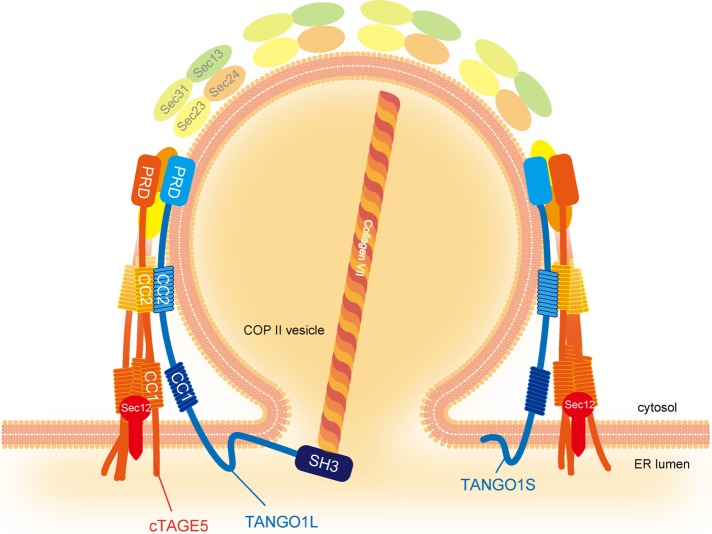
The short isoform of TANGO1, termed TANGO1S, is necessary for collagen secretion from the ER independently of TANGO1L. TANGO1L and TANGO1S form individual complexes at ER exit sites and cooperatively participate in collagen export from the ER.
Abstract
Collagens synthesized within the endoplasmic reticulum (ER) are too large to fit in conventional COPII-coated transport vesicles; thus their export from the ER requires specialized factors. TANGO1 (L) is an integral membrane protein that binds to collagen and the coatomer of vesicles and is necessary for collagen secretion from the ER. Here we characterized the short isoform of TANGO1 (TANGO1S), lacking the collagen-binding domain, and found that it was independently required for collagen export from the ER. Moreover, we found that each of the TANGO1 isoforms forms a stable protein complex with factors involved in collagen secretion: TANGO1L/cTAGE5/Sec12 (900 kDa) and TANGO1S/cTAGE5/Sec12 (700 kDa). Of interest, TANGO1S and TANGO1L seemed to be interchangeable in exporting collagen from the ER. Our results suggest that mammalian ER exit sites possess two different-sized membrane-bound macromolecular complexes that specifically function in large-cargo export from the ER.
INTRODUCTION
Collagens are among the most abundant secretory proteins, yet their mechanisms of secretion have not been fully elucidated (Malhotra and Erlmann, 2015). This is primarily because they form complexes within the ER that are too large to be accommodated by the conventional coat protein complex II (COPII)–coated vesicles of 60–90 nm in diameter (Miller and Schekman, 2013). Several lines of evidence suggest that modification of conventional COPII-mediated transport is important for collagen export from the endoplasmic reticulum (ER; Saito and Katada, 2015). Most prominently, Sec31 monoubiquitylation by CUL3-KLHL12 is required for large-carrier formation, which enables accommodation of collagen inside (Jin et al., 2012). Furthermore, there are several proteins that specifically participate in collagen export (Malhotra et al., 2015; McCaughey et al., 2016).
TANGO1 (L) is an integral membrane protein localized at the ER exit sites. TANGO1’s luminal SH3 domain interacts with collagen VII, and its cytoplasmic C-terminal proline-rich domain (PRD) binds to the inner COPII-coat proteins Sec23/Sec24. Depletion of TANGO1 leads to defects in collagen secretion in both cultured cells and mice (Saito et al., 2009; Wilson et al., 2011). Recently it was shown that the cytoplasmic first coiled-coil region of TANGO1 is capable of recruiting ER–Golgi intermediate compartment (ERGIC) membranes to the ER exit sites, which are proposed to act as sources for forming collagen-containing megacarriers (Santos et al., 2015). In addition, TANGO1 interacts with Sedlin and Sly1, and their roles in collagen secretion have been reported (Nogueira et al., 2014; Venditti et al., 2014).
Cutaneous T-cell lymphoma–associated antigen 5 (cTAGE5) is a close homologue of TANGO1, and its C-terminal PRD binds to Sec23/24 and is required for collagen export from the ER. We showed that the second coiled-coil region of cTAGE5 directly interacts with the corresponding region of TANGO1 (Saito et al., 2011). In addition, cTAGE5 interacts with Sec12, the guanine-nucleotide exchange factor for the small GTPase Sar1, which is involved in COPII-vesicle budding from the ER. Of interest, cTAGE5 is necessary for Sec12 concentration at the ER exit sites, and this concentration is required for collagen secretion by contributing to efficient activation of Sar1 in the vicinity of ER exit sites (Saito et al., 2014; Tanabe et al., 2016).
As mentioned earlier, the molecules involved in collagen secretion have been emerging in recent years, but little is known about how these molecules cooperate in the secretion of collagens from the ER.
Here we showed that the short isoform of TANGO1, termed TANGO1S, is also necessary for collagen secretion from the ER. We revealed that TANGO1L and TANGO1S form individual complexes at ER exit sites, and these complexes cooperatively participate in collagen export from the ER. Our results indicate that mammalian ER exit sites contain these protein complexes, which specifically function in large-cargo export from the ER.
RESULTS
TANGO1S is an integral membrane protein localized at ER exit sites
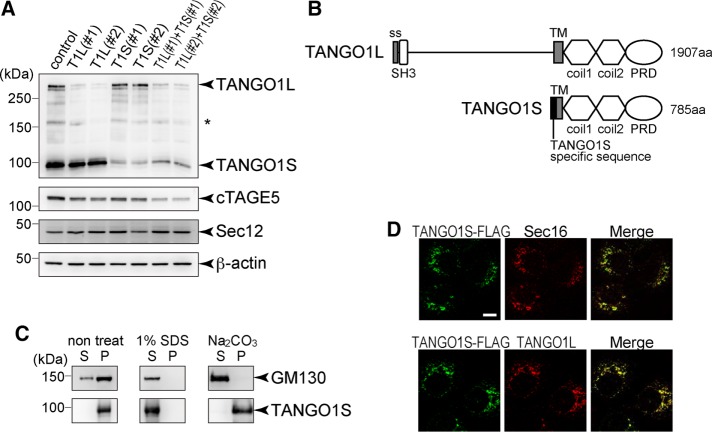
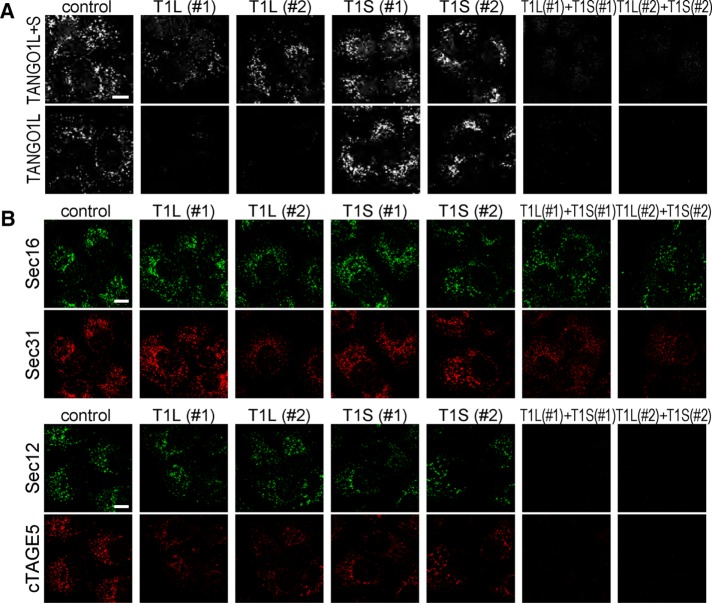
A previous report indicated that TANGO1 gives rise to an alternative splicing short variant lacking the luminal collagen-binding domain; however, its function has not been intensively explored (Wilson et al., 2011). Cell lysates from HSC-1 cells were blotted with TANGO1-CC1 antibody, which recognizes the cytoplasmic coiled-coil domain of TANGO1. In addition to the original 290-kDa TANGO1 isoform, an ∼100-kDa protein corresponding to the short isoform was detected (Figure 1A, lane 1). We refer to the short isoform as TANGO1S and the original isoform as TANGO1L. Isoform-specific small interfering RNA (siRNA) oligos specifically depleted either isoform (Figure 1A, lanes 2–5). Of note, depletion of both TANGO1L and TANGO1S reduced the expression of cTAGE5 without affecting Sec12 expression level (Figure 1A, lanes 6 and 7). TANGO1S lacks the well-defined signal peptide, as a previous report indicated (Figure 1B; Wilson et al., 2011), whereas TANGO1L contains a signal peptide in its N-terminus (Saito et al., 2009). Because TANGO1S also contains a putative transmembrane domain, next we investigated whether TANGO1S is a peripheral membrane protein or an integral membrane protein by checking the sensitivity to sodium carbonate. As shown in Figure 1C, TANGO1S was not extracted by sodium carbonate, indicating that TANGO1S is an integral membrane protein. It is difficult to produce a TANGO1S-specific antibody because TANGO1S shares predominantly an identical sequence with TANGO1L (Figure 1B). To check the localization of TANGO1S, we made a TANGO1S-FLAG–inducible cell line. FLAG-tagged TANGO1S expression was mildly induced by incubating cells with doxycycline for 24 h (Supplemental Figure S1A, lanes 5–8). Expressed TANGO1S extensively colocalized with Sec16, a bona fide ER exit site marker (Figure 1D, top). Costaining with a TANGO1L-specific antibody indicated that both TANGO1S and TANGO1L are localized at the same ER exit sites (Figure 1D, bottom). Knockdown of both TANGO1L and TANGO1S severely reduced the immunofluorescence by both TANGO1L and TANGO1-CT antibodies (Figure 2A). Conversely, TANGO1L-specific depletion only diminished the signals of TANGO1L, whereas the TANGO1-CT antibody gave substantial signals at ER exit sites (Figure 2A). These data suggest that endogenous TANGO1S is localized at ER exit sites in TANGO1L-depleted cells.
FIGURE 1:
TANGO1S is an integral membrane protein localized at ER exit sites. (A) HSC-1 cells were transfected with the indicated siRNA(s). After 72 h, proteins were extracted and subjected to SDS–PAGE, followed by Western blotting with anti–TANGO-CC1, cTAGE5, Sec12, and β-actin antibodies. *Nonspecific cross-reaction. (B) Schematic representation of human TANGO1L and TANGO1S domain organization. coil, coiled-coil; PRD, proline-rich domain; ss, signal sequence; TM, transmembrane. (C) Membrane fractions of HSC-1 cells were untreated or extracted with SDS or sodium carbonate, followed by centrifugation to separate the solubilized fractions (S) from the pelleted fractions (P). Both fractions were subjected to SDS–PAGE and Western blotting with anti-GM130 or anti–TANGO1-CC1 antibody. (D) TANGO1S-FLAG expression was induced by incubation with 10 ng/ml doxycycline for 24 h in an HSC-1 stable cell line. Cells were fixed and costained with anti-FLAG antibody and anti-Sec16 or anti-TANGO1L antibody. Scale bar, 10 μm.
FIGURE 2:
TANGO1S and TANGO1L are cooperatively required for correct localization of COPII components. (A) HSC-1 cells were transfected with the indicated siRNA(s). After 72 h, cells were fixed and stained with either anti–TANGO1-CT or anti-TANGO1L antibody. Scale bar, 10 μm. (B) HSC-1 cells were transfected with the indicated siRNA(s). After 72 h, cells were fixed and costained with either anti-Sec16 and anti-Sec31 antibodies or anti-Sec12 and anti-cTAGE5 antibodies. Scale bars, 10 μm.
Next we checked the localization of COPII components in TANGO1 siRNA–treated cells. As shown previously (Saito et al., 2009), Sec31 was partially dissociated from the ER exit sites marked by Sec16 upon knockdown of both TANGO1L and TANGO1S (Figure 2B, top). Knockdown of either TANGO1L or TANGO1S gave a milder phenotype, indicating that both TANGO1L and TANGO1S are necessary for Sec31 to localize at ER exit sites (Figure 2B, top). We previously showed that Sec12 is concentrated at the ER exit site via interaction with cTAGE5, and depletion of cTAGE5 leads to the reduction of Sec12 signals from ER exit sites without changing its protein expression level (Saito et al., 2014; Tanabe et al., 2016). In accordance with these reports, Sec12 was no longer localized at the ER exit site when cTAGE5 expression was reduced by both TANGO1L and TANGO1S depletion (Figure 2B, bottom). cTAGE5 and Sec12 were partially dispersed in either TANGO1L or TANGO1S siRNA-treated cells (Figure 2B, bottom). These data suggest that TANGO1L and TANGO1S are cooperatively required for correct localization of COPII components.
TANGO1S is required for collagen VII export from the ER
In our previous study, siRNAs targeted to both TANGO1L and TANGO1S were used for showing the involvement of TANGO1 in collagen VII secretion (Saito et al., 2009). Thus the phenotype may have been the accumulative effects of both isoforms and/or an indirect effect due to the cTAGE5 reduction. We then checked the isoform-specific effect on collagen VII secretion.
Although TANGO1S is incapable of interacting with collagen VII, as shown in Figure 3A, TANGO1S-specific depletion resulted in significant accumulation of collagen VII within the cells (Figure 3, B and C). The intracellular collagen VII showed a typical ER-like pattern and colocalized with protein disulfide isomerase (PDI) in TANGO1S-depleted cells (Figure 3B and Supplemental Figure S2). These results indicate that TANGO1L and TANGO1S are individually required for collagen VII secretion from the ER.
FIGURE 3:
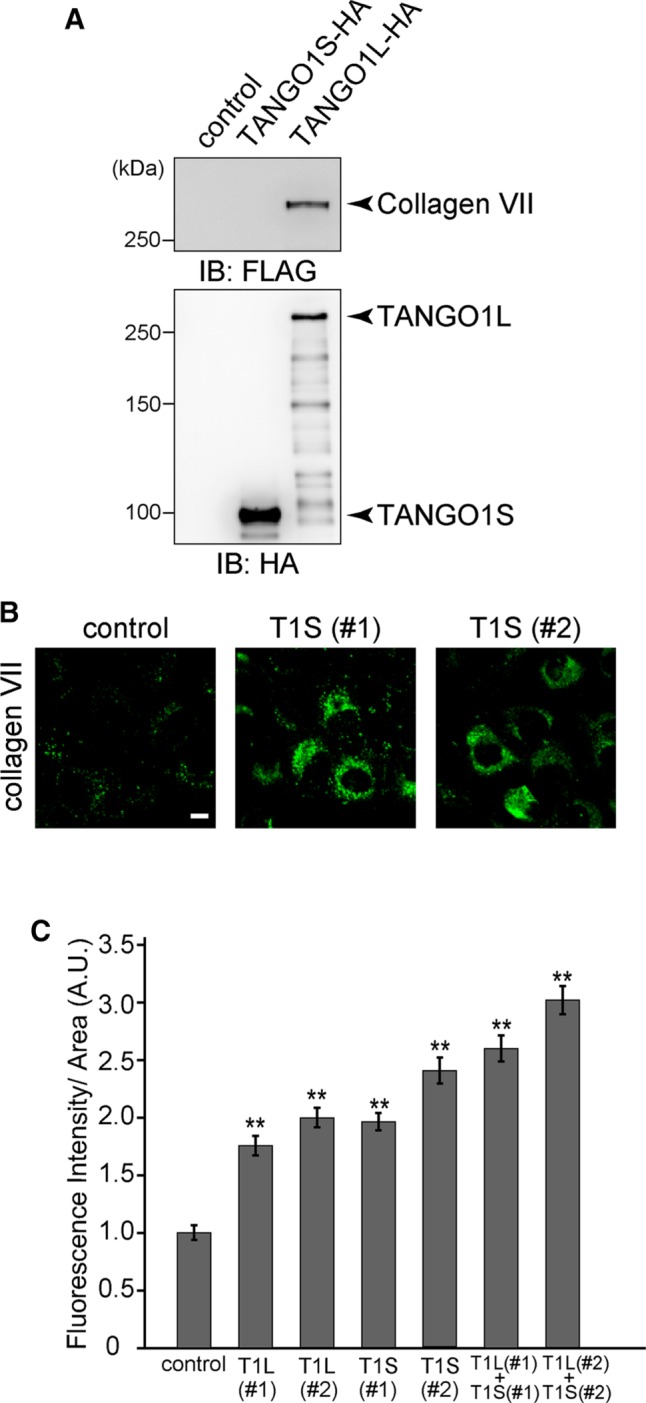
TANGO1S is required for collagen VII export from the ER. (A) HA tag alone, TANGO1S-HA, or TANGO1L-HA were expressed in 293T cells and immunoprecipitated with anti-HA antibody. Immunoprecipitants were further incubated with lysate prepared from 293T cells stably expressing FLAG-collagen VII. The beads were washed and subjected to SDS–PAGE, followed by Western blotting with anti-FLAG or anti-HA antibodies. (B) HSC-1 cells were transfected with the indicated siRNA. After 72 h, cells were fixed and stained with anti–collagen VII antibody. Scale bar, 10 μm. (C) HSC-1 cells were transfected with the indicated siRNA(s) and stained with anti–collagen VII antibody. The collagen VII immunofluorescence signal per cell was quantified (arbitrary units [A.U.]). Error bars represent mean ± SEM. **p < 0.005 compared with control siRNA. For cells treated with control siRNA, n = 92; with TANGO1L (#1), n = 93; with TANGO1L (#2), n = 102; with TANGO1S (#1), n = 109; with TANGO1S (#2), n = 84; with TANGO1L (#1) and TANGO1S (#1), n = 112; and with TANGO1L (#2) and TANGO1S (#2), n = 120.
TANGO1L and TANGO1S individually form a large complex with cTAGE5 and Sec12
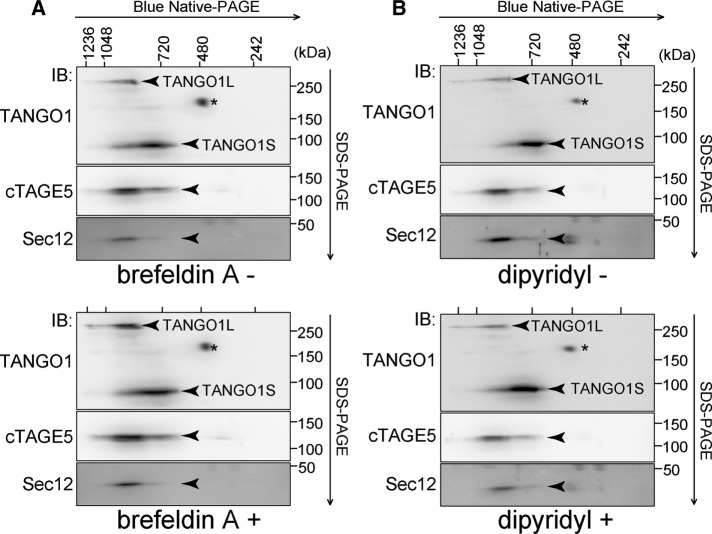
Our observation that TANGO1S is required for collagen secretion led to the question of whether TANGO1L and TANGO1S are functionally distinct or identical. To resolve this issue, we first tried to reveal the complex entity of TANGO1 and other molecules involved in collagen export from the ER. We used two-dimensional Blue-Native PAGE/SDS–PAGE analysis because this technique is comparatively suitable for detecting membrane-protein complexes. HSC-1 cell lysates were first resolved by Blue-Native PAGE and then subjected to SDS–PAGE, followed by Western blotting. We previously reported that TANGO1 interacts with the inner coat complex Sec23/24 and cTAGE5, which then associates with Sec12, the guanine-nucleotide exchange factor for Sar1 (Saito et al., 2009, 2011, 2014). As shown in Figure 4A, TANGO1L migrated at ∼900 kDa together with cTAGE5 and Sec12. Of interest, the major band of TANGO1S migrated at a different size from TANGO1L, at ∼700 kDa, where both cTAGE5 and a very faint signal of Sec12 were also present (Figure 4A). These data suggest that these molecules, which are involved in collagen secretion, are stably associated as high–molecular weight complexes.
FIGURE 4:
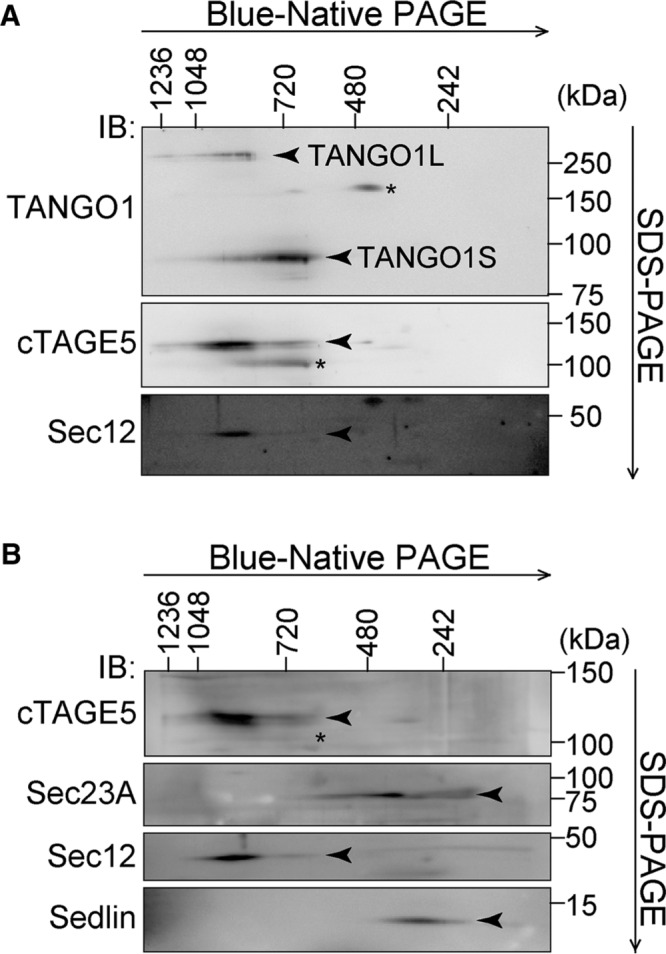
Two-dimensional Blue-Native PAGE/SDS–PAGE analysis of HSC-1 cell lysates. HSC-1 cell proteins extracted with 1% digitonin were subjected to 4–15% Blue-Native PAGE in the first dimension and SDS–PAGE in the second dimension, followed by Western blotting with (A) anti–TANGO1-CC1, anti-cTAGE5, and anti-Sec12 antibodies or (B) anti-cTAGE5, anti-Sec23A, anti-Sec12, and anti-Sedlin antibodies. *Nonspecific cross-reaction.
To check whether these proteins are within the same complexes, TANGO1S-FLAG was immunoprecipitated from a TANGO1S-FLAG–inducible cell line. Immunoprecipitants eluted by a FLAG peptide were subjected to Blue-Native PAGE/SDS–PAGE analysis. As shown in Figure 5A, TANGO1S, cTAGE5, and Sec12 comigrated at 700 kDa, strongly suggesting that these three proteins are within the same complex at the ER exit sites. Next we checked cTAGE5-FLAG immunoprecipitants isolated from a cTAGE5-FLAG–inducible cell line. Mildly induced cTAGE5-FLAG was also localized at ER exit sites (Supplemental Figure S1, A, lanes 1–4, and B). Along with the TANGO1S/cTAGE5/Sec12 complex at 700 kDa, TANGO1L, cTAGE5, Sec12, and possibly TANGO1S were detected at ∼900 kDa (Figure 5B). Because the sum of the molecular weights of these constituents (TANGO1L, 290 kDa; cTAGE5, 110 kDa; Sec12, 45 kDa; and TANGO1S, 100 kDa) does not add up to the observed complex size, we believed there might be (an) additional factor(s) within the complex. To check this possibility, we silver stained cTAGE5 immunoprecipitants (Figure 5C). Although we observed the bands corresponding to TANGO1L, TANGO1S, cTAGE5, and Sec12, no additional factors seemed to participate in these complexes. Indeed, Sec23A and Sedlin, previously reported as the interactors of TANGO1, did not migrate at the corresponding molecular size, indicating that these proteins are not within the complexes (Figure 4B).
FIGURE 5:
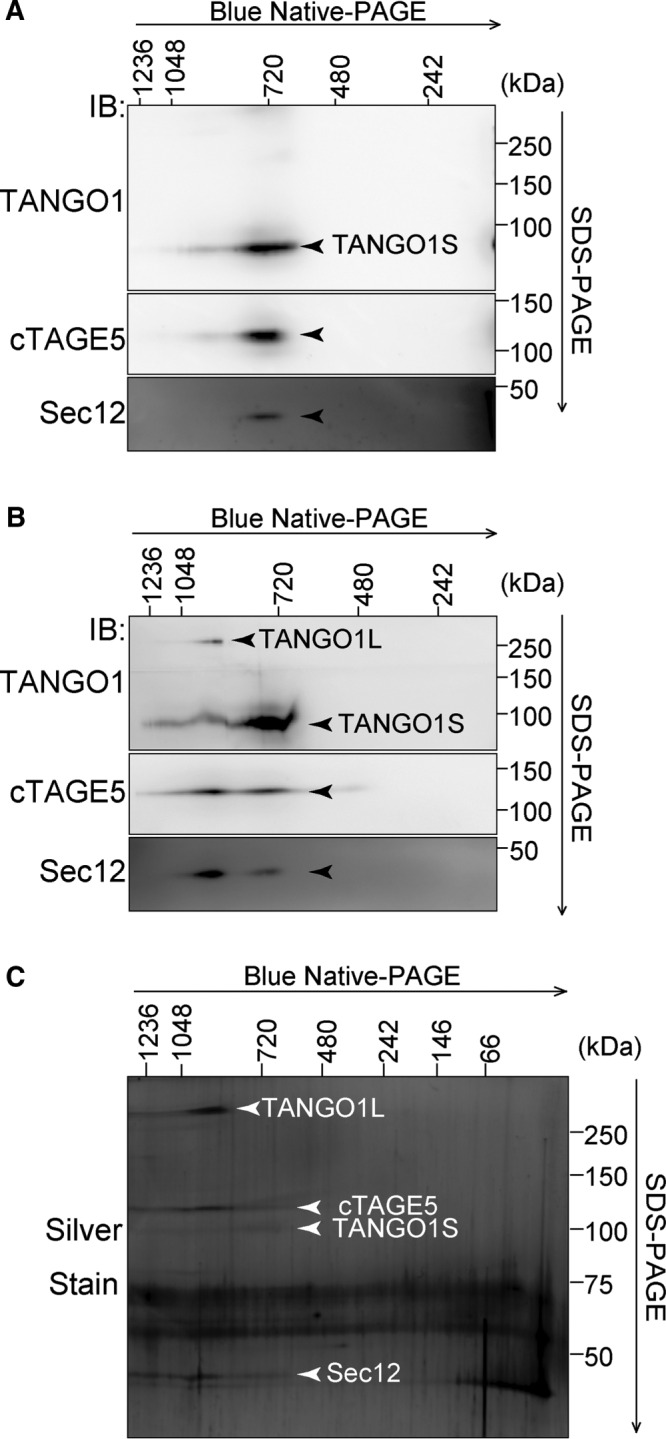
TANGO1S forms a 700-kDa complex with cTAGE5 and Sec12, and TANGO1L forms a 900-kDa complex with cTAGE5 and Sec12. Two-dimensional Blue-Native PAGE/SDS–PAGE analysis of immunoprecipitants from an HSC-1 inducible cell line. (A) TANGO1S-FLAG or (B, C) cTAGE5-FLAG expression was induced by incubation with 10 ng/ml doxycycline for 24 h in HSC-1 stable cell lines. Cell proteins extracted with 1% digitonin were immunoprecipitated with anti-FLAG antibody and eluted with a FLAG peptide. Eluates were analyzed by two-dimensional Blue-Native PAGE/SDS–PAGE analysis, followed by Western blotting with (A, B) anti–TANGO1-CC1, anti-cTAGE5, and anti-Sec12 antibodies or (C) silver staining.
Taken together, these data suggest that TANGO1L and TANGO1S mainly form distinct complexes at ER exit sites.
cTAGE5 and TANGO1S have the capacity to form a multimeric complex
Our observations indicated that four components (TANGO1L, TANGO1S, cTAGE5, and Sec12) are the only constituents of the two complexes. We next examined the possibility that these proteins form homomultimers. We transfected 293T cells with cTAGE5-FLAG, cTAGE5-hemagglutinin (HA), and cTAGE5-Myc constructs. We immunoprecipitated cell lysates with anti-FLAG antibody and subsequently immunoprecipitated the eluates with anti-HA antibody. cTAGE5-Myc was isolated by successive immunoprecipitation (Figure 6A), suggesting that cTAGE5 can at least form more than a homotrimer. Similar experiments with TANGO1S suggest that TANGO1S can also form more than a homotrimer (Figure 6B).
FIGURE 6:
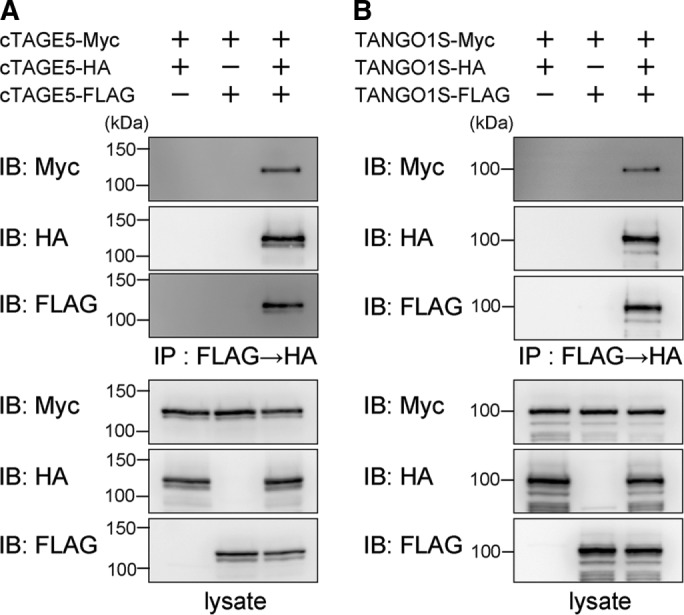
cTAGE5 and TANGO1S are capable of forming homotrimers. 293T cells were transfected with (A) cTAGE5-FLAG, cTAGE5-HA, and cTAGE5-Myc constructs or (B) TANGO1S-FLAG, TANGO1S-HA, and TANGO1S-Myc constructs as indicated. Cell lysates were immunoprecipitated with anti-FLAG antibody and eluted with a FLAG peptide. Eluates were then immunoprecipitated with anti-HA antibody, and immunoprecipitants were subjected to SDS–PAGE, followed by Western blotting with anti-FLAG, anti-HA, or anti-Myc antibodies.
The two individual complexes are stable irrespective of secretion status
Next we tried to address whether these complexes change their contents in accordance with the secretion status. Proteins from cells treated with brefeldin A, which inhibits general protein export from the ER, were extracted and subjected to Blue-Native PAGE/SDS–PAGE analysis. As shown in Figure 7A, both TANGO1L and TANGO1S complexes remained the same size with the same constituents, indicating that inhibition of secretion did not disrupt their formation. Treatment with dipyridyl, an inhibitor of collagen folding that then blocks its secretion, gave similar results (Figure 7B). These data indicate that these two complexes are rather stable components at the ER exit sites and do not change their contents according to the secretion conditions.
FIGURE 7:
TANGO1S/cTAGE5/Sec12, and TANGO1L/cTAGE5/Sec12 are stable complexes irrespective of the secretion status. HSC-1 cells were untreated or treated with (A) 5 μg/ml brefeldin A or (B) 0.6 mM dipyridyl. Cell proteins extracted with 1% digitonin were subjected to Blue-Native PAGE/SDS–PAGE analysis, followed by Western blotting with anti–TANGO1-CC1, anti-cTAGE5, and anti-Sec12 antibodies. *Nonspecific cross-reaction.
TANGO1 isoforms have exchangeable properties for secreting collagen VII
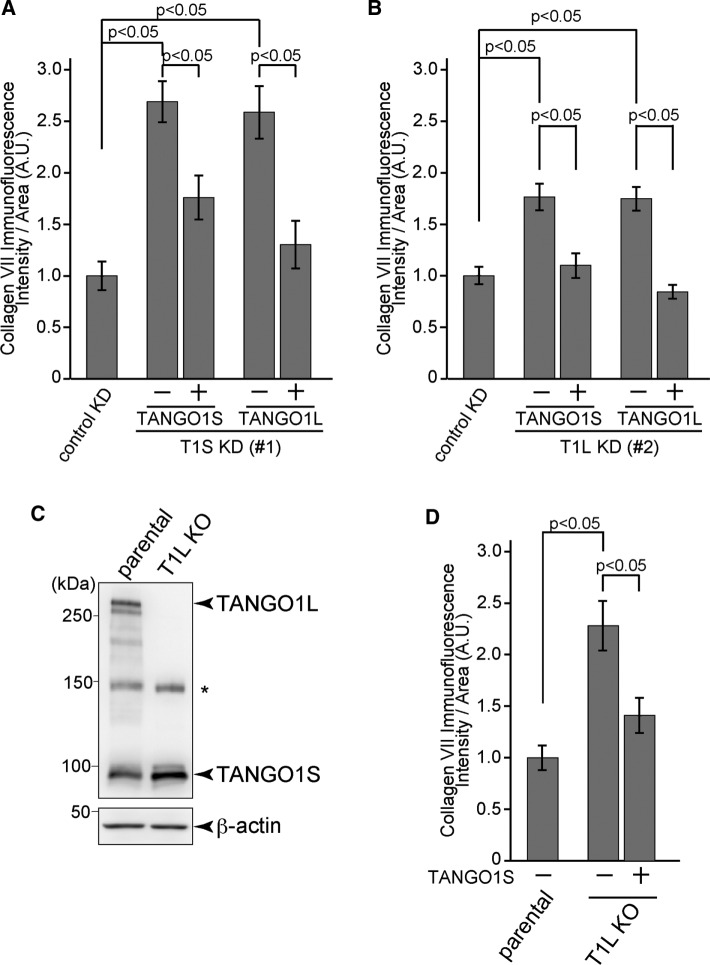
Although TANGO1S does not contain the luminal long stretch to which collagen VII binds, it is necessary for collagen VII export from the ER. To determine whether the TANGO1S complex is functionally distinct from the TANGO1L complex, we transfected cells depleted of either isoform with the other isoform and examined the status of collagen secretion. When TANGO1S-FLAG was expressed in TANGO1S-depleted cells, there was significantly less collagen VII accumulation than in nontransfected cells (Figure 8A). TANGO1L-FLAG expression in TANGO1S-depleted cells also decreased the accumulation, indicating that TANGO1L can substitute TANGO1S in collagen secretion (Figure 8A). Next we depleted TANGO1L and assessed the effects of TANGO1S expression. Of interest, not only the expression of TANGO1L, but also that of TANGO1S, efficiently rescued collagen VII accumulation in the ER (Figure 8B). This was a surprise, considering that TANGO1S is incapable of interacting with collagen VII, but there is a possibility that residual TANGO1L under siRNA-mediated depletion suffices for the function of cargo recognition with the help of expressed TANGO1S. To clarify this point, we generated TANGO1L-specific knockout (KO) cells by clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 genome editing on a TANGO1S-inducible HSC-1 cell line. As shown in Figure 8C, TANGO1L was completely absent in KO cells. It is noteworthy that the expression level of endogenous TANGO1S was slightly increased in TANGO1L KO cells. Collagen VII accumulated significantly in TANGO1L KO cells compared with parental cells. Moreover, TANGO1S induction significantly decreased intracellular collagen VII (Figure 8D). These data suggest that TANGO1S is interchangeable with TANGO1L in its role in collagen export from the ER.
FIGURE 8:
TANGO1S and TANGO1L have interchangeable properties for exporting collagen VII from the ER. HSC-1 cells were treated with control, (A) TANGO1S, or (B) TANGO1L siRNA and cultured for 24 h. For TANGO1 siRNA–treated cells, TANGO1S-FLAG or TANGO1L-FLAG was transfected and further cultured for 30 h. The cells were fixed and stained with collagen VII and FLAG antibodies. The collagen VII immunofluorescence signal per cell (A.U.) was quantified for each cell category described below. (A) For cells treated with control siRNA, n = 91; with TANGO1S siRNA in which TANGO1S-FLAG was not expressed, n = 182; with TANGO1S siRNA in which TANGO1S-FLAG was expressed, n = 53; with TANGO1S siRNA in which TANGO1L-FLAG was not expressed, n = 210; and with TANGO1S siRNA in which TANGO1L-FLAG was expressed, n = 57 (analysis of variance). KD, knockdown. (B) For cells treated with control siRNA, n = 101; with TANGO1L siRNA in which TANGO1S-FLAG was not expressed, n = 138; with TANGO1L siRNA in which TANGO1S-FLAG was expressed, n = 53; with TANGO1L siRNA in which TANGO1L-FLAG was not expressed, n = 153; and with TANGO1L siRNA in which TANGO1L-FLAG was expressed, n = 60 (analysis of variance). Data represent mean ± SEM. The data shown are from a single representative experiment out of three repeats. (C) TANGO1L-specific KO cells were generated by a CRISPR/Cas9 system on HSC-1 cells inducibly expressing TANGO1S-FLAG. Cell lysates were subjected to SDS–PAGE, followed by Western blotting with anti–TANGO1-CC1 and β-actin antibodies. *Nonspecific cross-reaction. KO, knockout. (D) TANGO1L KO cells were either untreated or incubated with 9 ng/ml doxycycline for 24 h to induce the expression of TANGO1S-FLAG. The cells were fixed and stained with collagen VII and FLAG antibodies. The collagen VII immunofluorescence signal per cell (A.U.) was quantified for each cell category described below. For parental cells, n = 111; for TANGO1L KO cells in which TANGO1S-FLAG was not expressed, n = 94; and for TANGO1L KO cells in which TANGO1S-FLAG was expressed, n = 112 (analysis of variance). Data represent mean ± SEM. The data shown are from a single representative experiment out of three repeats.
DISCUSSION
In this study, we found that TANGO1S is an integral membrane protein localized at ER exit sites. Because TANGO1S lacks characteristic signal peptide sequences, TANGO1S may be inserted in the membrane by a different mechanism than TANGO1L. TANGO1L contains two hydrophobic regions in the middle of the protein, and both are required for its correct topology. On the basis of these data, we proposed that one of the hydrophobic regions of TANGO1L spans the membrane, whereas the second may be partially inserted in either side of the membrane (Saito et al., 2009). TANGO1S fully shares one of the TANGO1L hydrophobic regions; however, the other one only shares half of the sequence, and its N-terminal is connected to the original sequence of TANGO1S. We presume that the full hydrophobic region acts as a transmembrane domain and the other sequence may be inserted into the membrane (Figure 9).
FIGURE 9:
Model of TANGO1L and TANGO1S complexes at ER exit sites. The 900-kDa TANGO1L/cTAGE5/Sec12 complex interacts with the COPII coat and collagen VII, whereas the 700-kDa TANGO1S/cTAGE5/Sec12 complex solely interacts with the COPII coat. The two complexes coordinately facilitate collagen VII export from the ER.
Consistent with previous report (Saito et al., 2009), we observed dissociation of Sec31 from ER exit sites by depletion of both TANGO1S and TANGO1L. In this study, we also found that knockdown of both isoforms decrease the cTAGE5 expression level, leading to Sec12 dispersion from the ER exit sites (Saito et al., 2014). Compared with these defects, depletion of either TANGO1S or TANGO1L gave a milder phenotype. These data indicate that two of the TANGO1 isoforms are individually involved in the correct localization of COPII components.
The present study suggests that mammalian ER exit sites enclose two differently sized membrane-spanning complexes specifically required for collagen export. These two complexes are highly similar in content. One complex is 900 kDa and mainly consists of TANGO1L (290 kDa), cTAGE5 (110 kDa), and Sec12 (45 kDa); the other is 700 kDa and consists of TANGO1S (100 kDa), cTAGE5 (110 kDa), and Sec12 (45 kDa). In addition, cTAGE5 and TANGO1S are capable of forming oligomers. The major difference appears to be the TANGO1 isoform within the complexes. Of interest, the difference in the size of the complexes (900 vs. 700 kDa) is well correlated with that of TANGO1 isoforms (290 vs. 100 kDa). On the basis of these results, we speculate that both complexes mainly consist of a cTAGE5 oligomer as the core, which then interacts with a corresponding amount of Sec12, and either TANGO1L (for 900 kDa) or TANGO1S (for 700 kDa; Figure 9). However, because of the similarity between TANGO1S and cTAGE5 in size, structure, and oligomer formation properties, it would be difficult to distinguish these two molecules by Blue-Native PAGE/SDS–PAGE analysis. Together with the fact that TANGO1S may also be present in the 900-kDa complex, even in a limited amount, we presume that the content of each of these complexes is not uniform, as there may be a certain amount of complexes in which cTAGE5 is replaced with TANGO1S. Although the preliminary biochemical analysis showed that TANGO1L could form at least dimers, taking into account the molecular weight of each constituent, it is hard to assume more than one TANGO1L molecule within a single 900-kDa complex. Furthermore, TANGO1-like (TALI), a fusion protein of cTAGE5 and MIA2, was recently reported to be expressed in a tissue-specific manner and form complexes with TANGO1(L) to export pre–chylomicron/very low density lipoproteins from the ER (Santos et al., 2016). Thus further characterization is needed to uncover the exact contents of these complexes. In any event, this is the first report suggesting that mammalian ER exit sites contain two differently sized built-in macromolecular structures that enable the export of large cargoes from the ER.
Our Blue-Native PAGE/SDS–PAGE analysis failed to detect cytoplasmic components previously reported as interactors of the complex constituents, such as Sec23/24, Sedlin, and sly1 (Saito et al., 2009, 2011; Venditti et al., 2012; Nogueira et al., 2014). Why did we not find these proteins in the complexes? It is interesting to speculate that the interactions with these components are transient and they have to be recruited to the ER exit sites by a signal or some other mechanisms. In addition, our analysis with inhibitors suggests that the complexes are fairly stable at the ER exit sites, irrespective of the secretion status of collagens and other molecules.
To our surprise, even though TANGO1S is incapable of interacting with collagen VII, TANGO1S is required for collagen VII secretion independently of TANGO1L. In addition, TANGO1S is not likely to be involved in general protein secretion because depletion of both TANGO1L and TANGO1S does not affect most cargo secretions (Saito et al., 2009). Thus we concluded that TANGO1S is specifically important for collagen export from the ER.
Moreover, each TANGO1 isoform fully substituted the function of the other isoform in collagen export under our assay conditions. One possible explanation is that collagen VII export requires a certain amount of TANGO1 localized at the ER exit site irrespective of isoforms. In this regard, TANGO1’s cytoplasmic portions shared by both isoforms have been reported to recruit ERGIC membranes, Sec23/Sec24, and cTAGE5 (Saito et al., 2009, 2011; Santos et al., 2015). These TANGO1 functions may be sufficient to produce transport carriers that can accommodate collagen VII inside. If the cytoplasmic TANGO1 domains (i.e., TANGO1S) are sufficient for their roles in collagen secretion, then what is the role of TANGO1L?
In mice, TANGO1L knockout leads to global defects in collagen secretion. These mice still possess intact TANGO1S (Wilson et al., 2011). In addition, an earlier report suggested that TANGO1L’s luminal SH3 domain directly interacts with collagen VII (Saito et al., 2009). Taking these observations into consideration, we believe that TANGO1L is indeed required for collagen VII export from the ER. We speculate that the interaction of TANGO1L’s luminal SH3 domain with collagen VII ensures either the efficiency or, of greater interest, the quality of collagens to be secreted. Although our assay is capable of measuring the dynamics of endogenous collagens and several proteins involved in collagen secretion have been identified by this assay (Saito et al., 2011, 2014), it failed to detect the folding status of collagen VII. To achieve this, it is vital to obtain collagen VII antibodies suitable for detection of endogenous collagen by Western blotting for checking the folding status. Further investigation is required to uncover how each TANGO1 isoform, that is, each complex, cooperatively achieves collagen VII export from the ER.
In summary, the present study found that mammalian ER exit sites possess two membrane-spanning complexes specifically required for collagen secretion from the ER.
MATERIALS AND METHODS
Antibodies
Anti–collagen VII monoclonal antibody (NP-185) was kindly provided by Lynn Sakai (Oregon Health and Science University, Portland, OR). Purified rabbit polyclonal antibodies against TANGO1 (TANGO1-CC1, 1211–1440 amino acids [aa]; and TANGO1-CT, 1884–1898 aa) recognizing both TANGO1L and TANGO1S were made as described previously (Saito et al., 2011). Antibody against TANGO1L (422–509 aa) was purchased from Sigma-Aldrich (St. Louis, MO). Anti-PDI antibody was purchased from Abcam (Cambridge, UK). Other antibodies were used as described previously (Saito et al., 2009, 2011, 2014; Tanabe et al., 2016).
siRNA oligos
Stealth select siRNAs for TANGO1S and TANGO1L were purchased from Thermo Fisher Scientific (Waltham, MA). The oligo sequences used were TANGO1S siRNA (#1), 5′-GAATTGTCGCTTGCGTTCAGCTGTT-3′; TANGO1S siRNA (#2), 5′-CAGTACCTGCCACTGTGCCTTCTAT-3′; TANGO1L siRNA (#1), 5′-CCTCAACTCTATGCCAGCTGCTGA A-3′; and TANGO1L siRNA (#2), 5′-CAACT CAGAGGAAAGTGATAGTGTA-3′.
Cell culture and transfection
HeLa, HSC-1, and 293T cells were cultured in DMEM supplemented with 10% fetal bovine serum. Lipofectamine RNAi max (Thermo Fisher Scientific) was used for transfecting siRNA. For plasmids transfection, polyethylenimine MAX (Polysciences, Warrington, PA) or FuGENE 6 (Promega, Madison, WI) were used. The doxycycline-inducible stable 293T cell line expressing FLAG-collagen VII was made with a Flp-In system (Thermo Fisher Scientific). Doxycycline-inducible stable HSC-1 cell lines expressing cTAGE5-FLAG or TANGO1S-FLAG were made with a lentivirus system described previously (Shin et al., 2006). TANGO1L-knockout cell lines were generated by the lentiCRISPR v2 system (Sanjana et al., 2014). The gRNA sequence used was 5′-TGGACCCCAGCACTGGCCGG-3′.
Extraction of membrane proteins
Extraction of membrane proteins was essentially performed as described previously (Nakamura et al., 1995; Cruz-Garcia et al., 2012). HSC-1 cells were passed through 23- and 30-gauge needles 10 times each in lysis buffer consisting of 320 mM sucrose, 20 mM Tris-HCl (pH 7.4), 1 mM EDTA-Na (pH 8.0), and protease inhibitors and then centrifuged at 900 × g for 10 min at 4°C. The supernatants were then centrifuged at 160,000 × g for 1 h at 4°C. The pellets were resuspended with lysis buffer, 0.2 M sodium carbonate (pH 11), or lysis buffer containing 1% SDS and further incubated for 30 min at 4°C. Extracts were centrifuged at 160,000 × g for 30 min at 4°C. The supernatants and pellets were processed for sample preparation for SDS–PAGE followed by Western blotting.
Immunoprecipitation and Western blotting
The experiments were performed essentially as described previously (Saito et al., 2014). Cells extracted with extraction buffer consisting of 20 mM Tris-HCl (pH 7.4), 100 mM NaCl, 1 mM EDTA, 1% Triton X-100, and protease inhibitors were centrifuged at 100,000 × g for 30 min at 4°C. Cell lysates were immunoprecipitated with FLAG M2 or HA antibodies. The beads were washed five times with Tris-buffered saline (TBS)/0.1% Triton X-100 and processed for sample preparation.
Collagen VII binding assay
The 293T cells transfected with HA tag alone, TANGO1S-HA, or TANGO1L-HA were cultured for 48 h, and cell lysates were immunoprecipitated with HA antibody. Immunoprecipitants were further incubated with cell lysates prepared from doxycycline-induced 293T cells stably expressing FLAG-tagged collagen VII for 2 h at 4°C. The beads were washed three times with TBS/0.1% Triton X-100 and prepared for Western blotting.
Sample preparation for Blue-Native PAGE
Blue-Native PAGE/SDS–PAGE analysis was conducted essentially as described previously (Swamy et al., 2006). HSC-1 cells extracted with BN buffer (20 mM Bis-Tris, 500 mM ε-amino capronic acid, pH 7.0, 20 mM NaCl, 2 mM EDTA, 10% glycerol, and protease inhibitors) containing 1% digitonin were centrifuged at 20,000 × g at 4°C. The cell lysates were supplemented with a final 0.25% of CBB G-250 for electrophoresis. For immunoprecipitated samples, cells extracted with lysis buffer (20 mM Tris-HCl, pH 7.4, 137 mM NaCl, 2 mM EDTA, 10% glycerol, 1% digitonin, and protease inhibitors) were centrifuged at 100,000 × g for 30 min at 4°C. The cell lysates were immunoprecipitated with anti-FLAG M2 agarose beads (Sigma-Aldrich) and washed with BN buffer containing 0.3% digitonin. The proteins were eluted with 200 μg/ml FLAG peptide in BN buffer and supplemented with 0.075% of CBB G-250 for electrophoresis.
Blue-Native PAGE
In the first dimension of Blue-Native PAGE, a 4–15% gradient gel was run at 4°C with cathode buffer (50 mM tricine, 15 mM Bis-Tris, pH 7.0) containing 0.02% CBB G-250 and anode buffer (50 mM Bis-Tris, pH 7.0). The cathode buffer was exchanged with cathode buffer without CBB once the dye front migrated one-third of the gel.
Two-dimensional SDS–PAGE
For further separation in second-dimension SDS–PAGE, the gel was cut off according to the lanes and boiled in Laemmli sample buffer. The gel strip was washed with SDS–PAGE buffer (25 mM Tris, 192 mM glycine, and 0.1% SDS) and placed in the stacking part of a SDS–PAGE gel. The second-dimension SDS–PAGE was run in SDS–PAGE buffer at room temperature. Immunoblotting and silver staining were performed according to the standard protocols.
Immunofluorescence microscopy
Immunofluorescence microscopy analysis was performed as described previously (Saito et al., 2014). Cells grown on coverslips were washed with phosphate-buffered saline (PBS), fixed with methanol (6 min at −20°C), and then washed with PBS and blocked in blocking solution (5% bovine serum albumin in PBS with 0.1% Triton X-100 for 15 min). After blocking, cells were stained with primary antibody for 1 h, followed by incubation with Alexa Fluor–conjugated secondary antibodies for 1 h at room temperature. Images were acquired with confocal laser scanning microscopy (LSM700; Plan-Apochromat 63×/1.40 numerical aperture [NA] oil immersion objective lens; Carl Zeiss, Oberkochen, Germany). The acquired images were processed with Zen 2009 software (Carl Zeiss). All imaging was performed at room temperature.
Quantification of collagen VII staining
Quantification of collagen VII accumulation was essentially performed as described previously (Saito et al., 2014). Stained cells were analyzed by epifluorescence microscopy (Axio Imager M1; EC Plan-Neofluar 40×/0.75 NA objective lens; Carl Zeiss) and processed with AxioVision software (Carl Zeiss). Area calculation and intensity scanning were done by ImageJ software (National Institutes of Health, Bethesda, MD).
Supplementary Material
Acknowledgments
We thank the members of the Katada lab for valuable discussions. We also thank Shotaro Tomoishi for drawing the model shown in Figure 9. This work is supported in part by research grants from the Japan Society for the Promotion of Science (K.S., T.K.).
Abbreviations used:
- COPII
coat protein complex II
- cTAGE5
cutaneous T-cell lymphoma–associated antigen 5
- ER
endoplasmic reticulum
- ERGIC
ER–Golgi intermediate compartment
- HA
hemagglutinin
- KO
knockout
- PRD
proline-rich domain
- siRNA
small interfering RNA.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E16-03-0196) on July 13, 2016.
REFERENCES
- Cruz-Garcia D, Diaz-Ruiz A, Rabanal-Ruiz Y, Peinado JR, Gracia-Navarro F, Castano JP, Montero-Hadjadje M, Tonon MC, Vaudry H, Anouar Y, et al. The Golgi-associated long coiled-coil protein NECC1 participates in the control of the regulated secretory pathway in PC12 cells. Biochem J. 2012;443:387–396. doi: 10.1042/BJ20110554. [DOI] [PubMed] [Google Scholar]
- Jin L, Pahuja KB, Wickliffe KE, Gorur A, Baumgartel C, Schekman R, Rape M. Ubiquitin-dependent regulation of COPII coat size and function. Nature. 2012;482:495–500. doi: 10.1038/nature10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V, Erlmann P. The pathway of collagen secretion. Annu Rev Cell Dev Biol. 2015;31:109–124. doi: 10.1146/annurev-cellbio-100913-013002. [DOI] [PubMed] [Google Scholar]
- Malhotra V, Erlmann P, Nogueira C. Procollagen export from the endoplasmic reticulum. Biochem Soc Trans. 2015;43:104–107. doi: 10.1042/BST20140286. [DOI] [PubMed] [Google Scholar]
- McCaughey J, Miller VJ, Stevenson NL, Brown AK, Budnik A, Heesom KJ, Alibhai D, Stephens DJ. TFG promotes organization of transitional ER and efficient collagen secretion. Cell Rep. 2016;15:1648–1659. doi: 10.1016/j.celrep.2016.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EA, Schekman R. COPII—a flexible vesicle formation system. Curr Opin Cell Biol. 2013;25:420–427. doi: 10.1016/j.ceb.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira C, Erlmann P, Villeneuve J, Santos AJ, Martinez-Alonso E, Martinez-Menarguez JA, Malhotra V. SLY1 and Syntaxin 18 specify a distinct pathway for Procollagen VII export from the endoplasmic reticulum. eLife. 2014;3:e02784. doi: 10.7554/eLife.02784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Chen M, Bard F, Chen S, Zhou H, Woodley D, Polischuk R, Schekman R, Malhotra V. TANGO1 facilitates cargo loading at endoplasmic reticulum exit sites. Cell. 2009;136:891–902. doi: 10.1016/j.cell.2008.12.025. [DOI] [PubMed] [Google Scholar]
- Saito K, Katada T. Mechanisms for exporting large-sized cargoes from the endoplasmic reticulum. Cell Mol Life Sci. 2015;72:3709–3720. doi: 10.1007/s00018-015-1952-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Yamashiro K, Ichikawa Y, Erlmann P, Kontani K, Malhotra V, Katada T. cTAGE5 mediates collagen secretion through interaction with TANGO1 at endoplasmic reticulum exit sites. Mol Biol Cell. 2011;22:2301–2308. doi: 10.1091/mbc.E11-02-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito K, Yamashiro K, Shimazu N, Tanabe T, Kontani K, Katada T. Concentration of Sec12 at ER exit sites via interaction with cTAGE5 is required for collagen export. J Cell Biol. 2014;206:751–762. doi: 10.1083/jcb.201312062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AJ, Nogueira C, Ortega-Bellido M, Malhotra V. TANGO1 and Mia2/cTAGE5 (TALI) cooperate to export bulky pre-chylomicrons/VLDLs from the endoplasmic reticulum. J Cell Biol. 2016;213:343–354. doi: 10.1083/jcb.201603072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AJ, Raote I, Scarpa M, Brouwers N, Malhotra V. TANGO1 recruits ERGIC membranes to the endoplasmic reticulum for procollagen export. Elife. 2015;4:e10982. doi: 10.7554/eLife.10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin KJ, Wall EA, Zavzavadjian JR, Santat LA, Liu J, Hwang JI, Rebres R, Roach T, Seaman W, Simon MI, et al. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc Natl Acad Sci USA. 2006;103:13759–13764. doi: 10.1073/pnas.0606179103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swamy M, Siegers GM, Minguet S, Wollscheid B, Schamel WW. Blue native polyacrylamide gel electrophoresis (BN-PAGE) for the identification and analysis of multiprotein complexes. Sci STKE. 2006;2006:pl4. doi: 10.1126/stke.3452006pl4. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Maeda M, Saito K, Katada T. Dual function of cTAGE5 in collagen export from the endoplasmic reticulum. Mol Biol Cell. 2016;27:2008–2013. doi: 10.1091/mbc.E16-03-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti R, Scanu T, Santoro M, Di Tullio G, Spaar A, Gaibisso R, Beznoussenko GV, Mironov AA, Jr, Zelante L, Piemontese MR, et al. Sedlin controls the ER export of procollagen by regulating the Sar1 cycle. Science. 2012;337:1668–1672. doi: 10.1126/science.1224947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venditti R, Wilson C, De Matteis MA. Exiting the ER: what we know and what we don’t. Trends Cell Biol. 2014;24:9–18. doi: 10.1016/j.tcb.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Wilson DG, Phamluong K, Li L, Sun M, Cao TC, Liu PS, Modrusan Z, Sandoval WN, Rangell L, Carano RA, et al. Global defects in collagen secretion in a Mia3/TANGO1 knockout mouse. J Cell Biol. 2011;193:935–951. doi: 10.1083/jcb.201007162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.