Abstract
Total pancreatectomy with islet autotransplantation (TPIAT) is being used increasingly as a definitive treatment for chronic pancreatitis. Patients with chronic pancreatitis have an elevated risk of pancreatic cancer, which can also masquerade as acute or chronic pancreatitis, making the diagnosis challenging. We describe here the first case of pancreatic ductal adenocarcinoma developing in the liver of a patient after TPIAT for presumed benign chronic pancreatitis. Retrospective analysis of the patient’s preoperative serum revealed normal carbohydrate antigen 19-9 and carcinoembryonic antigen levels but elevated levels of microRNAs -10b, -30c, and -106b compared with controls. Screening guidelines are important to reduce the risk of transplantation of malignant tissue. More sensitive screening tools, including the potential use of microRNAs, are needed to detect early preclinical disease, given the highly malignant nature of pancreatic cancer.
Introduction
Chronic pancreatitis is characterized pathologically by progressive destruction of pancreatic parenchyma and marked fibrosis. Clinically, it is marked by varying degrees of potentially debilitating pain, exocrine insufficiency, and endocrine dysfunction that can lead to pancreatogenic diabetes. In addition to the ramifications of these chronic conditions, patients with chronic pancreatitis are at an increased risk of pancreatic cancer. Therapies to relieve pain may include endoscopic and surgical attempts to relieve pressure in the pancreatic duct and parenchyma. Unfortunately, subsets of patients are not responsive to or appropriate for drainage procedures, limited resection, or other nonsurgical treatment modalities.
Total pancreatectomy with islet autotransplantation (TPIAT) is a treatment that is increasingly being used for patients with refractory disease. Because removal of the entire pancreas leads to complete insulin deficiency, the process of islet autotransplantation was developed (1). Classically, the processed islets are infused into the portal venous system with subsequent embolization into the liver for engraftment.
More than 1000 cases of TPIAT for benign chronic pancreatitis have been performed to date, with no reports of iatrogenic pancreatic cancer metastasis (2–9). In this paper, we report the first case of pancreatic ductal adenocarcinoma (PDAC) developing in the liver of a patient several months after undergoing TPIAT for chronic pancreatitis. Retrospective evaluation of the patient’s serum prior to pancreatectomy revealed the presence of elevated levels of three distinct microRNAs (miRNAs) known to be elevated in the plasma of patients with PDAC but not chronic pancreatitis, suggesting that this patient had early PDAC that was not detected by conventional cancer screening or markers.
Case Report
A 43-year-old male presented to the clinic with 5 years of abdominal pain secondary to chronic pancreatitis. He developed his first episode of acute pancreatitis in 2009 and required intensive care unit admission. This led to escalating episodic pain over several years that required multiple hospitalizations for pancreatitis. He began pancreatic enzyme therapy and underwent cholecystectomy and multiple endoscopic retrograde cholangiopancreatographies with more than five stents placed, all resulting in limited relief. At this point, he presented to the clinic for definitive therapy, given the failure of medical and endoscopic therapy to control his progressively worsening pancreatitis symptoms and significant debilitation from chronic pain. His medical history was otherwise unremarkable with pertinent endoscopic and surgical procedures, as listed above. He used a buprenorphine patch, tramadol, oxycodone and hydromorphone for pain relief. He reported tobacco smoking with a history of 13 pack-years but denied alcohol use since his first pancreatitis episode. His family history was negative for chronic pancreatitis or pancreatic malignancies.
His clinical examination was remarkable for epigastric tenderness. Preoperative laboratory test results were notable for elevated lipase (401 U/L); normal endocrine function (fasting glucose 73 mg/dL, stimulated C-peptide maximum 5.1 ng/mL, hemoglobin A1c [HgA1c] 4.9%, insulin antibodies <0.4); and normal liver, hematologic and coagulation profiles.
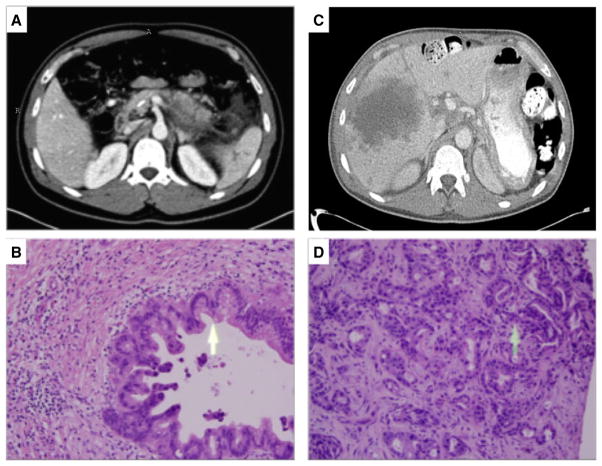
Computed tomography (CT) scan at the time of initial surgical evaluation (Figure 1A) demonstrated acute necrotizing pancreatitis in the setting of chronic pancreatitis with lack of enhancement in the body, along with pancreatic and peripancreatic necrotic collections. Magnetic resonance imaging (MRI) revealed mild dilation and irregularity of the main pancreatic duct and side branches, with no masses or strictures. Findings were consistent with chronic pancreatitis, more marked in the tail than the head. Endoscopic ultrasonography (EUS) revealed four of nine Rosemont criteria (10) for chronic pancreatitis with no masses identified.
Figure 1.
Imaging and microscopic examination of tissue before (A and B) and 11 mo after (C and D) total pancreatectomy with islet autotransplantation. (A) Cross-sectional image from a pancreas protocol computed tomography (CT) scan with intravenous contrast obtained at time of initial surgical evaluation, demonstrating acute necrotizing pancreatitis in the setting of chronic pancreatitis with pancreatic and peripancreatic necrotic collections as well as a pancreatic duct stent. (B) Microscopic examination of hematoxylin and eosin–stained pancreas tissue at the time of total pancreatectomy with islet autotransplantation, demonstrating chronic pancreatitis and pancreatic intraepithelial neoplasia II. (C) Follow-up CT at 1 year after surgery, demonstrating low-density liver lesions with ill-defined borders, the largest of which measured 10 cm in diameter. (D) Microscopic examination of hematoxylin and eosin–stained biopsy of liver lesion, consistent with metastatic pancreatic adenocarcinoma.
A multidisciplinary team (gastroenterologist, surgeon, endocrinologist, pain specialist, psychologist, and dietician) evaluated the patient, and he met institutional criteria, as published previously (2), for consideration for TPIAT without evidence of malignancy or other contraindications. He underwent TPIAT with splenectomy, appendectomy, feeding jejunostomy, and gastrostomy tube placement. Sections of pancreas, duodenum, spleen, appendix, and omentum were sent to pathology for evaluation (Figure 1B). The patient received an infusion of 2116 islet equivalents per kilogram into the portal venous system. The patient tolerated the procedure well, had an unremarkable hospital course, and was discharged from the hospital on postoperative day (POD) 10. At follow-up 3 mo after surgery, he had discontinued all narcotics, required 7 units of insulin glargine daily and small amounts of mealtime corrections. Laboratory tests reflected normal endocrine function (stimulated C-peptide maximum 1.4 ng/mL, HgA1c 5.5%).
Approximately 10 mo after surgery, the patient developed right upper quadrant pain, low-grade fevers, and malaise. Subsequent CT and MRI demonstrated several new necrotic liver lesions (Figure 1C). Ultrasound-guided core needle biopsy revealed liver parenchyma infiltrated by malignant epithelial cells (Figure 1D). The patient underwent two cycles of chemotherapy with gemcitabine and one cycle with gemcitabine and abraxane but ultimately died from his illness.
Methods
The University of Minnesota institutional review board (IRB) reviewed and approved the prospective study of patients undergoing TPIAT.
Clinical specimens
At the time of TPIAT, the surgically resected pancreas was processed with intact C1 collagenase (Vitacyte, Indianapolis, IN) and neutral protease (SERVA Electrophoresis, Heidelberg, Germany) in the islet-processing laboratory, as described previously (2). A volume of 15 cm3 of tissue, with a purity of 15% islets, was recovered from 116.5 g of the original resected pancreas, indicating 87.1% reduction of tissue volume during the isolation procedure. Tissue volume was appropriate for transfusion; therefore, it did not require purification based on our standard criteria (11). Nonprocessed pancreatic neck samples were sent to pathology, fixed in formalin and embedded in paraffin. Liver biopsies were performed with radiographic guidance 11 mo after surgery, fixed in formalin, and embedded in paraffin. The tissue samples were stained with hematoxylin and eosin. A battery of immunostaining of the liver tissue was performed, as described in the next section. Serum samples were saved as part of an ongoing prospective IRB approved study of TPIAT outcomes.
Analysis of miRNA
Assays for miRNAs -10b, -30c, and -106b were performed retrospectively. Serum samples were taken prior to surgery and on postoperative days 1 and 3. Samples were stored at −80°C prior to analysis. Assays were also performed on serum taken from four chronic pancreatitis patients at these same time points who underwent TPIAT and who have no evidence of pancreatic adenocarcinoma to date. To measure miRNA levels, total RNA was isolated from serum samples using Trizol-LS reagent (Life Technologies, Carlsbad, CA). RNA was then converted to cDNA for each TaqMan Gene Expression Assay target (miR-10b, -30c, and -106b) and the reference assay (miR-425-5p) using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). Assay-specific cDNA was then added to a master mix containing the assay-specific probe and TaqMan Fast Advanced Master Mix (Applied Biosystems), according to the manufacturer’s protocol, as previously reported (12). Quantitative real-time PCR (qRT-PCR) was performed with the ViiA7 system (Applied Biosystems) and ΔCT values for each miRNA were determined by subtracting the reference assay CT value from the target assay CT value. To calculate the ΔΔCT values, an average of the ΔCT for each miRNA of six normal control serum samples was used, and fold changes were determined as 2-ΔΔCT (13).
Results
Histology
Pancreas pathology from the index operation demonstrated pancreatic tissue with severe chronic pancreatitis as well as pancreatic intraepithelial neoplasia II (Figure 1B). Ampullary mucosa demonstrated acute inflammation and squamous metaplasia. One lymph node was extracted and was negative for malignancy. The pancreas, spleen, duodenum, appendix and omentum were also found to have no evidence of carcinoma.
Microscopic examination of the liver biopsies taken 11 mo after TPIAT demonstrated liver parenchyma infiltrated by malignant epithelial cells forming glands consistent with metastatic moderately differentiated adenocarcinoma. The malignant cells were reactive to cytokeratin 7, cytokeratin 20 and carbohydrate antigen (CA) 19-9. There were no reactions with antibodies to caudal type homeobox 2, prostate-specific antigen, prostate-specific acid phosphatase or thyroid transcription V1. A reticulin stain did not highlight a pattern of expansion or collapse or individual cell necrosis in the areas of uninvolved liver parenchyma. Masson’s trichrome stain demonstrated increased portal fibrosis in areas of uninvolved liver parenchyma.
Tumor markers
Retrospective analysis of the patient’s stored serum revealed normal preoperative tumor markers: CA 19-9 of 7 U/mL and carcinoembryonic antigen (CEA) <0.5 μg/L. At the time of pain recurrence 11 mo postoperatively, his levels had risen significantly to CA 19-9 of 39 872 U/mL and CEA 92.3 μg/L.
Plasma miRNA levels
Stored serum samples from the chronic pancreatitis patient who developed PDAC (chronic pancreatitis– PDAC) and four patients with chronic pancreatitis also treated with TPIAT were assayed retrospectively for miRNAs -10b, -30c and -106b. The chronic pancreatitis–PDAC patient’s preoperative serum levels were ≈20-fold, five-fold, and sevenfold higher, respectively, than in the corresponding chronic pancreatitis cases. Moreover, the elevated serum levels of all three microRNAs decreased rapidly following pancreas resection in the chronic pancreatitis– PDAC patient, whereas the normal levels in the four chronic pancreatitis patients did not change after resection (Table 1).
Table 1.
Results of miRNA -10b, -30c, and -106b analysis of serum samples taken prior to surgery and on PODs 1 and 3
| Serum | CP only (n = 4)
|
CP and PDAC (n = 1)
|
||||
|---|---|---|---|---|---|---|
| Preoperative | POD 1 | POD 3 | Preoperative | POD 1 | POD 3 | |
| miRNA 10b | 0.33 ± 0.18 | 0.41 ± 0.18 | 0.18 ± 0.12 | 6.53 ± 1.18** | 0.28 | 0.30 |
| miRNA 30c | 2.00 ± 0.82 | 2.91 ± 1.12 | 1.53 ± 0.68 | 9.65 ± 0.50** | 3.82 | 2.72 |
| miRNA 106b | 0.25 ± 0.04 | 0.25 ± 0.18 | 0.22 ± 0.04 | 1.79 ± 0.11* | 0.51 | 0.47 |
Data shown are relative expression levels by comparison to the mean values in 6 healthy controls. Please see changes to the Methods section for further clarification.
CP, chronic pancreatitis; miRNA, microRNA; PDAC, pancreatic ductal adenocarcinoma; POD, preoperative day.
Discussion
This report is the first of hepatic PDAC metastases after TPIAT for chronic pancreatitis. In this case, the patient had no radiographic evidence of malignancy prior to undergoing TPIAT, and retrospective analysis of his preoperative serum revealed normal CA 19-9 and CEA levels. Given the increasing use of TPIAT in the treatment armamentarium for chronic pancreatitis, it is important to establish guidelines for preoperative screening and to develop additional tools to help identify patients at increased PDAC risk.
With 5-year survival rates of approximately 7%, PDAC remains the fourth leading cause of death from cancer in the United States. In contrast to decreasing mortality trends for breast and colon cancer, yearly incidence and mortality for pancreatic cancer continue to rise (14). PDAC is projected to become the second leading cause of cancer deaths in adults within a decade (15). Advancing age, smoking, obesity, long-standing type 2 diabetes and family history are established risk factors for developing PDAC (16,17). In addition, several familial cancer syndromes, such as Li–Fraumeni syndrome, BRCA2, hereditary nonpolyposis colon cancer, and Peutz–Jeghers syndrome, have also been shown to confer varying degrees of increased risk of developing PDAC (18).
Numerous case–control, cohort, and record-linkage studies have demonstrated that chronic pancreatitis also confers an increased risk of developing PDAC, although reported risk rates vary across studies (16). Patients with inherited chronic pancreatitis have the highest risk of PDAC development. In a European study, researchers demonstrated that the risk of PDAC in hereditary chronic pancreatitis patients was about 50 times higher than in the corresponding population, with a 44% cumulative risk of PDAC by age 70 years (19). It has also been shown that patients with hereditary pancreatitis and current or former smoker status have a twofold increased risk of pancreatic cancer and develop cancer on average 20 years earlier compared with nonsmokers (20).
The U.S. Preventive Services Task Force does not currently recommend routine screening for PDAC in asymptomatic persons (21). This recommendation is based in part on the absence of sensitive and specific tests for early PDAC diagnosis. Case reports or limited series of TPIAT in the setting of known malignancy have been reported (22–24); however, this is not currently recommended in the United States, given the risk of iatrogenic metastasis of PDAC, as demonstrated in this report. Although the majority of exocrine tissue is enzymatically separated from the islet cells in preparation for transplantation, and the remaining nonendocrine tissue does not survive the initial 2-week engraftment period, this case demonstrates that malignant cells can survive enzymatic digestion and embolize to the liver during autotransfusion into the portal venous system, resulting in metastatic pancreatic cancer. This underscores why the noted risk factors for PDAC should be taken into consideration during preoperative screening of patients being evaluated for TPIAT.
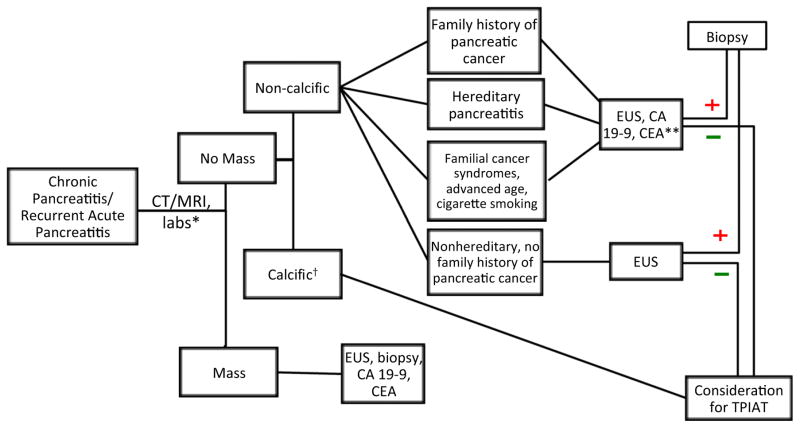
Because of the risk for preexisting subclinical malignancy in chronic pancreatitis patients, we developed a sequential algorithm for preoperative evaluation to selectively increase pancreatic cancer screening in high-risk persons (Figure 2). This scheme recognizes that CT or MRI will identify the majority of patients with large duct or calcific disease. Additional screening with EUS or tumor markers for high-risk patients should be used as indicated. Recent studies have explored the utilization of miRNAs as potential biomarkers for PDAC (25). These small nonprotein coding RNAs are involved in biological processes such as cell proliferation, differentiation, migration, invasion, and survival and have been implicated in the pathogenesis of numerous cancers through both up- and downregulation (25). A previous study demonstrated that levels of miRNA-10b, -30c, -155 and -106b were elevated in the plasma of patients with PDAC by comparison with chronic pancreatitis patients and healthy controls (25); however, miR-155 is expressed in the CD45+ T cells in PDAC and not in the pancreatic cancer cells (26). Consequently, retrospective analysis of stored serum samples were performed in this case and detected elevated miRNA -10b, -30c, and -106b levels in the chronic pancreatitis– PDAC patient in comparison to the four chronic pancreatitis patients also undergoing TPIAT. Clearly, neither CA 19-9 nor CEA are good markers for early PDAC diagnosis and would have failed to raise suspicion for malignancy in this case. In contrast, the inclusion of miRNA -10b, -30c, and -106b testing may provide a unique tool at this point in care for identification of patients with early preclinical PDAC not identified by conventional tumor markers.
Figure 2. TPIAT preoperative screening algorithm for patients with chronic pancreatitis or recurrent acute pancreatitis.
*Complete blood count, basic metabolic panel, liver function tests, amylase, lipase, endocrine function, coagulation profile, CT/MRI, EUS. †Patients with hereditary pancreatitis, family history of pancreatic cancer or familial cancer syndromes revert to respective pathway above. **Consider additional biomarker screening. CA, carbohydrate antigen; CEA, carcinoembryonic antigen; CT, computed tomography; EUS, endoscopic ultrasound; MRI, magnetic resonance imaging; TPIAT, total pancreatectomy with islet autotransplantation.
Conclusion
TPIAT has been performed worldwide in >1000 patients since 1977, and this report is the first of a case of autotransplantation of pancreatic malignancy after TPIAT for presumed benign chronic pancreatitis; malignancy was undetectable prior to surgery with conventional tumor markers or imaging. For patients at an increased risk of pancreatic cancer, additional screening may be indicated. Preoperative assays for miRNAs -10b, -30c, and -106b are potentially sensitive screening techniques; however, further study is needed. This case report points to the need for studies of larger populations of such patients and underscores the need to avoid TPIAT in the setting of known pancreatic cancer.
Acknowledgments
This work was supported, in part, by a National Institutes of Health (NIH) grant from the National Cancer Institute to M. Korc (CA-075059) and an NIH grant to M. Bellin (5K23DK084315).
Abbreviations
- CA
carbohydrate antigen
- CEA
carcinoembryonic antigen
- CP
chronic pancreatitis
- CT
computed tomography
- EUS
endoscopic ultrasound
- HgA1c
hemoglobin A1c
- IRB
institutional review board
- miRNA
microRNA
- MRI
magnetic resonance imaging
- PDAC
pancreatic ductal adenocarcinoma
- POD
postoperative day
- TPIAT
total pancreatectomy with islet autotransplantation
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Sutherland DE, Matas AJ, Najarian JS. Pancreatic islet cell transplantation. Surg Clin North Am. 1978;58:365–382. doi: 10.1016/s0039-6109(16)41489-1. [DOI] [PubMed] [Google Scholar]
- 2.Chinnakotla S, Beilman GJ, Dunn TB, et al. Factors predicting outcomes after a total pancreatectomy and islet autotransplantation lessons learned from over 500 cases. Ann Surg. 2015;262:610–622. doi: 10.1097/SLA.0000000000001453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcea G, Weaver J, Phillips J, Pollard CA, Ilouz SC. Total pancreatectomy with and without islet cell transplantation for chronic pancreatitis: A series of 85 consecutive patients. Pancreas. 2009;38:1–7. doi: 10.1097/MPA.0b013e3181825c00. [DOI] [PubMed] [Google Scholar]
- 4.Gruessner RW, Cercone R, Galvani C, Rana A, Porubsky M. Results of open and robot-assisted pancreatectomies with autologous islet transplantations: Treating chronic pancreatitis and preventing surgically induced diabetes. Transplant Proc. 2014;46:1978–1979. doi: 10.1016/j.transproceed.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Morgan K, Owczarski SM, Borckardt J, Madan A, Nishimura M. Pain control and quality of life after pancreatectomy with islet autotransplantation for chronic pancreatitis. J Gastrointest Surg. 2012;16:129–133. doi: 10.1007/s11605-011-1744-y. [DOI] [PubMed] [Google Scholar]
- 6.Takita M, Naziruddin B, Matsumoto S, Noguchi H, Shimoda M. Variables associated with islet yield in autologous islet cell transplantation for chronic pancreatitis. Proc (Bayl Univ Med Cent) 2010;23:115–120. doi: 10.1080/08998280.2010.11928597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walsh RM, Saavedra JR, Lentz G, Guerron AD, Scheman J. Improved quality of life following total pancreatectomy and auto-islet transplantation for chronic pancreatitis. J Gastrointest Surg. 2012;16:1469–1477. doi: 10.1007/s11605-012-1914-6. [DOI] [PubMed] [Google Scholar]
- 8.Wilson GC, Ahmad SA, Schauer DP, Eckman MH, Abbott DE. Cost-effectiveness of total pancreatectomy and islet cell autotransplantation for the treatment of minimal change chronic pancreatitis. J Gastrointest Surg. 2015;19:46–54. doi: 10.1007/s11605-014-2612-3. [DOI] [PubMed] [Google Scholar]
- 9.Dunderdale J, McAuliffe JC, McNeal SF, Bryant SM, Yancey BD. Should pancreatectomy with islet cell autotransplantation in patients with chronic alcoholic pancreatitis be abandoned? J Am Coll Surg. 2013;216:591–596. doi: 10.1016/j.jamcollsurg.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 10.Catalano MF, Sahai A, Levy M, et al. EUS-based criteria for the diagnosis of chronic pancreatitis: The rosemont classification. Gastrointest Endosc. 2009;69:1251–1261. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm JJ, Bellin MD, Dunn TB, et al. Proposed thresholds for pancreatic tissue volume for safe intraportal islet autotransplantation after total pancreatectomy. Am J Transplant. 2013;13:3183–3191. doi: 10.1111/ajt.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cote GA, Gore AJ, McElyea SD, et al. A pilot study to develop a diagnostic test for pancreatic ductal adenocarcinoma based on differential expression of select miRNA in plasma and bile. Am J Gastroenterol. 2014;109:1942–1952. doi: 10.1038/ajg.2014.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2012. Bethesda, MD: National Cancer Institute; 2015. Apr, Pancreas cancer. [cited 2016 Jan 13] Available from: http://seer.cancer.gov/csr/1975_2012/ [Google Scholar]
- 15.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the united states. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 16.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: An overview. Nat Rev Gastroenterol Hepatol. 2009;6:699–708. doi: 10.1038/nrgastro.2009.177. [DOI] [PubMed] [Google Scholar]
- 18.Lowenfels AB, Maisonneuve P, DiMagno EP, et al. Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group. J Natl Cancer Inst. 1997;89:442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 19.Howes N, Lerch MM, Greenhalf W, et al. Clinical and genetic characteristics of hereditary pancreatitis in Europe. Clin Gastroenterol Hepatol. 2004;2:252–261. doi: 10.1016/s1542-3565(04)00013-8. [DOI] [PubMed] [Google Scholar]
- 20.Lowenfels AB, Maisonneuve P, Whitcomb DC, Lerch MM, DiMagno EP. Cigarette smoking as a risk factor for pancreatic cancer in patients with hereditary pancreatitis. JAMA. 2001;286:169–170. doi: 10.1001/jama.286.2.169. [DOI] [PubMed] [Google Scholar]
- 21.Recommendations for Primary Care Practice-Pancreatic Cancer: Screening, February 2004. United States Preventive Services Task Force (USPSTF); Feb, 2004. [cited 2016 Jan 13]. Available from: http://www.uspreventiveservicestaskforce.org/Page/Document/ClinicalSummaryFinal/pancreatic-cancer-screening. [Google Scholar]
- 22.Iyegha UP, Asghar JA, Beilman GJ. Total pancreatectomy and islet auto-transplantation as treatment for ampullary adenocarcinoma in the setting of pancreatic ductal disruption secondary to acute necrotizing pancreatitis. A case report. JOP. 2012;13:239–242. [PubMed] [Google Scholar]
- 23.Dudeja V, Beilman GJ, Vickers SM. Total pancreatectomy with islet autotransplantation in patients with malignancy: Are we there yet? Ann Surg. 2013;258:219–220. doi: 10.1097/SLA.0b013e31829c4a1b. [DOI] [PubMed] [Google Scholar]
- 24.Balzano G, Maffi P, Nano R, et al. Autologous islet transplantation in patients requiring pancreatectomy: A broader spectrum of indications beyond chronic pancreatitis. Am J Transplant. 2016;16:1812–1826. doi: 10.1111/ajt.13656. [DOI] [PubMed] [Google Scholar]
- 25.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sempere LF, Preis M, Yezefski T, et al. Fluorescence-based codetection with protein markers reveals distinct cellular compartments for altered MicroRNA expression in solid tumors. Clin Cancer Res. 2010;16:4246–4255. doi: 10.1158/1078-0432.CCR-10-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]