Abstract
Objective
We sought to increase adolescent HIV testing across rural communities in east Africa and identify predictors of undiagnosed HIV.
Design
Hybrid mobile testing.
Methods
We enumerated 116,326 adolescents (10–24 years) in 32 communities of Uganda and Kenya (SEARCH:NCT01864603): 98,694 (85%) reported stable (≥6 months of prior year) residence. In each community we performed hybrid testing: 2- week multi-disease community health campaign (CHC) that included HIV testing, followed by home-based testing of CHC non-participants. We measured adolescent HIV testing coverage and prevalence, and determined predictors of newly-diagnosed HIV among HIV+ adolescents using multivariable logistic regression.
Results
86,421 (88%) stable adolescents tested for HIV; coverage was 86%, 90%, and 88% in early (10–14), mid (15–17) and late (18–24) adolescents, respectively. Self- reported prior testing was 9%, 26%, and 55% in early, mid and late adolescents tested, respectively. HIV prevalence among adolescents tested was 1.6% and 0.6% in Ugandan women and men, and 7.1% and 1.5% in Kenyan women and men, respectively. Prevalence increased in mid-adolescence for women, and late adolescence for men. Among HIV+ adolescents, 58% reported newly-diagnosed HIV. In multivariate analysis of HIV+ adolescents, predictors of newly-diagnosed HIV included male gender (OR=1.97 [95%CI: 1.42–2.73]), Ugandan residence (OR=2.63 [95%CI: 2.08–3.31]), and single status (OR=1.62 [95%CI: 1.23–2.14] vs. married).
Conclusions
The SEARCH hybrid strategy tested 88% of stable adolescents for HIV, a substantial increase over the 28% reporting prior testing. The majority (57%) of HIV+ adolescents were new diagnoses. Mobile HIV testing for adults should be leveraged to reach adolescents for HIV treatment and prevention.
Keywords: Adolescent, mobile HIV Testing, Uganda, Kenya, Community Health Campaign, home-based testing
INTRODUCTION
Among adolescents living with HIV, approximately 80% live in sub-Saharan Africa, and 15–24 year olds accounted for an estimated 39% of new HIV infections among people ≥15 years of age in 2012.[1] Despite the disproportionate share of incident infections occurring during adolescence compared with adulthood, national survey data across sub-Saharan Africa estimate that less than a third of adolescents have ever tested for HIV.[2, 3]
Adolescence is a critical time of physical, developmental and social change, and often includes sexual debut. Reaching adolescents for HIV testing offers an opportunity to establish HIV prevention behaviors, including routine testing and sexual education, and may reduce HIV incidence.[4] By late adolescence (18–24 years), HIV is a leading cause of preventable mortality in sub-Saharan Africa, and untreated infection contributes to ongoing transmission, at a time when youth are establishing regular sexual partnerships, having children, and entering the work force in greater numbers.[5] On this basis, population-wide HIV testing approaches that reach adolescents are needed.
The SEARCH Trial (NCT01864603) is a cluster-randomized trial evaluating the impact of population-based HIV testing and universal ART on HIV incidence and other health and economic outcomes, in 32 rural communities in Uganda and Kenya. In 2013–14, the baseline year of the trial, a hybrid mobile testing approach was used in an effort to achieve universal HIV testing in these communities. In this approach, census enumeration in each community was followed by multi-disease community health campaigns (CHC) to rapidly scale-up testing coverage.[6] CHC non-participants were then approached for home-based testing (HBT). We previously published that this approach achieved 89% HIV testing coverage among stable residents ≥15 years of age.[6] In the present analysis, we focus on adolescents (10–24 years) to describe HIV testing coverage in this vulnerable population by adolescent stage, characterize timing of increases in HIV prevalence by age and gender relative to reported prior HIV testing, and identify predictors of undiagnosed HIV among HIV-infected adolescents.
METHODS
In 2013–14, a door-to-door census was performed to enumerate all residents in the 32 communities, as previously described.[6] Each community is composed of one or more geopolitical units, just above the village level, with an approximate population of 10,000, within the catchment area of an HIV clinic in southwestern Uganda, eastern Uganda or western Kenya. Stable residence was defined as living in community for ≥6 months in the year prior to enumeration.
In all 32 communities a hybrid mobile testing strategy of multi-disease CHCs over 2 weeks per community, followed by HBT was implemented by SEARCH staff, as previously described (see annex).[6] HIV services included point-of-care (POC) HIV antibody testing according to national guidelines, followed by POC CD4+ T cell count measurement (PIMA, Alere Inc., Waltham, Massachusetts, USA) and prompt referral to care for HIV-infected participants. Non-HIV services included screening for hypertension, diabetes and malaria (annex). Enumerated residents who did not attend the CHC were approached for HBT over 1–2 months.
Adolescent stage was defined as early (10–14 years), mid (15–17) and late (18–24).[7] HIV prevalence was estimated with adolescents who tested for HIV as the denominator. HIV-infected adolescents were considered new diagnoses if they self-reported no prior HIV testing or that their last HIV test was negative.
Proportions were compared with a χ2 test or Fischer’s exact test, as appropriate. Multivariable logistic regression was used to determine predictors of HIV infection among all stable adolescents who participated in testing, with adolescent stage, sex, country of residence, marital status, self-reported prior HIV testing, and migration (months away from community, up to six months) as independent predictors. Multivariable logistic regression was also used to determine predictors of newly diagnosed HIV among stable, HIV-infected adolescents, with adolescent stage, sex, country, marital status and migration as independent predictors.
Ethics statement
The Makerere University School of Medicine Research and Ethics Committee and Ugandan National Council on Science and Technology (Uganda), the Kenya Medical Research Institute Scientific and Ethics Review Unit (Kenya), and the University of California San Francisco Committee on Human Research approved the consent procedures and the study. All participants, or their parent/guardian, provided verbal informed consent in their preferred language with biometric fingerprint confirmation of agreement. Adolescents 13–17 years could provide verbal consent if a parent/guardian was not present, whereas <13 year-olds could not participate without an assenting parent/guardian present, in accordance with Uganda and Kenya Ministry of Health guidelines.[8, 9]
RESULTS
A total of 116,326 adolescent residents (10–24 years) were enumerated in the 32 communities in Kenya (41,633) and Uganda (74,693) from April 2013 to June 2014: 56,880 (49%) were male, and 98,694 (85%) reported stable residence (see annex).
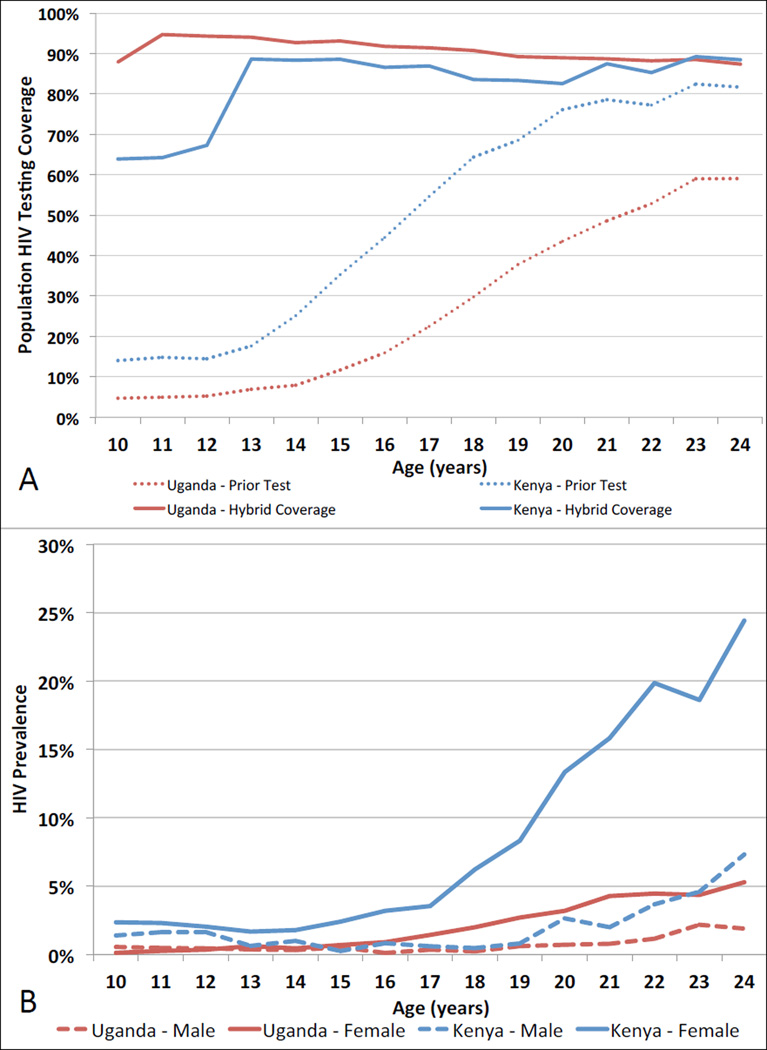
The hybrid strategy achieved 88% HIV testing coverage (range: 75–96% across communities) among stable adolescents (Table). Among those tested, 82% (range: 71–93%) tested via CHCs, and 18% (7–29%) via HBT. Ninety-one percent (37,621/41,563), 74% (11,871/16,057) and 45% (13,001/28,799) of early, mid and late stable adolescent residents who tested for HIV, respectively, reported never previously testing (Figure, Panel A). Ninety-six percent (71,154/73,939) of stable adolescents attending CHCs tested for HIV vs. 15,267/18,229 (84%) reached by HBT. Of 2,785 adolescent CHC participants who did not test for HIV, 2,171 (78%) were 10–12 year-old Kenyans.
Table.
HIV testing coverage following a hybrid mobile testing approach of multi-disease community health campaigns (CHC) followed by home-based testing (HBT) of CHC non-participants, among census-enumerated adolescents (10–24 years) living in 32 SEARCH trial communities in Uganda and Kenya.
| Enumerated Population |
Tested by CHC |
% | Tested by HBT |
% | Tested by Hybrid (CHC+HBT) Approach |
% | |
|---|---|---|---|---|---|---|---|
|
Stable*, 10–24 year old residents |
98,694 | 71,154 | 72% | 15,267 | 15% | 86,421 | 88% |
| Early (10–14 yrs) | 48,127 | 36,892 | 77% | 4,671 | 10% | 41,563 | 86% |
| Mid (15–17 yrs) | 17,756 | 12,574 | 71% | 3,483 | 20% | 16,057 | 90% |
| Late (18–24 yrs) | 32,811 | 21,688 | 66% | 7,113 | 22% | 28,801 | 88% |
| Uganda | 64,993 | 49,622 | 76% | 9,704 | 15% | 59,326 | 91% |
| Kenya | 33,701 | 21,532 | 64% | 5,563 | 17% | 27,095 | 80% |
| Male | 48,281 | 33,518 | 69% | 8,204 | 17% | 41,722 | 86% |
| Female | 50,413 | 37,636 | 75% | 7,063 | 14% | 44,699 | 89% |
| Non-stable residents | 17,632 | 2,835 | 16% | 1,539 | 9% | 4,374 | 25% |
Stable residence was defined as living in a study community for ≥6 months in the year prior to census enumeration.
Figure.
A) Adolescent resident population HIV testing coverage following the hybrid HIV testing approach (solid lines), and self-reported prior HIV testing among adolescents who tested for HIV via hybrid testing (dashed lines), by country of residence (Kenya [blue] or Uganda [red]) and age in 32 SEARCH trial communities. B) HIV prevalence in male (dashed line) and female (solid line) adolescents by country of residence (Kenya [blue] or Uganda [red]) and age.
HIV prevalence by age, gender and country is shown in Figure (Panel B). Prevalence among adolescent stable residents was 1.6% (503/30,827) and 0.6% (167/28,427) in Ugandan women and men, and 7.1% (978/13,836) and 1.5% (195/13,255) in Kenyan women and men, respectively. The number of adolescents needed to test to detect one HIV-infected adolescent was 88 in Uganda, and 23 in Kenya.
Odds of HIV infection among stable adolescent women who tested for HIV were significantly higher in mid- and late-adolescence vs. early adolescence in Uganda (mid: unadjusted OR=2.7, 95% CI: 1.9–4.0, p<0.001; and late: OR=10.0, 95% CI: 7.5–13.2, p<0.001) and Kenya (mid: OR=1.5, 95% CI: 1.1–2.0, p=0.004; and late: OR=8.8, 95% CI: 7.2–10.7, p<0.001). Odds of HIV infection among stable adolescent men were not significantly higher in mid vs. early adolescence in Uganda (OR=0.8, 95% CI: 0.5–1.3, p=0.36) and were lower in mid vs. early adolescence in Kenya (OR=0.5, 95% CI: 0.3–0.8, p=0.004). Odds of HIV infection in late vs. early adolescent men were higher in Uganda (OR=2.2, 9% CI: 1.6–3.0, p<0.001) and Kenya (OR=2.2, 95% CI: 1.6–2.9, p<0.001), among stable male residents tested.
Characteristics associated with increased HIV risk in a multivariable logistic regression model among stable adolescents tested included late adolescence (aOR=2.6, 95% CI: 1.9–3.5, p<0.001, vs. early), female gender (aOR=3.0, 95% CI: 2.6–3.5, p<0.001), Kenya residence (aOR=4.0, 95% CI: 3.5–4.4, p<0.001 vs. Uganda), non-single status (married aOR=2.7, 95% CI: 2.3–3.1, p<0.001; and widowed, divorced or separated aOR=7.4, 95% CI: 5.7–9.6, p<0.001; vs. single), prior HIV testing (aOr=1.6, 95% CI: 1.4–1.9, p<0.001 vs. no prior testing), and mobility in year prior to census (aOR=1.1 for each month away from community, 95% CI: 1.02–1.13, p=0.009, vs. no time away). In further modeling, we identified significant interactions in the effect of gender and adolescent stage, with female mid and late-adolescents having increased odds of HIV compared with early adolescents, as observed in univariate analysis.
Overall, 57% (1,058/1,843) of HIV-infected stable adolescent residents reported being unaware of their status prior to testing; of these, 51% (535/1,058) reported ever having a prior HIV test. In a multivariable logistic regression model, factors significantly associated with newly diagnosed HIV among HIV-infected stable adolescent residents included male gender (OR=2.0, 95% CI: 1.4–2.7, p<0.001), Ugandan residence (OR=2.6, 95% CI: 2.1–3.3, p<0.001), and single status (OR=1.6, 95% CI: 1.2–2.1, p=0.001, vs. married). In further modeling, we identified significant interactions in the effect of country and adolescent stage; Ugandan mid- and late HIV+ adolescents had significantly increased odds of newly diagnosed HIV vs. early adolescents, whereas adolescent stage was not significantly associated with newly diagnosed HIV in Kenyan HIV+ adolescents.
Discussion
The SEARCH hybrid mobile HIV testing strategy tested 88% of stable adolescent residents across 32 rural communities in Uganda and Kenya, a substantial increase over the 28% reporting prior testing. Cross-sectional trends in prevalence suggest an increased risk of HIV beginning in mid-adolescence among women, and late adolescence among men.
Our data suggest HIV testing in a multi-disease context may reduce anticipated stigma and allow adolescents to access testing without concern of being perceived as sexually active or at risk for HIV, in contrast to testing at a clinic.[10] This approach provided an opportunity for the majority of adolescent residents to access other prevention services, including sexual education and condoms , and could serve as a platform for introducing pre-exposure prophylaxis (PrEP) to at-risk youth. For HIV-infected adolescents, this approach offered a chance to ensure access to care and antiretroviral therapy (ART). Given the low rates of school attendance among adolescent women observed (annex), and relatively low use of clinic-based services by adolescents,[10, 11] approaches that are not dependent on accessing schools or clinics may be ideal for reaching a diverse group of adolescents in rural Africa. This approach reached early adolescents before a time of increased HIV risk - a critical window for prevention. Indeed, the 2012 Kenya AIDS Indicator Survey found that though 89% of 10–14 year olds have heard of HIV, only 17% had accurate knowledge about HIV prevention and treatment, and <25% of 12–14 year olds had used condoms at sexual debut.[12]
The trends in HIV prevalence and factors associated with HIV infection observed are consistent with prior studies from rural Africa.[12–15] Given the low prevalence among their male peers, our data are also consistent with prior research attributing incident HIV among adolescent women, in part, to sexual transmission from older men.[16, 17] These data highlight the importance of engaging adult men in HIV testing and treatment. Lower odds of HIV infection among Kenyan men in mid-adolescence may be a marker of decreased survival, or out-migration, of vertically-infected 15–17 year-old men.
Interestingly, we found that factors typically associated with lower HIV prevalence (e.g. male gender) were associated with higher odds of being unaware of one’s status among HIV-infected adolescents. This suggests prior testing initiatives may have targeted high-risk groups, but missed those at relatively lower risk, and universal testing might be needed, at least initially, if “test and treat” interventions to reduce HIV incidence are to succeed in generalized HIV epidemics.
This study has limitations. First, under or over enumeration of residents may have resulted in inaccurate estimates of population coverage. However, our census estimates are similar to national projections.[6] Second, adolescents who declined testing or could not be reached may have had a different HIV prevalence than those tested. Reassuringly, in a large sample of persons ≥15 years from SEARCH communities who did not test in baseline hybrid testing, but tested one year later, there was no difference in HIV prevalence between those who did vs. did not test in the baseline year.[18] Third, self-reported prior testing is subject to bias, but our findings are consistent with prior research.[10, 15] Lastly, in Uganda and Kenya adolescents >12 years can access HIV testing independent of parental consent; our findings may not be generalizable to settings where parental consent is required up to 18 or 21 years.
The SEARCH hybrid testing approach achieved high adolescent HIV testing coverage. Expanding this approach to include PrEP in the context of universal ART may provide a platform for reducing HIV incidence among adolescents in rural sub-Saharan Africa.
Supplementary Material
Acknowledgments
We thank the residents of the 32 SEARCH Trial communities for their participation. We also thank the Uganda and Kenya ministries of health, the Director of the Kenya Medical Research Institute (KEMRI) and the Director of KEMRI’s Centre for Microbiology. KK, TR and GC contributed to study design, data analysis and interpretation, literature search, figures, and writing of the manuscript. TC, EC, MP and DH contributed to study design, data analysis and interpretation, and writing of the manuscript. JK, ES, DK, NS, EB, CC, TL and MK contributed to study design, data interpretation, and writing of the manuscript. Research reported in this publication was supported by Division of AIDS, NIAID of the National Institutes of Health under award number U01AI099959 and in part by the President’s Emergency Plan for AIDS Relief and Gilead Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, PEPFAR, or Gilead.
References
- 1. [Last accessed on February 2, 2016];UNAIDS Fact Sheet: http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/factsheet/2012/20120417_FS_adolescentsyoungpeoplehiv_en.pdf. 2012
- 2.Idele P, Gillespie A, Porth T, Suzuki C, Mahy M, Kasedde S, et al. Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. Journal of acquired immune deficiency syndromes. 2014;66(Suppl 2):S144–S153. doi: 10.1097/QAI.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 3.Staveteig S, Wang S, Head S, Bradley S, Nybro E. DHS Comparative Reports No. 30. Calverton, Maryland, USA: ICF International; 2013. Demographic Patterns of HIV Testing Uptake in Sub-Saharan Africa. [Google Scholar]
- 4.Rosenberg NE, Westreich D, Barnighausen T, Miller WC, Behets F, Maman S, et al. Assessing the effect of HIV counselling and testing on HIV acquisition among South African youth. Aids. 2013;27:2765–2773. doi: 10.1097/01.aids.0000432454.68357.6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Health for the world's adolescents: a second chance in the second decade. Geneva: 2014. [Last accessed on February 2, 2016]. Available at: http://apps.who.int/adolescent/second-decade/files/1612_MNCAH_HWA_Executive_Summary.pdf. [Google Scholar]
- 6.Chamie G, Clark T, Kabami J, Kadede K, Ssemmondo E, Steinfeld R, et al. A hybrid mobile HIV testing approach for population-wide HIV testing in rural East Africa: an observational study. The Lancet HIV. 2016 doi: 10.1016/S2352-3018(15)00251-9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurth AE, Lally MA, Choko AT, Inwani IW, Fortenberry JD. HIV testing and linkage to services for youth. Journal of the International AIDS Society. 2015;18:19433. doi: 10.7448/IAS.18.2.19433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uganda. Uganda national policy guidelines for HIV voluntary counselling and testing. Kampala: Ministry of Health; 2005. Ministry of Health. [Google Scholar]
- 9.Guidelines for HIV Testing and Counselling and Kenya. Nairobi: NASCOP; 2008. National AIDS and STI Control Programme, Ministry of Public Health and Sanitation, Kenya. [Google Scholar]
- 10.Hampanda K, Ybarra M, Bull S. Perceptions of health care services and HIV-related health-seeking behavior among Uganda adolescents. AIDS care. 2014;26:1209–1217. doi: 10.1080/09540121.2014.894612. [DOI] [PubMed] [Google Scholar]
- 11.Otwombe K, Dietrich J, Laher F, Hornschuh S, Nkala B, Chimoyi L, et al. Health-seeking behaviours by gender among adolescents in Soweto, South Africa. Global health action. 2015;8:25670. doi: 10.3402/gha.v8.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National AIDS and STI Control Programme, Kenya AIDS Indicator Survey 2012: Preliminary Report. Kenya: Nairobi; 2012. [Google Scholar]
- 13.Uganda Ministry of Health and ICF International. 2011 Uganda AIDS Indicator Survey: Key Findings. Calverton, Maryland, USA: MOH and ICF International; 2012. [Google Scholar]
- 14.Pettifor AE, Rees HV, Kleinschmidt I, Steffenson AE, MacPhail C, Hlongwa-Madikizela L, et al. Young people's sexual health in South Africa: HIV prevalence and sexual behaviors from a nationally representative household survey. Aids. 2005;19:1525–1534. doi: 10.1097/01.aids.0000183129.16830.06. [DOI] [PubMed] [Google Scholar]
- 15.Wachira J, Ndege S, Koech J, Vreeman RC, Ayuo P, Braitstein P. HIV testing uptake and prevalence among adolescents and adults in a large home-based HIV testing program in Western Kenya. Journal of acquired immune deficiency syndromes. 2014;65:e58–e66. doi: 10.1097/QAI.0b013e3182a14f9e. [DOI] [PubMed] [Google Scholar]
- 16.Kelly RJ, Gray RH, Sewankambo NK, Serwadda D, Wabwire-Mangen F, Lutalo T, et al. Age differences in sexual partners and risk of HIV-1 infection in rural Uganda. Journal of acquired immune deficiency syndromes. 2003;32:446–451. doi: 10.1097/00126334-200304010-00016. [DOI] [PubMed] [Google Scholar]
- 17.Gregson S, Nyamukapa CA, Garnett GP, Mason PR, Zhuwau T, Carael M, et al. Sexual mixing patterns and sex-differentials in teenage exposure to HIV infection in rural Zimbabwe. Lancet. 2002;359:1896–1903. doi: 10.1016/S0140-6736(02)08780-9. [DOI] [PubMed] [Google Scholar]
- 18.Chamie G, Kabami J, Ssemmondo E, Clark T, Bukusi EA, Petersen ML, et al. 94% Population HIV Testing Coverage with Repeat Hybrid Mobile Testing in East Africa. Abstract 979; Presented at the Conference on Retroviruses and Opportunistic Infections (CROI); Boston. 2016. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.