SUMMARY
Stability of the genome is paramount to organisms. However, diverse eukaryotes carry out programmed DNA elimination, in which portions or entire chromsomes are lost in early development or during sex determination. During early development of the parasitic nematode, Ascaris suum, 13% of the genome is eliminated. How different genomic segments are reproducibly retained or discarded is unknown. Here we show that centromeric histone CENP-A localization plays a key role in this process. We show that Ascaris chromosomes are holocentric during germline mitoses, with CENP-A distributed along their length. Prior to DNA elimination in the 4-cell embryo, CENP-A is significantly diminished in chromosome regions that will be lost. This leads to the absence of kinetochores and microtubule attachment sites necessary for chromosome segregation, resulting in loss of these regions upon mitosis. Our data suggest that changes in CENP-A localization specify which portions of chromosomes will be lost during programmed DNA elimination.
Keywords: Programmed DNA elimination, centromere, holocentromere, CENP-A, kinetochore, CENP-C, NDC80, chromosome segregation, Ascaris
Graphical Abstract
INTRODUCTION
Maintenance of genome stability is an important process in all organisms. Genome instability can lead to cell death and disease. However, examples are known where genome instability through developmentally regulated DNA loss or rearrangements is integral in the biology of the organism. A well-known example is vertebrate immunoglobulin gene rearrangements that enable antibody and T-cell receptor diversification (Jung et al., 2006; Nishana and Raghavan, 2012). DNA rearrangement and elimination are also involved in the extensive remodeling of the somatic genome that occurs during development of the macronucleus in ciliates (Chalker and Yao, 2011). In addition, programmed DNA elimination also occurs during the development of diverse metazoa including some nematodes, copepod crustaceans, insects, lampreys, hagfish, zebra finches, and marsupials (Wang and Davis, 2014).
Programmed DNA elimination was first described in 1887 by Theodore Boveri in the intestinal nematode of horses, Parascaris (Boveri, 1887). In the related pig/human parasite Ascaris suum, DNA elimination occurs during the third through fifth cleavages (4 to 16 cell stage) of early development in five distinct somatic precursor cells that give rise to different cell lineages (Bonnevie, 1902; Meyer, 1895; Tobler et al., 1992). The genome in germline cells remains intact. During DNA elimination mitoses in the precursor somatic cells, chromosomes are broken and the fragments that undergo DNA elimination remain at the metaphase plate while the retained DNA is segregated into daughter cells (Niedermaier and Moritz, 2000). Using high-throughput sequencing, we previously compared the germline and somatic genomes of a single male Ascaris and found that 13% of the germline genome (43 Mb) is lost in forming the somatic genome including single-copy DNA for at least 685 germline-expressed genes (Wang et al., 2012). This suggests that DNA elimination is an irreversible mechanism for germline gene silencing in Ascaris somatic cells (Wang et al., 2012). A key question is what determines which chromosomes fragments will be kept, and which will be eliminated.
Caenorhabditis elegans has holocentric chromosomes and other nematode chromosomes may be holocentric (Albertson and Thomson, 1982; Goday et al., 1985; Maddox et al., 2004; Pimpinelli and Goday, 1989). A prevailing model of holocentric chromosomes is that multiple centromeric regions, kinetochores, and microtubule attachment sites are punctuated along the length of the chromosome (Drinnenberg et al., 2016; Maddox et al., 2004; McKinley and Cheeseman, 2015; Melters et al., 2012; Steiner and Henikoff, 2015). During nematode programmed DNA elimination, chromosomes break and some chromosome fragments are retained while others are lost. This raises a key question for what leads to the lack of segregation of those portions of Ascaris chromosomes that are eliminated.
We hypothesized that during DNA elimination, that either the centromere/kinetochore function of eliminated genomic regions or microtubule attachment to the centromere/kinetochore is compromised or inhibited leading to the lack of segregation and consequent loss of the DNA from these chromosome regions. To test whether the centromeres/kinetochores or microtubule attachment play a regulatory role during Ascaris DNA elimination, we generated antibodies to the histone H3 variant CENP-A (also known as CenH3), the epigenetic mark of centromeres (Black and Bassett, 2008; Chen and Mellone, 2016; De Rop et al., 2012; Drinnenberg et al., 2016; Earnshaw, 2015; Fukagawa and Earnshaw, 2014; McKinley and Cheeseman, 2015 ), and antibodies to components of the kinetochore. Our immunofluorescence staining data showed that CENP-A is reduced in chromosome regions that will be lost during DNA elimination, indicating centromere/kinetochore function is compromised in regions that will be eliminated. ChIP-seq data indicated that during Ascaris germline mitoses, CENP-A is deposited diffusely along Ascaris holocentric chromosomes and present within the chromosome regions that will be lost. However, CENP-A is later significantly reduced in these regions prior to DNA elimination. This CENP-A reduction leads to the loss of kinetochores and microtubule attachment sites necessary for chromosome segregation, and thus the DNA loss during elimination. Overall, our data suggest that Ascaris CENP-A localization contributes to the identification of regions to be retained and lost during DNA elimination. Thus, CENP-A localization plays a regulatory role and contributes to the mechanism of DNA elimination.
RESULTS
CENP-A is reduced on chromosome regions that are eliminated
To examine the contribution of centromeres/kinetochores in Ascaris DNA elimination, we identified several key protein components of the centromere and kinetochore in Ascaris (see Fig. S1A–C) (Cheerambathur and Desai, 2014; Chen and Mellone, 2016; Drinnenberg et al., 2016; Earnshaw, 2015; Fukagawa and Earnshaw, 2014; Hori and Fukagawa, 2012; Lampert and Westermann, 2011; McKinley and Cheeseman, 2015; Muller and Almouzni, 2014; Westhorpe and Straight, 2013). We generated polyclonal antibodies to the Ascaris histone H3 variant CENP-A, the inner kinetochore protein CENP-C, and the outer kinetochore protein NDC80 (see Supplemental Information). These three proteins represent the key regions of the centromere and kinetochore organization, and are required for chromosome segregation in the model nematode C. elegans (Buchwitz et al., 1999; Cheeseman et al., 2006; Cheeseman et al., 2004; Desai et al., 2003; Kitagawa, 2009; Maddox et al., 2004; Monen et al., 2005; Moore and Roth, 2001; Oegema et al., 2001).
Western blot analyses with these antibodies demonstrated that the antibodies recognize nuclear proteins of the expected molecular weight (see Fig. S1D). We then used these antibodies to examine mitoses and chromosome segregation in early Ascaris embryos. All three antibodies stained interphase nuclei and chromosomes during mitosis consistent with their known functions (Fig. S1E). Consistent with holocentric chromosome organization, CENP-A localized all along the outer surface of metaphase chromosomes facing the spindle poles (Buchwitz et al., 1999) (Fig. 1E). We next asked if these centromere/kinetochore components were present or absent at anaphase on chromosome regions destined for loss during DNA elimination where retained and eliminated chromosomes are easily identified. Two distinct CENP-A antibodies stain the chromosomes that will be retained during DNA elimination mitoses, but CENP-A staining is greatly reduced on chromosomes that will be eliminated (Fig. 1A–B red arrows) (CENP-A intensity is ~13% in the eliminated DNA compared to the retained DNA). Immunohistochemistry with CENP-C and NDC80 antibodies demonstrated that like CENP-A, these kinetochore components were present on chromosomes that will be retained during DNA elimination mitoses, but were greatly reduced on chromosomes that will be eliminated (Fig. 1C–D, red arrows). Overall, these data suggest that during Ascaris programmed DNA elimination, limited or no functional centromeres/kinetochores are assembled on the eliminated chromosome regions due to the lack of CENP-A localization.
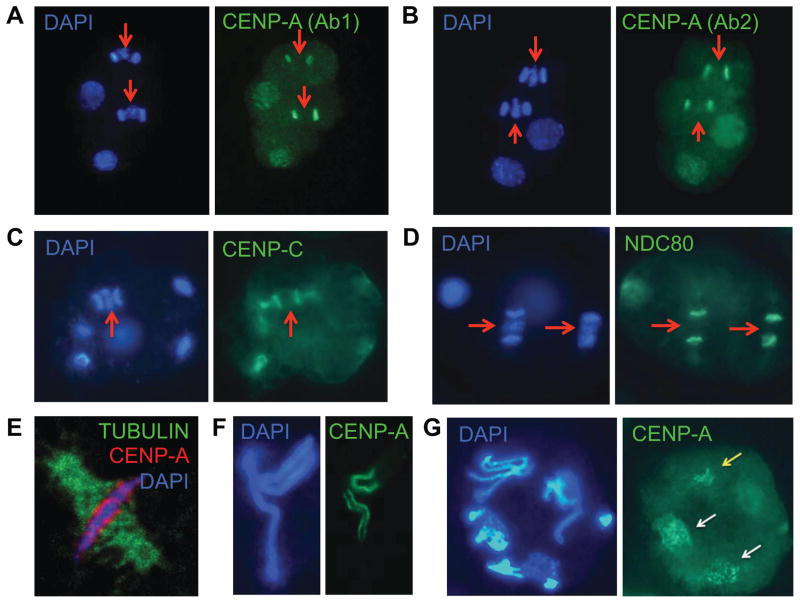
Figure 1. CENP-A is reduced in genomic regions lost during nematode programmed DNA elimination.
A and B. CENP-A is reduced in genomic regions that that remain at the metaphase plate and will be lost during Ascaris DNA elimination. Immunohistochemical (IHC) staining of CENP-A on a 4-cell Ascaris embryo with two cells undergoing DNA elimination mitoses (anaphase) indicates the DNA that wiil be lost (red arrows) has much less CENP-A than the DNA that will be segregated and retained. The staining observed is the same using two independently prepared antibodies (Ab1 and Ab2) made against a CENP-A peptide (A) or a fusion protein (B) (see Fig. S1). C and D. CENP-C (C) and NDC80 (D) are also reduced on Ascaris chromosome regions that remain at the metaphase plate and will be eliminated. E. IHC staining of microtubules and CENP-A in a 2-cell Ascaris embryo at metaphase illustrates that CENP-A extends along the length and is localized asymmetrically to the centrosome side of the chromosomes. F. IHC staining of CENP-A in a 1-cell Parascaris univalens embryo during prometaphase. Note that CENP-A is reduced on the long arms (arrows) of P. univalens chromosomes just before DNA elimination. These regions will be lost in subsequent cell divisions during DNA elimination, whereas the central chromosome region with CENP-A will form many new retained chromosomes (see G). G. IHC staining of CENP-A in a 4-cell embryo in P. univalens undergoing DNA elimination. Note CENP-A only stains the euchromatin regions (yellow arrows) and the retained genomic regions that form many new chromosomes (white arrows). The size of Ascaris and P. univalens embryos are ~ 70 × 45 μm and ~ 50 × 50 μm, respectively.
We next asked if chromosome regions that will be eliminated lack CENP-A in the cell cycle preceding the DNA elimination mitosis using a closely related nematode, Parascaris univalens. P. univalens undergoes DNA elimination in early embryos, has very high sequence similarity with Ascaris, and has been used in conjunction with Ascaris as a model for nematode DNA elimination. Parascaris has a single pair of large chromosomes that consist of long condensed and heterochromatic arms that are eliminated and a central region that is fragmented into smaller, retained chromosomes (Niedermaier and Moritz, 2000) (Fig. 1F–G). This chromosomal organization makes it easier to distinguish retained vs. eliminated chromosomal regions prior to DNA elimination. Consistent with their loss in elimination, the long arms have greatly reduced CENP-A at the 1-cell stage compared to the central region that will form new, retained chromosomes (Fig. 1F and Fig 1G, yellow arrow) that exhibit significant CENP-A staining (Fig. 1G, white arrows). These data suggest that CENP-A marks regions for retention prior to the DNA elimination mitosis in Parascaris. Overall these data indicate that CENP-A localization on particular regions of chromosomes immediately prior to and during DNA elimination likely determines which portions of chromosomes will be retained and eliminated in the somatic cells.
High-resolution CENP-A mapping reveals reduced CENP-A localization in the genomic regions that will be eliminated
We next carried out native CENP-A ChIP-seq to obtain whole-genome, base-pair resolution maps of CENP-A. CENP-A is organized into clusters, dispersed throughout the Ascaris genome, that extend along the length of the chromosomes (Fig. 2A). If the location of CENP-A in the genome represents functional centromeres/kinetochores, we would expect that the inner kinetochore component CENP-C to co-localize with CENP-A. ChIP-seq analyses demonstrate that CENP-C localization shows a strong correlation with CENP-A (Fig. 2A–B and Fig. S2A–C). These data strongly suggest that (1) our CENP-A and CENP-C ChIP-seq data represent centromeric regions with kinetochores; (2) Ascaris has holocentric chromosomes; and (3) that the holocentromeres typically extend along the length of the Ascaris chromosome.
Figure 2. Genome-wide mapping demonstrates Ascaris CENP-A is reduced in eliminated regions.
A, B. CENP-A and CENP-C genome-wide ChIP-seq co-localization. A. Representative region of an Ascaris chromosome (data from 32–64 cell embryos). B. Strong genome-wide correlation between CENP-A and CENP-C localization. C. Ascaris CENP-A nucleosomes are smaller than H3 nucleosomes (see Supplemental Experimental Procedures). D. CENP-A peaks in retained (ag85, blue) and eliminated (ag84, red) scaffolds just prior to DNA elimination (4-cell stage). Overall there is at least a 3-fold reduction of CENP-A peaks in eliminated regions (see Table S1). E. CENP-A is greatly reduced in genomic regions that will be eliminated (red) compared to retained (blue) regions. Representative scaffolds with data from 4-cell embryos.
We next characterized Ascaris CENP-A nucleosomes. We compared the size of the DNA from immunoprecipitated Ascaris CENP-A nucleosomes to all input nucleosomes (mostly H3 based nucleosomes) (Fig. 2C and Fig. S2D). Based on the observed DNA length for Ascaris CENP-A compared to the core H3 nucleosomes (Fig. 2C and Fig. S2D), the DNA size of an Ascaris CENP-A mono-nucleosome was ~ 6 bp smaller than for a core H3 nucleosome. This smaller DNA size for CENP-A nucleosomes is consistent with previous CENP-A nucleosome observations in other organisms (Hasson et al., 2013) further suggesting that our analyses are mapping octameric Ascaris CENP-A nucleosomes. Ascaris CENP-A is present primarily in mono- or di-nucleosomes flanked by H3 nucleosomes (Fig. 2C). Overall, these data suggest that our genome mapping of CENP-A and its localization likely corresponds to centromere/kinetochore sites in Ascaris.
We next carried out CENP-A ChIP-seq at the 4-cell stage (60 hr) to further examine the CENP-A reduction we observed by immunohistochemistry (Fig. 1) in regions that undergo DNA elimination. We found that compared to retained regions, the number of CENP-A peaks, the genomic area covered by CENP-A, and the level of CENP-A reads are all greatly reduced in DNA regions that will be eliminated (Fig. 2D–E). These data are consistent with a DNA elimination mechanism where CENP-A reduction leads to the absence of kinetochores and microtubule attachment sites necessary for chromosome segregation, thus leading to the loss of these DNA regions during Ascaris programmed DNA elimination.
Changes in CENP-A localization define DNA that will be retained and eliminated
To examine whether the reduced CENP-A in regions that undergo DNA elimination at the 4-cell stage is inherited from germ cells or re-organized during development, we carried out CENP-A ChIP-seq on the Ascaris mitotic germline and compared the CENP-A coverage in DNA regions destined for retention or elimination in the mitotic germline to that present in the 4-cell embryos. In contrast to the 4-cell stages where there is little CENP-A localization in regions that undergo DNA elimination, we found that CENP-A is abundant in the mitotic germline regions that will undergo DNA elimination later in embryos (Fig. 3A). We next compared CENP-A peaks numbers, the genomic regions covered by CENP-A peaks, and the CENP-A reads numbers in regions that will be eliminated (Fig. 3B–3C, Table S1 and Table S2) in different stages. All these features of CENP-A are reduced in 4-cell stages prior to DNA elimination compared to the germline tissues. The extensive and dynamic changes in CENP-A distribution in Ascaris suggests that this key epigenetic mark contributes to the specific loss of DNA sequences.
Figure 3. Dynamic CENP-A localization defines DNA to be retained and eliminated.
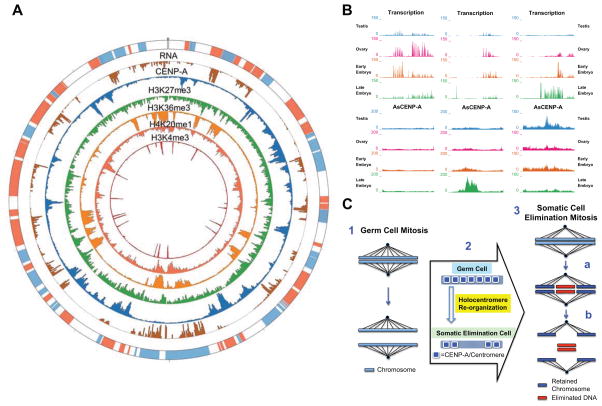
A. CENP-A localization in the mitotic regions of germline (testis and ovary) and 4-cell embryos. CENP-A enriched regions (positive values) are in green and CENP-A depleted regions (negative values) in red (z-scores; see Supplemental Experimental Procedures). The purple arc and light blue shading represent the genomic region that will be eliminated. Circles from outer to inner represent: (1) Genes (red + strand and blue – strand); (2) Testis CENP-A; (3) Ovary CENP-A; and (4) 4-cell embryo CENP-A. B. CENP-A peaks are reduced prior to DNA elimination in regions of the genome that will undergo elimination. Changes in CENP-A peaks between the germline and 4-cell embryos in a region eliminated (scaffold ag84) (see Fig. 3C for the overall changes). C. CENP-A is reduced before DNA elimination (see also Table S1). CENP-A is present at high levels in the mitotic germline (Testis-1 and Ovary-1) within regions that will become eliminated in embryos and then is greatly reduced before DNA elimination (4-cell).
CENP-A deposition, epigenetic marks, and transcription
To explore what factors might contribute to Ascaris CENP-A localization or its removal during DNA elimination, we examined the relationship between CENP-A, RNA expression (RNA-seq), and several active (H3K4me3, H3K36me3, and H4K20me1) or repressive histone marks (H3K9me2 and H3K27me3) (Fig. 4A, Fig. S4A–C and Table S3). CENP-A is primarily concentrated in lowly or non-transcribed genomic regions (including intergenic regions) (correlation coefficient, r = −0.546) (Fig. 4A and Fig. S4A), and it is not associated with repetitive sequence or enriched on promoter regions. In addition, CENP-A showed no significant correlation with either the active or repressive histone marks examined (Fig. 4A and Fig. S4B–C). Overall, these data indicate that CENP-A is in general inversely correlated with transcription and is not correlated with any of the specific histone marks examined.
Figure 4. Ascaris CENP-A deposition, histone marks, and transcription.
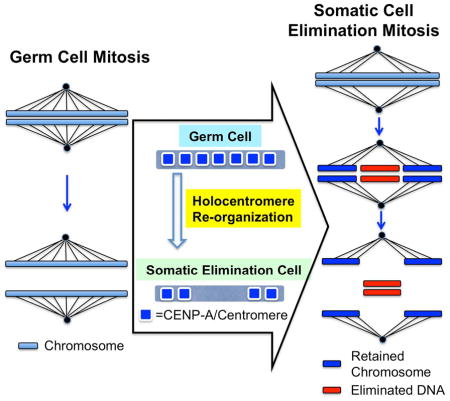
A. CENP-A, histone marks, and RNA (32–64 cell embryo) associated with a 1.0 Mb region of an Ascaris chromosome (ag1 5.5 – 6.5 Mb). Circles from outer to inner represent (1) Genes (red + strand and blue – strand); (2) RNA (brown); (3) CENP-A (blue); (4) H3K27me3 (green); (5) H3K36me3 (light orange); (6) H4K20me1 (dark orange) and (7) H3K4me3 (red). B. mRNA expression (RNA-seq) and CENP-A (ChIP-seq) in different stages and tissues. Three representative 50-kb-regions of the Ascaris chromosome (ag17). Data were averaged from 3 replicates with normalization (see Supplemental Experimental Procedures). Shown are transcriptionally active regions where there is lack of CENP-A (left and middle); CENP-A enriched regions where there is little transcription (middle); and a genomic region that illustrates both CENP-A localization as well as RNA transcription (right). CENP-A localization is in general not associated with transcription changes (left and middle), but is in some cases where transcription is highly dynamic CENP-A localization can be affected (right) (see also Fig. S4E). C. Model of programmed DNA elimination in nematodes. 1. Holocentric mitotic germline chromosomes. 2. Re-organization of CENP-A and centromeres/kinetochores during development in regions that will be eliminated. 3. DNA elimination mitosis. Chromosomes in early embryo somatic cells undergo chromosome breaks at metaphase (a). CENP-A is reduced in the regions to be eliminated prior to chromosome breakage. Retained chromosome fragments (blue) have centromere/kinetochore sites for microtubule attachment to facilitate chromosome segregation, whereas chromosomal fragments that will be eliminated (red) remain at the metaphase plate, are not segregated, and are lost (b).
In C. elegans, germline transcription plays a role in defining CENP-A deposition. Genomic regions that are transcribed in the C. elegans germline lack CENP-A deposition in the early embryo (Gassmann et al., 2012). To examine whether transcription plays a role in the Ascaris CENP-A deposition changes associated with DNA elimination, we compared RNA-seq and CENP-A data (Fig. 4B and Fig. S3A–B). CENP-A enriched regions and transcriptionally active regions are mutually exclusive throughout Ascaris development (Fig. 4B). Notably, transcriptionally active regions in all developmental stages examined have low levels of CENP-A (Fig. 4B [left and middle panels] and Fig. S4D) and developmental transcription changes have limited impact on the level of CENP-A deposition unless very large transcriptional changes occur (Fig. 4B Fig S4D–E). Overall, our data suggest that (1) CENP-A enrichment and transcribed regions are in general differentially organized in the genome, (2) only very large changes in RNA expression can impact the levels of CENP-A deposition, and 3) that germline or early embryo transcription does not appear to play a direct role in defining CENP-A deposition or removal for DNA elimination.
DISCUSSION
CENP-A localization in the Ascaris genome defines sequences for retention or elimination
During programmed DNA elimination in nematodes, chromosomes break and portions of chromosomes are segregated and retained in daughter cells while other parts of chromosomes remain at the metaphase plate, are not segregated, and are eventually degraded and lost (Fig. 4C, part 3). A key question is what determines which portions of chromosomes are retained and which will be lost during DNA elimination when all regions of a holocentric chromosomes (by definition) should be functional for segregation. We asked whether centromeres/kinetochore component assembly or microtubule attachment is compromised in the genomic regions lost during nematode DNA elimination using antibodies we generated to CENP-A, CENP-C (inner kinetochore), and NDC80 (outer kinetochore) proteins. CENP-A is in general uniformly localized along chromosomes in the mitotic germline consistent with a holocentric chromosome organization (Fig. 3). Immediately preceding and during DNA elimination, chromosome regions that will be lost have significantly reduced CENP-A, CENP-C and NDC80 immunohistochemical staining (Fig. 1). Early embryo ChIP-seq data also demonstrates that the regions that will be lost have significantly reduced levels of CENP-A. However, in contrast, genome regions in the mitotic germline chromosomes that will be lost in early embryos have significant CENP-A. During development, CENP-A localization is subsequently reduced or CENP-A is removed in specific chromosome regions thereby compromising centromere/kinetochore assembly and facilitating the loss of these chromosome regions during DNA elimination (Fig. 4C).
In the closely related parasitic nematode P. univalens, one pair of very large chromosomes undergoes DNA elimination in the second and third division of embryogenesis. The highly heterchromatic and condensed arms of these large chromosomes are lost during DNA elimination (Fig. 1F–G) (Niedermaier and Moritz, 2000; Pimpinelli and Goday, 1989). Pioneering cytological studies described a long kinetochore extending along the length of the mitotic germline chromosomes suggesting their holocentric nature (Goday et al., 1985). Just prior to DNA elimination, the heterochromatic arms were proposed to lose kinetochore activity and microtubule attachment leading to their loss in DNA elimination (Fig. 1F–G) (Goday et al., 1992). Our data provide a mechanistic explanation for the proposed change in the kinetochore distribution.
The reduction of CENP-A from Ascaris germline tissues to the 4-cell embryos could be due to a combination of defective CENP-A deposition (including during DNA replication) and active removal of CENP-A. CENP-A may not be completely lost from eliminated regions. We note that the minimal amount of CENP-A nucleosome(s) (and its organization) that is required to establish microtubule attachment on a holocentric chromosome, and how many of these functional centromere sites are needed to segregate chromosomes to the daughter cells during mitosis remains unknown.
Determinants of CENP-A deposition and CENP-A nucleosomes
As the presence of CENP-A appears to be a key event in defining the retention or elimination of portions of chromosomes during DNA elimination, we sought to understand where and how CENP-A is localized in the genome. Unlike in many organisms, Ascaris CENP-A deposition is not associated with repetitive sequences or with heterochromatin. In particular, there is no apparent CENP-A enrichment with the major 121 bp repetitive sequences (with >99% lost during DNA elimination) in the germline tissues and early embryos. In addition, no DNA sequence motifs or other attributes appear to be associated with CENP-A localization. These are also attributes observed for CENP-A deposition in the model nematode C. elegans (Gassmann et al., 2012; Steiner and Henikoff, 2014), and they are consistent with the observation that CENP-A deposition can occur and facilitate segregation of any extrachromosomal array in C. elegans (Stinchcomb et al., 1985; Yuen et al., 2011). The majority of Ascaris CENP-A is organized into peaks of 1–15 kb (median of ~3.5 kb) that appear uniformly distributed across Ascaris chromosomes. In general, Ascaris CENP-A deposition is inversely correlated with regions of the genome that are actively transcribed. However, this is not an exclusive relationship as there are regions of the genome with actively expressed genes where CENP-A can be deposited. These genes are expressed at relatively low levels, whereas genes that are expressed at very high levels in any stage generally preclude CENP-A deposition (Fig. 4B).
We used ChIP-seq to analyze the relationship between active and repressive histone marks (H3K4me3, H3K36me3, H4K20me1 and H3K27me3) and Ascaris CENP-A deposition. Despite the inverse correlation between CENP-A and transcription, we observed no correlations with any of these histone marks and CENP-A deposition. It remains to be determined in Ascaris whether there are specific histone marks that are strongly associated with CENP-A that may help establish or preclude CENP-A deposition and centromere/kinetochore assembly.
Nematode CENP-A deposition, centromeres/kinetochores, and holocentric chromosomes
Cytological data indicate that the model nematode C. elegans chromosomes are holocentric with centromere/kinetochore regions distributed along the length of the chromosomes in early embryos (Albertson and Thomson, 1982). Gassmann et al. demonstrated using CENP-A ChIP-chip that CENP-A diffusely occupies ~2,900 broad, low density domains of ~10–12 kb that cover about half of the genome (Gassmann et al., 2012). In contrast, in a more recent study using C. elegans CENP-A ChIP-seq, Steiner and Henikoff suggested that CENP-A is present in high density and discrete point-like peaks that are coincident with transcription factor hot spots (Steiner and Henikoff, 2014). It remains to be determined whether these observed differences are due to the different experimental conditions/methods used or data interpretation. In our Ascaris analyses, we used MNase digestion conditions and a native ChIP-seq protocol similar to those described by Steiner and Henikoff (Steiner and Henikoff, 2014). However, we did not observe any strong discrete point-like CENP-A peaks in Ascaris. We also found that the length of DNA wrapped around Ascaris CENP-A nucleosome is ~6 bp smaller than a canonical H3 nucleosome, consistent with the octameric nucleosome model that suggests CENP-A nucleosomes have loose DNA termini (Hasson et al., 2013). In addition, our CENP-C ChIP-seq data also showed a strong correlation with CENP-A data, in agreement with a diffuse, low-density CENP-A model (Gassmann et al., 2012). In Parascaris, electron microscopy analysis of the mitotic germline and early embryo chromosomes did not demonstrate regular interruptions along the length of the kinetochore regions, suggesting that the ascarid holocentric organization is more diffusely distributed, as classically described, rather than polycentric (Goday et al., 1985; Goday et al., 1992). Our CENP-A data in Ascaris appear consistent with these EM studies and thus favors a broad, diffusively distributed holocentric chromosome organization model.
CENP-A and ciliate DNA rearrangement
A recent study described the deletion of the CENP-A gene during DNA rearrangement in ciliates (Lhuillier-Akakpo et al., 2016). However, the loss of CENP-A in the formation of the ciliate macronucleus does not appear to lead to the direct loss of macronuclear chromosomes. In contrast, the nematode CENP-A gene is not lost, but CENP-A localization is regulated and reduced on chromosome regions that will undergo DNA elimination contributing to the loss of particular DNA regions.
CONCLUSION
Programmed DNA elimination in ascarid nematodes occurs during early embryo development. Chromosomes break, new chromosomes form, and regions of chromosomes are lost. Our data demonstrate that CENP-A, the key centromere component, extends along the length of the mitotic germline chromosomes, consistent with a holocentric organization of the germline chromosomes. CENP-A is then reduced in specific chromosome regions that will be eliminated in early development. Thus, regulation of CENP-A localization or its removal prior to DNA elimination defines and specifies which portions of somatic chromosomes will be lost during programmed DNA elimination. Our studies provide key insights into what determines which sequences are retained and which are eliminated in programmed DNA elimination, a phenomenon that occurs in a breadth of organisms including ciliates, nematodes, arthropods, crustraceans, and vertebrates.
MATERIALS AND METHODS
Ascaris, Antibodies, and Immunohistochemistry
Collection of Ascaris tissues, zygotes, and zygote embryonation were as previously described (Wang et al., 2011; Wang et al., 2014). Preparation of polyclonal antibodies and monoclonal histone antibodies used are described in Supplemental Experimental Procedures. Ascaris embryo immunohistochemistry was carried as described (Wang et al., 2014) using a modified freeze-crack method to permeabilize and fix embryos.
Nuclei Isolation and ChIP-seq
Nuclei were isolated from de-coated embryos or Ascaris germline tissues as described in Supplemental Experimental Procedures. For Native ChIP, a protocol modified from Steiner and Henikoff (Steiner and Henikoff, 2014) was used. For CENP-A ChIP, 5–10 million nuclei were isolated from germline tissues or different stages of embryos. CENP-A was immunoprecipitated from the extract by incubation with 15 μg affinity purified antibody and pre-cleared protein A beads. For CENP-C ChIP, a cross-linked method modified from Patel et al (Patel et al., 2014) was used with the 20 μg of affinity purified antibody. The details are described in Supplemental Experimental Procedures.
Sequencing and data analysis
Sequencing libraries and data analysis are described in Supplemental Experimental Procedures.
Data deposition, accession, and genome browser
All the data were deposited to NCBI GEO database and are available under the accession GSE76914. Data are also made available in the UCSC genome browser track data hubs via this link: http://amc-sandbox.ucdenver.edu/User14/hub.txt.
Supplementary Material
Acknowledgments
We thank Paul Megee and Chad Pearson for their input; Florian Steiner for sharing detailed protocols for nematode CENP-A ChIP-seq; Arshad Desai for help identifying Ascaris CENP-A and CENP-C; Martin Nielsen for Parascaris material; Richard Komuniecki, Amanda Ortega, Jeff Myers, and Routh Packing Co. for their support and hospitality in collecting Ascaris material; and Lee Niswander, David Bentley, Chad Pearson, and Mark Johnston for comments on the manuscript. This work was supported in part by NIH grants to R.E.D. (AI0149558 and AI114054).
Footnotes
AUTHOR CONTRIBUTIONS
YK, JW, and RED designed the experiments, YK, JW, SK, AN and RED conducted the experiments, and YK, JW, and RED analyzed and interpreted the data. JW carried out bioinformatics analyses and YK, JW, and RED wrote the manuscript. HK provided monoclonal histone antibodies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albertson DG, Thomson JN. The kinetochores of Caenorhabditis elegans. Chromosoma. 1982;86:409–428. doi: 10.1007/BF00292267. [DOI] [PubMed] [Google Scholar]
- Black BE, Bassett EA. The histone variant CENP-A and centromere specification. Curr Opin Cell Biol. 2008;20:91–100. doi: 10.1016/j.ceb.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Bonnevie K. Dber Chromatindiminution bei Nematoden. Jena Z Naturwiss. 1902;36:275–288. [Google Scholar]
- Boveri T. Ueber Differenzierung der Zellkerne wahrend der Furchung des Eies von Ascaris megalocephala. Anat Anz. 1887;2:688–693. [Google Scholar]
- Buchwitz BJ, Ahmad K, Moore LL, Roth MB, Henikoff S. A histone-H3-like protein in C. elegans. Nature. 1999;401:547–548. doi: 10.1038/44062. [DOI] [PubMed] [Google Scholar]
- Chalker DL, Yao MC. DNA elimination in ciliates: transposon domestication and genome surveillance. Annual review of genetics. 2011;45:227–246. doi: 10.1146/annurev-genet-110410-132432. [DOI] [PubMed] [Google Scholar]
- Cheerambathur DK, Desai A. Linked in: formation and regulation of microtubule attachments during chromosome segregation. Curr Opin Cell Biol. 2014;26:113–122. doi: 10.1016/j.ceb.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Niessen S, Anderson S, Hyndman F, Yates JR, 3rd, Oegema K, Desai A. A conserved protein network controls assembly of the outer kinetochore and its ability to sustain tension. Genes Dev. 2004;18:2255–2268. doi: 10.1101/gad.1234104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Mellone BG. Chromatin assembly: Journey to the CENter of the chromosome. J Cell Biol. 2016;214:13–24. doi: 10.1083/jcb.201605005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rop V, Padeganeh A, Maddox PS. CENP-A: the key player behind centromere identity, propagation, and kinetochore assembly. Chromosoma. 2012;121:527–538. doi: 10.1007/s00412-012-0386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A, Rybina S, Muller-Reichert T, Shevchenko A, Shevchenko A, Hyman A, Oegema K. KNL-1 directs assembly of the microtubule-binding interface of the kinetochore in C. elegans. Genes Dev. 2003;17:2421–2435. doi: 10.1101/gad.1126303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinnenberg IA, Henikoff S, Malik HS. Evolutionary Turnover of Kinetochore Proteins: A Ship of Theseus? Trends Cell Biol. 2016 doi: 10.1016/j.tcb.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw WC. Discovering centromere proteins: from cold white hands to the A, B, C of CENPs. Nat Rev Mol Cell Biol. 2015 doi: 10.1038/nrm4001. [DOI] [PubMed] [Google Scholar]
- Fukagawa T, Earnshaw WC. The Centromere: Chromatin Foundation for the Kinetochore Machinery. Dev Cell. 2014;30:496–508. doi: 10.1016/j.devcel.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann R, Rechtsteiner A, Yuen KW, Muroyama A, Egelhofer T, Gaydos L, Barron F, Maddox P, Essex A, Monen J, et al. An inverse relationship to germline transcription defines centromeric chromatin in C. elegans. Nature. 2012;484:534–537. doi: 10.1038/nature10973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goday C, Ciofi-Luzzatto A, Pimpinelli S. Centromere ultrastructure in germ-line chromosomes of Parascaris. Chromosoma. 1985;91:121–125. doi: 10.1007/BF00294055. [DOI] [PubMed] [Google Scholar]
- Goday C, Gonzalez-Garcia JM, Esteban MR, Giovinazzo G, Pimpinelli S. Kinetochores and chromatin diminution in early embryos of Parascaris univalens. J Cell Biol. 1992;118:23–32. doi: 10.1083/jcb.118.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson D, Panchenko T, Salimian KJ, Salman MU, Sekulic N, Alonso A, Warburton PE, Black BE. The octamer is the major form of CENP-A nucleosomes at human centromeres. Nat Struct Mol Biol. 2013;20:687–695. doi: 10.1038/nsmb.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori T, Fukagawa T. Establishment of the vertebrate kinetochores. Chromosome Res. 2012;20:547–561. doi: 10.1007/s10577-012-9289-9. [DOI] [PubMed] [Google Scholar]
- Jung D, Giallourakis C, Mostoslavsky R, Alt FW. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- Kitagawa R. Key players in chromosome segregation in Caenorhabditis elegans. Frontiers in bioscience. 2009;14:1529–1557. doi: 10.2741/3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert F, Westermann S. A blueprint for kinetochores - new insights into the molecular mechanics of cell division. Nat Rev Mol Cell Biol. 2011;12:407–412. doi: 10.1038/nrm3133. [DOI] [PubMed] [Google Scholar]
- Lhuillier-Akakpo M, Guerin F, Frapporti A, Duharcourt S. DNA deletion as a mechanism for developmentally programmed centromere loss. Nucleic Acids Res. 2016;44:1553–1565. doi: 10.1093/nar/gkv1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox PS, Oegema K, Desai A, Cheeseman IM. “Holo”er than thou: chromosome segregation and kinetochore function in C. elegans. Chromosome Res. 2004;12:641–653. doi: 10.1023/B:CHRO.0000036588.42225.2f. [DOI] [PubMed] [Google Scholar]
- McKinley KL, Cheeseman IM. The molecular basis for centromere identity and function. Nat Rev Mol Cell Biol. 2015;17:16–29. doi: 10.1038/nrm.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melters DP, Paliulis LV, Korf IF, Chan SW. Holocentric chromosomes: convergent evolution, meiotic adaptations, and genomic analysis. Chromosome Res. 2012;20:579–593. doi: 10.1007/s10577-012-9292-1. [DOI] [PubMed] [Google Scholar]
- Meyer Cellulare Untersuchungen an Nematoden-Eiern. Jena Z Naturwiss. 1895;29:391–410. [Google Scholar]
- Monen J, Maddox PS, Hyndman F, Oegema K, Desai A. Differential role of CENP-A in the segregation of holocentric C. elegans chromosomes during meiosis and mitosis. Nat Cell Biol. 2005;7:1248–1255. doi: 10.1038/ncb1331. [DOI] [PubMed] [Google Scholar]
- Moore LL, Roth MB. HCP-4, a CENP-C-like protein in Caenorhabditis elegans, is required for resolution of sister centromeres. J Cell Biol. 2001;153:1199–1208. doi: 10.1083/jcb.153.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller S, Almouzni G. A network of players in H3 histone variant deposition and maintenance at centromeres. Biochim Biophys Acta. 2014;1839:241–250. doi: 10.1016/j.bbagrm.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Niedermaier J, Moritz KB. Organization and dynamics of satellite and telomere DNAs in Ascaris: implications for formation and programmed breakdown of compound chromosomes. Chromosoma. 2000;109:439–452. doi: 10.1007/s004120000104. [DOI] [PubMed] [Google Scholar]
- Nishana M, Raghavan SC. Role of recombination activating genes in the generation of antigen receptor diversity and beyond. Immunology. 2012;137:271–281. doi: 10.1111/imm.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema K, Desai A, Rybina S, Kirkham M, Hyman AA. Functional analysis of kinetochore assembly in Caenorhabditis elegans. J Cell Biol. 2001;153:1209–1226. doi: 10.1083/jcb.153.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel B, Kang Y, Cui K, Litt M, Riberio MS, Deng C, Salz T, Casada S, Fu X, Qiu Y, et al. Aberrant TAL1 activation is mediated by an interchromosomal interaction in human T-cell acute lymphoblastic leukemia. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2014;28:349–361. doi: 10.1038/leu.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimpinelli S, Goday C. Unusual kinetochores and chromatin diminution in Parascaris. Trends in genetics : TIG. 1989;5:310–315. doi: 10.1016/0168-9525(89)90114-5. [DOI] [PubMed] [Google Scholar]
- Steiner FA, Henikoff S. Holocentromeres are dispersed point centromeres localized at transcription factor hotspots. eLife. 2014;3:e02025. doi: 10.7554/eLife.02025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner FA, Henikoff S. Diversity in the organization of centromeric chromatin. Curr Opin Genet Dev. 2015;31:28–35. doi: 10.1016/j.gde.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Stinchcomb DT, Shaw JE, Carr SH, Hirsh D. Extrachromosomal DNA transformation of Caenorhabditis elegans. Mol Cell Biol. 1985;5:3484–3496. doi: 10.1128/mcb.5.12.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler H, Etter A, Muller F. Chromatin diminution in nematode development. Trends in genetics. 1992;8:427–432. doi: 10.1016/0168-9525(92)90326-y. [DOI] [PubMed] [Google Scholar]
- Wang J, Czech B, Crunk A, Mitreva M, Hannon G, Davis RE. Deep small RNA sequencing from the nematode Ascaris reveals conservation, functional diversification, and novel developmental profiles. Genome Research. 2011;21:1462–1477. doi: 10.1101/gr.121426.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Davis RE. Programmed DNA elimination in multicellular organisms. Curr Opin Genet Dev. 2014;27C:26–34. doi: 10.1016/j.gde.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Garrey J, Davis RE. Transcription in Pronuclei and One- to Four-Cell Embryos Drives Early Development in a Nematode. Current biology : CB. 2014;24:124–133. doi: 10.1016/j.cub.2013.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Mitreva M, Berriman M, Thorne A, Magrini V, Koutsovoulos G, Kumar S, Blaxter ML, Davis RE. Silencing of germline-expressed genes by DNA elimination in somatic cells. Dev Cell. 2012;23:1072–1080. doi: 10.1016/j.devcel.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westhorpe FG, Straight AF. Functions of the centromere and kinetochore in chromosome segregation. Curr Opin Cell Biol. 2013;25:334–340. doi: 10.1016/j.ceb.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen KW, Nabeshima K, Oegema K, Desai A. Rapid de novo centromere formation occurs independently of heterochromatin protein 1 in C. elegans embryos. Curr Biol. 2011;21:1800–1807. doi: 10.1016/j.cub.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.