL6-low” asthmatics. Although the average BMI in IL6-high asthma is higher than in IL6-low asthma, the majority of obese asthmatics are IL6-low, and metabolic diseases such as hypertension and diabetes mellitus occur much more commonly in the IL6-high subgroups. Notably, asthma severity is much more severe in the subgroup of obese asthmatics who are IL6-high than in the subgroup who are IL6-low, and this association holds in the non-obese IL6-high and IL6-low asthmatics.
Implications of all the available evidence
Systemic IL6 levels, occurring independently of type 2 inflammation, is associated with a severe asthma phenotype. Variability in systemic IL6 levels in obese patients helps explain the heterogeneity of asthma severity in these patients. Systemic IL6 inflammation represents an extra-pulmonary mechanism of severe asthma, and treatment of low-grade systemic inflammation (including with IL6 inhibitors) or treatment of metabolic dysfunction represent rational clinical trials to pursue for a subset of patients with severe asthma. Plasma IL6 is a biomarker that could guide patient stratification in the trials.
Acknowledgements
We thank Patricia Noel, Ph.D. and Robert Smith, Ph.D. from the Division of Lung Diseases, National Heart Lung and Blood Institute for their support and leadership of the Severe Asthma Research Program. We also thank the following coordinators, research assistants, and clinicians at the UCSF and SARP centers for their work in making our study possible: (i) UCSF: Jennifer Soh, Zesemayat Mekonnen, Kelly Norsworthy, Sheena Kerr, Ph.D., Eleanor Dunican, M.D., Erin Gordon, M.D., and Charles McCulloch, Ph.D.; (ii) Brigham and Women's Hospital and Harvard Medical School: Nawal Ali, Carrie Nettles, and Gabriela Sauza; (iii) Cleveland Clinic: Elise Baldarelli, Marybeth Boyle, John Escano, and Michelle Koo; (iv) Wake Forest University: Regina Smith, Deborah Meyers, Ph.D., and Wendy Moore, M.D. (v) Washington University: Rebecca Schutz and Rachel Weaver; (vi) University of Pittsburgh: Louise Martin and Jenelle Mock; (vii) University of Wisconsin: Gina Crisafi, Holly Eversoll, Evelyne Falbene, Maranda Hyde, Michele Wolf, Ronald Sorkness, Ph.D., and Loren Denlinger, M.D.; (viii) Pennsylvania State University: Gail Snyder, Sara Marlin, Jennifer Zeller, Kelly Bixler-Nye, and Brenda Kline. We also thank Gabriella Sanchez and Carlos Iribarren, M.D., M.P.H., Ph.D. from the Kaiser Permanente Division of Research for assistance in subject recruitment at the UCSF center. Finally, we thank all of the volunteers who participated in these studies.
Funding: Supported by National Institutes of Health (NIH) PO1 HL107201, R01 HL080414, U19 AI077439, U10 HL109146, U10 HL109164, U10 HL109172, U10 HL109086, U10 HL109250, U10 HL109168, U10HL109257, and U10 HL109152; and by grants from the Parker B. Francis Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributions
MCP and JVF conceived and designed the study. KWM, GAH, ATH, BDL, EL, BRP, DTM, SAC, SCE, MWJ, NNJ, AMC, MC, FH, SEW, PGW, and ERB made substantial contributions to the design and analysis plan of the study. MCP, JVF, and BRP conducted the data analysis. MCP and JVF prepared the first draft of the manuscript and all authors revised the draft critically for important intellectual content.
Declaration of interests
MCP has served as a speaker for Amgen. EI has received research support from Genentech; non-financial support from Boehringer Ingelheim, GlaxoSmithKline, Merck, Sunovion, and Teva; served as a speaker for Merck; provided expert testimony on behalf of Campbell, Campbell, Edwards & Conroy, Crammer, Bishop & O'Brien, Ficksman & Conley, Fox Rothchild, LLP, Ryan Ryan Deluca LLP; served as a consultant for AstraZeneca, Novartis, Philips Respironics, and Regeneron Pharmaceuticals; and received royalties from UpToDate, and received travel/meeting expenses from Research in Real Life and TEVA Specialty Pharmaceuticals. DTM has received non-financial support from Boehringer Ingelheim, GlaxoSmithKline, Merck, Sunovion, and TEVA. SCE is Chair of the American Board of Internal Medicine Pulmonary Board. MC has received research support from Genentech, Amgen, Teva, Novartis, GlaxoSmithKline, Sanofi-Aventis, Vectura, Medimmune, Johnson & Johnson, Invion, Pfizer, and KaloBios; served as consultant for Boston Scientific, Holaira, Neostem, and Teva; served as speaker for GlaxoSmithKline, Genentech, Boston Scientific, Boehringer Ingelheim, and Teva; has received royalties from Elsevier; and holds stock in Sparo, INC. SEW has received research support from Amgen, AstraZeneca, GlaxoSmithKline, Sanofi Aventis, and Boehringer Ingelheim; and has received personal fees from Actelion, AstraZeneca, GlaxoSmithKline, Norvartis, and Boehringer Ingelheim. PGW has received research support from Genentech, served on advisory boards for Genentech, Johnson & Johnson, and Neostem; and served as consultant for Roche, AstraZeneca, and Novartis. ERB has served as consultant for Amgen, AstraZeneca, Medimmune, Boehringer Ingelheim, Pfizer, Forest, Genentech, Novartis, Roche, GlaxoSmithKline, Merck, Regeneron, and Sanofi Aventis; received research support from for Amgen, AstraZeneca, Medimmune, Boehringer Ingelheim, Pfizer, Forest, Genentech, Roche, Novartis, Roche, GlaxoSmithKline, Teva, Jenssen, Johnson & Johnson, and Sanofi Aventis. JVF has served as consultant for Boehringer Ingelheim and Dynavax and Medimmune and has received research support from Pfizer and Genentech. All other authors declare no competing interests.
References
- 1.Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015 Jan;15(1):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010 Feb 15;181(4):315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011 Jun;121(6):2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yokoyama A, Kohno N, Fujino S, Hamada H, Inoue Y, Fujioka S, et al. Circulating interleukin-6 levels in patients with bronchial asthma. Am J Respir Crit Care Med. 1995 May;151(5):1354–1358. doi: 10.1164/ajrccm.151.5.7735584. [DOI] [PubMed] [Google Scholar]
- 5.Ferreira MA, Matheson MC, Duffy DL, Marks GB, Hui J, Le Souef P, et al. Identification of IL6R and chromosome 11q13.5 as risk loci for asthma. Lancet. 2011 Sep 10;378(9795):1006–1014. doi: 10.1016/S0140-6736(11)60874-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014 Feb;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
- 7.Gershman NH, Wong HH, Liu JT, Mahlmeister MJ, Fahy JV. Comparison of two methods of collecting induced sputum in asthmatic subjects. Eur Respir J. 1996 Dec;9(12):2448–2453. doi: 10.1183/09031936.96.09122448. [DOI] [PubMed] [Google Scholar]
- 8.Hastie AT, Moore WC, Li H, Rector BM, Ortega VE, Pascual RM, et al. Biomarker surrogates do not accurately predict sputum eosinophil and neutrophil percentages in asthmatic subjects. J Allergy Clin Immunol. 2013 Jul;132(1):72–80. doi: 10.1016/j.jaci.2013.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, Fahy JV. Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol. 2014 Feb;133(2):388–394. doi: 10.1016/j.jaci.2013.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute, editor. Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline. 3rd ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2008. CLSI document C28-A3. [Google Scholar]
- 11.Castell JV, Gomez-Lechon MJ, David M, Fabra R, Trullenque R, Heinrich PC. Acute-phase response of human hepatocytes: regulation of acute-phase protein synthesis by interleukin-6. Hepatology. 1990 Nov;12(5):1179–1186. doi: 10.1002/hep.1840120517. [DOI] [PubMed] [Google Scholar]
- 12.Corren J, Lemanske RF, Hanania NA, Korenblat PE, Parsey MV, Arron JR, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med. 2011 Sep 22;365(12):1088–1098. doi: 10.1056/NEJMoa1106469. [DOI] [PubMed] [Google Scholar]
- 13.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009 Sep 1;180(5):388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, National Heart, Lung, and Blood Institute et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol. 2004 Feb;24(2):e13–8. doi: 10.1161/01.ATV.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 15.Tsai JC, Sheu SH, Chiu HC, Chung FM, Chang DM, Chen MP, et al. Association of peripheral total and differential leukocyte counts with metabolic syndrome and risk of ischemic cardiovascular diseases in patients with type 2 diabetes mellitus. Diabetes Metab Res Rev. 2007 Feb;23(2):111–118. doi: 10.1002/dmrr.647. [DOI] [PubMed] [Google Scholar]
- 16.Pearson TA, Mensah GA, Hong Y, Smith SC., Jr CDC, AHA. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: overview. Circulation. 2004 Dec 21;110(25):e543–4. doi: 10.1161/01.CIR.0000148979.11121.6B. [DOI] [PubMed] [Google Scholar]
- 17.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003 Dec;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neveu WA, Allard JB, Dienz O, Wargo MJ, Ciliberto G, Whittaker LA, et al. IL-6 is required for airway mucus production induced by inhaled fungal allergens. J Immunol. 2009 Aug 1;183(3):1732–1738. doi: 10.4049/jimmunol.0802923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes TC, Anderson ME, Moots RJ. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int J Rheumatol. 2011;2011:721608. doi: 10.1155/2011/721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007 Sep;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 21.Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, et al. The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005 Feb;115(2):313–325. doi: 10.1172/JCI22433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forno E, Han YY, Muzumdar RH, Celedon JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015 Mar 3; doi: 10.1016/j.jaci.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh S, Prakash YS, Linneberg A, Agrawal A. Insulin and the lung: connecting asthma and metabolic syndrome. J Allergy (Cairo) 2013;2013:627384. doi: 10.1155/2013/627384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rincon M. Interleukin-6: from an inflammatory marker to a target for inflammatory diseases. Trends Immunol. 2012 Nov;33(11):571–577. doi: 10.1016/j.it.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013 May 4;381(9877):1541–1550. doi: 10.1016/S0140-6736(13)60250-0. [DOI] [PubMed] [Google Scholar]
- 26.De Benedetti F, Brunner HI, Ruperto N, Kenwright A, Wright S, Calvo I, et al. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012 Dec 20;367(25):2385–2395. doi: 10.1056/NEJMoa1112802. [DOI] [PubMed] [Google Scholar]
- 27.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000 May;6(5):583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 28.Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a Therapeutic Target. Clin Cancer Res. 2015 Mar 15;21(6):1248–1257. doi: 10.1158/1078-0432.CCR-14-2291. [DOI] [PubMed] [Google Scholar]
- 29.Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015 Jan;108(1):32–37. doi: 10.1177/0141076814562718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.