Abstract
Purpose
The occupational risk to farmworkers, particularly chronic exposure to pesticides, is an acknowledged environmental and work-related health problem. Epigenetics has recently been shown to contribute to a number of complex diseases and traits, including measures of cognitive function and preclinical neurodegenerative disease. We sought to determine if changes in DNA methylation existed between farmworker and non-farmworker populations, and to identify the genes most likely involved in those changes.
Methods
Eighty-three farmworkers and 60 non-farmworkers were selected from PACE4, a community-based, participatory research project comparing occupational exposures between immigrant Latino farmworker and non-farmworker manual workers. Measurements of DNA methylation were performed with the Infinium HumanMethylation450 BeadChip, at the beginning and end of the 2012 growing season. Bonferroni adjustment was used to identify significant findings (p=1.03 × 10−7, based on 485,000 tested methylation sites), although less stringent criteria (i.e., p≤1 × 10−6) were used to identify sites of interest. Expression quantitative trait locus (eQTL) databases were used to help identify the most likely functional genes for each associated methylation site.
Results
Methylation at 36 CpG sites, located in or near 72 genes, differed between the two groups (p≤1 × 10−6). The difference between the two groups was generally due to an increase in methylation in the farmworkers, and a slight decrease in methylation in the non-farmworkers. Enrichment was observed in several biological pathways, including those involved in the immune response, as well as Growth Hormone Signaling, Role of BRCA1 in DNA Damage Response, p70S6K signaling, and PI3K Signaling in B Lymphocytes.
Conclusions
We identified considerable changes in DNA methylation at 36 CpG sites over the growing season that differed between farmworkers and non-farmworkers. Dominant pathways included immune-related (HLA) processes, as well as a number of diverse biological systems. Further studies are necessary to determine which exposures or behaviors are responsible for the observed changes, and whether these changes eventually lead to disease related phenotypes in this population.
Keywords: epigenetics, DNA methylation, farmworkers, pesticides
Introduction
Farmworker exposure to pesticides is a widely acknowledged environmental and occupational health problem (Arcury et al., 2006; Arcury et al., 2014; Arcury and Quandt, 2003; Calvert et al., 2008; Fenske et al., 2005; McCauley et al., 2006; Quandt et al., 2006; Reeves and Schafer, 2003). Organophosphorus insecticides are among the most widely used pesticides, and include acephate, chlorpyrifos, dimethoate, disulfoton, malathion, and phosmet. Farmworkers that experience chronic exposure to pesticides or their residues (active ingredients that persist on surfaces after the chemical has evaporated) may experience serious health problems, such as cancer and neurologic dysfunction (Alavanja et al., 2004; Hoppin et al., 2002; Kamel and Hoppin, 2004; McCauley et al., 2006; Mills and Yang, 2003). Epidemiological studies on the potential effects of organophosphate pesticide exposure have provided conflicting results, especially with chronic, low levels of exposures where immediate effects are absent (reviewed in (Ray, 1998; Ray and Richards, 2001).
Recent research suggests that epigenetics plays a role in a number of complex diseases, including depression, neurodegenerative diseases, and learning and memory formation (Barrett and Wood, 2008). The relevant mechanisms include histone modifications and DNA methylation that have been found to control hippocampal synaptic plasticity and memory processes (Barrett and Wood, 2008). Chronic abnormalities in histone acetylation may be related to aberrant expression of genes associated with learning and memory, synaptic plasticity, and synaptogenesis in mice. Such dysregulation may contribute to deregulated gene expression and learning impairment (Guan et al., 2009; Peleg et al., 2010). A number of recent studies have identified epigenetic changes in DNA from whole blood with phenotypes related to mental health (e.g., depression, schizophrenia, posttraumatic stress disorder) and cognitive function (Guidotti et al., 2014; Na et al., 2014; Thaler et al., 2014; Walton et al., 2014; Wang et al., 2014; Yehuda et al., 2014). It has also been suggested that both early and later life environmental exposures may have an epigenetic effect (Chin-Chan et al., 2015).
Studies demonstrating the direct effects of pesticides on epigenetic variation have involved in vitro, animal, and human studies, and these studies have shown that pesticides can alter DNA methylation patterns. In K562 cell lines, for example, exposure to fonofos, parathion, and terbufos, altered the methylation status of 712 genes (Zhang et al., 2012). In a study examining the effects of dichlorodiphenyltrichloroethane (DDT) on rats, six CpG islands from the hypothalamus were determined to be altered in exposed compared to control rats (Shutoh et al., 2009). In humans, organochlorines have been shown to result in global DNA hypomethylation (Kim et al., 2010). Together, these data support the hypotheses that changes in DNA methylation in blood can be associated with a number of complex disease and traits, including measures of cognitive function and preclinical neurodegenerative disease, and that these changes may be a result of persistent pesticide exposure.
Methods
Study population
The study population involved participants from the Preventing Agricultural Chemical Exposure (PACE4) study, a community-based participatory research project comparing occupational exposures, particularly pesticide exposure, among immigrant Latino farmworkers and immigrant Latino non-farmworker manual workers (Arcury et al., 2014). Briefly, participants were men aged 30 to 70 years, recruited from three agricultural counties in east central NC (Harnett, Johnston, Sampson), and an urban county in Piedmont NC (Forsyth). Farmworkers were currently employed in agriculture and worked in agriculture for at least three years. Non-farmworkers could not be employed for the past three years in jobs that exposed them to pesticides, including farm work, forestry, landscaping, grounds keeping, lawn maintenance, and pest control. Potential farmworker and non-farmworker participants were excluded if they reported being told by a healthcare provider that they had diabetes. Only limited data was available for other chronic diseases or medication use. Blood samples for DNA methylation (described below) were collected in 2012, generally at the beginning (clinic 1; June for farmworkers, July for non-farmworkers) and end of the growing season (clinic 2; September for farmworkers, October for non-farmworkers), with a few exceptions (three farmworkers who did their first clinic visit in July, nine non-farmworkers did their first clinic visit in August, and three non-farmworkers did their first clinic visit in September). Blood samples were stored at 4°C at the clinic site, and then transported to Wake Forest School of Medicine for DNA isolation. Sixty non-farmworkers and 83 farmworkers were selected for the DNA methylation study. All participants provided written consent, and this study was approved by the Wake Forest School of Medicine Institutional Review Board.
DNA methylation
DNA was isolated from whole blood using the AutoPure LS system (Qiagen, Inc.), and then bisulfite-converted using the EZ DNA Methylation Gold kit (Zymo, Irvine, CA). To quantify DNA methylation at each site, we used beta values, as determined with the HumanMethylation450 BeadChip (Illumina, Inc.), which targets over 485,000 CpG sites at single-nucleotide resolution, and the iScan Reader (Illumina, Inc.). The ChAMP program (Morris et al., 2014) in BioConductor was used to initially process the data, using the default import settings. These included removal of methylation probes with a detection p-value less than 0.01, a beadcount less than three, and on the X or Y chromosome. To adjust for the two distinct Infinium assays that are simultaneously measured on this microarray (Infinium I and Infinium II), we used the champ.norm command with BMIQ normalization (Teschendorff et al., 2013). The BMIQ-normalized data was used for all statistical analysis. Methylation sites with nearby SNPs (as defined by Illumina, dbSNP137, version 2) with a minor allele frequency greater than 0.05 in any population were not included in our results.
Statistical Analysis
The sample means and standard deviations were computed for the continuous demographic characteristics; counts and proportions were calculated for the discrete demographic characteristics by farmworker status. Linear regression models were used to study the association between the farmworkers and non-farmworkers, and CpG site methylation. The covariate of interest was the farmworker status. Participant age and position of the sample on each microarray were adjusted. Due to the small sample size, no adjustment was made for the chip itself. Four models were fitted. The first two models examined the cross-sectional analysis at each time point. The last two models examined the association between change of methylation site and farmworker status. First, the dependent variable was the CpG site methylation at clinic 1. This model could identify CpG site methylation differences between the farmworkers and non-farmworkers at clinic 1. Second, the dependent variable was the CpG site methylation at clinic 2. Third, the dependent variable was the change of CpG site methylation between clinic 2 and clinic 1. Fourth, the dependent variable was the change of CpG site methylation between clinic 2 and clinic 1. CpG site methylation at clinic 1 was additionally adjusted in the model. All analyses were performed using the R software package. Bonferroni adjustment was used to identify significant findings (p=1.03 × 10−7, based on 485,000 tested methylation sites), although less stringent criteria (i.e., p≤1 × 10−6) were used to identify sites of interest. This level of significance was assigned due to the conservative nature of the Bonferroni adjustment (given the likelihood that some methylation sites will be correlated and not completely independent) to provide a set of genes for follow up and pathway analysis.
Identification of expression quantitative trait loci (eQTLs) near associated CpG sites
There is now considerable eQTL data (which correlates gene expression with SNP alleles) publically available. We sought to use this data to determine if methylation sites of interest were in regions of the genome that might have regulatory effects. If eQTL SNPs were within 2kb of the methylation site (but not so close as to cause a problem with the assay), this indicated that the genomic region was regulatory, increasing the likelihood that the methylation difference was functional. Single nucleotide polymorphisms (SNPs) surrounding each associated CpG site were identified by searching 1kb upstream and 1kb downstream from each site. Each SNP was then searched to determine if it was an eQTL in any tissue, using two resources – the eQTL Browser from the University of Chicago (eqtl.uchicago.edu) and the Blood eQTL Browser (Westra et al., 2013). The eqtl.uchicago.edu database searches 17 data sets from six tissue types; the Blood eQTL Browser focuses on whole blood specific eQTLs.
Results
Some differences in the characteristics between the farmworker and non-farmworker populations were observed (Table 1). Marital status, education, and country of birth were all significantly different, most likely due to the ascertainment scheme instead of an artifact of the selected subset of participants from the parent study.
Table 1.
Participant Characteristics
| Participant Characteristics | Farmworkers n=83 | Non-farmworkers n=60 | p-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age | 0.31 | ||||
| 30–34 years | 35 | 42.2 | 18 | 30.0 | |
| 35–44 years | 26 | 31.3 | 21 | 35.0 | |
| 45+ years | 22 | 26.5 | 21 | 35.0 | |
| Marital Status | <0.0001 | ||||
| Never married/widowed/separated/divorced | 3 | 3.6 | 17 | 28.3 | |
| Married/living as married | 80 | 96.4 | 43 | 71.7 | |
| Education | 0.0065 | ||||
| 0–6 grade | 31 | 37.3 | 20 | 33.3 | |
| 7–11 grade | 42 | 50.6 | 20 | 33.3 | |
| 12 grade or more | 10 | 12.1 | 20 | 33.3 | |
| Country of birth | <0.0001 | ||||
| Mexico | 83 | 100 | 44 | 73.3 | |
| Other | 16 | 26.7 | |||
| H2A or H2B visa | NA | ||||
| Yes | 81 | 97.6 | |||
| No | 2 | 2.4 | |||
| Dominant language | 0.42 | ||||
| Spanish | 83 | 100 | 59 | 98.3 | |
| Other | 1 | 1.7 | |||
| Pack Years at Baseline* | 0.15 | ||||
| 0 years | 42 | 52.5 | 39 | 65.0 | |
| Less than 1 year | 25 | 31.25 | 12 | 20.0 | |
| 1 to 4 years | 9 | 11.25 | 3 | 5.0 | |
| 9 to 25 years | 4 | 5.00 | 6 | 10.0 | |
3 missing observations
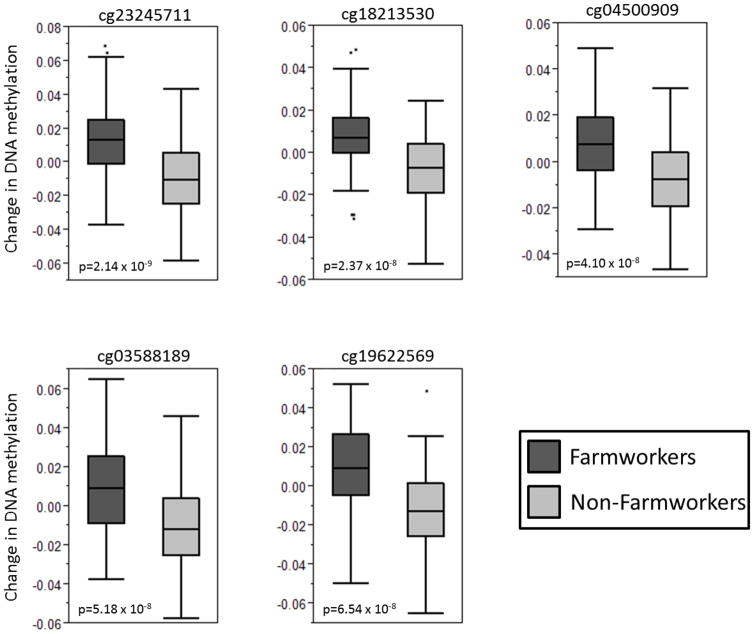
We compared the change in DNA methylation from whole blood, from the first and last time points collected (clinic 1 and clinic 2), between the farmworker and non-farmworker groups, adjusting for participant age. Thirty-six CpG sites, located in distinct genomic regions (with the exception of cg02001956 and cg04272615, which are 48kb apart on chromosome 6), had changes that were different between the two groups (p≤1 × 10−6; Table 2). Of these, 33 indicated an increase in methylation in farmworkers compared to non-farmworkers, suggestive of a general decrease is gene expression of the regulated genes. Five methylation sites met the more conservative Bonferroni-adjusted level of significance of p=1.03 × 10−7, based on 485,000 tested methylation sites (Figure 1; Supplementary Figure). The difference between the farmworkers and non-farmworker groups was generally due to an overall increase in methylation in the farmworkers over time, and a slight decrease in methylation in the non-farmworkers. The overall methylation level for each of these sites was relatively high (greater than 0.7), and the absolute change over time, within each group, was small (Supplementary Figure).
Table 2.
DNA methylation results for CpG sites with p≤1 × 10−6
| Methylation Site | Position (hg19) | Genes within 20kb | eQTL genes | P-value |
|---|---|---|---|---|
| cg23245711 | chr3:48413679 | FBXW12 | TREX1, ATRIP | 2.14E-09 |
| cg18213530 | chr8:101539164 | ANKRD46 | 2.37E-08 | |
| cg04500909 | chr5:159203122 | 4.10E-08 | ||
| cg03588189 | chr17:61988802 | CSHL1,GH1 | CD79B, DDX42, FTSJ3, SMARCD2 | 5.18E-08 |
| cg19622569 | chr4:1368561 | UVSSA | 6.54E-08 | |
| cg15423315 | chr10:93296555 | LOC100188947 | 1.45E-07 | |
| cg13452923 | chr3:196243269 | C3orf43 | 1.64E-07 | |
| cg10468951 | chr3:50315816 | SEMA3B,LSMEM2,IFRD2 | HYAL3,NAT6, HEMK, RBM6 | 1.69E-07 |
| cg03599575 | chr15:90893183 | GABARAPL3,ZNF774 | IQGAP1, CIB1 | 1.72E-07 |
| cg02001956 | chr6:32017261 | CYP21A2,TNXB | 1.91E-07 | |
| cg23201817 | chr19:40007923 | DLL3,SELV | 2.06E-07 | |
| cg04934652 | chr17:80608073 | WDR45B,RAB40B | RAB40B, FOXK2 | 2.14E-07 |
| cg13056222 | chr1:204219200 | PLEKHA6 | 2.27E-07 | |
| cg13595308 | chr9:128819106 | 2.35E-07 | ||
| cg20993403 | chr20:34700330 | EPB41L1 | SCAND1 | 2.80E-07 |
| cg04090349 | chr6:168426083 | KIF25 | 2.86E-07 | |
| cg04819337 | chr21:44982368 | HSF2BP,DQ577420 | HSF2BP | 2.97E-07 |
| cg06579354 | chr19:41618922 | CYP2F1 | 4.17E-07 | |
| cg26805528 | chr9:131872543 | CRAT,PPP2R4 | CRAT, PPP2R4 | 4.37E-07 |
| cg21295367 | chr17:80145563 | CCDC57 | 4.42E-07 | |
| cg08503994 | chr16:4585288 | CDIP1 | CDIP1, DNAJA3, NMRAL1 | 4.44E-07 |
| cg04272615 | chr6:32065703 | TNXB,ATF6B | SKIV2L, HLA-DRB5 | 4.95E-07 |
| cg21634628 | chr3:15687272 | BTD | ANKRD28 | 4.97E-07 |
| cg01049849 | chr1:10807676 | CASZ1 | 5.16E-07 | |
| cg24737570 | chr2:11727174 | GREB1 | 5.19E-07 | |
| cg17033684 | chr17:6588357 | SLC13A5 | 5.72E-07 | |
| cg16524733 | chr11:117070046 | SIDT2,TAGLN,PCSK7 | SIDT2,TAGLN,PCSK7 | 5.87E-07 |
| cg03440636 | chr15:66310729 | MEGF11 | 5.98E-07 | |
| cg06893362 | chr20:47897124 | ZFAS1,SNORD12C,SNORD12B,SNORD12 | 6.47E-07 | |
| cg21643547 | chr1:205240463 | TMCC2 | NUAK2,RIPK5 | 6.61E-07 |
| cg26354908 | chr12:49610040 | 6.89E-07 | ||
| cg23357805 | chr12:95135079 | 7.20E-07 | ||
| cg04166913 | chr6:32945336 | BRD2 | HLA-DPB1, HLA-DMB, PSMB9, HLA-DRB1, HLA-DRB5 | 8.81E-07 |
| cg11863863 | chr17:75706411 | 8.87E-07 | ||
| cg21028142 | chr17:79581711 | NPLOC4 | 8.93E-07 | |
| cg27061889 | chr17:37034885 | TRNA_Cys,LASP1 | CCDC49 | 9.82E-07 |
Figure 1.
Differences in DNA methylation for the five CpG sites that met Bonferroni-adjusted significance. The mean differences between the farmworkers and non-farmworkers are shown, with outliers shown as individual points.
Smoking in this population is modest (Table 1). However, to adjust for the potential effect of smoking on the difference in methylation, we ran a separate analysis including smoking (pack years) in the model. The DNA methylation results for the farmworker group effect with and without adjusting for smoking are consistent. The adjustment of smoking had minimal effect, with less than 5% change of the regression coefficient for the farmworker group.
To better identify the most likely functional genes located near the associated CpG sites, we determined if eQTLs were present nearby, based on SNPs within the same region. SNPs within 1 kilobase (kb) upstream and downstream of each CpG site were tested, and 34 unique genes were identified using an eQTL score (−log10(P-value)) threshold of 5 (Table 2). While all of the CpG sites did not have an associated eQTL nearby, this method identified more likely functional candidate genes when present, since several genes were identified relatively distant to the CpG. In some cases, eQTLs were identical to the nearby genes (e.g., CRAT, PPP2R4), whereas in others, the genes were completely distinct. In the latter case, we considered the eQTL gene to be the primary functional gene in the region. The 72 most likely functional genes (positional or eQTL genes, when available) were identified and carried forward for pathway analysis.
Pathway analysis was performed to identify biological pathways that were enriched with the identified functional genes. Sixty-six of the genes were available for pathway analysis, using Ingenuity Pathway Analysis (IPA; Qiagen, Inc.). The significantly enriched canonical pathways identified were antigen presentation (p=4.40 × 10−8), B cell development (p=1.69 × 10−6), allograft rejection signaling (p=7.56 × 10−5), CDC42 Signaling (p=8.38 × 10−5), and altered T cell and B cell signaling in rheumatoid arthritis (p=8.66 × 10−5). All of these were enriched due to the four HLA genes, all of which were regulated by the genomic region containing cg04166913. To eliminate the overwhelming effect of the immune-related pathways, we removed the HLA genes and ran the analysis with the 62 remaining genes. Enrichment of the canonical pathways was considerably reduced, although three pathways with at least two genes remained moderately significant (p<0.05). These included Growth Hormone Signaling, Role of BRCA1 in DNA Damage Response, p70S6K signaling, and PI3K Signaling in B Lymphocytes.
Discussion
Acute exposure to pesticides has a number of well-characterized physiological and clinical effects. The effects of chronic, long-term exposure, however, are unknown. As part of the ongoing PACE4 project to study the neurological and cognitive impacts of chronic pesticide exposure, we have examined DNA methylation levels of over 485,000 CpG sites in a subset of 143 individuals (83 farmworkers and 60 non-farmworkers). We identified considerable changes in DNA methylation at 36 CpG sites over the growing season that differed between farmworkers and non-farmworkers. These sites were mapped to the most likely functional genes using publically-available eQTL data, and a number of biological pathways were enriched for these genes. Dominant pathways included immune-related (HLA) processes, as well as a number of diverse biological systems. These data suggest that the farmworker environment leads to a unique epigenetic profile, further analysis of which will determine the potential long-term risks.
Initial pathway analysis was overshadowed by an abundance of immune-related pathways. This was primarily due to the inclusion of cg04166913, which is an eQTL for a number of HLA genes (Table 2). HLA genes are involved in the overall immune response, but more interestingly, HLA-DRB5 methylation was recently shown to be associated with pathological Alzheimer’s Disease (Yu et al., 2015). In addition, HLA-DR antigens, as detected by monoclonal antibodies using flow cytometry on peripheral blood mononuclear cells, were lower in individuals with acute organophosphate pesticide poisoning (Xia et al., 2014). These data provide initial support for a link between pesticide exposure, DNA methylation, and Alzheimer’s Disease.
Removal of the HLA genes was performed for additional pathway analysis to determine if an enrichment of other pathways was present. Four additional pathways were detected: Growth Hormone Signaling, Role of BRCA1 in DNA Damage Response, p70S6K signaling, and PI3K Signaling in B Lymphocytes. These additional pathways do not point to any key processes being affected, but rather indicate the potential diverse effects of DNA methylation differences.
The potential long-term effects of pesticide exposure are likely determined by the interaction between genetic susceptibility and the exposure itself. Much of the work previously performed in this area of research has focused on intrinsic genetic variability (e.g., SNPs), predominately in the paraoxonase 1 (PON1) gene, which encodes an enzyme known to metabolize organophosphorus pesticides (reviewed in Androutsopoulos et al. (Androutsopoulos et al., 2011). We have also previously reported that SNPs in BCHE, which encodes butyrylcholinesterase, were associated with levels of cholinesterase in a separate farmworker population (Howard et al., 2010). Cholinesterase is inhibited by organophosphorus pesticides, and depressed levels can be an indication of pesticide exposure. While these data provide some insight into potential gene-environment interactions, a better marker for a direct genetic effect of exposure can be observed by evaluating epigenetic marks, such as DNA methylation. Towards that end, our data are supportive of an in vitro study that examined the effects of individual pesticides on K562 cells (Zhang et al., 2012). As with our study, an abundance of hypermethylated CpG sites were observed in the cells treated with specific organophosphorus pesticides. In addition, two of the genes hypermethylated after treatment with fonofos, parathion, or turbufos in that study (SLC13A5 and EPB41L1) were also hypermethylated in our study (Table 2). These data support our hypothesis that pesticide exposure, perhaps in addition to other farmworker-specific environmental exposures, contribute to the epigenetic changes we observed.
We identified a change in 36 CpG sites that differed between a Latino farmworker and non-farmworker population. These sites led to 72 genes that may potentially be affected through epigenetic mechanisms. While the focus of the PACE4 study is the effect of pesticides on farmworker health, there are other differences that may have altered the DNA methylation profile between the farmworker and non-farmworker populations. First, the two populations were from different parts of North Carolina. It is possible that different environmental exposures, independent of occupation, led to a difference in methylation levels between the two time points. Second, the unique stresses of living in a migrant farmworker camp may have an impact on DNA methylation. Third, other farmworker-specific exposures besides pesticides (nicotine from tobacco plants, exhaust from machinery, etc.) may have contributed to the differences. While inclusion of smoking into the analysis model did not alter the methylation results in this population, it is possible that other tobacco-related products may contribute. And fourth, the DNA from this study was collected from whole blood samples. As each cell type has a distinct DNA methylation profile, it is possible that variation in cell types between the participants led to a perceived change in methylation.
In conclusion, we have identified changes in DNA methylation between farmworkers and non-farmworkers in a North Carolina Latino population. This is the first study to examine gene-specific, genome-wide DNA methylation in a pesticide-exposed population, and our data support that there is a unique epigenetic profile in farmworkers over the growing season, compared to non-farmworkers. A variety of genes were affected, suggesting that changes in DNA methylation may affect a number of different biological pathways and potential disease processes. Future work should be performed to determine if gene expression changes in these genes correlates with the methylation pattern, and to determine if these changes are transient or more long-standing. Additional investigation is necessary to delineate which specific exposures or behaviors are responsible for the observed changes, and whether or not these changes eventually lead to disease in this population.
Supplementary Material
Acknowledgments
This work was supported by NIEHS grant R01 ES008739.
Footnotes
Competing interests: The authors declare that they have no financial or non-financial competing interests.
Conflict of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Alavanja MC, Hoppin JA, Kamel F. Health effects of chronic pesticide exposure: cancer and neurotoxicity. Annu Rev Public Health. 2004;25:155–197. doi: 10.1146/annurev.publhealth.25.101802.123020. [DOI] [PubMed] [Google Scholar]
- 2.Androutsopoulos VP, Kanavouras K, Tsatsakis AM. Role of paraoxonase 1 (PON1) in organophosphate metabolism: implications in neurodegenerative diseases. Toxicol Appl Pharmacol. 2011;256:418–424. doi: 10.1016/j.taap.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Arcury TA, Nguyen HT, Summers P, Talton JW, Holbrook LC, Walker FO, Chen H, Howard TD, Galvan L, Quandt SA. Lifetime and current pesticide exposure among Latino farmworkers in comparison to other Latino immigrants. Am J Ind Med. 2014;57:776–787. doi: 10.1002/ajim.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arcury TA, Quandt SA. Pesticides at work and at home: exposure of migrant farmworkers. Lancet. 2003;362:2021. doi: 10.1016/S0140-6736(03)15027-1. [DOI] [PubMed] [Google Scholar]
- 5.Arcury TA, Vallejos QM, Marin AJ, Feldman SR, Smith G, Quandt SA. Latino farmworker perceptions of the risk factors for occupational skin disease. Am J Ind Med. 2006;49:434–442. doi: 10.1002/ajim.20311. [DOI] [PubMed] [Google Scholar]
- 6.Barrett RM, Wood MA. Beyond transcription factors: the role of chromatin modifying enzymes in regulating transcription required for memory. Learn Mem. 2008;15:460–467. doi: 10.1101/lm.917508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvert GM, Karnik J, Mehler L, Beckman J, Morrissey B, Sievert J, Barrett R, Lackovic M, Mabee L, Schwartz A, Mitchell Y, Moraga-McHaley S. Acute pesticide poisoning among agricultural workers in the United States, 1998–2005. Am J Ind Med. 2008;51:883–898. doi: 10.1002/ajim.20623. [DOI] [PubMed] [Google Scholar]
- 8.Chin-Chan M, Navarro-Yepes J, Quintanilla-Vega B. Environmental pollutants as risk factors for neurodegenerative disorders: Alzheimer and Parkinson diseases. Front Cell Neurosci. 2015;9:124. doi: 10.3389/fncel.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenske RA, Lu C, Curl CL, Shirai JH, Kissel JC. Biologic monitoring to characterize organophosphorus pesticide exposure among children and workers: an analysis of recent studies in Washington State. Environ Health Perspect. 2005;113:1651–1657. doi: 10.1289/ehp.8022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidotti A, Auta J, Davis JM, Dong E, Gavin DP, Grayson DR, Sharma RP, Smith RC, Tueting P, Zhubi A. Toward the identification of peripheral epigenetic biomarkers of schizophrenia. J Neurogenet. 2014;28:41–52. doi: 10.3109/01677063.2014.892485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoppin JA, Umbach DM, London SJ, Alavanja MC, Sandler DP. Chemical predictors of wheeze among farmer pesticide applicators in the Agricultural Health Study. Am J Respir Crit Care Med. 2002;165:683–689. doi: 10.1164/ajrccm.165.5.2106074. [DOI] [PubMed] [Google Scholar]
- 13.Howard TD, Hsu FC, Grzywacz JG, Chen H, Quandt SA, Vallejos QM, Whalley LE, Cui W, Padilla S, Arcury TA. Evaluation of candidate genes for cholinesterase activity in farmworkers exposed to organophosphorus pesticides: association of single nucleotide polymorphisms in BCHE. Environ Health Perspect. 2010;118:1395–1399. doi: 10.1289/ehp.0901764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamel F, Hoppin JA. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect. 2004;112:950–958. doi: 10.1289/ehp.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KY, Kim DS, Lee SK, Lee IK, Kang JH, Chang YS, Jacobs DR, Steffes M, Lee DH. Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environ Health Perspect. 2010;118:370–374. doi: 10.1289/ehp.0901131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCauley LA, Anger WK, Keifer M, Langley R, Robson MG, Rohlman D. Studying health outcomes in farmworker populations exposed to pesticides. Environ Health Perspect. 2006;114:953–960. doi: 10.1289/ehp.8526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mills PK, Yang R. Prostate cancer risk in California farm workers. J Occup Environ Med. 2003;45:249–258. doi: 10.1097/01.jom.0000058339.05741.0c. [DOI] [PubMed] [Google Scholar]
- 18.Morris TJ, Butcher LM, Feber A, Teschendorff AE, Chakravarthy AR, Wojdacz TK, Beck S. ChAMP: 450k Chip Analysis Methylation Pipeline. Bioinformatics. 2014;30:428–430. doi: 10.1093/bioinformatics/btt684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Na KS, Chang HS, Won E, Han KM, Choi S, Tae WS, Yoon HK, Kim YK, Joe SH, Jung IK, Lee MS, Ham BJ. Association between glucocorticoid receptor methylation and hippocampal subfields in major depressive disorder. PLoS ONE. 2014;9:e85425. doi: 10.1371/journal.pone.0085425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, Cota P, Wittnam JL, Gogol-Doering A, Opitz L, Salinas-Riester G, Dettenhofer M, Kang H, Farinelli L, Chen W, Fischer A. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328:753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- 21.Quandt SA, Hernandez-Valero MA, Grzywacz JG, Hovey JD, Gonzales M, Arcury TA. Workplace, household, and personal predictors of pesticide exposure for farmworkers. Environ Health Perspect. 2006;114:943–952. doi: 10.1289/ehp.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ray DE. Chronic effects of low level exposure to anticholinesterases--a mechanistic review. Toxicol Lett. 1998;102–103:527–533. doi: 10.1016/s0378-4274(98)00260-4. [DOI] [PubMed] [Google Scholar]
- 23.Ray DE, Richards PG. The potential for toxic effects of chronic, low-dose exposure to organophosphates. Toxicol Lett. 2001;120:343–351. doi: 10.1016/s0378-4274(01)00266-1. [DOI] [PubMed] [Google Scholar]
- 24.Reeves M, Schafer KS. Greater risks, fewer rights: U.S. farmworkers and pesticides. Int J Occup Environ Health. 2003;9:30–39. doi: 10.1179/107735203800328858. [DOI] [PubMed] [Google Scholar]
- 25.Shutoh Y, Takeda M, Ohtsuka R, Haishima A, Yamaguchi S, Fujie H, Komatsu Y, Maita K, Harada T. Low dose effects of dichlorodiphenyltrichloroethane (DDT) on gene transcription and DNA methylation in the hypothalamus of young male rats: implication of hormesis-like effects. J Toxicol Sci. 2009;34:469–482. doi: 10.2131/jts.34.469. [DOI] [PubMed] [Google Scholar]
- 26.Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D, Beck S. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics. 2013;29:189–196. doi: 10.1093/bioinformatics/bts680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thaler L, Gauvin L, Joober R, Groleau P, de Guzman R, Ambalavanan A, Israel M, Wilson S, Steiger H. Methylation of BDNF in women with bulimic eating syndromes: Associations with childhood abuse and borderline personality disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014 doi: 10.1016/j.pnpbp.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Walton E, Liu J, Hass J, White T, Scholz M, Roessner V, Gollub R, Calhoun VD, Ehrlich S. MB-COMT promoter DNA methylation is associated with working-memory processing in schizophrenia patients and healthy controls. Epigenetics. 2014;9 doi: 10.4161/epi.29223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Fang Y, Zhang F, Xu M, Zhang J, Yan J, Ju W, Brown WT, Zhong N. Hypermethylation of the enolase gene (ENO2) in autism. Eur J Pediatr. 2014 doi: 10.1007/s00431-014-2311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westra HJ, Peters MJ, Esko T, Yaghootkar H, Schurmann C, Kettunen J, Christiansen MW, Fairfax BP, Schramm K, Powell JE, Zhernakova A, Zhernakova DV, Veldink JH, Van den Berg LH, Karjalainen J, Withoff S, Uitterlinden AG, Hofman A, Rivadeneira F, ‘t Hoen PA, Reinmaa E, Fischer K, Nelis M, Milani L, Melzer D, Ferrucci L, Singleton AB, Hernandez DG, Nalls MA, Homuth G, Nauck M, Radke D, Volker U, Perola M, Salomaa V, Brody J, Suchy-Dicey A, Gharib SA, Enquobahrie DA, Lumley T, Montgomery GW, Makino S, Prokisch H, Herder C, Roden M, Grallert H, Meitinger T, Strauch K, Li Y, Jansen RC, Visscher PM, Knight JC, Psaty BM, Ripatti S, Teumer A, Frayling TM, Metspalu A, van Meurs JB, Franke L. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45:1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia C, Wang M, Liang Q, Yun L, Kang H, Fan L, Wang D, Zhang G. Changes in monoclonal HLA-DR antigen expression in acute organophosphorus pesticide-poisoned patients. Exp Ther Med. 2014;7:137–140. doi: 10.3892/etm.2013.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yehuda R, Flory JD, Bierer LM, Henn-Haase C, Lehrner A, Desarnaud F, Makotkine I, Daskalakis NP, Marmar CR, Meaney MJ. Lower Methylation of Glucocorticoid Receptor Gene Promoter 1 in Peripheral Blood of Veterans with Posttraumatic Stress Disorder. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Yu L, Chibnik LB, Srivastava GP, Pochet N, Yang J, Xu J, Kozubek J, Obholzer N, Leurgans SE, Schneider JA, Meissner A, De Jager PL, Bennett DA. Association of Brain DNA methylation in SORL1, ABCA7, HLA-DRB5, SLC24A4, and BIN1 with pathological diagnosis of Alzheimer disease. JAMA Neurol. 2015;72:15–24. doi: 10.1001/jamaneurol.2014.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Wallace AD, Du P, Kibbe WA, Jafari N, Xie H, Lin S, Baccarelli A, Soares MB, Hou L. DNA methylation alterations in response to pesticide exposure in vitro. Environ Mol Mutagen. 2012;53:542–549. doi: 10.1002/em.21718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.