Abstract
Background
Low-level light therapy (LLLT) with transcranial laser is a non-invasive form of neuroenhancement shown to regulate neuronal metabolism and cognition. Attention bias modification (ABM) is a cognitive intervention designed to improve depression by decreasing negative attentional bias, but to date its efficacy has been inconclusive. Adjunctive neuroenhancement to augment clinical effectiveness has shown promise, particularly for individuals who respond positively to the primary intervention.
Objective/Hypothesis
This randomized, sham-controlled proof-of-principle study is the first to test the hypothesis that augmentative LLLT will improve the effects of ABM among adults with elevated symptoms of depression.
Methods
Fifty-one adult participants with elevated symptoms of depression received ABM before and after laser stimulation and were randomized to one of three conditions: right forehead, left forehead, or sham. Participants repeated LLLT two days later and were assessed for depression symptoms one and two weeks later.
Results
A significant three-way interaction between LLLT condition, ABM response, and time indicated that right LLLT led to greater symptom improvement among participants whose attention was responsive to ABM (i.e., attention was directed away from negative stimuli). Minimal change in depression was observed in the left and sham LLLT.
Conclusions
The beneficial effects of ABM on depression symptoms may be enhanced when paired with adjunctive interventions such as right prefrontal LLLT; however, cognitive response to ABM likely moderates the impact of neuroenhancement. The results suggest that larger clinical trials examining the efficacy of using photoneuromodulation to augment cognitive training are warranted.
Keywords: Depression, brain stimulation, cognition, lasers, attention bias modification, photobiomodulation
Introduction
Individuals with depression experience biased cognitive processes that are theorized to facilitate the onset and maintenance of their symptoms [1]. Biased attention towards depression-relevant stimuli has been observed in clinical depression [1,2] and has been linked with vulnerability for clinical worsening [3–5]. In recent years, a growing number of techniques have set out to ameliorate negative cognitive biases with the goal of decreasing depression symptomatology.
One such technique is known as attention bias modification (ABM). ABM attempts to shift negatively biased attention in favor of more adaptive patterns of attention [6], which is theorized to decrease the cognitive and affective symptoms associated with depression [7]. ABM has been shown to decrease symptoms of anxiety by decreasing attention for threatening stimuli [8–10]. In depression, however, the results are less consistent. Previous research using ABM in individuals with clinically relevant depression showed greater decreases in symptoms compared to those who received no training [11–13]. However, meta-analyses quantifying the influence of ABM on depression symptoms have shown variable results and small effect sizes [10,14]. Considering the strong theoretical and empirical support linking attention biases to depression, there is a compelling rationale to augment ABM in order to improve its potential efficacy.
Neuroenhancement is a field that utilizes pharmacological or neuromodulatory interventions to improve cognitive capabilities [15]. This technique aims to directly influence cognitive systems either through the activation of adaptive processes or the inhibition of maladaptive ones. Though it is extensively researched in healthy individuals [16,17], neuroenhancement offers considerable promise as an adjunctive clinical intervention, particularly when cognitive systems are implicated in the etiology of the disorder [15,16].
Critically, recent findings suggest that improvement associated with adjunctive neuroenhancement may be contingent on the individual’s response to the primary intervention. In studies using adjunctive neuroenhancement such as d-cycloserine, yohimbine, and methylene blue, clinical improvement at follow-up was greatest for participants who showed the most improvement from exposures [18–21]. In contrast, neuroenhancement may lead to a worsening of clinical outcomes following an unsuccessful treatment session (e.g., an exposure session where end state fear remained high) [20,21]. These findings suggest that neuroenhancement may augment learning that takes place during the intervention, even if the learning was not necessarily therapeutic. As such, indicators of treatment response during the primary intervention should be considered a key moderator when investigating neuroenhancement outcomes.
A promising new option for adjunctive neuroenhancement is low-level light therapy (LLLT) using near-infrared transcranial lasers or light emitting diodes (LEDs). LLLT is a non-invasive intervention shown to regulate neuronal function in cell cultures, animal models, and clinical conditions [22]. The primary photoneuromodulation mechanism of action of LLLT is increased mitochondrial cytochrome oxidase [23], a respiratory enzyme that is commonly reduced in disorders involving cognitive impairment and decreased cognitive reserve [24–28]. Up-regulating cytochrome oxidase through neuroenhancement has been shown to improve oxygenation and metabolic efficiency in the brain [29,30], which stimulate ATP production and facilitate neuronal energy production [31–33]. Consequently, techniques that increase cytochrome oxidase, such as LLLT and methylene blue, have been shown to improve cognition in mice and rats [33,34]as well as in humans [35–37].
Although LLLT has FDA approval for peripheral pain management, it is growing in popularity as a brain research tool [35]. Specifically, LLLT with transcranial laser targeting the right prefrontal cortex has been shown to enhance sustained attention compared to sham LLLT [36], and LLLT with transcranial LEDs targeting the prefrontal cortex has been shown to decrease depression symptoms up to four weeks following treatment, although clinical studies to date have not used a sham control [38]. These findings suggest that LLLT may be an inexpensive, non-invasive, and promising neuroenhancement technique. Considering that hypoactivity in the prefrontal cortex has been associated with negative attentional bias [39–42], and considering that LLLT has been linked to increased cytochrome oxidase activity, improved cognitive function, and decreased negative affect [35,43], there is strong reason to believe that LLLT to the prefrontal cortex could improve the efficacy of ABM.
This proof-of-principle study uses LLLT with transcranial laser stimulation of the prefrontal cortex as an adjunctive neuroenhancement intervention with the goal of improving depression-related ABM outcomes. Participants completed ABM before and after a randomly-assigned session of right prefrontal LLLT, left prefrontal LLLT, or sham LLLT, then provided follow-up depression assessments over the ensuing two weeks. Administering LLLT separately from ABM is logistically necessary since laser safety regulations require participants to close their eyes and wear dark glasses during laser stimulation. However, it also allows us to assess each individual’s responsiveness to ABM independently from the influence of LLLT. Participants with adaptive attention bias change immediately before LLLT were expected to benefit from subsequent neuroenhancement. Such a finding would be consistent with the idea that neuroenhancement augments learning that occurs during the primary intervention and would also provide important information about who is likely to benefit from ABM with LLLT augmentation. This study could be used to lay the groundwork for larger, more robust neuroenhancement trials using clinically depressed participants.
Materials and Methods
Participants
Fifty-one adults with elevated symptoms of depression (31 female, mean age=19.37, SD=3.05) were recruited from the undergraduate research pool at the University of Texas at Austin. Participants who reported active neurological condition (e.g. stroke or epilepsy) were excluded from the study. Participants received course credit in exchange for their participation.
Racial and ethnic distribution in the sample was as follows: 24% Hispanic, 59% Caucasian, 23% Asian, 6% African-American, 4% American Indian or Alaska Native, 2% mixed race, and 6% unspecified. All participants provided written informed consent after receiving a complete description of the study.
Procedure
Participants were recruited based on their score on the Center for Epidemiologic Studies – Depression Scale (CES-D) from a prescreening questionnaire. Participants completed two study sessions scheduled to begin exactly 48 hours apart. The first session began with a CES-D (to confirm CES-D > 16 at the start of the experiment) and a demographic questionnaire, while session two only required the CES-D. In both sessions, participants then completed (in order) a negative bias assessment via the dot-probe task, one block of ABM, the LLLT session, an additional block of ABM, and a final negative bias assessment. Participants then completed follow-up CES-D questionnaires at one and two week intervals following enrollment (see Figure 1 for a depiction of study design).
Figure 1.
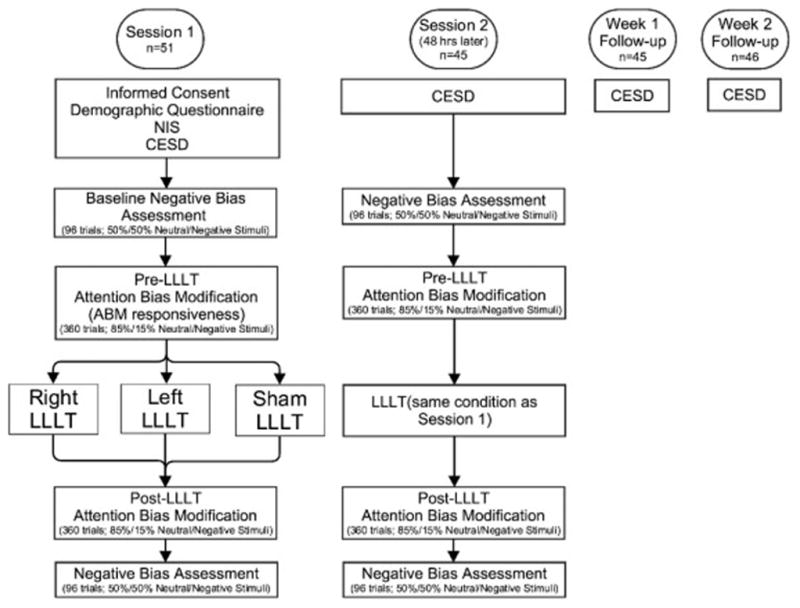
Flowchart of study procedures
LLLT was administered after and before blocks of ABM since the precise timing of optimal neuroenhancement with LLLT is currently unknown. Certain neuroenhancement mechanisms, e.g. transcranial magnetic stimulation, provide peak benefit when administered in advance of or simultaneous to the target task [17]. Other neuroenhancement mechanisms, particularly those that modulate cytochrome oxidase, yield optimal clinical impact when administered after the target task [20,21,43]. Simultaneous ABM/LLLT is unfeasible due to laser safety regulations requiring participants to keep their eyes closed and wear dark glasses during laser stimulation, but the current study design increases the probability of neuroenhancement through one of the proposed mechanisms.
Two participants were unable to attend their second study session but did complete follow-up CES-D questionnaires. As a result, these participants were only included in analyses that use depression symptoms as an outcome measure1. All study procedures and assessments were approved prior to participant enrollment by the Institutional Review Board of the University of Texas at Austin.
Measures
Center for Epidemiologic Studies – Depression Scale (CES-D)
The CES-D [44] is a self-report depression scale comprised of 20 items which assess symptoms over the previous week. A CES-D score of 16 or higher was required for eligibility. This cutoff has been shown to reflect elevated depression symptomatology [45,46] and has been validated as a screener for those at high risk for clinical depression [47]. The CES-D was completed in person at the first and second LLLT sessions, and at home via a secure online server at week 1 and week 2. All participants who completed the first LLLT session were asked to complete follow-up CES-D, regardless of whether they completed the second LLLT session.
New Immigrant Survey (NIS) Skin Color Scale
LLLT is administered by passing light through the skin, meaning that variable light absorption caused by differences in skin color can potentially impact the consistency of dosing. To account for the impact of individual differences, skin color was measured using the NIS skin color scale [48], an 11 point scale of skin color references ranging from 0 to 10, where 0 was total lack of skin color (i.e. albinism) and 10 was the darkest possible skin. The color reference was compared to the back of the participant’s hand to determine the appropriate score. NIS score was used as a covariate in all analyses.
Tasks
Dot Probe Task
Variants of the dot probe task were used to assess negative attention bias and to manipulate attention via ABM. All versions of the task initially presented a fixation cross for 500ms followed by presentation of two words concurrently on the left and right side of visual field on a 20 inch LCD screen. Words were selected from the Affective Norms for English Words (ANEW) list [49]. Each word pair consisted of one neutral (e.g. ship, chair, ink) and one negatively valenced word (e.g. hurt, grief, war), with the location varying randomly. Word pairs were matched for length and frequency of use in the English language. Mean valence strength on a 1–9 scale (where 1 is negative and 9 is positive) was 2.31 for negative words (SD=0.40) and 5.08 for neutral words (SD=0.54). Mean arousal on a 1–9 scale (where 1 is unarousing and 9 is arousing) was 5.70 for negative words (SD=1.14) and 4.26 for neutral words (SD=0.89).
Each word pair was presented for 1000 ms. Following stimulus offset, a probe (either the letter “O” or “Q”) immediately appeared in the same location as one of the word stimuli and remained on the screen until participants responded. Participants identified probe type by pressing as quickly as possible a corresponding button on a dedicated button box. Trials with incorrect responses or reaction times that were faster than 200ms or 2.5 standard deviations greater than each individual’s mean reaction time were excluded from analyses.
Mean reaction times were calculated for four distinct trial types: left sad word/right probe (left/right), right sad word/left probe (right/left), left sad word/left probe (left/left), and right sad word/right probe (right/right). Using these mean reaction times, negative attention bias was calculated using a standard formula for computing attention bias with the dot-probe task: ((left/right RT + right/left RT)−(left/left RT + right/right RT))/2
Negative Bias Assessment
For negative bias assessment, our intent was to measure bias rather than manipulate it. Therefore the probe appeared randomly and with equal frequency (50/50) in place of the negative or neutral word. The negative bias assessment consisted of 96 trials. All participants completed a negative bias assessment at the beginning and end of each session. All bias change models controlled for negative bias measured at the beginning of session 1, which we refer to as baseline negative bias. We also examined change in negative bias from baseline to the end of session 2.
Attention Bias Modification (ABM) & ABM Responsiveness
For each block of ABM, the probe appeared in place of the neutral word for 85% of trials and in place of the sad word for 15% of trials. This probe distribution was designed to promote preferential attention away from sad words and towards neutral words. Within each session, participants completed two blocks of ABM consisting of 360 trials each. One ABM block occurred immediately prior to LLLT administration and one occurred immediately after LLLT. Both ABM blocks contained the same word pairs, though the presentation order was randomized. Participants were allowed to rest briefly (1 min) halfway through each block. Across both sessions participants completed a total of 1440 trials of ABM, a moderate dosage of ABM for depression, which has ranged from 510 [50] to 2688 trials [51] in the published literature.
The use of an 85:15 probe distribution during ABM (i.e., 15% “catch trials”) rather than a 100:0 distribution allowed for the assessment of bias during ABM. This assessment of bias during ABM is referred to as “ABM responsiveness” because it putatively measures each individual’s response to ABM prior to the administration of LLLT. LLLT is expected to be most effective for participants who show greater responsiveness (i.e., attention away from negative stimuli) to ABM. ABM responsiveness was only measured during the first ABM block in session 1, as all other ABM blocks followed the administration of LLLT.
Pairs of neutral-negative stimuli were used to determine specifically whether decreasing attention for negative stimuli was beneficial (increased attention for neutral stimuli is not hypothesized to be therapeutic). This approach has been used in ABM protocols for depression (e.g. [11,50]) and anxiety (e.g. [8,52]). Training attention away from negative and toward positive stimuli would provide two potentially therapeutic mechanisms, and it would be more difficult to determine the locus of the therapeutic efficacy, should it be observed.
Low-Level Light Therapy (LLLT)
An LLLT session involves applying a specific wavelength of light (1064 nanometers) using the CG-5000 high density laser (Cell Gen Therapeutics, Dallas, TX, USA; see Figure 2). The laser aperture has a diameter of 4 centimeters (13.6 cm2 beam area) and a continuous power output of 3.4 watts, resulting in an irradiance (or power density) of 250 milliwatts/cm2 (3,400 mW/13.6 cm2 = 250 mW/cm2) for 4 min (3.4 W × 240 s = 816 J/location) and a cumulative fluence (or energy density) of 60 Joules/cm2 (0.25 W/cm2 × 240 s = 60 J/cm2). These power and energy density parameters are identical to those that have been previously shown to improve cognition and psychological well-being [36,37,53].
Figure 2. Laser system.
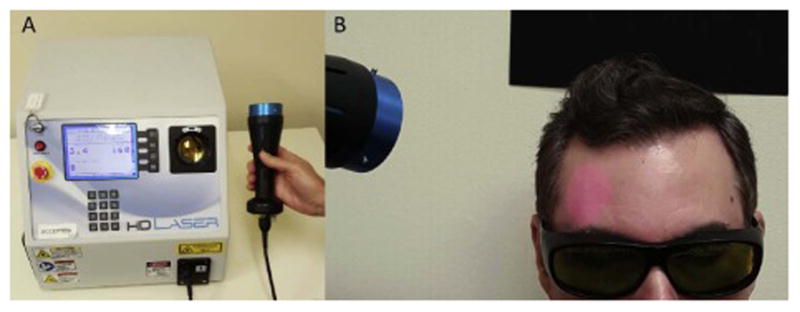
A. The FDA-cleared Class IV laser device (HD Laser, Cell Gen Therapeutics, Dallas, Texas) consisted of a control unit (16″×14″×13″) with a fiber optic cable coupled to a handpiece (4 cm laser beam size). The left part of the unit has on/off controls and multiple safety interlocks, including key and emergency stop. The center part has a screen display and keypad to program the unit output power, number of LLLT administrations (also known as treatment counts), and exposure time. Output is programmable between 100 mW and 20 W, and for this application we used 3.4 W and 8 counts of 60 seconds. On screen messages and instructions confirm correct handling, calibration and use of laser. The right side of the unit has a calibration port that securely locks the handpiece in place while the laser is being calibrated or not in use. Beam output characteristics are continuously monitored while laser is active. B. The handpiece is aimed at the forehead using an internal red diode aiming light. Since the 1064 nm laser is invisible, the beam area provides visual confirmation to facilitate precise tissue targeting. During laser operation, participants are instructed to keep their eyes closed, and experimenters and participants wear dark safety glasses that block the specific infrared wavelengths from reaching the eyes, as required by the laser manufacturer and the University of Texas Laser Safety Program.
At the power level used, this dose is safe, exposure to it is not harmful to tissue, and it causes negligible heat and no physical damage. Due to possible adverse events associated with laser exposure to the eye, participants and laser operators wore protective eyewear during all LLLT sessions and participants were instructed to keep their eyes closed for the duration of stimulation. LLLT was delivered in between ABM sessions since it is theorized to have the greatest impact when administered before or after the target task [33], and since participants were instructed to keep their eyes closed while the laser was in use.
Participants were randomized to receive left active, right active, or right sham LLLT. The randomization distribution was as follows: 18 left active, 18 right active, 15 sham (a priori power analysis, based on effect size f2 of 0.3 and power of 0.95, suggested 48 participants equally distributed across groups). There were no significant differences in age, gender, baseline mood, or ABM responsiveness (all p>0.05; see Table 1). All sessions consisted of 8 consecutive minute-long applications of LLLT, which alternated between the medial (4 min) and lateral (4 min) parts of the left or right side of the forehead (depending on condition).
Table 1. Participant demographics by group.
Participants were randomized to receive sham LLLT, right active LLLT, or left active LLLT. There were no significant differences in gender, age, baseline depression symptoms (measured using the CES-D), or ABM responsiveness across groups. There was a significantly higher rate of medication usage in the sham condition relative to left LLLT and marginally higher rate relative to right LLLT. As a result, medication status was controlled for in all subsequent analyses.
| Sham | Right LLLT | Left LLLT | |
|---|---|---|---|
| Randomized | 15 | 18 | 18 |
| Male/Female | 5/10 | 6/12 | 9/9 |
| Age; Mean (SD) | 20.33 (5.29) | 18.72 (0.89) | 19.22 (1.47) |
| % Medicated | 46.67% | 16.67%† | 11.11% * |
| Baseline CES-D; Mean (SD) | 32.2 (10.54) | 29.61 (9.49) | 27.17 (8.50) |
| ABM Responsiveness; Mean (SD) | −1.20 ms (19.00) | 3.40 ms (12.81) | −2.83 ms (22.88) |
p<0.1 vs. sham
p<0.05 vs. sham
For the active conditions, participants received LLLT for the full 60 seconds per block. For the sham condition, participants received LLLT to the right side for the first 5 seconds before the operator covertly disabled the power while keeping the laser in place for the final 55 seconds. These parameters are comparable to previous LLLT research [36,37]. Upon being prompted after two weeks to guess whether they received either active or sham LLLT, 43% of participants guessed correctly, which did not significant differ from chance (p>0.05). There was no significant difference across groups, with 38.46% of the sham group, 41.18% of the right LLLT group, and 31.25% of the left LLLT group guessing that they received active LLLT. However, the duration of time that had elapsed between the guess and the final LLLT session may have decreased its reliability.
Psychotropic Medication Usage
Participants were permitted to be using psychotropic medication, provided that they had been on a stable dose for at least two months prior to enrollment. Thirty-nine participants (76%) were unmedicated and no participants adjusted their psychiatric medication during their two weeks of follow-up. Amongst medicated participants, the most commonly prescribed family of medications was selective serotonin reuptake inhibitors (e.g. fluoxetine; 8 participants), followed by mood stabilizers (e.g. lamotrigine; 4 participants), atypical antidepressants (e.g. bupropion; 2 participants), and anxiolytics (e.g. alprazolam; 2 participants). Randomized group assignments resulted in the sham group having significantly more medicated participants than the left LLLT group (p=0.03), and marginally more than the right LLLT group (p=0.07). To address this imbalance, all subsequent analyses controlled for current psychotropic medication use.
Statistical Analysis
Mixed effect regression models were used to predict change in depression symptoms. The first assessed the three-way interaction between LLLT condition (active left, active right, or sham), ABM responsiveness (negative bias during the first ABM block), and time (days from baseline) when predicting CES-D score while controlling for baseline negative attention bias. Controlling for baseline negative attention bias allowed for a more interpretable assessment of the shift between baseline and ABM attentional bias levels. When the three-way interaction was significant, it was broken down into three models assessing the two-way interaction between ABM responsiveness and time for each of the three LLLT conditions.
Results
Sample attrition
Of the 51 participants to complete session 1 (15 sham, 18 right active LLLT, 18 left active LLLT), 45 completed session 2 (12 sham, 16 right, 17 left), 45 completed the one week follow-up (13 sham, 16 right, 16 left), and 46 completed the two week follow-up (13 sham, 17 right, 16 left).
Effects of combined ABM and LLLT
We first examined whether depression symptoms changed over time. Mean CES-D score at baseline was 29.51 (SD=9.51). Controlling for medication use, CES-D score decreased significantly, approximately 3.5 points on the CES-D per week, β=−0.50, SE=0.10, p<0.01, Cohen’s f2=0.150, across the 14 day follow-up period.
We next examined whether LLLT condition and responsiveness to ABM moderated the effect of time on depression symptom change. There was a significant three-way interaction between LLLT condition, ABM responsiveness, and time for the prediction of CES-D score, F(2, 47)=4.31, p=0.01, Cohen’s f2=0.062 (baseline negative attention bias was included as a covariate). To understand the form of this three-way interaction, three subsequent models examined the two-way interactions between ABM responsiveness and time for each of the three LLLT conditions.
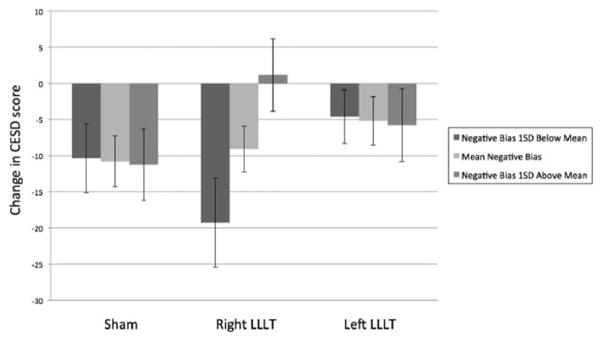
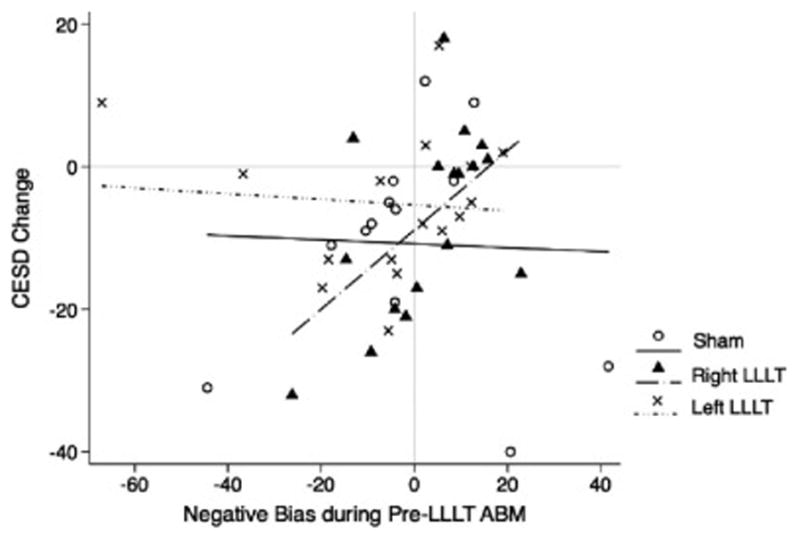
For the right prefrontal LLLT group, there was a significant two-way interaction between ABM responsiveness and time, F(1,16)=8.10, p<0.01, Cohen’s f2=0.158. Specifically, individuals with a stronger bias away from negative words during ABM prior to LLLT showed greater subsequent symptom improvement than individuals with no bias or bias towards negative words. That is, in the right prefrontal LLLT condition, those with a stronger response to ABM prior to LLLT benefited most from LLLT (see Figure 3 for observed outcomes and figure 4 for predicted outcomes at varying levels of ABM responsivity). This finding suggests that right LLLT neuroenhancement was particularly helpful for people who were responsive to ABM, but was not helpful to those who did not show an initial improvement in attention bias.
Figure 3.
Interaction between LLLT condition, negative bias during pre-LLLT ABM, and mood change
Note: All presented models used time as a predictor in the interaction. However, for the sake of clarity, figures 3 and 4 use CES-D change (CES-D score at 2 week follow-up minus baseline CES-D) in order to represent the effect of time on mood.
Figure 4.
Predicted CES-D change from baseline to two-week follow-up based on LLLT condition and negative attention bias during pre-LLLT ABM session
The ABM responsiveness and time interaction was not significant for sham LLLT, F(1,13)=0.84, p=0.36, Cohen’s f2=0.016, or left LLLT, F(1,16)=0.05, p=0.82, Cohen’s f2=0.001. The regression lines for left and sham LLLT are relatively flat (Figure 3), suggesting that ABM responsiveness did not influence the effect of LLLT on subsequent symptom change. Comparisons of the interactions indicated that the ABM responsiveness x time interaction for right LLLT was significantly stronger than sham LLLT, β=−0.05, SE=0.02, p<0.01, and left LLLT, β=−0.04, SE=0.02, p=0.01. There was no difference between the interaction between sham and left LLLT, β=0.01, SE=0.01, p=0.50. Depression symptom improvement in left and sham was not dependent upon initial responsiveness to ABM.
Finally, we examined whether the change in depression symptoms coincided with a comparable change in negative bias across the two treatment sessions. Mean negative bias at baseline was 1.63 ms (SD=24.03 ms). Baseline negative bias was not significantly different across LLLT groups, F(2,46)=1.88, p=0.16, η2=0.075. For the entire sample, there was a marginal decrease in negative bias following session 2, β=−9.24, SE=4.84, p=0.06, to a mean negative bias of −7.71 ms (SE=29.59 ms). Using analyses parallel to those used for mood change, there was no significant interaction between LLLT condition, ABM responsiveness, and time when comparing baseline to post-session 2, F(2, 40)=0.39, p=0.67, Cohen’s f2=0.022. Similarly, the interaction between LLLT condition and time (without including ABM responsiveness) was also non-significant, F(2, 40)=1.57, p=0.21, Cohen’s f2=0.080. ABM responsiveness did significantly predict negative bias at the end of session 1, F(1,48)=6.91, p<0.01, Cohen’s f2=0.068, but did not predict negative bias at the end of session 2, F(1,41)=0.10, p=0.75, Cohen’s f2=0.008. This finding suggests that the marginal change in negative bias that occurred following two sessions of ABM and LLLT was not influenced by LLLT condition or ABM responsiveness.
High Influence Observation
In order to identify data points whose leverage and residual may unduly influence the model predicting mood change, Cook’s d was calculated for each observation. Based on the convention of excluding observations with d>1 [54], one participant was dropped from the left LLLT condition. In Figure 3, this data point is the outlier on the far left of the figure. With this observation removed, the three-way interaction between LLLT condition, ABM responsiveness, and time remained significant, F(2, 46) = 4.22, p=0.01, Cohen’s f2=0.061. In the left LLLT condition, the two-way interaction between ABM responsiveness and time remained not significant, F(1, 15) = 1.73, p=0.18, Cohen’s f2=0.033, although the effect was stronger than observed in the full sample and more closely resembled the effect observed in right LLLT. Further, the two-way interaction for the left LLLT condition was no longer significantly different from the same two-way interaction in the right LLLT condition, β=0.02, SE=0.02, p=0.18, although the comparison was generally consistent with that observed in the full sample. There is still no difference between the interactions for the left LLLT and sham conditions, β=−0.02, SE=0.02, p=0.12.
Discussion
Findings from this proof-of-principle study indicate that LLLT augmentation of ABM was dependent on the location of the stimulation and whether participants displayed an initial response to ABM. Specifically, right prefrontal LLLT stimulation leads to a greater reduction of depression symptoms in participants with better response to ABM compared to sham and also compared to participants who did not respond to ABM. These findings suggest that right LLLT facilitates treatment outcome following ABM, but not sham or left prefrontal LLLT, neither of which showed an interaction with ABM responsiveness.
The relationship between right LLLT, ABM responsiveness, and depression symptoms is consistent with the “for better or worse” pattern of adjunctive neuroenhancement seen in the treatment of anxiety using d-cycloserine, yohimbine, and methylene blue. Specifically, for all four neuroenhancers, increases in clinical improvement are contingent upon the successful response to the primary intervention prior to the onset of the enhancer, while unsuccessful response to the primary intervention led to worse clinical outcomes [18,20,21]. One possible mechanism for this response is that increased right anterior prefrontal activity has been associated with improvements in reappraisal following cognitive-behavioral therapy [55]. It is plausible that enhancement of this region by LLLT helps participants receiving cognitive interventions (in this case, ABM) more effectively utilize adaptive regulatory strategies such as reappraisal.
The recruitment of adaptive regulatory strategies could also help to explain why the impact of LLLT and ABM on mood change was not accompanied by a significant impact on negative bias. Specifically, it is possible that the augmentative effect of right LLLT is on the appraisal of incoming stimuli, rather than on the attentional orientation towards or away from incoming stimuli. It is also possible that the early change in bias indicated by higher ABM responsivity may reflect a greater sensitivity to environmental manipulation, which contributes to a stronger beneficial impact of right LLLT. Future research would benefit from more focused and sensitive assessments of cognitive and affective systems before and after the intervention to better explore the mechanisms of improvement.
These results have the potential to contribute to a new and improved formulation for personalized psychological care and may provide a guideline for identifying who is most likely to benefit from neuroenhancement treatment. Specifically, a Sequential Multiple Assignment Randomized Trial (SMART) study design [56,57] would personalize treatment by measuring ABM responsiveness in real time and administering LLLT (or equivalent neuroenhancement) only for participants who demonstrate a positive response to ABM. Positive ABM responsiveness could therefore be considered an indication for adjunctive neuroenhancement, which would help identify patients with greater chance of seeing optimal improvement. Such an approach would be consistent with the development of precision medicine interventions.
These results may help explain the inconsistent findings related to ABM in depression [10,11,13,14]. The impact of ABM on clinical symptoms likely depends on a host of factors that modulate the extent of learning and generalization of the participant experiences. The current findings suggest that prefrontal metabolism may play a causal role in the response (or lack thereof) to ABM in depression. Similar adjunctive neuroenhancement using transcranial direct current stimulation in participants with anxiety has shown that stimulating the left dorsolateral prefrontal cortex during ABM leads to greater modification of attention bias for threat stimuli compared to sham [58]. Together with the current study, these results indicate that ABM efficacy can be improved by augmenting prefrontal functioning.
Limitations of the current study include the relatively short follow-up period. There is significant heterogeneity in the naturalistic course of depression symptoms [59] and limiting the follow-up to only two weeks may not be capturing the full extent of symptom change. Furthermore, attention bias was not assessed during the follow-up period, making it difficult to determine whether changes in attention bias might serve as a mediator for clinical improvement. Future iterations of this study would benefit by expanding the follow-up period to at least six months while collecting mood and cognition assessments at various points during that time. This would allow us to plot the trajectory of bias change relative to mood change, which would facilitate modeling of the interaction between these systems. Finally, the current study recruited a convenient sample based on elevated symptoms of depression, but did not conduct comprehensive diagnostic assessments to determine which participants met criteria for clinical depression (i.e. major depressive disorder; MDD) and which were subclinical. Given the positive signal from this small trial, future tests should recruit individuals who meet criteria for MDD from the community.
Conclusions
The results from this proof-of-principle study serve as a promising indicator that neuroenhancement could be used to augment the benefits of bias modification techniques such as ABM in depression. LLLT, which has been shown to increase cytochrome oxidase activity and improve sustained attention [23,30,35,36], appears to enhance learning acquired during ABM. Future LLLT research can build on this finding in numerous domains, including other forms of bias modification (e.g. ABM for anxiety, interpretation bias modification for depression, cognitive remediation for schizophrenia, etc.) and other learning-based clinical protocols. Adjunctive neuroenhancement should ideally be applied after the patient displays therapeutic change during the primary intervention. Although research that pairs cognitive interventions with neuroenhancers is still in its infancy, these results suggest that it is a promising and important direction for further study, with the potential to improve treatment matching and clinical outcomes for the treatment of depression and other disorders.
Highlights.
LLLT using transcranial laser stimulation is a novel neuromodulatory technique.
LLLT can improve cognition, suggesting utility as adjunctive neuroenhancement.
Sham-controlled LLLT was applied to augment ABM in depressed adults.
Right LLLT led to greater improvement in those who showed initial response to ABM.
Left LLLT and sham LLLT showed no impact on clinical symptoms.
Acknowledgments
Research reported in this publication was supported by the National Institute of Health (grant R21MH092430 to CGB) and the National Institute on Drug Abuse (grant R01DA032457 to CGB). FGL gratefully acknowledges support from an institutional research fellowship from the College of Liberals Arts of the University of Texas at Austin. FGL holds the George I. Sanchez Centennial Endowed Professorship in Liberal Arts and Sciences and CGB is the Wayne H. Holtzman Regents Chair in Psychology. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Removal of these participants would not have significantly impacted the direction or significance of the subsequent analyses.
ClinicalTrials.gov registration
Study: Behavioral Study of Effects of Low-Level Light Therapy on Mood and Reaction Time Identifier: NCT02390076
URL: clinicaltrials.gov/ct2/show/NCT02390076
Financial Disclosures
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beck AT. Cognitive Models of Depression. The Journal of Cognitive Psychotherapy: an International Quarterly. 1987;1:5–37. [Google Scholar]
- 2.Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113:121–35. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- 3.Beevers CG, Carver CS. Attentional bias and mood persistence as prospective predictors of dysphoria. Cognitive Therapy and Research. 2003;27:619–37. [Google Scholar]
- 4.Ingram RE, Miranda J, Segal Z. Cognitive Vulnerability to Depression. New York: The Guilford Press; 1998. [Google Scholar]
- 5.McCabe SB, Gotlib IH, Martin RA. Cognitive Vulnerability for Depression: Deployment of Attention as a Function of History of Depression and Current Mood State. Cognitive Therapy and Research. 2000;24:427–44. doi: 10.1023/A:1005579719849. [DOI] [Google Scholar]
- 6.Koster EHW, Fox E, MacLeod C. Introduction to the special section on cognitive bias modification in emotional disorders. J Abnorm Psychol. 2009;118:1–4. doi: 10.1037/a0014379. [DOI] [PubMed] [Google Scholar]
- 7.MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. J Abnorm Psychol. 2002;111:107–23. doi: 10.1037/0021-843X.111.1.107. [DOI] [PubMed] [Google Scholar]
- 8.Amir N, Weber G, Beard C, Bomyea J, Taylor CT. The effect of a single-session attention modification program on response to a public-speaking challenge in socially anxious individuals. Journal of Abnormal Psychology. 2008;117:860–8. doi: 10.1037/a0013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amir N, Bomyea J, Beard C. The effect of single-session interpretation modification on attention bias in socially anxious individuals. Journal of Anxiety Disorders. 2010;24:178–82. doi: 10.1016/j.janxdis.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychol Bull. 2011;137:940–58. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- 11.Wells TT, Beevers CG. Biased attention and dysphoria: Manipulating selective attention reduces subsequent depressive symptoms. Cognition & Emotion. 2010;24:719–28. [Google Scholar]
- 12.Yang W, Ding Z, Dai T, Peng F, Zhang JX. Attention Bias Modification training in individuals with depressive symptoms: A randomized controlled trial. Journal of Behavior Therapy and Experimental Psychiatry. 2014 doi: 10.1016/j.jbtep.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Baert S, De Raedt R, Schacht R, Koster EH. Attentional bias training in depression: Therapeutic effects depend on depression severity. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41:265–74. doi: 10.1016/j.jbtep.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Mogoaşe C, David D, Koster EHW. Clinical Efficacy of Attentional Bias Modification Procedures: An Updated Meta-Analysis. J Clin Psychol. 2014:n/a–n/a. doi: 10.1002/jclp.22081. [DOI] [PubMed] [Google Scholar]
- 15.Clark VP, Parasuraman R. Neuroenhancement: Enhancing brain and mind in health and in disease. Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.08.071. [DOI] [PubMed] [Google Scholar]
- 16.Normann C, Nissen C, Frase L. Neuroenhancement strategies for psychiatric disorders: rationale, status quo and perspectives. European Archives of Psychiatry and Clinical Neuroscience. 2012;262:113–6. doi: 10.1007/s00406-012-0356-1. [DOI] [PubMed] [Google Scholar]
- 17.Luber B, Lisanby SH. Enhancement of human cognitive performance using transcranial magnetic stimulation (TMS) Neuroimage. 2013 doi: 10.1016/j.neuroimage.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smits JAJ, Rosenfield D, Davis ML, Julian K, Handelsman PR, Otto MW, et al. Yohimbine Enhancement of Exposure Therapy for Social Anxiety Disorder: A Randomized Controlled Trial. Biological Psychiatry. 2014;75:840–6. doi: 10.1016/j.biopsych.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Smits JAJ, Rosenfield D, Otto MW, Marques L, Davis ML, Meuret AE, et al. d-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. Journal of Psychiatric Research. 2013;47:1455–61. doi: 10.1016/j.jpsychires.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smits JA, Rosenfield D, Otto MW, Powers MB, Hofmann SG, Telch MJ, et al. D-Cycloserine enhancement of fear extinction is specific to successful exposure sessions: evidence from the treatment of height phobia. Bps. 2013;73:1054–8. doi: 10.1016/j.biopsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Telch MJ, Bruchey AK, Rosenfield D, Cobb AR, Smits J, Pahl S, et al. Effects of post-session administration of methylene blue on fear extinction and contextual memory in adults with claustrophobia. American Journal of Psychiatry. 2014;171:1091–8. doi: 10.1176/appi.ajp.2014.13101407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eells JT, Wong-Riley MTT, VerHoeve J, Henry M, Buchman EV, Kane MP, et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559–67. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 23.Wong-Riley MTT, Liang HL, Eells JT, Chance B, Henry MM, Buchmann E, et al. Photobiomodulation Directly Benefits Primary Neurons Functionally Inactivated by Toxins. J Biol Chem. 2005;280:4761–71. doi: 10.1074/jbc.M409650200. [DOI] [PubMed] [Google Scholar]
- 24.Parker WD. Cytochrome Oxidase Deficiency in Alzheimer’s Diseasea. Ann N Y Acad Sci. 1991;640:59–64. doi: 10.1111/j.1749-6632.1991.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 25.Swerdlow RH. Pathogenesis of Alzheimer’s disease. Clinical Interventions in Aging. 2007 [PMC free article] [PubMed] [Google Scholar]
- 26.Mutisya EM, Bowling AC, Beal MF. Cortical Cytochrome Oxidase Activity Is Reduced in Alzheimer’s Disease. J Neurochem. 1994;63:2179–84. doi: 10.1046/j.1471-4159.1994.63062179.x. [DOI] [PubMed] [Google Scholar]
- 27.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu W, Yu J-T, Tan M-S, Tan L. Cognitive Reserve and Alzheimer’s Disease. Mol Neurobiol. 2014;51:187–208. doi: 10.1007/s12035-014-8720-y. [DOI] [PubMed] [Google Scholar]
- 29.Tian F, Hase SN, Gonzalez-Lima F, Liu H. Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg Med. 2016:n/a–n/a. doi: 10.1002/lsm.22471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rojas JC, Bruchey AK, Gonzalez-Lima F. Low-level light therapy improves cortical metabolic capacity and memory retention. Journal of Alzheimer’s Disease. 2012 doi: 10.3233/JAD-2012-120817. [DOI] [PubMed] [Google Scholar]
- 31.Lapchak PA, De Taboada L. Transcranial near infrared laser treatment (NILT) increases cortical adenosine-5′-triphosphate (ATP) content following embolic strokes in rabbits. Brain Res. 2010 doi: 10.1016/j.brainres.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Uozumi Y, Nawashiro H, Sato S, Kawauchi S, Shima K, Kikuchi M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg Med. 2010;42:566–76. doi: 10.1002/lsm.20938. [DOI] [PubMed] [Google Scholar]
- 33.Rojas JC, Gonzalez-Lima F. Neurological and psychological applications of transcranial lasers and LEDs. Biochemical Pharmacology. 2013;86:447–57. doi: 10.1016/j.bcp.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Michalikova S, Ennaceur A, van Rensburg R, Chazot PL. Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: Effects of low infrared light. Neurobiology of Learning and Memory. 2008;89:480–8. doi: 10.1016/j.nlm.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Lima F, Barrett DW. Augmentation of cognitive brain functions with transcranial lasers. Front Syst Neurosci. 2014:8. doi: 10.3389/fnsys.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrett DW, Gonzalez-Lima F. Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience. 2013;230:13–23. doi: 10.1016/j.neuroscience.2012.11.016. [DOI] [PubMed] [Google Scholar]
- 37.Blanco NJ, Maddox WT, Gonzalez-Lima F. Improving executive function using transcranial infrared laser stimulation. J Neuropsychol. 2015:n/a–n/a. doi: 10.1111/jnp.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassano P, Cusin C, Mischoulon D, Hamblin MR. Near-Infrared Transcranial Radiation for Major Depressive Disorder: Proof of Concept Study. Psychiatry …. 2015 doi: 10.1155/2015/352979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beevers CG, Clasen P, Stice E, Schnyer D. Depression symptoms and cognitive control of emotion cues: a functional magnetic resonance imaging study. 2010;167:97–103. doi: 10.1016/j.neuroscience.2010.01.047. S0306-4522(10)00095-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Disner SG, Beevers CG, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–77. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- 41.Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biol Psychiatry. 2008;63:377–84. doi: 10.1016/j.biopsych.2007.06.012. S0006-3223(07)00565-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Soc Cogn Affect Neurosci. 2009;4:387–98. doi: 10.1093/scan/nsp029. nsp029 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rojas JC, Bruchey AK, Gonzalez-Lima F. Neurometabolic mechanisms for memory enhancement and neuroprotection of methylene blue. Progress in Neurobiology. 2012;96:32–45. doi: 10.1016/j.pneurobio.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- 45.Comstock GW, Helsing KJ. Symptoms of depression in two communities. Psychol Med. 1976;6:551–63. doi: 10.1017/s0033291700018171. [DOI] [PubMed] [Google Scholar]
- 46.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. American Journal of Epidemiology. 1977;106:203–14. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 47.Lewinsohn PM, Hoberman HM, Rosenbaum M. A prospective study of risk factors for unipolar depression. 1988;97:251–64. doi: 10.1037//0021-843x.97.3.251. [DOI] [PubMed] [Google Scholar]
- 48.Massey D, Martin JA. The NIS skin color scale. Office of Population Research, Princeton University; 2003. [Google Scholar]
- 49.Bradley MM, Lang PJ. Affective norms for English words (ANEW): Instruction manual and affective ratings. 1999. [Google Scholar]
- 50.Tsumura H, Shimada H, Nomura K, Sugaya N, Suzuki K. The effects of attention retraining on depressive mood and cortisol responses to depression-related stimuli1. Japanese Psychological Research. 2012;54:400–11. doi: 10.1111/j.1468-5884.2012.00523.x. [DOI] [Google Scholar]
- 51.Browning M, Holmes EA, Charles M, Cowen PJ, Harmer CJ. Using Attentional Bias Modification as a Cognitive Vaccine Against Depression. Biological Psychiatry. 2012;72:572–9. doi: 10.1016/j.biopsych.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amir N, Beard C, Burns M, Bomyea J. Attention modification program in individuals with generalized anxiety disorder. Journal of Abnormal Psychology. 2009;118:28. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schiffer F, Johnston AL, Ravichandran C, Polcari A, Teicher MH, Webb RH, et al. Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct. 2009;5:46. doi: 10.1186/1744-9081-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cook RD, Weisberg S. Residuals and influence in regression. 1982. [Google Scholar]
- 55.Goldin PR, Ziv M, Jazaieri H, Weeks J, Heimberg RG, Gross JJ. Impact of cognitive-behavioral therapy for social anxiety disorder on the neural bases of emotional reactivity to and regulation of social evaluation. Behaviour Research and Therapy. 2014;62(IS):97–106. doi: 10.1016/j.brat.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lei H, Nahum-Shani I, Lynch K, Oslin D, Murphy SA. A “SMART” Design for Building Individualized Treatment Sequences. Annu Rev Clin Psychol. 2012;8:21–48. doi: 10.1146/annurev-clinpsy-032511-143152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murphy SA, Collins LM, Rush AJ. Customizing treatment to the patient: Adaptive treatment strategies. Drug and Alcohol Dependence. 2007;88:S1–S3. doi: 10.1016/j.drugalcdep.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clarke PJF, Browning M, Hammond G, Notebaert L, MacLeod C. The Causal Role of the Dorsolateral Prefrontal Cortex in the Modification of Attentional Bias: Evidence from Transcranial Direct Current Stimulation. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Posternak MA, Miller I. Untreated short-term course of major depression: a meta-analysis of outcomes from studies using wait-list control groups. Journal of Affective Disorders. 2001;66:139–46. doi: 10.1016/S0165-0327(00)00304-9. [DOI] [PubMed] [Google Scholar]