Abstract
Background
Synovial sarcoma is a soft tissue malignancy with a predilection for adolescents and young adults. Despite recent improvements in the understanding of its character and etiology, few therapeutic advances have been made. The mortality rate is high among the young population it affects. The low incidence of most subtypes of sarcoma, such as synovial sarcoma, makes disease-specific trials difficult to organize. The biological differences between sarcoma subtypes make inclusion of multiple types in general trials unsatisfactory as well.
Methods
A review of the literature regarding targetable pathways in synovial sarcoma was undertaken. A strategy has been devised to utilize available technologies in order to prioritize drug trial planning.
Results
Cell culture and xenograft research with synovial sarcoma cell lines have identified some critical pathways that may be targetable. Promising therapeutic strategies include newer cytotoxic chemotherapies, antiangiogenic agents, anti-IGF1R pathway agents, anti-Bcl-2/proapoptotic agents, and histone deacetylase complex inhibitors.
Conclusions
We propose to prioritize potential therapeutic strategies via preclinical testing of agents in a genetic mouse model of synovial sarcoma. Preclinical optimization of treatment regimens can guide the development of more focused patient trials.
Introduction
Synovial sarcoma remains a deadly soft tissue mesenchymal malignancy that primarily afflicts adolescents and young adults. Although it is a rare tumor in terms of numbers of patients diagnosed with synovial sarcoma, its societal impact is magnified by the young age group affected and the high mortality associated with synovial sarcoma. Much has been learned in recent years about its biology, but as with most soft tissue sarcomas, therapeutic advances have been limited.1 Metastatic disease still portends a poor prognosis.2 Long-term follow-up of patients with initial localized disease reveals an increasing proportion with systemic relapse.3
Recurrent balanced translocations between the X and 18 chromosomes were identified in synovial sarcoma cells in the 1980s.4 It was later noted that these translocations generated fusion oncoproteins composed of the amino-terminus of the SYT gene and the carboxy-terminus of one of the SSX genes, most commonly SSX1 or SSX2.5,6 The discovery of this, and many other balanced translocation fusion oncogenes in sarcomas, began as associations but quickly came to define each tumor type, even at times trumping histologic diagnosis.7 Modeling attempts have even proven the sufficiency of some of these fusion oncoproteins to drive subtype-specific sarcomagenesis, arguing for an initiatory biological role of these translocations.8-10 Nonetheless, molecular diagnostic advances and improved understanding of transformative synovial sarcomagenesis itself have not yet materially changed therapeutic strategies or success rates.
Identifying the Problem
Sarcoma, which is Greek for “fleshy tumor,” includes a group of malignancies with a broad variety of biological behaviors. As in any area of therapeutic knowledge, the definition of a treatable diagnostic entity requires a combination of “lumping and splitting” to define distinct, smaller groups with more consistent biology on which to focus treatment. Sarcoma presents major challenges to “lumping and splitting” by virtue of the many different characteristics by which subgroups may be divided, few of which create corroborative or overlapping subgroup distinctions. About one-third of sarcomas have subtype-specific balanced translocations, while the remaining two-thirds have more complex karyotypes. Sarcomas have a bimodal age distribution, most frequently arising in adolescents and young adults and the others occurring in the middle-aged and elderly. While any mesenchymal tissue can be involved by sarcomas, about one-third occurs in the skeleton and two-thirds in the soft tissues. Dividing sarcomas generally into two groups by any of these methods still renders heterogeneous groups, with wide biological variation within each group. Histologic subtyping provides another method of defining sarcomas, by which approximately 100 different groups can be identified. Molecular subtyping can now divide each histologic group even further. The greatest challenge to these more biologically specific divisions is the small numbers in any group created.11 Just over 10,000 sarcomas of any type occur in the United States each year.12 The incidence of any specific subtype is far smaller.
The Children’s Oncology Group (COG), the largest sarcoma trial-organizing entity in North America, divides sarcomas into Ewing sarcoma, osteosarcoma, rhabdomyosarcoma, and nonrhabdomyosarcoma soft tissue sarcoma (which includes synovial sarcoma). COG trials accrue approximately 100 patients per year to this last group. Sarcoma Alliance for Research through Collaboration (SARC), the largest group focused on trials for sarcomas in adults, has trials specific for bone sarcomas, soft tissue sarcomas, uterine leiomyosarcoma, dermatofibrosarcoma protuberans, gastrointestinal stromal tumor, malignant peripheral nerve sheath tumor, and osteosarcoma. The SARC, which includes 36 cancer centers, recruits approximately 300 patients each year to their varied protocols.
While the small numbers of new diagnoses per year make lumping into groups by type an attractive practice for these trials, lumping sarcoma types together compromises the validity of the outcomes in important ways. First, each sarcoma subtype has unique prognostic implications with regard to the expected natural history. The common algorithms and nomograms used to prognosticate sarcoma relapse or disease-specific death include subtype as a major factor.13-15 Second, trials have already shown some clear disparities between subtypes in terms of responses to given chemotherapeutic regimens. For example, randomized trials testing the addition of ifosfamide to the standard treatment regimens for osteosarcoma and Ewing sarcoma generated opposite results for each subtype, improving survival for Ewing sarcoma patients and worsening it for osteosarcoma patients.16,17 These predictive implications of subtype might mask a significant benefit from a specific regimen for a specific sarcoma subtype, especially if tested within a single group of combined diagnoses.
Such a masking of therapeutic benefit already has been proposed for synovial sarcoma. Although trials of regimens including doxorubicin and/or ifosfamide demonstrated no survival benefit for large, deep-location, high-grade soft tissue sarcomas as a whole, retrospective reviews of the synovial sarcoma patients treated in those trials have shown variable survival benefit.18-27 Partly through strong, vocal opinions and partly through a desire to conquer such a deadly cancer, the standard of care adopted throughout most of the United States and Canada is to offer combination doxorubicin/ifosfamide chemotherapy for patients with synovial sarcoma despite the absence of any controlled, prospective evidence of benefit. Practitioners trust that the randomized controlled trials’ negative results cannot be applied to the subgroup of synovial sarcomas.28
The collection of the ideal randomized controlled evidence is challenging. Approximately 300 patients in the United States are diagnosed with synovial sarcoma each year. Unlike patients with Ewing sarcoma or osteosarcoma, whose natural histories yield 80% to 95% death by disease in the absence of systemic treatments, those with synovial sarcoma have 40% to 60% survival following effective local control without systemic therapies. That somewhat more favorable (although hardly favorable in any absolute sense) natural history demands higher numbers for statistical detection of benefit. To have sufficient power to rule out a difference of at least 20% (eg, an increase from 50% survival to 60% survival) between two treatment arms, every synovial sarcoma patient in North America would have to be recruited to a single two-arm trial for an entire year. Such recruitment success is clearly not possible. More realistic recruitment might require 5 years for each trial as well as cross-continental recruitment support. Obtaining a real answer in 7 years (5 years of recruitment and 2 years of follow-up) is not unreasonable, but such a protocol can answer only a single question at a time, necessitating the prioritization of the most clinically critical questions. In addition to the time and expense of organizing such trials, the patients available for recruitment are a scarce resource, which becomes the rate-limiting step in advancing treatment for synovial sarcoma.
Multiplicity of Potential Agents
As mentioned above, the utility of standard cytotoxic chemotherapy is not well established for synovial sarcoma, and the particular cytotoxic regimens that might provide the most benefit have not yet been established.18-27 Anthracycline antibiotics and alkylating agents are most commonly used today to treat synovial sarcoma. Retrospective data are conflicting about the utility of either class in general for synovial sarcoma,29-32 but there are more favorable reports of ifosfamide with or without doxorubicin.18,20,22,24,26,33,34 Tumor response rates are typically reported to be around 50%. Other cytotoxic agents, including trabectedin and tasidotin (used only in xenografts so far), have been studied, most with mixed results.35-37
Another class of chemotherapeutic agents suspected to have some activity against synovial sarcomas is the newer group of antiangiogenesis agents.38-40 Synovial sarcoma displays a characteristic hemangiopericytomatous vascular pattern under the microscope, suggestive that angiogenesis is critical to its establishment and growth.41 A few synovial sarcoma patients have been included in general trials of antiangiogenesis agents for solid tumors. Some responses have been reported from these patients. Among 37 patients with synovial sarcoma who were treated with pazopanib, 18 experienced progression-free survival at 12 weeks.38 One of 2 patients noted to respond to a phase I trial of squalamine was a synovial sarcoma patient.40 These case reports are sufficient to suggest that this class of antiangiogenic agents merits further investigation, but no follow-up studies have yet been reported.
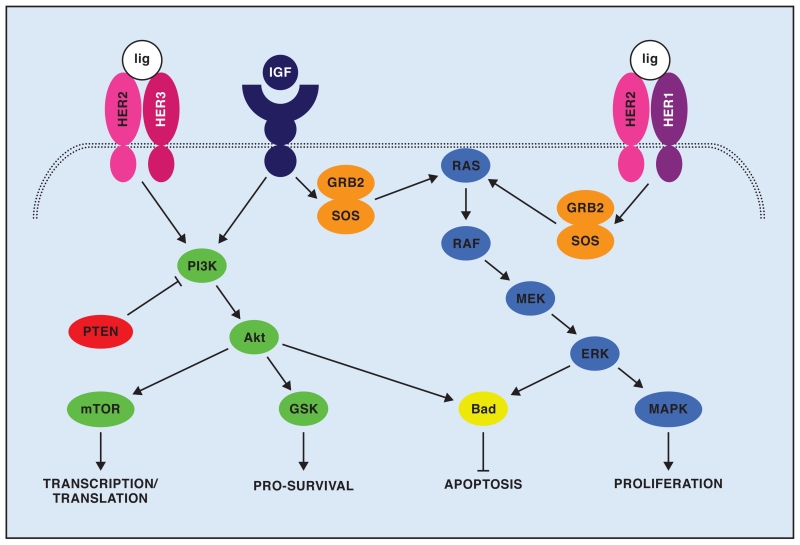
As with other sarcoma subtypes, there has been recent interest in druggable targets in the IGF1R/mTOR/Akt pathway (Fig 1).42 Akt and mTOR have been noted to be constitutively active in some synovial sarcomas, and their activation correlates with metastasis.43 Synovial sarcomas positive for HER1 did not respond to gefitinib,44 but HER2 activation has been noted in head and neck cases of synovial sarcoma.45 One patient with advanced synovial sarcoma treated with figitumumab demonstrated stable disease for 4 months.46 Sorafenib has been effective on synovial sarcoma cell lines in culture and has been tried in patients with metastatic disease.47,48 While preliminary clinical data are scant, IGF1R/mTOR/Akt is an active pathway in synovial sarcoma for which many new agents are becoming available.
Fig 1.
Schematic representing IGF1R/HER2 intersecting pathways. Either IGF signaling or HER2 dimerizing with HER3 on ligand (lig) binding will lead to activation of phosphoinositol 3 kinase (PI3K), which then activates Akt and its downstream effectors. Either IGF signaling or HER2 dimerizing with HER1 (also EGFR) on ligand binding will activate GRB2 and SOS, which then activate RAS/RAF signaling.
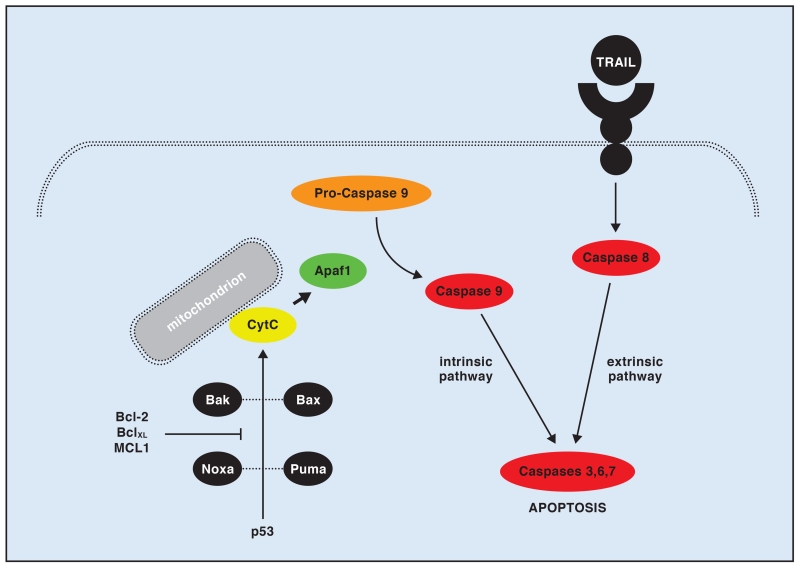
Inhibitors of antiapoptotic pathways (Fig 2) such as Bcl-2 inhibitors also show theoretical promise for synovial sarcoma.49 Although no pertinent trials have been reported to include synovial sarcoma patients, Bcl-2 is upregulated in the common expression profile of synovial sarcoma.50 High levels of Bcl-2 in synovial sarcoma are thought to be a major mechanism of chemoresistance by allowing the tumor to escape the apoptotic drive from usual cytotoxic agents. Specific in vitro experiments to inhibit Bcl-2 have proven successful at slowing the growth of synovial sarcoma and inducing apoptosis in its cell lines in conjunction with cytotoxic agents.51 Other apoptotic pathways remain intact in synovial sarcoma cells, including tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL),52 and may provide other candidate loci that can be targeted.
Fig 2.
Schematic representing intrinsic and extrinsic apoptosis initiation. When activated, p53 activates Bax and Bak, then Suma and Nova, leading to Apaf1 cleavage of pro-caspase 9 into active caspase 9. Bcl-2, along with BclXL and MCL1, can downregulate the activation of cytochrome C, halting proapoptotic signals from the intrinsic pathway. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) and potentially other activation methods of extrinsic apoptosis remain active in synovial sarcoma.
Finally, inhibitors of the histone deacetylase complex (HDACi) are of interest to the management of synovial sarcoma. SYT-SSX proteins have been shown to associate with polycomb group proteins, regulating expression of certain targets by epigenetic modifications of histones. One of the highlighted targets of these epigenetic silencing effects of SYT-SSX is the tumor suppressor early growth response 1 (EGR1).53 Increased expression of EGR1 and subsequent phosphatase and tensin homolog (PTEN)-mediated apoptosis can be achieved in synovial sarcoma cell lines by the administration of FK228, an HDACi.54,55 Additionally, synergism has been noted between an HDACi and the proapoptotic 17-AAG compound, which downregulates heat shock protein 90.56 This area of histone deactylation is enhanced by recent basic research findings and holds promise for therapeutic benefits yet to materialize.
Prioritizing Agents
One common method of prioritizing agents for a given disease is to pilot them in small numbers in patients who already have failed standard treatment regimens and are moribund with progressively advancing disease. The search for a response to any agent can be desperate in this setting for both the patient and the practitioner. Knowledge gained from the slowing of progression — or lack thereof — of widely metastatic, multiple, chemo-exposed and resistant aggressive tumors in patients with often poor functional status may be difficult to apply to the management of otherwise healthy patients with treatment-naive, microscopic, systemic disease that is hoped to be eradicated in the primary tumor setting. Nonetheless, this remains one utilized method for identifying effective agents.
While a number of molecular methods are available to identify critical pathways and potentially druggable targets, an agent ultimately must prove to be able to kill the tumor in question. Three principle strategies are most commonly employed to this end: culture of human tumor cell lines, xenografts of human tumor cell lines into immunocompromised mice, and genetic models of disease in model organisms. Each approach has benefits and detriments both practical and theoretical.
Culture of Tumor Cell Lines
Tumor cell lines that have been passaged multiple times in culture have accrued through such processing a definite but not entirely discernible variety of phenotypic and genetic changes. Their ultimate mimic of tumor cells in a native tumor environment is also challenged by the lack of the natural intratumor polyclonality of supporting cells, vasculature, paracrine signaling, and cell-to-cell signaling. Further, cultured cells lack the natural contribution to, or selective pressure from, ongoing immune surveillance, which likely has a heavy impact on tumors in a native host. Finally, the interactions of the drugs themselves with the host are difficult to model in culture systems. Some biological agents are cleaved or otherwise processed by the nontumor organ systems of hosts but will not be so processed in culture. The pharmacokinetics and distribution of other agents can invalidate the translation of in vitro data into the in vivo setting.
Xenografts of Human Tumor Cell Lines
Xenograft experiments for preclinical testing can address some of these pitfalls by better modeling some of the host processing of therapeutic agents and their delivery and distribution. These experiments even provide some of the host-tumor interactions missing from cell culture. Nonetheless, such host-tumor interactions, when based in immunocompromised mice with tumors implanted either subcutaneously or in the renal capsule, may have limited relation to the host-tumor interactions of a tumor arising in its natural primary or metastatic foci. In some ways, in terms of preclinical testing of drugs, xenografts become a more expensive version of cell-culture experiments. Xenograft experiments remain focused on cell lines but grow those cell lines in an environment that more closely resembles their growth in a tumor than a tissue flask can.
Genetic Models of Disease in Model Organisms
Genetic animal models of disease correct many of the challenges presented by cell culture and xenograft experiments, but they create other challenges. First, these animal tumors arise in another species, which limits some of the validity in translation to humans. Despite 98% coding sequence homology between the mouse and human genomes, there are obvious differences.57 Second, while a much closer to natural host-tumor interaction is created in genetically modified mice, it does not completely follow the natural history of the human tumor. Typically, genes will be activated or inactivated across an entire tissue type, meaning that some of the nontumor host cells will have genetic derangements that may or may not be important to their host function. Developmental effects of such alterations can arise that affect the lifespan and health of the host independent from tumorigenesis. Means to limit such effects are available, by temporal regulation of activation and density of activation in a tissue, but they cannot be removed completely. The greatest challenge to the use of genetic models of disease for preclinical testing is their expense. Generation of each model costs hundreds of thousands of dollars, well beyond the cost of media and culture plates.
Synovial Sarcoma Mouse Model
The Mario Capecchi Laboratory at the University of Utah, which participated in the discovery of many of the technologies used to generate genetically modified mice, has recently developed two mouse models of synovial sarcoma.10,58 Both models offer different strengths for preclinical testing. Mice from both models develop sarcomas with complete penetrance. The sarcomas from these models mimic human synovial sarcoma both histologically and by expression profile (comparing mouse to human homologs). These mice form no other tumors above background rates. The only genetic alteration in the mouse is the expression of human SYT-SSX2 in Myf5-expressing cells, without the need for additional genetic disruptions for potentiation. Sarcomas are fully developed in one of the models consistently by about 3 months of age,10 minimizing expensive cage costs of a large colony awaiting sarcomagenesis.
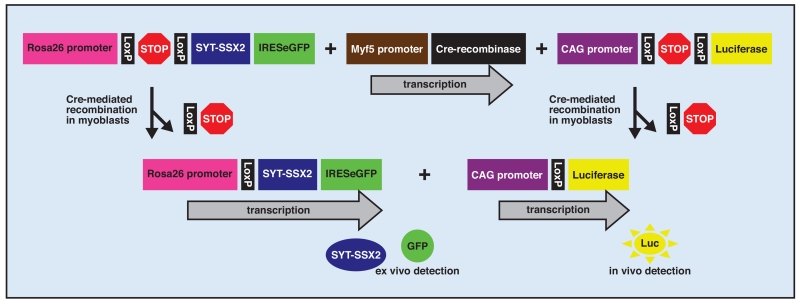
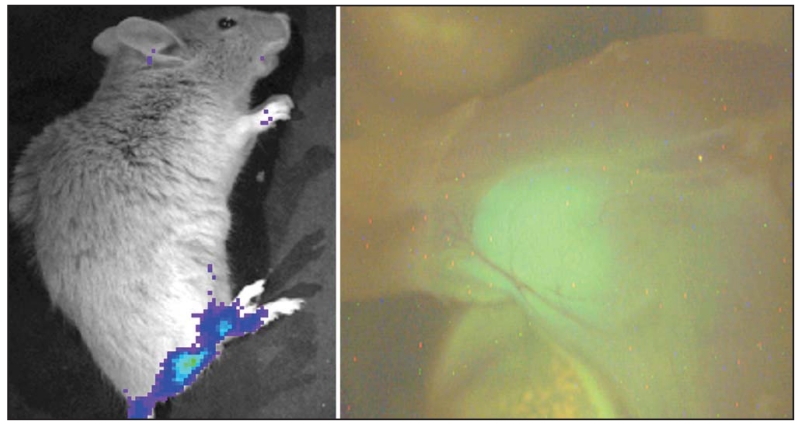
These mouse models have been recently modified further to include a luciferase reporter that is activated in the tumor cells but not the host background (Fig 3).59 This enables in vivo monitoring of tumor size and viability. The proposed experimental plan will be to expose groups of 3-month-old mice, with imaging-confirmed tumors, to a variety of chemotherapeutic or biological agents. Noninvasive monitoring (Fig 4), with luciferase imaging coordinated onto an overlaid plain radiograph anatomical reference, will measure the effects of each agent or combination of agents over time, followed by a histologic assessment (Fig 5) of tumors at necropsy.
Fig 3.
Schematic of the targeted alleles present in the synovial sarcoma mouse, as well as their activation by Cre-mediated stop-cassette excision in Myf5 expressing myoblasts.
Fig 4.
Luciferase imaging of a 15-week-old mouse with an apparent synovial sarcoma in the hind limb (left). The image at right shows fluorescein isothiocyanate (FITC) gross imaging of the same limb at necropsy, demonstrating the green fluorescent protein (GFP) signal from the tumor embedded within the muscle (right).
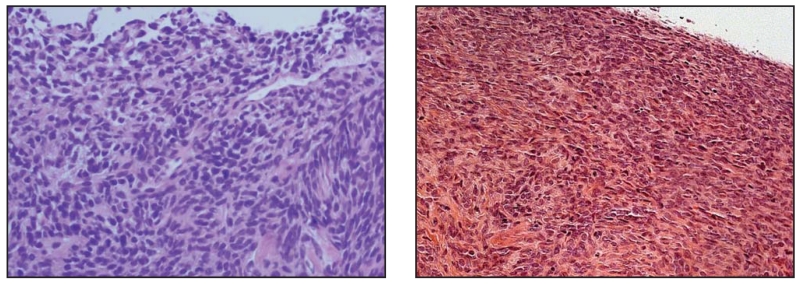
Fig 5.
Photomicrographs of sections stained with hematoxylin and eosin from a human synovial sarcoma (left), followed by a mouse synovial sarcoma (right).
Conclusions
Preclinical strategies for synovial sarcoma include cell-line culture and xenograft experiments, as well as genetically modified model organisms. These approaches continue to improve our understanding and the number of potentially beneficial treatment strategies available to patients. However, rather than driving each preclinically identified therapeutic strategy forward into a prolonged, frustrating, and underpowered clinical trial, we hope to provide a means of prioritizing these strategies. The use of genetic models of disease is expensive and time-consuming, but far less so than the running of human clinical trials. These mouse trials can not only establish priorities for human clinical trials, but also provide valuable information regarding optimal dosing and drug delivery methods to guide human trial planning. The precious and limited resource of patients with synovial sarcoma requires forethought and planning to organize the maximal benefit to them and to future patients. It is hoped that prospective assessment of the effects of a variety of agents and combinations of agents, including cytotoxic, antiangiogenic, proapoptotic, and antideacetylase compounds in the mouse model of synovial sarcoma, will help to prioritize these multiplied therapeutic strategies in order to guide the planning of future randomized controlled trials.
Acknowledgments
Dr Jones receives grant support from the National Cancer Institute (NCI K08138764) and Dr Sharma receives grant/research support from Novartis Pharmaceuticals and serves as a consultant for Novartis Pharmaceuticals and Pfizer Inc. The other authors report no significant relationship with the companies/organizations whose products or services may be referenced in this article.
This research is partly supported by an intramural grant from the Huntsman Cancer Institute Nuclear Control Program (Drs Jones, Lessnick, Sharma, Capecchi, and Randall) and ongoing support from the Paul Nabil Bustany Fund for Synovial Sarcoma Research (Drs Jones, Capecchi, and Haldar).
References
- 1.Weitz J, Antonescu CR, Brennan MF. Localized extremity soft tissue sarcoma: improved knowledge with unchanged survival over time. J Clin Oncol. 2003;21(14):2719–2725. doi: 10.1200/JCO.2003.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Brennan B, Stevens M, Kelsey A, et al. Synovial sarcoma in childhood and adolescence: a retrospective series of 77 patients registered by the Children’s Cancer and Leukaemia Group between 1991 and 2006. Pediatr Blood Cancer. 2010;55(1):85–90. doi: 10.1002/pbc.22453. [DOI] [PubMed] [Google Scholar]
- 3.Guadagnolo BA, Zagars GK, Ballo MT, et al. Long-term outcomes for synovial sarcoma treated with conservation surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69(4):1173–1180. doi: 10.1016/j.ijrobp.2007.04.056. [DOI] [PubMed] [Google Scholar]
- 4.Turc-Carel C, Dal Cin P, Limon J, et al. Involvement of chromosome X in primary cytogenetic change in human neoplasia: nonrandom translocation in synovial sarcoma. Proc Natl Acad Sci U S A. 1987;84(7):1981–1985. doi: 10.1073/pnas.84.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark J, Rocques PJ, Crew AJ, et al. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nat Genet. 1994;7(4):502–508. doi: 10.1038/ng0894-502. [DOI] [PubMed] [Google Scholar]
- 6.Fligman I, Lonardo F, Jhanwar SC, et al. Molecular diagnosis of synovial sarcoma and characterization of a variant SYT-SSX2 fusion transcript. Am J Pathol. 1995;147(6):1592–1599. [PMC free article] [PubMed] [Google Scholar]
- 7.Coindre JM, Pelmus M, Hostein I, et al. Should molecular testing be required for diagnosing synovial sarcoma? A prospective study of 204 cases. Cancer. 2003;98(12):2700–2707. doi: 10.1002/cncr.11840. [DOI] [PubMed] [Google Scholar]
- 8.Pérez-Losada J, Pintado B, Gutiérrez-Adán A, et al. The chimeric FUS/TLS-CHOP fusion protein specifically induces liposarcomas in transgenic mice. Oncogene. 2000;19(20):2413–2422. doi: 10.1038/sj.onc.1203572. [DOI] [PubMed] [Google Scholar]
- 9.Keller C, Arenkiel BR, Coffin CM, et al. Alveolar rhabdomyosarcomas in conditional Pax3:Fkhr mice: cooperativity of Ink4a/ARF and Trp53 loss of function. Genes Dev. 2004;18(21):2614–2626. doi: 10.1101/gad.1244004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haldar M, Hancock JD, Coffin CM, et al. A conditional mouse model of synovial sarcoma: insights into a myogenic origin. Cancer Cell. 2007;11(4):375–388. doi: 10.1016/j.ccr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Verweij J, Baker LH. Future treatment of soft tissue sarcomas will be driven by histological subtype and molecular aberrations. Eur J Cancer. 2010;46(5):863–868. doi: 10.1016/j.ejca.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 12.Cancer Facts and Figures 2009. American Cancer Society; Atlanta, GA: 2009. [Google Scholar]
- 13.Singer S, Corson JM, Gonin R, et al. Prognostic factors predictive of survival and local recurrence for extremity soft tissue sarcoma. Ann Surg. 1994;219(2):165–173. doi: 10.1097/00000658-199402000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kattan MW, Leung DH, Brennan MF. Postoperative nomogram for 12-year sarcoma-specific death. J Clin Oncol. 2002;20(3):791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 15.Eilber FC, Brennan MF, Eilber FR, et al. Validation of the postoperative nomogram for 12-year sarcoma-specific mortality. Cancer. 2004;101(10):2270–2275. doi: 10.1002/cncr.20570. [DOI] [PubMed] [Google Scholar]
- 16.Meyers PA, Schwartz CL, Krailo MD, et al. Osteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival. A report from the Children’s Oncology Group. J Clin Oncol. 2008;26(4):633–638. doi: 10.1200/JCO.2008.14.0095. [DOI] [PubMed] [Google Scholar]
- 17.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 18.Sleijfer S, Ouali M, van Glabbeke M, et al. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STB-SG) Eur J Cancer. 2010;46(1):72–83. doi: 10.1016/j.ejca.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 19.Minchom A, Jones RL, Fisher C, et al. Clinical benefit of second-line palliative chemotherapy in advanced soft-tissue sarcoma. Sarcoma. 2010;2010:264360. doi: 10.1155/2010/264360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canter RJ, Qin LX, Maki RG, et al. A synovial sarcoma-specific preoperative nomogram supports a survival benefit to ifosfamide-based chemotherapy and improves risk stratification for patients. Clin Cancer Res. 2008;14(24):8191–8197. doi: 10.1158/1078-0432.CCR-08-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karavasilis V, Seddon BM, Ashley S, et al. Significant clinical benefit of first-line palliative chemotherapy in advanced soft-tissue sarcoma: retrospective analysis and identification of prognostic factors in 488 patients. Cancer. 2008;112(7):1585–1591. doi: 10.1002/cncr.23332. [DOI] [PubMed] [Google Scholar]
- 22.Eilber FC, Brennan MF, Eilber FR, et al. Chemotherapy is associated with improved survival in adult patients with primary extremity synovial sarcoma. Ann Surg. 2007;246(1):105–113. doi: 10.1097/01.sla.0000262787.88639.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pappo AS, Devidas M, Jenkins J, et al. Phase II trial of neoadjuvant vincristine, ifosfamide, and doxorubicin with granulocyte colony-stimulating factor support in children and adolescents with advanced-stage nonrhabdomyosarcomatous soft tissue sarcomas: a Pediatric Oncology Group Study. J Clin Oncol. 2005;23(18):4031–4038. doi: 10.1200/JCO.2005.03.209. [DOI] [PubMed] [Google Scholar]
- 24.Spurrell EL, Fisher C, Thomas JM, et al. Prognostic factors in advanced synovial sarcoma: an analysis of 104 patients treated at the Royal Marsden Hospital. Ann Oncol. 2005;16(3):437–444. doi: 10.1093/annonc/mdi082. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari A, Gronchi A, Casanova M, et al. Synovial sarcoma: a retrospective analysis of 271 patients of all ages treated at a single institution. Cancer. 2004;101(3):627–634. doi: 10.1002/cncr.20386. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen OS, Judson I, van Hoesel Q, et al. Effect of high-dose ifosfamide in advanced soft tissue sarcomas: a multicentre phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 2000;36(1):61–67. doi: 10.1016/s0959-8049(99)00240-3. [DOI] [PubMed] [Google Scholar]
- 27.Van Glabbeke M, van Oosterom AT, Oosterhuis JW, et al. A European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group Study Prognostic factors for the outcome of chemotherapy in advanced soft tissue sarcoma: an analysis of 2,185 patients treated with anthracycline-containing first-line regimens. J Clin Oncol. 1999;17(1):150–157. doi: 10.1200/JCO.1999.17.1.150. [DOI] [PubMed] [Google Scholar]
- 28.Wilson RE, Wood WC, Lerner HL, et al. Doxorubicin chemotherapy in the treatment of soft-tissue sarcoma. Combined results of two randomized trials. Arch Surg. 1986;121(11):1354–1359. doi: 10.1001/archsurg.121.11.1354. [DOI] [PubMed] [Google Scholar]
- 29.Palmerini E, Staals EL, Alberghini M, et al. Synovial sarcoma: retrospective analysis of 250 patients treated at a single institution. Cancer. 2009;115(13):2988–2998. doi: 10.1002/cncr.24370. [DOI] [PubMed] [Google Scholar]
- 30.Italiano A, Penel N, Robin YM, et al. Neo/adjuvant chemotherapy does not improve outcome in resected primary synovial sarcoma: a study of the French Sarcoma Group. Ann Oncol. 2009;20(3):425–430. doi: 10.1093/annonc/mdn678. [DOI] [PubMed] [Google Scholar]
- 31.Okcu MF, Despa S, Choroszy M, et al. Synovial sarcoma in children and adolescents: thirty three years of experience with multimodal therapy. Med Pediatr Oncol. 2001;37(2):90–96. doi: 10.1002/mpo.1175. [DOI] [PubMed] [Google Scholar]
- 32.Keizer HJ, Crowther D, Nielsen OS, et al. EORTC Group Phase II Study of Oral Etoposide for Pretreated Soft Tissue Sarcoma. Sarcoma. 1997;1(2):99–101. doi: 10.1080/13577149778371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosen G, Forscher C, Lowenbraun S, et al. Synovial sarcoma. Uniform response of metastases to high dose ifosfamide. Cancer. 1994;73(10):2506–2511. doi: 10.1002/1097-0142(19940515)73:10<2506::aid-cncr2820731009>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 34.Kampe CE, Rosen G, Eilber F, et al. Synovial sarcoma. A study of intensive chemotherapy in 14 patients with localized disease. Cancer. 1993;72(7):2161–2169. doi: 10.1002/1097-0142(19931001)72:7<2161::aid-cncr2820720716>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 35.Schöffski P, Dumez H, Wolter P, et al. Clinical impact of trabectedin (ecteinascidin-743) in advanced/metastatic soft tissue sarcoma. Expert Opin Pharmacother. 2008;9(9):1609–1618. doi: 10.1517/14656566.9.9.1609. [DOI] [PubMed] [Google Scholar]
- 36.Garg V, Zhang W, Gidwani P, et al. Preclinical analysis of tasidotin HCl in Ewing’s sarcoma, rhabdomyosarcoma, synovial sarcoma, and osteosarcoma. Clin Cancer Res. 2007;13(18 pt 1):5446–5454. doi: 10.1158/1078-0432.CCR-06-2661. [DOI] [PubMed] [Google Scholar]
- 37.Fayette J, Coquard IR, Alberti L, et al. ET-743: a novel agent with activity in soft-tissue sarcomas. Curr Opin Oncol. 2006;18(4):347–353. doi: 10.1097/01.cco.0000228740.70379.3f. [DOI] [PubMed] [Google Scholar]
- 38.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009;27(19):3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]
- 39.Benesch M, Windelberg M, Sauseng W, et al. Compassionate use of bevacizumab (Avastin) in children and young adults with refractory or recurrent solid tumors. Ann Oncol. 2008;19(4):807–813. doi: 10.1093/annonc/mdm510. [DOI] [PubMed] [Google Scholar]
- 40.Bhargava P, Marshall JL, Dahut W, et al. A phase I and pharmacokinetic study of squalamine, a novel antiangiogenic agent, in patients with advanced cancers. Clin Cancer Res. 2001;7(12):3912–3919. [PubMed] [Google Scholar]
- 41.Zito RA. Synovial sarcoma: an Australian series of 48 cases. Pathology. 1984;16(1):45–52. doi: 10.3109/00313028409067910. [DOI] [PubMed] [Google Scholar]
- 42.Friedrichs N, Küchler J, Endl E, et al. Insulin-like growth factor-1 receptor acts as a growth regulator in synovial sarcoma. J Pathol. 2008;216(4):428–439. doi: 10.1002/path.2438. [DOI] [PubMed] [Google Scholar]
- 43.Dobashi Y, Suzuki S, Sato E, et al. EGFR-dependent and independent activation of Akt/mTOR cascade in bone and soft tissue tumors. Mod Pathol. 2009;22(10):1328–1340. doi: 10.1038/modpathol.2009.104. [DOI] [PubMed] [Google Scholar]
- 44.Ray-Coquard I, Le Cesne A, Whelan JS, et al. A phase II study of gefitinib for patients with advanced HER-1 expressing synovial sarcoma refractory to doxorubicin-containing regimens. Oncologist. 2008;13(4):467–473. doi: 10.1634/theoncologist.2008-0065. [DOI] [PubMed] [Google Scholar]
- 45.Olsen RJ, Lydiatt WM, Koepsell SA, et al. C-erb-B2 (HER2/neu) expression in synovial sarcoma of the head and neck. Head Neck. 2005;27(10):883–892. doi: 10.1002/hed.20267. [DOI] [PubMed] [Google Scholar]
- 46.Olmos D, Postel-Vinay S, Molife LR, et al. Safety, pharmacokinetics, and preliminary activity of the anti-IGF-1R antibody figitumumab (CP-751,871) in patients with sarcoma and Ewing’s sarcoma: a phase 1 expansion cohort study. Lancet Oncol. 2010;11(2):129–135. doi: 10.1016/S1470-2045(09)70354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng CL, Guo W, Ji T, et al. Sorafenib induces growth inhibition and apoptosis in human synovial sarcoma cells via inhibiting the RAF/MEK/ERK signaling pathway. Cancer Biol Ther. 2009;8(18):1729–1736. doi: 10.4161/cbt.8.18.9208. [DOI] [PubMed] [Google Scholar]
- 48.Basso U, Brunello A, Bertuzzi A, et al. Sorafenib is active on lung metastases from synovial sarcoma. Ann Oncol. 2009;20(2):386–387. doi: 10.1093/annonc/mdn685. [DOI] [PubMed] [Google Scholar]
- 49.Albritton KH, Randall RL. Prospects for targeted therapy of synovial sarcoma. J Pediatr Hematol Oncol. 2005;27(4):219–222. doi: 10.1097/01.mph.0000163713.46762.72. [DOI] [PubMed] [Google Scholar]
- 50.Mancuso T, Mezzelani A, Riva C, et al. Analysis of SYT-SSX fusion transcripts and bcl-2 expression and phosphorylation status in synovial sarcoma. Lab Invest. 2000;80(6):805–813. doi: 10.1038/labinvest.3780085. [DOI] [PubMed] [Google Scholar]
- 51.Joyner DE, Albritton KH, Bastar JD, et al. G3139 antisense oligonucleotide directed against antiapoptotic Bcl-2 enhances doxorubicin cytotoxicity in the FU-SY-1 synovial sarcoma cell line. J Orthop Res. 2006;24(3):474–480. doi: 10.1002/jor.20087. [DOI] [PubMed] [Google Scholar]
- 52.Tomek S, Koestler W, Horak P, et al. Trail-induced apoptosis and interaction with cytotoxic agents in soft tissue sarcoma cell lines. Eur J Cancer. 2003;39(9):1318–1329. doi: 10.1016/s0959-8049(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 53.Lubieniecka JM, de Bruijn DR, Su L, et al. Histone deacetylase inhibitors reverse SS18-SSX-mediated polycomb silencing of the tumor suppressor early growth response 1 in synovial sarcoma. Cancer Res. 2008;68(11):4303–4310. doi: 10.1158/0008-5472.CAN-08-0092. [DOI] [PubMed] [Google Scholar]
- 54.Ito T, Ouchida M, Morimoto Y, et al. Significant growth suppression of synovial sarcomas by the histone deacetylase inhibitor FK228 in vitro and in vivo. Cancer Lett. 2005;224(2):311–319. doi: 10.1016/j.canlet.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 55.Su L, Cheng H, Sampaio AV, et al. EGR1 reactivation by histone deacetylase inhibitors promotes synovial sarcoma cell death through the PTEN tumor suppressor. Oncogene. 2010;29(30):4352–4361. doi: 10.1038/onc.2010.204. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen A, Su L, Campbell B, et al. Synergism of heat shock protein 90 and histone deacetylase inhibitors in synovial sarcoma. Sarcoma. 2009;2009:794901. doi: 10.1155/2009/794901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeBry RW, Seldin MF. Human/mouse homology relationships. Genomics. 1996;33(3):337–351. doi: 10.1006/geno.1996.0209. [DOI] [PubMed] [Google Scholar]
- 58.Haldar M, Hedberg ML, Hockin MF, et al. A CreER-based random induction strategy for modeling translocation-associated sarcomas in mice. Cancer Res. 2009;69(8):3657–3664. doi: 10.1158/0008-5472.CAN-08-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haldar M, Hedberg M, Tvrdik P, et al. Bioluminescence based in vivo monitoring of synovial sarcomas in a genetically engineered mouse model. Proc Am Assoc Cancer Res. 2009 Abstract LB-67. [Google Scholar]