Abstract
Purpose
Boron neutron capture therapy (BNCT) has the potential to become a viable cancer treatment modality, but its clinical translation has been limited by the poor tumor selectivity of agents. To address this unmet need, a boronated 2-nitroimidazole derivative (B-381) was synthesized and evaluated for its capability of targeting hypoxic glioma cells.
Methods
B-381 has been synthesized from a 1-step reaction. Using D54 and U87 glioma cell lines, the in vitro cytotoxicity and cellular accumulation of B-381 has been evaluated under normoxic and hypoxic conditions compared to L-boronophenylalanine (BPA). Furthermore, tumor retention of B-381 was evaluated in vivo.
Results
B-381 had low cytotoxicity in normal and cancer cells. Unlike BPA, B-381 illustrated preferential retention in hypoxic glioma cells compared to normoxic glioma cells and normal tissues in vitro. In vivo, B-381 illustrated significantly higher long-term tumor retention compared to BPA, with 9.5-fold and 6.5-fold higher boron levels at 24 and 48 h, respectively.
Conclusions
B-381 represents a new class of BNCT agents in which their selectivity to tumors is based on tumor hypoxic metabolism, and further studies are warranted to evaluate this compound and similar compounds as preclinical candidates for future BNCT clinical trials for the treatment of glioma.
Keywords: Boron neutron capture therapy, BNCT, Glioma, Hypoxia, Tumor Targeting
INTRODUCTION
Glioblastoma multiforme (GBM) is an aggressive astrocytoma and is the most common primary brain tumor in adults (1). The current standard of care for treating GBM is surgical resection (when operable) followed by radiation therapy (XRT) with concomitant and adjuvant temozolomide treatment (2, 3). Even with this aggressive therapeutic regimen, GBM patients still have a poor prognosis: 2- and 5- year survival rates of 10% and 1%, respectively (4). Current treatment provides only a modest boost to patient survival, and a myriad of radiation- and chemotherapy-induced side effects plague the quality of life for these patients. Even with therapy, patients experience a high recurrence rate of approximately 90% (5). Therefore, new approaches that reduce off-target side effects, improve efficacy and extend patient survival are direly needed.
Boron neutron capture therapy (BNCT) is an emerging treatment modality with the potential to minimize side effects and improve GBM patient survival (6). BNCT utilizes the neutron capture reaction of boron-10 (10B) and its subsequent nuclear fission reaction to produce cellular death (7). After a 10B atom absorbs a neutron, the resulting unstable 11B isotope undergoes a nuclear fission reaction releasing an alpha particle, lithium-7 ion and gamma radiation (8). The path lengths of these newly generated linear energy transfer particles are typically 5-9 microns, thereby localizing the cytotoxic effect (8). Additionally, the cytotoxic effect is further localized since the nuclear fission reaction will only occur in boron-containing cells that fall within the neutron irradiation field.
Unlike radiation therapy, BNCT uses a non-ionizing neutron beam for irradiation. Therefore, if boron selectively accumulates in the tumor and minimally in the surrounding tissue, the off-target radiation effects common to traditional radiation therapy will be mitigated in BNCT. To date, L-boronophenylalanine (BPA) and sodium borocaptate (BSH) are the most commonly investigated BNCT agents in clinical studies. The challenge in developing an efficacious BNCT agent is to achieve adequate tumor/normal tissue (T/N) and tumor/blood concentration ratios (ideally greater than 3:1). Not only must an agent have a preferential tumor accumulation, it must also have limited systemic cytotoxicity.
High grade glioma patients enrolled in BNCT clinical trials have affirmed that BNCT is tolerated well and has comparable (or fewer) side effects than conventional XRT (9, 10). However, the median survival times of these trials was comparable to the standard of care (XRT and temozolomide). BPA and BSH typically have T/N ratios < 3 clinically which limits their therapeutic efficacy (7). These results emphasize the need to develop novel compounds with higher tumor specificity and improved T/N ratios.
GBM consists of a heterogeneous tumor microenvironment containing areas of differing oxygenation levels which reflect unique metabolic patterns (11). Highly oxygenated regions (close proximity to blood vessels) are characterized with fast tumor proliferation and oxidative metabolism (12), while hypoxic regions (with low oxygenation) are characterized with low proliferation and reductive metabolism (13). Hypoxic conditions in GBM tumors have been shown to decrease cell proliferation (14), induce metastasis (15), promote angiogenesis (16), and confer resistance to chemotherapy (17) and XRT (18). Resistance has been attributed to the development of a subpopulation of cancer stem-like cells (19) that contribute to relapse in GBM (20, 21).
It has long been recognized that 2-nitroimidazole derivatives are capable of selectively accumulating in hypoxic cells (22, 23).
In the current study, we hypothesized that a boronated nitroimidazole derivative will preferentially accumulate in the tumor while sparing the surrounding (normoxic) healthy tissue, thereby improving the boron T/N ratio. In this study, we present the chemical synthesis and biological evaluation of the boronated nitroimidazole derivative B-381. This derivative had low toxicity and a preferential accumulation in hypoxic glioma cells, making it a suitable candidate for future BNCT studies.
MATERIALS AND METHODS
2.1 Reagents and Cell Culture
All synthetic reagents for the chemical synthesis were purchased from Sigma Aldrich (St. Louis, MO). Glioma (D54 and U87) and hippocampus (HT22) cell lines were a kind gift from Dr. Dinesh Thotala (Department of Radiation Oncology, Cancer Biology Division, Washington University in Saint Louis School of Medicine). All cell lines were cultured in Dulbecco's Modified Eagle's Medium (DMEM, Corning CellGro, Mediatech, Manassas, VA) supplemented with 20% fetal bovine serum (FBS, Gibco, Life Technologies, Grand Island, NY), 2 mmol/L of L-glutamine, 100 U/mL Penicillin and 100 μg/mL Streptomycin (CellGro, Mediatech, Manassas, VA). Before plating, cells were washed with phosphate-buffered saline (PBS, Corning CellGro, Mediatech, Manassas, VA), trypsinized with 0.05% Trypsin-EDTA 1x (Gibco, Life Technologies, Grand Island, NY), spun for 5 minutes (1000 RPM) and resuspended in fresh DMEM media. Peripheral blood mononuclear cells (PBMCs) were isolated from pheresis leukopaks from the Siteman Cancer Center (Washington University in Saint Louis). Red Blood Cell Lysis Buffer 1x (BioLegend, San Diego, CA) was added to whole blood, gently vortexed and incubated at room temperature for 15 minutes (protected from light). PBMCs were washed with PBS and resuspended in fresh DMEM media. For normoxic conditions, cells were cultured at 37°C (5% CO2) in a NuAire water jacket incubator (Plymouth, MN). For hypoxic conditions, cells were cultured at 37°C with 0.5% O2 concentration in a hypoxic chamber (Coy Laboratory Products, Grass Lake, MI).
2.2 Synthesis of boronated 2-nitroimidazole derivative B-381
Piperidine-4-boronic acid pinacol ester hydrochloride (73 mg, 0.296 mmol) was dissolved in saturated sodium bicarbonate solution (1 mL). Ethanol (50 mL) was added, mixed for several minutes, and was followed by addition of sodium sulfate (until no clumping was observed). This mixture was filtered and transferred to a 100 mL round bottom flask. Thereafter 1-(2,3-Epoxypropyl)-2-nitroimidazole (50 mg, 0.296 mmol) was added and the mixture refluxed for 5 h. After the starting material was consumed, the mixture was concentrated on a rotary evaporator. Methanol was added to the crude oil resulting in precipitation of the product B-381 (IUPAC name: 1-(2-nitro-1H-imidazol-1-yl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)piperidin-1-yl)propan-2-ol). The precipitate was isolated and the chemical structure was confirmed by liquid chromatography-mass spectrometry and proton nuclear magnetic resonance (1H-NMR) spectroscopy. The molecular weight of the product was confirmed to be 381 g/mole, giving rise to the compound abbreviation B-381.
2.3 Cell viability assay
Cell viability was assessed by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay as described previously (24). Briefly, HT22, PBMCs, D54 and U87 cell lines were cultured in normoxia (21% O2) or hypoxia (0.5% O2) and treated for 24 h or 72 h with B-381 or BPA (0, 0.01, 0.1, 1, 10, 100 μM). After treatment, MTT solution was added for 3 h followed by the addition of 10% sodium dodecyl sulfate solution. The absorbance was read the following day at 570 nm using a plate reader.
2.4 HPLC assay for B-381 detection
B-381 was analyzed using high performance liquid chromatography (HPLC, Agilent 1100 series, Santa Clara, CA) with a reverse phase C-18 column (Agilent Zorbax Eclipse XDB C18), at a flow rate of 1 mL/min and operating pressure range of 70-85 Barr. An acetonitrile gradient containing 0.1% trifluoroacetic acid was used as the mobile phase: the gradient was increased from 0 to 10% acetonitrile (from time 0 to 7 minutes) and then decreased back to 0% acetonitrile (from time 7 to 14 minutes). A calibration curve was formed by plotting the area under curve (AUC) of the B-381 HPLC peak (at retention time = 5 min, λ = 330 nm) for the concentration range of B-381 (0 to 10 μg/mL). The linear correlation for the curve had a R2 = 1, with a limit of detection approximately 0.1 μg/mL.
2.5 Cellular uptake of B-381 in vitro
PBMCs, D54, U87 or HT22 cells (1 × 106 cells/well) were cultured overnight under normoxia. The following day, cells were incubated in normoxic or hypoxic conditions for 4 h in serum free media, then B-381 was added with a final concentration of 10 μg/mL for 48 h. Additionally, B-381 solution was added to wells with no cells to serve as a no-cellular uptake control. It was observed that the AUC remained constant for this control over the experimental timeline. Media samples were collected from each well at 0 and 48 h, and analyzed by the aforementioned HPLC assay (section 2.4) for B-381 concentration. Percent uptake of B-381 was calculated as % Uptake = [(AUCControl-AUCSample)/AUCControl]*100.
2.6 Cellular uptake of BPA in vitro
PBMCs, D54, U87 or HT22 cells (1 × 106 cells/well) were cultured overnight under normoxia. The following day, cells were incubated in normoxic or hypoxic conditions for 4 h in serum free media, then BPA was added with a final concentration of 10 μg/mL for 48 h. Also, BPA solution was added to wells with no cells to serve as a no-cellular uptake control. Media samples were collected from each well at 0 and 48 h, and then were digested with concentrated nitric acid for two days. Samples were diluted with deionized water to a final acid concentration of 5% (v/v) and were filtered through a 0.22 micron polyethersulfone syringe filter (DiKMA Technologies, Lake Forest, CA) and analyzed using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES, Optima 7300 V series, Perkin Elmer, Waltham, MA). Samples were analyzed for boron content (λ = 249.677 nm) against a calibration curve of boron standards of 0, 5.2, 15.625, 31.25, 62.5, 125 and 250 parts per billion (ppb) prepared from a 10 parts per million boron standard solution (Inorganic Ventures, Christiansburg, VA). BPA percent uptake was calculated as % Uptake = [(BoronControl-BoronSample)/BoronControl]*100.
2.7 Tumor retention study of BPA and B-381 in vivo
Approval for all animal studies was obtained from the Ethical Committee for Animal Experiments at Washington University in St. Louis Medical School. Athymic Nude-Foxn1nu mice (N=10, females, 6 week old) were obtained from Envigo (Indianapolis, IN). Mice were anesthetized with ketamine/xylazine and bilaterally injected with 3.5 × 106 D54 glioma cells under the skin of each hindlimb (two injections per mouse). Two weeks post-injection both tumors were palpable under the skin. The mice were split into control (N=1) or treatment (N=9) groups. Each mouse in the treatment group was anesthetized and received two intratumoral injections: the left tumor received BPA (23.3 mg/kg mouse, equivalent to 0.02 mmol boron/mouse) while the right tumor received B-381 (42.9 mg/kg mouse, equivalent to 0.02 mmol boron/mouse). Mice were sacrificed at 8 (N=3), 24 (N=3) and 48 (N=3) h post-injection, and tumors were excised, weighed and digested in concentrated nitric acid. Samples were diluted with deionized water to a final acid concentration of 5% (v/v) and analyzed by ICP-OES for boron content which was normalized to tumor weight (reported as ng of boron/g of tumor (ppb)).
2.8 B-381 in vivo biodistribution study
Five (N=5) athymic Nude-Foxn1nu mice were subcutaneously injected with 3.5 × 106 D54 glioma cells in their back. When the subcutaneous tumors were large and visible, four mice each received a 200 μL intravenous (i.v.) tail vein injection of B-381 (dose 100 mg/kg in 10% w/v captisol solution). The fifth mouse did not receive an injection and was used as control. After 24 h, the mice were anesthetized, blood samples were collected, and the tumors were resected. Blood and tumor samples were weighed and digested in nitric acid. After diluting to a final acid concentration of 5% (v/v) with deionized water, samples were analyzed for boron concentration by ICP-OES and normalized to blood or tumor mass.
2.9 Plasma half-life study of B-381 in vivo
Six C57BL/6 mice (N=6) were intravenously injected with B-381 (50 mg/kg in 10% w/v captisol). Blood was collected from the mice under anesthesia using a submandibular bleeding technique (25) at 5 min, 1, 2, 4, 6 and 24 h (n ≥ 3 for each time point). The blood samples were weighed, digested in nitric acid and analyzed by ICP-OES for boron concentration (normalized to mass of each blood sample).
RESULTS
3.1 Synthesis and characterization of boronated nitroimidazole derivative B-381
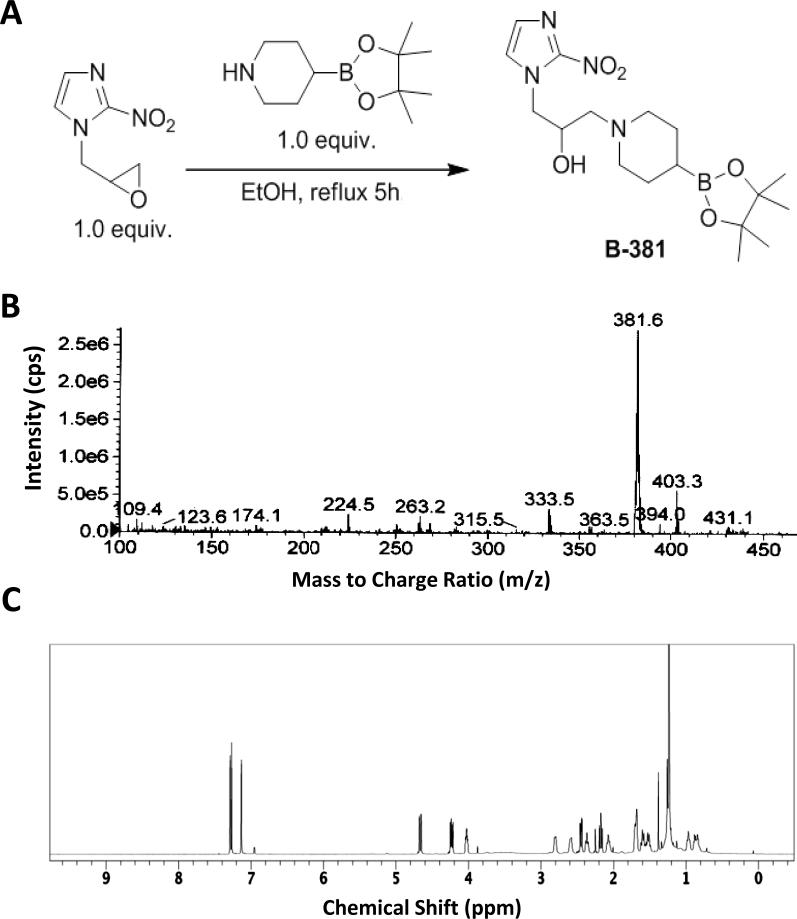
In an effort to design a BNCT agent capable of targeting the hypoxic tumor microenvironment, a facile 1-step synthesis of a boronated 2-nitroimidazole derivative was envisioned. In short, commercially available piperidine-4-boronic acid pinacol ester and 1-(2,3-epoxypropyl)-2-nitroimidazole were refluxed in anhydrous ethanol to afford the boronated 2-nitroimidazole derivative termed B-381 (Figure 1 A). The resulting product had a corresponding mass to charge (m/z) ratio of 381.6 (Figure 1 B), and the structure was further validated by 1H NMR spectroscopy (Figure 1 C).
Figure 1. Synthesis and characterization of boronated nitroimidazole derivative B-381:
Chemical synthesis (A), mass spectrum (B) and 1H nuclear magnetic resonance spectrum in deuterated chloroform (C) of B-381 (IUPAC name: 1-(2-nitro-1H-imidazol-1-yl)-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)piperidin-1-yl)propan-2-ol).
3.2 The effect of B-381 and BPA on viability of normoxic and hypoxic cells in vitro
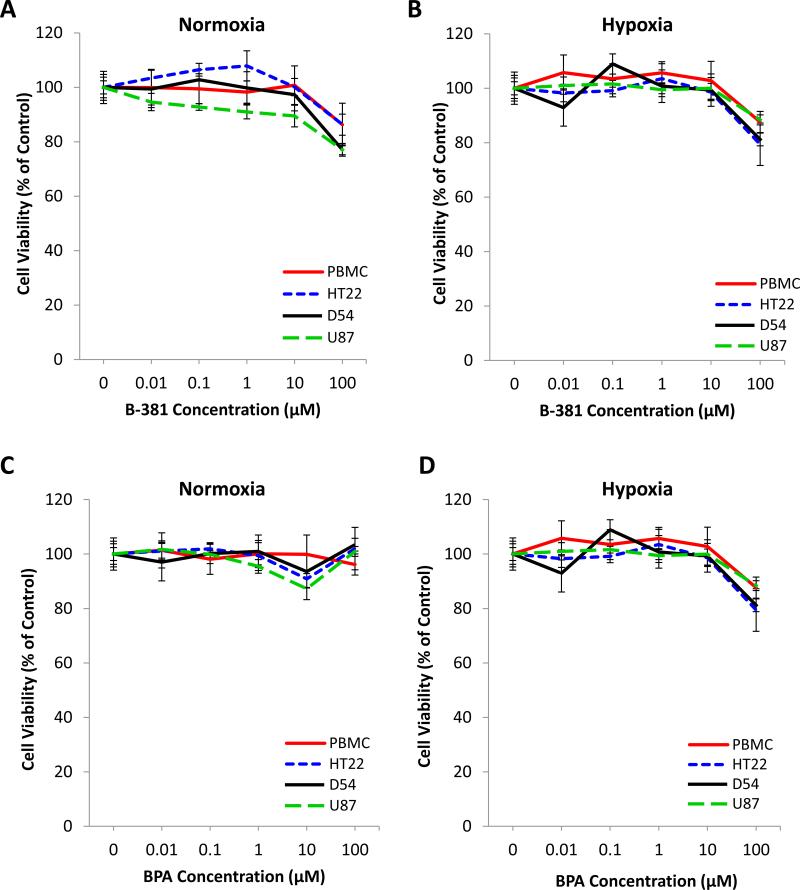
We next evaluated the effect of B-381 compared to the extensively studied BNCT agent BPA on cell viability. In normoxia (21% O2) B-381 illustrated minimal cytotoxicity in all cell lines evaluated (D54, U87, HT22 and PBMCs) up to concentrations of 100 μM (Figure 2 A). The cytotoxicity profile of B-381 was nearly identical under hypoxia (0.5% O2) (Figure 2 B). In comparison, BPA showed minimal cytotoxicity at concentrations up to 100 μM in all cell lines evaluated in both normoxic (Figure 2 C) and hypoxic (Figure 2 D) conditions. Additionally, the long-term cytotoxicity of B-381 was evaluated in normoxia and hypoxia for the D54 cell line. Even after a 72 h treatment of B-381 up to concentrations of 100 μM, no cytotoxicity was observed (Supplementary Figure 1).
Figure 2. The effect of B-381 and BPA on viability of normoxic and hypoxic cells in vitro.
The effect of a 24 h treatment with B-381 (A and B) and BPA (C and D) on the viability of PBMCs from healthy subjects, hippocampal cell line HT22, and glioma cell lines D54 and U87 when cultured in normoxia (A and C) and in hypoxia (B and D). Viability was analyzed using MTT assay normalized to untreated control.
3.3 Cellular uptake of B-381 and BPA in normoxic and hypoxic cells in vitro
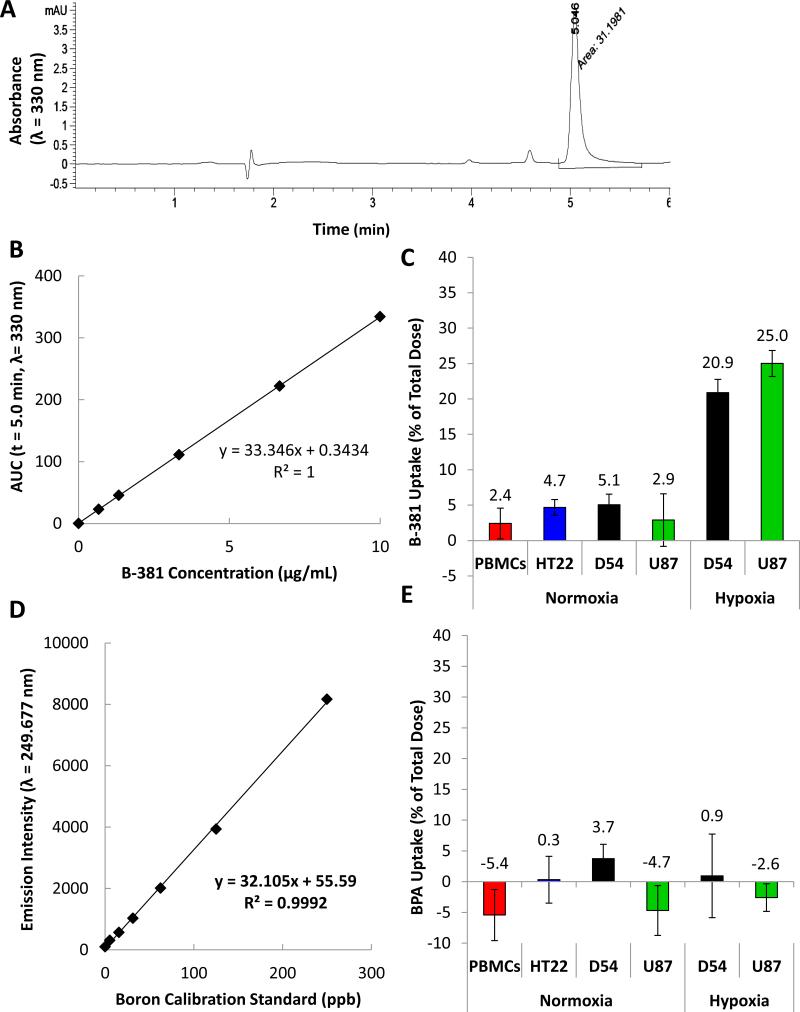
For evaluating the in vitro cellular uptake of B-381, a reverse phase HPLC assay was developed. B-381 had a retention time of 5 minutes (Figure 3 A) and illustrated a linear dynamic range in the concentration range between 0-10 μg/mL with a R2 = 1 (Figure 3 B). Cells were treated for 48 h with 10 μg/mL of B-381 under normoxia (PBMCs, HT22, D54 and U87) or hypoxia (D54 and U87). Percent uptake was calculated by comparing the AUC values for B-381 in media aliquots at 0 and 48 h time points. In all cell lines treated under normoxic conditions (PBMCs, HT22, D54 and U87), B-381 had a low percent cellular uptake of < 5 % after 48 h. In contrast, D54 and U87 glioma cell lines treated under hypoxia had significantly higher B-381 uptake of 21% and 25%, respectively (Figure 3C). Subsequently the in vitro cellular uptake of BPA was evaluated using ICP-OES. Boron standards between 0-250 ppb had a linear dynamic range with a R2 = 0.9992 (Figure 3 D). Using 10 μg/mL of BPA under normoxia (PBMCs, HT22, D54 and U87) or hypoxia (D54 and U87), BPA had a low percent uptake of approximately < 5% in all conditions evaluated (Figure 3 E). These results indicate that B-381 preferentially accumulates in hypoxic glioma cells compared to BPA.
Figure 3. Cellular uptake of B-381 and BPA in normoxic and hypoxic cells in vitro.
Representative HPLC chromatogram for B-381 on a C-18 column with a 0-10% acetonitrile gradient (containing 0.1% trifluoroacetic acid) in water over 7 minutes with a retention time = 5 min (λ = 330 nm) (A). Calibration curve of B-381 for HPLC cellular uptake study (B). Cellular uptake of B-381 in normoxic PBMCs from normal subjects, normoxic hippocampal cell line HT22, and in normoxic and hypoxic glioma cell lines D54 and U87 (C). Calibration curve of BPA for detection of boron using ICP-OES (D). Cellular uptake studies of BPA in normoxic PBMCs from normal subjects, normoxic hippocampal cell line HT22, and in normoxic and hypoxic glioma cell lines D54 and U87 (E).
3.4 Tumor retention, biodistribution and pharmacokinetics of B-381 in vivo
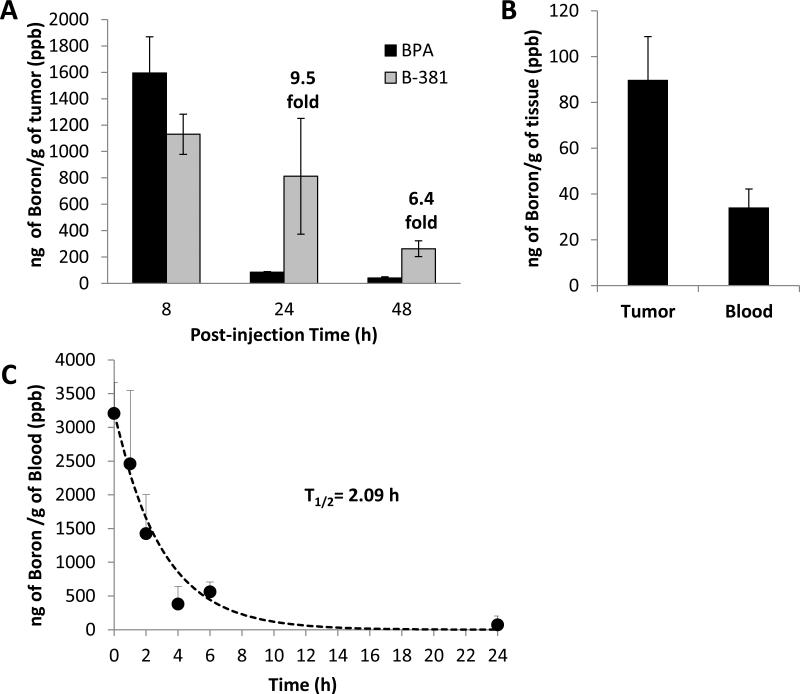
To eliminate the biodistribution and metabolic components associated with B-381 administration, an intratumoral injection of B-381 was compared to BPA in order to verify the tumor retention of B-381 due to its presumed formation of protein conjugates in a hypoxic microenvironment. To compare the in vivo tumor accumulation of B-381 and BPA, a D54 glioma xenograft mouse model was utilized. Mice containing bilateral hindlimb D54 glioma tumors were intratumorally injected with equimolar concentrations of BPA or B-381. Following injection, mice were sacrificed at 8, 24 or 48 h. Tumors were excised, digested and boron concentration was determined with ICP-OES. The tumor concentration of BPA and B-381 was nearly identical 8 h post-injection (1,595 ± 274 ppb and 1,130 ± 152 ppb, respectively, Figure 4 A). However, at 24 and 48 h post-injection, the concentration of BPA was almost undetectable. In contrast to BPA, the tumor demonstrated a long-term retention of B-381, with 9.5-fold and 6.4-fold higher boron levels at 24 and 48 h, respectively.
Figure 4. Tumor retention, biodistribution and pharmacokinetics of B-381 in vivo.
Tumor boron concentration analyzed by ICP-OES after intratumoral injection of left tumor with BPA (23.3 mg/kg mouse, equivalent to 0.02 mmol boron/mouse) and intratumoral injection of right tumor with B-381 (42.9 mg/kg mouse, equivalent to 0.02 mmol boron/mouse) (A). Biodistribution of boron 24 h after intravenous injection of 100 mg/kg B-381 into D54 glioma bearing mice, sacrificed 24 h post-injection and boron content determined by ICP-OES (B). Pharmacokinetic analysis of boron blood levels after intravenous injection of 50 mg/kg of B-381 using naïve mice analyzed by ICP-OES (C).
Following the observed preferential hypoxic tumor accumulation of B-381 in vivo (Figure 4 A), the biodistribution of B-381 was investigated. Five (N=5) athymic nude mice containing subcutaneous D54 glioma tumors were treated with B-381. Four mice received an i.v. injection of B-381, while the fifth mouse did not receive an injection and was used as a control. After 24 h, the boron content in tumor and blood was determined by ICP-OES. B-381 had preferential tumor accumulation, in which average tumor boron levels were 89.9 ± 18.9 ppb, while blood levels were 34.2 ± 8.0 ppb (Figure 4 B). This correlated to a tumor/blood ratio of 2.6.
An in vivo pharmacokinetic study was performed to determine the plasma half-life (T1/2) for B-381. In brief, C57BL/6 mice received an i.v. injection of B-381. Blood was collected at 5 min, 1 h, 2 h, 4 h, 6 h and 24 h post-injection, and boron levels were determined by ICP-OES (Figure 4 C). Immediately following i.v. injection, a maximal boron level of 3,205 ± 458 ppb was detected (time = 5 min). Based on first order elimination kinetics, it was determined that B-381 was quickly eliminated from the blood with a T1/2 = 2.09 h.
DISCUSSION
BNCT is a promising therapeutic approach based on the nuclear fission reaction of boron that is triggered by neutron irradiation. The resulting intracellular production of high-energy alpha particles can target tumor cells for destruction while having less off-target associated cytotoxicity compared to XRT and chemotherapy (6, 7). However, the potential of BNCT to have a targeted tumoricidal effect is limited by the ability of a boronated agent to accumulate specifically in the tumor (ideally T/N > 3). Clinical trial agents BPA and BSH in glioma have suffered from poor tumor selectivity, with T/N ratios of 1.1 – 2.9 for BPA (10, 26, 27) and 0.7 - 3.6 for BSH (28-30). Therefore, in order for the full therapeutic potential of BNCT to be realized, there is an urgent need to develop novel boronated tumor selective compounds.
To improve tumor selectivity in the setting of glioma, we envisioned that the hypoxic tumor microenvironment could be exploited as a targeting strategy. Hypoxic tumor cells contribute to chemotherapy (17) and XRT (18) resistance. Various factors such as increased expression of drug efflux pumps, decreased cell proliferation and oxygen-dependent cytotoxicity all play important factors in hypoxia-mediated drug resistance (13). It was previously shown that these hypoxic areas have more reductive rather than the normal-cell oxidative metabolism (13), and we hypothesized that this property could be used to specifically target these tumor regions.
It has long been recognized that 2-nitromidazole derivatives can selectively accumulate in hypoxic cells. The most recognized 2-nitroimidazole derivative is pimonidazole, which is a gold-standard immunohistochemical marker of hypoxia (31). In hypoxia, the nitro functional group undergoes a series of reductions and is converted to an amine (32). This thereby makes the nitroimidazole ring susceptible to forming intracellular protein conjugates with thiol-containing proteins such as glutathione, which in turn causes accumulation of the nitroimidazole derivative in hypoxic cells. In an oxygen rich (normoxic) environment, the nitro functional group remains in its oxidized form, thereby preventing the formation of the aforementioned protein conjugates. This differential metabolism in normoxic and hypoxic cells has been utilized to develop a PET imaging agent for hypoxic tumor regions. 18F-Fluoromisonidazole is a clinically used fluorinated-2-nitroimidazole PET agent for monitoring tumor hypoxia in glioma patients (33). Therefore, we hypothesized that synthesizing a boronated 2-nitromidazole derivative should be able to selectively deliver boron to hypoxic glioma cells.
Herein we report the synthesis of B-381, which is a new boronated derivative of 2-nitroimidazole (Figure 1 A). B-381 is readily synthesized from a one-step reaction with commercially available precursors and is easily purified as a precipitate in methanol. After precipitation, B-381 has a clear mass spectrum (Figure 1 B) with a single major peak observed on the HPLC chromatogram (Figure 3 A). Consistent with the structure of B-381, we observed a characteristic singlet peak in the 1H NMR spectrum (integrating to 12 protons) that is a result of 4 methyl groups found in the pinacol ester group. Additionally, the aromatic region contained 2 nitroimidazole ring protons, while the remaining NMR peaks in the aliphatic region accounted for the final 14 protons (Figure 1 C).
In order to be a suitable drug candidate for BNCT, the boronated agent must have low systemic cytotoxicity (7). Dose-limiting toxicities could prevent sufficient tumor boron levels being reached which are required for BNCT to achieve a therapeutic effect. The cytotoxicity profile of B-381 in both normoxia and hypoxia is analogous to routinely studied BPA, and concentrations up to 100 μM can be studied with minimal cytotoxicity (Figure 2). Additionally, B-381 exhibited a superior cytotoxic profile compared to BSH, which has an IC50 value of 2.5 μM (34).
While BPA and BSH are the most extensively studied BNCT agents, their suboptimal T/N ratios observed in patients (usually T/N < 3) limits the efficacy of BNCT (7). An ideal BNCT agent would have minimal systemic cytotoxicity and most importantly selective tumor accumulation with a T/N ratio of 3:1 or greater (6, 8, 35-40). In vitro cellular uptake studies show that B-381 selectively accumulated in a hypoxic tumor environment. Specifically, B-381 accumulated 4.1-fold higher in hypoxic D54 and 8.6-fold higher in hypoxic U87 cells compared to their normoxic controls. Additionally, compared to HT22, cellular uptake of B-381 in hypoxic D54 and U87 translated into T/N ratios of 4.4 and 5.3, respectively. On the other hand, the clinically used compound BPA showed very low uptake of boron in the cells, and showed poor tumor selectivity. The level of B-381 tumor selectivity satisfies requirements for effective BNCT and should be adequate to minimize off-target side effects to normal brain cells. Furthermore, the tumor selectivity of B-381 is higher than clinical T/N ratios achieved with BPA and BSH, which commonly have a T/N ratio between 0.7 – 3.6 (10, 26-30).
Intratumoral injection of B-381 in vivo showed that it was selectively retained in the tumor significantly longer than BPA. While tumor boron levels were almost undetectable in the tumor at 24 and 48 h post-injection of BPA, B-381 had significantly longer tumor retention with values at 24 h similar to boron levels at 8 h. These results may be a direct result of B-381 forming intracellular protein conjugates in the hypoxic tumor microenvironment, which would be consistent with the in vitro results and with the known mechanism of 2-nitroimidazole compound accumulation. The long-term retention of B-381 can offer a clinical advantage compared to BPA, providing a longer therapeutic window for neutron irradiation.
In addition to the selective tumor retention, the tumor/blood ratio is an important factor to demonstrate selective uptake in the tumor and prevent damage to normal blood vessels during BNCT. To determine the tumor/blood ratio of B-381, mice received an i.v. injection of B-381 and boron levels were detected in the tumor and blood 24 h post-injection. We found that B-381 accumulated in the tumor against the concentration gradient, in which the tumor boron level was about 3-fold higher than the blood. These findings again indicate that B-381 is a good candidate for use in BNCT. However, we observed relatively low levels of tumor boron accumulation following i.v. injection of B-381, which can be attributed to fast elimination of the drug from the plasma. Therefore, we performed a pharmacokinetic analysis of B-381 following i.v. injection which found that it was quickly eliminated from the plasma (T1/2 = 2.09 h). Thus, it is not surprising that the tumor boron content was lower following the i.v. injection. This is a classic drug delivery problem, where a drug is effective in the tumor environment but suffers from poor pharmacokinetics. Therefore, to maximize its tumor efficacy, B-381 can benefit from a secondary drug delivery system to bring the drug to the vicinity of the tumor and reduce pharmacokinetic elimination. Such studies are ongoing for developing a drug delivery system to improve the tumor delivery of B-381.
CONCLUSIONS
In conclusion, we have reported the synthesis and preliminary biological evaluation of B-381 as a novel agent for BNCT. B-381 had minimal cytotoxic ity, preferentially accumulated in hypoxic cells, and demonstrated longer tumor retention in an in vivo glioma model compared to BPA. It achieved significant tumor/normal tissue ratios as well as tumor/blood ratios which are in compliance with the requirements for selective and successful BNCT. However, it presented relatively poor pharmacokinetics. B-381 presents a new class of BNCT agents in which their selectivity to tumors is based on tumor metabolism and biology. Future studies are warranted to synthesize similar compounds with better pharmacokinetics, or for the development of drug delivery systems to improve boron delivery to the tumor environment.
Supplementary Material
ACKNOWLEDGEMENTS
The first author thanks the N.I.H. Training Grant T32 GM007200 for research support.
ABBREVIATIONS
- 1H-NMR
Proton nuclear magnetic resonance spectroscopy
- 10B
Boron-10
- AUC
Area under curve
- BNCT
Boron neutron capture therapy
- BPA
L-boronophenylalanine
- BSH
Sodium borocaptate
- DMEM
Dulbecco's modified eagle's medium
- FBS
Fetal bovine serum
- GBM
Glioblastoma multiforme
- HPLC
High performance liquid chromatography
- ICP-OES
Inductively coupled plasma optical emission spectrometry
- i.v.
Intravenous
- m/z
Mass to charge (m/z)
- PBMC
Peripheral blood mononuclear cells
- PBS
Phosphate-buffered saline
- PET
Positron emission tomography
- ppb
Parts per billion
- XRT
Radiation therapy
- T1/2
Half-life
- T/N
Tumor/normal tissue
Footnotes
CONFLICT OF INTERESTS
Mr. Luderer and Dr. Azab have a pending provisional patent application describing the work reported in this manuscript. Moreover, Dr. Azab receives research support from Verastem, Selexys, Karyopharm, Cell Works, Cleave Bioscience, and Glycomimetics; and is the founder and owner of Targeted Therapeutics LLC and Cellatrix LLC. Dr. de la Puente is co-founder of Cellatrix LLC. Other authors state no conflicts of interest.
REFERENCES
- 1.Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nature clinical practice Neurology. 2006;2(9):494–503. doi: 10.1038/ncpneuro0289. quiz 1 p following 16. Epub 2006/08/26. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009;10(5):459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. New England Journal of Medicine. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Rij Cv, Wilhelm A, Sauerwein WG, Loenen Av. Boron neutron capture therapy for glioblastoma multiforme. Pharm World Sci. 2005;27(2):92–5. doi: 10.1007/s11096-004-2850-7. [DOI] [PubMed] [Google Scholar]
- 5.Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma—are we there yet? Neuro-Oncology. 2012 doi: 10.1093/neuonc/nos273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azab AK, Abu Ali H, Srebnik M. Chapter 5 Boron neutron capture therapy. In: Hijazi Abu Ali VMD, Morris S, editors. Studies in Inorganic Chemistry. Elsevier; 2006. pp. 337–66. [Google Scholar]
- 7.Luderer M, de la Puente P, Azab A. Advancements in Tumor Targeting Strategies for Boron Neutron Capture Therapy. Pharm Res. 2015:1–13. doi: 10.1007/s11095-015-1718-y. [DOI] [PubMed] [Google Scholar]
- 8.Barth RF, Coderre JA, Vicente MGH, Blue TE. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clinical Cancer Research. 2005;11(11):3987–4002. doi: 10.1158/1078-0432.CCR-05-0035. [DOI] [PubMed] [Google Scholar]
- 9.Chandra S, Barth RF, Haider SA, Yang W, Huo T, Shaikh AL, et al. Biodistribution and subcellular localization of an unnatural boron-containing amino acid (cis-ABCPC) by imaging secondary ion mass spectrometry for neutron capture therapy of melanomas and gliomas. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0075377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pellettieri L, H-Stenstam B, Rezaei A, Giusti V, Sköld K. An investigation of boron neutron capture therapy for recurrent glioblastoma multiforme. Acta Neurologica Scandinavica. 2008;117(3):191–7. doi: 10.1111/j.1600-0404.2007.00924.x. [DOI] [PubMed] [Google Scholar]
- 11.Brat DJ, Mapstone TB. Malignant glioma physiology: cellular response to hypoxia and its role in tumor progression. Annals of internal medicine. 2003;138(8):659–68. doi: 10.7326/0003-4819-138-8-200304150-00014. Epub 2003/04/16. [DOI] [PubMed] [Google Scholar]
- 12.Snajdr I, Janousek Z, Takagaki M, Cisarova I, Hosmane NS, Kotora M. Alpha (alpha-) and beta (beta- carboranyl-C-deoxyribosides: syntheses, structures and biological evaluation. European journal of medicinal chemistry. 2014;83:389–97. doi: 10.1016/j.ejmech.2014.06.005. Epub 2014/07/02. [DOI] [PubMed] [Google Scholar]
- 13.Muz B, de la Puente P, Azab F, Luderer M, Azab AK. The role of hypoxia and exploitation of the hypoxic environment in hematologic malignancies. Molecular cancer research : MCR. 2014;12(10):1347–54. doi: 10.1158/1541-7786.MCR-14-0028. [DOI] [PubMed] [Google Scholar]
- 14.Oliver L, Olivier C, Marhuenda FB, Campone M, Vallette FM. Hypoxia and the malignant glioma microenvironment: regulation and implications for therapy. Current molecular pharmacology. 2009;2(3):263–84. doi: 10.2174/1874467210902030263. Epub 2009/12/22. [DOI] [PubMed] [Google Scholar]
- 15.Fu Y, Zheng S, Zheng Y, Huang R, An N, Liang A, et al. Glioma derived isocitrate dehydrogenase-2 mutations induced up-regulation of HIF-1alpha and beta-catenin signaling: possible impact on glioma cell metastasis and chemo-resistance. The international journal of biochemistry & cell biology. 2012;44(5):770–5. doi: 10.1016/j.biocel.2012.01.017. Epub 2012/02/09. [DOI] [PubMed] [Google Scholar]
- 16.Greenfield JP, Cobb WS, Lyden D. Resisting arrest: a switch from angiogenesis to vasculogenesis in recurrent malignant gliomas. The Journal of clinical investigation. 2010;120(3):663–7. doi: 10.1172/JCI42345. Epub 2010/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haar CP, Hebbar P, Wallace GCt, Das A, Vandergrift WA, 3rd, Smith JA, et al. Drug resistance in glioblastoma: a mini review. Neurochemical research. 2012;37(6):1192–200. doi: 10.1007/s11064-011-0701-1. Epub 2012/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amberger-Murphy V. Hypoxia helps glioma to fight therapy. Current cancer drug targets. 2009;9(3):381–90. doi: 10.2174/156800909788166637. Epub 2009/05/16. [DOI] [PubMed] [Google Scholar]
- 19.Rycaj K, Tang DG. Cancer stem cells and radioresistance. International journal of radiation biology. 2014;90(8):615–21. doi: 10.3109/09553002.2014.892227. Epub 2014/02/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bell C, Dowson N, Fay M, Thomas P, Puttick S, Gal Y, et al. Hypoxia Imaging in Gliomas With F Fluoromisonidazole PET: Toward Clinical Translation. Seminars in nuclear medicine. 2015;45(2):136–50. doi: 10.1053/j.semnuclmed.2014.10.001. Epub 2015/02/24. [DOI] [PubMed] [Google Scholar]
- 21.Parker NR, Khong P, Parkinson JF, Howell VM, Wheeler HR. Molecular heterogeneity in glioblastoma: potential clinical implications. Frontiers in oncology. 2015;5:55. doi: 10.3389/fonc.2015.00055. Epub 2015/03/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernsen HJ, Rijken PF, Peters H, Raleigh JA, Jeuken JW, Wesseling P, et al. Hypoxia in a human intracerebral glioma model. Journal of neurosurgery. 2000;93(3):449–54. doi: 10.3171/jns.2000.93.3.0449. Epub 2000/09/02. [DOI] [PubMed] [Google Scholar]
- 23.Olive PL, Durand RE, Raleigh JA, Luo C, Aquino-Parsons C. Comparison between the comet assay and pimonidazole binding for measuring tumour hypoxia. Br J Cancer. 2000;83(11):1525–31. doi: 10.1054/bjoc.2000.1489. Epub 2000/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de la Puente P, Azab F, Muz B, Luderer M, Arbiser J, Azab AK. Tris DBA palladium overcomes hypoxia-mediated drug resistance in multiple myeloma. Leukemia & Lymphoma. 2015:1–10. doi: 10.3109/10428194.2015.1099645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.William T, Golde PG, Luis L. Rodriguez. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Animal. 2005;34(9):4. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 26.Liberman SJ, Dagrosa A, Jimenez Rebagliati RA, Bonomi MR, Roth BM, Turjanski L, et al. Biodistribution studies of boronophenylalanine-fructose in melanoma and brain tumor patients in Argentina. Applied radiation and isotopes : including data, instrumentation and methods for use in agriculture, industry and medicine. 2004;61(5):1095–100. doi: 10.1016/j.apradiso.2004.05.013. Epub 2004/08/17. [DOI] [PubMed] [Google Scholar]
- 27.Aihara T, Hiratsuka J, Morita N, Uno M, Sakurai Y, Maruhashi A, et al. First clinical case of boron neutron capture therapy for head and neck malignancies using 18F-BPA PET. Head & neck. 2006;28(9):850–5. doi: 10.1002/hed.20418. Epub 2006/05/25. [DOI] [PubMed] [Google Scholar]
- 28.Wittig A, Malago M, Collette L, Huiskamp R, Buhrmann S, Nievaart V, et al. Uptake of two 10B- compounds in liver metastases of colorectal adenocarcinoma for extracorporeal irradiation with boron neutron capture therapy (EORTC Trial 11001). International journal of cancer Journal international du cancer. 2008;122(5):1164–71. doi: 10.1002/ijc.23224. Epub 2007/11/07. [DOI] [PubMed] [Google Scholar]
- 29.Neumann M, Bergmann M, Gabel D. Cell type selective accumulation of mercaptoundecahydro- closo-dodecaborate (BSH) in glioblastoma multiforme. Acta neurochirurgica. 2003;145(11):971–5. doi: 10.1007/s00701-003-0117-z. Epub 2003/11/25. [DOI] [PubMed] [Google Scholar]
- 30.Wittig A, Collette L, Appelman K, Buhrmann S, Jackel MC, Jockel KH, et al. EORTC trial 11001: distribution of two 10B-compounds in patients with squamous cell carcinoma of head and neck, a translational research/phase 1 trial. Journal of cellular and molecular medicine. 2009;13(8B):1653–65. doi: 10.1111/j.1582-4934.2009.00856.x. Epub 2009/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varia MA, Calkins-Adams DP, Rinker LH, Kennedy AS, Novotny DB, Fowler WC, et al. Pimonidazole: A Novel Hypoxia Marker for Complementary Study of Tumor Hypoxia and Cell Proliferation in Cervical Carcinoma. Gynecologic Oncology. 1998;71(2):270–7. doi: 10.1006/gyno.1998.5163. [DOI] [PubMed] [Google Scholar]
- 32.Varghese AJ, Gulyas S, Mohindra JK. Hypoxia-dependent Reduction of 1-(2-Nitro-1-imidazolyl)-3- methoxy-2-propanol by Chinese Hamster Ovary Cells and KHT Tumor Cells in Vitro and in Vivo. Cancer Research. 1976;36(10):3761–5. [PubMed] [Google Scholar]
- 33.Bell C, Dowson N, Fay M, Thomas P, Puttick S, Gal Y, et al. Hypoxia Imaging in Gliomas With 18F Fluoromisonidazole PET: Toward Clinical Translation. Seminars in Nuclear Medicine. 2015;45(2):136–50. doi: 10.1053/j.semnuclmed.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Genady AR, Ioppolo JA, Azaam MM, El-Zaria ME. New functionalized mercaptoundecahydrododecaborate derivatives for potential application in boron neutron capture therapy: Synthesis, characterization and dynamic visualization in cells. European Journal of Medicinal Chemistry. 2015;93:574–83. doi: 10.1016/j.ejmech.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 35.Pisarev MA, Dagrosa MA, Juvenal GJ. Boron neutron capture therapy in cancer: past, present and future. Arq Bras Endocrinol Metabol. 2007;51(5):852–6. doi: 10.1590/s0004-27302007000500024. [DOI] [PubMed] [Google Scholar]
- 36.Barth RF, Vicente MG, Harling OK, Kiger WS, 3rd, Riley KJ, Binns PJ, et al. Current status of boron neutron capture therapy of high grade gliomas and recurrent head and neck cancer. Radiat Oncol. 2012;7(146):7–146. doi: 10.1186/1748-717X-7-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soloway AH, Tjarks W, Barnum BA, Rong F-G, Barth RF, Codogni IM, et al. The Chemistry of Neutron Capture Therapy. Chemical Reviews. 1998;98(4):1515–62. doi: 10.1021/cr941195u. [DOI] [PubMed] [Google Scholar]
- 38.Hawthorne MF, Lee M. A critical assessment of boron target compounds for boron neutron capture therapy. J Neurooncol. 2003;62(1-2):33–45. doi: 10.1007/BF02699932. [DOI] [PubMed] [Google Scholar]
- 39.Hosmane NS. Boron and Gadolinium Neutron Capture Therapy for Cancer Treatment. World Scientific Publishing Co.; Singapore, SGP: 2012. [Google Scholar]
- 40.Hosmane NS. Boron science : new technologies and applications. CRC Press; Boca Raton. FL: 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.