Abstract
Using eight hour human laboratory experiments, we evaluated the analgesic efficacy of vaporized cannabis in patients with neuropathic pain related to injury or disease of the spinal cord, the majority of whom were experiencing pain despite traditional treatment. After obtaining baseline data, 42 participants underwent a standardized procedure for inhaling 4 puffs of vaporized cannabis containing either placebo, 2.9%, or 6.7% delta-9-tetrahydrocannabinol on three separate occasions. A second dosing occurred 3 hours later; participants chose to inhale 4 to 8 puffs. This flexible dosing was utilized to attempt to reduce the placebo effect. Using an 11-point numerical pain intensity rating scale as the primary outcome, a mixed effects linear regression model demonstrated a significant analgesic response for vaporized cannabis. When subjective and psychoactive side effects (e.g., good drug effect, feeling high, etc.) were added as covariates to the model, the reduction in pain intensity remained significant above and beyond any effect of these measures (all p<0.0004). Psychoactive and subjective effects were dose dependent. Measurement of neuropsychological performance proved challenging because of various disabilities in the population studied. As the two active doses did not significantly differ from each other in terms of analgesic potency, the lower dose appears to offer the best risk-benefit ratio in patients with neuropathic pain associated with injury or disease of the spinal cord.
Keywords: neuropathic pain, analgesia, cannabis, clinical trial, neuropsychological testing
INTRODUCTION
The mechanisms involved in neuropathic pain arising from spinal cord pathology are numerous 37. Hypothetically, injury to spinal cord structures may alter sensory processing generating a central pain state. There may be decreased inhibition of neuronal activity through deafferentation of interneurons and/or sensitization such that sensory input is amplified and sustained via intact circuitry 89. Whatever the etiology, new approaches to treatment are constantly being sought. In 2012, pregabulin was approved by the Food and Drug Administration (FDA) for the treatment of neuropathic pain related to spinal cord central pain. The number needed to treat (NNT) for 50% pain relief (7.1 (95% confidence interval 3.9–37)) was higher than in most peripheral neuropathic pain studies 38. Thus, although pregabulin has been approved by the FDA and should be considered a first-line therapy, there still exists an unmet need to improve pain relief in spinal cord central pain 37.
Neuropathic pain of spinal cord origin is a major cause of suffering adding to the physical, emotional, and societal impact of loss or impairment in motor movement, bowel and bladder function, digestion, and breathing. In one survey, respondents specified the extent of their dissatisfaction with analgesic medications 19. While opioids produced the greatest degree of pain relief, these medications were often discontinued because of side effects, primarily constipation. Although 44 of 117 (38%) respondents tried gabapentin, only 20 (17%) continued to use it, and their pain relief was only moderate. A majority of respondents eventually tried several alternative treatments. Interestingly, maximum relief was provided by massage or cannabis.
In general, current therapeutic strategies to treat neuropathic pain aim to reduce the excitability of neurons by increasing activity of ion channels (e.g., gabapentin, pregabalin, carbamazepine, lidocaine and capsaicin) or by modulating endogenous descending inhibitory tracts (e.g., tricyclic antidepressants, duloxetine and opioids) 92. In the last decade, another therapeutic option has been recognized involving non-neuronal cells (e.g., spinal microglia and astrocytes) 92. Inflammatory mediators released by activated glial cells include tumor necrosis factor-a and interleukin-1b; these cytokines are believed to trigger neuropathic pain by acting upon spinal cord dorsal horn neurons 98. Modulation of glial cell activation and blockade of signaling pathways between neuronal and non-neuronal cells offers new opportunities for more effective treatment of neuropathic pain.
Given the need for therapeutic advancement, it is surprising that few clinical studies have examined drugs altering glial function 92. One such class of medications are the cannabinoids 97, 98, 110. Several clinical studies involving administration of cannabis have been indicative of an analgesic effect in neuropathic pain 2, 33, 104, 105, 107, 112, 113. A recent meta-analysis supported the use of cannabinoids for the treatment of chronic pain by demonstrating that the average number of patients who reported a reduction in pain of at least 30% was greater with cannabinoids than with placebo. (OR, 1.41 [95% CI, 0.99–2.00]) 109. These findings are confirmatory of animal research that demonstrated a potent analgesic effect of cannabinoids 60, 91, 115.
The purpose of the present study was to compare the analgesic efficacy of different strengths of vaporized cannabis in participants with pathology of the spinal cord related to traumatic injury or disease. Secondary outcomes included neuropsychological and psychomimetic effects. It was hypothesized that pain relief could be achieved using whole plant cannabis while side-effects would prove to be tolerable.
MATERIALS AND METHODS
Regulatory Approvals
The study was registered using Clinical Trials.gov identifier NCT01555983. The Human Subjects Institutional Review Boards at the UC Davis Medical Center and the Veterans Affairs of Northern California Health Care System approved the protocol. Mandated federal reviews for investigation of cannabis included submissions to the Drug Enforcement Administration, the Food and Drug Administration and the National Institute of Drug Abuse 1. The FDA approval process included an Investigational New Drug Application (IND number assigned: 102,847). The Research Advisory Panel of California also evaluated and approved the protocol.
Participants
Data in the present study was obtained from two populations; individuals with injury and disease of the spinal cord (see Table 1). Such a pooling of conditions has been done by other investigators looking at diverse neuropathic pain conditions 44, 90, 107, 112, 113. Studying closely related painful neuropathic conditions is valid because their clinical course are considered related and receive similar treatment recommendations 7, 83.
Table 1.
Demographics and Characteristics of Patients (N=42)
| Sex (no.) | |
| Male | 29 (69%) |
| Female | 13 (31%) |
| Age, mean (SD) | 46.4 (13.6) |
| Educational level | |
| Some high school | 1 (2%) |
| High school graduate | 9 (22%) |
| Some college | 21 (51%) |
| College graduate | 10 (24%) |
| Ethnicity | |
| Caucasian | 26 (62%) |
| Hispanic | 7 (17%) |
| African American | 5 (12%) |
| Asian American | 2 (5%) |
| American Indian | 1 (2%) |
| Other | 1 (2%) |
| LANSS Score, mean (SD) | 16.0 (2.8) |
| Etiology of Spinal Cord Pain | |
| Trauma | 29 |
| Automobile Accident | 11 |
| Recreational Sporting Injury (All-Terrain Vehicle, Diving, Snowboard, Snowmobile, Trampoline) | 5 |
| Motorcycle Accident | 3 |
| Fall Injury | 3 |
| Sports Injury (Football, Martial Arts) | 2 |
| Firearm Injury | 2 |
| Assault with Blunt Object | 1 |
| Osteomyelitis with Epidural Abscess | 1 |
| Post-Surgical Wound Hematoma | 1 |
| Disease | 13 |
| Multiple Sclerosis | 6 |
| Cervical Disc Disease | 3 |
| Spinal Cord Tumor | 1 |
| Vertebral Artery Occlusion | 1 |
| Arachnoid Cysts | 1 |
| Syringomyelia | 1 |
| Neurological Level | |
| Cervical | 22 |
| Thoracic | 14 |
| Lumbar | 6 |
| Sacral | 0 |
| Duration of Pain, mean years (SD) | 11.6 (10.1) |
Participants were enrolled via recruitment from the UC Davis Medical Center Spinal Cord Injury Clinic, IRB-approved recruitment letters and newspaper advertisements. Screening for inclusion criteria (e.g., age > 18 and ≤ 70, pain intensity ≥ 4/10) was conducted via telephone. The 11-point pain intensity numerical rating scale, anchored at 0 equals no pain and 10 equals worst possible pain, was selected because of the ability to administer this instrument by telephone during the screening process 30. A level of ≥ 4/10 was chosen as this is a commonly utilized cut-off point in randomized clinical trials involving neuropathic pain 82. Qualified candidates were subsequently consented, interviewed and examined by the principal investigator who administered the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS), a pain scale based on analysis of sensory description and bedside examination of sensory dysfunction 15, 65. A threshold of ≥12 on this instrument was utilized to substantiate neuropathic pain.
Because there is evidence that cannabis may exacerbate bipolar depression 73, 75 or increase suicide risk in patients with schizophrenia 50, candidates with these diagnoses were excluded. The Patient Health Questionnaire-9 (PHQ-9), a nine-item depression scale based directly on the diagnostic criteria for major depressive disorder in the Diagnostic and Statistical Manual Fourth Edition (DSM-IV) 72, was administered. As cannabis use may on occasion worsen depressive symptomatology 74, individuals with severe depression (PHQ-9 ≥ 15) were excluded and referred for psychiatric consultation, if not already in progress. Because exposure to cannabis may be associated with suicidal thoughts and attempts 87, the Center for Epidemiological Studies-Depression Scale (CES-D) was administered using the three item subscale measuring suicidal ideation proposed by Garrison et al. 42, 43 and others 22. If any of the items (“I felt life was not worth living”; “I felt like hurting myself”; “I felt like killing myself”) were answered affirmatively, the patient was excluded.
Comorbidities were also evaluated; potential participants were excluded if a medical condition was present that, in the opinion of the investigator, would potentially lead to a deleterious effect on the patient’s well-being (e.g., coronary artery disease, obstructive pulmonary disease, severe liver disease or impaired renal function). A history and physical, chest X-ray, electrocardiogram and laboratory analysis (e.g., hematology screen, blood chemistries, and urinalysis) were performed to screen for medical illnesses. The Substance Abuse Module of the Diagnostic Interview Schedule for DSM-IV 88 was administered to exclude individuals with a current substance use disorder. Urine drug immunoassay for opioids, benzolyecgonine (cocaine metabolite), cannabinoids, and amphetamines were also performed to confirm historical information obtained by interview. Participants without a medical indication for these substances were excluded.
All participants were required to refrain from smoking cannabis or taking oral synthetic delta 9-THC medications (i.e. Marinol®) for 7 days before study sessions to reduce residual effects. To further reduce unsystematic variation, participants were instructed to take all other concurrent medications as per their normal routine during the 3 to 4 week study period.
Design
A randomized, double-blind, placebo-controlled, crossover design study employing three different strengths of cannabis (placebo, 2.9%, and 6.7% delta 9-THC) was performed. The cannabis was harvested at the University of Mississippi under the supervision of the National Institute on Drug Abuse (NIDA). NIDA provides bulk cannabis for experimental use subject to existing concentrations of delta 9-THC in their marijuana plant material supply program. Placebo cannabis was derived from whole plant material with extraction of delta 9-THC. Following overnight delivery, the cannabis was stored in a freezer at the Sacramento VA Medical Center Research Pharmacy using precautionary measures (e.g., limited access to the pharmacy with an alarm system to protect against diversion).
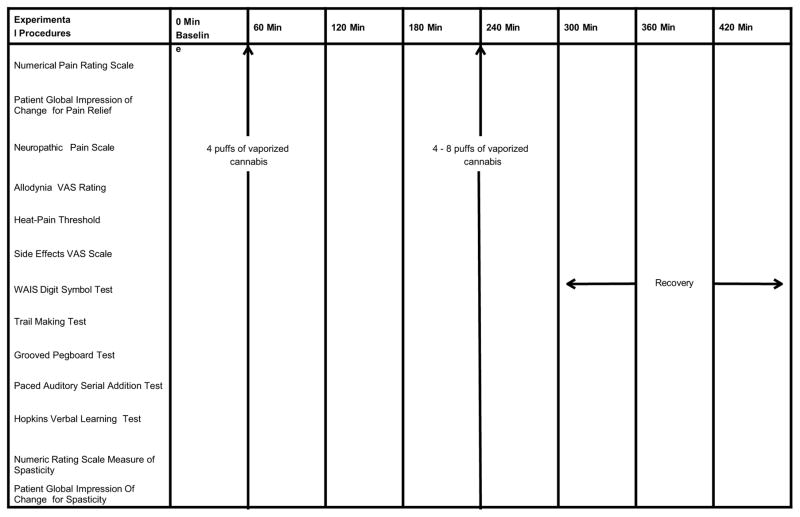
A cumulative dosing scheme was employed to determine dosing relationships for analgesia, psychoactive and cognitive effects as recommended by previous investigators 23, 46. Participants inhaled four puffs after baseline data were obtained (Figure 1). They then inhaled four to eight puffs of cannabis (or placebo) during the second administration of study medication at 240 minutes. The second dosing was purposefully flexible as to the number of puffs permitted; experience has shown that clinical trials with adaptable dose designs are almost twice more likely to demonstrate significant differences between medications and placebo than fixed dose trials 68,69. One explanation for this is that fixed dose trials probably involve an expectation of benefit. As a result, the placebo effect may increase thereby reducing the ability of a clinical trial to differentiate between an effective treatment (e.g., an active drug) and a less effective or ineffective treatment (e.g., placebo). The flexible dose design emulates clinical practice whereby the clinician, in cooperation with the patient, adjusts dosages to optimize efficacy and tolerability according to distinct patient conditions. Such a technique may also offer an advantage in the research setting by preventing withdrawal due to an adverse event when the therapeutic window is surpassed in some individuals, or withdrawals due to lack of efficacy in other participants in whom a fixed dose is insufficient 31. To enhance the sensitivity to differences among the treatment doses, participants served as their own controls.
Figure 1.
Experimental procedures and timing of cannabis vaporization sessions
Procedures
Participants were scheduled for three, 8-hour experimental sessions at the UC Davis Clinical Translational Science Center Clinical Research Center. The sessions were separated by at least 3 days to permit the metabolic breakdown of delta 9-THC metabolites 48. The median (interquartile range) interval between sessions was 7 (0.75) days. Participants received placebo, 2.9%, or 6.7% delta 9-THC in random order utilizing a web-based random number-generating program, Randomization.com 〈http://www.randomization.com〉 to determine the sequence of administration. The allocation schedule was maintained by a research pharmacist and concealed from other study personnel.
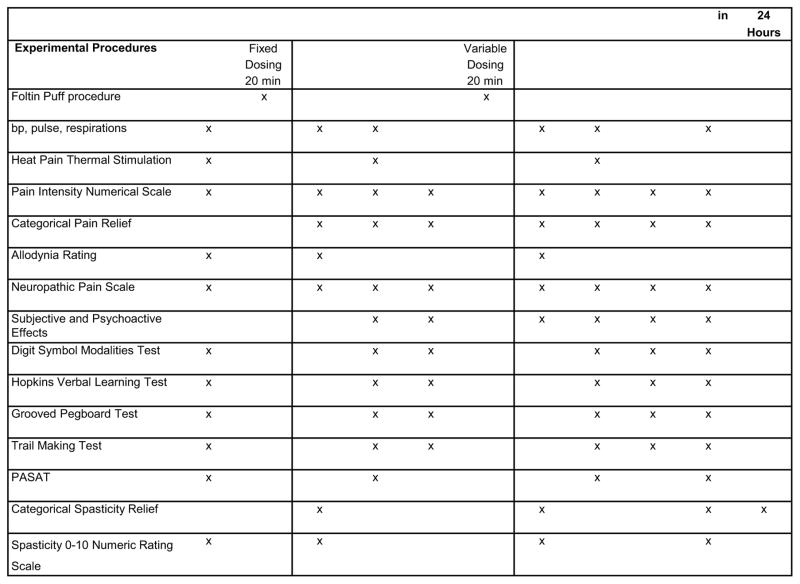
Cannabis was stored in a freezer at −20°C until the day before use when it was thawed and humidified by placing the medication above a saturated NaCl solution in a closed humidifier at room temperature for twelve hours. The Volcano® vaporizer (Storz & Bickel America, Inc., Oakland, CA) was used to administer study medication to participants. Four hundred milligrams of NIDA cannabis with either placebo, 2.9%, or 6.7% delta 9-THC was loaded into the filling chamber, the temperature set at 185 degrees centigrade, and the machine was switched on. A balloon bag collected the vapor; this commercial device was fitted with a specially designed mouthpiece that allowed one to willfully interrupt inhalation repeatedly without loss of vapor to the atmosphere. To prevent contamination of the breathing space of observers, vaporization was conducted under a standard laboratory fume hood with constant ventilation. This was performed in a room with an ambient temperature of 22°C and a humidity of 40% to 60% that was outfitted with an exhaust system. This area was physically separate from the UC Davis Clinical Research Center and required that participants travel to an adjoining building. This necessitated a time delay of approximately 20 minutes at times 60 and 240 min before the onset of study assessments. Because of the time involved in the dispensing of vaporized cannabis, we did not perform the neuropsychological examinations, subjective and psychoactive effects, or heat pain threshold at Times 60 and 240 min. These tests were delayed until the subsequent evaluation periods (Figure 2 provides details of assessment schedule).
Figure 2.
Schedule of Experimental Procedures
A cued-puff procedure known as the “Foltin Puff Procedure” standardized the administration of the cannabis 23. Participants were verbally signaled to “hold the vaporizer bag with one hand and put the vaporizer bag mouthpiece in their mouth” (30 seconds), “get ready” (5 seconds), “inhale” (5 seconds), “hold vapor in lungs” (10 seconds), “exhale and wait” before repeating puff cycle (40 seconds). Assessments were performed before the administration of vaporized cannabis or placebo and hourly thereafter for seven hours for outcome variables.
Vital signs (blood pressure, respiratory rate, and heart rate) were recorded at baseline and at every hour to ensure well-being of participants while under the influence of cannabis. Participants were allowed to engage in normal activities, such as reading, watching television, or listening to music, between puff cycles and assessment periods. After each session, participants were accompanied home by a responsible adult. Upon completion of study sessions, participants were compensated with a modest stipend for their participation (prorated at $25 per hour).
Outcome Measurements
Pain intensity, the primary outcome variable, was assessed by asking participants to indicate their current pain on an 11-point numerical rating scale anchored between 0 (no pain) and 10 (worst possible pain) 30, 32. As a secondary measure of pain relief, the Patient Global Impression of Change was administered 30. This 7-point ordinal scale has the following range of values: very much worse, much worse, minimally worse, unchanged, minimally improved, much improved, very much improved. The Neuropathic Pain Scale 41, an 11-point ordinal scale, was another secondary outcome. It includes a series of items that assess two global pain domains (pain intensity and unpleasantness), six specific pain qualities (sharp, dull, sensitive, hot, cold, and itchy pain), and two spatial qualities (deep and surface pain) 65. Allodynia, pain due to a stimulus that does not normally provoke pain, was measured by tangentially stroking a painful area with a foam paint brush. The response was recorded using a 100-mm visual analogue scale (VAS) anchored between 0 (no pain) and 100 (severely painful). Baseline levels were obtained from the same painful area of the body prior to administration of study medications. Heat-pain threshold was determined at baseline and again after vaporization sessions by applying mild-to-moderately painful heat stimuli to skin on the non-dominant C2–C3 level, where heat sensitivity could be assured to be present, using the commercially available Medoc TSA 2001 Peltier thermode 52. This device applied a constant 1-degree centigrade per second increasing thermal stimulus until the patient pressed the response button, indicating that the temperature change was considered painful. The heat pain threshold (mean of three attempts) was recorded in degrees centigrade.
Separate subjective intensities for “any drug effect,” “good drug effect,” and “bad drug effect,” were measured using a 100-mm VAS anchored by “not at all” at 0 and “extremely” at 100. In addition, psychoactive effects were measured by inquiring “Do you feel ______ right now?” and filling in the blank with “high”; “drunk”; “impaired”; “stoned”; “like the drug effect”; “sedated”; “confused”; “nauseated”; “desire more of the drug”; “hungry”; “changes perceiving time”; “changes perceiving space”; “difficulty paying attention”; and “difficulty remembering things”. Similar VAS questions have been shown to be sensitive and reliable subjective measures of cannabis intoxication 8, 56, 57, 67.
Spasticity assessments, manifested as spasms, pain, and muscle stiffness, were performed using the Numeric Rating Scale Measure of Spasticity (bordered by 0=no spasticity to 10=worst possible spasticity) and the Patient’s Global Impression of Change, a 7-point ordinal scale with the following range of values: very much worse, much worse, minimally worse, unchanged, minimally improved, much improved, very much improved 34. The Modified Ashworth Scale was not used as it has been called into question by several observers for assessment of spasticity 39, 79, 114. One recognized problem is that this instrument has inadequate reliability for determining lower extremity spasticity between raters (inter-rater) or over time (inter-session) 25. Furthermore, while such clinician-rated measures of spasticity are purported to be objective, they do not measure the patient’s experience and may not be sensitive to changes that are meaningful to the patient 34.
Neurocognitive assessments focused on several domains: attention and concentration, fine motor speed, processing speed and learning and memory. Participants completed the Wechsler Adult Intelligence Scale Digit Symbol Test (DST) 108, a test of concentration, psychomotor speed, and graphomotor abilities. This pen and paper test involved having participants substitute a series of symbols with numbers as quickly and accurately as possible during a 120-second period. The results were expressed as the number of correct substitutions.
The Trail Making Test (TMT), a test of processing speed, visual attention and task switching consists of two parts; Part A had consecutive numbers and Part B had a combination of numbers and letters of the alphabet 6, 7. Participants were instructed to connect a set of 25 circles containing the numbers and letters with a pencil as fast as possible while maintaining accuracy. The scores are recorded as the time in seconds required to complete testing.
For the Grooved Pegboard Test (GPT) 70, a test of fine motor coordination and speed, participants were asked to place 25 small metal pegs into holes on a 3″ × 3″ metal board as quickly as possible. The score is the total time for each test (using the dominant and non-dominant hand); a five-minute limit was employed for those unable to complete the task. Quadriplegic participants were instructed to use their best grip to hold pens (digit symbol and trail making test) or pins (grooved pegboard) where applicable. However, some participants were not able to complete testing because of the severity of their disability, a problem identified in the literature 59.
The Paced Auditory Serial Addition Test (PASAT) 47, a measure of auditory information processing speed and working memory, was also administered. A series of single digit numbers were presented via a digital recording where the two most recent digits had to be summed. The scores reported for the Paced Auditory Serial Addition Test were the number of correct responses for each trial 28, 29, 101.
The Hopkins Verbal Learning Test Revised (HVLT) assessed learning and immediate recall of verbal information, as well as the ability to retain, reproduce, and recognize this information after a delay 13. Alternate forms (A through F) were used to minimize practice effects 12, 14. A list of 12 words was presented, using 4 words from each of three semantic categories. The participant was asked to recall as many words as possible in any order. This was repeated for three trials. After a 20-minute delay, the participant was asked to recall the original words once again (delayed recall). To test delayed recognition, the participant was asked to listen to 24 words (which included the original 12 words, and 12 distractors) and say whether the word was in the original list or not. The True Positive score reflects the number of words correctly identified as being in the original list, whereas the False Positive score indicates the number of words the participant incorrectly identified as being in the first list.
In order to estimate the level of functioning at baseline, the raw scores on each test were converted to demographically-corrected T-scores adjusting for age, gender, highest educational level achieved, and ethnicity 54, 55. Baseline neuropsychological test performance was summarized using the global deficit score (GDS), a validated approach for detecting neuropsychological impairments across multiple measures 20.
Statistical Methodology
For most outcomes, mixed effects models with random intercepts were generated for testing the main effects of time, dose, and time × dose interaction. Initial models also included the baseline value of the outcome if measurements were taken at time 0 at the study visit. Treatment sequence, the order in which the treatments were administered, was tested and retained in the model if significant. Then, potential confounding variables (i.e., pain condition coded as spinal cord injury vs. disease; level of injury coded as cervical vs. below cervical; and cannabis use coded as current [within 30 days prior to randomization] vs. not current), specified a priori, were added to the model and retained individually and, if applicable, in combination, if significant. For models that did not test for cannabis use, cannabis use was added to the main effects model to determine if main effects changed with the addition of that term. To account for the possibility that the level of spinal cord pathology (cervical vs. below cervical) might affect performance on tests in which upper extremity dexterity was required (i.e., Grooved Pegboard, Trail Making, and Digit Symbol Tests), a covariant term for the level of injury was evaluated in the same way. For the models described above, time was coded as a continuous variable and centered on its mean, and a Tukey’s honestly significant difference test which restricts the Type I error rate was performed to identify which doses differed from each other when a significant main dose effect was found. Dose effects at each individual timepoint were tested using contrasts for the main effect models generated above but with the time variable coded categorically. In some cases, only the largest p-value for comparing two doses may be indicated when reporting significant results for multiple pairwise comparisons in the text. Effect sizes for neuropsychological testing results were estimated using Glass’s delta (i.e., estimator of the effect size) 45 and classified as either positive or negative centered on the placebo mean. For all statistical testing, a 5% significance level was used, and unless indicated otherwise, no adjustments for multiple testing were applied. For each visit, participants who reported no pain at baseline were omitted from analyses using pain intensity, pain relief and Neuropathic Pain Scale (NPS) intensity as outcomes. For other NPS outcomes (e.g., sharpness, burning, etc.), allodynia, and spasticity, participants who were not experiencing that particular outcome at the baseline for that visit were also excluded from analyses of that outcome. If excluded due to no baseline spasticity, then the Patient’s Global Impression of Change in spasticity was also not analyzed.
To determine whether or not a psychomimetic side effect had an influence on the primary outcome of pain intensity, an additional term measuring each side effect (e.g., feeling high, stoned, drunk) was separately forced into the final mixed effects model developed for the main effect of pain intensity as described above, and the impact of the psychoactive side effect on the treatment dose effect was tested. The proportion of participants with at least a 30% pain reduction rate were estimated for each treatment dose with 95% score confidence intervals and compared among all three doses with a Cochran Armitage Trend Test 4, 24 and pairwise using a χ2 likelihood ratio test. The number of individuals needed to be treated during the 8-hour study visit for one additional person to achieve 30% pain reduction relative to a comparison treatment was calculated as 1÷(Absolute Risk reduction), rounded up to the nearest integer, and 95% confidence intervals were estimated according to Altman (1998) 3. The number of flexible dosing puffs, both within visit and combined for all visits, were compared among the three treatment levels using Kruskal-Wallis tests.
RESULTS
Recruitment and Withdrawals
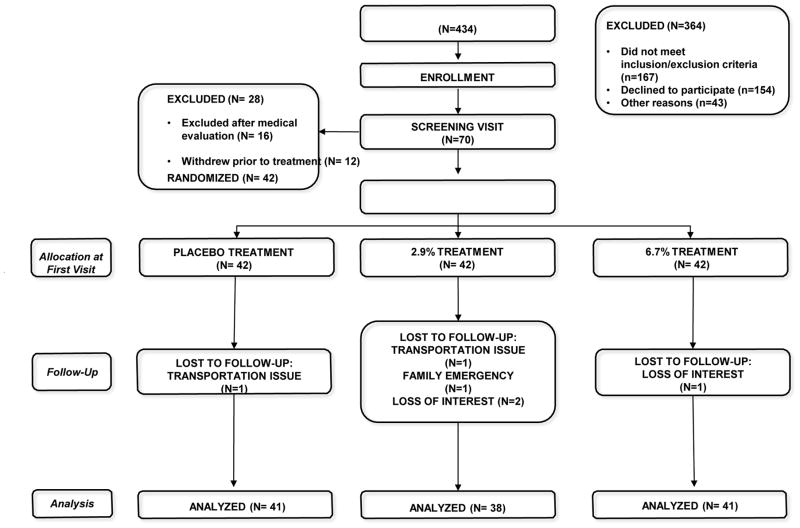
Four hundred thirty four patients were evaluated for eligibility between July 2012 and May 2014 (Figure 3 Consort Flow Chart). Following telephone vetting by a research associate, seventy patients were consented and then interviewed by the principal investigator. Sixteen participants were excluded following a medical evaluation and 12 other participants withdrew for various reasons (e.g., loss of interest, logistic difficulties, inability to curtail medicinal cannabis, etc.). The remaining 42 participants participated in 120 eight-hour human laboratory experimental sessions. No participant withdrew from the study due to an experimental intervention. One participant felt syncopal and was found to be slightly hypotensive; this responded promptly to his mechanical wheelchair being adjusted from the sitting to the reclining position. There were no study related serious adverse events. Consistent with the notion that cannabis is well tolerated, the majority of participants took 8 puffs during flexible dosing sessions (23 of 41, 24 of 38, and 24 of 41 after receiving placebo, 2.9% and 6.7% delta 9-THC respectively) with no significant difference among the doses (p > 0.3).
Figure 3.
Consort Flow Diagram
The demographic make-up of participants is presented in Table 1. Of the 42 participants who completed at least one study visit, 17 (40%) were current cannabis users defined as reporting that they had used cannabis within 30 days prior to randomization. Twenty one (50%) were ex-users and 4 (10%) had never been exposed to cannabis previously.
Primary Efficacy Measurement: Pain Intensity
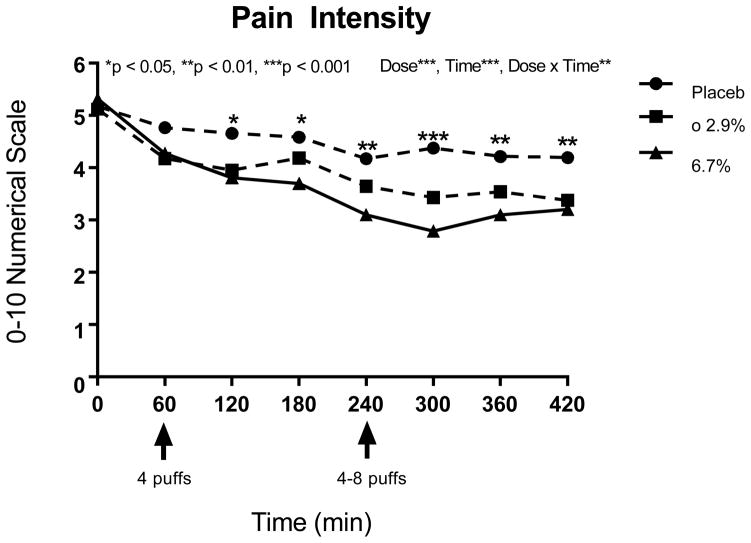
The primary analysis compared patients’ mean numeric pain intensity scale before and after consuming vaporized marijuana or placebo (Figure 4). The mean (SD) pain intensities at baseline were 5.3 (1.8) prior to administration of placebo, and 5.0 (1.8) and 5.2 (2.1) for the lower (2.9%) and higher (6.7%) delta 9-THC doses of cannabis, respectively, on an 11-point numerical pain rating scale. These baseline values were not significantly different (p > 0.6). Overall, after controlling for baseline pain, a significant dose effect on pain intensity occurred (p<0.0001, see Table 2). According to the Tukey test, there was a significant stairstep effect where the most pain occurred with placebo, significantly less pain was measured at the 2.9% delta 9-THC dose, and at the highest delta 9-THC dose, significantly less pain was experienced compared to the lower dose and placebo. Recent cannabis use did not affect these results, and neither the sequence of treatment nor the presence of either spinal cord injury or disease had a significant effect on response to treatment. One hour after the first treatment dose (120 minutes), pain intensity was significantly lower for both doses of active treatment compared to placebo (p<0.05), but the effects of only the higher delta 9-THC dose retained significance over the next two hours (p<0.01, see Table 2). One hour after the variable dose at 240 minutes, the dose-response effect was evident in separation between the two active doses in addition to their showing significantly better pain control over placebo (p<0.05), and both active doses continued to be associated with significantly less pain compared to placebo for the next two hours (p<0.05), although the impact on pain showed no distinction between the lower and higher doses of delta 9-THC (p>0.11).
Figure 4.
11-point numerical pain intensity rating scale anchored between 0 (no pain) and 10 (worst possible pain)
Table 2.
Main overall dose effect (left of dotted line) and dose effects at specified timepoints (right of dotted line) for Pain Intensity and related measures. Inequalities show the direction of significant dose effects, 0 = placebo, 2.9 = 2.9% THC, 6.7 = 6.7% THC. Higher intensity and allodynia values reflect more pain, higher pain relief values reflect more relief from pain.
| Measure* | Main | Timepoint (minutes) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dose Effect | 0 | 60 | 120 | 180 | 240 | 300 | 360 | 420 | |
| Intensity**,n | 0 > 2.9 > 6.7 | ns | ns | 0>2.9,6.7 | 0>6.7 | 0>6.7 | 0>2.9>6.7 | 0>2.9,6.7 | 0>2.9,6.7 |
| Pain Relief | 0<2.9,6.7 | --- | 0<2.9,6.7 | 0<2.9,6.7 | ns | 0<2.9,6.7 | 0<2.9,6.7 | 0<2.9 | 0<6.7 |
| Allodynia** | ns | ns | 0>6.7 | --- | --- | ns | --- | --- | --- |
Abbreviations: ns, not significant; ---, not measured
Treatment sequence was tested but dropped in all models since no significance was identified; Intensity and allodynia had a significant time effect (p<0.0001); Intensity had a significant dose x time interaction effect (p < 0.01); Recent cannabis use did not alter main effect results.
Adjusted for baseline values (p < 0.0001).
Neuropathic pain condition (spinal cord injury vs. disease) was tested but dropped since no significance was identified.
When each of the seventeen subjective and psychoactive side effects (e.g., good drug effect, feeling high, etc.) were added as a covariate to the mixed effects model that included pain intensity in order to evaluate the mechanisms of the analgesic treatment effects, four side effects showed no significant effect on pain (“bad drug effect,” “nauseous,” “changes perceiving time,” and “difficulty remembering things”), but the others did (p ranged from <0.0001 to p=0.02). However, the main effect for delta 9-THC treatment remained significant (all p<0.0004) above and beyond any effect of the psychomimetic measures. Therefore, an independent effect of study medication was evident. In most cases, the stairstep direction of the effect was present. For a few of the side effects, the effects of both active treatments were indistinguishable from each other but evidenced less pain than with placebo.
The number of participants achieving a reduction of pain intensity of 30% or more, a level believed to be clinically important 35, was estimated for each treatment dose. Eighteen participants (45%, 95% confidence interval: 31–60%) reached that level when on placebo compared to 26 (70%, 95% CI: 54–83%) and 35 (88%, 95% CI: 74–95%) on the lower and higher active doses of cannabis, respectively. A significant dose-response effect was realized for these three treatment doses according to the Cochran-Armitage trend test (p<0.0001) and, pairwise, placebo differed from 2.9% (p=0.0242) and 6.7% (p<0.0001) but the two active doses did not significantly differ from each other on this measure (p=0.0606).
The number needed to treat (NNT) to achieve 30% pain reduction during the 8-hour period was 4 (95% CI: 2.1–25.3) for the lower dose vs. placebo, and 3 (95% CI: 1.6–4.2) for the higher dose versus placebo. For the higher dose compared to the lower dose, the NNT was 6, but because the 95% confidence interval for the absolute risk reduction ranged between a negative number (treatment may harm) and a positive number (treatment may benefit), division by zero would be required to report conventional confidence intervals. Instead, we report that with 95% confidence, when compared to the lower dose, either the higher dose is helpful and the number need to help is greater than 2.8 or the higher dose is harmful and the number needed to harm is greater than 140.33
Secondary Outcomes
Global Impression of Change
In addition to VAS ratings for pain intensity, the degree of relief was monitored by a seven-point ordinal scale of patient global impression of change. On average over the entire eight hour visit, there was significantly more pain relief with active cannabis compared to the placebo (p<0.0001, Table 2). This effect was observed immediately after the first vaporization (p<0.005) and one hour (p<0.04), but not two hours (p>0.2), later. Once the second vaporization took place, both delta 9-THC doses provided greater pain relief than placebo immediately (p<0.001) and one hour later (p<0.05). However, only one dose (2.9% at Time 360, p=0.03; 6.7% at Time 420, p=0.03) remained effective in reducing pain significantly compared to placebo. At no time was there a significant difference in pain relief between the two active doses.
Neuropathic Pain Scale
Measurements from the Neuropathic Pain Scale (NPS) over all timepoints indicate that vaporized cannabis positively and significantly affected all of the measured multidimensional pain descriptors associated with neuropathic pain, even after controlling for baseline levels (p<0.0001 for all except itching which had p=0.04, see Table 3). At one hour after the first vaporization session and maintaining throughout the duration of the visit, modeling of intensity, cold and superficial pain revealed significantly reduced pain levels after both active treatments compared with placebo (p-values ranged from <0.0001 to 0.048). The sequence of treatments was significant for intensity and superficial pain, but even after controlling for sequence, the dose effects remained. NPS outcomes that showed a dose effect immediately after the first vaporization were burning, cold, itching, deep pain, and superficial pain (p<0.05). No results for any of the NPS measures were changed when the statistical models controlled for recent cannabis use.
Table 3.
Main overall dose effect (left of dotted line) and dose effects at specified timepoints (right of dotted line) for Neuropathic Pain Scale measures. Inequalities show the direction of significant dose effects, 0 = placebo, 2.9 = 2.9% THC, 6.7 = 6.7% THC.
| Main | Timepoint (minutes) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Measure* | Dose Effect | 0 | 60 | 120 | 180 | 240 | 300 | 360 | 420 |
| Intensity** | 0>2.9,6.7 | ns | ns | 0>2.9,6.7 | 0>2.9,6.7 | 0>2.9,6.7 | 0>2.9>6.7 | 0>2.9,6.7 | 0>2.9,6.7 |
|
| |||||||||
| Sharpness | 0>2.9>6.7 | ns | ns | 0>6.7 | 0>2.9,6.7 | 0>6.7 | 0,2.9>6.7 | 0>6.7 | ns |
|
| |||||||||
| Burning | 0>2.9,6.7 | ns | 0>2.9,6.7 | 0>2.9 | 0>6.7 | ns | 0>2.9,6.7 | 0>6.7 | 0>6.7 |
|
| |||||||||
| Aching | 0>2.9,6.7 | ns | ns | ns | 0>6.7 | 0>6.7 | 0>6.7 | 0>6.7 | ns |
|
| |||||||||
| Cold | 0>2.9,6.7 | ns | 0>2.9,6.7 | 0>2.9,6.7 | 0>2.9,6.7 | 0>2.9,6.7 | 0>2.9,6.7 | 0>2.9,6.7 | 0>2.9,6.7 |
|
| |||||||||
| Sensitivity | 0>2.9,6.7 | ns | ns | 0>6.7 | 0>6.7 | 0>6.7 | 0>6.7 | 0>2.9,6.7 | ns |
|
| |||||||||
| Itching | 0>6.7 | ns | 2.9>6.7 | ns | ns | ns | ns | ns | ns |
|
| |||||||||
| Unpleasantness | 0>2.9,6.7 | ns | ns | 0>6.7 | 0>6.7 | 0>6.7 | 0>2.9,6.7 | ns | 0>2.9 |
|
| |||||||||
| Deep Pain | 0>2.9,6.7 | ns | 0>2.9 | 0>2.9 | 0>2.9,6.7 | 0>6.7 | 0>6.7 | ns | 0>2.9 |
|
| |||||||||
| Superficial Pain** | 0>2.9,6.7 | ns | 0>2.9 | 0>2.9,6.7 | 0>2.9,6.7 | 0>2.9,6.7 | 0>2.9,6.7 | 0>2.9,6.7 | 0>2.9,6.7 |
Abbreviation: ns, not significant
All models included an adjustment for the baseline values (p<0.0001) and had a significant time effect (p<0.0001); Only Intensity and sensitivity had a significant dose x time interaction effect (p < 0.04); Recent cannabis use did not alter main effect results.
Adjusted for treatment sequence effect
Allodynia
There was no general effect of treatment on allodynia after adjusting for baseline levels, although immediately after the first vaporization, lower measurements were observed for the higher dose cannabis than for the placebo (p=0.01, see Table 2). Treatment sequence was not significant and recent cannabis use did not alter the results.
Heat Pain Threshold
Neither time (p>0.05) nor treatment condition (p>0.05) at any timepoint was associated with change in pain response to mild-to-moderately painful heat stimuli delivered to the non-dominant C2–C3 level (data not shown).
Subjective and Psychoactive Effects
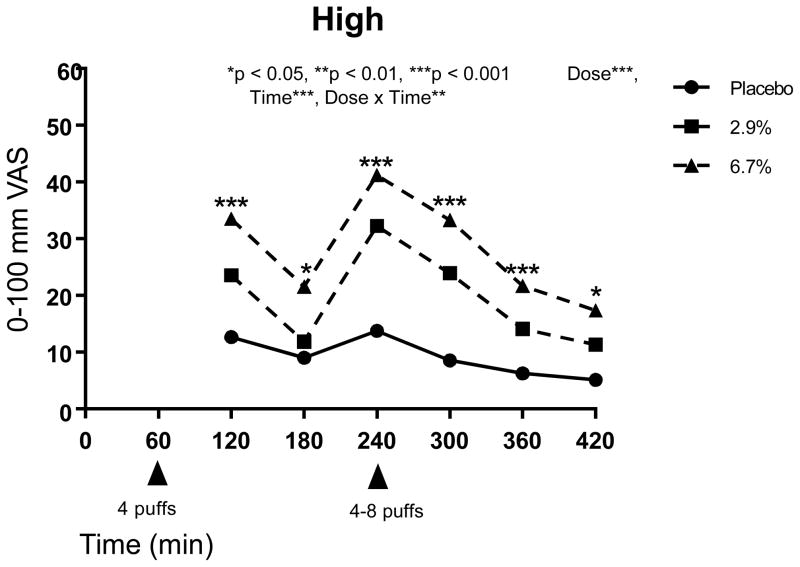
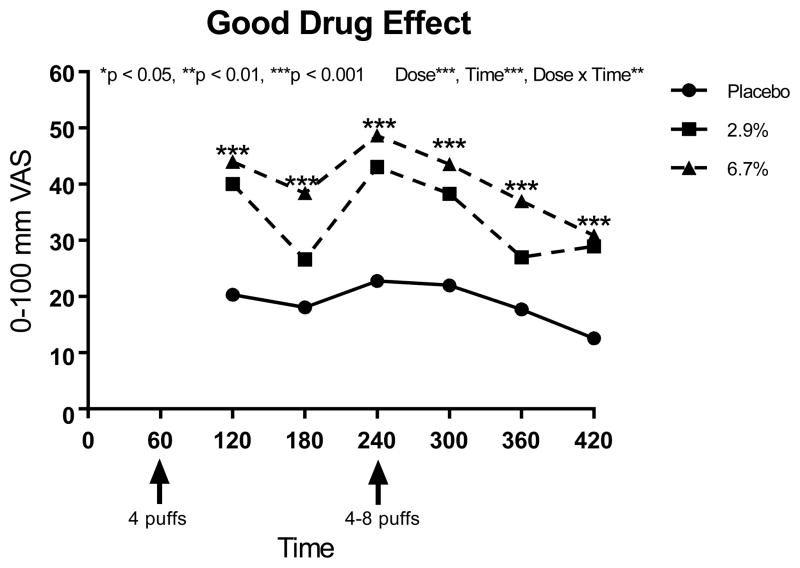
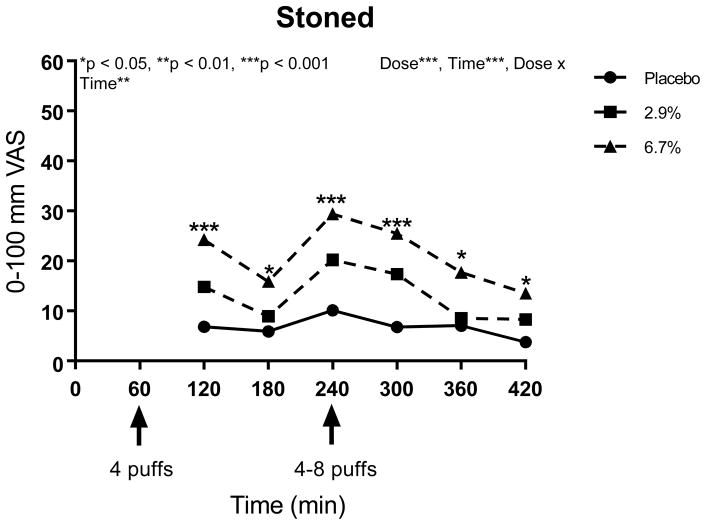
Using several variables to explore side effects, the main effect of treatment (placebo vs. 2.9% delta 9-THC vs. 6.7% delta 9-THC) as well as treatment by time interaction effects were considered in the modeling. For each of the 17 psychoactive side effects analyzed, a significant main treatment effect was detected (p-values ranged from <0.0001 to 0.026, see Table 4). The lower dose produced effects lower than that for the higher dose, and placebo was lower than both active doses for “any drug effect,” “good drug effect,” “high,” “impaired,” “stoned,” “sedated,” and “changes perceiving space” (p<0.0001). For “bad drug effect,” “like the drug effect,” “nauseous,” and “changes perceiving time,” placebo levels were significantly lower than both 2.9% and 6.7% delta 9-THC doses overall, but there were no differences between the two active treatment doses (p<0.01). The higher delta 9-THC dose was associated with significantly higher levels of “desires more,” “hungry,” “difficulty remembering things,” (p<0.03) “drunk,” “confused,” and “difficulty paying attention” compared to placebo, but only the last three side effects showed significantly stronger effects on the higher dose compared to the lower THC dose overall (p<0.0001).
Table 4.
Main overall dose effect (left of dotted line) and dose effects at specified timepoints (right of dotted line) for Subjective and Psychoactive Side Effects. Inequalities show the direction of significant dose effects, 0 = placebo, 2.9 = 2.9% THC, 6.7 = 6.7% THC.
| Main | Timepoint (minutes) | ||||||
|---|---|---|---|---|---|---|---|
| Measure* | Dose effect | 120 | 180 | 240 | 300 | 360 | 420 |
| Any Drug Effect | 0<2.9<6.7 | 0<2.9,6.7 | 0,2.9<6.7 | 0<2.9<6.7 | 0<2.9<6.7 | 0<2.9<6.7 | 0<2.9<6.7 |
|
| |||||||
| Good Drug Effect | 0<2.9<6.7 | 0<2.9,6.7 | 0,2.9<6.7 | 0<2.9,6.7 | 0<2.9,6.7 | 0<2.9<6.7 | 0<2.9,6.7 |
|
| |||||||
| Bad Drug Effect | 0<2.9,6.7 | 0<2.9,6.7 | ns | ns | 0<2.9,6.7 | ns | ns |
|
| |||||||
| High | 0<2.9<6.7 | 0<2.9<6.7 | 0,2.9<6.7 | 0<2.9<6.7 | 0<2.9<6.7 | 0<2.9,6.7 | 0<6.7 |
|
| |||||||
| Drunk | 0,2.9<6.7 | 0<2.9 | ns | 2.9<6.7 | 0,2.9<6.7 | ns | 2.9<6.7 |
|
| |||||||
| Impaired | 0<2.9<6.7 | 0<6.7 | 0<6.7 | 0<2.9<6.7 | 0<2.9<6.7 | 0,2.9<6.7 | 0,2.9<6.7 |
|
| |||||||
| Stoned | 0<2.9<6.7 | 0<2.9<6.7 | 0<6.7 | 0<2.9<6.7 | 0<2.9<6.7 | 0,2.9<6.7 | 0<6.7 |
|
| |||||||
| Like the Drug Effect | 0<2.9,6.7 | 0<2.9,6.7 | 0<2.9,6.7 | 0<2.9,6.7 | 0<2.9<6.7 | 0<2.9,6.7 | 0<2.9,6.7 |
|
| |||||||
| Sedated | 0<2.9<6.7 | 0<2.9,6.7 | 0<6.7 | 0<2.9,6.7 | 0<2.9,6.7 | 0,2.9<6.7 | 0<6.7 |
|
| |||||||
| Confused | 0,2.9<6.7 | ns | ns | 0<6.7 | 0,2.9<6.7 | ns | 0<6.7 |
|
| |||||||
| Nauseous | 0<2.9,6.7 | 0<2.9 | 0<2.9 | ns | ns | ns | ns |
|
| |||||||
| Desires More | 0<6.7 | ns | ns | ns | ns | ns | ns |
|
| |||||||
| Hungry | 0<6.7 | 0<2.9 | 2.9>6.7 | ns | 0,2.9<6.7 | ns | ns |
|
| |||||||
| Changes Perceiving Time | 0<2.9,6.7 | ns | ns | 0<6.7 | 0<6.7 | ns | ns |
|
| |||||||
| Changes Perceiving Space | 0<2.9<6.7 | ns | ns | 0<6.7 | 0,2.9<6.7 | 0<6.7 | 0<6.7 |
|
| |||||||
| Difficulty Paying Attention | 0,2.9<6.7 | 0<6.7 | ns | 0<6.7 | ns | 0<6.7 | ns |
|
| |||||||
| Difficulty Remembering Things | 0<6.7 | ns | ns | 0<6.7 | ns | ns | ns |
Abbreviation: ns, not significant
Treatment sequence and recent cannabis use were tested but dropped since no significance was identified; Significant time effects for any drug effect, high, sedated, and hungry had p<0.0001, time effects for good drug effect, impaired, and stoned had p<0.01, and bad drug effect and like the drug effect had p<0.04, with no significant time effects for the other side effects; Only hungry had a significant dose x time interaction effect (p<0.01).
When testing contrasts at each timepoint, the previously described stairstep dose response occurred for “high” and “stoned” one hour after each vaporization (0% < 2.9% < 6.7%, p<0.05), and those effects plus “any drug effect” and “impaired” were significantly affected immediately after the flexible dose inhalation, too, and continued for at least one additional hour (p<0.05). The maximum mean effect for “any drug effect” and “good drug effect” for all three dose levels was attained immediately after the flexible dose at Time 240 (52 and 49 out of 100 mm, respectively, for the 6.7% delta 9-THC dose). Starting with the second THC dose and continuing throughout the rest of the study visits for “any drug effect,” there was a clear stairstep dose response over all three treatments (p<0.05), with a stronger effect as the dose increased. For “bad drug effect,” the only times showing significant dose effects (both active doses resulted in stronger effects than placebo) occurred at one hour post-first and post-second vaporizations, respectively (p<0.05). However, the differences of the mean effects found to be present were less than 6 and 7 out of 100 mm, respectively, which are considered likely to be clinically unimportant. Similarly, the differences for all subjective and psychomimetic side effects were of small magnitude. Compared to placebo, the mean values were 6 mm and 9 mm on 100 mm visual analogue scales, respectively, for the 2.9% and 6.7% delta 9-THC. Significant dose effects (p<0.05) were detected one hour after both of the cannabis vaporizations for all psychomimetic side effects except “desires more” and “difficulty remembering things” and dose effects were also absent at one hour after just the first inhalation for “confused,” “changes perceiving time,” and “changes perceiving space,” and one hour after just the second dosing for “nauseous” and “difficulty paying attention.”
Spasticity
Spasticity showed an overall response to delta 9-THC (p=0.036), with less spasticity reported for the lower dose compared to placebo (Table 5). However, statistical significance was not reached until 3 hours after the second dosing with cannabis (Time 420, p=0.03). The categorical spasticity relief measure reflected more relief at that same timepoint, with the 2.9% dose providing more relief than either of the other two doses (p<0.05).
Table 5.
Main overall dose effect (left of dotted line) and dose effects at specified timepoints (right of dotted line) for Spasticity. Inequalities show the direction of significant dose effects, 0 = placebo, 2.9 = 2.9% THC, 6.7 = 6.7% THC.
| Main | Timepoint (minutes) | ||||
|---|---|---|---|---|---|
|
| |||||
| Measure* | Dose Effect | 0 | 60 | 240 | 420 |
| Spasticity Numerical Scale** | 0>2.9 | ns | ns | ns | 0>2.9 |
| Categorical Spasticity Relief | ns | --- | ns | ns | 0,6.7<2.9 |
Abbreviations: ns, not significant; ---, not measured
Terms for treatment sequence and neuropathic pain condition were included and tested for significance in initial models but were dropped since no significance was identified; There were significant time effects (p<0.0001 for spasticity numerical scale and p=0.0227 for categorical spasticity relief); There were no dose x time interaction effects and recent cannabis use did not alter main effect results.
Adjusted for baseline value (p < 0.0001).
Vital Signs
There was an effect of treatment on heart rate immediately after both vaporization sessions (Table 6, p < 0.01). At Time 60 minutes, the average pulse was 70, 80, and 79 beats per minute for placebo, 2.9% and 6.7% delta 9-THC sessions, respectively. The corresponding values at Time 240 were 70, 77, and 80 beats per minute. There was no treatment effect on respiratory rate; there was a clinically insignificant difference of 1 breath per minute at baseline between the active medications. There were no treatment effects for systolic and diastolic blood pressure. At Time 300 both of these values were lower with the 6.7% delta 9-THC than with placebo (p < 0.05). The differences were clinically insignificant. The average systolic difference was 6 mm Hg (placebo: 120 mmHg vs. 6.7% delta 9-THC: 114 mm Hg). The average diastolic difference was 1 mm Hg (placebo: 72 mm Hg vs. 6.7% delta 9-THC: 71 mm Hg). Current cannabis use did not have an effect on any of the measures.
Table 6.
Main overall dose effect (left of dotted line) and dose effects at specified timepoints (right of dotted line) for Vital Signs. Inequalities show the direction of significant dose effects, 0 = placebo, 2.9 = 2.9% delta 9-THC, 6.7 = 6.7% delta 9-THC.
| Main | Timepoint (minutes) | |||||||
|---|---|---|---|---|---|---|---|---|
| Measure* | Dose Effect | 0 | 60 | 120 | 180 | 240 | 300 | 360 |
| Heart rate | 0<2.9,6.7 | ns | 0<2.9,6.7 | ns | ns | 0<2.9,6.7 | ns | ns |
| Respiration Rate | ns | 2.9>6.7 | ns | ns | ns | ns | ns | ns |
| Systolic Blood Pressure** | ns | ns | ns | ns | ns | ns | 0>6.7 | ns |
| Diastolic Blood Pressure | ns | ns | ns | ns | ns | ns | 0>6.7 | ns |
All models included an adjustment for the baseline values (p<0.0001) and had no dose x time interaction effects; There were time effects for heart and respiration rates (p<0.0001), systolic blood pressure (p<0.01), and diastolic blood pressure (p=0.02); A term for level of injury was included and tested for significance in initial models but was dropped since no significance was identified; Recent cannabis use did not alter main effect results.
Adjusted for treatment sequence effect
Neuropsychological Testing
Based on neuropsychological test results at participants’ first visit before initial treatment, 73% of participants (N=30 of 41) evidenced global cognitive impairment prior to initiating the study, with the mean (standard deviation) GDS being 1.28 (0.96), indicative of an overall moderate-to-high level of cognitive impairment.
The main effects of dose and time model the cognitive effects over the course of the experimental sessions. To account for possible residual neuropsychological effects of cannabis in chronic users, the covariate of cannabis use (current vs. not current) was added to the main effects model for all of the neuropsychological tests to determine if there were any changes in the main results. Data from specific tests for five participants were removed from consideration due to the participant’s inability to validly perform the tests as a result of their disability (as determined by the research associate).
Overall, there were no significant differences between study medications on neuropsychological testing (Table 7). The results of the individual tests are described below:
Table 7.
Main overall dose effect (left of dotted line) and dose effects at specified timepoints (right of dotted line) for raw NP test measures. Inequalities show the direction of significant dose effects, 0 = placebo, 2.9 = 2.9% delta 9-THC, 6.7 = 6.7% delta 9-THC.
| Main | Timepoint (minutes) | ||||||
|---|---|---|---|---|---|---|---|
| Measure* | Dose Effect | 0 | 120 | 180 | 300 | 360 | 420 |
| Digit Symbol | ns | ns | ns | ns | ns | ns | ns |
|
| |||||||
| HVLT Sum | ns | ns | ns | ns | ns | ns | ns |
|
| |||||||
| HVLT Delay | ns | ns | ns | ns | ns | ns | ns |
|
| |||||||
| HVLT True Positive | ns | ns | 0,2.9>6.7 | 0,2.9>6.7 | ns | ns | ns |
|
| |||||||
| HVLT False Positive | 0<6.7 | ns | ns | ns | 0<6.7 | ns | ns |
|
| |||||||
| Pegboard D TimeI | ns | ns | ns | ns | ns | ns | ns |
|
| |||||||
| Pegboard ND Time I | ns | ns | ns | ns | 0<6.7 | 2.9<0,6.7 | ns |
|
| |||||||
| PASAT Correctc | ns | ns | ns | --- | ns | --- | 2.9<6.7 |
|
| |||||||
| Trails A Time | *** | ns | ns | ns | ns | ns | 2.9>6.7 |
|
| |||||||
| Trails B Time** | ns | ns | ns | ns | ns | ns | ns |
Abbreviations: ns, not significant; ---, not measured.
All models included an adjustment for the baseline values (all p < 0.0001); Time effects were significant for digit symbol, HVLT delay, PASAT correct, and Trails A and B (p<0.0001), HVLT sum, HVLT true positive, and pegboard ND time (p<0.02), and pegboard D time (p<0.05), but not for HVLT false positive; There were no significant dose x time interaction effects.
Adjusted for treatment sequence effect
Significant overall dose effect (p=0.047) but no pairwise differences found with Tukey HSD test.
Shorter time with injury below cervical level, but main effects unchanged.
More correct with recent cannabis use, but main effects unchanged
Grooved Pegboard Test
After controlling for baseline values, no significant treatment effect was seen on the Grooved Pegboard dominant subtests, even after controlling for the level of spinal cord pathology. On the non-dominant subtests, at time 360, participants at 2.9% delta-9 THC took less time to complete the task than when on placebo or at the high dose.
Digit Symbol Test
Dose effect differences were not significant throughout the 8 hour visit, although there was improvement across all visits for all conditions (i.e., placebo, 2.9% and 6.7% delta 9-THC, time p-value<0.0001), consistent with practice effects.
Trail Making Test
There was an overall dose effect on Trailmaking Part A, although in the pairwise comparison participants only took longer to complete Part A at 420 when on 2.9, compared to 6.7 delta 9-THC. There were no significant dose effect differences on Part B.
Hopkins Verbal Learning Test
There were no significant dose effect differences for either immediate or delayed recall. True positive responses at 6.7% were less than 2.9% and placebo at 120 and 180 minutes (p<0.05); false positive results were higher at 6.7% compared to placebo at 300 minutes (p=0.023).
Paced Auditory Serial Addition Test
This examination exhibited non-significant dose effect differences except at Time 420, where the number of correct responses at this time for the higher active dose was greater than for the lower active dose (p<0.05).
In summary, cannabis generally did not result in reduced cognitive performance, as indicated by the lack of dissimilarities in scores over time between treatment groups.
DISCUSSION
Analgesia
The primary outcome variable, numerical pain intensity, provides verification that vaporized cannabis can impart relief from neuropathic pain related to spinal cord injury or disease. Concordant with this proposition are the results of the Patient Global Impression of Change and Neuropathic Pain Scale. In addition, the NNT to achieve 30% pain reduction was 4 (95% CI: 2.1–25.3) for the lower dose vs. placebo, and 3 (95% CI: 1.6–4.2) for the higher dose vs. placebo, These values are similar to those found in previous studies evaluating smoked cannabis 2, 33 and are in the range of two commonly deployed anticonvulsants used to treat neuropathic pain (pregabalin, NNT = 3.9; gabapentin, NNT = 3.8) 9, 78.
It is noteworthy that the two active doses did not significantly differ from each other (p=0.0606, χ2 likelihood ratio test). Likewise, the difference in the NNT of 6 between the two active doses does not indicate a definite dose difference as the confidence interval comparing the higher with the lower dose is wide and encompasses the possibility that 6.7% delta 9-THC is not more effective as an analgesic when compared to 2.9% delta 9-THC. Furthermore, there were no differences on the Patient Global Impression of Change between the two active doses.
Allodynia and Heat Pain Threshold
Abnormal thresholds to sensory stimuli are common in patients with neuropathic pain arising from lesions of the spinothalamic pathways 84. We therefore sought evidence for an effect of cannabis on allodynia as well as changes in thresholds for sensory perception using the heat pain threshold. However, substantiation of reversal of sensory sensitization or an increase in heat pain threshold by cannabis was not a consistent finding. Although lower measurements for allodynia were observed for the higher dose of delta 9-THC compared to placebo, this only occurred after the first vaporization session and not after the second session. The lack of an effect on the experimental heat pain threshold suggests that the analgesic effect of cannabis in treating acute pain would be less than ideal; this is consistent with the recommendation that cannabinoids are not suitable for postoperative pain 18.
Psychoactive and Subjective Side Effects
Many of the psychoactive side effects were delta 9-THC concentration-dependent with greater effects seen in the higher compared to the lower dose, with both active doses inducing a higher response than placebo. Similar assessments of “high,” “good drug effect,” and “stoned” have been described in the literature 62. The magnitude of the effects were modest, especially for the lower dose (Figures 5, 6 and 7). Given the aforementioned evidence that the two active doses did not significantly differ from each other in terms of analgesic potency, it appears appropriate for patients with spinal cord injury or disease who wish to avoid psychomimetic effects to consider the lower dose.
Figure 5.
“Do you feel high right now?” a 100-mm VAS anchored by “not at all” at 0 and “extremely” at 100
Figure 6.
“Do you feel a good drug effect right now?” a 100-mm VAS anchored by “not at all” at 0 and “extremely” at 100
Figure 7.
“Do you feel stoned right now?” a 100-mm VAS anchored by “not at all” at 0 and “extremely” at 100
Spasticity
Relief from spasticity utilizing an 11-point numerical rating scale was not statistically significant until just prior to discharge at Time 420 minutes. Unexpectedly, the relief was greater with the 2.9% than after 6.7% delta-9-THC. In a recent meta-analysis, cannabinoids were associated with a greater improvement in spasticity (secondary to multiple sclerosis or paraplegia) compared to placebo when assessed using numerical rating scales (mean difference, −0.76 [95% CI, −1.38 to −0.14]) 109. Assessments in the studies in the meta-analysis followed two weeks of treatment; therefore, a direct comparison with an 8 hour human laboratory experiment is not feasible.
Vital Signs
The approximate 10% increase in heart rate that was seen immediately after cannabis vaporization is a well-recognized side-effect. Cannabis can result in a rapid and substantial dose-dependent increase in heart rate by as much as 20–100%, as well as supine hypertension and orthostatic hypotension 5, 77. Although the hemodynamic effects of cannabis are generally not problematic for most young, healthy users, the orthostatic hypotension induced by cannabis can be symptomatic and incapacitating 66. As mentioned earlier, one participant with quadriplegia had near syncope associated with a low blood pressure. This was consistent with the autonomic instability with loss of descending autonomic control seen in patients with interruption of spinal tracts 71. For the most part, however, blood pressure was stable following administration of cannabis in the present study.
Neuropsychological Testing
The lack of treatment effects on cognition was surprising, given previous findings from our group 112, 113 and others 17, 26, 105. Although the reasons for this unanticipated finding are unclear, there are a few possible explanations. Administration of the cognitive measures instruments was delayed for 60 minutes after each dose (until 120 minutes and 300 minutes) (Figure 2). This was necessary because participants received treatments in a separate location. However, since peak levels of intoxication typically occur after approximately 30 minutes and last several hours 48, reductions in cognitive performance would still have been expected to be present at subsequent evaluations.
Perhaps of more significance, numerous patients in this study had spinal cord involvement of their upper extremities. The challenge of assessing neuropsychological functioning using tests requiring the use of upper extremities in persons with paralyzed limbs has been reported in the literature 59. However, non-significant results were still seen when excluding patients with upper limb limitations. In addition, measures involving word recall (Hopkins Verbal Learning Test) and verbal calculations (Paced Auditory Serial Addition Test) also did not show performance decrements, which is inconsistent with previous reports of dose-related neurocognitive effects of marijuana 26. Of note, many of the participants had spinal cord injuries as a result of trauma (Table 1), including causes that may also have resulted in traumatic brain injury as well (i.e., motorized vehicle accidents). Indeed, a significant proportion were identified as having cognitive impairment (73%) at study initiation. Hypothetically, it is possible that the effects of delta 9-THC in the individuals who already have significant cognitive deficits are of insufficient magnitude to be detectable On the other hand, others have suggested that the use of cannabis in impaired individuals might significantly amplify such deficits 61, 86. The current study is not adequately powered to address which of these hypotheses is most plausible.
Daily smokers may develop tolerance and demonstrate smaller effect sizes on cognitive tests compared to less frequent users 53. Tolerance probably factored into a recent cohort study of chronic pain patients who used 12.5% delta-9 THC (median dose 2.5 grams/day) and found improvements in neurocognitive function over a one year period 106. Seventeen of the 42 participants in the present study used cannabis regularly; we thus entered recent use as a covariate in modeling. But a majority of participants were ex-users or naïve to cannabis. Other potential confounding factors might include fatigability as well as depressed mood, as described in the multiple sclerosis literature 11, 36, 93. However, we excluded individuals with severe depression (PHQ-9 ≥ 15), as cannabis may worsen depressive symptomatology, so this may not have been that relevant to our study 74. And although the neuropsychological battery was relatively brief (taking approximately 20–30 minutes), it was administered repeatedly so physical and psychological/cognitive fatigability may have factored into the results of this study. Thus, factors in acute cognitive changes must be understood in the context of underlying chronic diseases, with attendant fatigue and its possible influence on neuropsychological testing.
Recommendations for Future Research
In the future, it will be important to explore the relative merits of different methods of delivery of cannabinoids and whether oral delta-9 THC or sublingual nabixamols would have comparable efficacy in the treatment of neuropathic pain as vaporized cannabis. Pharmacologic oral preparations avoid deleterious effects upon the respiratory system and have deservedly warranted attention from the scientific community for this reason27, 40, 49, 64, 80, 96, 99. However, some experts have opined that whole plant cannabis is superior to the FDA approved oral cannabinoid preparations, e.g., synthetic tetrahydrocannabinol (dronabinol, Marinol®) and/or the synthetic analog of Δ9-THC (nabilone, Cesamet®) 76. As testament to this belief, oral cannabinoids have been on the market in the United States for many years and are not widely used 76. In order to definitively describe the relative merits of cannabis and oral cannabinoids, it will be necessary to conduct randomized, controlled trials comparing the two methods of administration and interpret the results. The same might be said for the comparison of cannabis and nabixamols.
In addition, the optimization of dosing should be evaluated for all cannabinoid preparations when utilized for the treatment of neuropathic pain. It has been suggested that a patient-specific, self-titrating model is a useful prescription paradigm for medicinal cannabis 21. The rationale for this arises from the considerable variability in response of different individuals when balancing efficacy with side-effects 21.
An additional issue is whether or not vaporization alters the risk of long-term negative pulmonary sequelae. While it is known that cannabis smoking increases cough, sputum production, hyperinflation, and upper lobe emphysematous changes 85, 100; the effect of vaporization on these pulmonary outcomes will require more investigative work. Also, many individuals are incidentally exposed to secondhand cannabis smoke, but little has been known about the effects of this exposure until recently. Under extreme, unventilated conditions, secondhand cannabis smoke exposure can produce detectable levels of delta-9 THC in blood and urine, minor physiological and subjective drug effects, and minor impairment on a task requiring psychomotor ability and working memory 58. To our knowledge, exposure to secondhand vaporized cannabis has not been studied. An effort needs to be made to find out if this is a preventable problem perhaps by the use of an active exhaust or a hepa filter.
Another objective of future studies will be to evaluate all other short- and long-term side-effects known to result from cannabis. When used by patients as part of a monitored treatment program in 7 pain clinics in Canada over 1 year, medicinal whole plant cannabis, appeared to have a reasonable safety profile 106. An average dose of 2.5 g cannabis per day resulted in a higher rate of adverse events (AE) among cannabis users compared with controls; there were no differences for serious adverse events (SAE). In contradistinction, a recent meta-analysis found that cannabinoids (e.g., cannabis, oral delta-9 THC and/or sublingual nabixamols) were associated with a greater risk of not only AE but also SAE in comparison with controls. Presumably, the difference between the two studies was related to the different study designs (i.e., types of study medications, blinding, use of controls, duration of studies, etc.) Common AE listed among both studies included headache, nasopharyngititis, dizziness, dry mouth, nausea, fatigue, somnolence, euphoria, vomiting, disorientation, drowsiness, confusion, loss of balance, and hallucination. Obviously, longer-term monitoring for adverse outcomes is needed. The epidemiological literature concerning recreational cannabis reveals that cannabis use increases the risk of motor vehicle accidents and can produce dependence, and that there are consistent associations between regular cannabis use and poor psychosocial outcomes and mental health in adulthood 51. It remains to be seen whether these longer term consequences will be similar for medicinal cannabis.
Another existing knowledge gap that should be evaluated in the future is the potential of the non-psychoactive cannabinoid, cannabidiol (CBD). The synergistic contributions of CBD to the analgesia produced by Δ9-THC have been explored in studies involving Sativex®, an extract of Δ9-THC and CBD manufactured by GW Pharmaceuticals in Great Britain. Sativex® alleviates spasticity, overactive bladder, and neuropathic pain associated with multiple sclerosis 10, 16, 63, 94, 103. CBD can counteract some of the negative effects of Δ9-THC (e.g., anti-psychotic effects 111, anti-anxiety effects 116), although such findings have not always been consistent 81.
The amount of CBD in the stock supplies provided by the National Institute of Drug Abuse (NIDA) at the time the present study was initiated were minimal. In the interim, the availability of research grade cannabis plant material available from NIDA has undergone substantial upgrading in terms of variability. CBD can each be provided in low (<1%), medium (1–5%), high (5–10%), and very high (>10%) concentrations 95. Nora Volkow M.D., Director of NIDA, has stated, “CBD appears to be a safe drug with no addictive effects, and the preliminary data suggest that it may have therapeutic value for a number of medical conditions. Addressing barriers that slow clinical research with CBD would accelerate progress. NIDA will do what we can to address such barriers and expedite the study of this potentially valuable compound, as well as other components of the marijuana plant” 102.
CONCLUSION
The present study complements previous investigative work that cannabis is a promising treatment in selected pain syndromes caused by injury or diseases of the nervous system 2, 33, 104, 105, 107, 112, 113. Due to the large number of statistical tests in the present study, a recognition of the potential of an elevated Type I error rate is warranted. However, at least for analgesic outcomes, the consistency of findings mitigates this concern. As this was an eight hour human laboratory experiment, additional studies are needed to examine this type of treatment over a more prolonged period (e.g., several weeks to months) to insure that an analgesic response will be sustained. In addition to measuring pain relief, such studies would also involve the impact of cannabis on daily functioning, cognition, and mood to adequately evaluate the real world impact of cannabis on spinal cord injury and disease. As this was a preliminary, phase I study, it does not justify the routine clinical use of medicinal cannabis until further research is conducted. Such research should include direct comparisons of cannabinoids both for efficacy and adverse effects over time.
Table 8.
Neuropsychological Instrument Effect Sizes* Compared to Placebo.
| Timepoint (minutes) | |||||||
|---|---|---|---|---|---|---|---|
| Test Measure | delta 9-THC Dose | 0 | 120 | 180 | 300 | 360 | 420 |
| Digit Symbol | 2.9% | 0.08 | 0.02 | −0.02 | 0.05 | 0.07 | 0.08 |
| 6.7% | 0.11 | 0.03 | 0.05 | −0.06 | −0.00 | 0.05 | |
|
| |||||||
| HVLT, Sum | 2.9% | 0.02 | 0.03 | −0.01 | −0.04 | 0.15 | 0.07 |
| 6.7% | −0.04 | −0.12 | −0.14 | −0.09 | 0.08 | 0.16 | |
|
| |||||||
| HVLT, Delay | 2.9% | −0.02 | 0.24 | −0.07 | 0.02 | −0.11 | 0.09 |
| 6.7% | −0.07 | 0.02 | −0.07 | −0.23 | −0.22 | 0.05 | |
|
| |||||||
| HVLT, True Positive | 2.9% | −0.11 | 0.12 | 0.10 | −0.03 | −0.14 | 0.03 |
| 6.7% | −0.25 | −0.46 | −0.44 | −0.08 | −0.09 | 0.06 | |
|
| |||||||
| HVLT, False Positive | 2.9% | 0.09 | 0.17 | 0.06 | 0.22 | 0.23 | 0.31 |
| 6.7% | 0.35 | 0.14 | −0.04 | 0.45 | 0.38 | 0.39 | |
|
| |||||||
| Grooved Pegboard, D | 2.9% | −0.06 | −0.03 | 0.03 | 0.00 | 0.07 | 0.10 |
| 6.7% | −0.02 | 0.10 | 0.07 | 0.04 | 0.06 | 0.01 | |
|
| |||||||
| Grooved Pegboard, ND | 2.9% | −0.02 | −0.05 | −0.02 | 0.02 | −0.14 | 0.07 |
| 6.7% | −0.01 | 0.03 | −0.05 | 0.17 | 0.01 | 0.11 | |
|
| |||||||
| PASAT | 2.9% | 0.09 | 0.20 | --- | 0.02 | --- | 0.01 |
| 6.7% | 0.09 | 0.18 | --- | 0.07 | --- | 0.23 | |
|
| |||||||
| Trails Making A Time | 2.9% | −0.11 | −0.10 | −0.02 | 0.01 | −0.04 | 0.09 |
| 6.7% | 0.17 | 0.01 | −0.13 | 0.02 | −0.08 | −0.06 | |
|
| |||||||
| Trails Making B Time | 2.9% | −0.15 | −0.16 | −0.18 | −0.07 | −0.16 | −0.10 |
| 6.7% | −0.13 | −0.01 | −0.20 | −0.03 | −0.21 | −0.07 | |
Abbreviations: HVLT, Hopkins Verbal Learning Test Revised; D, dominant hand; ND, non-dominant hand; PASAT, Paced Auditory Serial Addition Test; ---, not measured (PASAT only performed 4 times during the visit)
Bold type represents significant difference between active dose and placebo (Positive values: active dose mean is larger than the placebo mean; Negative values: active dose mean is smaller than the placebo mean.)
PERSPECTIVE.
A cross-over, randomized, placebo-controlled human laboratory experiment involving administration of vaporized cannabis was performed in patients with neuropathic pain related to spinal cord injury and disease. This study supports consideration of future research that would include longer duration studies over weeks to months in order to evaluate the efficacy of medicinal cannabis in patients with central neuropathic pain.
Highlights.
Vaporized cannabis positively affected all of the neuropathic pain descriptors
There was significantly more pain relief with active cannabis compared to placebo
The two active doses did not significantly differ from each other in terms of analgesic potency
Many of the psychoactive side effects were delta 9-THC concentration-dependent
Footnotes
Disclosures
No conflicts of interest declared. The research reported in this publication was supported by the National Institute of Drug Abuse (R01DA030424). The project described was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR000002. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, Department of Veterans Affairs or US Government. This material is the result of work supported with resources and the use of facilities at the Sacramento VA Medical Center.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Barth Wilsey, Email: blwilsey@ucdavis.edu.
Thomas D. Marcotte, Email: tmarcotte@ucsd.edu.
Reena Deutsch, Email: redeutsch@ucsd.edu.
Holly Zhao, Email: holly.zhao@ucdmc.ucdavis.edu.
Hannah Prasad, Email: hannahprasad89@gmail.com.
Amy Phan, Email: atphan07@gmail.com.
References
- 1.Announcement of the Department Of Health And Human Services’ Guidance On Procedures for the Provision of Marijuana For Medical Research. National Institutes of Health Release Date; May 21, 1998. https://grants.nih.gov/grants/guide/notice-files/not99-091.html. [Google Scholar]
- 2.Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- 3.Altman DG. Confidence intervals for the number needed to treat. BMJ (Clinical research ed. 1998;317:1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armitage P. Tests for Linear Trends in Proportions and Frequencies. Biometrics. 1955;11:375–386. [Google Scholar]
- 5.Aryana A, Williams MA. Marijuana as a trigger of cardiovascular events: speculation or scientific certainty? International journal of cardiology. 2007;118:141–144. doi: 10.1016/j.ijcard.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Ashendorf L, Jefferson AL, O’Connor MK, Chaisson C, Green RC, Stern RA. Trail Making Test errors in normal aging, mild cognitive impairment, and dementia. Arch Clin Neuropsychol. 2008;23:129–137. doi: 10.1016/j.acn.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attal N, Cruccu G, Baron R, Haanpaa M, Hansson P, Jensen TS, Nurmikko T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–e1188. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 8.Azorlosa JL, Heishman SJ, Stitzer ML, Mahaffey JM. Marijuana smoking: effect of varying delta 9-tetrahydrocannabinol content and number of puffs. The Journal of pharmacology and experimental therapeutics. 1992;261:114–122. [PubMed] [Google Scholar]
- 9.Backonja MM. Use of anticonvulsants for treatment of neuropathic pain. Neurology. 2002;59:S14–17. doi: 10.1212/wnl.59.5_suppl_2.s14. [DOI] [PubMed] [Google Scholar]
- 10.Barnes MP. Sativex: clinical efficacy and tolerability in the treatment of symptoms of multiple sclerosis and neuropathic pain. Expert Opin Pharmacother. 2006;7:607–615. doi: 10.1517/14656566.7.5.607. [DOI] [PubMed] [Google Scholar]
- 11.Beatty WW, Goodkin DE, Hertsgaard D, Monson N. Clinical and demographic predictors of cognitive performance in multiple sclerosis. Do diagnostic type, disease duration, and disability matter? Archives of neurology. 1990;47:305–308. doi: 10.1001/archneur.1990.00530030081019. [DOI] [PubMed] [Google Scholar]
- 12.Beglinger LJ, Gaydos B, Tangphao-Daniels O, Duff K, Kareken DA, Crawford J, Fastenau PS, Siemers ER. Practice effects and the use of alternate forms in serial neuropsychological testing. Arch Clin Neuropsychol. 2005;20:517–529. doi: 10.1016/j.acn.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Benedict R, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test ? Revised: Normative Data and Analysis of Inter-Form and Test-Retest Reliability. The Clinical Neuropsychologist. 1998;12(1):43–55. [Google Scholar]
- 14.Benedict RH, Zgaljardic DJ. Practice effects during repeated administrations of memory tests with and without alternate forms. Journal of clinical and experimental neuropsychology. 1998;20:339–352. doi: 10.1076/jcen.20.3.339.822. [DOI] [PubMed] [Google Scholar]
- 15.Bennett M. The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92:147–157. doi: 10.1016/s0304-3959(00)00482-6. [DOI] [PubMed] [Google Scholar]
- 16.Berman JS, Symonds C, Birch R. Efficacy of two cannabis based medicinal extracts for relief of central neuropathic pain from brachial plexus avulsion: results of a randomised controlled trial. Pain. 2004;112:299–306. doi: 10.1016/j.pain.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- 18.Campbell FA, Tramer MR, Carroll D, Reynolds DJ, Moore RA, McQuay HJ. Are cannabinoids an effective and safe treatment option in the management of pain? A qualitative systematic review. BMJ (Clinical research ed. 2001;323:13–16. doi: 10.1136/bmj.323.7303.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardenas DD, Jensen MP. Treatments for chronic pain in persons with spinal cord injury: A survey study. J Spinal Cord Med. 2006;29:109–117. doi: 10.1080/10790268.2006.11753864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, Heaton RK. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of clinical and experimental neuropsychology. 2004;26:307–319. doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 21.Carter GT, Weydt P, Kyashna-Tocha M, Abrams DI. Medicinal cannabis: rational guidelines for dosing. IDrugs. 2004;7:464–470. [PubMed] [Google Scholar]
- 22.Chabrol H, Choquet M. Relationship between depressive symptoms, hopelessness and suicidal ideation among 1547 high school students. L’Encephale. 2009;35:443–447. doi: 10.1016/j.encep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Chait LD, Corwin RL, Johanson CE. A cumulative dosing procedure for administering marijuana smoke to humans. Pharmacology, biochemistry, and behavior. 1988;29:553–557. doi: 10.1016/0091-3057(88)90019-6. [DOI] [PubMed] [Google Scholar]
- 24.Cochran WG. Some methods for strengthening the common chi-squared tests. Biometrics. 1954:417–451. [Google Scholar]
- 25.Craven BC, Morris AR. Modified Ashworth scale reliability for measurement of lower extremity spasticity among patients with SCI. Spinal cord. 2010;48:207–213. doi: 10.1038/sc.2009.107. [DOI] [PubMed] [Google Scholar]
- 26.Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. Journal of addiction medicine. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Vries M, van Rijckevorsel DC, Wilder-Smith OH, van Goor H. Dronabinol and chronic pain: importance of mechanistic considerations. Expert Opin Pharmacother. 2014;15:1525–1534. doi: 10.1517/14656566.2014.918102. [DOI] [PubMed] [Google Scholar]
- 28.Diehr MC, Cherner M, Wolfson TJ, Miller SW, Grant I, Heaton RK. The 50 and 100-item short forms of the Paced Auditory Serial Addition Task (PASAT): demographically corrected norms and comparisons with the full PASAT in normal and clinical samples. Journal of clinical and experimental neuropsychology. 2003;25:571–585. doi: 10.1076/jcen.25.4.571.13876. [DOI] [PubMed] [Google Scholar]
- 29.Diehr MC, Heaton RK, Miller W, Grant I. The Paced Auditory Serial Addition Task (PASAT): norms for age, education, and ethnicity. Assessment. 1998;5:375–387. doi: 10.1177/107319119800500407. [DOI] [PubMed] [Google Scholar]
- 30.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Dworkin RH, Turk DC, Peirce-Sandner S, Baron R, Bellamy N, Burke LB, Chappell A, Chartier K, Cleeland CS, Costello A, Cowan P, Dimitrova R, Ellenberg S, Farrar JT, French JA, Gilron I, Hertz S, Jadad AR, Jay GW, Kalliomaki J, Katz NP, Kerns RD, Manning DC, McDermott MP, McGrath PJ, Narayana A, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Reeve BB, Rhodes T, Sampaio C, Simpson DM, Stauffer JW, Stucki G, Tobias J, White RE, Witter J. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain. 2010;149:177–193. doi: 10.1016/j.pain.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 32.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthwaite JA, Jensen MP, Kerns RD, Ader DN, Brandenburg N, Burke LB, Cella D, Chandler J, Cowan P, Dimitrova R, Dionne R, Hertz S, Jadad AR, Katz NP, Kehlet H, Kramer LD, Manning DC, McCormick C, McDermott MP, McQuay HJ, Patel S, Porter L, Quessy S, Rappaport BA, Rauschkolb C, Revicki DA, Rothman M, Schmader KE, Stacey BR, Stauffer JW, von Stein T, White RE, Witter J, Zavisic S. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 33.Ellis RJ, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, Bentley H, Atkinson JH. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672–680. doi: 10.1038/npp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrar JT, Troxel AB, Stott C, Duncombe P, Jensen MP. Validity, reliability, and clinical importance of change in a 0–10 numeric rating scale measure of spasticity: a post hoc analysis of a randomized, double-blind, placebo-controlled trial. Clin Ther. 2008;30:974–985. doi: 10.1016/j.clinthera.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 35.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 36.Feinstein A, Magalhaes S, Richard JF, Audet B, Moore C. The link between multiple sclerosis and depression. Nature reviews. 2014;10:507–517. doi: 10.1038/nrneurol.2014.139. [DOI] [PubMed] [Google Scholar]
- 37.Finnerup NB. Pain in patients with spinal cord injury. Pain. 2013;154(Suppl 1):S71–76. doi: 10.1016/j.pain.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Finnerup NB, Jensen TS. Clinical use of pregabalin in the management of central neuropathic pain. Neuropsychiatric disease and treatment. 2007;3:885–891. doi: 10.2147/ndt.s1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleuren JF, Voerman GE, Erren-Wolters CV, Snoek GJ, Rietman JS, Hermens HJ, Nene AV. Stop using the Ashworth Scale for the assessment of spasticity. Journal of neurology, neurosurgery, and psychiatry. 2010;81:46–52. doi: 10.1136/jnnp.2009.177071. [DOI] [PubMed] [Google Scholar]
- 40.Frank B, Serpell MG, Hughes J, Matthews JN, Kapur D. Comparison of analgesic effects and patient tolerability of nabilone and dihydrocodeine for chronic neuropathic pain: randomised, crossover, double blind study. BMJ (Clinical research ed. 2008;336:199–201. doi: 10.1136/bmj.39429.619653.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Galer BS, Jensen MP. Development and preliminary validation of a pain measure specific to neuropathic pain: the Neuropathic Pain Scale. Neurology. 1997;48:332–338. doi: 10.1212/wnl.48.2.332. [DOI] [PubMed] [Google Scholar]
- 42.Garrison CZ, Addy CL, Jackson KL, McKeown RE, Waller JL. A longitudinal study of suicidal ideation in young adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1991;30:597–603. doi: 10.1097/00004583-199107000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Garrison CZ, Jackson KL, Addy CL, McKeown RE, Waller JL. Suicidal behaviors in young adolescents. American journal of epidemiology. 1991;133:1005–1014. doi: 10.1093/oxfordjournals.aje.a115809. [DOI] [PubMed] [Google Scholar]
- 44.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. The New England journal of medicine. 2005;352:1324–1334. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 45.Glass GV, McGraw B, Smith ML. Meta-analysis in social research. Sage; Beverly Hills, CA: 1981. p. 120. [Google Scholar]
- 46.Greenwald MK, Stitzer ML. Antinociceptive, subjective and behavioral effects of smoked marijuana in humans. Drug and alcohol dependence. 2000;59:261–275. doi: 10.1016/s0376-8716(99)00128-3. [DOI] [PubMed] [Google Scholar]
- 47.Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44:367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- 48.Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clinical pharmacokinetics. 2003;42:327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- 49.Grotenhermen F, Muller-Vahl K. The therapeutic potential of cannabis and cannabinoids. Deutsches Arzteblatt international. 2012;109:495–501. doi: 10.3238/arztebl.2012.0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gut-Fayand A, Dervaux A, Olie JP, Loo H, Poirier MF, Krebs MO. Substance abuse and suicidality in schizophrenia: a common risk factor linked to impulsivity. Psychiatry research. 2001;102:65–72. doi: 10.1016/s0165-1781(01)00250-5. [DOI] [PubMed] [Google Scholar]
- 51.Hall W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction. 2015;110:19–35. doi: 10.1111/add.12703. [DOI] [PubMed] [Google Scholar]
- 52.Hansson P, Backonja M, Bouhassira D. Usefulness and limitations of quantitative sensory testing: clinical and research application in neuropathic pain states. Pain. 2007;129:256–259. doi: 10.1016/j.pain.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 53.Hart CL, Ilan AB, Gevins A, Gunderson EW, Role K, Colley J, Foltin RW. Neurophysiological and cognitive effects of smoked marijuana in frequent users. Pharmacology, biochemistry, and behavior. 2010;96:333–341. doi: 10.1016/j.pbb.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heaton R, Grant I, Matthews C. Comprehensive Norms for an expanded Halstead-Reitan Battery: demographic corrections, research findings, and clinical applications. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- 55.Heaton RK, Miller SW, Taylor MJ, Grant I. Revised Comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources, Inc; Lutz, FL: 2004. [Google Scholar]
- 56.Heishman SJ, Arasteh K, Stitzer ML. Comparative effects of alcohol and marijuana on mood, memory, and performance. Pharmacology, biochemistry, and behavior. 1997;58:93–101. doi: 10.1016/s0091-3057(96)00456-x. [DOI] [PubMed] [Google Scholar]
- 57.Heishman SJ, Stitzer ML, Bigelow GE. Alcohol and marijuana: comparative dose effect profiles in humans. Pharmacology, biochemistry, and behavior. 1988;31:649–655. doi: 10.1016/0091-3057(88)90244-4. [DOI] [PubMed] [Google Scholar]
- 58.Herrmann ES, Cone EJ, Mitchell JM, Bigelow GE, LoDico C, Flegel R, Vandrey R. Non-smoker exposure to secondhand cannabis smoke II: Effect of room ventilation on the physiological, subjective, and behavioral/cognitive effects. Drug and alcohol dependence. 151:194–202. doi: 10.1016/j.drugalcdep.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hill-Briggs F, Dial JG, Morere DA, Joyce A. Neuropsychological assessment of persons with physical disability, visual impairment or blindness, and hearing impairment or deafness. Arch Clin Neuropsychol. 2007;22:389–404. doi: 10.1016/j.acn.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 60.Hohmann AG, Suplita RL., 2nd Endocannabinoid mechanisms of pain modulation. The AAPS journal. 2006;8:E693–708. doi: 10.1208/aapsj080479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Honarmand K, Tierney MC, O’Connor P, Feinstein A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology. 2011;76:1153–1160. doi: 10.1212/WNL.0b013e318212ab0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ilan AB, Gevins A, Coleman M, ElSohly MA, de Wit H. Neurophysiological and subjective profile of marijuana with varying concentrations of cannabinoids. Behavioural pharmacology. 2005;16:487–496. doi: 10.1097/00008877-200509000-00023. [DOI] [PubMed] [Google Scholar]
- 63.Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Current medical research and opinion. 2007;23:17–24. doi: 10.1185/030079906x158066. [DOI] [PubMed] [Google Scholar]
- 64.Issa MA, Narang S, Jamison RN, Michna E, Edwards RR, Penetar DM, Wasan AD. The subjective psychoactive effects of oral dronabinol studied in a randomized, controlled crossover clinical trial for pain. The Clinical journal of pain. 30:472–478. doi: 10.1097/AJP.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jensen MP. Review of measures of neuropathic pain. Current pain and headache reports. 2006;10:159–166. doi: 10.1007/s11916-006-0041-z. [DOI] [PubMed] [Google Scholar]
- 66.Jones RT. Cardiovascular system effects of marijuana. Journal of clinical pharmacology. 2002;42:58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 67.Kelly TH, Foltin RW, Fischman MW. Effects of smoked marijuana on heart rate, drug ratings and task performance by humans. Behavioural pharmacology. 1993;4:167–178. [PubMed] [Google Scholar]
- 68.Khan A, Kolts RL, Thase ME, Krishnan KR, Brown W. Research design features and patient characteristics associated with the outcome of antidepressant clinical trials. The American journal of psychiatry. 2004;161:2045–2049. doi: 10.1176/appi.ajp.161.11.2045. [DOI] [PubMed] [Google Scholar]
- 69.Khan A, Schwartz K. Study designs and outcomes in antidepressant clinical trials. Essential psychopharmacology. 2005;6:221–226. [PubMed] [Google Scholar]
- 70.Klove H. Clinical neuropsychology. In: Forster FE, editor. Medical Clinics of North America. New York, NY: Saunders; 1963. [PubMed] [Google Scholar]
- 71.Krassioukov A. Which pathways must be spared in the injured human spinal cord to retain cardiovascular control? Progress in brain research. 2006;152:39–47. doi: 10.1016/S0079-6123(05)52003-X. [DOI] [PubMed] [Google Scholar]
- 72.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lev-Ran S, Le Foll B, McKenzie K, George TP, Rehm J. Bipolar disorder and co-occurring cannabis use disorders: characteristics, co-morbidities and clinical correlates. Psychiatry research. 2013;209:459–465. doi: 10.1016/j.psychres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 74.Lev-Ran S, Roerecke M, Le Foll B, George TP, McKenzie K, Rehm J. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychological medicine. 2014;44:797–810. doi: 10.1017/S0033291713001438. [DOI] [PubMed] [Google Scholar]
- 75.Leweke FM, Koethe D. Cannabis and psychiatric disorders: it is not only addiction. Addiction biology. 2008;13:264–275. doi: 10.1111/j.1369-1600.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- 76.Mechoulam R, Parker L. Towards a better cannabis drug. British journal of pharmacology. 2013;170:1363–1364. doi: 10.1111/bph.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805–2809. doi: 10.1161/01.cir.103.23.2805. [DOI] [PubMed] [Google Scholar]
- 78.Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. The Cochrane database of systematic reviews. 2009:CD007076. doi: 10.1002/14651858.CD007076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mutlu A, Livanelioglu A, Gunel MK. Reliability of Ashworth and Modified Ashworth scales in children with spastic cerebral palsy. BMC musculoskeletal disorders. 2008;9:44. doi: 10.1186/1471-2474-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS, Jamison RN. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain. 2008;9:254–264. doi: 10.1016/j.jpain.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 81.Niesink RJ, van Laar MW. Does Cannabidiol Protect Against Adverse Psychological Effects of THC? Frontiers in psychiatry. 2013;4:130. doi: 10.3389/fpsyt.2013.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Connor A, Dworkin R. Understanding and Interpreting Neuropathic Pain Clinical Trials. In: Simpson D, McArthur J, Dworkin R, editors. Neuropathic Pain: Mechanisms, Diagnosis and Treatment. Oxford University Press; New York, NY: 2012. p. 136. [Google Scholar]
- 83.O’Connor AB, Dworkin RH. Treatment of neuropathic pain: an overview of recent guidelines. The American journal of medicine. 2009;122:S22–32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 84.Osterberg A, Boivie J. Central pain in multiple sclerosis - sensory abnormalities. European journal of pain (London, England) 2010;14:104–110. doi: 10.1016/j.ejpain.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Owen KP, Sutter ME, Albertson TE. Marijuana: respiratory tract effects. Clinical reviews in allergy & immunology. 2014;46:65–81. doi: 10.1007/s12016-013-8374-y. [DOI] [PubMed] [Google Scholar]
- 86.Pavisian B, MacIntosh BJ, Szilagyi G, Staines RW, O’Connor P, Feinstein A. Effects of cannabis on cognition in patients with MS: a psychometric and MRI study. Neurology. 2014;82:1879–1887. doi: 10.1212/WNL.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pedersen W. Does cannabis use lead to depression and suicidal behaviours? A population-based longitudinal study. Acta psychiatrica Scandinavica. 2008;118:395–403. doi: 10.1111/j.1600-0447.2008.01259.x. [DOI] [PubMed] [Google Scholar]
- 88.Robins LN, Helzer JE, Croughan J, Ratcliff KS National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Archives of general psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 89.Rowbotham MC. Mechanisms of neuropathic pain and their implications for the design of clinical trials. Neurology. 2005;65:S66–73. doi: 10.1212/wnl.65.12_suppl_4.s66. [DOI] [PubMed] [Google Scholar]
- 90.Rowbotham MC, Twilling L, Davies PS, Reisner L, Taylor K, Mohr D. Oral opioid therapy for chronic peripheral and central neuropathic pain. The New England journal of medicine. 2003;348:1223–1232. doi: 10.1056/NEJMoa021420. [DOI] [PubMed] [Google Scholar]
- 91.Sagar DR, Jhaveri M, Chapman V. Targeting the cannabinoid system to produce analgesia. Curr Top Behav Neurosci. 2009;1:275–287. doi: 10.1007/978-3-540-88955-7_11. [DOI] [PubMed] [Google Scholar]
- 92.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nature neuroscience. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 93.Schwid SR, Covington M, Segal BM, Goodman AD. Fatigue in multiple sclerosis: current understanding and future directions. Journal of rehabilitation research and development. 2002;39:211–224. [PubMed] [Google Scholar]
- 94.Selvarajah D, Gandhi R, Emery CJ, Tesfaye S. Randomized placebo-controlled double-blind clinical trial of cannabis-based medicinal product (Sativex) in painful diabetic neuropathy: depression is a major confounding factor. Diabetes care. 2010;33:128–130. doi: 10.2337/dc09-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singh H, Gormley K. NIDA Drug Supply Program. National Institute of Drug Abuse; Bethesda, MD: 2015. http://www.drugabuse.gov/researchers/research-resources/nida-drug-supply-program and http://www.drugabuse.gov/researchers/research-resources/nida-drug-supply-program-dsp/marijuana-plant-material-available-nida-drug-supply-program. [Google Scholar]
- 96.Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J Pain. 2008;9:164–173. doi: 10.1016/j.jpain.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 97.Stella N. Cannabinoid signaling in glial cells. Glia. 2004;48:267–277. doi: 10.1002/glia.20084. [DOI] [PubMed] [Google Scholar]
- 98.Suter MR, Wen YR, Decosterd I, Ji RR. Do glial cells control pain? Neuron glia biology. 2007;3:255–268. doi: 10.1017/S1740925X08000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ (Clinical research ed. 2004;329:253. doi: 10.1136/bmj.38149.566979.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tetrault JM, Crothers K, Moore BA, Mehra R, Concato J, Fiellin DA. Effects of marijuana smoking on pulmonary function and respiratory complications: a systematic review. Archives of internal medicine. 2007;167:221–228. doi: 10.1001/archinte.167.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tombaugh TN. A comprehensive review of the Paced Auditory Serial Addition Test (PASAT) Arch Clin Neuropsychol. 2006;21:53–76. doi: 10.1016/j.acn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 102.Volkow N. Researching Marijuana for Therapeutic Purposes: The Potential Promise of Cannabidiol (CBD) https://www.drugabuse.gov/about-nida/noras-blog/2015/07/researching-marijuana-therapeutic-purposes-potential-promise-cannabidiol-cbd.
- 103.Wade DT, Makela PM, House H, Bateman C, Robson P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 2006;12:639–645. doi: 10.1177/1352458505070618. [DOI] [PubMed] [Google Scholar]
- 104.Wallace M, Schulteis G, Atkinson JH, Wolfson T, Lazzaretto D, Bentley H, Gouaux B, Abramson I. Dose-dependent effects of smoked cannabis on capsaicin-induced pain and hyperalgesia in healthy volunteers. Anesthesiology. 2007;107:785–796. doi: 10.1097/01.anes.0000286986.92475.b7. [DOI] [PubMed] [Google Scholar]
- 105.Wallace MS, Marcotte TD, Umlauf A, Gouaux B, Atkinson JH. Efficacy of Inhaled Cannabis on Painful Diabetic Neuropathy. J Pain. 2015 doi: 10.1016/j.jpain.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ware MA, Wang T, Shapiro S, Collet JP. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS) J Pain. 2015;16:1233–1242. doi: 10.1016/j.jpain.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 107.Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, Gamsa A, Bennett GJ, Collet JP. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. Cmaj. 2010;182:E694–701. doi: 10.1503/cmaj.091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wechsler D. Wechsler Adult Intelligence Scale. 3. Administration and Scoring Manual; San Antonio, TX: The Psychological Corporation, Harcourt Brace & Co; 1997. [Google Scholar]
- 109.Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, Kleijnen J. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. Jama. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 110.Wieseler-Frank J, Maier SF, Watkins LR. Glial activation and pathological pain. Neurochemistry international. 2004;45:389–395. doi: 10.1016/j.neuint.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 111.Wilkinson ST, Radhakrishnan R, D’Souza DC. Impact of Cannabis Use on the Development of Psychotic Disorders. Current addiction reports. 2014;1:115–128. doi: 10.1007/s40429-014-0018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain. 2013;14:136–148. doi: 10.1016/j.jpain.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, Fishman S. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain. 2008;9:506–521. doi: 10.1016/j.jpain.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zajicek JP, Hobart JC, Slade A, Barnes D, Mattison PG. Multiple sclerosis and extract of cannabis: results of the MUSEC trial. Journal of neurology, neurosurgery, and psychiatry. 2012;83:1125–1132. doi: 10.1136/jnnp-2012-302468. [DOI] [PubMed] [Google Scholar]
- 115.Zogopoulos P, Vasileiou I, Patsouris E, Theocharis SE. The role of endocannabinoids in pain modulation. Fundam Clin Pharmacol. 2013;27:64–80. doi: 10.1111/fcp.12008. [DOI] [PubMed] [Google Scholar]
- 116.Zuardi AW, Shirakawa I, Finkelfarb E, Karniol IG. Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology. 1982;76:245–250. doi: 10.1007/BF00432554. [DOI] [PubMed] [Google Scholar]