Abstract
Background
Deep brain stimulation (DBS) of the anterior thalamic nucleus (ATN) exerts its effects by modulating neural circuits involved in seizures. However, these networks remain incompletely characterized.
Objective
Investigate the effects of ATN DBS on network activity in a large animal model using 3-T fMRI.
Methods
Anesthetized swine underwent ATN DBS using stimulation parameters applied in the Stimulation of the Anterior Thalamus for the Treatment of Epilepsy (SANTE) trial. Stimulation amplitude, frequency, and temporal paradigm were varied and the resulting blood oxygen level-dependent signal was measured.
Results
ATN DBS resulted in activation within temporal, prefrontal, and sensorimotor cortex. An amplitude-dependent increase in cluster volume was observed at 60 Hz and 145 Hz stimulation.
Conclusion
ATN DBS in swine induced parameter-dependent activation in cortical regions including but not limited to the Papez circuit. These findings may hold clinical implications for treatment of epilepsy in patients with temporal or extratemporal seizure foci.
Keywords: deep brain stimulation, anterior thalamic nucleus, epilepsy, fMRI
Introduction
While surgical resection of epileptogenic brain regions is the first option for treatment-refractory epilepsy patients, an estimated 40% of patients with localized epilepsy are not surgical candidates due to eloquence of the epilepsy onset zone. Increasingly, deep brain stimulation (DBS) is being considered as an alternative option, as it can modulate the epileptic network and is non-ablative. The anterior thalamic nucleus (ATN) is a DBS target in epilepsy due to its established connectivity within the Papez circuit and its widespread thalamocortical projections. The multicenter randomized double-blind Stimulation of the Anterior Nucleus of the Thalamus (SANTE) trial was recently conducted [1], in which ATN DBS resulted in a 40% decline in median seizure frequency after the blinded phase, and a 69% reduction after five years of unblinded follow-up [2]. While the mechanisms that mediate this therapeutic effect are not completely understood, it is known that ATN DBS exerts its effects, at least in part, by modulating mesial temporal circuitry. The anterior thalamus connects with hippocampus, parahippocampal gyrus and entorhinal cortex by way of both the cingulum bundle and the mammillothalamic tract and fornix: a network known as the Papez Circuit [3]. Indeed, both functional neuroimaging [4] and electrophysiological data [5] support the notion that DBS works by modulating networks distal to the site of stimulation, rather than simply inducing a local functional lesion. Our group has recently used functional magnetic resonance imaging (fMRI) in a swine model to investigate DBS in two other brain regions that have been targeted to treat epilepsy, the subthalamic nucleus [6] and centromedian nucleus of the thalamus [7]. Here, we used fMRI in swine implanted with ATN DBS to map the networks that may mediate the effects of this treatment.
Material and Methods
All study procedures were performed in accordance with the National Institutes of Health Guidelines for Animal Research and approved by Mayo Clinic Institutional Animal Care and Use Committee. The subject group consisted of three normal domestic male swine (30 ± 3 kg). An MR image-guided Leksell stereotactic targeting system (Elekta, Stockholm, Sweden) modified for large animals was used for DBS electrode targeting and implantation [6]. Imaging was conducted by a 3-Tesla MR scanner (Signa HDx, General Electric, Fairfield, Connecticut) with a custom, in-house designed radiofrequency coil (Mayo Clinic, Rochester, Minnesota) [6]. Subjects were implanted with a quadripolar (contacts labeled 0, 1, 2, and 3) DBS electrode (Model 3389, Medtronic, Minneapolis, Minnesota). The electrode contacts were positioned such that contacts 0 and 1 were located within the left ATN on the basis of the pig atlas (Supplementary Figure 1A) [8], with contact 0 residing in the same coronal plane as the mammillothalamic tract (Supplementary Figure 1C). The location of the electrode was confirmed by a postsurgical computed tomography (Dual Source Somatom Definition, Siemens, Munich, Germany) scan (image resolution: 0.3×0.3×0.3 mm), which was co-registered to the pre-MRI magnetization prepared rapid acquisition gradient-echo (MPRAGE) scan (Supplementary Figure 1B,C).
The fMRI experiment for each animal consisted of seven conditions (one run per condition) in which the stimulation frequency and amplitude were varied, with a 10 minute rest interval between each condition. During each run, unilateral bipolar stimulation (0-1+) was applied in a block paradigm (5 consecutive blocks of 6 sec ON, 1 min OFF) at 145 Hz, or 60 Hz; 90 μsec pulse-width; 2 V, 5 V, or 8 V. In addition, the temporal cycling paradigm used in the SANTE trial (145 Hz, 5 V, 90 μsec, 1 min ON, 5 min OFF) was applied in a single fMRI run consisting of two stimulation blocks. The multi-subject dataset was preprocessed and analyzed with general linear model using previously described methods [6].
Results
ATN DBS at identical stimulation parameters to those used in the SANTE trial (5V 145Hz 90us) resulted in significant blood oxygen level-dependent (BOLD) activation in a range of ipsilateral structures within the Papez circuit, including cingulate cortex, anterior thalamus, amygdala, and hippocampus. (Figure 1A, Table 1). In addition, other regions of cortex including insula, prefrontal, premotor, motor, and somatosensory cortex were activated. Subcortical regions of activation included ipsilateral putamen, nucleus accumbens, thalamus, and lateral habenula. Stimulation using these same parameters but with the temporal cycling paradigm used in the SANTE trial (1 minute on, 5 minutes off) resulted in similar patterns of activation across the Papez circuit, prefrontal, and eloquent cortical regions (Figure S2, Table S1).
Figure 1.
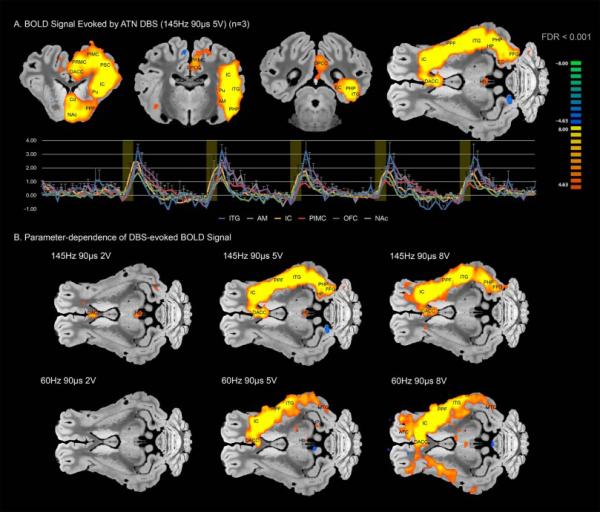
A) Multi-subject functional activation map (n=3) normalized with respect to a 3D pig brain template [9] showing areas activation resulting from ATN stimulation at 145Hz, 90μs, and 5V. Time course blood oxygen level-dependent (BOLD) signal data from select brain regions is shown below. B) Multi-subject functional activation maps showing amplitude- (2V, 5V, and 8V) and frequency-dependence of DBS-evoked BOLD signal. Abbreviations: AFC: anterior frontal cortex; AM: amygdala; Cd: caudate; DACC: dorsal anterior cingulate cortex; DPCC: dorsal posterior cingulate cortex; EC: entorhinal cortex; FFG: fusiform gyrus; Hb: habenular nucleus; HP: hippocamus; IC: insular cortex; ITG: inferior temporal gyrus; MTG: middle temporal gyrus; NAc: nucleus accumbens; PHP: parahippocampal cortex; PIMC: primary motor cortex; PPF: prepyriform area; PRMC: premotor cortex; PSC: primary somatosensory cortex; Pu: putamen
Table 1.
| Peak coordinate (mm) | |||||
|---|---|---|---|---|---|
| Average T-statistic | Structure | Cluster Size (mm3) | x | y | z |
| 9.73 | Insula (I) | 2122 | −16 | 18 | 9 |
| 9.65 | Putamen (I) | 777 | −11 | 18 | 5 |
| 8.79 | Inferior temporal gyrus (I) | 564 | −20 | 2 | 4 |
| 8.09 | Somatosensory association (I) | 260 | −17 | 6 | 16 |
| 8.01 | Orbitofrontal (I) | 217 | −1 | 20 | 2 |
| 7.76 | Parahippocampal gyrus (I) | 814 | −18 | −3 | 2 |
| 7.74 | Amygdala (I) | 577 | −14 | 10 | 1 |
| 7.68 | Primary motor (I) | 1044 | −8 | 18 | 20 |
| 7.59 | Premotor (I) | 896 | −5 | 20 | 17 |
| 7.48 | Nucleus accumbens (I) | 510 | −1 | 20 | −5 |
| 7.08 | Dorsal anterior cingulate (I) | 812 | −2 | 24 | 10 |
| 7.07 | Primary somatosensory (I) | 555 | −15 | 24 | 18 |
| 6.96 | Auditory (I) | 453 | −19 | 2 | 13 |
| 6.8 | Anterior prefrontal (I) | 286 | −2 | 27 | −5 |
| 6.41 | Hippocampus (I) | 295 | −12 | −4 | 5 |
| 6.25 | Globus pallidus (I) | 149 | −7 | 17 | −1 |
| 6.17 | Dorsolateral prefrontal (I) | 210 | −6 | 31 | 14 |
| 5.54 | Dorsal posterior cingulate (I) | 210 | −1 | 4 | 16 |
| 5.13 | Posterior cingulate (I) | 240 | −3 | −6 | 13 |
| 5.1 | Prepyriform area (I) | 50 | −11 | 33 | −4 |
| 5.06 | Ventral anterior thalamus (I) | 55 | −2 | 6 | −1 |
| 4.92 | Ectosplenial area (I) | 28 | −1 | 0 | 8 |
| 4.9 | Primary somatosensory (C) | 36 | 13 | 22 | 13 |
| 4.85 | Geniculate nucleus (I) | 57 | −9 | −3 | 0 |
| 4.77 | Lateral habenula (I) | 11 | −1 | 3 | 5 |
| 4.77 | Mediodorsal thalamus (I) | 28 | −3 | 2 | 1 |
| 4.67 | Zona incerta/Subthalamic nucleus (I) | 20 | −7 | 5 | −4 |
| 4.66 | Interpeduncular nucleus (I) | 13 | −1 | −2 | −8 |
| 4.59 | Cerebellum (C) | 15 | 9 | −24 | 0 |
By varying the stimulation voltage between 2V, 5V, and 8V, and by alternating the frequency between 145Hz and 60Hz, we observed strong amplitude-dependent effects on the BOLD signal (Figure 1B). While stimulation at 2V resulted in negligible effects on BOLD, at 5V and 8V, both 145Hz and 60Hz stimulation resulted in activation of temporal, prefrontal, and motor/sensory cortex. At 8V 60Hz, DBS resulted in activation of bilateral temporal and prefrontal cortices.
Discussion
In these experiments, we observed that ATN DBS resulted in robust activation of ipsilateral Papez structures including entorhinal cortex, hippocampus, parahippocampal gyrus, cingulate and inferior temporal gyrus. This result, coupled with the fact that the most significant therapeutic effects in the SANTE trial were observed in patients with seizures of temporal origin (76% median reduction in seizure frequency at 5 years) [1,2], supports the theory that ATN DBS achieves its effects in part through modulation of the mesial temporal cortex by way of the Papez circuit. In addition, our data suggest that ATN DBS may activate a range of prefrontal and eloquent cortical areas, including orbitofrontal, motor, somatosensory, and insular cortex. This is an intriguing result, since long-term follow-up of patients in the SANTE trial revealed that patients with seizures originating in the frontal lobe and other extratemporal origin experienced median seizure reductions of 59% and 68%, respectively. The notion that ATN DBS may affect the extratemporal cortex is supported by a human low resolution electromagnetic tomography study in which ATN DBS resulted in cortical responses in insula and parietal operculum [10], and a TMS study in which ATN DBS increased short-interval intra-cortical inhibition in motor cortex [11]. In addition, recruiting rhythms induced by low frequency ATN DBS have been shown to be strongest in frontal and frontopolar regions [12]. Our results may therefore be particularly relevant for patients suffering from extratemporal epilepsy that includes motor cortex and other eloquent foci, for whom current strategies are limited. We have previously shown that DBS of the centromedian thalamic nucleus [7] and subthalamic nucleus [6], both investigational targets for the treatment of epilepsy, can also result in modulation of mesial temporal, prefrontal, and motor circuits, perhaps suggesting that these targets may also hold therapeutic potential for these patients.
Due to several limitations inherent in our experimental paradigm, caution must be exercised when drawing parallels between our findings and the effects of ATN DBS in human subjects. First, our study was conducted in healthy animals that were under general anesthesia. While our laboratory is currently investigating the effects of general anesthesia on the DBS-evoked BOLD signal, this relationship has yet to be fully characterized. Therefore, we cannot exclude the possibility that anesthesia may have affected the observed patterns of cortical activation. Second, the potential effect of current spread on adjacent thalamic nuclei must be considered. In the SANTE trial, the majority of patients were implanted with Medtronic 3387 leads, and were stimulated in a monopolar configuration. In order to mitigate the effect of current spread in the smaller swine brain, animals were implanted with Medtronic 3389 leads, which have shorter (0.5 mm vs. 1.5 mm) inter-contact spacing, and were stimulated using a bipolar configuration. Nevertheless, results from previous studies in which computational models of the volume of tissue activated by DBS were generated [13] suggest that neural elements several millimeters from the DBS lead may have be affected. In particular, it is possible that DBS-evoked activation of the mediodorsal (MD) thalamic nucleus may have contributed to the observed patterns of cortical activation. The MD is located immediately ventral and posterior to the ATN, and was therefore likely to be affected by our ventral stimulation localization (0-1+). This nucleus is thought to play a role in mood regulation [14, 15], and it is connected with mood-related brain regions that were activated in our study, including orbitofrontal cortex, amygdala, nucleus accumbens, and lateral habenula [16]. Notably, 15% of subjects in the active arm of the SANTE trial experienced depression. We therefore speculate that inappropriate DBS-evoked modulation of mood-related prefrontal circuits may play a role in the emergence of mood-related side effects. The block stimulation paradigm employed in this study (6 sec on, 1 min off) was based on our previous work, which has consistently shown this paradigm to allow for robust detection of DBS-evoked perturbations in the BOLD signal [6, 7, 15, 17, 18]. In this study, we also applied the temporal cycling paradigm used in the SANTE trial (1 minute ON, 5 minutes OFF) during fMRI, and found that we were able to detect similar patterns of DBS-evoked BOLD activation (Supplementary Figure 2).
Finally, we observed that cortical activation patterns were strongly dependent on stimulation amplitude. It is important to note that all patients in the active arm of the SANTE trial were assigned the same stimulation parameters (5V, 145Hz, 90us). Our results suggest that DBS parameter optimization may have a large impact on the modulated network(s), and therefore further work may be needed to arrive at optimal stimulation parameters for this treatment.
Supplementary Material
Highlights.
Anterior thalamic nucleus deep brain stimulation (145Hz, 90μs, 5V) in a large animal model resulted in activation of ipsilateral temporal, prefrontal, sensorimotor cortex
Amplitude- (2V, 5V, and 8V) and frequency- (60Hz and 145Hz) dependent effects cortical activation were observed
Anterior thalamic DBS likely results in widespread, parameter-dependent effects on cortical activity
Acknowledgements
This work was supported by the National Institutes of Health (R01 NS 70872 awards to KHL) and the The Grainger Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. doi:10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 2.Salanova V, Witt T, Worth R, Henry TR, Gross RE, Nazzaro JM, et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology. 2015;84:1017–25. doi: 10.1212/WNL.0000000000001334. doi:10.1212/WNL.0000000000001334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fisher RS, Theodore WH. Brain stimulation for epilepsy. Lancet Neurol. 2004 doi: 10.1016/s1474-4422(03)00664-1. [DOI] [PubMed] [Google Scholar]
- 4.Kahan J, Urner M, Moran R, Flandin G, Marreiros A, Mancini L, et al. Resting state functional MRI in Parkinson's disease: the impact of deep brain stimulation on ‘effective’ connectivity. Brain. 2014;137:1130–44. doi: 10.1093/brain/awu027. doi:10.1093/brain/awu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McIntyre CC, Hahn PJ. Network perspectives on the mechanisms of deep brain stimulation. Neurobiology of Disease. 2010;38:329–37. doi: 10.1016/j.nbd.2009.09.022. doi:10.1016/j.nbd.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Min H-K, Hwang S-C, Marsh MP, Kim I, Knight E, Striemer B, et al. Deep brain stimulation induces BOLD activation in motor and non-motor networks: An fMRI comparison study of STN and EN/GPi DBS in large animals. NeuroImage. 2012;63:1408–20. doi: 10.1016/j.neuroimage.2012.08.006. doi:10.1016/j.neuroimage.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JP, Min H-K, Knight EJ, Duffy PS, Abulseoud OA, Marsh MP, et al. Centromedian-Parafascicular Deep Brain Stimulation Induces Differential Functional Inhibition of the Motor, Associative, and Limbic Circuits in Large Animals. Biological Psychiatry. 2013;74:917–26. doi: 10.1016/j.biopsych.2013.06.024. doi:10.1016/j.biopsych.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Félix B, Léger M-E, Albe-Fessard D, Marcilloux JC, Rampin O, Laplace JP, et al. Stereotaxic atlas of the pig brain. Brain Research Bulletin. 1999;49:1–137. doi: 10.1016/s0361-9230(99)00012-x. doi:10.1016/S0361-9230(99)00012-X. [DOI] [PubMed] [Google Scholar]
- 9.Saikali S, Meurice P, Sauleau P, Eliat P-A, Bellaud P, Randuineau G, et al. A three-dimensional digital segmented and deformable brain atlas of the domestic pig. J Neurosci Methods. 2010;192:102–9. doi: 10.1016/j.jneumeth.2010.07.041. doi:10.1016/j.jneumeth.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 10.Zumsteg D, Lozano A, Wieser H, Wennberg R. Cortical activation with deep brain stimulation of the anterior thalamus for epilepsy. Clinical Neurophysiology. 2006;117:192–207. doi: 10.1016/j.clinph.2005.09.015. doi:10.1016/j.clinph.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 11.Molnar GF, Sailer A, Gunraj CA, Cunic DI, Wennberg RA, Lozano AM, et al. Changes in motor cortex excitability with stimulation of anterior thalamus in epilepsy. Neurology. 2006;66:566–71. doi: 10.1212/01.wnl.0000198254.08581.6b. doi:10.1212/01.wnl.0000198254.08581.6b. [DOI] [PubMed] [Google Scholar]
- 12.Kerrigan JF, Litt B, Fisher RS, Cranstoun S, French JA, Blum DE, et al. Electrical stimulation of the anterior nucleus of the thalamus for the treatment of intractable epilepsy. Epilepsia. 2004;45:346–54. doi: 10.1111/j.0013-9580.2004.01304.x. doi: 10.1111/j.0013-9580.2004.01304. [DOI] [PubMed] [Google Scholar]
- 13.Lujan JL, Chaturvedi A, Malone DA, Rezai AR, Machado AG, McIntyre CC. Axonal pathways linked to therapeutic and nontherapeutic outcomes during psychiatric deep brain stimulation. Hum Brain Mapp. 2012;33:958–68. doi: 10.1002/hbm.21262. doi: 10.1002/hbm.21262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Gibson WS, Cho SH, Abulseoud OA, Gorny KR, Felmlee JP, Welker KM, et al. The Impact of Mirth-Inducing Ventral Striatal Deep Brain Stimulation on Functional and Effective Connectivity. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw074. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell AS, Chakraborty S. What does the mediodorsal thalamus do? Front Syst Neurosci. 2013;7:37. doi: 10.3389/fnsys.2013.00037. doi: 10.3389/fnsys.2013.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paek SB, Min HK, Kim I, Knight EJ, Baek JJ, Bieber AJ, et al. Frequency-dependent functional neuromodulatory effects on the motor network by ventral lateral thalamic deep brain stimulation in swine. Neuroimage. 2015;105:181–8. doi: 10.1016/j.neuroimage.2014.09.064. doi: 10.1016/j.neuroimage.2014.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ross EK, Kim JP, Settell ML, Han SR, Blaha CD, Min HK, Lee KH. Fornix deep brain stimulation circuit effect is dependent on major excitatory transmission via the nucleus accumbens. Neuroimage. 2016;128:138–48. doi: 10.1016/j.neuroimage.2015.12.056. doi: 10.1016/j.neuroimage.2015.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


